The Alleviating Effect of Brassinosteroids on Cadmium Stress in Potato Plants: Insights from StDWF4 Gene Overexpression
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Observation of Roots Ultrastructure
2.3. MDA Content and Enzymatic Antioxidant Activities Determination
2.4. Ion Concentrations Detection
2.5. Identification and Quantification of Plant Hormones
2.6. Isolation and Analysis of Total RNA by Illumina Sequencing
2.7. Filtering of Sequences, Sequence Assembly, and Analysis of Unigene Expression
2.8. Annotation and Clustering Analysis of DEGs
2.9. Weighted Gene Co-Expression Network (WGCNA) Analysis
2.10. Assessment of Transcriptome Accuracy Through qRT-PCR
2.11. Statistical Analysis
3. Results
3.1. Phenotypic Comparison of the Potatoes Under Cd Stresses
3.2. Cell Ultrastructure Changes of WT and OE Potato Roots Under Cd Stresses
3.3. Physiological Response of WT and OE Potatoes to Cd Stress Conditions
3.4. Analysis of Cd2+ Concentrations
3.5. Changes in Phytohormone Levels
3.6. Analysis of Transcriptome Sequencing and Comprehensive Gene Expression Profiling
3.7. Differentially Expressed Gene Analysis
3.8. GO and KEGG Analysis of DEGs in OE and WT Potatoes
3.9. TFs in DEGs of Potato Roots Under Cd Stress
3.10. DEGs Related to Phytohormone Signaling of Potato Plants Responded to Cd Stresses
3.11. WGCNA Analysis of OE and WT Potatoes Under Cd Stresses
3.12. qRT-PCR Validation
4. Discussion
4.1. Physiological Levels Response to Cd Stress of OE and WT Plants
4.2. Phytohormone Levels Regulation of Cd Stresses in OE and WT Plants
4.3. Transcriptional Levels Regulation of Potato Response to Cd Stresses
4.4. A Plausible Network Responding to Cd Stress in Potatoes Transgenic for the StDWF4 Gene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Q.; Guo, J.J.; He, T.C.; Shen, C.; Huang, Y.Y.; Chen, J.X.; Guo, J.H.; Yuan, J.G.; Yang, Z.Y. Comparative transcriptome analysis between low- and high-cadmium-accumulating genotypes of Pakchoi (Brassica chinensis L.) in response to cadmium stress. Environ. Sci. Technol. 2016, 50, 6485–6494. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Nie, Z.; Liu, H.; Zhao, P.; Qin, S.Y.; Shi, Z.W. Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ. Exp. Bot. 2018, 150, 232–239. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.f.; Ok, S.Y.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Li, Y.; Wen, Y.; Zhao, H.; Wang, Q.; Li, S.W. Transcriptome analysis provides molecular evidences for growth and adaptation of plant roots in cadmium-contaminated environments. Ecotoxicol. Environ. Saf. 2020, 204, 111098. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ullah, E.; Wang, L.C. Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. Clean-Soil Air Water 2016, 44, 29–36. [Google Scholar] [CrossRef]
