The Influence of Microbial Community on Soybean Cyst Nematode Under the Condition of Suppressive Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Cyst Collection and Population Estimation
2.3. Biolog Analysis
2.4. Soil Chemical Properties
2.5. Statistical Analysis
3. Results
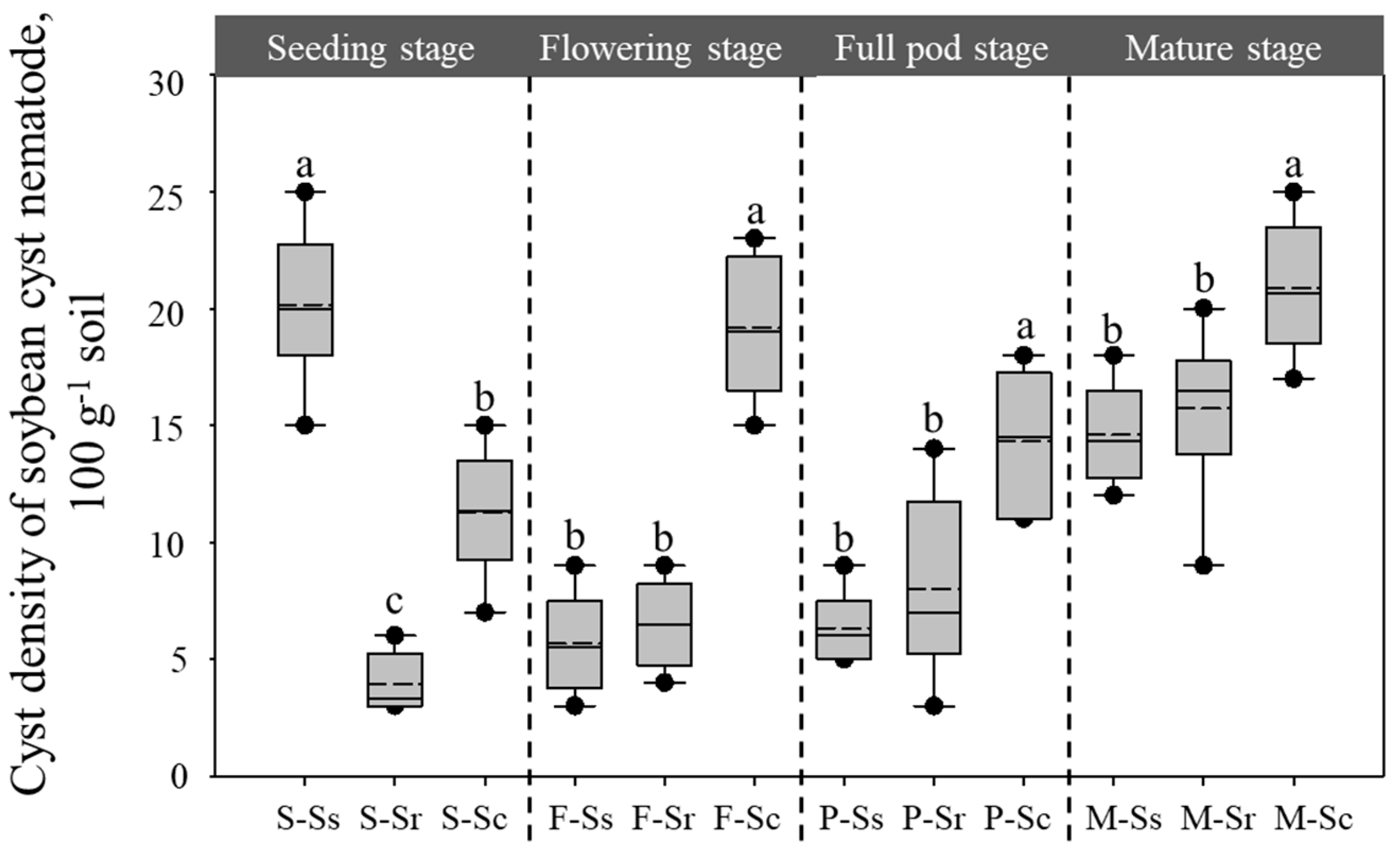
3.1. Population Densities of SCN Cysts and Parasitic Microorganisms
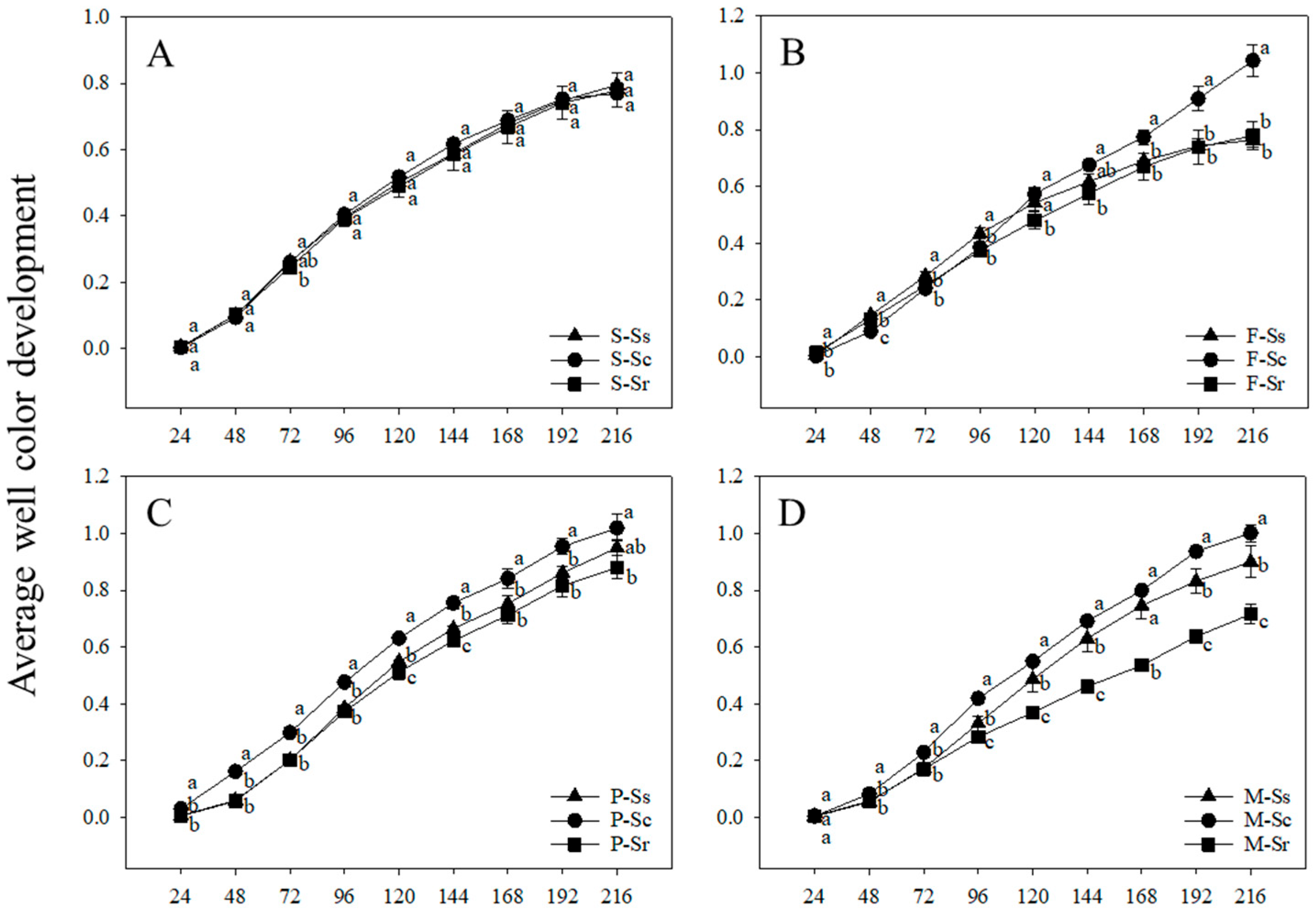
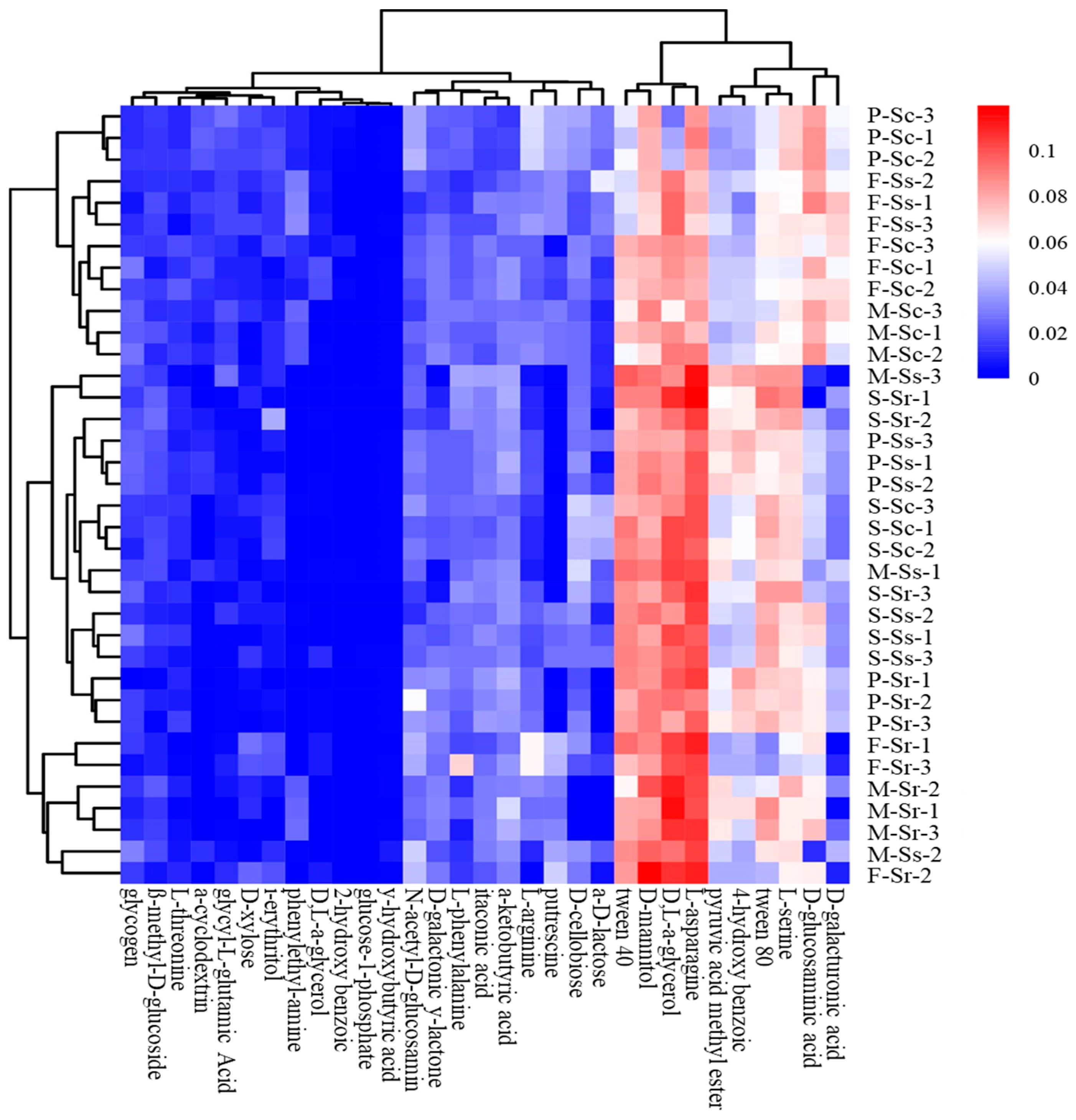
3.2. Metabolic Activity of Parasitic Microbes in SCN Cysts
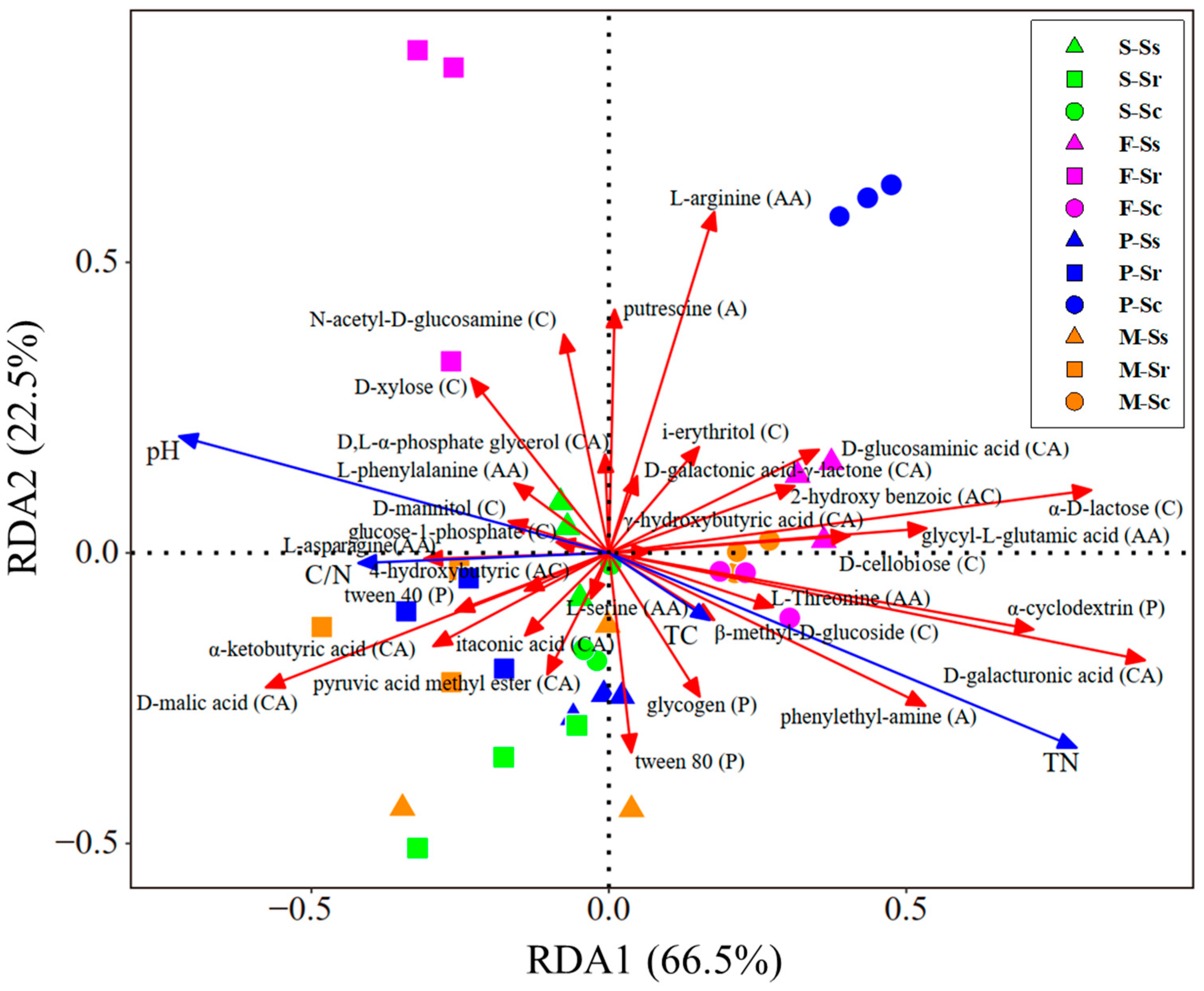
3.3. Community Structure of Parasitic Microbes in SCN Cysts
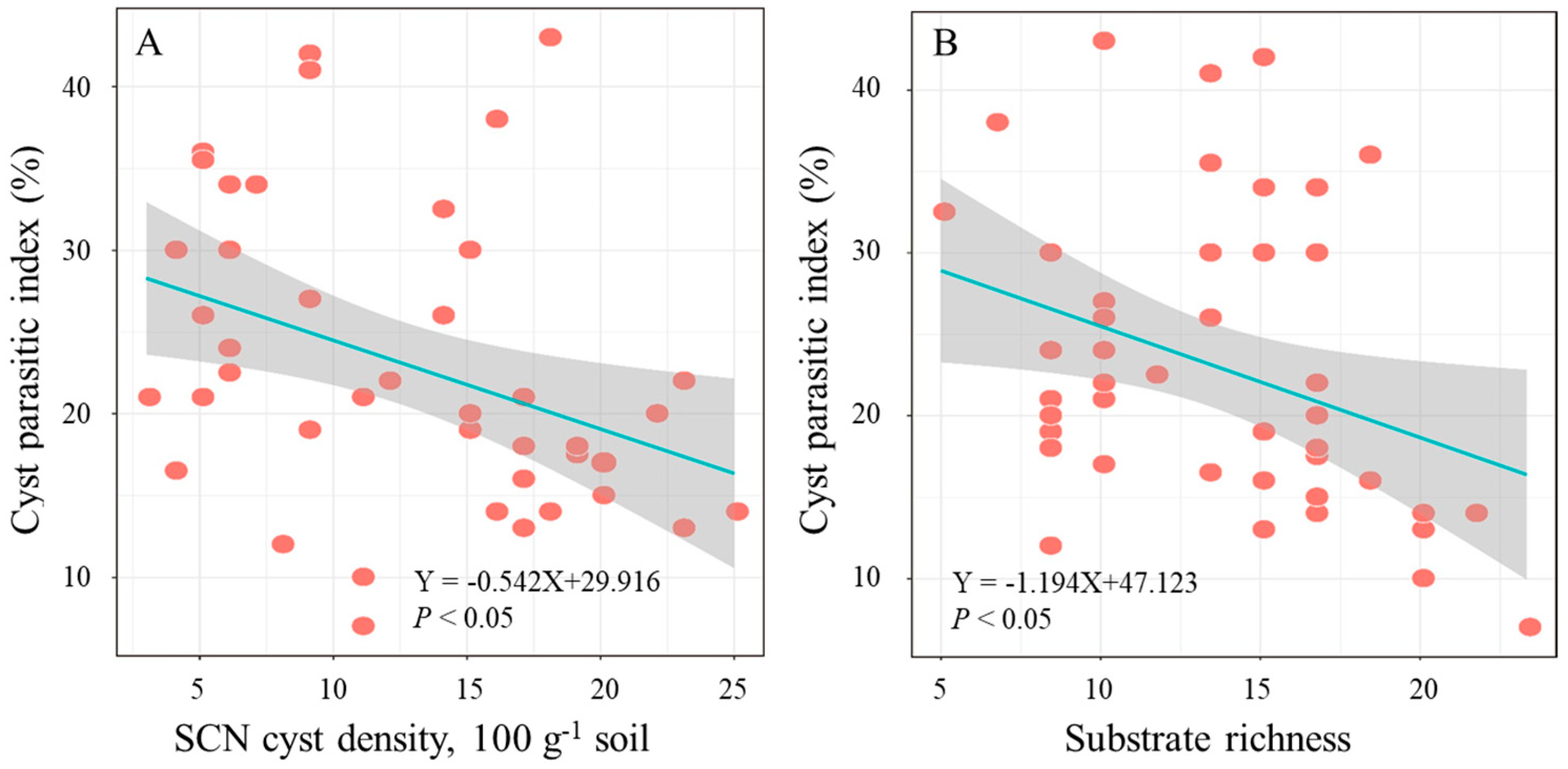
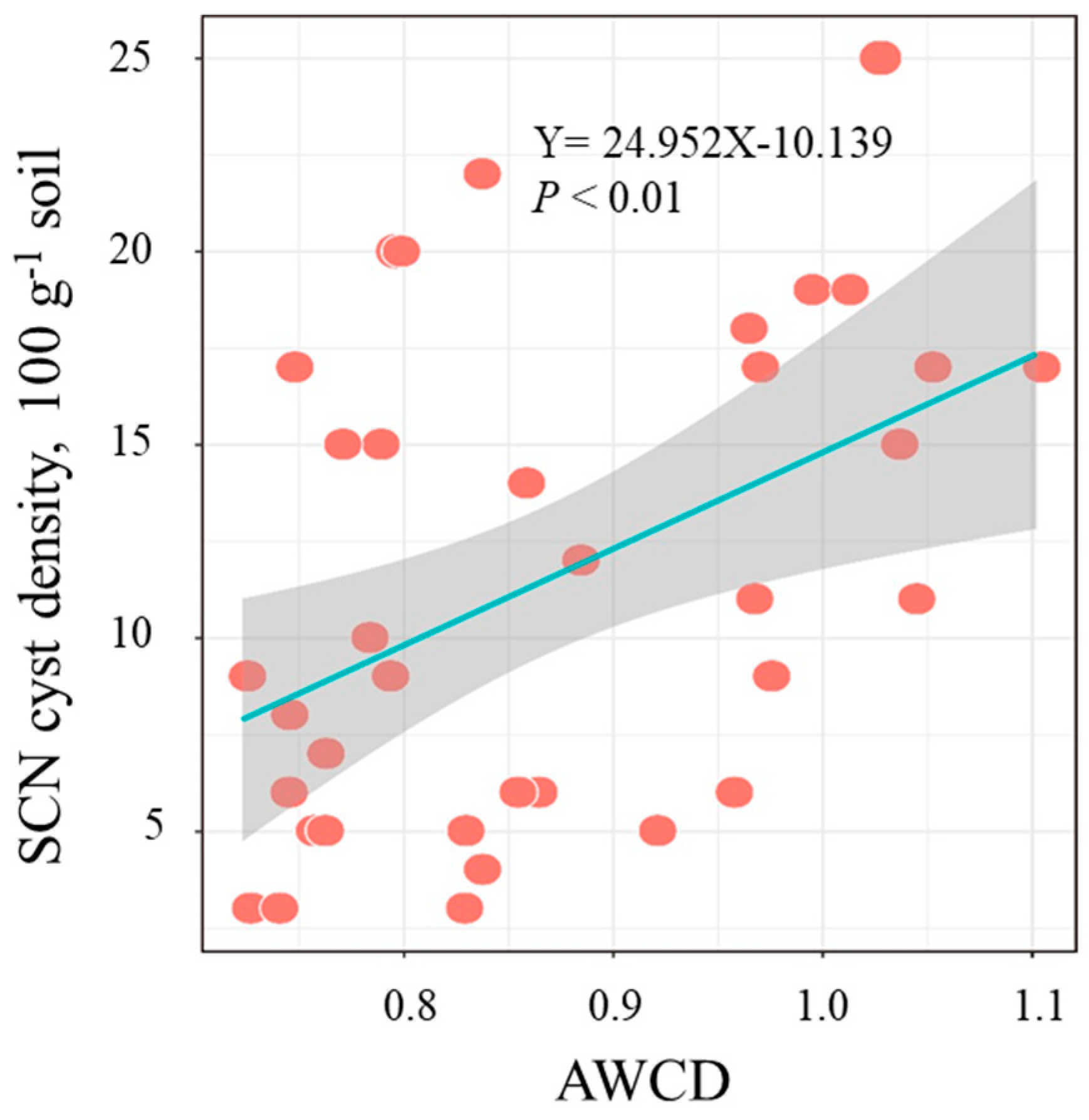
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.Y.; Porter, P.M.; Reese, C.D.; Stienstra, W.C. Crop sequence effects on soybean cyst nematode and soybean and corn yields. Crop. Sci. 2001, 41, 1843–1849. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Wu, J.N.; Perry, R.N.; Toyota, K. Evaluation of soil suppressiveness of various Japanese soils against the soybean cyst nematode Heterodera glycines and its relation with the soil chemical and biological properties. Agron. J. 2023, 13, 2826. [Google Scholar] [CrossRef]
- Zheng, J.W.; Chen, S.Y. Estimation of virulence type and level of soybean cyst nematode field populations in response to resistant cultivars. J. Entomol. Nematol. 2011, 3, 37–43. [Google Scholar]
