ZIF-93-Based Nanomaterials as pH-Responsive Drug Delivery Systems for Enhanced Antibacterial Efficacy of Kasugamycin in the Management of Pear Fire Blight
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of KSM@ZIF-93
2.2.1. Synthesis of ZIF-93
2.2.2. Synthesis of KSM-Linked ZIF-93 (KSM@ZIF-93)
2.3. Characterization of KSM@ZIF-93
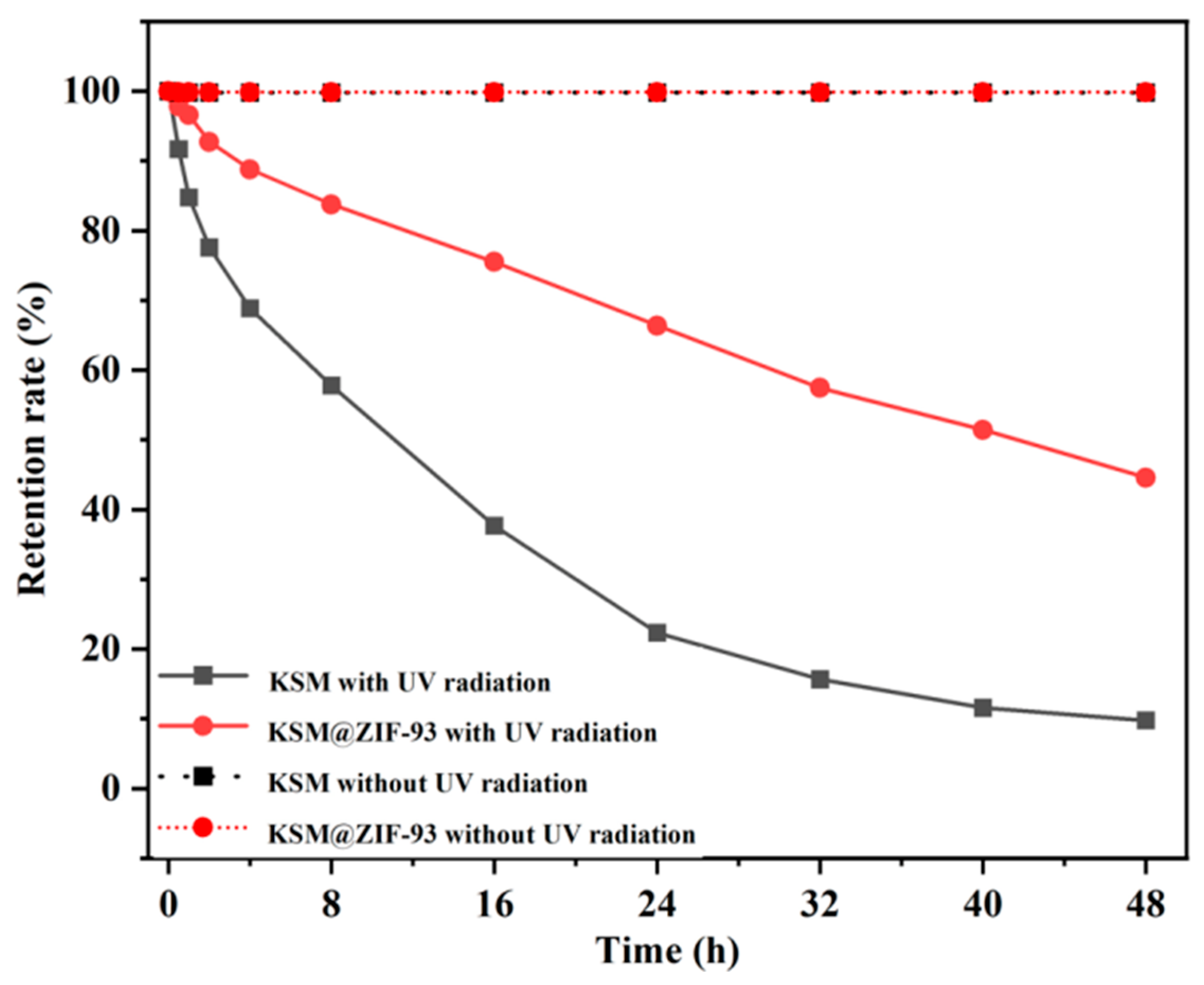
2.4. Light Stability of KSM@ZIF-93
2.5. Controlled Release Kinetics
2.6. Antimicrobial Assays In Vitro
2.7. Field Experiment
| Progression of Disease | Symptom |
|---|---|
| 0 | Leaves without diseased spots. |
| 1 | The area of leaf diseased spots accounted for 1–5% of the inoculated leaf area. |
| 3 | The area of leaf diseased spots accounted for 6–15% of the inoculated leaf area. |
| 5 | The area of leaf diseased spots accounted for 16–30% of the inoculated leaf area. |
| 7 | The area of leaf diseased spots accounted for 31–50% of the inoculated leaf area. |
| 9 | The area of leaf diseased spots accounted for more than 50% of the inoculated leaf area. |
2.8. Safety Evaluation
2.9. Data Processing
3. Results
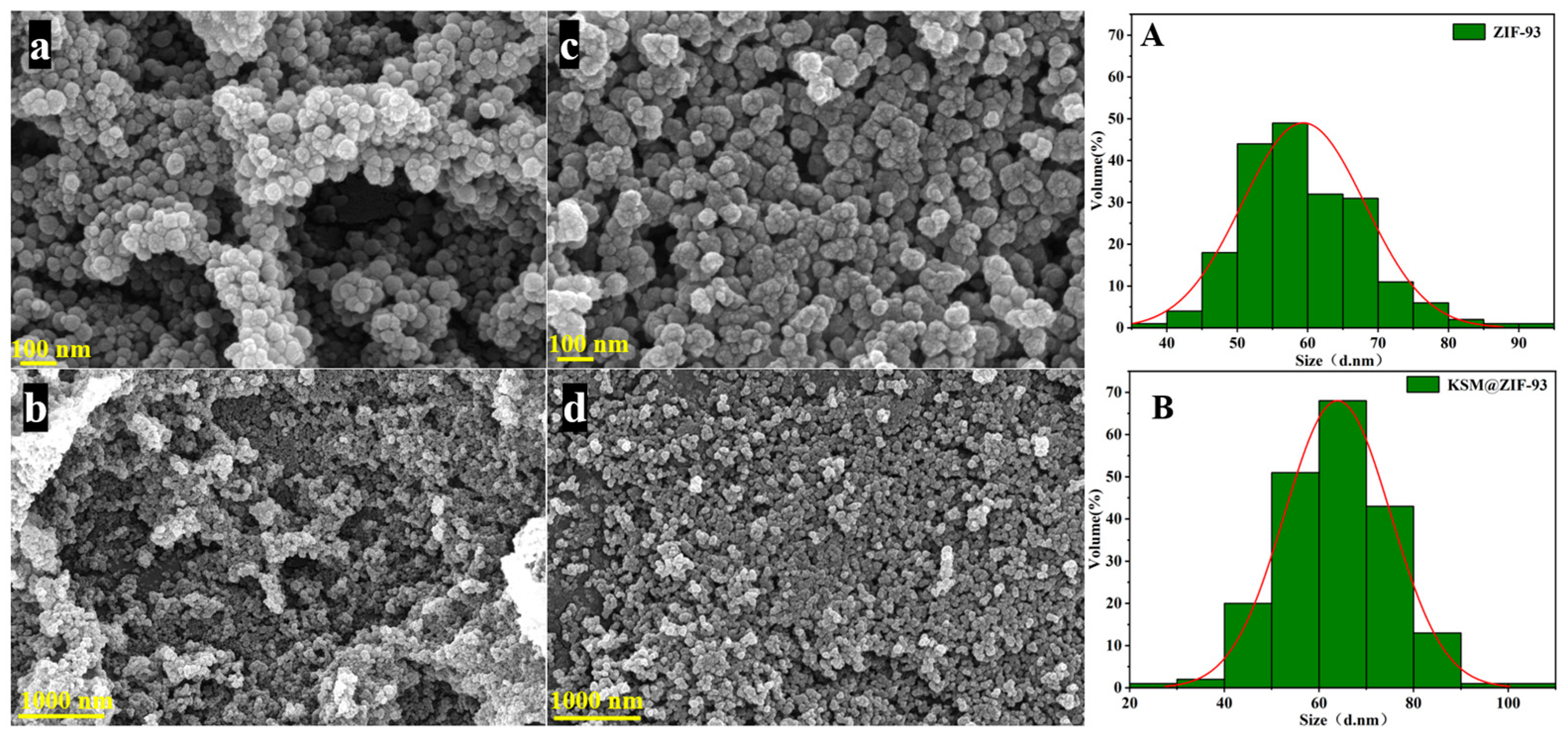
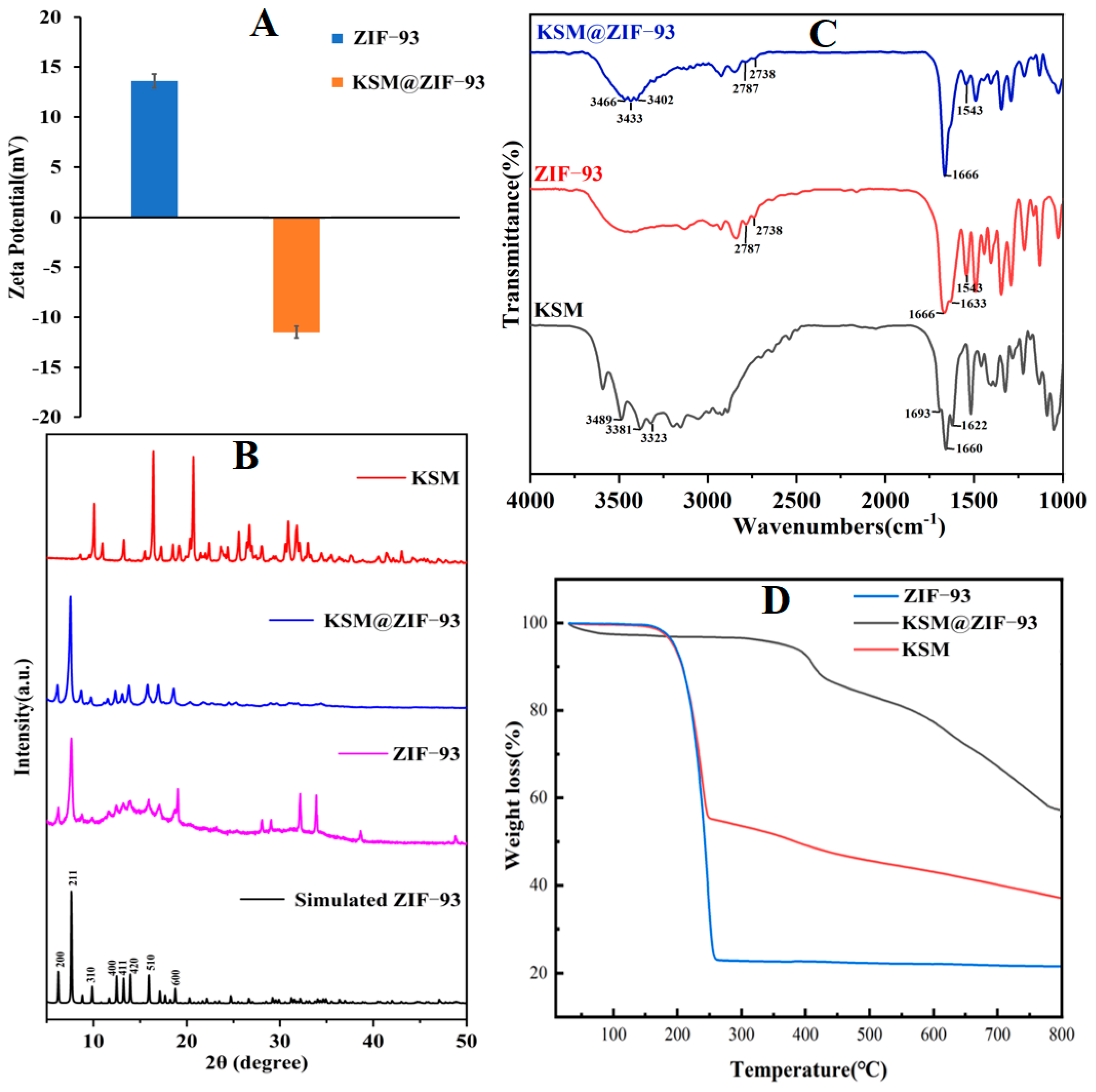
3.1. Preparation and Characterization of KSM@ZIF-93
3.2. Release Kinetics
3.3. Light Stability of KSM@ZIF-93
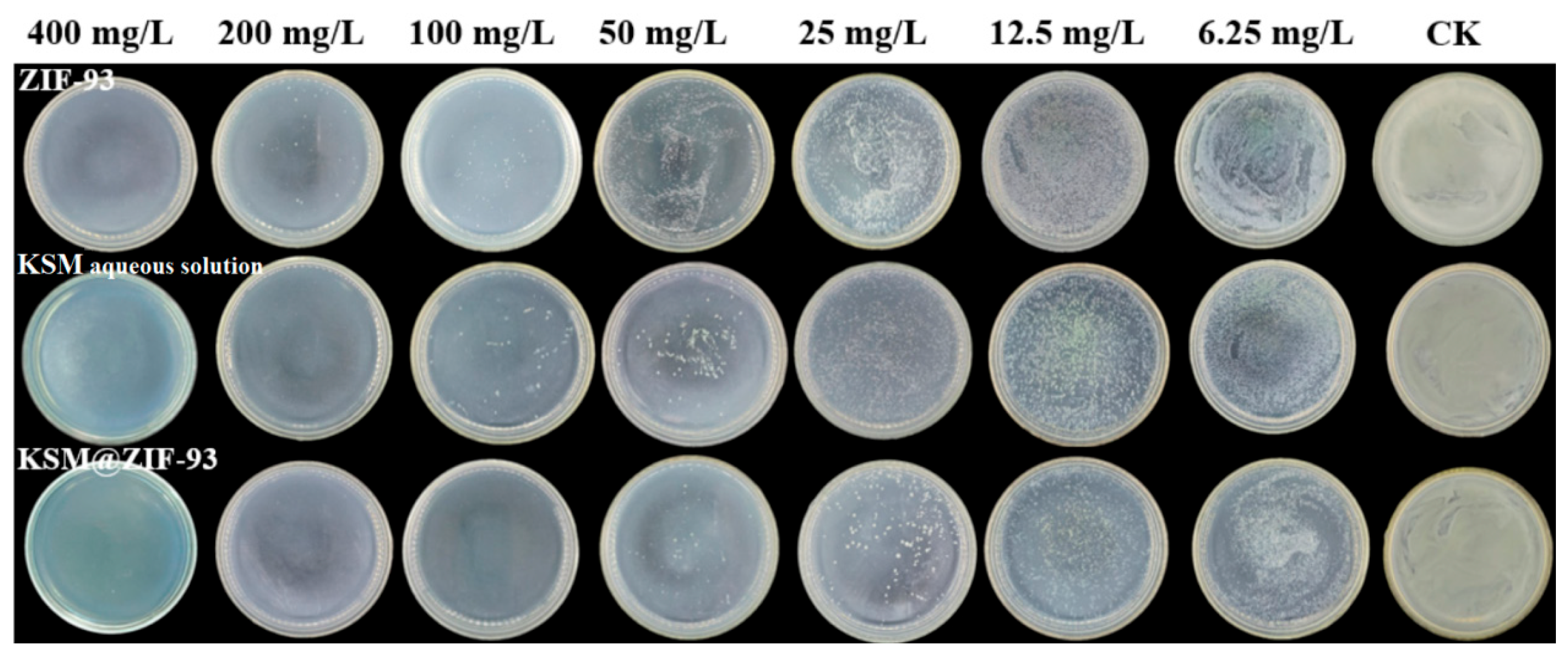
3.4. Antimicrobial Assays In Vitro
3.5. Field Experiment
3.6. Safety Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Psallidas, P.; Tsiantos, J. Chemical control of fire blight. In Fire Blight: The Disease and Its Causative Agent. Erwinia amylovora; CABI: Wallingford, UK, 2000; pp. 199–234. [Google Scholar]
- Laaziza, D.; Nabil, R.; Said, E.; Abdessalem, T.; Bouchra, T.; Farhate, G.; Said, A.; Rachid, L. Lessons learnt from the fire blight epidemics: A mini review. Indian Phytopathol. 2022, 75, 611–625. [Google Scholar]