- Chen, L.; Long, C.; Wang, D.; Yang, J.Y. Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere 2020, 242, 125112. [Google Scholar] [CrossRef]
- Li, B.B.; Fu, Y.S.; Li, X.X.; Yin, H.N.; Xi, Z.M. Brassinosteroids alleviate cadmium phytotoxicity by minimizing oxidative stress in grape seedlings: Toward regulating the ascorbate-glutathione cycle. Sci. Hortic. 2022, 299, 11100. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S.C. Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Guo, J.J.; Qin, S.Y.; Rengel, Z.; Gao, W.; Nie, Z.J.; Li, C. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol. Environ. Saf. 2019, 172, 380–387. [Google Scholar] [CrossRef]
- Pan, C.L.; Lu, H.L.; Liu, J.C.; Wang, Q.; Li, J.W.; Yang, J.J.; Hang, H.L.; Yan, C.L. SODs involved in the hormone mediated regulation of H2O2 content in Kandelia obovata root tissues under cadmium stress. Environ. Pollut. 2020, 256, 113272. [Google Scholar] [CrossRef]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.D.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40 (Suppl. S1), 373–386. [Google Scholar] [CrossRef] [PubMed]
- Demecsova, L.; Zelinov, V.; Liptáková, Ľ.; Tamás, L. Mild cadmium stress induces auxin synthesis and accumulation, while severe cadmium stress causes its rapid depletion in barley root tip. Environ. Exp. Bot. 2020, 175, 104038. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.T.; Li, L.Y.; Duan, Q.X.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant Signal. Behav. 2021, 16, 1836884. [Google Scholar] [CrossRef]
- Gao, M.Y.; Wang, Z.T.; Jia, Z.Z.; Zhang, H.Y.; Wang, T. Brassinosteroids alleviate nanoplastic toxicity in edible plants by activating antioxidant defense systems and suppressing nanoplastic uptake. Environ. Int. 2023, 174, 107901. [Google Scholar] [CrossRef]
- Sharma, A.; Ramakrishnan, M.; Khanna, K.; Landi, M.; Prasad, R.; Bhardwaj, R.; Zheng, B.S. Brassinosteroids and metalloids: Regulation of plant biology. J. Hazard. Mater. 2022, 424, 127518. [Google Scholar] [CrossRef]
- Sousa, V.Q.; Messias, W.F.S.; Pereira, Y.C.; Silva, B.R.S.; Lobato, E.F.S.; Alyemeni, M.N.; Ahmad, P.; Lobato, A.K.S. Pretreatment with 24-epibrassinolide synergistically protects root structures and chloroplastic pigments and upregulates antioxidant enzymes and biomass in Na+-stressed tomato plants. J. Plant Growth Regul. 2022, 41, 2869–2885. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The role of nitrate reductase in brassinosteroid-induced endogenous nitric oxide generation to improve cadmium stress tolerance of pepper plants by up-regulating the ascorbate-glutathione cycle. Ecotoxicol. Environ. Saf. 2020, 196, 110483. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef]
- Miao, R.; Li, C.J.; Liu, Z.L.; Zhou, X.Y.; Chen, S.J.; Zhang, D.; Luo, J.Q.; Tang, W.H.; Wang, C.L.; Wu, J.L.; et al. The role of endogenous brassinosteroids in the mechanisms regulating plant reactions to various abiotic stresses. Agronomy 2024, 14, 356. [Google Scholar] [CrossRef]
