Study on the Response of Cotton Leaf Color to Plant Water Content Changes and Optimal Irrigation Thresholds
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.2.1. Experiment 1
2.2.2. Experiment 2
2.2.3. Cotton Leaf Color Value Collection
2.3. Cotton Moisture Determination
2.4. Agronomic Traits and Yield
2.5. Data Processing and Analysis
3. Results and Analysis
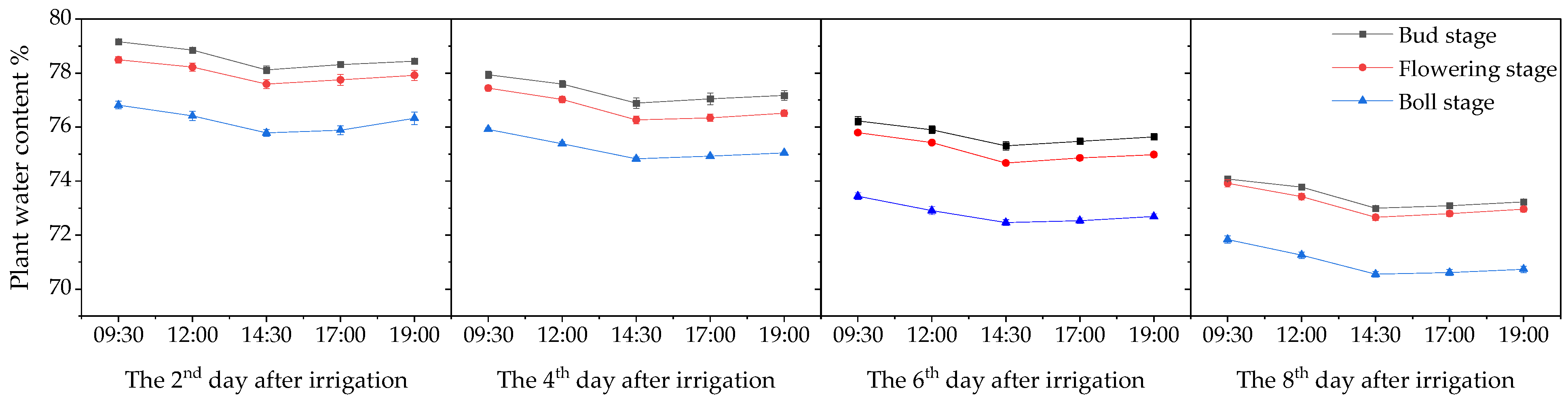
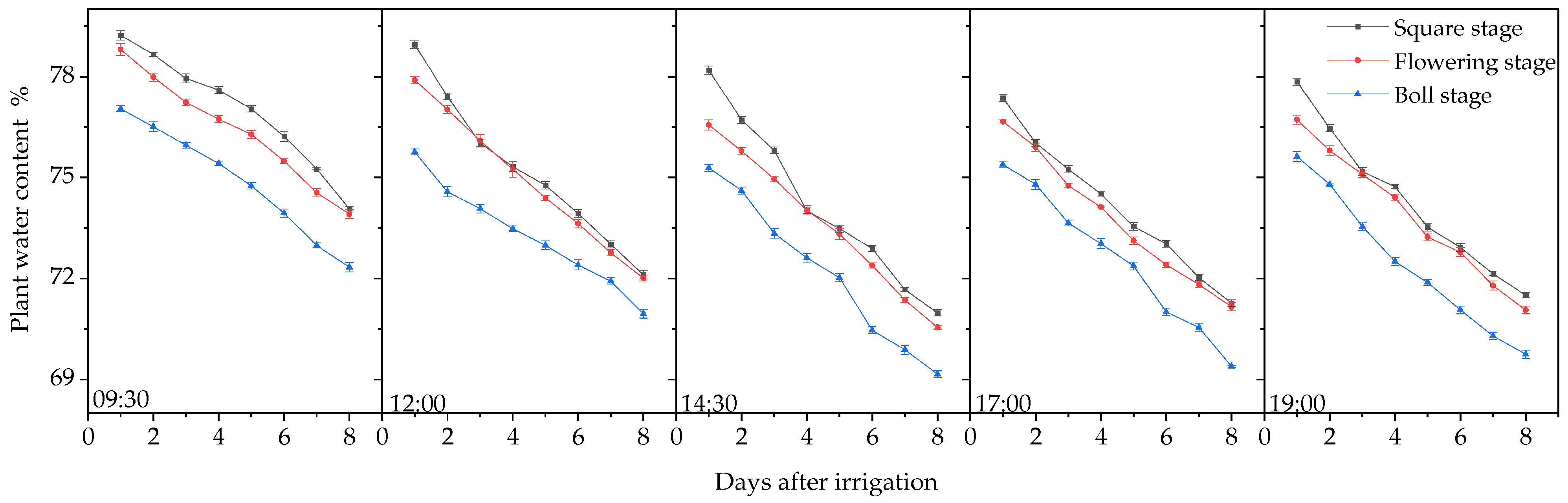
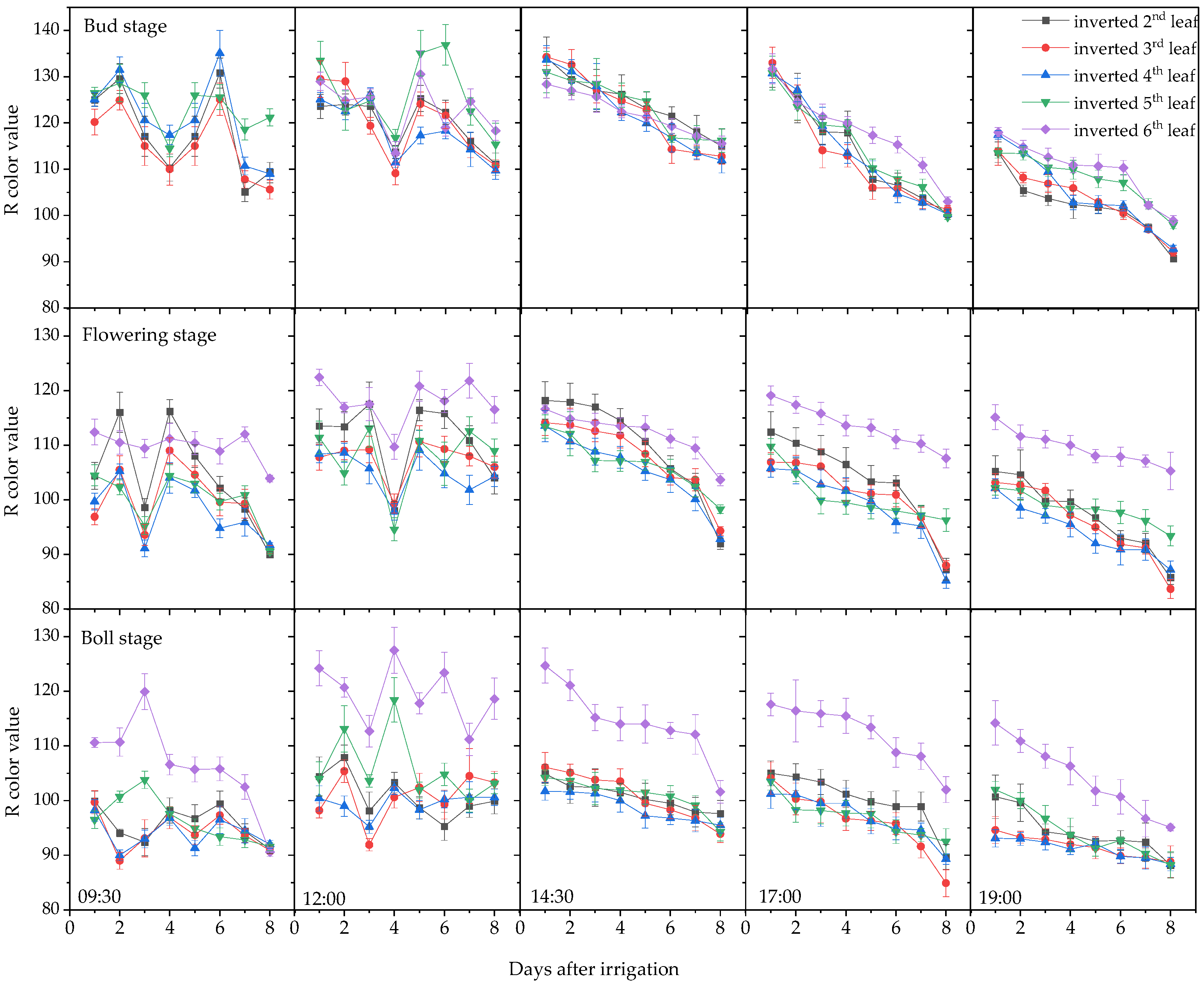
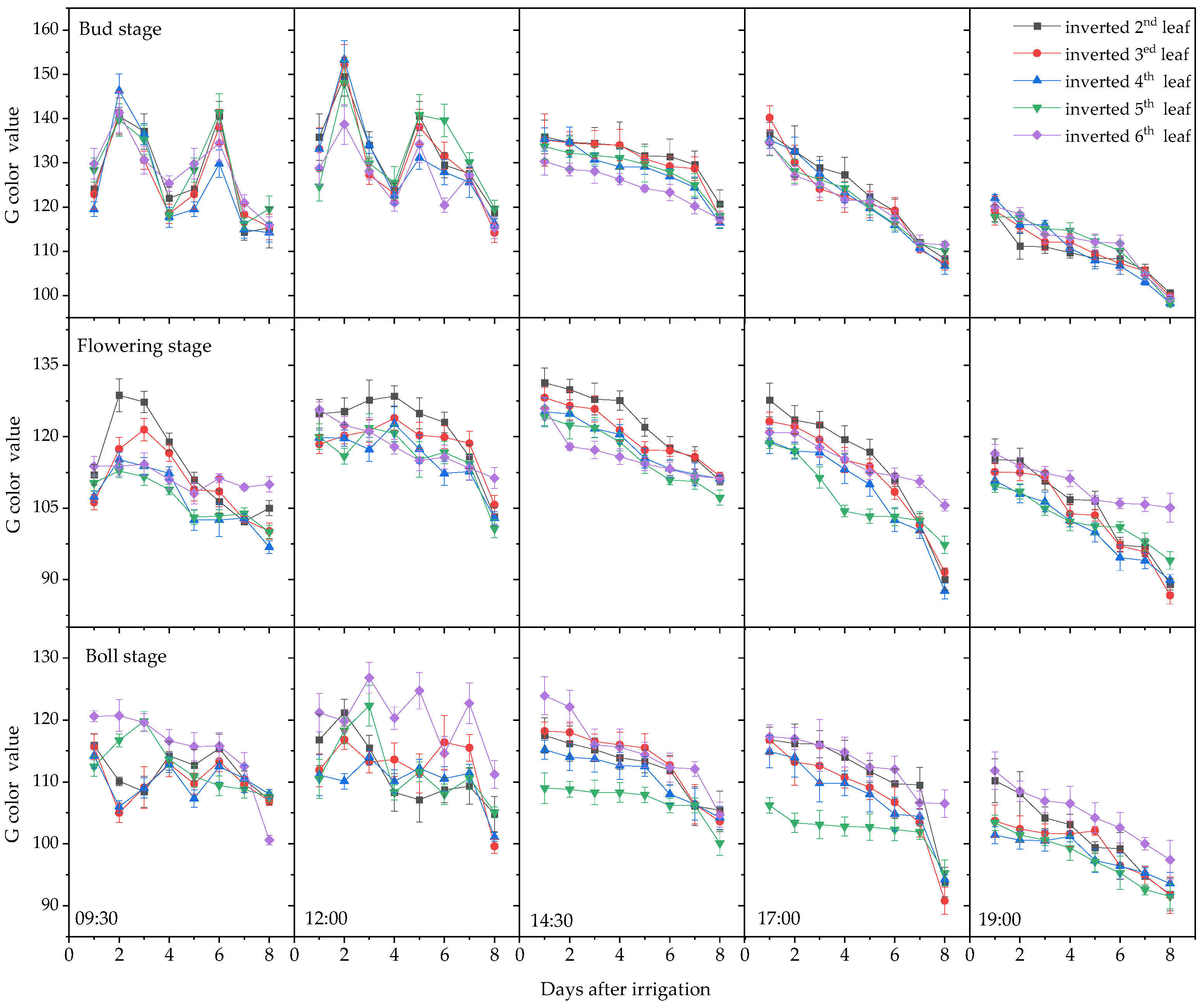
3.1. Plant Moisture Content and Leaf Color Change Trends
3.1.1. Changes in Plant Water Content
3.1.2. Variation in Leaf RGB Values
3.1.3. Correlation Analysis Between Leaf RGB Values and Plant Water Content
3.1.4. Linear Regression of Leaf Color Values and Plant Water Content
3.2. Effects of Different Water Thresholds on Cotton Growth
3.2.1. Effects of Different Water Thresholds on Cotton Agronomic Traits
3.2.2. Effects of Different Water Thresholds on Boll Number and Individual Boll Weight in Cotton
3.2.3. Effects of Different Water Thresholds on Cotton Yield and Its Components
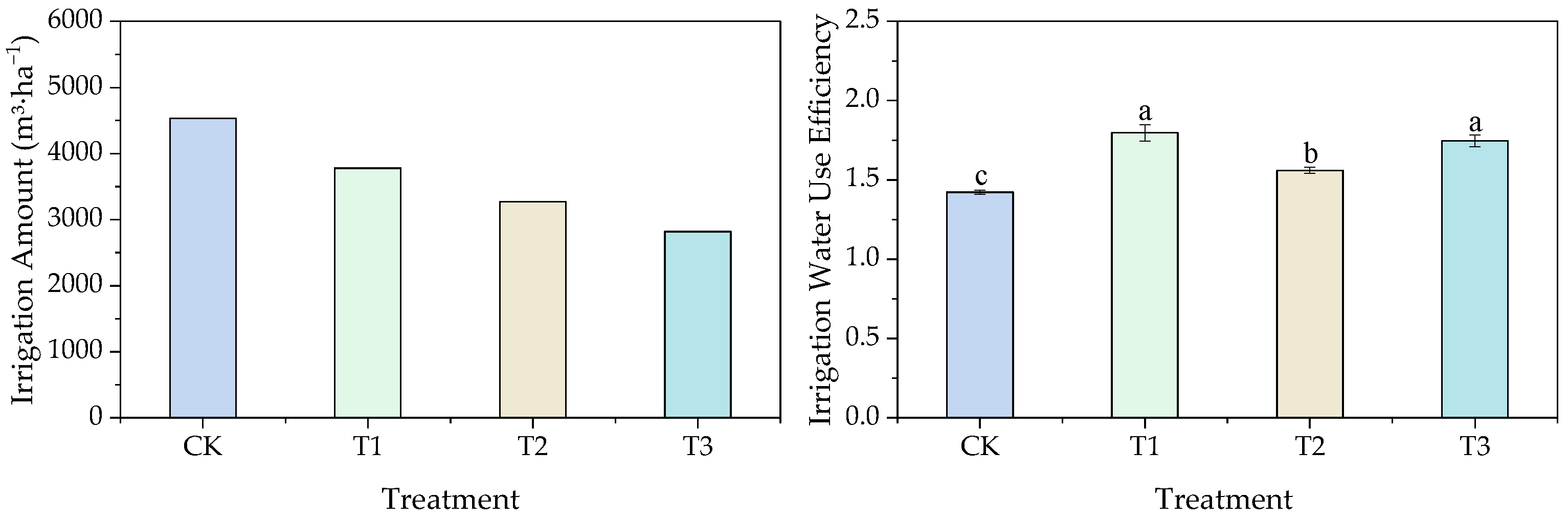
3.2.4. Effects of Different Water Thresholds on Cotton Water Application and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tlatlaa, J.S.; Tryphone, G.M.; Nassary, E.K. Unexplored Agronomic, Socioeconomic and Policy Domains for Sustainable Cotton Production on Small Landholdings: A Systematic Review. Front. Agron. 2023, 5, 1281043. [Google Scholar] [CrossRef]
- Chabi Simin Najib, D.; Fei, C.; Dilanchiev, A.; Romaric, S. Modeling the Impact of Cotton Production on Economic Development in Benin: A Technological Innovation Perspective. Front. Environ. Sci. 2022, 10, 926350. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publ.: Paris, France, 2023. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. China Statistical Yearbook 2024; China Statistics Press: Beijing, China, 2024. Available online: https://www.stats.gov.cn/sj/ndsj/2024/indexch.htm (accessed on 27 April 2025).
