Abstract
Rice–fish coculture, a traditional integrated agriculture–aquaculture system, has been recognized as a “Globally Important Agricultural Heritage System” due to its ecological and socio-economic benefits. However, the impact of rice–fish coculture on greenhouse gas emissions remains controversial. This study investigated the effects of rice–fish coculture on methane (CH4) and nitrous oxide (N2O) emissions in the Qingtian rice–fish system, a 1200-year-old terraced paddy field system in Zhejiang Province, China. A field experiment with two treatments, rice–fish coculture (RF) and rice monoculture (RM), was conducted to examine the relationships between fish activities, water and soil properties, microbial communities, and greenhouse gas fluxes. Results showed that the RF system had significantly higher CH4 emissions, particularly during the early rice growth stage, compared to the RM system. This increase was attributed to the lower dissolved oxygen levels and higher methanogen abundance in the RF system, likely driven by the grazing, “muddying”, and burrowing activities of fish. In contrast, no significant differences in N2O emissions were observed between the two systems. Redundancy analysis revealed that water variables contributed more to the variation in greenhouse gas emissions than soil variables. Microbial community analysis indicated that the RF system supported a more diverse microbial community involved in methane cycling processes. These findings provide new insights into the complex interactions between fish activities, environmental factors, and microbial communities in regulating greenhouse gas emissions from rice–fish coculture systems. The results suggest that optimizing water management strategies and exploring the potential of microbial community manipulation could help mitigate greenhouse gas emissions while maintaining the ecological and socio-economic benefits of these traditional integrated agriculture–aquaculture systems.
1. Introduction
Agricultural activities, including livestock farming, fertilizer application, and rice cultivation, represent primary sources of methane (CH4) and nitrous oxide (N2O) emissions globally [1]. In China, agricultural CH4 emissions constitute the second-largest emission source, predominantly originating from rice cultivation. According to the latest data from the Emissions Database for Global Atmospheric Research [2], CH4 emissions from rice cultivation in China account for 21% of total CH4 production, substantially higher than the 1% in the United States and 10% in Europe. N2O ranks as the third-most significant greenhouse gas in the atmosphere after carbon dioxide (CO2) and CH4, with agricultural emissions contributing over 60% of global anthropogenic N2O emissions, and cropland emissions representing approximately 30% [3]. Although N2O emissions from rice cultivation systems remain relatively low due to the anaerobic conditions resulting from year-round flooding [4], its 100-year global warming potential (GWP) significantly exceeds that of CO2 (273 ± 130 vs. 1 [5]). Therefore, a comprehensive assessment of non-CO2 greenhouse gas emissions remains crucial in climate change mitigation research.
Rice–fish coculture, a traditional integrated agriculture–aquaculture system, has been practiced for centuries throughout Asia, particularly in China [6]. This system has been recognized as a “Globally Important Agricultural Heritage System” (GIAHS) by the Food and Agriculture Organization of the United Nations (FAO) due to its substantial ecological and socio-economic benefits [7]. In 2023, rice–fish coculture systems occupy approximately ~0.934 million hectares in China alone, with expanding implementation in Vietnam, Indonesia, and other Southeast Asian countries [8]. Compared to rice monoculture, the rice-fish coculture system has demonstrated potential for greenhouse gas emissions reduction and has been proposed as a lower-carbon agricultural model [9,10]. However, the impact of rice–fish coculture on greenhouse gas emissions remains controversial, with some studies suggesting that it may not necessarily reduce emissions and could potentially increase them compared to rice monoculture [11].
The introduction of fish into rice fields can influence greenhouse gas emissions through direct or indirect modification of the micro-environment (encompassing both biotic and abiotic factors) within the rice–fish system. For instance, the foraging, stirring, and burrowing activities of omnivorous fish [12] can enhance soil–water gas exchange or directly release CH4 gas trapped in the soil matrix [13].
Furthermore, fish activities can alter the redox conditions of bottom sediments, dissolved oxygen (DO) concentrations in the water column, pH levels, and nutrient dynamics in the rice–fish system, thereby influencing greenhouse gas emissions. However, the magnitude and mechanisms of these effects exhibit considerable variation across different studies, potentially attributable to differences in geographical location, experimental conditions, fish species, and management practices employed in the coculture systems [9,13,14,15].
The majority of researchers attribute changes in CH4 and N2O emissions in rice–fish coculture systems to alterations in water DO or soil redox potential (Eh) induced by fish activities. Some researchers have reported that fish activities can increase water DO or soil Eh, thereby enhancing CH4 oxidation and consequently reducing CH4 emissions from the system [9]. Conversely, others have observed decreased water DO resulting from fish activities [13,14], creating an anaerobic environment conducive to methanogenesis and leading to increased CH4 release. Additionally, fish bioturbation can promote the release of CH4 in the form ebullition (bubbles) into the atmosphere [13]. Several studies have reported that rice–fish coculture does not significantly alter CH4 emissions compared to rice monoculture or fish monoculture [16,17].
Regarding N2O emissions, most studies have demonstrated that rice–fish coculture systems can mitigate N2O fluxes through two primary mechanisms: reduced dissolved oxygen concentrations in water suppressing nitrification rates, and enhanced nitrogen utilization efficiency decreasing bioavailable nitrogen pools for N2O-producing microbial processes [9,13,18,19]. However, contrasting evidence exists, with some studies reporting non-significant but slight increases [16] relative to rice monoculture systems. A recent meta-analysis based on 247 pairwise comparisons revealed that rice–aquaculture animal coculture systems did not significantly alter N2O emissions overall [20]. In terraced rice–fish systems specifically, the interplay between unique hydrological regimes and fish-induced bioturbation may create distinct nitrogen cycling patterns compared to flat paddies [21,22], yet the mechanistic links to N2O fluxes under these conditions remain poorly understood and warrant further investigation.
The Qingtian rice–fish coculture system in Zhejiang Province, China, represents one of the regions with the longest documented history of integrated rice–fish cultivation, dating back more than 1200 years [23]. This system, which involves the cultivation of rice concurrently with the indigenous Qingtian carp (Cyprinus carpio var. qingtianensis), was the first in China to receive GIAHS designation from the FAO [7]. Despite its historical and cultural significance, systematic studies examining greenhouse gas emissions from this traditional system remain surprisingly scarce, particularly in the context of terraced paddy fields that dominate the landscape of Qingtian County. According to recent reports, the rice–fish coculture system in Qingtian covered approximately 3700 hectares of paddy fields as of 2021 [24], with terraced fields constituting a significant portion due to the region’s mountainous topography and sufficient water resources.
To address the existing knowledge gaps and inconsistencies in the literature, we conducted a comprehensive field experiment in the Qingtian rice–fish coculture system to investigate the effects of fish activities on CH4 and N2O emissions in terraced paddy fields. We hypothesized that: (i) the presence of Qingtian carp in rice fields would significantly alter water physicochemical properties, particularly dissolved oxygen levels, thereby affecting CH4 production and oxidation processes; (ii) fish activities would modify soil properties and nutrient dynamics, influencing microbial communities responsible for N2O production and consumption; (iii) changes in microbial community structure and functional gene abundance would serve as primary mechanisms mediating greenhouse gas flux differences between rice–fish coculture and rice monoculture systems. By examining the relationships between fish activities, environmental parameters, microbial communities, and greenhouse gas dynamics, this study aims to provide novel insights into the biogeochemical mechanisms regulating CH4 and N2O emissions in traditional terraced rice-fish systems. The findings will contribute to the development of sustainable management practices for integrated agriculture–aquaculture systems, optimizing their ecological and socio-economic benefits while minimizing their contribution to climate change.