- Chen, F.J.; Chen, S.Y. Mycofloras in cysts, females, and eggs of the soybean cyst nematode in Minnesota. Appl. Soil Ecol. 2002, 19, 35–50. [Google Scholar] [CrossRef]
- Meyer, S.L.; Huettel, R.N.; Sayre, R.M. Isolation of fungi from Heterodera glycines and in vitro bioassays for their antagonism to eggs. J. Nematol. 1990, 22, 532–537. [Google Scholar]
- Xu, Y.L.; Chen, Y.L.; Si, Z.S.; Li, Z.L.; Li, C.J.; Wen, G.S. The effects of the root diffusate of different crops from different rotation systems on the egg hatch of soybean cyst nematode Heterodera glycines. Acta Phytopathol. Sin. 2004, 34, 481–486. (In Chinese) [Google Scholar] [CrossRef]
- Neher, D.A.; Nishanthan, T.; Grabau, Z.J.; Chen, S.Y. Crop rotation and tillage affect nematode communities more than biocides in monoculture soybean. Appl. Soil Ecol. 2019, 140, 89–97. [Google Scholar] [CrossRef]
- Hu, W.M.; Samac, D.A.; Liu, X.Z.; Chen, S.Y. Microbial communities in the cysts of soybean cyst nematode affected by tillage and biocide in a suppressive soil. Appl. Soil Ecol. 2017, 119, 396–406. [Google Scholar] [CrossRef]
- Sun, M.H.; Liu, X.Z. Suppressive soils of soybean cyst nematode in China. Acta Phytopathol. Sin. 2000, 30, 353–356. [Google Scholar] [CrossRef]
- Song, J.; Li, S.X.; Wei, W.; Xu, Y.L.; Yao, Q. Assessment of parasitic fungi for reducing soybean cyst nematode with suppressive soil in soybean fields of northeast China. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 730–736. [Google Scholar] [CrossRef]