- Shamim, N.; Asim, M.; Baker, T.; Awad, A.I. Efficient Approach for Anomaly Detection in IoT Using System Calls. Sensors 2023, 23, 652. [Google Scholar] [CrossRef]
- Qi, Q.; Yang, H.; Zhou, Q.; Han, X.; Jia, Z.; Jiang, Y.; Chen, Z.; Hou, L.; Mei, S. Performance of Soil Moisture Sensors at Different Salinity Levels: Comparative Analysis and Calibration. Sensors 2024, 24, 6323. [Google Scholar] [CrossRef]
- Quemada, C.; Pérez-Escudero, J.M.; Gonzalo, R.; Ederra, I.; Santesteban, L.G.; Torres, N.; Iriarte, J.C. Remote Sensing for Plant Water Content Monitoring: A Review. Remote Sens. 2021, 13, 2088. [Google Scholar] [CrossRef]
- Pagano, M.; Baldacci, L.; Ottomaniello, A.; de Dato, G.; Chianucci, F.; Masini, L.; Carelli, G.; Toncelli, A.; Storchi, P.; Tredicucci, A.; et al. THz Water Transmittance and Leaf Surface Area: An Effective Nondestructive Method for Determining Leaf Water Content. Sensors 2019, 19, 4838. [Google Scholar] [CrossRef]
- Moyankova, D.; Stoykova, P.; Veleva, P.; Christov, N.K.; Petrova, A.; Atanassova, S. An Aquaphotomics Approach for Investigation of Water-Stress-Induced Changes in Maize Plants. Sensors 2023, 23, 9678. [Google Scholar] [CrossRef]
- Thalheimer, M. A Leaf-Mounted Capacitance Sensor for Continuous Monitoring of Foliar Transpiration and Solar Irradiance as an Indicator of Plant Water Status. J. Agric. Eng. 2023, 54. [Google Scholar] [CrossRef]
- Hama, A.; Matsumoto, Y.; Matsuoka, N. Estimating Leaf Water Content Through Low-Cost LiDAR. Agronomy 2022, 12, 1183. [Google Scholar] [CrossRef]
- Li, Y.; Song, X.; Li, S.; Salter, W.T.; Barbour, M.M. The Role of Leaf Water Potential in the Temperature Response of Mesophyll Conductance. New Phytol. 2020, 225, 1193–1205. [Google Scholar] [CrossRef]
- Ahmad, U.; Alvino, A.; Marino, S. A Review of Crop Water Stress Assessment Using Remote Sensing. Remote Sens. 2021, 13, 4155. [Google Scholar] [CrossRef]