- Shtienberg, D.; Manulis-Sasson, S.; Zilberstaine, M.; Oppenheim, D.; Shwartz, H. The incessant battle against fire blight in pears: 30 years of challenges and successes in managing the disease in Israel. Plant Dis. 2015, 99, 1048–1105. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gong, P.; Zhao, Y.; Ming, L.; Zeng, Q.; Liu, F. Current situation of fire blight in China. Phytopathology 2023, 113, 2143–2151. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural. Affairs of the People’s Republic of China. Announcement No. 333 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Available online: http://www.moa.gov.cn/govpublic/ZZYGLS/202009/t20200917_6352227.htm (accessed on 11 November 2023).
- Kharadi, R.; Schachterle, J.; Yuan, X.; Castiblanco, L.; Peng, J.; Slack, S.; Zeng, Q.; Sundin, G. Genetic dissection of the Erwinia amylovora disease cycl. Annu. Rev. Phytopathol. 2021, 59, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, Y.; Wang, L.; Geng, G.; ZHAO, W.; HU, S.; Zhao, Y. Fire blight disease, a fast-approaching threat to apple and pear production in China. J. Integr. Agric. 2019, 18, 815–820. [Google Scholar] [CrossRef]
- Chiou, C.; Jones, A. Molecular analysis of high level streptomycin resistance in Erwinia amylovora. Phytopathology 1995, 85, 324–328. [Google Scholar] [CrossRef]
- McGhee, G.; Sundin, G. Evaluation of kasugamycin for fire blight management, effect on nontarget bacteria, and assessment of kasugamycin resistance potential in Erwinia amylovora. Phytopathology 2011, 101, 192–204. [Google Scholar] [CrossRef]
- Al-Daoude, A.; Ammouneh, M. Studying Erwinia amylovora isolates from syria for copper resistance and streptomycin sensitivity. J. Plant Pathol. 2009, 91, 203–205. [Google Scholar]