- Fujita, S.; Ohnishi, T.; Watanabe, B.; Yokta, T.; Takatsuto, S.; Fujioka, S.; Yoshida, S.; Sakata, K.; Mizutani, M. Arabidopsis CYP90B1 catalyses the early C-22 hydroxylation of C27, C28 and C29 sterols. Plant J. 2006, 45, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.F.; Han, C.Y.; Chen, X.X.; Wu, X.L.; Mi, J.X.; Wan, X.Q.; Liu, Q.L.; He, F.; Chen, L.H.; Yang, H.B.; et al. PscCYP716A1-mediated brassinolide biosynthesis increases cadmium tolerance and enrichment in poplar. Front. Plant Sci. 2022, 13, 919682. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Renou, J.P.; Berthome, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, H.J.; Chen, Y.T.; Pan, H.W.; Wang, S.F. Transcriptome sequencing and expression analysis of cadmium (Cd) transport and detoxification related genes in Cd-accumulating Salix integra. Front. Plant Sci. 2016, 7, 1577. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhang, N.; Yang, J.W.; Tang, X.; Wen, Y.K.; Si, H.J. Functional analysis of StDWF4 gene in response to salt stress in potato. Plant Physiol. Biochem. 2018, 125, 63–73. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Ma, Y.M.; Miao, R.; Li, C.J.; Liu, Z.L.; Zhang, D.; Chen, S.J.; Luo, J.Q.; Tang, W.H. Physiological responses and transcriptomic analysis of StCPD gene overexpression in potato under salt stresses. Front. Plant Sci. 2024, 15, 1297812. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhang, N.; Yang, J.W.; Si, H.J. Functional analysis of potato CPD gene: A rate-limiting enzyme in brassinosteroid biosynthesis under polyethylene glycol-induced osmotic stress. Crop Sci. 2016, 56, 2675–2687. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, H.Q.; Nie, Z.J.; Tao, Z.K.; Peng, H.Y.; Shi, H.Z.; Zhao, P.; Liu, H.E. Combined application of arbuscular mycorrhizal fungi and selenium fertilizer increased wheat biomass under cadmium stress and shapes rhizosphere soil microbial communities. BMC Plant Biol. 2024, 24, 359. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, Y.; Li, W.L.; Li, G.C.; Jin, N.; Zhao, X.Q.; Zhang, D. Development and comprehensive SPE-UHPLC-MS/MS analysis optimization, comparison, and evaluation of 2,4-epibrassinolide in different plant tissues. Molecules 2022, 27, 831. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef]
- Bhandari, S.; Nailwal, T.K. Role of brassinosteroids in mitigating abiotic stresses in plants. Biologia 2020, 75, 2203–2230. [Google Scholar] [CrossRef]
- Rahman, S.U.; Li, Y.L.; Hussain, S.; Hussain, B.; Khan, W.; Riaz, L.; Ashraf, M.N.; Du, Z.J.; Cheng, H.F. Role of phytohormones in heavy metal tolerance in plants: A review. Ecol. Indic. 2023, 146, 109844. [Google Scholar] [CrossRef]