2. Materials and Methods
2.1. Studying Site
The study was conducted in the Qingtian rice–fish system, a globally important agricultural heritage site located in Shangen Village, Fangshan Town, Qingtian County, Zhejiang Province, China (28°2′17.77″ N, 120°19′36.95″ E) (Figure 1). This system, designated as a Globally Important Agricultural Heritage System (GIAHS) by the Food and Agriculture Organization (FAO), represents a traditional integrated agriculture–aquaculture practice with over 1200 years of history. In this system, rice paddies simultaneously function as fish habitats, allowing for a symbiotic relationship between rice and fish. The rice provides shade and organic matter for the fish, while the fish helps to control pests, fertilize the soil, and oxygenate the water [23]. The area features a subtropical monsoon climate with an average annual rainfall of 1400–1450 mm and an average annual temperature of 17–18 °C. The study was conducted during the 2023 rice growing season. The predominant rice variety cultivated is “Zhongzheyou 8” (refer to Supplementary Materials for more details), a local hybrid that requires a single growing season of approximately 160–170 days. The fish species raised is the indigenous Qingtian paddy carp (C. carpio var. qingtianensis).
Figure 1.
Geographic location of the experimental site.
2.2. Experiment Design
A field experiment with two treatments, (1) rice–fish coculture (RF) and (2) rice monoculture (RM), was conducted using the hybrid rice “Zhongzheyou 8” and Qingtian paddy carp. Each treatment had five replicates (n = 5), with irregular individual plot sizes of 300–500 m2. The experimental plots were arranged in a randomized block design to account for potential environmental gradients within the study area. All experimental plots were surrounded by 40–50 cm high clay barriers to prevent fish movement between plots and cross-contamination of treatments. Additionally, plots were separated by a buffer zone of 0.5–1 m to minimize edge effects. Each plot was equipped with its own isolated inlet water pipe and drainage pipe to ensure independent water management.
In both treatments, rice seedlings were transplanted at a density of 40 cm × 40 cm with one seedling per hill. Rice transplantation was performed on 15 June 2023, following local agricultural practices. No chemical fertilizers were applied during the rice growing season to minimize their potential influence on greenhouse gas emissions. All plots received the same irrigation water source to ensure treatment differences were attributable to the presence or absence of fish rather than to differences in water quality.
For the RF treatment, Qingtian paddy carp with an individual weight of 100–200 g were stocked at a density of approximately ~0.2 individuals m−2. Fish were introduced to the RF plots on 25 June 2023, ten days after rice transplantation to allow rice seedlings to establish. The fish were fed twice a week with a wheat and rapeseed meal mixture at a 1:1 weight ratio at rates varying according to the growth stage: 53–60 kg ha−1 per feeding in the early and late growth stages, and 98–106 kg ha−1 in the mid-growth stage. This feeding regime follows standard local practices for rice–fish coculture systems in the region.
Water depth in the RF treatment was maintained at 10–25 cm and adjusted based on the growth dynamics of rice and fish. During the rice tillering stage (15 July to 15 August 2023), the water level was kept at 10–15 cm; after the effective tillering stage until rice heading and flowering (16 August to 15 September 2023), the water depth was gradually increased but did not exceed 35 cm [25]. During the maturation stage (16 September to 31 October 2023), water depth was gradually reduced to 5–10 cm following local practices. Flowing irrigation was used to control the water level by adjusting the outlet height throughout the experiment. The same water depth management protocol was applied to the RM treatment to ensure comparability between treatments.
2.3. Measurement of Water and Soil Properties
Water properties, including dissolved oxygen (DO), electrical conductivity (EC), total dissolved solids (TDS), pH, and oxidation–reduction potential (ORP), were measured in situ at the rice tillering stage using a calibrated YSI ProDSS multifunctional water quality meter (YSI Inc., Yellow Springs, OH, USA). Measurements were taken between 9:00–11:00 a.m. and 14:00–16:00 p.m. to capture diurnal variations. At each sampling time, three readings were taken per plot at a depth of 5 cm below the water surface and averaged to obtain a representative value for each plot. Temperature compensation was automatically applied by the instrument for all measurements.
Soil samples were collected at three critical growth stages: tillering (11 August 2023), heading (10 September 2023), and maturity (15 October 2023). At each sampling stage, a composite soil sample was collected for every treatment plot. A total of 10 composite samples were collected from all 10 experimental plots (5 for RM treatment and 5 for RF treatment) at each growth stage. For each composite sample, soil was collected from the 0–15 cm depth at three randomly selected locations within the plot and mixed thoroughly to form a representative sample for that plot. Sampling was conducted between 9:00 and 11:00 a.m. to minimize diurnal variations in soil properties. The samples were then air-dried in a well-ventilated area at room temperature for 7 days, grounded, and passed through a 100-mesh sieve (0.15 mm) for subsequent analyses [26].
Soil physicochemical variables, including soil organic matter (SOM), total nitrogen (TN), alkali-hydrolyzable nitrogen (AN), total phosphorus (TP), and available phosphorus (AP), were determined using standard methods. SOM content was measured using the potassium dichromate oxidation–external heating method [27]. TN content was determined by the Kjeldahl method using a Foss Kjeltec™ 8100 analyzer (Foss Analytical AB, Höganäs, Sweden) [28]. AN content was quantified using the alkaline hydrolysis diffusion method [26]. TP was measured by the NaOH alkaline fusion and molybdenum–antimony anti-spectrophotometric method, while AP content was determined using the molybdenum blue colorimetric method after extraction with 0.5 mol L−1 sodium bicarbonate [26]. For each parameter, analytical-grade reagents were used, and quality control was ensured by including standard reference materials and procedural blanks in each batch of analyses.
2.4. Measurement of Soil Microbial Groups
At the rice maturity stage (5 October 2023), composite soil samples from the 0–5 cm layer were collected for microbial analysis. The samples were sealed in sterile 50 mL plastic bags and immediately transported to the laboratory in an ice box maintained at 4 °C, where they were stored at −80 °C until further processing.
DNA extraction from the soil samples was performed using the DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. Approximately 0.25 g of soil was used for each extraction. The quality and quantity of the extracted DNA were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively [29]. Only DNA samples with A260/A280 ratios between 1.8 and 2.0 were used for subsequent analyses. The quality-verified DNA was then used for quantitative PCR (qPCR) and Illumina MiSeq sequencing.
The abundance of specific microbial functional groups was assessed by quantifying the following functional genes: mcrA (methanogenesis), pmoA (methane oxidation), amoA (nitrification), and nirS (denitrification). The primer sets used for the amplification of these functional genes are listed in Table 1. qPCR was performed using a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Each 20 μL reaction mixture contained 10 μL of 2× SYBR Premix Ex Taq (TaKaRa, Dalian, China), 0.5 μL of each primer (10 μM), 1 μL of template DNA (10–20 ng), and 8 μL of sterile water. The thermal cycling conditions were as follows: initial denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 30 s, annealing at primer-specific temperatures (mcrA: 55 °C, pmoA: 56 °C, amoA: 54 °C, nirS: 57 °C) for 30 s, and extension at 72 °C for 30 s. Melting curve analysis was performed to confirm the specificity of the amplification. Standard curves were generated using serial dilutions of plasmids containing the target gene fragments. All samples were analyzed in triplicate, and the results were expressed as gene copy numbers per gram of dry soil.