- Johnson, K.; Temple, T.; Kc, A. Acidifying spray suspensions of oxytetracycline and kasugamycin enhances their effectiveness for fire blight control in apple and pear. Phytopathology 2023, 113, 2205–2214. [Google Scholar] [CrossRef]
- Adaskaveg, J.; Förster, H.; Wade, M.L. Effectiveness of kasugamycin against Erwinia amylovora and its potential use for managing fire blight of pear. Plant Dis. 2011, 95, 448–454. [Google Scholar] [CrossRef]
- Fan, C.; Guo, M.; Liang, Y.; Dong, H.; Ding, G.; Zhang, W.; Tang, G.; Yang, J.; Kong, D.; Cao, Y. Pectin-conjugated silica microcapsules as dual-responsive carriers for increasing the stability and antimicrobial efficacy of kasugamycin. Carbohydr. Polym. 2017, 172, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Suhara, Y.; Maeda, K.; Umezawa, H.; Ohno, M. Chemical studies on kasugamycin. V. The structure of kasugamycin. Tetrahedron Lett. 1966, 7, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Slack, S.; Walters, K.; Outwater, C.; Sundin, G. Effect of kasugamycin, oxytetracycline, and streptomycin on in-orchard population dynamics of Erwinia amylovora on apple flower stigmas. Plant Dis. 2021, 105, 1843–1850. [Google Scholar] [CrossRef]
- Guan, Y.; Lin, M.; Shen, P.; Zou, Z. Alanine-mediated P cycle boosting enhances the killing efficiency of kasugamycin on antibiotic-resistant Xanthomonas oryzae. Front. Microbiol. 2023, 14, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Ding, G.; Geng, Q.; Zhu, J.; Guo, M.; Duan, Y.; Wang, B.; Cao, Y. Synthesis, characterization, and application of microbe-triggered controlled-release kasugamycin-pectin conjugate. J. Agric. Food Chem. 2015, 63, 4263–4268. [Google Scholar] [CrossRef]
