Abstract
A bioassay study was conducted to determine the differences in the sensitivity of selected crops to simulated clomazone residues (nine concentrations were used ranging from 5.625 to 1440 μg a.i./kg soil). White mustard was the most susceptible as measured by shoot fresh weight (SFW) and shoot dry weight (SDW) inhibition, with EC50 values of 94.6 and 128.2 μg a.i./kg soil, respectively. Regarding the EC50 values for the inhibition of pigment content (carotenoids, chlorophyll a and chlorophyll b), sugar beet and white mustard showed a high sensitivity, as the EC50 values for all three pigments were in the range of 45.8–47.4 and 57.5–63.3 μg a.i./kg soil, respectively. However, as the SFW and SDW of sugar beet were only reduced at the three highest clomazone concentrations applied, white mustard proved to be the most sensitive crop. Wheat was less sensitive (EC50 = 214.4–243.8 μg a.i./kg soil, for all three pigments), while sunflower and maize were the least sensitive (EC50 = 359.7–417.5 and 456.1–535.8 μg a.i./kg soil, respectively). Field trials were conducted for two years in the Srem region to study the dynamics of clomazone degradation in sandy loam soil. Clomazone was applied pre-plant incorporated (PPI) and post-emergence (POST-EM) in three doses: 480, 720 and 960 g a.i./ha. Soil samples were taken at regular intervals from the day of herbicide application until one year after application and residue concentrations were determined using the white mustard bioassay (based on the measurement of carotenoid content inhibition). The application rate had no consistent effect on the persistence of clomazone. Slower degradation was observed in the PPI treatment than in the POST-EM treatment (8.5 and 15 days longer average half-lives in the first and second year, respectively). Persistence was affected by lower rainfall, resulting in a longer half-life in the second year (12 days on average). Herbicide residues caused no visible injury to white mustard one year after application, while the reduction in carotenoid content ranged from 0.37 to 22.89%, indicating that no injury can occur to any of the tested crops one year after application of clomazone in sandy loam soil.
1. Introduction
Clomazone 2-[(2-chlorophenyl) methyl]-4,4-dimethyl-3-isoxazolidinone is a selective herbicide of the isoxazolidinone group (HRAC F4/13) commonly used to control broadleaf and annual grass weeds in various crops (soybeans, tobacco, cotton, peas, beans, rapeseed, rice, sugarcane, poppy, watermelon and several vegetable crops, including pumpkins, peppers and cabbage) [1,2,3,4,5,6,7,8]. It is also effective against important weed species that compete strongly with crops, such as Abutilon theophrasti Medic, Ambrosia artemisiifolia L., Chenopodium album L., Echinochloa cruss-galli L. and Digitaria spp. At higher application rates, it controls Datura stramonium L. and Amaranthus retroflexus L. and suppresses Amaranthus hybridus L. and Xanthium strumarium L. [9,10,11,12,13]. Clomazone is usually applied (depending on the crop) before sowing/planting (pre-plant incorporated, PPI), before the plants emerge (pre-emergence, PRE-EM) or after the plants have emerged (post-emergence, POST-EM). The plants absorb it via the roots and leaves and translocate it to the plastids (the site of action). Inhibition of carotenoid biosynthesis occurs in the methylerythritol 4-phosphate (MEP) biosynthetic pathway by inhibiting the enzyme deoxyxylulose 5-phosphate synthase (DXS). This leads to chlorosis and bleaching of the new leaves and ultimately to the death of the plant [14].
The physico-chemical properties of clomazone determine its fate and behavior in the soil and in the environment. They show that this herbicide has a relatively high water solubility (1102 mg/L) and moderate evaporation (Vp: 19.2 mPa) and yet is not rapidly degraded by processes such as photolysis or hydrolysis in a wide pH range. Its properties such as moderate mobility in soil (Koc: 150–562 mL/g) and persistence (DT50: 30–135 days) indicate a potentially harmful effect on plants in crop rotation, which emphasizes its agricultural importance [15]. It is a non-ionic herbicide with moderate soil movement potential, which additionally depends on the soil type, application method, presence of ground cover and environmental conditions [16]. The persistence of clomazone is influenced by the adsorption intensity [17], which correlates strongly with the soil organic matter content, to a lesser extent with the clay content and negatively with the cation exchange capacity (CEC) [18,19,20,21,22,23,24,25]. The bioavailability of its residues in the soil is strongly influenced by desorption hysteresis [24,26], which increases the risk of injury to subsequent crops in the rotation, especially in fine-textured soils, in dry years and in short rotation periods [27,28,29]. Persistence may also depend on the time and type of application (vapor loss was observed in PRE-EM but not in PPI treatments) [28,30,31,32,33,34] and on the activity of soil microorganisms [35,36,37]. Due to its moderate to long persistence, residues of clomazone were found in soils in different amounts and at different depths: 1–218 μg/kg soil (with an average residue concentration of 56 μg/kg soil) [38] or in 5.5% of soil samples with an average concentration of 8 μg/kg soil [29]. In the province of Vojvodina, significant concentrations of clomazone were detected when monitoring the occurrence of the most commonly used herbicides (in soils with intensive crop cultivation), which was attributed to the application practice (i.e., PRE-EM or PPI). Clomazone was found in 20–50% of soil samples at 0–30 cm and 30–60 cm soil depths, with an average concentration of 11.856 ± 6.213 and 4.763 ± 2.176 mg/kg soil in 2013 and also in 2023 with concentrations of 2.672 ± 5.708 and 0.983 ± 1.260 mg/kg soil, respectively [39].
Soon after the introduction of clomazone, the first reports of injuries to subsequent crops due to carryover were documented. Most studies focused on the detection of injury in wheat, with results varying widely over many years [11,19,27,28]. However, no reduction in wheat yield was observed in any case [1,40,41]. Studies on the effects of clomazone residues on maize as a subsequent crop in the rotation also gave contradictory results: no injury [32,40,42] to slight injury (visual and mostly in the early stages of development) but no yield loss [27,33,34,43,44] and extremely severe injury if the maize was sown within a short time after clomazone application [45]. Renner and Powell [46] investigated the effects of clomazone residues on sugar beet, and found injury in the form of chlorosis without yield reduction. In alfalfa [27,47], as well as in cotton and sorghum [43,44,48], different results were obtained regarding the injury caused by residues. Miller [1] investigated the effects of clomazone carryover on different crops grown in the fall of the same year or in the spring of the following year. No harmful effects of possible residues were observed in the fall crops, while some injury was observed in the spring crops, but this was not statistically significant.
In the available literature, plant responses to clomazone carryover were mainly assessed visually based on the intensity or percentage of chlorosis on leaves or by measuring morphological parameters (fresh weight of shoots and roots) [18,20,34,49,50]. This resulted in a lack of data on EC50 values, which are required to compare and categorize different plant species in terms of their sensitivity to clomazone. The new studies have emphasized the importance of physiological parameters such as the content of carotenoids, chlorophyl a and chlorophyl b when using herbicides that inhibit carotenoid biosynthesis [51,52,53]. It is well justified, as the highest bioassay sensitivity is achieved when the selected measurement parameter is directly related to the mode of action of the herbicide in question.
The Srem region in the province of Vojvodina is known for intensive agriculture, where herbicides are unavoidable. The most widespread and most frequently cultivated crop is maize, followed by soybeans, wheat, sunflowers and sugar beet. The most common crop rotation consists of maize and soybeans, but it is also common for all other crops to be sown after the soybeans. Although there is indication of crop injury in the Srem region, there are no data on the persistence of clomazone and the susceptibility of crops depending on the prevailing soil type. As residues of clomazone have been found in some parts of Vojvodina [39] and farmers are concerned about safe production, the trials were conducted to (1) investigate the susceptibility of selected crops (maize, wheat, sunflower, sugar beet and white mustard) to clomazone by bioassay (white mustard was used as the test crop as it was assumed to be highly susceptible and therefore suitable for the next objective of this study); (2) investigate the influence of weather conditions, application type and rate on the persistence of clomazone in sandy loam soil (as the predominant soil type) with a bioassay using white mustard as the test crop; (3) compare the residue data with the susceptibility data of sugar beet, wheat, maize and sunflower and estimate the injury potential for these crops when sown in sandy loam soil one year after clomazone application.
2. Materials and Methods
2.1. Plant Material, Soil and Herbicide
The following plant species/varieties were used for all bioassays performed under controlled conditions: maize (Zea mays L. var. NS 444), sunflower (Helianthus annuus L. var. Bačvanin), sugar beet (Beta vulgaris ssp. saccharifera var. Lara), wheat (Triticum aestivum L. var. Venera) and white mustard (Sinapis alba L. var. NS bela). White mustard was used as a model test plant, as it was assumed to have a higher sensitivity than other plant species. The seeds were obtained from the Scientific Institute of Field and Vegetable Crops in Novi Sad.