Table 1.
The primer sets used for the amplification of these functional genes.
For Illumina MiSeq sequencing, the amplified target gene samples were sent to Wuhan Benagen Technology Co., Ltd. (Wuhan, China) for library preparation and sequencing on the Illumina MiSeq platform using the 2 × 300 bp paired-end sequencing protocol on an Illumina MiSeq platform with an average insert size of 450 bp. Libraries were prepared using the Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA, USA) following the manufacturer’s instructions. Quality control was performed using Qubit fluorometric quantification, and library concentrations were normalized to 4 nM before pooling. Sequencing aimed for a target coverage of 50,000 raw reads per sample to ensure adequate depth for diversity analysis. The raw sequencing data were processed using the QIIME2 pipeline (version 2023.2) for quality control, chimera removal, and taxonomic assignment [30]. Specifically, raw sequences were demultiplexed and quality-filtered using the DADA2 plugin in QIIME2 (version 2023.2). Forward and reverse reads were truncated at positions 280 and 250 bp, respectively, and sequences with more than 2 expected errors were discarded for both forward and reverse reads. Chimeric sequences were identified and removed using the consensus method. Amplicon sequence variants (ASVs) were generated and taxonomically classified. The SILVA SSU database (release 138) was used as the reference for taxonomic classification of the functional gene sequences [31]. Alpha diversity indices (Ace, Chao1, Shannon, and Simpson) were calculated using the q2-diversity plugin in QIIME2. The obtained microbial community data were further analyzed to explore the structure and diversity of the respective functional groups.
2.5. Greenhouse Gas Sampling and Measurement
Greenhouse gas (GHG) emissions from the paddy fields were measured using static transparent chambers, following the method described by Li et al. [32] with minor modifications. The chambers (dimensions: 50 cm × 50 cm × 120 cm, L × W × H) were constructed from 5 mm thick transparent Perspex, with a removable top and an open bottom. Each chamber was equipped with a small electric fan powered by a 12V battery for air circulation and a rubber tube (20 cm in length, 0.5 cm in inner diameter) with a three-way valve for gas sampling. All rubber hoses and joints were sealed with silica gel to ensure airtightness during gas collection. The chambers were installed at fixed locations within each plot at least 5 m away from the plot boundaries to avoid edge effects.
Gas samples were collected on clear or cloudy days at three rice growth stages: tillering (11–13 August 2023), heading (10–12 September 2023), and maturity (15–17 October 2023). Sampling was conducted twice daily, from 9:00 to 11:00 a.m. and from 14:00 to 16:00 p.m., for 30 min each time. To minimize gas leakage, the chambers were inserted into U-shaped stainless-steel grooves that were pre-installed in the soil and filled with water to create an airtight seal [33]. During each sampling event, 50 mL gas samples were withdrawn from the chambers using gas-tight syringes at 0, 10, 20, and 30 min after chamber closure and transferred into Fluode gas sampling bags (FLU11-0.05, Dalian Hede Technologies LTD., Dalian, China). The chamber temperature was recorded at each sampling time to account for temperature effects on gas fluxes.
The concentrations of CH4 and N2O in the gas samples were determined within 24 h of collection using an Agilent 7820A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA). For CH4 analysis, a flame ionization detector (FID) (Agilent Technologies, Santa Clara, CA, USA) was used, with the detector temperature set at 200 °C. The separation was achieved using a 2 m long packed column (2 mm inner diameter) filled with 60–80 mesh 13XMS molecular sieve (Sigma-Aldrich, St. Louis, MO, USA). The column temperature was maintained at 55 °C, and high-purity nitrogen (N2) was used as the carrier gas at a flow rate of 30 cm3 min−1. For N2O analysis, an electron capture detector (ECD) (ECD; Agilent Technologies, Santa Clara, CA, USA) was employed, with the detector temperature set at 330 °C for enhanced sensitivity. The separation and backflushing of N2O were performed using two packed columns (1 m and 3 m in length, 2 mm inner diameter) filled with 80–100 mesh PORAPAKOQ. The column temperature was set at 55 °C, and high-purity N2 was used as the carrier gas at a flow rate of 35 cm3 min−1. Standard gas mixtures (National Center for Standard Materials, Beijing, China) containing 2.0, 5.0, 10.0, and 20.0 μL L−1 CH4 and 0.2, 0.5, 1.0, and 2.0 μL L−1 N2O were used for calibration before each set of measurements. The detection limits were 0.1 μL L−1 for CH4 and 0.01 μL L−1 for N2O.
GHG fluxes (mg·m−2·h−1) were calculated using the linear regression method based on the change in gas concentrations over time [34]. The flux was determined as follows:
where ΔC/Δt is the change in the concentration of GHG over time (ppm h−1), V is the volume of the enclosure (L), A is the cross-sectional area (m2) of the enclosure, and T is the mean air temperature inside the chamber during the sampling period (°C). The term 273/(273 + T) is the temperature correction factor. M is the molar mass of target gas (g mol−1) (16.04 g mol−1 for CH4 and 44.01 g mol−1 for N2O). V0 is the molar volume of target gas at a standard atmosphere (22.41 L mol−1). We checked for the data quality due to potential leakage or saturation by implementing a linear regression. The flux values were discarded when the correlation coefficient of the regression is lower than R2 = 0.85.
2.6. Data Analysis
All statistical analyses were performed using SPSS software (version 19.0, IBM Inc., Armonk, NY, USA). Prior to analysis, the normality of the data was assessed using the Kolmogorov–Smirnov test at α = 0.05 significance level. All data groups met the assumptions of normality, and therefore, parametric tests were employed for subsequent analyses.
Independent-sample t-tests were conducted to compare the differences in water properties, soil physicochemical characteristics, and microbial functional gene abundances between the rice monoculture (RM) and rice–fish coculture (RF) systems. For each parameter, the mean and standard deviation (SD) were calculated and presented in tables and figures. Significant differences were determined at p < 0.05 and p < 0.01 levels.
To investigate the relationships between environmental variables (water and soil properties) and greenhouse gas (GHG) fluxes, redundancy analysis (RDA) was performed using CANOCO software (version 4.5, Microcomputer Power, Ithaca, NY, USA) [35]. Prior to RDA, the GHG flux data were normalized using the min–max normalization method to ensure comparability among different flux components (CH4 and N2O). This normalization was performed using the following equation,
where X′ is the normalized value, X is the original value, and Xmin and Xmax are the minimum and maximum values of the variable, respectively. The normalized flux data were used as response variables, while the water and soil properties were used as explanatory variables in the RDA model, respectively.