- Wei, W.; Xu, Y.L.; Li, S.X.; Zhu, L.; Song, J. Developing suppressive soil for root diseases of soybean with continuous long-term cropping of soybean in black soil of Northeast China. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 279–285. [Google Scholar] [CrossRef]
- Wang, S.; Hafeez, A.; Zhang, T.; Rao, M.J.; Li, S.; Cai, K. Silicon-modified Solidago canadensis L. biochar suppresses soilborne disease and improves soil quality. Biochar 2025, 7, 3. [Google Scholar] [CrossRef]
- Hamid, M.I.; Hussain, M.; Wu, Y.; Zhang, X.; Xiang, M.; Liu, X. Successive soybean-monoculture cropping assembles rhizosphere microbial communities for the soil suppression of soybean cyst nematode. FEMS Microbiol. Ecol. 2017, 93, fiw222. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pan, F.J.; Han, X.Z.; Song, F.B.; Zhang, Z.M.; Yan, J.; Xu, Y.L. Response of soil fungal community structure to long-term continuous soybean cropping. Front. Microbiol. 2019, 9, 3316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.B.; Shi, F.Y.; Tian, J.Q.; Liu, J.B.; Chen, S.Y.; Xiang, M.C.; Liu, X.Z. Effect of soybean monoculture on the bacterial communities associated with cysts of Heterodera glycines. J. Nematol. 2013, 45, 228–235. [Google Scholar]
- Liu, X.; Zhang, J.L.; Gu, T.Y.; Zhang, W.M.; Shen, Q.R.; Yin, S.X.; Qiu, H.Z. Microbial community diversities and taxa abundances in soils along a seven-year gradient of potato monoculture using high throughput pyrosequencing approach. PLoS ONE 2014, 9, e86610. [Google Scholar] [CrossRef]