- Gentile, A.; Vaughan, A.; Pfeiffer, D. Cucumber pollen germination and tube elongation inhibited or reduced by pesticides and adjuvants. Environ. Entomol. 1978, 7, 689–691. [Google Scholar] [CrossRef]
- Gentile, A.; Vaughan, A.; Richman, S.; Eaton, A. Corn pollen germination and tube elongation inhibited or reduced by commercial and experimental formulations of pesticides and adjuvants. Environ. Entomol. 1973, 2, 473–476. [Google Scholar] [CrossRef]
- Oscar, C.; Diego, E.; Jorge, L.; Gildardo, S.; Vicente, R.; Angelica, L.; Araceli, S.; Sergio, M.; Karla, J.; Naveen, T.; et al. Enhancing antioxidant properties of CeO2 nanoparticles with Nd3+ doping: Structural, biological, and machine learning insights. Biomater. Sci. 2024, 12, 2108–2120. [Google Scholar]
- Dong, H.; He, Y.; Fan, C.; Zhu, Z.; Zhang, C.; Liu, X.; Qian, K.; Tang, T. Encapsulation of imazalil in HKUST-1 with versatile antimicrobial activity. Nanomaterials 2022, 12, 3879. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Jia, H.; Yao, Y.; Song, J.; Dong, H.; Cao, Y.; Zhu, F.; Huo, Z. Pectin functionalized metal-organic frameworks as dual-stimuli-responsive carriers to improve the pesticide targeting and reduce environmental risks. Colloids Surf. B 2022, 219, 112796. [Google Scholar]
- Dong, H.; Guo, M.; Liang, Y.; Fan, C.; Ding, G.; Zhang, W.; Tang, G.; Yang, J.; Kong, D.; Cao, Y. Preparation and characterization of indole-3-butyric acid nanospheres for improving its stability and utilization. Mater. Sci. Eng. C 2018, 89, 175–181. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liang, Y.; Zhou, Z.; Yang, J.; Tian, Y.; Niu, J.; Tang, G.; Tang, J.; Chen, X.; Li, Y.; et al. Metal-organic framework nanohybrid carrier for precise pesticide delivery and pest management. Chem. Eng. J. 2021, 422, 130143. [Google Scholar] [CrossRef]
