Zinc Finger-Homeodomain Transcription Factor: A New Player in Plant Growth, Stress Response, and Quality Regulation
Abstract
1. Introduction
2. The Structural Characteristics of ZF-HD Transcription Factors
3. Roles of ZF-HD Transcription Factors in Plants
3.1. Abiotic Stress Response
3.1.1. Temperature
3.1.2. Water Stress
3.1.3. Light
3.1.4. Salt and Alkali Stress
3.1.5. Heavy Metal
3.2. Biotic Stress
3.3. Plant Hormone Signaling Pathway
3.4. Plant Growth and Development
3.4.1. Root
3.4.2. Stem and Leaf
3.4.3. Flowering and Fruit
3.5. Regulation of Plant Quality
3.5.1. Appearance
3.5.2. Nutrient Accumulation
4. The Regulatory Mechanisms of ZF-HD Transcription Factors
5. Prospects
6. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munns, R.; Millar, A.H. Seven plant capacities to adapt to abiotic stress. J. Exp. Bot. 2023, 74, 4308–4323. [Google Scholar] [CrossRef]
- Strader, L.; Weijers, D.; Wagner, D. Plant transcription factors—being in the right place with the right company. Curr. Opin. Plant Biol. 2022, 65, 102136. [Google Scholar] [CrossRef] [PubMed]
- Dhatterwal, P.; Sharma, N.; Prasad, M. Decoding the functionality of plant transcription factors. J. Exp. Bot. 2024, 75, 4745–4759. [Google Scholar] [CrossRef]
- Bollier, N.; Gonzalez, N.; Chevalier, C.; Hernould, M. Zinc Finger-Homeodomain and Mini Zinc Finger proteins are key players in plant growth and responses to environmental stresses. J. Exp. Bot. 2022, 73, 4662–4673. [Google Scholar] [CrossRef]
- Windhövel, A.; Hein, I.; Dabrowa, R.; Stockhaus, J. Characterization of a novel class of plant homeodomain proteins that bind to the C4 phosphoenolpyruvate carboxylase gene of Flaveria trinervia. Plant Mol. Biol. 2001, 45, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Hong, W.J.; Park, Y.S.; Jung, K.H.; Kim, S. Genomic basis of multiphase evolution driving divergent selection of zinc-finger homeodomain genes. Nucleic Acids Res. 2023, 51, 7424–7437. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, L.; An, M.; Wang, Y.; Li, S.; Dong, Y.; Yang, S.; Shi, K.; Fan, S.; Chen, X.; et al. Zinc finger-homeodomain transcriptional factors (ZHDs) in cucumber (Cucumis sativus L.): Identification, evolution, expression profiles, and function under abiotic stresses. Int. J. Mol. Sci. 2024, 25, 4408. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zheng, C.; Shi, C.; Lu, X.; She, Z.; Jiang, S.; Tian, D.; Qin, Y. The OsZHD1 and OsZHD2, two zinc finger homeobox transcription factor, redundantly control grain size by influencing cell proliferation in rice. Rice 2025, 18, 20. [Google Scholar] [CrossRef]
- Liu, M.D.; Liu, H.; Liu, W.Y.; Ni, S.F.; Wang, Z.Y.; Geng, Z.H.; Zhu, K.Y.; Wang, Y.F.; Zhao, Y.H. Systematic analysis of zinc finger-homeodomain transcription factors (ZF-HDs) in barley (Hordeum vulgare L.). Genes 2024, 15, 578. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, K.; Ngea, G.L.N.; Godana, E.A.; Ackah, M.; Dhanasekaran, S.; Zhang, Y.; Su, Y.; Yang, Q.; Zhang, H. Recent advances in the multifaceted functions of Cys2/His2-type zinc finger proteins in plant growth, development, and stress responses. J. Exp. Bot. 2024, 75, 5501–5520. [Google Scholar] [CrossRef]
- Moulick, D.; Bhutia, K.L.; Sarkar, S.; Roy, A.; Mishra, U.N.; Pramanick, B.; Maitra, S.; Shankar, T.; Hazra, S.; Skalicky, M.; et al. The intertwining of Zn-finger motifs and abiotic stress tolerance in plants: Current status and future prospects. Front. Plant Sci. 2022, 13, 1083960. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Chan, Z.; Wei, P.; Mao, Y.; Bartels, D.; Liu, X. PHD finger proteins function in plant development and abiotic stress responses: An overview. Front. Plant Sci. 2023, 14, 1297607. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Shen, T.; Guo, Q.; Zhang, R.; Chen, Y.; Zhang, Y.; Luo, K. Molecular characterization of cassava zinc finger-homeodomain (ZF-HD) transcription factors reveals their role in disease resistance. Int. J. Biol. Macromol. 2024, 279, 134846. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.F. Homeobox gene-encoded transcription factors in development and mature circadian function of the rodent pineal gland. J. Pineal Res. 2024, 76, e12950. [Google Scholar] [CrossRef]