- Hussain, M.; Xuan, P.; Xin, Y.; Ma, H.; Zhou, Y.; Wen, S.; Hamid, M.I.; Wan, T.; Hu, J.; Li, Y. Redundancy in microbiota-mediated suppression of the soybean cyst nematode. Microbiome 2024, 12, 125. [Google Scholar] [CrossRef]
- Nadarajah, K. Role of microbiome in the generation of disease-suppressive soil for sustainable agriculture. ScienceAsia 2024, 50, 1–8. [Google Scholar] [CrossRef]
- Hu, W.M.; Strom, N.; Haarith, D.; Chen, S.Y.; Bushley, K.E. Mycobiome of cysts of the soybean cyst nematode under long term crop rotation. Front. Microbiol. 2018, 368, 386. [Google Scholar] [CrossRef]
- Nour, S.M.; Lawrence, J.R.; Zhu, H.; Swerhone, G.D.W.; Welsh, M.; Welacky, T.W.; Topp, E. Bacteria associated with cysts of the soybean cyst nematode (Heterodera glycines). Appl. Environ. Microb. 2003, 69, 607–615. [Google Scholar] [CrossRef]
- Song, J.; Li, S.X.; Xu, Y.L.; Wei, W.; Yao, Q.; Pan, F.J. Diversity of parasitic fungi from soybean cyst nematode associated with long-term continuous cropping of soybean in black soil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 432–442. [Google Scholar] [CrossRef]
- Morgan-Jones, G.; Gintis, B.O.; Rodriguez-Kabana, R. Fungal colonisation of Heterodera glycines cysts in Arkansas, Florida, Mississippi and Missouri soils. Nematropica 1981, 11, 155–163. [Google Scholar]