The soil for the bioassays was collected in Sremska Mitrovica (45°00′06.6″ N; 19°37′50.3″ E; altitude: 96 ± 5 m) from a field where no herbicides had been applied before. The soil was a sandy loam with the following properties: pH 7.43, 50.76% sand, 38.2% silt, 11.04% clay and 2.7% organic matter. The soil was air dried, passed through a 3 mm sieve and divided into 600 g portions (one portion representing one replication for each herbicide concentration used).
A commercial formulation of clomazone (Gamit 480 EC, with 480 g active ingredient (a.i.)/L, FMC International, Switzerland) was used, and standard solutions of the herbicide in aqueous solutions were prepared. The application rates of clomazone were established at 0, 5.625, 11.25, 22.5, 45, 90, 180, 360, 720 and 1440 µg a.i./kg soil.
2.2. Bioassay Under Controlled Conditions
Each sample of 600 g of soil was treated with 6 mL of an appropriately diluted herbicide solution for each test plant and herbicide dose. A thin-layer chromatography sprinkler connected to a compressor delivered the herbicide solution at a constant pressure of 120 kPa. Immediately after application of the herbicide, each soil sample was mixed manually and then placed in a rotating mixer where it was mixed for a further 7 min at a speed of 60 rpm. After homogenization, the soil was divided into three 200 mL plastic pots (dimensions: h = 9 cm, d(bottom) = 7 cm, d(top) = 5 cm) and sown with the seeds of the tested plant species. For maize and sunflower, there were three seeds per pot; for sugar beet and wheat, five seeds per pot; and for white mustard, seven seeds per pot. After sowing, the soil moisture was brought to field capacity (24% vol) and then the pots were placed in a growth chamber where the plants were grown for 14 days at 25 °C/18 °C (day/night) with a 16 h photoperiod of 300 μE/m2s. During this time, pots were irrigated daily up to 70% of field capacity by adding water up to a predetermined weight. Plants of each species grown in untreated soil served as controls. Treatments were arranged in a completely randomized block design with four replications and the entire experiment was repeated twice for each plant species. After 14 days plants were cut at soil level to measure morphological and physiological parameters. The shoot fresh weight (SFW) was measured. Leaf sections with a diameter of 5 mm and a total weight of 0.1 g per treatment (required for the determination of the pigment content) were taken from the intact leaves. The shoots were then dried at room temperature and subsequently in a drying oven at 105 °C so that the shoot dry weight (SDW) could be measured.
The pigment content was determined using the dimethylformamide (DMF) extraction method [54]. Leaf pieces with a diameter of 5 mm up to a total weight of 0.1 g were taken from intact leaves of plants grown in clomazone-treated soil (for each replication of each clomazone concentration used) and placed in vials containing 3 mL DMF each. Pigment extraction was performed for 24 h in the dark at a temperature of up to 4 °C. After extraction, the absorbance of the extract was measured using a spectrophotometer Camspec M107 (Spectronic Camspec Ltd., Leeds, UK; range 325–1000 nm) at specific wavelengths: for chlorophyll a at 664 nm, for chlorophyll b at 647 nm and for carotenoids at 480 nm. Wellburn’s formulas [54] were used to calculate the pigment concentration in μg/mL as follows:
Chlorophyll a: ca = 11.65A664 − 2.69A647
Chlorophyll b: cb = 20.81A647 − 4.53A664
Carotenoids: cc = (1000A480 − 0.89 ca − 52.02 cb)/245
The pigment concentration in mg/g fresh mass was then recalculated using the following equation (Equation (1)):
where C is the pigment concentration (mg/g); c is the pigment concentration (μg/mL); V is the total volume of the extract; R is the dilution factor (in cases where the extract was diluted); m is the mass of the fresh material (g); 1000 is the factor for converting μg to mg.
2.3. Field Trials: Clomazone Degradation and Residue Levels in the Soil
The field trials were conducted in two separate years, 2015/2016 and 2017/2018, to determine the dynamics of clomazone degradation from the time of application to the beginning of the next growing season (one year after application). The trials were conducted in Sremska Mitrovica. The experimental field was located at 45°00′13.2″ N; 19°38′16.8″ E; and altitude: 96 ± 5 m. The soil was sandy loam with the following properties: pH 7.69, 53.48% sand, 38% silt, 8.52% clay and 2.47% organic matter (very similar to the soil used for the bioassays).
In both years of this study, the experimental field was sown with “Wendy” soybeans after herbicide application (PPI treatments) on 9 April 2015 and 23 April 2017 at a depth of 4 cm, with a row spacing of 50 cm between the rows and 4 cm in the rows. In the intervening year, which was deliberately omitted to ensure that no clomazone residues were present in the second year of this study, the maize hybrid DKC 5031 was sown and cultivated in accordance with good agricultural practice. Dicamba and nicosulfuron were used as herbicides (to ensure good weed control with different modes of action compared with clomazone).
Clomazone was applied as a pre-plant incorporated (PPI) and post-emergence (POST-EM) treatment. For each application method, three herbicide doses were applied in four randomized replications. The plots were 7.5 by 7.5 m in size (Figure 1). The PPI treatments were incorporated into the soil to a depth of 5 cm immediately after application using a rolling cultivator. The POST-EM treatments were applied when the soybean was in the third trifoliate stage. The application rates were 480, 720 and 960 g a.i./ha, corresponding to 1, 1.5 and 2 L/ha of the product Gamit 4-EC. The first two doses are the highest recommended depending on the soil properties, while the third is the double recommended dose, which is the most commonly used in soybeans and represents the case of overlap. The application was carried out using a hand-held CO2 sprayer with flat fan nozzles (XR Tee Jet 11002), which delivered a spray volume of 240 L/ha. The plots were kept weed-free throughout the growing season by hand hoeing.
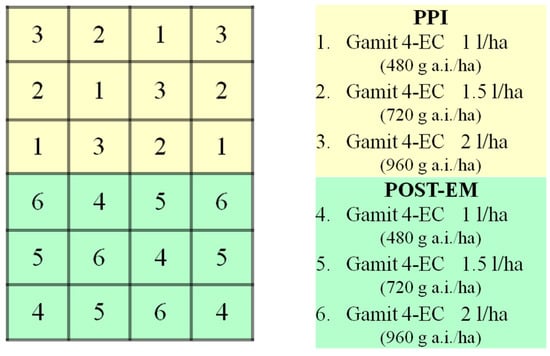
Figure 1.
Schematic representation of the field trial design for clomazone degradation dynamics. There were two application types (PPI—pre-plant incorporated, POST-EM—post-emergence) and three application rates (480, 720 and 960 g a.i./ha) within each of these types.
Soil samples were taken from the top soil layer (0–7.5 cm depth) immediately after application and at predetermined intervals up to one year after application (Table 1). Eight random soil cores of 5 cm diameter from each replication (for each treatment) were combined, and after sieving, the soil was divided into 200 mL pots and sown with white mustard (the same variety as in the bioassay above), watered and transferred to the growth chamber under the conditions described above. For each treatment, there were four replications at each sampling date. For each sampling date, there was also a control (pots with untreated soil sown with white mustard). After 14 days, the plants were removed intact and leaf sections were taken for further analysis to determine the pigment content, which was used to recalculate the level of bioavailable clomazone residues in the soil.

Table 1.
Soil sampling dates.
The weather conditions (i.e., the sum of precipitation as the most important factor) for both years and the average of the 50-year period are shown in Table 2.

Table 2.
Monthly and quarterly average precipitation levels in Sremska Mitrovica.
2.4. Statistical Analyses
All data were subjected to ANOVA using IBM SPSS software (Statistical Package for the Social Sciences, Version 25). The significance of the differences between the treatments and the control was determined using Tukey’s test at a 95% significance level (p < 0.05). Data on shoot fresh weight and shoot dry weight (where applicable) and pigment content (for all plant species tested) were converted to the percentage of inhibition compared to the control treatment. Two data sets (as each bioassay was repeated twice) were pooled and subjected to non-linear regression analysis to calculate EC50, EC20 and EC10. The log-logistic model with four parameters was used (Equation (2)):
where Y is the response (i.e., the inhibition of the measured parameter) of the test plant as a function of the herbicide dose X; C is the lower limit of the plant response (lower asymptote); D is the upper limit of plant response (upper asymptote); b is the proportional slope of the curve around ED50 (the inflexion point); and E is the herbicide dose required to obtain half of the plant response between the upper and lower limits, i.e., ED50. All statistical analysis and graphs were performed in R Software Program (version 3.1.1.) using the dose–response curve (drc) statistic package [55].