The statistical significance of the RDA was tested using Monte Carlo permutation tests (999 permutations). The relative contributions of each environmental variable to the variation in GHG fluxes were calculated based on the partial correlation coefficients between the environmental variables and the RDA axes. All graphical presentations were generated using Origin Pro 2022 (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. Water and Soil Physiochemical Properties
At the tillering stage, significant differences were observed in several water quality parameters between treatments (Figure 2). The rice monoculture (RM) system had significantly higher dissolved oxygen (DO) (p = 0.001) but lower electrical conductivity (EC) (p = 0.002) and total dissolved solids (TDS) (p = 0.002) compared to the rice–fish coculture (RF) system. The mean DO concentration in the RF system was approximately 34.0% lower than in the RM system. Conversely, EC and TDS values were 29.9% and 30.4% higher in the RF system compared to the RM system, respectively. No significant differences were observed in pH and oxidation–reduction potential (ORP) between the two systems (p > 0.05).
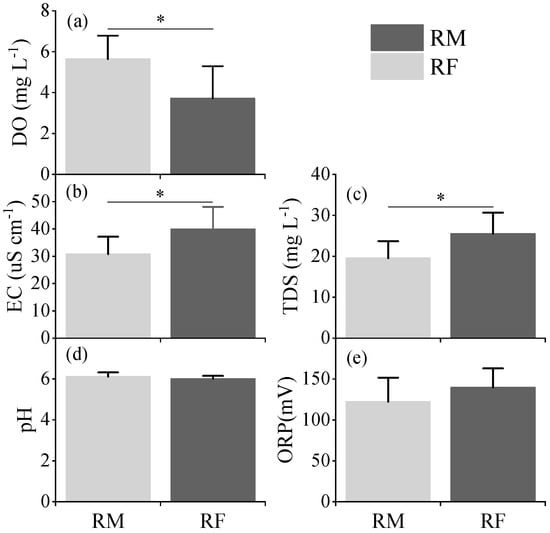
Figure 2.
Water variables including dissolved oxygen (DO) (a), electrical conductivity (EC) (b), total dissolved solids (TDS) (c), pH (d), and oxidation–reduction potential (ORP) (e) in rice monoculture (RM) and rice–fish (RF) coculture systems at tillering stage. The asterisks above the horizontal lines indicate significant differences (* p < 0.05) between RM and RF systems.
Temporal analysis of soil physicochemical properties throughout the rice growing season revealed treatment-specific patterns between treatments (Figure 3). Soil physicochemical properties did not differ significantly between the RM and RF systems throughout the study period (p > 0.05), except for total phosphorus (TP) at the heading stage where RF was significantly lower than RM (p < 0.05). Throughout the rice growing period, the RM system consistently had higher soil organic matter (SOM), total nitrogen (TN), TP, and available phosphorus (AP) contents, while the RF system had higher alkali-hydrolyzable nitrogen (AN) content, although these differences were not statistically significant. A clear temporal trend was observed across both treatments, with SOM, TN, and AP showing progressive decreases with the progression of the rice growing season in both systems. Specifically, from the tillering to maturation stage, SOM decreased by 13.7% and 23.5% in the RM and RF systems, respectively, while TN decreased by 15.2% and 24.5%, and AP decreased by 45.2% and 50.0%.
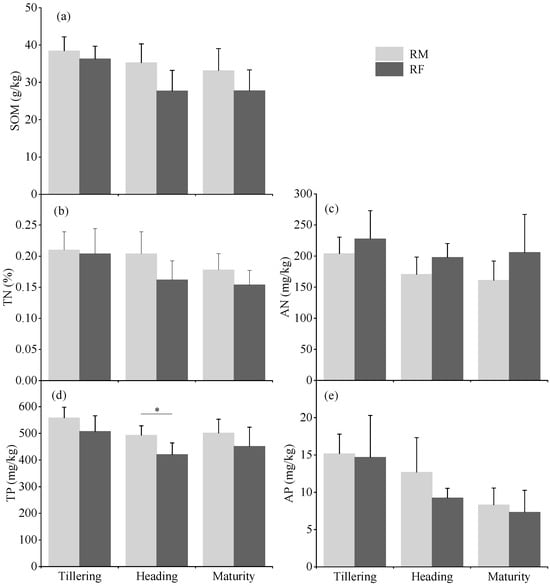
Figure 3.
Soil variables including soil organic matter (SOM) (a), total nitrogen (TN) (b), alkali-hydrolyzable nitrogen (AN) (c), total phosphorus (TP) (d), and available phosphorus (AP) (e) in rice monoculture (RM) and rice–fish (RF) coculture systems at tillering, heading, and maturation stage. The asterisks above the horizontal lines indicate significant differences (* p < 0.05) between RM and RF systems.
3.2. Microbial Properties
The abundances in the methanogenesis-related gene mcrA and the methane oxidation-related gene pmoA were significantly higher in the rice–fish coculture (RF) system compared to the rice monoculture (RM) system (p < 0.01) (Figure 4a,b). Quantitatively, the mcrA gene abundance in the RF system was approximately 2.5-fold higher than that in the RM system, while the pmoA gene abundance was twice as high in the RF system compared to the RM system. In contrast, no significant differences were observed in the abundances of the nitrification-related gene amoA and the denitrification-related gene nirS between the two systems (p > 0.05) (Figure 4c,d). Despite the lack of statistical significance, the RF system exhibited a 13.12% higher amoA gene abundance and a 16.91% lower nirS gene abundance compared to the RM system.
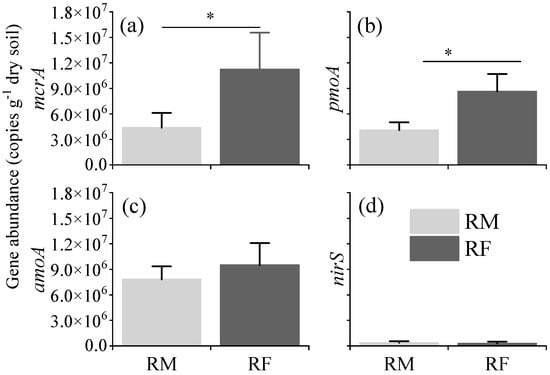
Figure 4.
Abundances of methanogen (mcrA) (a), methanotroph (pmoA) (b), ammonia-oxidizing bacteria (amoA) (c), and denitrifying bacteria (nirS) (d), in different treatments (RF vs. RM) at maturity stage. The asterisks above the horizontal lines indicate significant differences (* p < 0.05) between RM and RF systems.
Microbial community alpha diversity was assessed across multiple indices for the functional gene groups (Table 2). The alpha diversity indices (Ace, Chao1, Shannon, and Simpson) of the selected microbial functional groups were consistently higher in the RF system than in the RM system, although the differences were not statistically significant (p > 0.05) (Table 2). This trend suggests that the rice–fish coculture system may support a more diverse microbial community involved in methane and nitrogen cycling processes.

Table 2.
Community diversity index (Average ± SD) of methanogen (mcrA), methanotroph (pmoA), ammonia-oxidizing bacteria (amoA), and denitrifying bacteria (nirS) in different treatments (RF vs. RM) at maturity stage.