- Carris, L.M.; Glawe, D.A.; Smyth, C.A.; Edwards, D.I. Fungi associated with populations of Heterodera glycines in two Illinois soybean fields. Mycologia 1989, 81, 66–75. [Google Scholar] [CrossRef]
- Li, C.G.; Li, X.M.; Kong, W.D.; Wu, Y.; Wang, J.G. Effect of monoculture soybean on soil microbial community in the Northeast China. Plant Soil. 2010, 330, 423–433. [Google Scholar] [CrossRef]
- Sekiguchi, H.; Tomioka, N.; Nakahara, T.; Uchiyama, H. A single band does not always represent single bacterial strains in denaturing gradient gel electrophoresis analysis. Biotechnol. Lett. 2001, 23, 1205–1208. [Google Scholar] [CrossRef]
- Smalla, K.; Wieland, G.; Buchner, A.; Zock, A.; Parzy, J.; Kaiser, S.; Roskot, N.; Heuer, H.; Berg, G. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: Plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microb. 2001, 67, 4742–4751. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wang, J.H.; Zhu, L.S.; Wang, J.; Mao, S.S.; Yan, X.J.; Wen, S.F.; Wang, L.J.; Dong, Z.K.; Kim, Y.M. New insights into the effects of antibiotics and copper on microbial community diversity and carbon source utilization. Environ. Geochem. Health 2023, 45, 4779–4793. [Google Scholar] [CrossRef]
- Qiao, Y.F.; Miao, S.J.; Li, N.; Xu, Y.L.; Han, X.Z.; Zhang, B. Crop species affect soil organic carbon turnover in soil profile and among aggregate sizes in a Mollisol as estimated from natural 13C abundance. Plant Soil. 2015, 392, 163–174. [Google Scholar] [CrossRef]