- Dong, H.; Dong, J.; Sun, S.; Bai, T.; Zhao, D.; Yin, Y.; Wang, Y. Crop Water Stress Detection Based on UAV Remote Sensing Systems. Agric. Water Manag. 2024, 303, 109059. [Google Scholar] [CrossRef]
- Lloret, J.; Sendra, S.; Garcia, L.; Jimenez, J.M. A Wireless Sensor Network Deployment for Soil Moisture Monitoring in Precision Agriculture. Sensors 2021, 21, 7243. [Google Scholar] [CrossRef]
- Le, M.T.; Pham, C.D.; Nguyen, T.P.T.; Nguyen, T.L.; Nguyen, Q.C.; Hoang, N.B.; Nghiem, L.D. Wireless Powered Moisture Sensors for Smart Agriculture and Pollution Prevention: Opportunities, Challenges, and Future Outlook. Curr. Pollut. Rep. 2023, 9, 646–659. [Google Scholar] [CrossRef]
- Özreçberoğlu, N.; Kahramanoğlu, İ. Mathematical Models for the Estimation of Leaf Chlorophyll Content Based on RGB Colours of Contact Imaging with Smartphones: A Pomegranate Example. Folia Hortic. 2020, 32, 57–67. [Google Scholar] [CrossRef]
- Yadav, S.P.; Ibaraki, Y.; Gupta, D. Estimation of the Chlorophyll Content of Micropropagated Potato Plants Using RGB-Based Image Analysis. Plant Cell Tissue Organ Cult. 2010, 100, 183–188. [Google Scholar] [CrossRef]
- Bodor-Pesti, P.; Taranyi, D.; Nyitrainé Sárdy, D.Á.; Le Phuong Nguyen, L.; Baranyai, L. Correlation of the Grapevine (Vitis vinifera L.) Leaf Chlorophyll Concentration with RGB Color Indices. Horticulturae 2023, 9, 899. [Google Scholar] [CrossRef]
- González-Espíndola, L.Á.; Pedroza-Sandoval, A.; Trejo-Calzada, R.; Jacobo-Salcedo, M.d.R.; de los Santos, G.G.; Quezada-Rivera, J.J. Relative Water Content, Chlorophyll Index, and Photosynthetic Pigments on Lotus corniculatus L. in Response to Water Deficit. Plants 2024, 13, 961. [Google Scholar] [CrossRef]
- Zhang, Y.; Ru, G.; Zhao, Z.; Wang, D. Hyperspectral Prediction Models of Chlorophyll Content in Paulownia Leaves Under Drought Stress. Sensors 2024, 24, 6309. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Guo, J.; Wei, C.; Han, D. Effects of Different Imaging Methods on Spectral Parameters of Cotton Canopy Characteristics. J. Shihezi Univ. (Nat. Sci.) 2006, 24, 397–400. [Google Scholar]