Taxonomic analysis at the genus level revealed substantial microbial community restructuring beyond functional gene abundance changes (Figure S1). The RF system supported 20 unique genera, including novel colonization by Microlunatus sp. (associated with amoA pathways) and enhanced Methylosinus abundance (linked to methanotrophic processes), while the RM system maintained 26 distinct genera comprising traditional soil microorganisms such as Actinomycetia, Planctomycetaceae (nirS-associated), and Staphylococcus (pmoA-associated). This taxonomic differentiation indicates that fish activities induced environmental filtering that selectively favored specific microbial taxa, leading to functionally distinct microbial communities between the two systems. The emergence of RF-specific genera provides mechanistic insight into the specialized metabolic capabilities that likely contribute to the altered biogeochemical processes observed in rice–fish coculture systems.
3.3. Greenhouse Gas Emission
Greenhouse gas emissions showed distinct temporal patterns and treatment effects throughout the rice growing season (Figure 5). The RF system had consistently higher CH4 fluxes compared to the RM system, with a significant difference observed at the tillering stage (p < 0.05) (Figure 5a). During this stage, CH4 emissions from the RF system were 4.7 times those from the RM system. When averaged across all growth stages, the CH4 flux in the RF system was more than three times higher than that in the RM system.
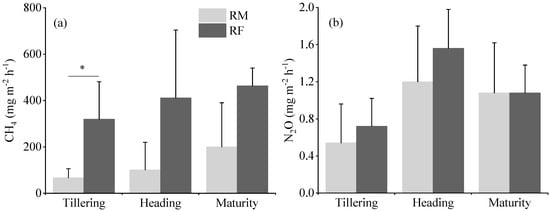
Figure 5.
CH4 (a) and N2O flux (b) in rice monoculture (RM) and rice–fish (RF) coculture systems at tillering, heading, and maturation stages. The asterisks above the horizontal lines indicate significant differences (* p < 0.05) between RM and RF systems.
N2O fluxes peaked at the heading stage and were lowest at the tillering stage in both the RM and RF systems (Figure 5b). Although the N2O fluxes in the RF system were 17.71% higher than those in the RM system when averaged across all growth stages, the differences were not statistically significant (p > 0.05).
These results suggest that the rice–fish coculture system may have a higher potential for CH4 emissions, particularly during the early growth stages of rice, while its impact on N2O emissions appears to be less pronounced compared to the rice monoculture system.
3.4. Effects of Environmental Factors on GHG Emission
To elucidate the key environmental drivers of greenhouse gas emissions, redundancy analysis (RDA) was performed separately to investigate the relationships between GHG emissions and both water and soil variables (Figure 6). For the RDA with water variables, the first two canonical axes explained 55.4% of the total variation in GHG emissions (Figure 6a). In contrast, the first two canonical axes of the RDA with soil variables accounted for 29.5% of the total variation in GHG emissions (Figure 6b). This indicates that water variables had a stronger explanatory power for GHG emissions than soil variables in the current study system.
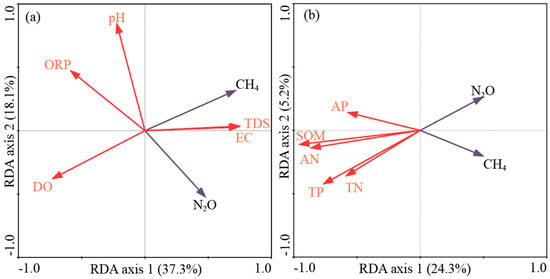
Figure 6.
Ordination diagram based on the redundancy analysis (RDA) of the CH4 and N2O flux with respect to the water (a) and soil variables (b). Note: A vector angle between treatments and variables of less than 90° indicates a positive correlation, whereas an angle more than 90° indicates a negative correlation. Perpendicular vectors (~90°) indicate weak correlation between treatments and variables.
Among the water variables, dissolved oxygen (DO) emerged as the most significant factor affecting GHG emissions (Table 3). DO contributed the most to the variation in GHG emissions, followed by total dissolved solids (TDS), electrical conductivity (EC), oxidation–reduction potential (ORP), and pH. DO and ORP were negatively correlated with GHG emissions, while TDS and EC exhibited positive correlations. This suggests that lower oxygen levels and redox potentials, coupled with higher ionic content in the water, created conditions favorable for enhanced GHG production, particularly CH4.

Table 3.
Explained variance and relative contributions of water physicochemical index to the emission of GHG (CH4 and N2O) during the tillering and heading stage. Tests are redundancy analysis (RDA).
For soil parameters, organic matter content was identified as the main driver of GHG emissions (Table 4). Soil organic matter (SOM) had the largest contribution to the variation in GHG emissions, followed by alkali-hydrolyzable nitrogen (AN), total phosphorus (TP), total nitrogen (TN), and available phosphorus (AP) (Table 4). All soil variables showed negative correlations with GHG emissions.

Table 4.
Explained variance and relative contributions of soil physicochemical index to emission of the GHG (CH4 and N2O) during the tillering, heading, and maturity stages. Tests are redundancy analysis (RDA).
4. Discussion
4.1. Fish-Induced Water Quality Alterations as Primary Drivers of GHG Emissions
Our investigation in the traditional Qingtian rice–fish coculture system revealed that water quality parameters, particularly dissolved oxygen (DO), were the predominant environmental factors influencing greenhouse gas emissions. The rice–fish (RF) system exhibited significantly higher CH4 flux than the rice monoculture (RM) system, especially during the tillering stage, while N2O emissions showed no significant differences between treatments. Notably, the tillering stage represents a particularly sensitive period for CH₄ emission responses to fish presence, likely due to the following: (1) peak root exudation providing methanogenic substrates, (2) optimal soil anaerobic conditions for methane production, and (3) active fish bioturbation enhancing microbial activity. The absence of significant differences during later growth stages suggests that fish effects on CH₄ emissions may be most pronounced during periods of rapid plant growth and high soil organic matter turnover.
Redundancy analysis (RDA) demonstrated that water variables explained 55.4% of the total variation in GHG emissions, substantially more than soil variables (29.5%). Among water parameters, DO emerged as the most critical factor to the variation in GHG emissions and showed strong negative correlation with methane production. The RF system exhibited 36.7% lower DO levels compared to the RM system, directly attributable to the grazing, “muddying”, and burrowing activities of Qingtian paddy carp [12].
This DO reduction can be attributed to multiple mechanisms: (i) direct oxygen consumption through fish respiration, (ii) increased oxygen demand from enhanced microbial activity stimulated by fish excreta, and (iii) oxygen consumption during the decomposition of uneaten fish feed. These findings align with Bhattacharyya et al. [13] and Frei et al. [14], who reported similar fish-induced DO reductions leading to enhanced methane emissions. However, our results contrast with Yuan et al. [9], who found that fish activities increased DO and reduced CH4 emissions in a rice–fish–duck system, suggesting that system design and management practices significantly influence outcomes.
Interestingly, despite significant alterations in water quality, soil physicochemical properties showed minimal differences between treatments, with only total phosphorus (TP) at the heading stage showing significant variation. This indicates that fish activities primarily influence GHG emissions through immediate water quality modifications rather than through long-term soil alterations, at least within the timeframe of our experiment.
4.2. Microbial Mechanisms Underlying Differential CH4 and N2O Responses
Our microbial analysis provided crucial insights into the biological mechanisms driving the observed GHG emission patterns. The RF system exhibited significantly higher abundances of both methanogens (mcrA gene) and methanotrophs (pmoA gene), with methanogen abundance increasing by 156.2% compared to 110.9% for methanotrophs. This disproportionate enhancement of methanogenic activity relative to methane oxidation explains the net increase in CH4 emissions from the RF system.