- Muthalagu, S.; Natarajan, S. Deciphering the antimicrobial and antibiofilm efficiency of thyme essential oil encapsulated zeolitic imidazole framework-8 against foodborne pathogens. Curr. Microbiol. 2025, 82, 1–17. [Google Scholar] [CrossRef]
- Huang, W.; Chang, Y.; Wang, J.; Tsai, R.; Wu, K. Comparison of the stability of one-pot synthesis of acetylcholinesterase-embedded zeolitic imidazolate frameworks (AChE/ZIFs) with different morphologies. Inorg. Chem. Commun. 2025, 178, 114662. [Google Scholar] [CrossRef]
- Wang, T.; Kou, Z.; Mu, S.; Liu, J.; He, D.; Amiinu, I.; Meng, W.; Zhou, K.; Lou, Z.; Chaemchuen, S.; et al. 2D dual-metal zeolitic-imidazolate-framework-(ZIF)-derived bifunctional air electrodes with ultrahigh electrochemical properties for rechargeable zinc-air batteries. Adv. Funct. Mater. 2018, 28, 1705048. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Chen, F.; Yan, Y.; Li, Y.; Zhang, Y.; Ma, Y.; Wan, H.; Xue, Z.; Wang, Q. In situ synthesis of zeolitic imidazolate framework-11@ZnO heterostructures for enhanced antimicrobial activity and biological preservation. Chem. Mater. 2024, 36, 10. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, Y.S.; Ban, Y.J.; Peng, Y.; Jin, H.; Yang, W.; Li, K. Synthesis of zeolitic imidazolate framework nanocrystals. Mater. Lett. 2014, 136, 341–344. [Google Scholar] [CrossRef]
- Luque-Alled, J.; Martinez-Izquierdo, L.; Gorgojo, P.; Téllez, C.; Coronas, J. Organic solvent-free fabrication of thin film polyamide/zeolitic imidazolate framework membranes for removal of dyes from water. Chem. Eng. J. 2023, 470, 17. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, K.; Guo, W.; Liang, K.; Li, J.; Li, X.; Deng, Q.; Xu, X.; Chao, H.; Xi, H.; et al. Room-temperature rapid synthesis of hierarchically porous ZIF-93 for effective adsorption of volatile organic compounds. Industrial Chemistry & Materials. 2024, 3, 109–121. [Google Scholar]