- Wang, J.; Wei, C.; Wang, X.; Zhu, Q.; Zhu, J.; Wang, J. Prediction of Cotton Chlorophyll Content Using Computer Vision Corrected by Gray Board. Trans. Chin. Soc. Agric. Eng. 2013, 29, 173. [Google Scholar]
- Qi, W.; Wang, H.; Xue, H.; Wang, Z.; Ma, X.; Wang, J.; Xiao, J. Effects of Irrigation Lower Limit on Photosynthetic Physiology and Yield of Cotton in Northern Xinjiang. Water Sav. Irrig. 2024, 12, 95–101+110. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Y.; Zhang, Y.; Wang, J.; Li, J.; Gao, Y.; Wang, X.; Wu, L. Rapid Nondestructive Detection of Chlorophyll Content in Muskmelon Leaves Under Different Light Quality Treatments. Agronomy 2022, 12, 3223. [Google Scholar] [CrossRef]
- Li, W.; Pan, K.; Huang, Y.; Fu, G.; Liu, W.; He, J.; Xiao, W.; Fu, Y.; Guo, J. Monitoring the Maize Canopy Chlorophyll Content Using Discrete Wavelet Transform Combined with RGB Feature Fusion. Agronomy 2025, 15, 212. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, R.; Mi, T.; Song, Y.; Shi, F.; Liu, X.; Wang, Y.; Sun, F.; Xi, Y.; Zhang, C. Rapid and Nondestructive Evaluation of Wheat Chlorophyll Under Drought Stress Using Hyperspectral Imaging. Int. J. Mol. Sci. 2023, 24, 5825. [Google Scholar] [CrossRef]
- Yan, W.; Lu, Y.; Guo, L.; Liu, Y.; Li, M.; Zhang, B.; Zhang, L.; Qin, D.; Huo, J. Effects of Drought Stress on Photosynthesis and Chlorophyll Fluorescence in Blue Honeysuckle. Plants 2024, 13, 2115. [Google Scholar] [CrossRef]
- Ye, X.; Kitaya, M.; Abe, S.; Sheng, F.; Zhang, S. A New Small-Size Camera with Built-In Specific-Wavelength LED Lighting for Evaluating Chlorophyll Status of Fruit Trees. Sensors 2023, 23, 4636. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, E.; Saponaro, V.; Hoshika, Y.; Arab, L.; Rennenberg, H.; Paoletti, E. Hyperspectral Reflectance of Light-Adapted Leaves Can Predict Both Dark- and Light-Adapted Chl Fluorescence Parameters, and the Effects of Chronic Ozone Exposure on Date Palm (Phoenix dactylifera). Int. J. Mol. Sci. 2020, 21, 6441. [Google Scholar] [CrossRef]