This imbalance in the methanogen-to-methanotroph ratio is consistent with the findings of Sun et al. [11] and Zhao et al. [17], who reported similar patterns in rice–fish systems. Notably, Zhao et al. [17] further elucidated the underlying ecological mechanisms, demonstrating that fish presence increases both methanogens and aerobic methanotrophs through feeding interactions that trigger trophic cascades in the paddy ecosystem. The enhanced methanogenic potential we observed was further supported by higher alpha diversity indices across all microbial functional groups in the RF system, suggesting that fish activities not only increase microbial abundance but also promote community diversity [25].
Although we observed numerical trends toward higher amoA gene abundance (13.12% increase) and lower nirS gene abundance (16.91% decrease) in the RF system, these changes were not statistically significant (p > 0.05), indicating that fish activities did not substantially alter the functional capacity of key nitrogen-transforming microbial communities. This absence of significant shifts in nitrogen-cycling gene abundances provides a mechanistic explanation for the similar N2O emission rates observed between RF and RM systems, contrasting with the pronounced differences in methane-cycling genes that corresponded with elevated CH4 emissions. Our findings align with the broader scientific literature on rice–fish systems and N2O dynamics. Zhan et al. [16] similarly reported that rice–fish coculture did not significantly alter N2O emissions compared to rice monoculture in Hunan Province, China. More conclusively, Wang et al. [20] conducted a comprehensive meta-analysis of 247 pairwise comparisons and determined that rice–aquaculture animal coculture systems generally do not significantly affect N2O emissions, providing strong support for our observations.
These microbial findings support a conceptual model where fish activities initiate a cascade of environmental changes primarily through water quality alterations, which subsequently influence microbial community dynamics, ultimately resulting in enhanced CH4 emissions. The more complex pathway for N2O emissions, involving counterbalancing microbial processes, explains the absence of significant treatment effects on N2O flux.
4.3. Implications of Terraced Field Systems for Rice–Fish Coculture GHG Dynamics
A critical distinguishing feature of our study is the use of terraced paddy fields, which represent a unique agroecosystem with distinct hydrological and topographical characteristics. Unlike the plain paddy fields commonly used in previous studies [9,13], terraced fields create stepped microenvironments with varied oxygen gradients and water flow patterns.
These unique features may intensify the impact of fish activities on GHG emissions through several mechanisms: (i) the stepped structure may create zones of differential oxygen availability, potentially amplifying anaerobic conditions in lower terraces; (ii) subsurface seepage from higher elevation plots to lower terraces may create indirect influences, where dissolved nutrients and oxygen-depleted water from fish-inhabited upper plots gradually infiltrate into lower terraces, potentially affecting water chemistry and biogeochemical processes across the elevation gradient; (iii) the traditional water management practices in terraced systems may interact differently with fish bioturbation compared to plain fields.
The discrepancies between our findings and some previous studies may thus be partially attributable to these field-type differences. For instance, the contrasting results of Yuan et al. [9], who found reduced CH4 emissions in rice–fish systems, were obtained from plain paddy fields where water circulation and oxygenation patterns likely differ substantially from our terraced system.
This highlights the importance of considering field geometry and hydrology when evaluating GHG emissions from rice–fish systems. Future research should systematically compare GHG dynamics across different paddy field types to better understand how physical field characteristics interact with biological processes to influence emissions.
4.4. Ecological Implications and Trade-Offs in Agricultural Heritage Systems
The observed increase in CH4 emissions from the Qingtian rice–fish system must be contextualized within the broader ecological framework of agricultural heritage systems, where greenhouse gas dynamics represent one component of complex multifunctional landscapes. While our study documented elevated CH4 fluxes during early growth stages, research across multiple heritage rice–fish systems demonstrates that these emissions occur within agroecosystems that provide substantial compensatory ecological benefits, including enhanced biodiversity conservation, improved soil health, and reduced reliance on synthetic inputs [9,23]. Meta-analyses of ecological rice-cropping systems reveal that rice–fish configurations can reduce overall global warming potential by 11.1% compared to conventional systems when accounting for reduced pesticide use (68% reduction) and fertilizer requirements (24% reduction) [11].
The ecological trade-offs inherent in heritage systems differ fundamentally from those in industrial agriculture due to their integrated management approaches and millennial-scale sustainability records. Recent studies demonstrate that coculturing rice with aquatic animals can reduce methane emissions by 23% in pond systems through enhanced aerobic conditions, suggesting that optimization of traditional practices rather than abandonment may offer pathways for emission mitigation [36]. The FAO’s expanded GIAHS network now encompasses 89 systems across 28 countries, reflecting growing recognition that agricultural heritage systems provide essential adaptation strategies for climate resilience while maintaining cultural landscapes that support both biodiversity and rural livelihoods [37].
From a climate policy perspective, agricultural methane mitigation strategies increasingly emphasize “alternate wetting and drying (AWD)” approaches that could halve emissions while maintaining productivity, approaches that align with traditional water management knowledge embedded in heritage systems [38]. For the Qingtian system specifically, the challenge lies in developing adaptive management protocols that honor the 1200-year cultural legacy while incorporating climate-smart modifications such as optimized fish stocking densities and precision water management. This approach aligns with climate-smart agriculture principles that systematically consider synergies and trade-offs between productivity, adaptation, and mitigation, ensuring that heritage preservation and climate action remain complementary rather than competing objectives in these globally significant agricultural landscapes [39].
4.5. Management Implications for Sustainable Rice–Fish Coculture
Our integrated understanding of the key factors influencing GHG emissions provides valuable insights into developing targeted mitigation strategies. Given that water quality parameters, particularly DO, emerged as the primary drivers of increased CH4 emissions in rice–fish systems, water management practices represent the most promising avenue for emission mitigation.
Potential strategies include: (i) implementing intermittent irrigation or AWD to periodically increase DO levels [40,41]; (ii) optimizing fish stocking densities to balance ecological benefits with emission concerns [19,23]; (iii) adjusting feeding protocols to minimize oxygen demand from decomposing feed [42]; (iv) exploring mechanical aeration during critical growth stages when methane emissions peak.
These management interventions should be carefully designed to maintain the ecological and economic benefits of rice–fish coculture while minimizing its contribution to greenhouse gas emissions. The enhanced microbial diversity observed in RF systems suggests potential resilience benefits that should be preserved in any mitigation approach.
When evaluating the environmental impact of rice–fish systems, it is important to consider that RF systems produce fish protein in addition to rice. A complete environmental assessment should compare the GHG emissions from rice–fish coculture with those from separate rice cultivation and fish production systems (e.g., pond aquaculture, recirculating aquaculture systems, or flow-through systems). This integrated perspective may reveal that despite higher CH₄ emissions per unit area of paddy field, the overall carbon footprint per unit of total protein production could be more favorable in rice–fish systems [43]. Future life cycle assessment studies should quantify the environmental trade-offs between integrated rice–fish production and conventional separate production systems, considering not only GHG emissions but also land-use efficiency, water consumption, and biodiversity impacts.
Future research should focus on long-term monitoring across multiple growing seasons to capture cumulative effects on soil properties, investigating the role of different fish species and stocking densities, and developing integrated management protocols that optimize both productivity and environmental sustainability in these traditional agricultural heritage systems.