- Zheng, H.; Xing, L.; Cao, Y.; Che, S. Coordination bonding based pH-responsive drug delivery systems. Coord. Chem. Rev. 2013, 257, 1933–1944. [Google Scholar] [CrossRef]
- Sahajpal, K.; Shekhar, S.; Kumar, A.; Sharma, B.; Meena, M.; Bhagi, A.; Sharma, S. Dynamic protein and polypeptide hydrogels based on Schiff base co-assembly for biomedicine. J. Mater. Chem. B 2022, 10, 3173–3198. [Google Scholar] [CrossRef]
- Jin, H.; Li, Y.S.; Liu, X.L.; Ban, Y.; Peng, Y.; Jiao, W.; Yang, W. Recovery of HMF from aqueous solution by zeolitic imidazolate frameworks. Chem. Eng. Sci. 2015, 124, 170–178. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Paprstein, F.; Sedlak, J.; Korba, J.; Sillerova, J. Testing of resistance to Erwinia amylovora in an in vitro culture assay. Acta Hortic. Sin. 2010, 896, 381–384. [Google Scholar] [CrossRef]
- Hurch, R.M.C.; Williams, R.R. The toxicity to apple pollen of several fungicides, as demonstrated by in vivo and in vitro techniques. J. Hortic. Sci. 1977, 52, 429–436. [Google Scholar] [CrossRef]
- Vasconcelos, I.B.; da Silva, T.G.; Militão, G.C.G.; Soares, T.A.; Rodrigues, N.M.; Rodrigues, M.O.; Costa Jr, N.B.; Freire, R.O.; Junior, S.A. Cytotoxicity and slow release of the anti-cancer drug doxorubicin from ZIF-8. RSC Adv. 2012, 2, 9437–9442. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Dong, H.; Yu, S.; Jia, H.; Wang, J.; Yao, Y.; Wang, Y.; Song, J.; Huo, Z. Zeolitic imidazole framework-90-based pesticide smart-delivery system with enhanced antimicrobial performance. Nanomaterials 2022, 12, 3622. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Ren, X.; Ye, X.; Li, W.; Yuan, S.; Sun, M.; Sheng, G.; Yu, H.; Wang, X. pH dependence of structure and surface properties of microbial EPS. Environ. Sci. Technol. 2012, 46, 737–744. [Google Scholar] [CrossRef]