- Frac, M.; Oszust, K.; Lipiec, J.; Jezierska-Tys, S.; Nwaichi, E.O. Soil microbial functional and fungal diversity as influenced by municipal sewage sludge accumulation. nt. J. Environ. Res. Public Health 2014, 11, 8891–8908. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil Agricultural Chemistry, 1st ed.; China Agricultural Science and Technology Press: Beijing, China, 2000; (In Chinese). [Google Scholar] [CrossRef]
- Xu, Y.L.; Lu, J.C.; Song, J. Virulence and control effect of parasitic fungi on race 3 of soybean cyst nematode. Soy Sci. 2020, 39, 595–604. (In Chinese) [Google Scholar]
- Strom, N.; Hu, W.M.; Chen, S.Y.; Bushley, K. Continuous monoculture shapes root and rhizosphere fungal communities of corn and soybean in soybean cyst nematode-infested soil. Phytobiomes J. 2019, 3, 300–314. [Google Scholar] [CrossRef]
- Urbanová, M.; Kopecký, J.; Valásková, V.; Ságová-Marecková, M.; Elhottová, D.; Kyselková, M.; Ságová-Marečková, M.; Elhottová, D.; Kyselková, M.; Moënne-Loccoz, Y.; et al. Development of bacterial community during spontaneous succession on spoil heaps after brown coal mining. FEMS Microbiol. Ecol. 2011, 78, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.V.; Meriles, J.; Conforto, C.; Basanta, M.; Radl, V.; Hagn, A.; Schloter, M.; Marchl, G.J. Response of soil microbial communities to different management practices in surface soils of a soybean agroecosystem in Argentina. Eur. J. Soil Biol. 2011, 47, 55–60. [Google Scholar] [CrossRef]