5. Conclusions
This study revealed that rice–fish coculture in Qingtian’s terraced paddies significantly increased CH4 emissions without substantially affecting N2O emissions. Water quality parameters—primarily dissolved oxygen—were the dominant drivers of GHG emissions (55.4% of variation) compared to soil variables (29.5%). The enhanced CH4 emissions resulted from disproportionate increases in methanogens over methanotrophs (156.2% vs. 110.9%), creating favorable conditions for methanogenesis.
Terraced field characteristics likely influenced GHG dynamics differently than in previously studied plain paddies. Despite increased CH4 emissions, targeted strategies including intermittent irrigation, optimized fish stocking, and refined feeding protocols could mitigate emissions while maintaining the traditional system’s benefits.
Future assessments should adopt a holistic approach, comparing the environmental footprint of rice–fish coculture systems with that of separate rice and fish production systems to provide a more comprehensive evaluation of their sustainability benefits.
Future research should address long-term assessment across multiple seasons and develop integrated protocols balancing GHG mitigation with productivity. This study enhances our understanding of GHG regulation in rice–fish systems, contributing to climate-friendly practices that preserve agricultural heritage while addressing climate challenges.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15061480/s1, Figure S1. Genus-level taxonomic composition of microbial functional genes in rice monoculture (RM) and rice-fish (RF) systems. The mcrA, pmoA, amoA, and nirS represent functional genes for methanogenesis, methane oxidation, ammonia oxidation, and denitrification, respectively. Note: The top eight most abundant genera for each functional gene are displayed individually, with remaining genera grouped as “Others”.
Author Contributions
Conceptualization, Q.L. (Qixuan Li), L.X., S.L., Q.L. (Qigen Liu) and Y.L.; Methodology, Q.L. (Qixuan Li), S.L., X.C., Q.L. (Qigen Liu) and Y.L.; Software, Q.L. (Qixuan Li), L.X. and Y.L.; Validation, L.X. and Y.L.; Formal analysis, L.X., S.L., X.C. and Y.L.; Investigation, Q.L. (Qixuan Li), L.X., S.L., X.C. and Y.L.; Writing—original draft, Q.L. (Qixuan Li), L.X. and Y.L.; Writing—review & editing, Q.L. (Qigen Liu) and Y.L.; Visualization, Y.L.; Supervision, Q.L. (Qigen Liu); Project administration, Y.L.; Funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Provincial Natural Science Foundation of China, grant number LQ24C030004 and Lishui Science and Technology Bureau, grant number 2024GYX15.
Data Availability Statement
All relevant data is contained within the article: The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the four anonymous reviewers and the editor for their valuable comments that greatly improved this manuscript. During the preparation of this manuscript, the author(s) used Claude 3.7 for the purposes of linguistic improvement. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CH4 | Methane |
| N2O | Nitrous oxide |
| RF | Rice–fish coculture |
| RM | Rice monoculture |
| GWP | Global warming potential |
| GIAHS | Globally Important Agricultural Heritage System |
| FAO | Food and Agriculture Organization of the United Nations |
References
- Liebig, M.A.; Bergh, E.L.; Archer, D.W. Variation in methodology obscures clarity of cropland global warming potential estimates. J. Environ. Qual. 2023, 52, 549–557. [Google Scholar] [CrossRef] [PubMed]
- EDGAR (Emissions Database for Global Atmospheric Research). Community GHG Database (a Collaboration Between the European Commission, Joint Research Centre (JRC), the International Energy Agency (IEA), and Comprising IEA-EDGAR CO2, EDGAR CH4, EDGAR N2O, EDGAR F-GASES Version 7.0, 2022. European Commission, JRC (Datasets). Available online: https://edgar.jrc.ec.europa.eu/dataset_ghg70 (accessed on 8 May 2025).
- Yan, S.; Shang, Z.; Deng, A.; Zhang, W. Spatiotemporal Characteristics and Reduction Approaches of Farmland N2O Emission in China. Crops 2022, 38, 582–591. (In Chinese) [Google Scholar]
- Gerber, J.S.; Carlson, K.M.; Makowski, D.; Mueller, N.D.; Garcia de Cortazar-Atauri, I.; Havlík, P.; Herrero, M.; Launay, M.; O’Connell, C.S.; Smith, P.; et al. Spatially explicit estimates of N2O emissions from croplands suggest climate mitigation opportunities from improved fertilizer management. Glob. Change Biol. 2016, 22, 3383–3394. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; p. 184. [Google Scholar]
- Hu, L.L.; Zhang, J.; Ren, W.Z.; Guo, L.; Cheng, Y.X.; Li, J.Y.; Li, K.X.; Zhu, Z.W.; Zhang, J.E.; Luo, S.M.; et al. Can the co-cultivation of rice and fish help sustain rice production? Sci. Rep. 2016, 6, 28728. [Google Scholar] [CrossRef]
- Lu, J.B.; Li, X. Review of rice–fish-farming systems in China—One of the globally important ingenious agricultural heritage systems (GIAHS). Aquaculture 2006, 260, 106–113. [Google Scholar] [CrossRef]
- National Aquatic Technology Promotion Station, Report on the development of rice and fishery integrated breeding industry in China (2024). China Fish. 2024, 12–17. (In Chinese) [CrossRef]
- Yuan, W.L.; Cao, C.G.; Li, C.F.; Zhan, M.; Cai, M.L.; Wang, J.P. Methane and nitrous oxide emissions from rice-duck and rice-fish complex ecosystems and the evaluation of their economic significance. Agric. Sci. China 2009, 8, 1246–1255. (In Chinese) [Google Scholar] [CrossRef]
- Cui, W.C.; Jiao, W.; Min, Q.W. Environmental impact assessment of rice-fish culture with different land management models. China J. Eco Agric. 2022, 30, 630–640. (In Chinese) [Google Scholar]
- Sun, G.; Sun, M.; Du, L.S.; Zhang, Z.; Wang, Z.C.; Zhang, G.B.; Nie, S.A.; Xu, H.Q.; Wang, H. Ecological rice-cropping systems mitigate global warming—A meta-analysis. Sci. Total Environ. 2021, 789, 147900. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, L.F.; Ye, J.L.; Ji, Z.J.; Tang, J.J.; Bai, K.Y.; Zheng, S.J.; Hu, L.L.; Chen, X. Using aquatic animals as partners to increase yield and maintain soil nitrogen in the paddy ecosystems. Elife 2022, 11, e73869. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Sinhababu, D.P.; Roy, K.S.; Dash, P.K.; Sahu, P.K.; Dandapat, R.; Neogi, S.; Mohanty, S. Effect of fish species on methane and nitrous oxide emission in relation to soil C, N pools and enzymatic activities in rainfed shallow lowland rice-fish farming system. Agric. Ecosyst. Environ. 2013, 176, 53–62. [Google Scholar] [CrossRef]