- Jia, Y.; Lin, Z.; He, H.; Zhou, Z.; Gao, K.; Du, K.; Zhang, R. Comprehensive analysis and identification of the WOX gene family in Schima superba and the key gene SsuWOX1 for enhancing callus regeneration capacity. BMC Plant Biol. 2025, 25, 367. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.; Shi, L.; Winarsih, C.; Jakada, B.H.; Chai, R.; Huang, H. Plant growth regulators: An overview of WOX gene family. Plants 2024, 13, 3108. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Zhang, L. Zinc finger-homeodomain transcriptional factors (ZF-HDs) in wheat (Triticum aestivum L.): Identification, evolution, expression analysis and response to abiotic stresses. Plants 2021, 10, 593. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Yang, Q.; Xu, J.; Han, Z.; Ma, W.; Zhang, X.; Xu, K.; Zhao, J.; Chen, X. Evolution of HD-ZIP transcription factors and their function in cabbage leafy head formation. Front. Plant Sci. 2025, 16, 1583110. [Google Scholar] [CrossRef]
- He, X.; Zhao, X.; Zheng, Q.; Zhang, M.M.; Huang, Y.; Liu, Z.J.; Lan, S. Whole-genome analysis of ZF-HD genes among three Dendrobium species and expression patterns in Dendrobium chrysotoxum. Horticulturae 2024, 10, 610. [Google Scholar] [CrossRef]
- Jing, X.; Li, C.; Luo, C.; Yao, C.; Zhang, J.; Zhu, T.; Wang, J.; Liu, C. Identification and characterization of ZF-HD genes in response to abscisic acid and abiotic stresses in maize. Phyton-Int. J. Exp. Bot. 2022, 92, 707–723. [Google Scholar] [CrossRef]
- Islam, M.A.U.; Nupur, J.A.; Shafiq, M.; Ali, Q.; Sami, A.; Shahid, M.A. In silico and computational analysis of zinc finger motif-associated homeodomain (ZF-HD) family genes in chilli (Capsicum annuum L). BMC Genom. 2023, 24, 603. [Google Scholar] [CrossRef]
- Xing, L.; Peng, K.; Xue, S.; Yuan, W.; Zhu, B.; Zhao, P.; Wu, H.; Cheng, Y.; Fang, M.; Liu, Z. Genome-wide analysis of zinc finger-homeodomain (ZF-HD) transcription factors in diploid and tetraploid cotton. Funct. Integr. Genom. 2022, 22, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Zhu, W.; Liu, J.; Cheng, F. Non-coding RNAs fine-tune the balance between plant growth and abiotic stress tolerance. Front. Plant Sci. 2022, 13, 965745. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ response to abiotic stress: Mechanisms and strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Rizwan, H.M.; He, J.; Nawaz, M.; Lu, K.; Wang, M. The members of zinc finger-homeodomain (ZF-HD) transcription factors are associated with abiotic stresses in soybean: Insights from genomics and expression analysis. BMC Plant Biol. 2025, 25, 56. [Google Scholar] [CrossRef]
- Wu, R.; Wang, C.; Yin, L.; Ran, K.; Wang, L. Zinc finger–homeodomain gene family in apple and their expression analysis in apple rootstock Malus hupehensis under abiotic stress. J. Am. Soc. Hortic. Sci. 2022, 147, 312–321. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Zhao, L.; Wu, H.; Zhu, Y.; Ahmad, I.; Zhou, G. Abiotic stress responses in crop plants: A multi-scale approach. J. Integr. Agr. 2024, in press. [CrossRef]
- Huang, F.; Wang, J.; Tang, C. Genome-wide Identification and analysis of ZF-HD gene family in moso bamboo (Phyllostachys edulis). Plants 2023, 12, 4064. [Google Scholar] [CrossRef]