First order kinetics (integrated form) was used to determine the dissipation kinetic of clomazone and to calculate the half-lives (Equation (3)):
where C is the herbicide concentration in time t; C0 is the herbicide concentration at time of application; t is time in days; and k is the first order constant or the rate constant. The first-order rate constant was estimated by regression analysis by plotting ln(C/C0) as a function of time. The slope of the resulting line was considered the first-order rate constant. The degradation half-lives of clomazone were calculated using the following equation:
where DT50 is the half-life in days; ln is the natural logarithm; and k is the first-order dissipation rate constant.
DT50 = ln2/k = 0.693/k
3. Results
3.1. Effect of Clomazone on Morphological Parameters
The application of clomazone at the concentrations tested (5.625–1440 μg a.s./kg soil) did not result in a corresponding inhibition of the shoot fresh and/or dry weight in most of the plant species tested.
No inhibitory effect was observed in maize plants at concentrations of 5.625–180 μg a.i./kg soil. At concentrations of 360–1440 μg a.i./kg soil, the inhibition of shoot fresh weight (SFW) was in the range of 3.21–4.73% and shoot dry weight (SDW) in the range of 2.70–20.04%.
In sunflower, the inhibition of SFW was below 10% at concentrations of 5.625–720 μg a.i./kg soil, and at the highest concentration tested, an inhibition of 29.99% was observed. The shoot dry weight was higher in most treatments than in the control, i.e., an inhibition of 10.11% was only achieved at the highest concentration.
In wheat plants, an inhibition of less than 10% was observed at concentrations of 5.625–180 μg a.i./kg soil, while at concentrations of 360–1440 μg a.i./kg soil, inhibition was in the range of 16.27–45.88% for SFW and 20.98–40.30% for SDW.
In sugar beet, no inhibitory effect was observed for treatments with 5.625–180 μg a.i./kg soil. At the three highest concentrations tested, inhibition was consistently higher in both shoot fresh weight (20.87–47.76%) and shoot dry weight (14.39–40.34%).
Although no inhibitory effect on white mustard plants (especially with respect to SDW) was observed at clomazone concentrations of 5.625–22.5 μg a.i./kg soil, an increasing inhibitory effect was observed for both SFW (18.44–85.21%) and SDW (2.31–80.15%) at herbicide concentrations in the range of 45–1440 μg a.i./kg soil (Figure 2a). Statistically significant differences were found between concentrations of 45 μg a.i./kg soil and higher and the control for SFW (F = 146.79, p < 0.05); and between concentrations of 90 μg a.i./kg soil and higher and the control for SDW (F = 57.1, p < 0.05). Based on the results obtained, a greater sensitivity of the fresh weight of the shoots was observed.
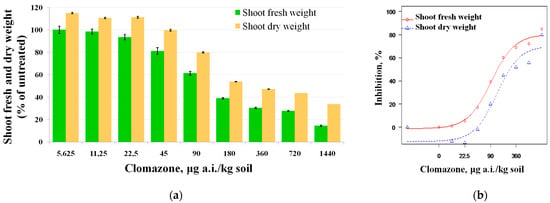
Figure 2.
(a) Effect of clomazone on shoot fresh weight and shoot dry weight of white mustard. Data are expressed as percentage relative to control (±SE); (b) dose–response curves for the sensitivity of white mustard to clomazone. The regression lines were plotted using Equation (2) and the parameter values are shown in Table 3.
Since the measured morphological parameters did not show sufficient sensitivity and the inhibition of these parameters did not increase with increasing clomazone concentration (except for white mustard), the results obtained were not suitable for further regression analysis and are therefore not graphically presented.
A non-linear regression (Equation (2)) was used to describe the dependence of the inhibition of SFW and SDW of white mustard on clomazone concentration (Figure 2b). The EC50, EC20 and EC10 values of SFW and SDW, as well as the coefficients of the regression equation for the measured parameters, are shown in Table 3.

Table 3.
Regression parameters (Equation (2)) and clomazone concentrations (μg a.i./kg soil) that caused 50%, 20% and 10% inhibition [EC50 (±SE), EC20 (±SE) and EC10 (±SE)] of shoot fresh and dry weight for white mustard.
3.2. Effect of Clomazone on Pigment Content
The application of clomazone at concentrations of 5.625–1440 μg a.i./kg soil led to a corresponding inhibition of carotenoids, chlorophyll a and chlorophyll b content in most of the plant species tested.
In maize plants, the lowest concentration of clomazone applied did not lead to a decrease in any pigment content, nor did the next concentration of 11.25 μg a.i./kg soil for chlorophylls a and b. For the clomazone concentration range of 22.5–90 μg a.i./kg soil, an inhibition of the pigment content of less than 10% was observed, while consistently higher effects were only achieved at concentrations of 180–1440 μg a.i./kg soil (20.13–90.98% for carotenoids, 22.58–96.47% for chlorophyll a and 20.32–95.42% for chlorophyll b). Statistically significant differences were observed between concentrations of 180 μg a.i./kg soil and higher and the control for both replications of the experiment and all parameters: for carotenoid content F = 108.025 and F = 110.503, p < 0.05; for chlorophyll a F = 128.517 and F = 140.038, p < 0.05; and chlorophyll b F = 92.591 and F = 120.044, p < 0.05 (two F-values for each pigment refer to two separate bioassays).
Application of the lowest clomazone concentration did not inhibit the content of carotenoids and chlorophyll b in sunflower, while the content of chlorophyll a was reduced by only 2.88%. The next higher concentration led to a decrease in the content of carotenoids (11.52%) and chlorophyll a (15.25%), but not in chlorophyll b. However, only the application of clomazone at concentrations of 22.5–1440 μg a.i/kg soil led to a gradually increasing inhibition of all three parameters: carotenoids from 11.52 to 86.50%, chlorophyll a from 15.25 to 89.02% and chlorophyll b from 16.97 to 87.77%. Statistically significant differences were observed between concentrations of 22.5 μg a.i./kg soil and higher and the control for both replications of the experiment and all parameters: for carotenoids F = 156.52 and F = 177.378, p < 0.05; for chlorophyll a F = 206.312 and F = 163.787, p < 0.05; and for chlorophyll b F = 95.531 and F = 154.655, p < 0.05.
In wheat plants, clomazone concentrations of 5.625–22.5 μg a.i./kg soil caused an inhibition of less than 10%. With increasing herbicide concentration, i.e., in the range of 45–1440 μg a.i./kg soil, an increase in inhibition of pigment content was observed: carotenoids from 15.96 to 88.86%, chlorophyll a from 15.64 to 94.66% and chlorophyll b from 11.18 to 94.99%. Statistically significant differences were found between concentrations of 90 μg a.i./kg soil and higher and the control, for both replications of the experiment and all parameters: for carotenoids F = 56.845 and F = 427.164, p < 0.05; for chlorophyll a F = 55.473 and F = 538.929, p < 0.05; and chlorophyll b F = 56.392 and F = 381.585, p < 0.05.
In sugar beet, the two lowest concentrations had no effect on the content of carotenoids and chlorophyll a, while the inhibition of chlorophyll b was 11.88%. With increasing clomazone concentration in the range of 22.5–1440 μg a.i./kg soil, the inhibitory effect increased: for carotenoids from 31.11 to 92.56%, for chlorophyll a from 33.17 to 98.76% and chlorophyll b from 34.49 to 98.47%. Statistically significant differences were found between concentrations of 22.5 μg a.i./kg soil and higher and the control for both replications of the experiment and all measured parameters: for carotenoids F = 183.076 and F = 118.562, p < 0.05; for chlorophyll a F = 167.998 and F = 164.663, p < 0.05; and for chlorophyll b F = 163.409 and F = 161.026, p < 0.05.
No inhibitory effect on the pigment content of white mustard was observed at the two lowest clomazone concentrations tested. Only at higher concentrations (22.5–1440 μg a.i./kg soil) was an increasing inhibitory effect on carotenoids (21.87–89.87%), chlorophyll a (29.87–97.78%) and chlorophyll b (30.36–98.85%) observed. Statistically significant differences were found between concentrations of 22.5 μg a.i./kg soil and higher and the control for both replications of the experiment and all three measured parameters: for carotenoids F = 144.907 and F = 205.667, p < 0.05; for chlorophyll a F = 157.442 and F = 311.958, p < 0.05; and for chlorophyll b F = 148.496 and F = 317.578, p < 0.05.