- Frei, M.; Razzak, M.A.; Hossain, M.M.; Oehme, M.; Dewan, S.; Becker, K. Methane emissions and related physicochemical soil and water parameters in rice–fish systems in Bangladesh. Agric. Ecosyst. Environ. 2007, 120, 391–398. [Google Scholar] [CrossRef]
- Wang, J.; Smith, P.; Hergoualc’h, K.; Zou, J. Direct N2O emissions from global tea plantations and mitigation potential by climate-smart practices. Resour. Conserv. Recycl. 2022, 185, 106501. [Google Scholar] [CrossRef]
- Zhan, M.; Cao, C.G.; Wang, J.P.; Cai, M.L.; Yuan, W.L. Greenhouse gases exchange of integrated paddy field and their comprehensive global warming potentials. Acta Ecol. Sin. 2008, 28, 5461–5468. (In Chinese) [Google Scholar]
- Zhao, L.F.; Dai, R.X.; Zhang, T.J.; Guo, L.; Luo, Q.Y.; Chen, J.X.; Zhu, S.Y.; Xu, X.C.; Tang, J.J.; Hu, L.L.; et al. Fish mediate surface soil methane oxidation in the agriculture heritage rice–fish system. Ecosystems 2023, 26, 1656–1669. [Google Scholar] [CrossRef]
- Datta, A.; Nayak, D.R.; Sinhababu, D.P.; Adhya, T.K. Methane and nitrous oxide emissions from an integrated rainfed rice–fish farming system of Eastern India. Agric. Ecosyst. Environ. 2009, 129, 228–237. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, T.; Liu, Y.B.; Li, F.B.; Xu, C.C.; Fang, F.P.; Feng, J.F. High fish stocking density weakens the effects of rice-fish co-culture on water eutrophication and greenhouse gas emissions. Water Air Soil Pollut. 2022, 233, 222. [Google Scholar] [CrossRef]
- Wang, C.; Shi, X.Y.; Qi, Z.M.; Xiao, Y.Q.; Zhao, J.; Peng, S.; Chu, Q.Q. How does rice-animal co-culture system affect rice yield and greenhouse gas? A meta-analysis. Plant Soil 2023, 493, 325–340. [Google Scholar] [CrossRef]
- Onishi, T.; Nakamura, K.; Horino, H.; Adachi, T.; Mitsuno, T. Evaluation of the denitrification rate of terraced paddy fields. J. Hydrol. 2012, 436, 111–119. [Google Scholar] [CrossRef]
- Baranov, V.; Lewandowski, J.; Krause, S. Bioturbation enhances the aerobic respiration of lake sediments in warming lakes. Biol. Lett. 2016, 12, 20160448. [Google Scholar] [CrossRef]
- Xie, J.; Hu, L.L.; Tang, J.J.; Wu, X.; Li, N.; Yuan, Y.; Yang, H.; Zhang, J.; Luo, S.; Chen, X. Ecological mechanisms underlying the sustainability of the agriculture heritage rice–fish coculture system. Proc. Natl. Acad. Sci. USA 2011, 108, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Xinhua. In Pics: Rice-Fish Co-Culture System in East China’s Zhejiang. Xinhua News Agency, 20 July 2022. Available online: https://english.news.cn/20220720/a83e8735f42b4565991d1d9a93c4470d/c.html (accessed on 8 May 2025).
- Chen, J.; Zhao, L.F.; Dai, R.X.; Zhang, T.J.; Tang, J.J.; Hu, L.L.; Chen, X. Soil microbial communities of methanogens and methanotrophs in the rice-fish coculture ecosystem. China J. Ecol. 2023, 42, 2961–2971. (In Chinese) [Google Scholar]
- Lu, R.K. Soil Argrochemistry Analysis Protocoes; China Agriculture Science Press: Beijing, China, 1999. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 539–579. [Google Scholar]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis: Part 3 Chemical Methods; American Society of Agronomy: Madison, WI, USA, 1996; Volume 5, pp. 1085–1121. [Google Scholar]
- Guo, J.J.; Ling, N.; Chen, Z.J.; Xue, C.; Li, L.; Liu, L.S.; Gao, L.M.; Wang, M.; Ruan, J.Y.; Guo, S.W.; et al. Soil fungal assemblage complexity is dependent on soil fertility and dominated by deterministic processes. New Phytol. 2020, 226, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Li, Y.L.; Guo, H.Q.; Ge, Z.M.; Wang, D.Q.; Liu, W.L.; Xie, L.N.; Li, S.H.; Tan, L.S.; Zhao, B.; Li, X.Z.; et al. Sea-level rise will reduce net CO2 uptake in subtropical coastal marshes. Sci. Total Environ. 2020, 747, 141214. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Jiang, J.; Zheng, X.; Sass, R.L. A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: Effects of water regime, crop residue, and fertilizer application. Glob. Biogeochem. Cycles 2005, 19, 1–9. [Google Scholar] [CrossRef]
- Hutchinson, G.L.; Mosier, A.R. Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci. Soc. Am. J. 1981, 45, 311–316. [Google Scholar] [CrossRef]
- CanoDraw for Windows, Version 4.5; Software for Canonical Community Ordination. Microcomputer Power: Ithaca, NY, USA, 2002.
- Yang, T.; Wang, X.D.; Wang, M.J.; Li, F.B.; Barthel, M.; Six, J.H.; Feng, J.F.; Fang, F.P. Impact of rice-crab and rice-fish co-cultures on the methane emission and its transport in aquaculture ponds. Agric. Ecosyst. Environ. 2024, 378, 109281. [Google Scholar] [CrossRef]
- FAO. Agricultural Heritage: Tackling Climate Challenges and Building Resilient Communities. Members Gateway News. 2024. Available online: https://www.fao.org/members-gateway/news/detail/en/c/1731201 (accessed on 8 June 2025).
- UNEP. Methane Emissions Are Driving Climate Change. Here’s How to Reduce Them. UNEP News Stories. 2021. Available online: https://www.unep.org/news-and-stories/story/methane-emissions-are-driving-climate-change-heres-how-reduce-them (accessed on 8 June 2025).
- World Bank. Climate-Smart Agriculture. World Bank Topics. 2024. Available online: https://www.worldbank.org/en/topic/climate-smart-agriculture (accessed on 8 June 2025).
- Cai, Z.C.; Xing, G.X.; Yan, X.Y.; Xu, H.; Tsuruta, H.; Yagi, K.; Minami, K. Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilisers and water management. Plant Soil 1997, 196, 7–14. [Google Scholar] [CrossRef]
- Zhang, B.W.; Tian, H.Q.; Ren, W.; Tao, B.; Lu, C.Q.; Yang, J.; Banger, K.; Pan, S.F. Methane emissions from global rice fields: Magnitude, spatiotemporal patterns, and environmental controls. Glob. Biogeochem. Cycles 2016, 30, 1246–1263. [Google Scholar] [CrossRef]
- Sun, Z.C.; Guo, Y.; Li, C.F.; Cao, C.G.; Yuan, P.L.; Zou, F.L.; Wang, J.H.; Jia, P.G.; Wang, J.P. Effects of straw returning and feeding on greenhouse gas emissions from integrated rice-crayfish farming in Jianghan Plain, China. Environ. Sci. Pollut. Res. 2019, 26, 11710–11718. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.L.; Liu, H.B.; Wang, H.Y.; Wu, S.X.; Bashir, M.A.; Reis, S.; Sun, Q.Y.; Xu, J.M.; Gu, B.J. Rice-animal co-culture systems benefit global sustainable intensification. Earth Future 2023, 11, e2022EF002984. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).