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Huang, X.; Sha, A.J.; He, J.; Chen, X.D.; Xiao, W.Q.; Peng, L.X.; Zou, L.; Liu, B.L.; Li, Q. Analysis of growth physiological changes and metabolome of highland barley seedlings under cadmium (II) stress. Environ. Pollut. 2025, 367, 125664. [Google Scholar] [CrossRef]
- Basit, F.; Liu, J.X.; An, J.Y.; Chen, M.; He, C.; Zhu, X.B.; Li, Z.; Hu, J.; Guan, Y.J. Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ. Sci. Pollut. Res. 2021, 28, 44768–44779. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N. Ahmad P24-Epibrassinolide alleviates the injurious effects of Cr(VI) toxicity in tomato plants: Insights into growth, physio-biochemical attributes, antioxidant activity and regulation of Ascorbate-glutathione and Glyoxalase cycles. J. Plant Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]
- Hayat, S.; Hasan, S.A.; Hayat, Q.; Ahmad, A. Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma 2010, 239, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, S.; Rao, S. The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil Environ. 2018, 53, 465–472. [Google Scholar] [CrossRef]
- An, S.M.; Liu, Y.; Sang, K.Q.; Wang, T.; Yu, J.Q.; Zhou, Y.H.; Xia, X.J. Brassinosteroid signaling positively regulates abscisic acid biosynthesis in response to chilling stress in tomato. J. Integr. Plant Biol. 2023, 65, 10–24. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegon-Putze, I.; Bosc, N.; Ibanes, M.; Cano-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef]
- Tong, H.N.; Chu, C.C. Functional specificities of brassinosteroid and potential utilization for crop improvement. Trends Plant Sci. 2018, 23, 1016–1028. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, H.Y.; Zhang, S.; Gao, F.W.; Sun, W.; Ren, X.K. Interactive effect of 24-epibrassinolide and silicon on the alleviation of cadmium toxicity in rice (Oryza sativa L.) plants. Environ. Technol. 2024, 45, 4725–4736. [Google Scholar] [CrossRef]
- Popova, L.P.; Maslenkova, L.T.; Ivanova, A.; Stoinova, Z. Role of salicylic acid in alleviating heavy metal stress. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 447–466. [Google Scholar] [CrossRef]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmad, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef]
- Han, Z.X.; Wei, X.; Wan, D.J.; He, W.X.; Wang, X.J.; Xiong, Y. Effect of molybdenum on plant physiology and cadmium uptake and trans-location in rape (Brassica napus L.) under different levels of cadmium stress. Int. J. Environ. Res. Public Health 2020, 17, 2355. [Google Scholar] [CrossRef]
- Huang, Q.N.; Zhang, Y.; Liu, S.; Wang, H.; Gao, F.Y.; Shao, G.S. 24-epibrassinolide mitigates cadmium toxicity in rice plants by activating the plant detoxification system and regulating genes expression involved in Cd/Fe uptake and translocation. Plant Stress 2024, 12, 100485. [Google Scholar] [CrossRef]