- Hendrix, S.; Dard, A.; Meyer, A.J.; Reichheld, J.P. Redox-mediated responses to high temperature in plants. J. Exp. Bot. 2023, 74, 2489–2507. [Google Scholar] [CrossRef]
- Qian, Z.; He, L.; Li, F. Understanding cold stress response mechanisms in plants: An overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Thomas, H.R.; Xia, X.; Shi, H.; Zhang, L.; Hong, J.; Shi, K.; Zhou, J.; Yu, J.; Zhou, Y. An integrative overview of cold response and regulatory pathways in horticultural crops. J. Integr. Plant Biol. 2025, 67, 1028–1059. [Google Scholar] [CrossRef]
- Yong, Y.B.; Li, W.Q.; Wang, J.M.; Zhang, Y.; Lu, Y.M. Identification of gene co-expression networks involved in cold resistance of Lilium lancifolium. Biol. Plant. 2018, 62, 287–298. [Google Scholar] [CrossRef]
- Yong, Y.; Zhang, Y.; Lyu, Y. Functional characterization of Lilium lancifolium cold-responsive Zinc Finger Homeodomain (ZFHD) gene in abscisic acid and osmotic stress tolerance. PeerJ 2021, 9, e11508. [Google Scholar] [CrossRef]
- Zhang, R.; Xi, X.; Chen, X.; Wang, Y.; Zhou, M. Comparing time-series transcriptomes between chilling-resistant and -susceptible rice reveals potential transcription factors responding to chilling stress. Front. Plant Sci. 2024, 15, 1451403. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Haq, I.U.; Fiaz, S.; Alharthi, B.; Xu, M.L.; Wang, J.L.; Hou, W.H.; Feng, X.B. Genome-wide identification and expression analysis of the ZF-HD gene family in pea (Pisum sativum L.). Front. Genet. 2023, 13, 1089375. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Dwivedi, S.; Bhagavatula, L.; Datta, S. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep. 2023, 42, 829–841. [Google Scholar] [CrossRef]
- Kompas, T.; Che, T.N.; Grafton, R.Q. Global impacts of heat and water stress on food production and severe food insecurity. Sci. Rep. 2024, 14, 14398. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef]
- Tran, L.-S.P.; Nakashima, K.; Sakuma, Y.; Osakabe, Y.; Qin, F.; Simpson, S.D.; Maruyama, K.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Co-expression of the stress-inducible zinc finger homeodomain ZFHD1 and NAC transcription factors enhances expression of the ERD1 gene in Arabidopsis. Plant J. 2007, 49, 46–63. [Google Scholar] [CrossRef]
- Lai, W.; Zhu, C.; Hu, Z.; Liu, S.; Wu, H.; Zhou, Y. Identification and transcriptional analysis of zinc finger-homeodomain (ZF-HD) family genes in cucumber. Biochem. Genet. 2021, 59, 884–901. [Google Scholar] [CrossRef]
- Li, M.; Dong, H.; Li, J.; Dai, X.; Lin, J.; Li, S.; Zhou, C.; Chiang, V.L.; Li, W. PtrVCS2 regulates drought resistance by changing vessel morphology and stomatal closure in Populus trichocarpa. Int. J. Mol. Sci. 2023, 24, 4458. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xie, M.; Li, X.; Li, Z.; Wang, Q.; Ding, A.; Wang, W.; Sun, Y. Systematic investigations of the ZF-HD gene family in tobacco reveal their multiple roles in abiotic stresses. Agronomy 2021, 11, 406. [Google Scholar] [CrossRef]
- Sun, W.; Wei, J.; Wu, G.; Xu, H.; Chen, Y.; Yao, M.; Zhan, J.; Yan, J.; Wu, N.; Chen, H.; et al. CqZF-HD14 enhances drought tolerance in quinoa seedlings through interaction with CqHIPP34 and CqNAC79. Plant J. 2022, 323, 111406. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.K.; Jha, S.K.; Agarwal, P.; Mallick, N.; Niranjana, M.; Vinod. Leaf rolling in bread wheat (Triticum aestivum L.) is controlled by the upregulation of a pair of closely linked/duplicate zinc finger homeodomain class transcription factors during moisture stress conditions. Front. Plant Sci. 2022, 13, 1038881. [Google Scholar] [CrossRef]