A non-linear regression (Equation (2)) was used to describe the dependence of the changes in pigment content on the clomazone concentration (Figure 3). The EC50 values were calculated as indicators of the sensitivity of the plants tested, the scientific significance of which lies in the comparisons between different plant species. The EC10 and EC20 values are indicators of concentrations that have no statistically significant effects. The EC10 value is considered a NOEL (no observable effect level), i.e., a herbicide residue level that is safe for future sowings [56,57,58], while the EC20 value has been proposed as an ARL (acceptable residue level) [59] or herbicide residue level that could cause an effect that does not necessarily significantly affect the further development of the plant. These values for all pigments and the coefficients of the regression equation for the measured parameters are listed in Table 4.
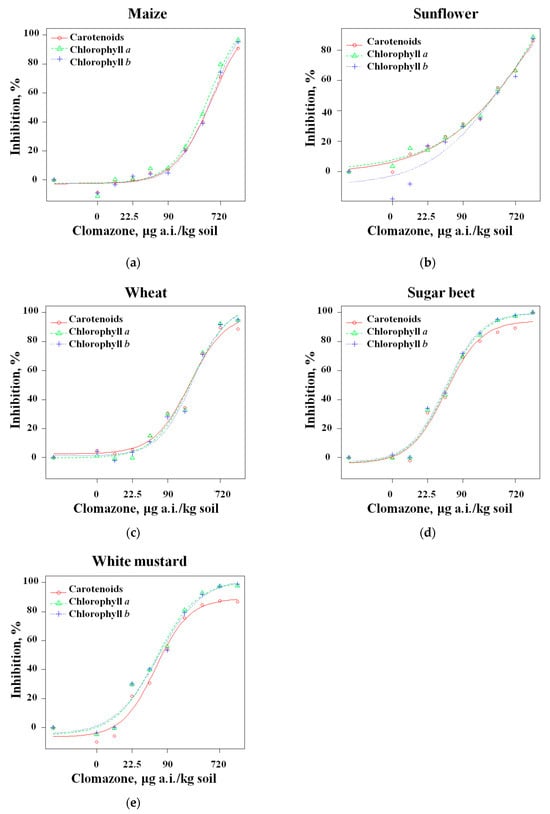
Figure 3.
Dose–response curves for crop sensitivity to clomazone ((a) maize, (b) sunflower, (c) wheat, (d) sugar beet, (e) white mustard). The regression lines were plotted using Equation (2) and the parameter values are shown in Table 4.

Table 4.
Regression parameters (Equation (2)) and clomazone doses (μg a.i./kg soil) that caused 50%, 20% and 10% inhibition [EC50 (±SE), EC20 (±SE) and EC10 (±SE)] of all pigments for all crops tested.
Of the species tested here, sugar beet and white mustard showed the highest susceptibility, in that order, with EC50 values of 45.8–47.4 and 57.5–63.3 μg a.i./kg soil, respectively (for all three pigments). These crops also had very similar EC20 and EC10 values. In comparison, wheat showed a lower susceptibility, with EC50 values of 214.4–243.8 μg a.i./kg soil for all three pigments (which is about five times the EC50 for sugar beet and white mustard). Sunflower and maize showed even lower susceptibility (i.e., their EC50 values were 359.7–417.5 and 456.1–535.8 μg a.i./kg soil for all three pigments (or about ten times the values determined for the most susceptible plant species)).
3.3. Clomazone Degradation Dynamics and Residue Levels in the Soil
The dynamics of clomazone degradation were determined using the white mustard bioassay, which measured the decreasing inhibition of carotenoid content due to reduced clomazone residues in the soil (Figure 4 and Figure 5). The degradation of clomazone followed first-order kinetics in both experimental years and for all treatments (Figure 6 and Figure 7), which was confirmed by R2 values of 0.96 (p = 0.05) or higher for all PPI treatments and 0.67–0.96 (p = 0.05) for POST-EM treatments (Table 5).
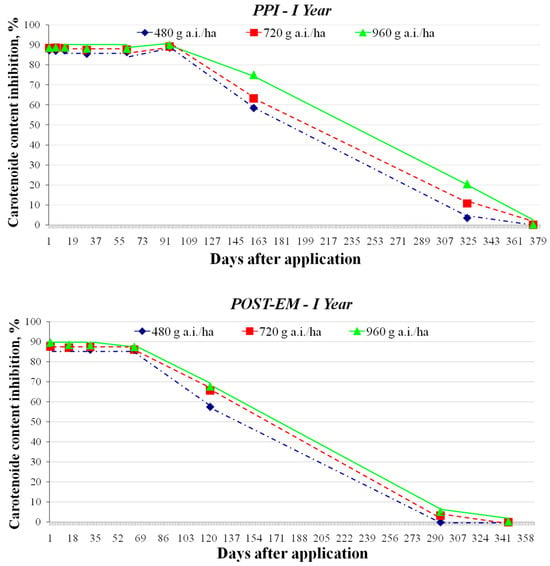
Figure 4.
Effect of clomazone residues from the top 7.5 cm of sandy loam soil on the inhibition of carotenoid content of white mustard (sampling at specific intervals after application in year I of the study, with PPI treatments shown at the top and POST-EM treatments at the bottom).
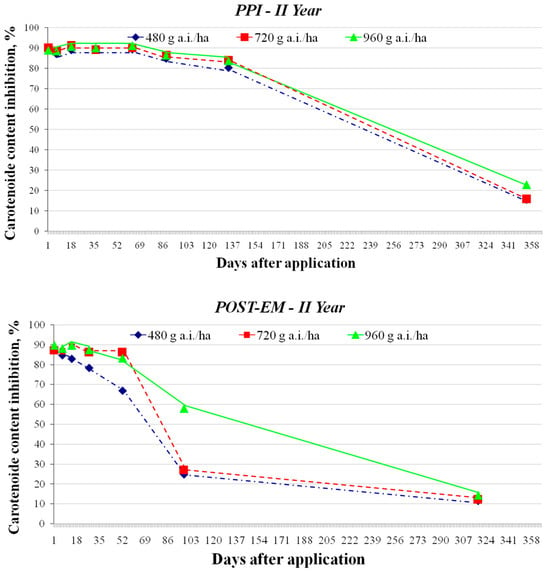
Figure 5.
Effect of clomazone residues from the top 7.5 cm of sandy loam soil on the inhibition of carotenoid content of white mustard (sampling at specific intervals after application in year II of the study, with PPI treatments shown at the top and POST-EM treatments at the bottom).
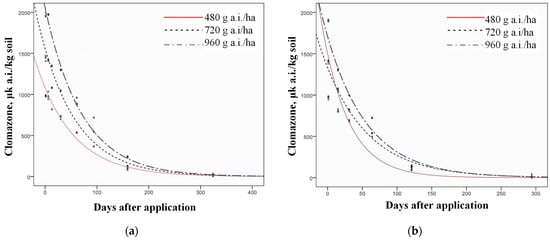
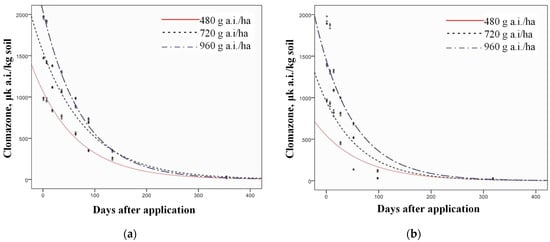

Table 5.
First-order kinetics and R2 for all treatments in both years of examination.
The half-lives of clomazone were calculated and are listed in Table 6. Clomazone residues decreased significantly with elapsed time in all treatments, as shown by both the inhibition of carotenoid content and the estimated clomazone concentration (Table 7).

Table 6.
Clomazone half-life values and averages for different rate and type of application and years of examination.

Table 7.
The effect of clomazone residues on white mustard carotenoid content inhibition and clomazone concentrations (μg a.i./kg) one year after application.
4. Discussion
4.1. Effect of Clomazone on Shoot Fresh and Dry Weight
This study has shown that in sunflower, maize, wheat and even sugar beet there is no change/increase in the inhibition of fresh weight and/or dry weight of shoots; so, these crops can be categorized as less sensitive to clomazone. In these plant species, inhibition of SFW and SDW was observed either only for the highest applied concentration (1440 μg a.i./kg soil in sunflower) or for the last two or three concentrations in a range of applied concentrations (720 and 1440 μg a.i./kg soil in maize and 360–1440 μg a.i./kg soil in wheat and sugar beet). These results are well supported by the definition of the sensitive test plant, which should be sensitive to small amounts of herbicide, with the sensitivity gradually increasing with an increasing concentration of the compound and the response being clear, obvious and measurable [60]. In addition, Gallandt et al. [18] showed that the shoot dry weight response to clomazone (concentration range of 0–1600 μg a.i./kg soil) using sunflower, radish and maize as test plants showed no regression dependence between the observed effect and herbicide concentration. This is consistent with the results of the present study, i.e., there is no possibility of regression modeling of the response (SFW or SDW) of maize, sunflower, wheat and sugar beet to the given concentration range of clomazone. Loux et al. [19] found the same growth inhibition in roots and shoots of wheat for the clomazone concentration range of 0–1200 μg a.i./kg soil, but no dependence on herbicide concentration was found, so these parameters could not be used to develop a suitable bioassay. The opinion that sunflower, maize, wheat and sugar beet can be characterized as less sensitive plants to clomazone on the basis of the measured morphological parameters alone is also confirmed by the fact that these parameters were actually used to determine the tolerance of the plants to clomazone. Indeed, Weston and Barrett [61] have clearly shown that higher concentrations are required when morphological parameters are used in less sensitive plant species, i.e., above the level of manifestation of visible symptoms.