- Atibalentja, N.; Noel, G.R.; Liao, T.F.; Gertner, G.Z. Population changes in Heterodera glycines and its bacterial parasite Pasteuria spp. in naturally infested soil. J. Nematol. 1998, 30, 81–92. [Google Scholar]
- Jiang, Y.J.; Liu, M.Q.; Zhang, J.B.; Chen, Y.; Chen, X.Y.; Chen, L.J.; Li, H.X.; Zhang, X.X.; Sun, B. Nematode grazing promotes bacterial community dynamics in soil at the aggregate level. ISME J. 2017, 11, 2705–2717. [Google Scholar] [CrossRef]
- Li, B.; Yang, P.; Feng, Y.; Du, C.; Qi, G.; Zhao, X. Rhizospheric microbiota of suppressive soil protect plants against Fusarium solani infection. Pest. Manag. Sci. 2024, 80, 4186–4198. [Google Scholar] [CrossRef]
- Trived, P.; Leach, J.E.; Tringe, S.G.; Sa, T.M.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Singh, U.B.; Sahu, A.; Sahu, N.; Singh, B.P.; Singh, R.K.; Renu; Singh, D.P.; Jaiswal, R.K.; Sarma, B.K.; Singh, H.B.; et al. Can endophytic Arthrobotrys oligospora modulate accumulation of defence related biomolecules and induced systemic resistance in tomato (Lycopersicon esculentum Mill.) against root knot disease caused by Meloidogyne incognita. Appl. Soil Ecol. 2013, 63, 45–56. [Google Scholar] [CrossRef]
- Haarith, D.; Bushley, K.E.; Chen, S.Y. Fungal communities associated with Heterodera glycines and their potential in biological control: A current update. J. Nematol. 2020, 52, e2020-22. [Google Scholar] [CrossRef]