- Dai, F.W.; Luo, G.Q.; Li, Z.Y.; Wei, X.; Wang, Z.J.; Lin, S.; Tang, C.M. Physiological and transcriptomic analyses of mulberry (Morus atropurpurea) response to cadmium stress. Ecotoxicol. Environ. Saf. 2020, 205, 111298. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.Y.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.L.; Sun, S.X.; Li, Y.M.; Dossa, K.; Luan, Y.P. Physiological and transcriptional mechanisms associated with cadmium stress tolerance in Hibiscus syriacus L. BMC Plant Biol. 2023, 23, 286. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmed, S.; Abbas, M.; Yasin, N.A. Seed priming with 3-epibrassinolide alleviates cadmium stress in Cucumis sativus through modulation of antioxidative system and gene expression. Sci. Hortic. 2020, 265, 109203. [Google Scholar] [CrossRef]
- Shafi, Z.; Shahid, M.; AlGarawi, A.M.; Zeyad, M.T.; Marey, S.A.; Hatamleh, A.A.; Wang, S.; Singh, U.B. The exogenous application of 24-epibrassinolide (24-EBL) increases the Cd and Pb resilience in Zea mays (L.) by regulating the growth and physiological mechanism. Appl. Biochem. Biotechnol. 2024, 196, 3949–3973. [Google Scholar] [CrossRef]
- Rolón-Cárdenas, G.A.; Arvizu-Gómez, J.L.; Soria-Guerra, R.E.; Pacheco-Aguilar, J.R.; Alatorre-Cobos, F.; Hernández-Morales, A. The role of auxins and auxin-producing bacteria in the tolerance and accumulation of cadmium by plants. Environ. Geochem. Health 2022, 44, 3743–3764. [Google Scholar] [CrossRef]
- Palusińska, M.; Barabasz, A.; Kozak, K.; Papierniak, A.; Maślińska, K.; Antosiewicz, D.M. Zn/Cd status-dependent accumulation of Zn and Cd in root parts in tobacco is accompanied by specific expression of ZIP genes. BMC Plant Biol. 2020, 20, 37. [Google Scholar] [CrossRef]
- Sun, L.N.; Zhang, X.H.; Ouyang, W.K.; Yang, E.D.; Cao, Y.Y.; Sun, R.B. Lowered Cd toxicity, uptake and expression of metal transporter genes in maize plant by ACC deaminase-producing bacteria Achromobacter sp. J. Hazard. Mater. 2022, 423, 127036. [Google Scholar] [CrossRef]
- Liu, Z.G.; Wu, X.Z.; Hou, L.; Ji, S.Z.; Zhang, Y.; Fan, W.R.; Li, T.; Zhang, L.; Liu, P.; Yang, L. Effects of cadmium on transcription, physiology, and ultrastructure of two tobacco cultivars. Sci. Total Environ. 2023, 869, 161751. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, T.; Niu, K.J.; Ma, H.L. Root cell wall polysaccharides and endodermal barriers restrict long-distance Cd translocation in the roots of Kentucky bluegrass. Ecotoxicol. Environ. Saf. 2024, 281, 116633. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, M.; Charfeddine, S.; Bouaziz, D.; Messaoud, R.B.; Bouzid, R.G. The effect of cadmium on transgenic potato (Solanum tuberosum) plants overexpressing the StDREB transcription factors. Plant Cell Tissue Organ Cult. 2017, 128, 521–541. [Google Scholar] [CrossRef]