Of all the plant species tested, only white mustard showed a change in the measured parameters, when the clomazone concentration was increased in the range of 45–1440 μg a.i./kg soil. The EC50 values determined (94.6 and 128.2 μg a.i./kg soil for SFW and SDW, respectively) are well below the clomazone concentration that caused a visible decrease in SFW/SDW in the other plant species tested (360 μg a.i./kg soil or higher), so it can be said with certainty that white mustard is the most sensitive of all the species tested.
The sensitivity of the different plant species to herbicides is determined by their genetic characteristics (the genome determines the morphological, physiological and biochemical characteristics of the plants). However, it is not clear whether the differences in sensitivity are due to micromorphological characteristics, as in some weeds [62], subtle differences in physiology, gene expression or other factors, making this topic an interesting opportunity for further investigation.
4.2. Effect of Clomazone on Pigment Content (Carotenoids, Chlorophyll a and Chlorophyll b)
The results obtained show that the EC values for the content of carotenoids, chlorophyll a and chlorophyll b do not differ significantly from each other in any of the plant species tested; thus, susceptibility tests and comparisons of plants can be carried out on the basis of one of these parameters. This was to be expected considering that clomazone inhibits the synthesis of the common precursor of all three pigments [14]. Therefore, it can be concluded that all further susceptibility tests can be performed with only one of these pigments, and, considering the classification of herbicides according to their mode of action, this would be the inhibition of carotenoid content.
The results obtained in this study show a clear and pronounced difference in the sensitivity of the tested plant species, according to which sugar beet and white mustard can be categorized as very sensitive (with EC50 values for all three pigments of 45.8–47.4 and 57.5–63.3 μg a.i/kg soil, respectively), wheat as moderately sensitive (EC50 = 214.4–243.8 μg a.i/kg soil) and maize and sunflower as less sensitive (with EC50 values of 456.1–535.8 and 359.7–409.1 μg a.i/kg soil, respectively) to clomazone.
For the less sensitive plant species, there are no data from bioassays on their susceptibility and therefore no EC50 values. The only confirmation for the categorization of sunflower as a less sensitive plant is the fact that no damage has been observed on this plant so far. The data for maize are numerous and clearly conclude that visible injury (in the form of chlorosis) can occur early in the vegetation without affecting yield [27,32,33,34,42,43,44], or when maize is sown in a very short period after clomazone application [45]. These findings could support the results obtained here on the low sensitivity of sunflower and maize to clomazone.
In the available scientific literature, there are EC50 values for wheat, but only for the assessment of injury (chlorosis and leaf bleaching). Loux et al. [19] reported values of 180 and 600 μg a.i/kg soil determined for different soil types (with 1.3 and 5.8% organic matter content, respectively). Gallandt et al. [18] graphically illustrated the dependence of leaf chlorosis in wheat on increasing clomazone concentration for two soil types and showed that the EC50 values were about 480 μg a.i./kg soil and about 520 μg a.i./kg soil (for soils with an organic matter content of 1.6 and 2.3%, respectively). These data are comparable with the value determined here, as the EC50 value for the carotenoids (214 μg a.i./kg soil) is within the range of the data given. In addition, a statistically significant difference in the inhibition of pigment content in wheat in the present study occurs at a concentration of 90 μg a.i/kg soil, which is consistent with the detection limit of 80 μg a.i/kg soil in a bioassay with visual assessment of wheat leaf bleaching [34]. The differences between these data are most likely due to different soil types and different wheat varieties used, which, on the other hand, could be seen as confirmation of the validity of the results obtained here. The conclusion that wheat is only moderately sensitive to clomazone could also be supported by the results of numerous field trials in which no damage to wheat plants grown in a rotation after soybean [1,40,41] or no effects on yield [20,27,28] were observed. However, it should be taken into account that many researchers emphasize that different results regarding the sensitivity of wheat to clomazone are obtained from soils with different organic matter content, which has a direct impact on the bioavailability of clomazone. In addition, plants that develop deep taproots generally have fewer problems with clomazone residues [49]. The root of wheat is located in shallow soil layers that could also contain clomazone residues (due to its low mobility and the fact that clomazone movement below 10 cm under field conditions is negligible [34,42]), which, in combination with a certain soil type and certain meteorological conditions, can lead to significantly greater injury than expected.
As no data on EC50 values are available for sugar beet, its sensitivity can only be confirmed by studies on possible carry-over effects of clomazone. Renner and Powell [46] observed visual damage from clomazone residues, but this did not affect yield. In sugar beet, there was a weak dependence regarding the inhibition of morphological parameters (inhibition of SFW and SDW at clomazone concentrations > 360 μg a.i./kg soil), but obviously a higher sensitivity regarding the inhibition of pigment content (statistically significant differences in the inhibition of pigment content were observed for clomazone concentrations ≥ 22.5 μg a.i./kg soil). The fact that there is a clear and pronounced difference between physiological and morphological parameters shows that sugar beet cannot be classified as very sensitive. On the basis of physiological parameters, it is a good indicator of the presence of low clomazone concentrations in the soil, but, due to its lower sensitivity, it has no effect on growth and development. On this basis, sugar beet can be characterized as a moderately sensitive plant species to clomazone.
In these tests, white mustard was used as a model test plant, as it was assumed to have a higher sensitivity than other plant species. The results obtained confirmed the proposed hypothesis, as it showed an adequate response in terms of morphological (EC50 values of 94.6 and 128.2 μg a.i./kg soil for SFW and SDW, respectively) and physiological parameters (EC50 values of 57.5–63.3 μg a.i./kg soil for all three pigments). This allowed the use of white mustard as the most reliable and sensitive plant species in tests to determine the dynamics of clomazone degradation.
4.3. Clomazone Degradation Dynamics and Residue Levels in the Soil
The dynamics of clomazone degradation shown in Figure 6 and Figure 7 followed a similar pattern for both application times and application rates. However, the half-lives differed, although not consistently. A comparison of DT50 values for different application rates showed no differences for the PPI treatments in the first year, while the differences for the POST-EM treatments were 16.6 days (due to a large deviation at the application rate of 480 g a.i./ha and with the highest value for the medium application rate). In the second year, the differences were 9.7 days for the PPI treatments (with the highest application rate having the shortest half-life and the medium application rate having the longest half-life) and 8.3 days for the POST-EM treatments (the lowest application rate had the longest half-life, while the other two application rates had the same DT50 values). This is consistent with the findings of other studies where the initial application rate of clomazone within the same time of application does not necessarily have a significant effect on the rate of degradation [32,42].
The half-life of clomazone was on average (for all three application rates) longer in the PPI treatments than in the POST-EM treatments, i.e., 12.5 and 6 days longer in the first and second year, respectively (Table 6). The longer half-life of clomazone in the PPI treatments compared with the POST-EM treatments in the first year was due to the fact that the PPI treatments were followed by a period without rainfall, which favored a greater adsorption capacity of clomazone, but also reduced microbiological activity, resulting in significantly slower degradation in the first month after application. In contrast, POST-EM treatments were followed by rainfall (about 50 mm in the first ten days of May), which favored greater volatilization of clomazone, as previously established by Mervosh et al. [35]. The importance of incorporation of clomazone after application to reduce its losses due to volatilization has been confirmed in several studies [31,42,63]. In the second year, the precipitation distribution was much more uniform, both after the PPI treatments (which stimulated the decomposition processes) and after the POST-EM treatments. The results of Curran et al. [34] (the discrepancy in DT50 values of 48 days between PPI and PRE-EM treatments in a year with extremely low rainfall) are also consistent with the results of the present study.