- Park, B.; Wi, S.; Chung, H.; Lee, H. Chlorophyll Fluorescence Imaging for Environmental Stress Diagnosis in Crops. Sensors 2024, 24, 1442. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Dai, T. Physiological and Biochemical Changes During Drought and Recovery Periods at Tillering and Jointing Stages in Wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Bian, M.; Lv, T.; Li, W.; Chen, C.; Zhang, X.; Wang, M. Effect of Irrigation Amount on Cotton Growth and Optimization of Irrigation Regime Using AquaCrop in Southern Xinjiang. Agronomy 2025, 15, 1101. [Google Scholar] [CrossRef]
- Koudahe, K.; Sheshukov, A.Y.; Aguilar, J.; Djaman, K. Irrigation-Water Management and Productivity of Cotton: A Review. Sustainability 2021, 13, 10070. [Google Scholar] [CrossRef]
- Lin, M.; Wang, L.; Lv, G.; Gao, C.; Zhao, Y.; Li, X.; He, L.; Sun, W. Deficit Irrigation Effects on Cotton Growth Cycle and Preliminary Optimization of Irrigation Strategies in Arid Environment. Plants 2024, 13, 1403. [Google Scholar] [CrossRef]
- Guedes, W.A.; Nobre, R.G.; Soares, L.A.d.A.; de Lima, G.S.; Gheyi, H.R.; Fernandes, P.D.; Ferreira, A.P.N.; da Silva, A.A.R.; de Azevedo, C.A.V.; Silva, D.V.; et al. Irrigation Strategies with Controlled Water Deficit in Two Production Cycles of Cotton. Plants 2023, 12, 2892. [Google Scholar] [CrossRef]
- Patil, A.M.; Pawar, B.D.; Wagh, S.G.; Shinde, H.; Shelake, R.M.; Markad, N.R.; Bhute, N.K.; Červený, J.; Wagh, R.S. Abiotic Stress in Cotton: Insights into Plant Responses and Biotechnological Solutions. Agriculture 2024, 14, 1638. [Google Scholar] [CrossRef]
- Eid, M.A.M.; El-hady, M.A.A.; Abdelkader, M.A.; Abd-Elkrem, Y.M.; El-Gabry, Y.A.; El-Temsah, M.E.; El-Areed, S.R.M.; Rady, M.M.; Alamer, K.H.; Alqubaie, A.I.; et al. Response in Physiological Traits and Antioxidant Capacity of Two Cotton Cultivars Under Water Limitations. Agronomy 2022, 12, 803. [Google Scholar] [CrossRef]