- Baghel, M.; Nagaraja, A.; Srivastav, M.; Meena, N.K.; Kumar, M.S.; Kumar, A.; Sharma, R.R. Pleiotropic influences of brassinosteroids on fruit crops: A review. Plant Growth Regul. 2019, 87, 375–388. [Google Scholar] [CrossRef]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and response of plants to heavy metals action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Xu, D.; Yang, C.; Li, C.; Luo, K. Molecular cloning and characterization of a brassinosteriod biosynthesis-related gene PtoDWF4 from Populus tomentosa. Tree Physiol. 2018, 38, 1424–1436. [Google Scholar] [CrossRef]
- Si, J.; Sun, Y.; Wang, L.U.; Qin, Y.; Wang, C.; Wang, X. Functional analyses of Populus euphratica brassinosteroid biosynthesis enzyme genes DWF4 (PeDWF4) and CPD (PeCPD) in the regulation of growth and development of Arabidopsis thaliana. J. Biosci. 2016, 41, 727–742. [Google Scholar] [CrossRef]
- Liu, M.Y.; Huang, Z.R.; Xie, K.X.; Guo, C.C.; Wang, Y.D.; Wang, X. Mitostasis is the central biological hub underlying the response of plants to cadmium stress. J. Hazard. Mater. 2023, 441, 129930. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.X.; Shang, E.; Hawar, A.; Ito, T.; Sun, B. Brassinosteroid signaling represses ZINC FINGER PROTEIN11 to regulate ovule development in Arabidopsis. Plant Cell 2024, 36, 5004–5022. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, T.T.; Lu, L.; Qiu, S.J.; Huang, Z.C.; Wang, Y.; Chen, X.Y.; Li, L.; Sun, Y.Y.; Zhang, R.J.; et al. Synergistic interaction between brassinosteroid and jasmonate pathways in rice response to cadmium toxicity. Sci. Total Environ. 2024, 954, 176369. [Google Scholar] [CrossRef]
- Anwar, A.; Yuan, C.; Cui, B.; Wang, L.X.; He, L.L.; Gao, J.W. BrMYB116 transcription factor enhances Cd stress tolerance by activating FIT3 in yeast and Chinese cabbage. Front. Plant Sci. 2024, 15, 1388924. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid signaling, crosstalk and, physiological functions in plants under heavy metal stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, T.; Niu, K.J.; Ma, H.L. Integrated proteomics, transcriptomics, and metabolomics offer novel insights into Cd resistance and accumulation in Poa pratensis. J. Hazard. Mater. 2024, 474, 134727. [Google Scholar] [CrossRef] [PubMed]
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Penna, S.; Kumar, V. Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Opin. Plant Biol. 2021, 27, 100213. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Pandey, P.; Rajpoot, R.; Rani, A.; Dubey, R.S. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 2014, 251, 1047–1065. [Google Scholar] [CrossRef]
- Casanova-Sáez, R.; Voß, U. Auxin metabolism controls developmental decisions in land plants. Trends Plant Sci. 2019, 24, 741–754. [Google Scholar] [CrossRef]