Comparing the two years, the DT50 values showed an overall longer half-life of clomazone in the second year: 8.5 days longer in the PPI treatments and 15 days longer in the POST-EM treatments (Table 6), indicating a slower degradation of the herbicide in the second year. Comparing the overall average of the two application times to facilitate comparison, the DT50 value was found to be higher in the second year (55 days) than in the first year (43 days). This difference in the persistence of clomazone in these two years can only be explained by the environmental conditions, as both trials were conducted in the same experimental field. As shown in Table 2, the two growing seasons differed in terms of total precipitation, especially for the period from May to October (i.e., the part of the growing season when microbiological activity is highest). In the first year, 146.9 mm more precipitation was recorded than in the second year. Very similar are the results of Cumming et al. [26], who found a significant decrease in clomazone concentration in light clay soil between 114 and 140 days after application, when more than 130 mm or 25% of the annual precipitation fell. Wang et al. [29] attributed the slower clomazone degradation to the difference between the average annual precipitation of 486.8 and 512.8 mm in the first and second year of the study. Other results also confirm the importance of soil moisture on the degradation rate of clomazone, i.e., its longer persistence under conditions with less precipitation in the year after application [26,27,28,34]. Basham and Lavy [64] studied persistent herbicides and found that the microbial activity and volatility of herbicides are always greater in moist soils, whereas, in dry soils, the rate of biodegradation and chemical degradation is higher in a year with more rainfall than in a year with less rainfall.
Previous studies on the persistence of clomazone are showing numerous and quite different DT50 values. The shortest persistence was found in silt loam soils with an organic matter (OM) content of 2.9 and 3.4%, where the half-life of clomazone was 8 and 11 days, respectively [32], and in clay soil with an OM content of 1.5%, where the half-life was 9.5 days [65]. Kirskey et al. [21] determined an average DT50 of 19 days for clay loam (19–24 days) and loam (5–29 days) with PRE-EM treatment and extremely high soil moisture (85% of field capacity) at the time of application. They hypothesized that higher DT50 values would be achieved in the PPI treatments. These researchers also investigated the persistence of clomazone under laboratory conditions in the same soils and obtained average half-lives of 34 days. Quayle et al. [17] confirmed that the half-life of clomazone was shorter under anaerobic conditions (14.6 days), similar to previous results from China (5.7–22 days) and California (38 days). Mills et al. [42] determined DT50 values between 6 and 34 days for silt loam with 2.9 and 3.4% OM content, respectively, and attributed the differences mainly to environmental conditions. Similarly, Gallandt et al. [18] found that the half-life of clomazone was 33 and 37 days (in loam with an OM content of 1.6% and silty clay loam with an OM content of 2.3%, respectively). Loux et al. [19] found larger differences in DT50: 22 days (silt loam with 1.3% OM), 49 and 58 days (silty clay loam with 5.8% OM, but depending on the year of investigation). Gallaher and Mueller [66] reported that the half-life of clomazone in loam soil with 1.6% OM was 55 days on average and concluded that the DT50 values determined indicate a possible carry-over to rotational crops. Curran et al. [34] found average half-lives of 59 and 93 days in silt loam soils with 4% OM over a two-year period, with the highest DT50 value to date for field trials in their studies, being 117 days. All these researchers used the first-order kinetics equation to describe the dynamics of clomazone degradation and found that the coefficients of determination confirmed the suitability of the chosen statistical model. In contrast, Cumming et al. [26] investigated the persistence of clomazone in clay loam, loamy sand, silty loam and light clay soil with different clay and organic matter contents (2.1–4.3% OM). Due to the different desorption hysteresis observed, they applied Hoerl’s equation and obtained DT50 values in the range of 6–59 days. A different statistical model was also used by Mervosh et al. [36] to estimate the half-life of clomazone under laboratory conditions and they obtained a DT50 value of 154 days. All these data indicate that the DT50 for clomazone in field studies is in the range of 5–117 days. The mean value of the half-life of clomazone determined in the present study in sandy loam soil with an OM content of 2.8% in two years with different rainfall amounts is 50 days (with R2 values confirming the suitability of the chosen statistical model). This value is within the previously defined range and is consistent with the results of certain studies [26,34,65]. The existing differences are mainly due to large differences in soil properties (as not one soil in all cited studies was a sandy loam), weather conditions during the studies and the method used (bioassay or chemical extraction).
The dynamics of clomazone degradation showed a consistent trend of decreasing herbicide concentrations in the soil in the PPI and POST-EM treatments and at all application rates, with differences in residue levels between treatments decreasing over time. In the first year, the differences in residue levels resulting from the different initial application rates for the PPI treatment were evident at the penultimate sampling date (27 February 2016) or 324 days after application (DAA) (Table 7). The inhibition of carotenoid content of white mustard was 20.57% at the highest application rate (960 g a.i./ha). This value is slightly higher than the EC20 value for the carotenoid content of sugar beet (17.295 μg a.i./kg soil). Considering that sugar beet in the Srem region can be sown around 1 March, this means that clomazone residues at this level could cause slight chlorosis on the youngest leaves at early growth stages. On the same day (293 DAA), the inhibition of carotenoid content in the POST-EM treatments was well below 10% at all application rates. On the last sampling date (18 April 2016), no inhibition of carotenoid content in white mustard was observed in either the PPI treatment (375 DDA) or the POST-EM treatment (345 DAA) at clomazone application rates of 480 and 720 g a.i./ha. The highest application rate resulted in negligible inhibition of carotenoid content in both the PPI and POST-EM treatments. The estimated concentrations of clomazone residues, one year after application, were either undetectable or <1 μg a.i./kg soil (Table 7). Since maize and sunflowers in the Srem region are usually sown in early April, it is obvious that the residues detected cannot pose a risk to them.
As far as wheat is concerned, only winter wheat is grown in the Srem region. The optimum time for sowing is between 5 and 25 October, but it is often sown at a later date. The soil samples were taken in September (157 DAA), more than a month before the optimal sowing date (Table 7). The clomazone residues led to an inhibition of the carotenoid content of white mustard in the range of 67.16–86.25%, which means that the residues were in the range of 70.76–90.88 μg a.i./kg soil (for all three application rates). These values correspond to the EC20 determined for wheat for carotenoids (80.4 μg a.i./kg soil). The clomazone residues were lower one month later, so that no harmful effects on the wheat were to be expected. In the POST-EM treatments, residue levels at 126 DAA were even lower (69.79–79.2 μg a.i./kg soil) and are not expected to exceed the EC10 value for wheat (for carotenoids) at the optimum sowing date.
In the second year, all three application rates were characterized by residues that caused a decrease in the carotenoid content of white mustard by 15.53–22.89% and 11.53–14.27% in the PPI (356 DDA) and POST-EM (324 DDA) treatments, respectively (Table 7). The estimated clomazone concentrations in the PPI treatments for the clomazone application rate of 720 g a.i./ha would be sufficient to cause phytotoxicity of more than 20% but less than 50% (in terms of carotenoid content) in the sugar beet plants, which would certainly be manifested by visible chlorosis. However, even this amount of clomazone residue is far from the concentration required to produce an effect in terms of a reduction in SFW or SDW in sugar beet (according to the bioassay, no reduction in SFW and SDW was observed at concentrations < 180 μg a.i./kg soil). The POST-EM treatments resulted in lower clomazone residues, which could ultimately lead to an inhibition of carotenoid content in sugar beet of 10–20%, with possibly minimal visual symptoms in the form of chlorosis of the youngest leaves (for all three application rates). In any case, there is no risk of phytotoxic effects in maize and sunflower, as these crops have been shown to be less sensitive.
As far as the wheat is concerned, the soil samples were taken at the beginning of September, more than two months before the optimum sowing date for wheat. Not only were they taken earlier than in the previous year, but clomazone persistence was also longer, resulting in higher residue levels of the herbicide. In the PPI treatments, the inhibition of carotenoid content in white mustard at 135 DAA was 83.7–84%, which corresponds to 97.42–101.78 μg a.i./kg soil (for all three application rates). These values are higher than EC20, but much lower than EC50 for wheat. Within two months, these residues continued to decrease and it can be assumed that they were even below the EC20 (80.4 μg a.i./kg soil). In any case, more than a slight chlorosis is not to be expected. In the POST-EM treatments (102 DAA), the residue content was harmless, as it was below EC20 even at the highest application rate.
5. Conclusions
When testing the sensitivity of plants to clomazone, the bioassay used must be based on the parameters resulting from the mode of action of the herbicide. Obvious differences in the sensitivity of the tested plant species were found when the inhibition of pigment content was measured as a function of clomazone concentration. White mustard was the most sensitive plant. Sugar beet and wheat were moderately sensitive (with sugar beet being five times more sensitive than wheat in terms of physiological parameters, but showing similar sensitivity in terms of morphological parameters). Sunflower and maize were the least sensitive.
White mustard can be used as a reliable and suitable test plant for the determination of clomazone residues by the bioassay method.
The rate of degradation of clomazone is not consistently dependent on the application rate, but is influenced by the time of application. In addition to soil factors, climatic and meteorological factors have the greatest influence on the dynamics of degradation, especially the amount of precipitation.