- Mahmoud, A.; Qi, R.; Chi, X.; Liao, N.; Malangisha, G.K.; Ali, A.; Moustafa-Farag, M.; Yang, J.; Zhang, M.; Hu, Z. Integrated bulk segregant analysis, fine mapping, and transcriptome revealed QTLs and candidate genes associated with drought adaptation in wild watermelon. Int. J. Mol. Sci. 2023, 25, 65. [Google Scholar] [CrossRef]
- Xin, G.Y.; Li, L.P.; Wang, P.T.; Li, X.Y.; Han, Y.J.; Zhao, X. The action of enhancing weak light capture via phototropic growth and chloroplast movement in plants. Stress Biol. 2022, 2, 50. [Google Scholar] [CrossRef]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef]
- Perrella, G.; Davidson, M.L.H.; O’Donnell, L.; Nastase, A.M.; Herzyk, P.; Breton, G.; Pruneda-Paz, J.L.; Kay, S.A.; Chory, J.; Kaiserli, E. ZINC-FINGER interactions mediate transcriptional regulation of hypocotyl growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E4503–E4511. [Google Scholar] [CrossRef]
- Feng, Z.; Zioutopoulou, A.; Xu, T.; Li, J.; Kaiserli, E. TANDEM ZINC-FINGER/PLUS3: A multifaceted integrator of light signaling. Trends Plant Sci. 2024, 30, P654–P664. [Google Scholar] [CrossRef]
- Li, R.; Ma, R.; Zheng, Y.; Zhao, Q.; Zong, Y.; Zhu, Y.; Chen, W.; Li, Y.; Guo, W. A study of the molecular regulatory network of VcTCP18 during blueberry bud dormancy. Plants 2023, 12, 2595. [Google Scholar] [CrossRef]
- He, K.; Li, C.; Zhang, Z.; Zhan, L.; Cong, C.; Zhang, D.; Cai, H. Genome-wide investigation of the ZF-HD gene family in two varieties of alfalfa (Medicago sativa L.) and its expression pattern under alkaline stress. BMC Genom. 2022, 23, 150. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Song, H.; Zhang, L. New insight into plant saline-alkali tolerance mechanisms and application to breeding. Int. J. Mol. Sci. 2022, 23, 16048. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Kim, M.L.; Kim, H.S.; Park, J.H.; Jung, M.S.; Shen, M.; Kang, C.H.; Kim, M.C.; Lee, S.Y.; Cho, M.J.; et al. Specificity of DNA sequences recognized by the zinc-finger homeodomain protein, GmZF-HD1 in soybean. Phytochemistry 2010, 71, 1832–1838. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, R.; Feng, X.; Zhang, J.; Jiang, Y.; Li, L.; Guo, J.; Zhang, X. Genome-wide identification and expression analysis of ZF-HD family in sunflower (Helianthus annuus L.) under drought and salt stresses. BMC Plant Biol. 2025, 25, 140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Xu, L.; Zhang, H.; Xing, H.; Fu, Y.; Zhu, L. PUB30-mediated downregulation of the HB24-SWEET11 module is involved in root growth inhibition under salt stress by attenuating sucrose supply in Arabidopsis. New Phytol. 2023, 237, 1667–1683. [Google Scholar] [CrossRef]
- Yong, Y.; Zhang, Y.; Lyu, Y. A stress-responsive NAC transcription factor from tiger lily (LlNAC2) interacts with LlDREB1 and LlZHFD4 and enhances various abiotic stress tolerance in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 3225. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y. How do plants maintain pH and ion homeostasis under saline-alkali stress? Front. Plant Sci. 2023, 14, 1217193. [Google Scholar] [CrossRef]
- Niekerk, L.-A.; Gokul, A.; Basson, G.; Badiwe, M.; Nkomo, M.; Klein, A.; Keyster, M. Heavy metal stress and mitogen activated kinase transcription factors in plants: Exploring heavy metal-ROS influences on plant signalling pathways. Plant Cell Environ. 2024, 47, 2793–2810. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress induced and nuclear localized HIPP26 from Arabidopsis thaliana interacts via its heavy metal associated domain with the drought stress related zinc finger transcription factor ATHB29. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, R.; Shokat, S.; Iqbal, N.; Kocsy, G.; Pérez-Pérez, J.M.; Riyazuddin, R. Ascorbate, plant hormones and their interactions during plant responses to biotic stress. Physiol. Plant. 2024, 176, e14388. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Jordá, L.; Torres, M.Á.; Martín-Dacal, M.; Berlanga, D.J.; Fernández-Calvo, P.; Gómez-Rubio, E.; Martín-Santamaría, S. Plant cell wall-mediated disease resistance: Current understanding and future perspectives. Mol. Plant 2024, 17, 699–724. [Google Scholar] [CrossRef]