- Bashir, W.; Anwar, S.; Zhao, Q.; Hussain, I.; Xie, F.T. Interactive effect of drought and cadmium stress on soybean root morphology and gene expression. Ecotoxicol. Environ. Saf. 2019, 175, 90–101. [Google Scholar] [CrossRef]
- Liu, C.; Chang, J.B.; Yang, J.X.; Li, H.C.; Wu, J.; Wu, J.L.; Dai, X.Y.; Wei, F.J.; Zhang, X.Q.; Su, X.H.; et al. Overexpression of NtDOGL4 improves cadmium tolerance through abscisic acid signaling pathway in tobacco. J. Hazard. Mater. 2024, 465, 133462. [Google Scholar] [CrossRef]
- Li, Z.W.; Zhang, R.S.; Zhang, H.M. Effects of plant growth regulators (DA-6 and 6-BA) and EDDS chelator on phytoextraction and detoxification of cadmium by Amaranthus hybridus Linn. Int. J. Phytoremediation 2018, 20, 1121–1128. [Google Scholar] [CrossRef]
- Xian, J.P.; Wang, Y.; Niu, K.J.; Ma, H.L.; Ma, X. Transcriptional regulation and expression network responding to cadmium stress in a Cd-tolerant perennial grass Poa pratensis. Chemosphere 2020, 250, 126158. [Google Scholar] [CrossRef]
- Wu, X.Z.; Yan, J.Y.; Qin, M.Z.; Li, R.Z.; Jia, T.; Liu, Z.G.; Ahmad, P.; El-Sheikh, M.A.; Yadav, K.K.; Rodríguez-Díaz, J.M.; et al. Comprehensive transcriptome, physiological and biochemical analyses reveal that key role of transcription factor WRKY and plant hormone in responding cadmium stress. J. Environ. Manag. 2024, 367, 121979. [Google Scholar] [CrossRef]
- Yao, X.N.; Cai, Y.R.; Yu, D.Q.; Liang, G. bHLH104 confers tolerance to cadmium stress in Arabidopsis thaliana. J. Integr. Plant Biol. 2018, 60, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Z.; Li, M.Y.; Guo, Z.T.; Chen, J.F.; Wu, J.Y.; Xia, Z.L. The transcription factor ZmbHLH105 confers cadmium tolerance by promoting abscisic acid biosynthesis in maize. J. Hazard. Mater. 2024, 480, 135826. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Ahmed, K.B.M.; Alam, P.; Hayat, S. 24-Epibrassinolide-mediated mitigation of Cd-induced toxicity in hyperaccumulator—Brassica juncea: Influence on photosynthesis, cell death, redox, and elemental status. J. Plant Growth Regul. 2023, 42, 2646–2661. [Google Scholar] [CrossRef]
- Peng, R.N.; Sun, W.Y.; Jin, X.X.; Yu, L.J.; Chen, C.; Yue, Z.H.; Dong, Y.L. Analysis of 2,4-epibrassinolide created an enhancement tolerance on Cd toxicity in Solanum nigrum L. Environ. Sci. Pollut. Res. 2020, 27, 16784–16797. [Google Scholar] [CrossRef]
- Duan, F.; Ding, J.; Lee, D.S.; Lu, X.L.; Feng, Y.Q.; Song, W.W. Overexpression of SoCYP85A1, a spinach cytochrome p450 gene in transgenic tobacco enhances root development and drought stress tolerance. Front. Plant Sci. 2017, 8, 1909. [Google Scholar] [CrossRef]