The clomazone residues in sandy loam were below the sensitivity limits of maize, sunflower, wheat and sugar beet at all application rates, for both types of application and in both test years. The application of the recommended application rates of clomazone on sandy loam soil in a year with average meteorological conditions or even with lower rainfall after application does not pose a risk for maize, sunflower, wheat or sugar beet as the next crops in the crop rotation.
For sandy loam soils with a higher organic matter content and soils with a different texture, further investigations are needed, especially considering that summers have been hotter and rainfall has been lower in the last ten years.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [KJ-R], upon reasonable request.
Acknowledgments
The author would like to thank Tajma Hodžić for her assistance with the statistics.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PPI | Pre-Plant Incorporated |
| POST-EM | Post-Emergence |
| SFW | Shoot Fresh Weight |
| SDW | Shoot Dry Weight |
| NOEL | No Observable Effect Level |
| ARL | Acceptable Residue Level |
| DT50 | Degradation Time (50%) i.e., half-life |
References
- Miller, T.W. Effects of several herbicides on green pea (Pisum sativum) and subsequent crops. Weed Technol. 2003, 17, 731–737. [Google Scholar] [CrossRef]
- Scherder, E.F.; Talbert, R.T.; Clark, S.D. Rice (Oryza sativa) cultivar tolerance to clomazone. Weed Technol. 2004, 18, 140–144. [Google Scholar] [CrossRef]
- Mudge, C.R.; Webster, E.P.; Leon, C.T.; Zhang, W. Rice (Oryza sativa) cultivar tolerance to clomazone in water-seeded production. Weed Technol. 2005, 19, 907–911. [Google Scholar] [CrossRef]
- Zhang, W.; Webster, E.P.; Blouin, D.C. Response of rice and barnyardgrass (Echinochloa crus-galli) to rates and timmings of clomazone. Weed Technol. 2005, 19, 528–531. [Google Scholar] [CrossRef]
- Brandenberger, L.P.; Shrefler, J.W.; Webber III, C.L.; Talbert, R.E.; Payton, M.E.; Wells, L.K.; McClelland, M. Injury potential from carryover of watermelon herbicide residues. Weed Technol. 2007, 21, 473–476. [Google Scholar] [CrossRef]
- Sikkema, P.H.; Shropshire, C.; Soltani, N. Effect of clomazone on various market classes of dry beans. Crop Prot. 2007, 26, 943–947. [Google Scholar] [CrossRef]
- Andres, A.; Concenço, G.; Theisen, G.; Vidotto, F.; Ferrero, A. Selectivity and weed control efficacy of pre- and post-emergence applications of clomazone in Southern Brazil. Crop Prot. 2013, 53, 103–108. [Google Scholar] [CrossRef]
- Carvalho, D.R.d.; Lins, H.A.; Souza, M.d.F.; Silva, T.S.; Porto, M.A.F.; Mendonça, V.; Silva, D.V. Weed control in melon with pre emergence herbicides. Pesqui. Agropecu. Bras. 2022, 57, e02334. [Google Scholar] [CrossRef]
- Westberg, D.E.; Oliver, L.R.; Frans, R.E. Weed control with clomazone alone and with other herbicides. Weed Technol. 1989, 3, 678–685. [Google Scholar] [CrossRef]
- Porter, W.C. Clomazone for weed control in sweet potatoes (Ipomea batatas). Weed Technol. 1990, 4, 648–651. [Google Scholar] [CrossRef]
- Renner, K.A.; Powell, G.E. Response of Navy bean (Phaseolus vulgaris) and wheat (Triticum aestivum) grown in rotation to clomazone, imazethapyr, bentazon and acifluorfen. Weed Sci. 1992, 40, 127–133. [Google Scholar] [CrossRef]
- Burnside, O.C.; Ahrens, W.H.; Holder, B.J.; Wiens, M.J.; Johnson, M.M.; Ristan, E.A. Efficacy and economics of various mechanical plus chemical weed control systems in dry beans (Phaseolus vulgaris). Weed Technol. 1994, 8, 238–244. [Google Scholar] [CrossRef]
- Scott, J.E.; Weston, L.A.; Jones, R.T. Clomazone for weed control in transplanted cole crops (Brassica oleracea). Weed Sci. 1995, 43, 121–127. [Google Scholar] [CrossRef]
- Ferhatogly, Y.; Barrett, M. Studies of clomazone mode of action. Pestic. Biochem. Physiol. 2006, 85, 7–14. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Conclusion regarding the peer review of the pesticide risk assessment of the active substance clomazone. EFSA J. 2007, 109, e9206. [Google Scholar] [CrossRef]
- Antonius, G.F. Clomazone residues in soil and runoff: Measurement and mitigation. Bull. Environ. Contam. Toxicol. 2000, 64, 168–175. [Google Scholar] [CrossRef]
- Quayle, W.C.; Oliver, D.P.; Zrna, S. Field dissipation and environmental hazard assessment of clomazone, molinate, and thiobencarb in Australian rice culture. J. Agric. Food Chem. 2006, 54, 7213–7220. [Google Scholar] [CrossRef] [PubMed]
- Gallandt, E.R.; Fay, P.K.; Inskeep, W.P. Clomazone dissipation in two Montana soils. Weed Technol. 1989, 3, 146–150. [Google Scholar] [CrossRef]
- Loux, M.M.; Liebl, R.A.; Slife, F.W. Availability and persistence of imazaquin, imazethapyr and clomazone in soil. Weed Sci. 1989, 37, 259–267. [Google Scholar] [CrossRef]
- Loux, M.M.; Liebl, R.A.; Slife, F.W. Adsorption of clomazone on soils, sediments and clays. Weed Sci. 1989, 37, 440–444. [Google Scholar] [CrossRef]
- Kirskey, K.B.; Hayes, R.M.; Krueger, W.A.; Mullins, C.A.; Mueller, T.C. Clomazone dissipation in two Tennessee soils. Weed Sci. 1996, 44, 959–963. [Google Scholar] [CrossRef]
- Lee, D.J.; Senseman, S.A.; O’Barr, J.H.; Chandler, J.M.; Krutz, L.J.; McCauley, G.N.; Kuk, Y.I. Soil characteristics and water potential effects on plant-available clomazone in rice. Weed Sci. 2004, 52, 310–318. [Google Scholar] [CrossRef]
- Gunasekara, A.S.; Cruz, I.D.P.d.; Curtis, M.J.; Claassen, V.P.; Tjeerdema, R.S. The behavior of clomazone in the soil environment. Pest Manag. Sci. 2009, 65, 711–716. [Google Scholar] [CrossRef]
- Đurović-Pejčev, R.; Radmanović, S.B.; Tomić, Z.P.; Kaluđerović, L.M.; Bursić, V.P.; Šantrić, L.J.R. Adsorption-desorption behavior of clomazone in Regosol and Chernozem agricultural soils. J. Serb. Chem. Soc. 2020, 85, 809–819. [Google Scholar] [CrossRef]
- Cao, D.; Peng, W.; Xu, H.; Fu, X.; Gong, X.; Yu, S.; Wei, H.; Zhou, Q.; Huang, Y. Bioavailability and phytotoxicity of clomazone to corn depend on soil characteristics and can be estimated by in situ pore water. Pest Manag. Sci. 2025, 81, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Cumming, J.P.; Doyle, R.B.; Brown, P.H. Clomazone dissipation in four Tasmanian topsoils. Weed Sci. 2002, 50, 405–409. [Google Scholar] [CrossRef]
- Gunsolus, J.L.; Bahrens, R.; Lueschen, W.E.; Warnes, D.D.; Wiersma, J.V. Carryover potential of AC-263,449, DPX-F6025, FMC-57020 and imazaquin in Minnesota. Proc. North Cent. Weed Sci. Soc. 1986, 41, 52. [Google Scholar]
- Ahrens, W.H.; Fuerst, P. Carryover injury of clomazone applied in soybeans (Glycine max) and fallow. Weed Technol. 1990, 4, 855–861. [Google Scholar] [CrossRef]
- Wang, K.; Ren, Y.; Pan, X.; Wu, X.; Xu, J.; Zheng, Y.; Dong, F. Insights on persistent herbicides in cropland soils in northern China: Occurrence, ecological risks, and phytotoxicity to subsequent crops. J. Hazard. Mater. 2025, 490, 137794. [Google Scholar] [CrossRef]
- Halsted, S.J.; Harvey, R.G. Effect of rate and carrier on clomazone movement off-site. Weed Technol. 1988, 2, 179–182. [Google Scholar] [CrossRef]