- Cheaib, A.; Killiny, N. Photosynthesis responses to the infection with plant pathogens. Mol. Plant Microbe Interact. 2025, 38, 9–29. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, Á.; Cobos, A.; López-Herranz, M.; Canto, T.; Pagán, I. Environmental conditions modulate plant virus vertical transmission and survival of infected seeds. Phytopathology 2023, 113, 1773–1787. [Google Scholar] [CrossRef]
- Park, H.C.; Kim, M.L.; Lee, S.M.; Bahk, J.D.; Yun, D.J.; Lim, C.O.; Hong, J.C.; Lee, S.Y.; Cho, M.J.; Chung, W.S. Pathogen-induced binding of the soybean zinc finger homeodomain proteins GmZF-HD1 and GmZF-HD2 to two repeats of ATTA homeodomain binding site in the calmodulin isoform 4 (GmCaM4) promoter. Nucleic Acids Res. 2007, 35, 3612–3623. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Kang, J.Y.; Park, M.Y.; Song, M.r.; Kim, Y.C.; Kim, S.Y. Arabidopsis zinc finger homeodomain protein ZHD5 promotes shoot regeneration and confers other cytokinin-related phenotypes when overexpressed. Plant Cell Tissue Organ Cult. 2019, 137, 181–185. [Google Scholar] [CrossRef]
- Rattan, U.K.; Kumar, S.; Kumari, R.; Bharti, M.; Hallan, V. Homeobox 27, a homeodomain transcription factor, confers tolerances to CMV by associating with cucumber mosaic virus 2b protein. Pathogens 2022, 11, 788. [Google Scholar] [CrossRef]
- Kumari, M.; Yagnik, K.N.; Gupta, V.; Singh, I.K.; Gupta, R.; Verma, P.K.; Singh, A. Metabolomics-driven investigation of plant defense response against pest and pathogen attack. Physiol. Plant. 2024, 176, e14270. [Google Scholar] [CrossRef]
- Lohn, A.F.; Trtikova, M.; Chapela, I.; van den Berg, J.; du Plessis, H.; Hilbeck, A. Effect of herbivore stress on transgene behaviour in maize crosses with different genetic backgrounds: cry1Ab transgene transcription, insecticidal protein expression and bioactivity against insect pests. Environ. Sci. Eur. 2023, 35, 106. [Google Scholar] [CrossRef]
- Zhang, Y.; Berman, A.; Shani, E. Plant hormone transport and localization: Signaling molecules on the move. Annu. Rev. Plant Biol. 2023, 74, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Nazir, F.; Maheshwari, C.; Kaur, H.; Gupta, R.; Siddique, K.H.M.; Khan, M.I.R. Plant hormones and secondary metabolites under environmental stresses: Enlightening defense molecules. Plant Physiol. Biochem. 2024, 206, 108238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, S.; Qi, Y. Advances in structure and function of auxin response factor in plants. J. Integr. Plant Biol. 2023, 65, 617–632. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Zhao, S.; Fu, Y.; Zhu, L. HOMEOBOX PROTEIN 24 mediates the conversion of indole-3-butyric acid to indole-3-acetic acid to promote root hair elongation. New Phytol. 2021, 232, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pan, Y.; Cui, J.; Lu, X.; Yu, W. Mechanism of ABA in plants exposed to cold stress. Agronomy 2025, 15, 403. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The interaction of ABA and ROS in plant growth and stress resistances. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef]
- Abu-Romman, S. Molecular cloning and expression analysis of zinc finger-homeodomain transcription factor TaZFHD1 in wheat. S. Afr. J. Bot. 2014, 91, 32–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, X.; Zhao, F.; Che, Y.; Liu, X.; Hou, L. Cloning and expression characteristics analysis of VvZF-HD11 gene in grape (Vitis vinifera). J. Agric. Biotechnol. 2025, 33, 547–560. [Google Scholar] [CrossRef]