- Ma, H.; Ge, C.; Liu, R.; Zhang, S.; Liu, S.; Shen, Q.; Chen, J.; Wan, S.; Pang, C. Effects of Different Irrigation Water Volumes with 1,1-Dimethyl-Piperidinium Chloride (DPC) on Cotton Growth and Yield. Agronomy 2024, 14, 1656. [Google Scholar] [CrossRef]
- Mahler, E. Innovations in Clay-Based Irrigation Technologies—A Systematic Review. Sustainability 2024, 16, 7029. [Google Scholar] [CrossRef]
- Devi, M.J.; Reddy, V.R. Transpiration Response of Cotton to Vapor Pressure Deficit and Its Relationship with Stomatal Traits. Front. Plant Sci. 2018, 9, 1572. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, Z.; Feng, K.; Chen, Z.; Cao, C.; Huang, J.; Ye, H.; Gao, Y. Influencing factors for transpiration rate: A numerical simulation of an individual leaf system. Therm. Sci. Eng. Prog. 2022, 27, 101110. [Google Scholar] [CrossRef]
- Huang, C.W.; Chu, C.R.; Hsieh, C.I.; Palmroth, S.; Katul, G.G. Wind-induced leaf transpiration. Adv. Water Resour. 2015, 86, 240–255. [Google Scholar] [CrossRef]

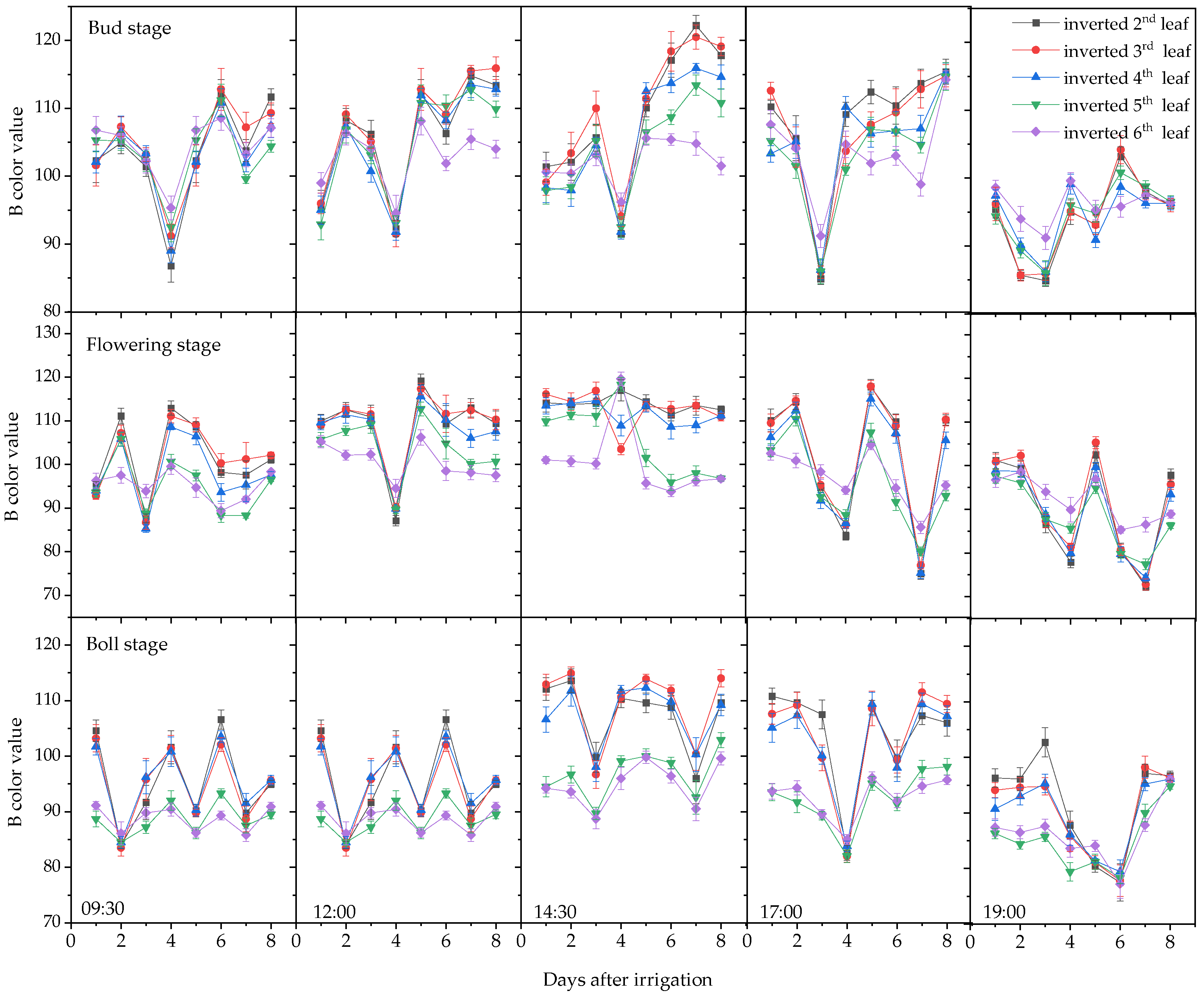
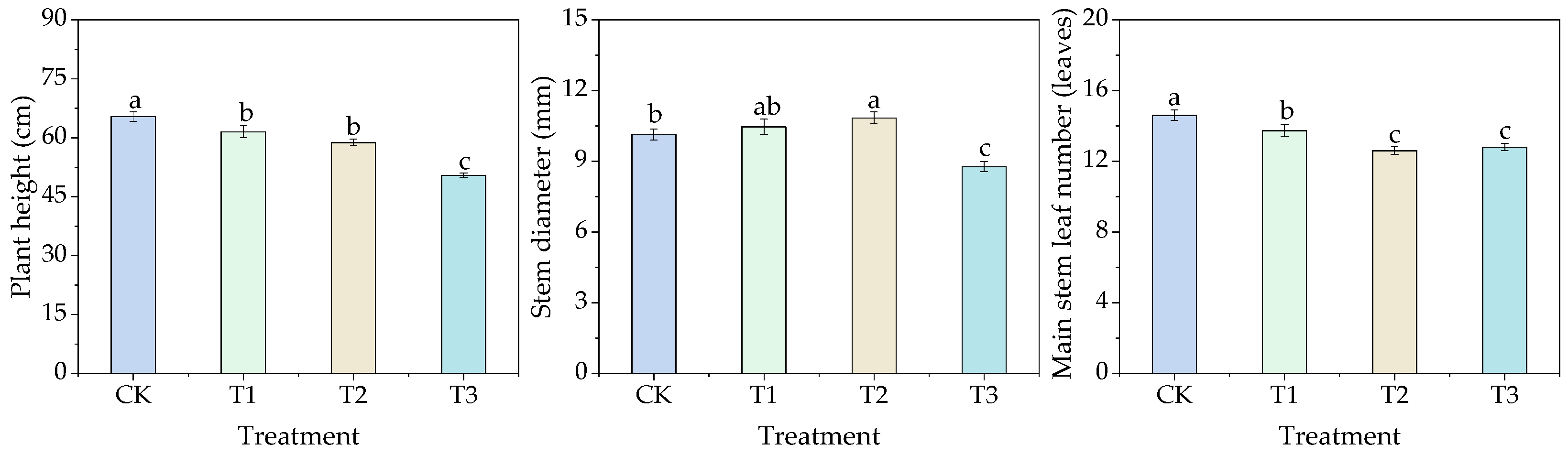
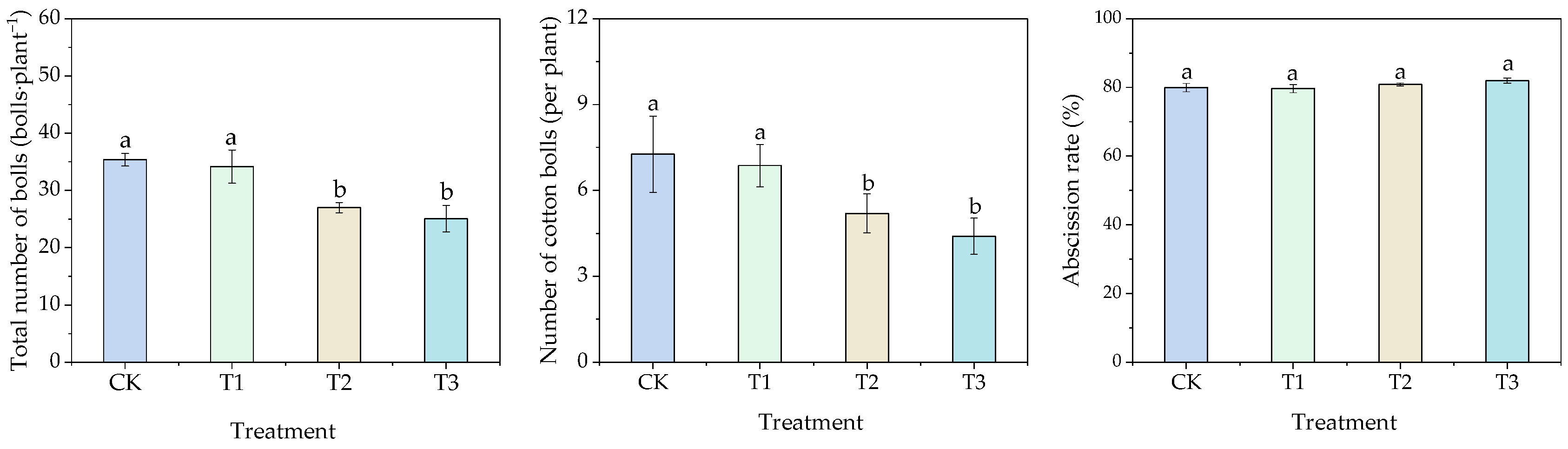
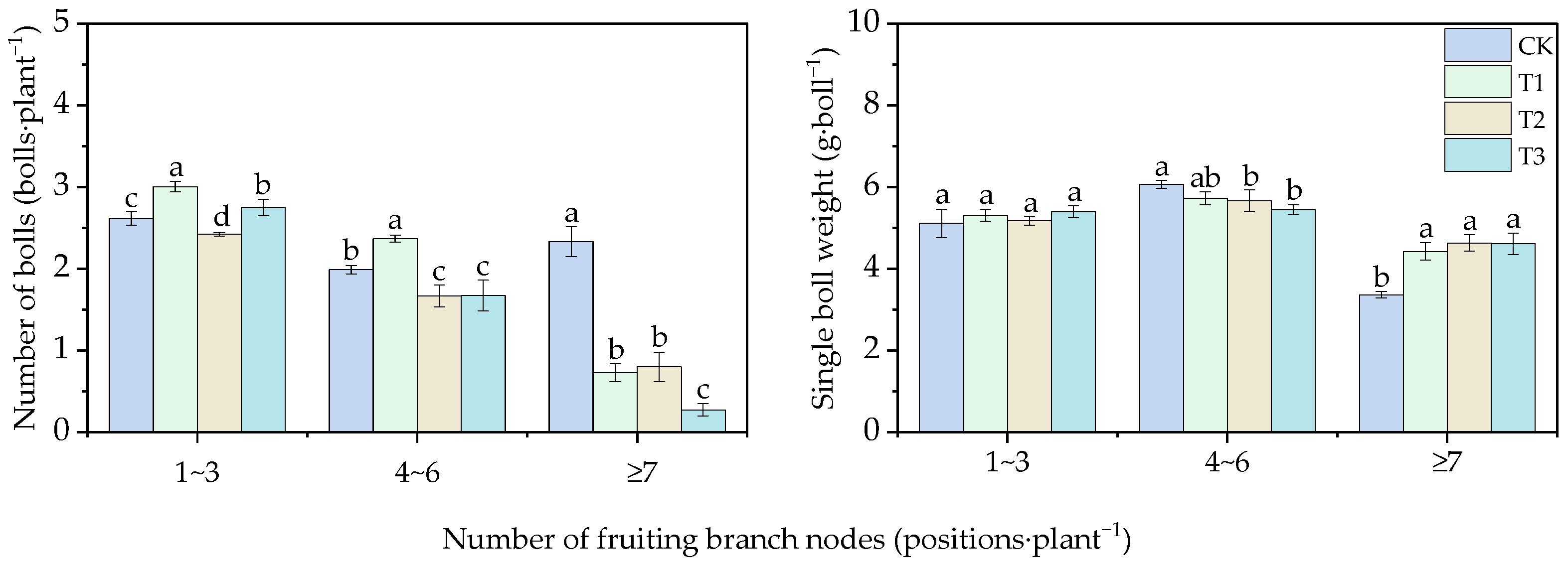
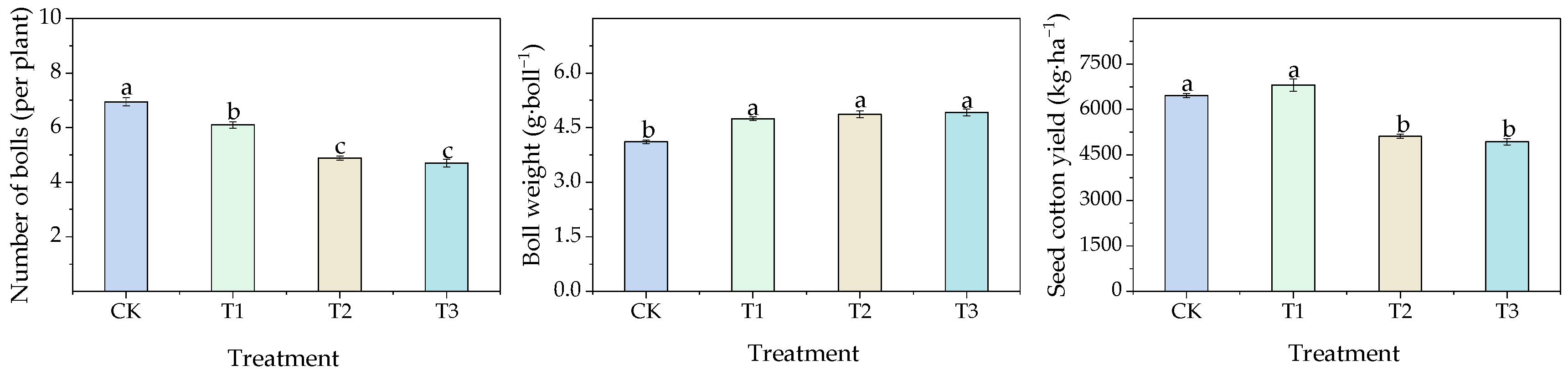
| Stages | Times of Day | Inverted Second Leaf | Inverted Third Leaf | Inverted Fourth Leaf | Inverted Fifth Leaf | Inverted Sixth Leaf |
|---|---|---|---|---|---|---|
| Bud Stage | 09:30 | 0.56 | 0.52 | 0.58 | 0.59 | 0.53 |
| 12:00 | 0.58 | 0.63 | 0.63 | 0.28 | 0.52 | |
| 14:30 | 0.79 * | 0.82 * | 0.83 * | 0.79 * | 0.74 * | |
| 17:00 | 0.79 * | 0.82 * | 0.82 * | 0.79 * | 0.76 * | |
| 19:00 | 0.69 | 0.82 * | 0.80 * | 0.73 * | 0.74 * | |
| Flowering Stage | 09:30 | 0.36 | −0.87 | 0.34 | 0.27 | 0.24 |
| 12:00 | 0.62 | 0.63 | 0.58 | 0.63 | 0.60 | |
| 14:30 | 0.85 * | 0.90 ** | 0.90 ** | 0.87 * | 0.86 * | |
| 17:00 | 0.80 * | 0.82 * | 0.83 * | 0.79 * | 0.77 * | |
| 19:00 | 0.74 * | 0.80 * | 0.79 * | 0.76 * | 0.72 * | |
| Boll Stage | 09:30 | 0.69 | 0.62 | 0.69 | 0.69 | 0.57 |