- Thelen, K.D.; Kells, J.J.; Penner, D. Comparison of application methods and tillage practices on volatilization of clomazone. Weed Technol. 1988, 2, 323–326. [Google Scholar] [CrossRef]
- Mills, J.A.; Witt, W.W. Efficacy, phytotoxicity and persistence of imazaquin, imazethapyr and clomazone in no-till double-crop soybean (Glycine max). Weed Sci. 1989, 37, 353–359. [Google Scholar] [CrossRef]
- Curran, W.S.; Knake, E.L.; Liebl, R.A. Corn (Zea mays) injury following use of clomazone, chlorimuron, imazaquin and imazethapyr. Weed Technol. 1991, 5, 539–544. [Google Scholar] [CrossRef]
- Curran, W.S.; Loux, M.M.; Liebl, R.A.; Simmons, F.W. Effect of tillage and application method on clomazone, imazaquine and imazethapyr persistence. Weed Sci. 1992, 40, 482–489. [Google Scholar] [CrossRef]
- Mervosh, T.L.; Sims, G.K.; Stoller, E.W.; Ellsworth, T.R. Clomazone sorption in soil: Incubation time, temperature and soil moisture effects. J. Agric. Food Chem. 1995, 43, 2295–2300. [Google Scholar] [CrossRef]
- Mervosh, T.L.; Sims, G.K.; Stoller, E.W. Clomazone fate in soil as affected by microbial activity, temperature and soil moisture. J. Agric. Food Chem. 1995, 43, 537–543. [Google Scholar] [CrossRef]
- Liu, S.Y.; Shocken, M.; Rosazza, J.P.N. Microbial transformation of clomazone. J. Agric. Food Chem. 1996, 44, 313–319. [Google Scholar] [CrossRef]
- Wang, H.; Ren, W.; Xu, Y.; Wang, X.; Ma, J.; Sun, Y.; Hu, W.; Chen, S.; Dai, S.; Song, J.; et al. Long-term herbicide residues affect soil multifunctionality and the soil microbial community. Ecotoxicol. Environ. Saf. 2024, 283, 116783. [Google Scholar] [CrossRef]
- Šunjka, D.; Pucarević, M.; Lazić, S.; Stojić, N.; Milošević, L.; El Bilali, H.; Berjan, S.; Šušnjar, A.; Ećimović, J. Monitoring of herbicide residues in agricultural soils in Vojvodina Province (Northern Serbia). Land 2024, 13, 1347. [Google Scholar] [CrossRef]
- Krausz, R.F.; Kapusta, G.; Knake, E.L. Soybean (Glycine max) and rotational crop tolerance to chlorimuron, clomazone, imazaquin and imazethapyr. Weed Technol. 1992, 6, 77–80. [Google Scholar] [CrossRef]
- Krausz, R.F.; Kapusta, G.; Matthews, J.L. Soybean (Glycine max) and rotational crop response to PPI chlorimuron, clomazone, imazaquin and imazethapyr. Weed Technol. 1994, 8, 224–230. [Google Scholar] [CrossRef]
- Mills, J.A.; Witt, W.W.; Barrett, M. Effects of tillage on the efficacy and persistence of clomazone in soybean (Glycine max). Weed Sci. 1989, 37, 217–222. [Google Scholar] [CrossRef]
- Monks, C.D.; Banks, P.A. Rotational crop response to chlorimuron, clomazone and imazaquin applied the previous year. Weed Sci. 1991, 39, 629–633. [Google Scholar] [CrossRef]
- Walsh, J.D.; Defelice, M.S.; Sims, B.D. Soybean (Glycine max) herbicide carryover to grain and fiber crops. Weed Technol. 1993, 7, 625–632. [Google Scholar] [CrossRef]
- Gheno, E.A.; Oliveira JR, R.S.; Constantin, J.; Takano, H.K.; Gemelli, A. Residual activity of herbicides applied to cotton on crops cultivated in succession. Rev. Caatinga 2016, 29, 143–152. [Google Scholar] [CrossRef][Green Version]
- Renner, K.A.; Powell, G.E. Response of sugar beet (Beta vulgaris) to herbicide residues in soil. Weed Technol. 1991, 5, 622–627. [Google Scholar] [CrossRef]
- Walsh, J.D.; Defelice, M.S.; Sims, B.D. Influence of tillage on soybean (Glycine max) herbicide carryover to grass and legume forage crops in Missouri. Weed Sci. 1993, 41, 144–149. [Google Scholar] [CrossRef]
- Gheno, E.A.; Oliveira JR, R.S.; Constantin, J.; Biffe, D.F.; Menezes, C.C.E.; Franchini, L.H.M.; Osipe, J.B.; Raimondi, R.T. Carryover of herbicides applied in the pre-emergence of cotton on the corn grown in succession. Rev. Bras. Herb. 2015, 14, 155–163. [Google Scholar] [CrossRef]
- Mervosh, T.L.; Stoller, E.W.; Simmons, F.W.; Ellsworth, T.R.; Sims, G.K. Effects of starch encapsulation on clomazone and atrazine movement in soil and clomazone volatilization. Weed Sci. 1995, 43, 445–453. [Google Scholar] [CrossRef]
- Johnson, D.H.; Beaty, J.D.; Horton, D.K.; Talbert, R.E.; Guy, C.B.; Mattice, J.D.; Lavy, T.L.; Smith, R.J., Jr. Effects of rotational crop herbicides on rice (Oryza sativa). Weed Sci. 1995, 43, 648–654. [Google Scholar] [CrossRef]
- Pintar, A.; Stipičeić, S.; Lakić, J.; Barić, K. Phytotoxicity of Mesotrione Residues on Sugar Beet (Beta vulgaris L.) in Agricultural Soils Differing in Adsorption Affinity. Sugar Tech. 2020, 22, 137–142. [Google Scholar] [CrossRef]
- Pintar, A.; Svečnjak, Z.; Lakić, J.; Magdić, I.; Brzoja, D.; Barić, K. The Susceptibility of Pea (Pisum sativum L.) to Simulated Mesotrione Residues as Affected by Soil pH Manipulation. Agriculture 2021, 11, 688. [Google Scholar] [CrossRef]
- Pismarović, L.; Milanović-Litre, A.; Kljak, K.; Lazarević, B.; Šćepanović, M. Soil solution pH can affect the response of the common bean (Phaseolus vulgaris L.) to mesotrione residues. Plant Soil Environ. 2022, 68, 237–244. [Google Scholar] [CrossRef]
- Wellburn, A.R. The special determinations of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 15 April 2025).
- Pestemer, W.; Günther, P. No-observable Effect Level (NOEL). In Herbicide Bioassays; Streibig, J.C., Kudsk, P., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 137–152. [Google Scholar]
- Onofri, A. Biological activity, field persistence and safe recroping intervals for imazethapyr and rimsulfuron on a silty-clay soil. Weed Res. 1996, 36, 73–83. [Google Scholar] [CrossRef]
- Pannacci, E.; Onofri, A.; Covarelli, G. Biological activity, availability and duration of phytotoxicity for imazamox in four different soils of central Italy. Weed Res. 2006, 46, 243–250. [Google Scholar] [CrossRef]
- Jovanović-Radovanov, K.; Rančić, D. Susceptibility of selected crops to simulated imazethapyr carryover: A morpho-anatomical analysis. Agronomy 2023, 13, 1857. [Google Scholar] [CrossRef]
- Horowitz, M. Application of bioassay techniques to herbicide investigations. Weed Res. 1976, 16, 209–215. [Google Scholar] [CrossRef]
- Weston, L.A.; Barrett, M. Tolerance of tomato (Lycopersicon esculentum) and bell pepper (Capsicum annum) to clomazone. Weed Sci. 1989, 37, 285–289. [Google Scholar] [CrossRef]
- Vrbničanin, S.; Božić, D.; Rančić, D.; Jovanović-Radovanov, K. Susceptibility of different varieties of Canada thistle (Cirsium arvense (L.) Scop.) to some herbicides. Acta Herbol. 2004, 13, 457–464. [Google Scholar]
- Locke, A.M.; Smeda, R.J.; Howard, K.D.; Reddy, K.N. Clomazone volatilization under varying environmental conditions. Chemosphere 1996, 33, 1213–1225. [Google Scholar] [CrossRef]
- Basham, G.; Lavy, T.L. Microbial and photolytic dissipation of imazaquin in soil. Weed Sci. 1987, 35, 865–870. [Google Scholar] [CrossRef]
- Szpyrka, E.; Słowik-Borowiec, M.; Książek, P.; Zwolak, A.; Podbielska, M. The difference in dissipation of clomazone and metazachlor in soil under field and laboratory conditions and their uptake by plants. Sci. Rep. 2020, 10, 3747. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, K.; Mueller, T.C. Effects of crop presence on persistence of atrazine, metribuzin and clomazone in surface soil. Weed Sci. 1996, 44, 698–703. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).