- Hu, Y.; Shani, E. Cytokinin activity—Transport and homeostasis at the whole plant, cell, and subcellular levels. New Phytol. 2023, 239, 1603–1608. [Google Scholar] [CrossRef]
- Aydin, A.; Yerlikaya, B.A.; Yerlikaya, S.; Yilmaz, N.N.; Kavas, M. CRISPR-mediated mutation of cytokinin signaling genes (SlHP2 and SlHP3) in tomato: Morphological, physiological, and molecular characterization. Plant Genome 2025, 18, e20542. [Google Scholar] [CrossRef]
- Hasegawa, R.; Fujita, K.; Tanaka, Y.; Takasaki, H.; Ikeda, M.; Yamagami, A.; Mitsuda, N.; Nakano, T.; Ohme-Takagi, M. Arabidopsis zinc finger homeodomain transcription factor BRASSINOSTEROID-RELATED HOMEOBOX 2 acts as a positive regulator of brassinosteroid response. Plant Biotechnol. 2022, 39, 185–189. [Google Scholar] [CrossRef]
- Zheng, X.B.; Wu, Y.; Wang, H.; Song, S.W.; Bai, T.H.; Jiao, J.; Song, C.H.; Pang, H.G.; Wang, M.M. Genome-wide investigation of the zinc finger-homeodomain family genes reveals potential roles in apple fruit ripening. Front. Genet. 2021, 12, 783482. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.U.; Nupur, J.A.; Khalid, M.H.B.; Din, A.M.U.; Shafiq, M.; Alshegaihi, R.M.; Ali, Q.; Ali, Q.; Kamran, Z.; Manzoor, M.; et al. Genome-wide identification and in silico analysis of ZF-HD transcription factor genes in Zea mays L. Genes 2022, 13, 2112. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, H.; Yang, Q.; Yang, Y.; Pu, X. ZF-HD gene family in rapeseed (Brassica napus L.): Genome-wide identification, phylogeny, evolutionary expansion and expression analyses. BMC Genom. 2024, 25, 1181. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wu, L.; Miao, X.; Zhang, S.; Wei, N.; Zhao, S.; Shang, X.; Hu, H.; Xue, J.; Zhang, T.; et al. A dynamic regulome of shoot-apical-meristem-related homeobox transcription factors modulates plant architecture in maize. Genome Biol. 2024, 25, 245. [Google Scholar] [CrossRef]
- Ferela, A.; Debernardi, J.M.; Rosatti, S.; Liebsch, D.; Schommer, C.; Palatnik, J.F. Interplay among ZF-HD and GRF transcription factors during Arabidopsis leaf development. Plant Physiol. 2023, 191, 1789–1802. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Long, Q.; Huang, J.; Wang, Y.; Zhou, K.; Zheng, M.; Sun, J.; Chen, H.; Chen, S.; et al. Overexpression of OsZHD1, a zinc finger homeodomain class homeobox transcription factor, induces abaxially curled and drooping leaf in rice. Planta 2014, 239, 803–816. [Google Scholar] [CrossRef]
- Khatun, K.; Nath, U.K.; Robin, A.H.K.; Park, J.I.; Lee, D.J.; Kim, M.B.; Kim, C.K.; Lim, K.B.; Nou, I.S.; Chung, M.Y. Genome-wide analysis and expression profiling of zinc finger homeodomain (ZHD) family genes reveal likely roles in organ development and stress responses in tomato. BMC Genom. 2017, 18, 695. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Kumari, S.; Olson, A.; Hauser, F.; Ware, D. Role of a ZF-HD Transcription factor in miR157-mediated feed-forward regulatory module that determines plant architecture in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 8665. [Google Scholar] [CrossRef]
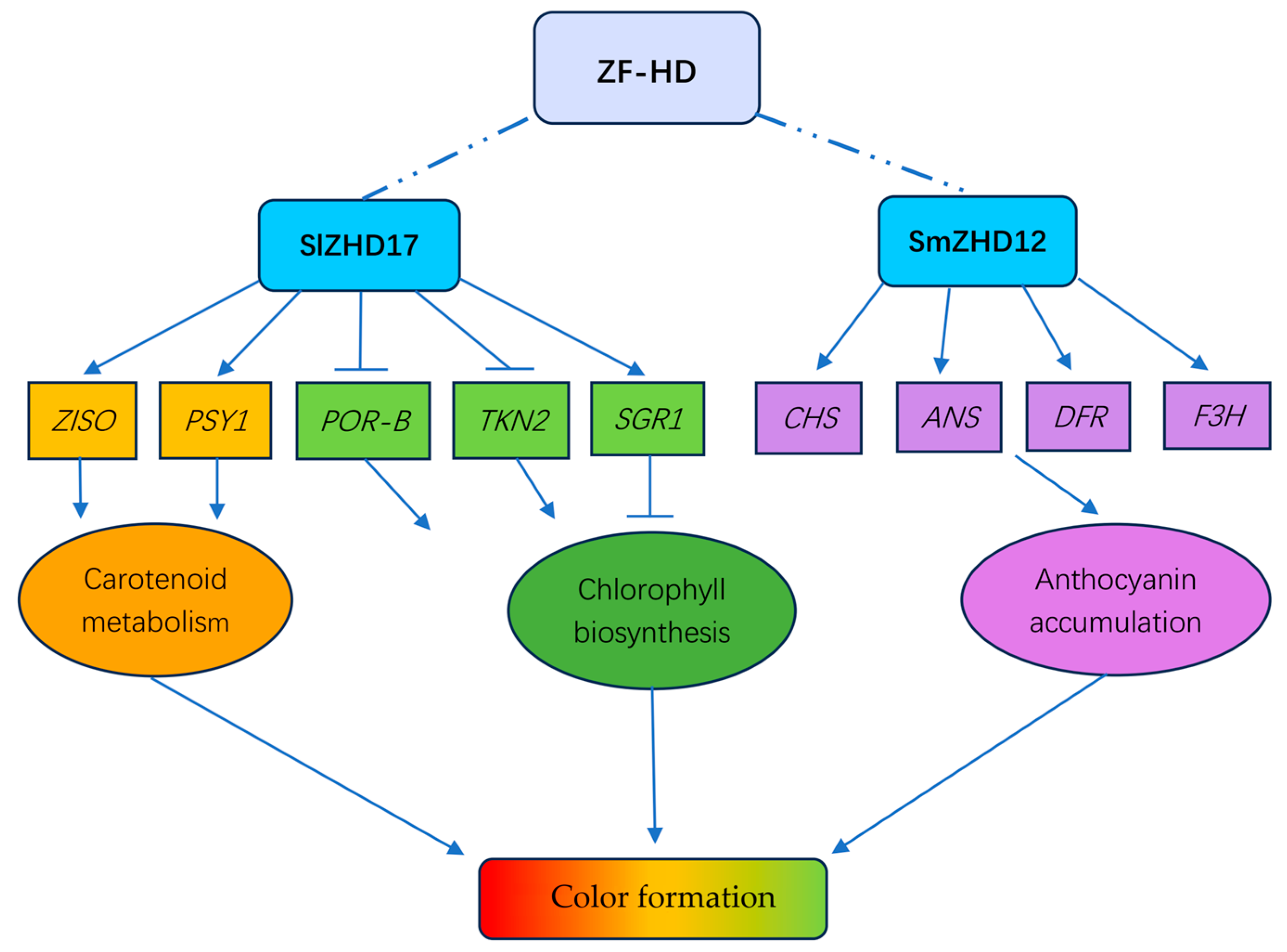
- Shi, Y.; Pang, X.; Liu, W.; Wang, R.; Su, D.; Gao, Y.; Wu, M.; Deng, W.; Liu, Y.; Li, Z. SlZHD17 is involved in the control of chlorophyll and carotenoid metabolism in tomato fruit. Hortic. Res. 2021, 8, 259. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Zhan, W.; Wang, H.; Chen, M.; Li, T.; Bai, T.; Jiao, J.; Song, C.; Song, S.; et al. The apple transcription factor MdZF-HD11 regulates fruit softening by promoting Mdβ-GAL18 expression. J. Exp. Bot. 2024, 75, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, S.; Wei, H.; Yu, R.; Wang, X.; Zeng, B.; Liu, G.; Fan, C. Cloning and functional analysis of EgrZHD5, a ZF-HD transcription factor in Eucalyptus, reveals its role in flowering time and xylem development in Arabidopsis. Plant Mol. Biol. Rep. 2025, 1–10. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, K.; Ware, D. Role of moss and Arabidopsis zinc-finger homeodomain transcription factors in regulating plant architecture. Plant Biotechnol. Rep. 2024, 18, 223–231. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Zhang, H.; Wang, J.; Du, L.; Zhao, Z.; Li, S.; He, Y. Genome-wide characterization of SmZHD gene family and the role of SmZHD12 in regulating anthocyanin biosynthesis in eggplant (Solanum melongena L.). Plant Cell Rep. 2024, 43, 114. [Google Scholar] [CrossRef]
- Abdullah, M.; Cheng, X.; Cao, Y.; Su, X.; Manzoor, M.A.; Gao, J.; Cai, Y.; Lin, Y. Zinc finger-homeodomain transcriptional factors (ZHDs) in upland cotton (Gossypium hirsutum): Genome-wide identification and expression analysis in fiber development. Front. Genet. 2018, 9, 357. [Google Scholar] [CrossRef]
- Kathi, S.; Laza, H.; Singh, S.; Thompson, L.; Li, W.; Simpson, C. A decade of improving nutritional quality of horticultural crops agronomically (2012−2022): A systematic literature review. Sci. Total Environ. 2024, 911, 168665. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Qu, C.; Ling, W.; Wang, T.; Zhang, Y.; Fang, Y.; Guo, Y.; He, M.; Zou, J.; Zhao, S.; et al. A model for the adaptation of Euryale ferox leaves to aquatic environments through EfCGT1-controlled flavonoid C-glycoside-specific accumulation in epidermis cells. Plant Biotechnol. J. 2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, X.; Sun, W.; Ma, Z.; Zheng, T.; Huang, L.; Wu, Q.; Tang, Z.; Bu, T.; Li, C.; et al. Genome-wide investigation of the ZF-HD gene family in Tartary buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2019, 19, 248. [Google Scholar] [CrossRef]
- Tan, Q.; Jiang, S.; Wang, N.; Liu, X.; Zhang, X.; Wen, B.; Fang, Y.; He, H.; Chen, X.; Fu, X.; et al. OVATE family protein PpOFP1 physically interacts with PpZFHD1 and confers salt tolerance to tomato and yeast. Front. Plant Sci. 2021, 12, 759955. [Google Scholar] [CrossRef]
- Thiaw, M.R.N.; Gantet, P. The emerging functions of mini zinc finger (MIF) microproteins in seed plants: A minireview. Biochimie 2024, 218, 69–75. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, C.; Xie, S.; Weng, J.; Lin, Y.; Lai, Z.; Guo, Y. Genome-wide analysis of zinc finger motif-associated homeodomain (ZF-HD) family genes and their expression profiles under abiotic stresses and phytohormones stimuli in tea plants (Camellia sinensis). Sci. Hortic. 2021, 281, 109976. [Google Scholar] [CrossRef]

| Gene | Species | Function | Reference |
|---|---|---|---|
| TaZHD4 | Triticum aestivum | High-temperature acclimation | [17] |
| TaZHD28 | Triticum aestivum | High-temperature acclimation | [17] |
| GHIR_A05G037870.1 | Gossypium hirsutum | Responses to heat, cold, and salinity stress/Fiber development | [22] |
| Ghir_A13G007510.1 | Gossypium hirsutum | Heat-stress tolerance/Response to nematode infection | [22] |
| Ghir_D11G019490.1 | Gossypium hirsutum | Heat-stress tolerance | [22] |
| ZFHD1 | Lilium lancifolium | Cold-stress resistance | [33] |
| LlZFHD4 | Lilium lancifolium | Responses to cold, salt, and water stress | [34,57] |
| OsZHD8 | Oryza sativa | Chilling-stress resistance | [35] |
| PsZHD10 | Pisum sativum | Response to low temperature | [36] |
| ZFHD1 | Arabidopsis thaliana | Drought-stress tolerance | [40] |
| CsZF-HDs | Cucumis sativus | Response to drought stress | [41] |
| CsZHD9 | Cucumis sativus | Drought-stress tolerance | [7] |
| CsZHD10 | Cucumis sativus | Drought-stress tolerance | [7] |