| 12:00 | 0.52 | 0.76 * | 0.72 * | 0.63 | 0.65 | |
| 14:30 | 0.81 ** | 0.85 ** | 0.84 ** | 0.83 ** | 0.82 ** | |
| 17:00 | 0.82 * | 0.86 ** | 0.84 ** | 0.84 ** | 0.83 * | |
| 19:00 | 0.78 * | 0.82 * | 0.80 * | 0.79 * | 0.78 * |
| Stages | Times of Day | Inverted Second Leaf | Inverted Third Leaf | Inverted Fourth Leaf | Inverted Fifth Leaf | Inverted Sixth Leaf |
|---|---|---|---|---|---|---|
| Bud Stage | 09:30 | 0.39 | 0.39 | 0.45 | 0.50 | 0.50 |
| 12:00 | 0.56 | 0.43 | 0.57 | 0.08 | 0.48 | |
| 14:30 | 0.82 * | 0.86 * | 0.88 * | 0.80 * | 0.79 * | |
| 17:00 | 0.80 * | 0.83 * | 0.83 * | 0.74 * | 0.76 * | |
| 19:00 | 0.77 * | 0.82 * | 0.81 * | 0.77 * | 0.75 * | |
| Flowering Stage | 09:30 | 0.30 | −0.05 | 0.29 | 0.52 | 0.59 |
| 12:00 | 0.69 | 0.69 | 0.60 | 0.53 | 0.57 | |
| 14:30 | 0.81 * | 0.88 ** | 0.86 ** | 0.76 * | 0.87 ** | |
| 17:00 | 0.80 * | 0.82 * | 0.82 * | 0.79 * | 0.79 * | |
| 19:00 | 0.76 * | 0.79 * | 0.78 * | 0.77 * | 0.71 * | |
| Boll Stage | 09:30 | 0.67 | 0.72 * | 0.71 * | 0.67 | 0.63 |
| 12:00 | 0.67 | 0.76 * | 0.81 * | 0.69 | 0.66 | |
| 14:30 | 0.81 ** | 0.85 ** | 0.84 ** | 0.83 ** | 0.81 ** | |
| 17:00 | 0.81 * | 0.85 ** | 0.85 ** | 0.82 * | 0.81 * | |
| 19:00 | 0.78 * | 0.81 * | 0.82 * | 0.79 * | 0.77 * |
| Stages | Times of Day | Inverted Second Leaf | Inverted Third Leaf | Inverted Fourth Leaf | Inverted Fifth Leaf | Inverted Sixth Leaf |
|---|---|---|---|---|---|---|
| Bud Stage | 09:30 | 0.39 | 0.39 | 0.46 | 0.50 | 0.50 |
| 12:00 | 0.56 | 0.43 | 0.57 | 0.08 | 0.48 | |
| 14:30 | −0.67 | −0.72 * | −0.72 * | −0.69 | −0.34 | |
| 17:00 | −0.27 | −0.15 | −0.26 | −0.22 | 0.33 | |
| 19:00 | −0.43 | −0.42 | −0.07 | −0.47 | 0.15 | |
| Flowering Stage | 09:30 | 0.30 | −0.05 | 0.29 | 0.52 | 0.59 |
| 12:00 | −0.08 | −0.12 | 0.07 | 0.28 | 0.52 | |
| 14:30 | 0.18 | 0.28 | 0.76 * | 0.70 | 0.26 | |
| 17:00 | 0.21 | 0.21 | 0.23 | 0.57 | 0.61 | |
| 19:00 | 0.35 | 0.40 | 0.45 | 0.69 | 0.73 * | |
| Boll Stage | 09:30 | 0.05 | 0.17 | 0.03 | −0.26 | 0.48 |