- Cui, T.; Wang, Y.; Niu, K.J.; Dong, W.K.; Zhang, R.; Ma, H.L. Auxin alleviates cadmium toxicity by increasing vacuolar compartmentalization and decreasing long-distance translocation of cadmium in Poa pratensis. J. Plant Physiol. 2023, 282, 153919. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, D.; Niu, D.; Deng, J.; Ma, F.W.; Liu, C.H. Overexpression of auxin response gene MdIAA24 enhanced cadmium tolerance in apple (Malus domestica). Ecotoxicol. Environ. Saf. 2021, 225, 112734. [Google Scholar] [CrossRef]
- Ren, H.; Gray, W.M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Huang, L.P.; Liu, X.; Liu, Y.; Tanveer, M.; Chen, W.; Fu, W.X.; Wang, Q.Q.; Guo, Y.J.; Shabala, S. Revealing mechanistic basis of ameliorating detrimental effects of cadmium in cherry tomatoes by exogenous application of melatonin and brassinosteroids. Ecotoxicol. Environ. Saf. 2024, 283, 116768. [Google Scholar] [CrossRef]
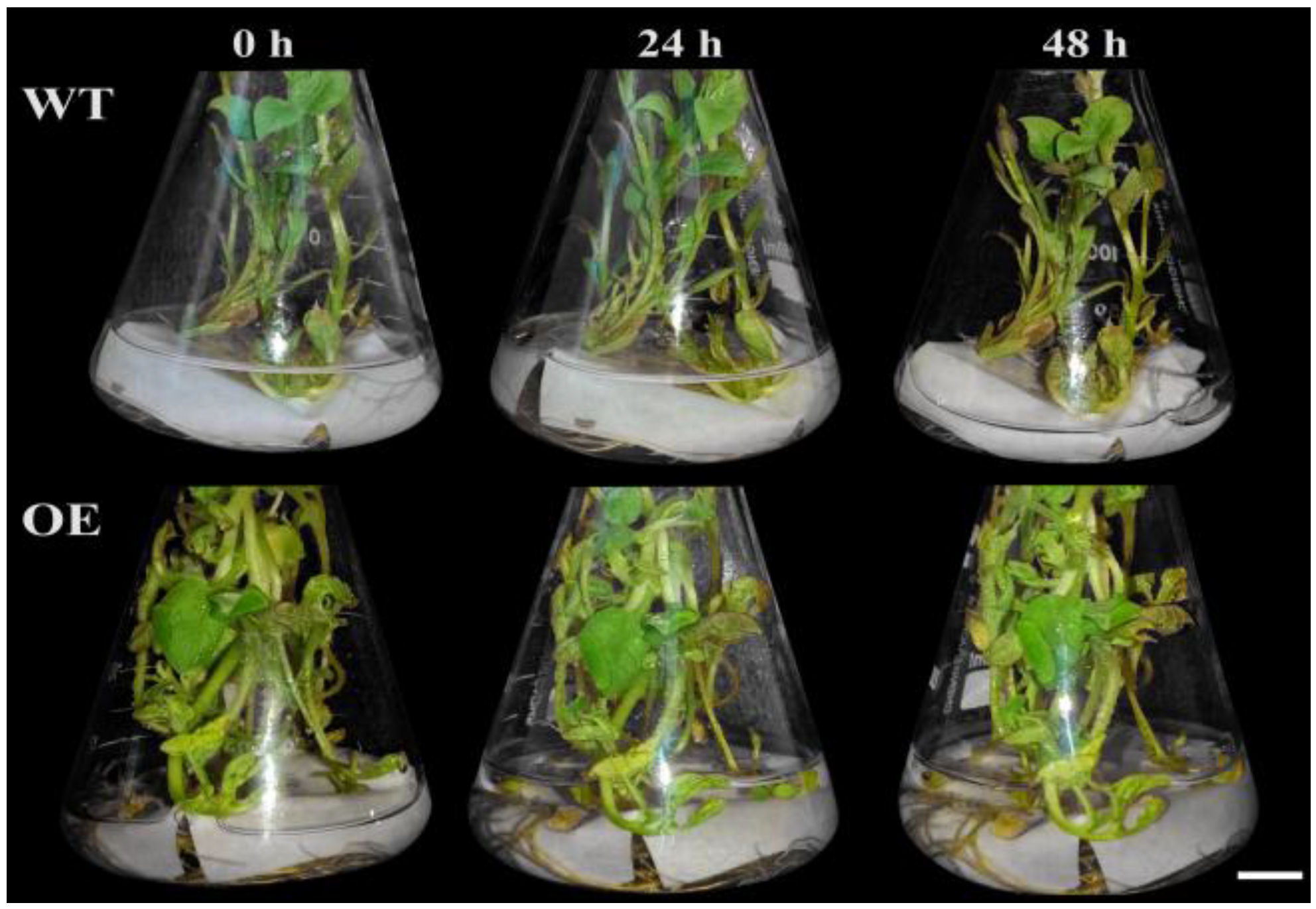
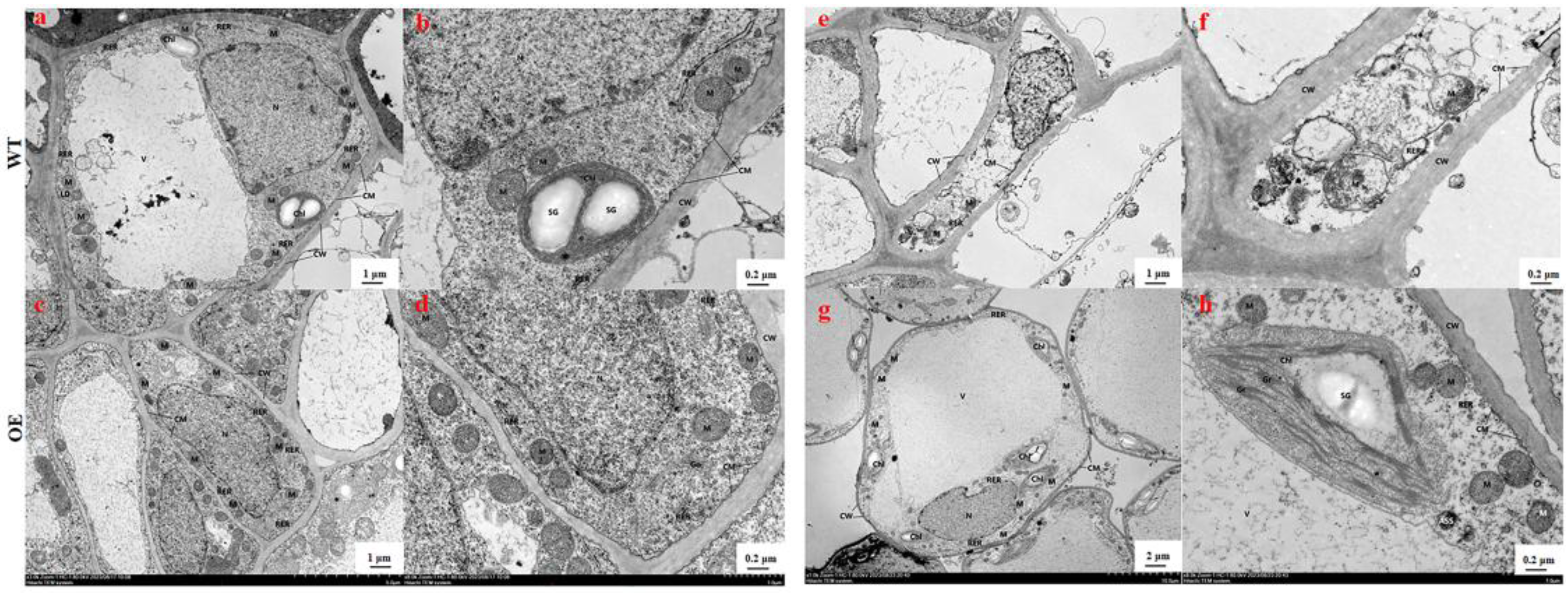

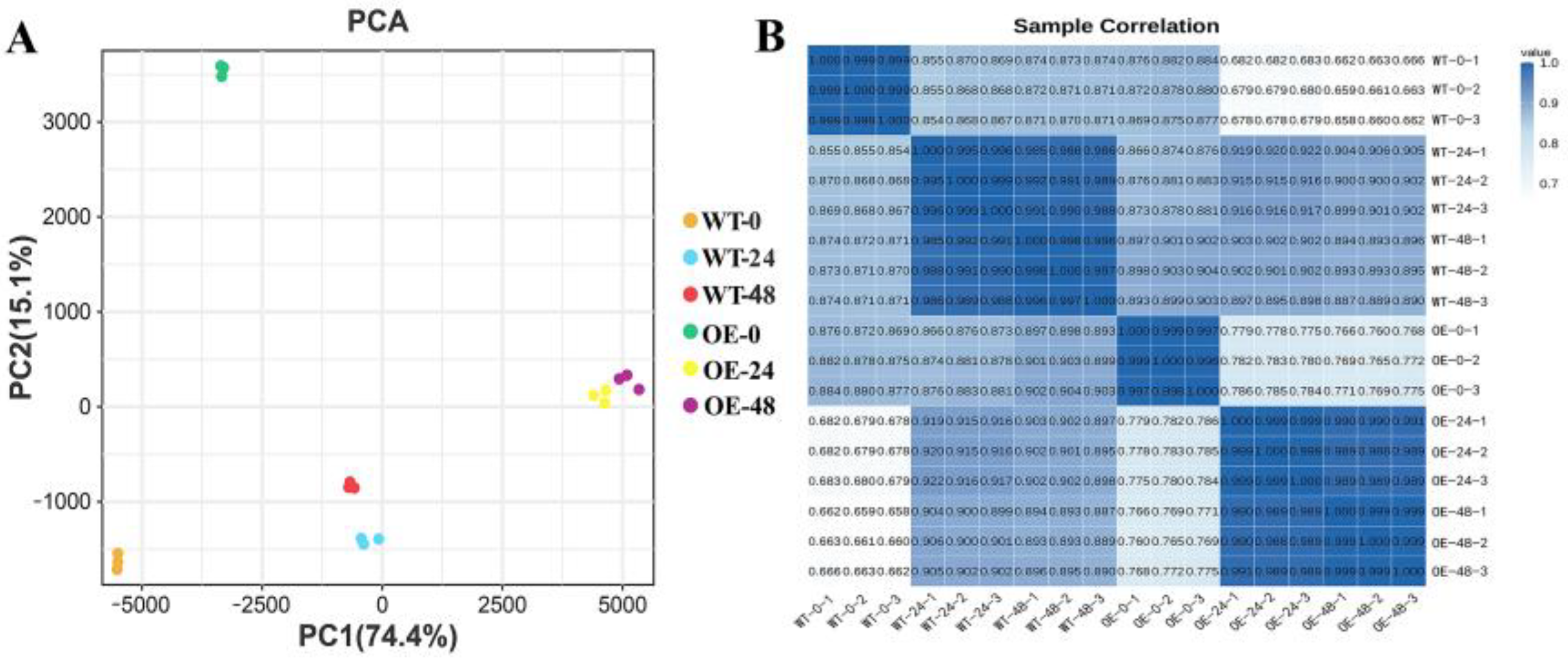
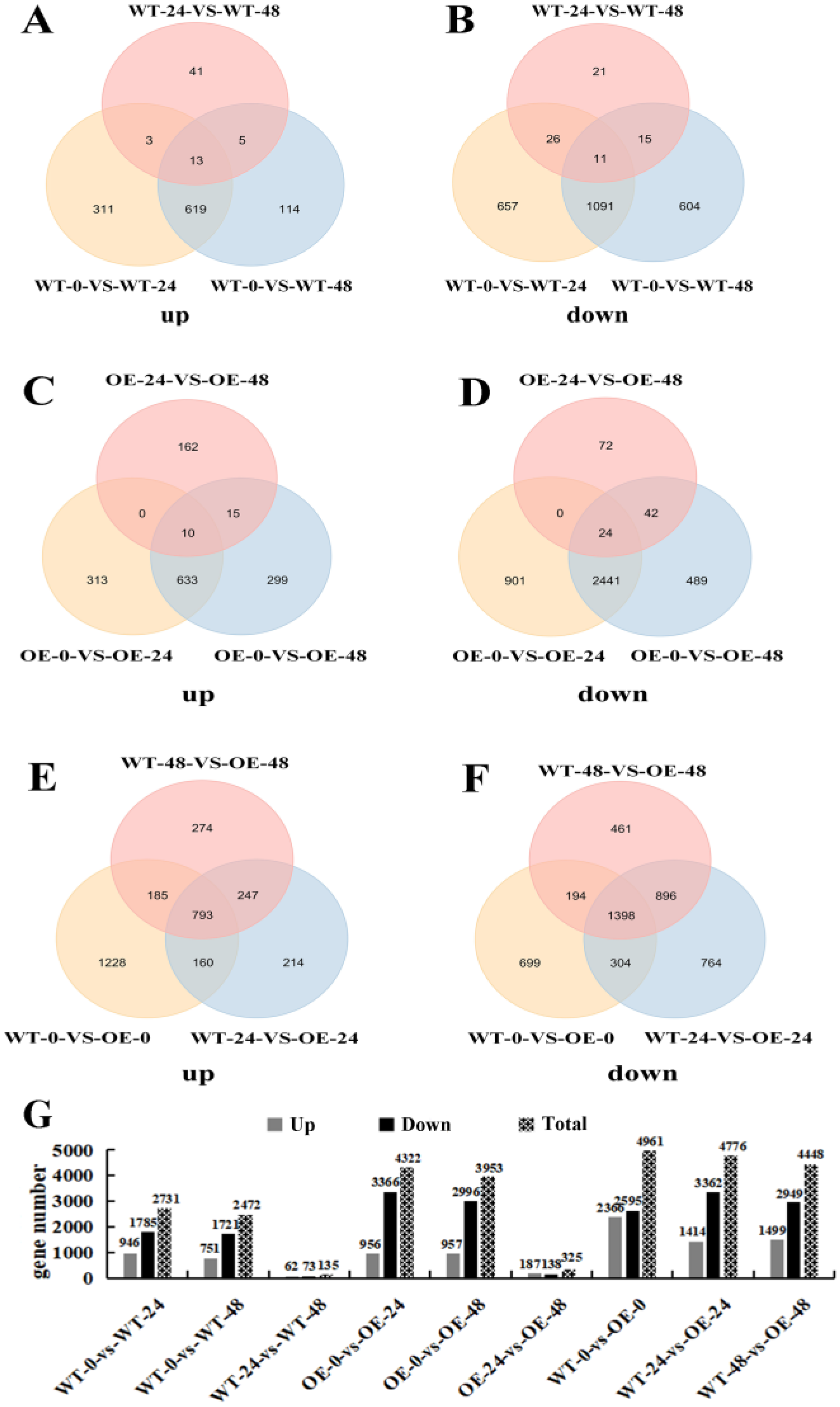




| Treatment Time (h) | Leaf Fresh Weight (g/plant) | Leaf Dry Weight (g/plant) | ||
|---|---|---|---|---|
| WT | OE | WT | OE | |
| 0 | 0.869 ± 0.054 c | 1.309 ± 0.037 ab | 0.086 ± 0.009 ab | 0.096 ± 0.011 ab |
| 24 | 1.036 ± 0.220 bc | 1.592 ± 0.205 a | 0.115 ± 0.027 ab | 0.192 ± 0.023 a |
| 48 | 0.709 ± 0.122 c | 1.469 ± 0.183 a | 0.072 ± 0.013 c | 0.136 ± 0.020 b |
| Treatment Time (h) | Plant Height (cm) | Root Length (cm) | Stem Diameter (mm) | |||
|---|---|---|---|---|---|---|
| WT | OE | WT | OE | WT | OE | |
| 0 | 11.54 ± 0.350 b | 14.22 ± 0.561 a | 9.90 ± 0.503 bc | 11.68 ± 0.493 ab | 1.36 ± 0.084 bc | 1.56 ± 0.063 ab |
| 24 | 9.42 ± 0.524 c | 11.98 ± 0.831 b | 9.06 ± 0.322 c | 12.24 ± 0.684 a | 1.16 ± 0.088 c | 1.59 ± 0.078 ab |
| 48 | 12.74 ± 0.567 ab | 14.08 ± 0.546 a | 9.42 ± 0.706 c | 11.56 ± 0.555 ab | 1.18 ± 0.030 c | 1.71 ± 0.138 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Miao, R.; Luo, J.; Tang, W.; Liu, K.; Li, C.; Zhang, D. The Alleviating Effect of Brassinosteroids on Cadmium Stress in Potato Plants: Insights from StDWF4 Gene Overexpression. Agronomy 2025, 15, 1503. https://doi.org/10.3390/agronomy15071503
Zhou X, Miao R, Luo J, Tang W, Liu K, Li C, Zhang D. The Alleviating Effect of Brassinosteroids on Cadmium Stress in Potato Plants: Insights from StDWF4 Gene Overexpression. Agronomy. 2025; 15(7):1503. https://doi.org/10.3390/agronomy15071503
Chicago/Turabian StyleZhou, Xiangyan, Rong Miao, Jiaqi Luo, Wenhui Tang, Kexin Liu, Caijuan Li, and Dan Zhang. 2025. "The Alleviating Effect of Brassinosteroids on Cadmium Stress in Potato Plants: Insights from StDWF4 Gene Overexpression" Agronomy 15, no. 7: 1503. https://doi.org/10.3390/agronomy15071503
APA StyleZhou, X., Miao, R., Luo, J., Tang, W., Liu, K., Li, C., & Zhang, D. (2025). The Alleviating Effect of Brassinosteroids on Cadmium Stress in Potato Plants: Insights from StDWF4 Gene Overexpression. Agronomy, 15(7), 1503. https://doi.org/10.3390/agronomy15071503