| PtrVCS2 | Populus trichocarpa | Drought-stress resistance | [42] |
| NtZF-HD21 | Nicotiana tabacum | Drought-stress tolerance | [43] |
| CqZF-HD14 | Chenopodium quinoa | Drought-stress tolerance | [44] |
| TaZHD1 | Triticum aestivum | Response to moisture stress/Leaf rolling | [45] |
| TaZHD10 | Triticum aestivum | Response to moisture stress/Leaf rolling | [45] |
| ZHD2 | Citrullus lanatus | Drought adaptation | [46] |
| ZFHD10 | Arabidopsis thaliana | Response to low blue light/Hypocotyl elongation/Flowering | [49,50] |
| VcZF-HD1/4/5/9 | Vaccinium spp. | Bud Dormancy | [51] |
| MsZF-HDs | Medicago sativa | Light response | [52] |
| GmZF-HD1 | Glycine max | Response to salt stress and pathogens | [54,66] |
| GmZF-HD2 | Glycine max | Response to pathogens | [66] |
| HB24 | Arabidopsis thaliana | Salt-stress resistance/Sucrose supply/Auxin metabolism/Root hair elongation | [56,74] |
| ATHB29 | Arabidopsis thaliana | Response to heavy metal stress | [61] |
| MeZHD7 | Manihot esculenta | Resistance against bacterial blight | [13] |
| ZHD5 | Arabidopsis thaliana | Resistance to pathogens/Shoot regeneration | [67] |
| HB27 | Arabidopsis thaliana | Tolerance to cucumber mosaic virus | [68] |
| VvZF-HD11 | Vitis vinifera | Resistance to high temperatures | [78] |
| SlHB25 | Solanum lycopersicum | Stomatal formation | [80] |
| SlHB31 | Solanum lycopersicum | Stomatal formation | [80] |
| BHB2 | Arabidopsis thaliana | Hypocotyl elongation | [81] |
| MdZF-HD1/2/6/7/10 | Malus domestica | Ethylene-induced fruit ripening | [82] |
| MdZF-HD11 | Malus domestica | Fruit ripening and softening | [82,91] |
| OsZHD1 | Oryza sativa | Cell proliferation/Plant height/Grain size formation/Leaf rolling | [8,87] |
| OsZHD2 | Oryza sativa | Cell proliferation/Plant height/Grain size formation | [8] |
| HB33 | Arabidopsis thaliana | Leaf development/Flower development | [86,89] |
| SlZHD | Solanum lycopersicum | Flower development | [88] |
| HB31 | Arabidopsis thaliana | Flower development | [89] |
| HB34 | Arabidopsis thaliana | Flower development | [89] |
| SlZHD17 | Solanum lycopersicum | Chlorophyll and carotenoid metabolism | [90] |
| EgrZHD5 | Eucalyptus grandis | Plant height/Stem thickness | [92] |
| PpZF-HD1 | Physcomitrella patens | Plant architecture formation | [93] |
| SmZHD12 | Solanum melongena | Anthocyanin biosynthesis | [94] |
| GhZHDs | Gossypium hirsutum | Proanthocyanidin accumulation | [95] |
| EfZHD17 | Euryale ferox | Flavonoid C-glycoside accumulation | [97] |
| EfZHD19 | Euryale ferox | Flavonoid C-glycoside accumulation | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, A.-Q.; Lv, M.-Y.; Ge, Y.-X.; Zhou, J.; Hu, Z.-Z.; Ren, X.-Q.; Xiong, A.-S.; Wang, G.-L. Zinc Finger-Homeodomain Transcription Factor: A New Player in Plant Growth, Stress Response, and Quality Regulation. Agronomy 2025, 15, 1522. https://doi.org/10.3390/agronomy15071522
Shen A-Q, Lv M-Y, Ge Y-X, Zhou J, Hu Z-Z, Ren X-Q, Xiong A-S, Wang G-L. Zinc Finger-Homeodomain Transcription Factor: A New Player in Plant Growth, Stress Response, and Quality Regulation. Agronomy. 2025; 15(7):1522. https://doi.org/10.3390/agronomy15071522
Chicago/Turabian StyleShen, An-Qing, Mei-Yan Lv, Yan-Xin Ge, Jin Zhou, Zhen-Zhu Hu, Xu-Qin Ren, Ai-Sheng Xiong, and Guang-Long Wang. 2025. "Zinc Finger-Homeodomain Transcription Factor: A New Player in Plant Growth, Stress Response, and Quality Regulation" Agronomy 15, no. 7: 1522. https://doi.org/10.3390/agronomy15071522
APA StyleShen, A.-Q., Lv, M.-Y., Ge, Y.-X., Zhou, J., Hu, Z.-Z., Ren, X.-Q., Xiong, A.-S., & Wang, G.-L. (2025). Zinc Finger-Homeodomain Transcription Factor: A New Player in Plant Growth, Stress Response, and Quality Regulation. Agronomy, 15(7), 1522. https://doi.org/10.3390/agronomy15071522