- Cleymand, F.; Zhang, H.; Dostert, G.; Menu, P.; Arab-Tehrany, E.; Velot, E.; Mano, J. Membranes combining chitosan and natural-origin nanoliposomes for tissue engineering. RSC Adv. 2016, 6, 83626–83637. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Zornoza, B.; Orsi, A.; Łozińska, M.; Dawson, D.; Ashbrook, S.; Francis, D.; Wright, P.; Llewellyn, D.; Coronas, J. Synthesis of ZIF-93/11 Hybrid Nanoparticles via Post-Synthetic Modification of ZIF-93 and Their Use for H2/CO2 Separation. Chem.–A Eur. J. 2018, 24, 11211–11219. [Google Scholar] [CrossRef] [PubMed]
- Cacho-Bailo, F.; Caro, G.; Etxeberría-Benavides, M.; Karvan, O.; Téllez, C.; Coronas, J. High selectivity ZIF-93 hollow fiber membranes for gas separation. Chem. Commun. 2015, 51, 11283–11285. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Leung, B.; Furukawa, H.; Yaghi, O.; He, N.; Hayashi, H.; Houndonougbo, Y.; Asta, M.; Laird, B.; Yaghi, O. A combined experimental-computational investigation of carbon dioxide capture in a series of isoreticular zeolitic imidazolate frameworks. J. Am. Chem. Soc. 2010, 132, 11006–11008. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.C.; Hsiao, Y.M. High-performance capillary electrophoretic method for the determination of antibiotic fungicide kasugamycin in formulated products. J. Agric. Food Chem. 1996, 44, 2231–2234. [Google Scholar] [CrossRef]


| Treatment Group | Effective Component Concentration (mg/L) | Disease Index | Control Effect (%) |
|---|---|---|---|
| Blank control | 0 | 83.67 ± 1.27a | - |
| ZIF-93 | 50 | 71.10 ± 2.20b | 15.03 ± 1.64h |
| 100 | 53.33 ± 2.25d | 36.27 ± 1.92f | |