- Chu, H.; Tang, M.; Wang, H.; Wang, C. Pinewood nematode infection alters root mycoflora of Pinus tabulaeformis Carr. J. Appl. Microbiol. 2018, 125, 554–563. [Google Scholar] [CrossRef]
- Spooren, J.; Bentum, S.V.; Thomashow, L.S.; Pieterse, C.M.J.; Weller, D.M.; Berendsen, R.L. Plant-driven assembly of disease-suppressive soil microbiomes. Annu. Rev. Phytopathol. 2024, 62, 1–30. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Liu, M.; Yao, Q.; Zhang, X.; Zhang, Z.; Pan, F.; Xu, Y. The Influence of Microbial Community on Soybean Cyst Nematode Under the Condition of Suppressive Soil. Agronomy 2025, 15, 1496. https://doi.org/10.3390/agronomy15061496
Song J, Liu M, Yao Q, Zhang X, Zhang Z, Pan F, Xu Y. The Influence of Microbial Community on Soybean Cyst Nematode Under the Condition of Suppressive Soil. Agronomy. 2025; 15(6):1496. https://doi.org/10.3390/agronomy15061496
Chicago/Turabian StyleSong, Jie, Meiqi Liu, Qin Yao, Xiaoyu Zhang, Zhiming Zhang, Fengjuan Pan, and Yanli Xu. 2025. "The Influence of Microbial Community on Soybean Cyst Nematode Under the Condition of Suppressive Soil" Agronomy 15, no. 6: 1496. https://doi.org/10.3390/agronomy15061496
APA StyleSong, J., Liu, M., Yao, Q., Zhang, X., Zhang, Z., Pan, F., & Xu, Y. (2025). The Influence of Microbial Community on Soybean Cyst Nematode Under the Condition of Suppressive Soil. Agronomy, 15(6), 1496. https://doi.org/10.3390/agronomy15061496