| 200 | 35.93 ± 1.33f | 57.06 ± 0.94d | |
| 400 | 23.30 ± 1.10h | 72.16 ± 0.97b | |
| KSM | 50 | 61.43 ± 3.42c | 26.56 ± 4.41g |
| 100 | 42.20 ± 2.20e | 49.55 ± 2.72e | |
| 200 | 30.37 ± 1.68g | 63.70 ± 1.96c | |
| 400 | 21.10 ± 1.91h | 74.80 ± 1.91b | |
| KSM@ZIF-93 | 50 | 58.53 ± 3.36c | 30.03 ± 3.92g |
| 100 | 32.20 ± 1.10g | 61.52 ± 0.86c | |
| 200 | 20.73 ± 2.77h | 75.19 ± 3.63b | |
| 400 | 15.57 ± 2.25i | 81.37 ± 2.95a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Hao, B.; Shen, J.; Liu, S.; Feng, H.; Zhang, J.; Liu, C.; Li, Y.; Dong, H. ZIF-93-Based Nanomaterials as pH-Responsive Drug Delivery Systems for Enhanced Antibacterial Efficacy of Kasugamycin in the Management of Pear Fire Blight. Agronomy 2025, 15, 1535. https://doi.org/10.3390/agronomy15071535
Chen C, Hao B, Shen J, Liu S, Feng H, Zhang J, Liu C, Li Y, Dong H. ZIF-93-Based Nanomaterials as pH-Responsive Drug Delivery Systems for Enhanced Antibacterial Efficacy of Kasugamycin in the Management of Pear Fire Blight. Agronomy. 2025; 15(7):1535. https://doi.org/10.3390/agronomy15071535
Chicago/Turabian StyleChen, Chunli, Bin Hao, Jincheng Shen, Shuren Liu, Hongzu Feng, Jianwei Zhang, Chen Liu, Yong Li, and Hongqiang Dong. 2025. "ZIF-93-Based Nanomaterials as pH-Responsive Drug Delivery Systems for Enhanced Antibacterial Efficacy of Kasugamycin in the Management of Pear Fire Blight" Agronomy 15, no. 7: 1535. https://doi.org/10.3390/agronomy15071535
APA StyleChen, C., Hao, B., Shen, J., Liu, S., Feng, H., Zhang, J., Liu, C., Li, Y., & Dong, H. (2025). ZIF-93-Based Nanomaterials as pH-Responsive Drug Delivery Systems for Enhanced Antibacterial Efficacy of Kasugamycin in the Management of Pear Fire Blight. Agronomy, 15(7), 1535. https://doi.org/10.3390/agronomy15071535