| 12:00 | 0.27 | −0.26 | −0.24 | −0.52 | −0.49 | |
| 14:30 | 0.18 | −0.03 | −0.20 | −0.59 | −0.53 | |
| 17:00 | 0.13 | −0.13 | −0.12 | −0.45 | −0.24 | |
| 19:00 | 0.48 | 0.37 | 0.34 | 0.15 | 0.43 |
| Stages | Functional Leaf | Color Value | Regression Equation | p | R2 |
|---|---|---|---|---|---|
| Bud Stage | Inverted third leaf | R | y = 0.25x + 40.97 | <0.05 | 0.77 |
| G | y = 0.34x + 28.34 | <0.05 | 0.65 | ||
| Inverted fourth leaf | R | y = 0.24x + 41.79 | <0.05 | 0.69 | |
| G | y = 0.51x + 4.66 | <0.05 | 0.69 | ||
| Flowering Stage | Inverted third leaf | R | y = 0.32x + 35.42 | <0.05 | 0.73 |
| G | y = 0.20x + 45.82 | <0.05 | 0.53 | ||
| Inverted fourth leaf | R | y = 0.25x + 44.13 | <0.05 | 0.64 | |
| G | y = 0.18x + 49.11 | <0.05 | 0.56 | ||
| Boll Stage | Inverted third leaf | R | y = 0.15x + 52.71 | <0.05 | 0.77 |
| G | y = 0.36x + 28.24 | <0.05 | 0.75 | ||
| Inverted fourth leaf | R | y = 0.19x + 48.68 | <0.05 | 0.77 | |
| G | y = 0.37x + 26.79 | <0.05 | 0.62 |
| Stages | Functional Leaf | Color Value | Regression Equation | p | R2 |
|---|---|---|---|---|---|
| Bud Stage | Inverted third leaf | R: x1, G: x2 | y = 0.18x1 + 0.12x2 + 33.57 | <0.05 | 0.80 |
| Inverted fourth leaf | R: x1, G: x2 | y = 0.14x1 + 0.29x2 + 16.82 | <0.05 | 0.78 | |
| Flowering Stage | Inverted third leaf | R: x1, G: x2 | y = 0.83x1 − 0.39x2 + 27.66 | <0.05 | 0.87 |
| Inverted fourth leaf | R: x1, G: x2 | y = 0.08x1 + 0.22x2 + 36.01 | <0.05 | 0.87 | |
| Boll Stage | Inverted third leaf | R: x1, G: x2 | y = 0.10x1 + 0.16x2 + 40.41 | <0.05 | 0.89 |
| Inverted fourth leaf | R: x1, G: x2 | y = 0.11x1 + 0.19x2 + 34.8 | <0.05 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, B.; Wang, L.; Cheng, J.; Chen, B.; Wang, J.; Zhang, K.; Liu, X. Study on the Response of Cotton Leaf Color to Plant Water Content Changes and Optimal Irrigation Thresholds. Agronomy 2025, 15, 1477. https://doi.org/10.3390/agronomy15061477
Mao B, Wang L, Cheng J, Chen B, Wang J, Zhang K, Liu X. Study on the Response of Cotton Leaf Color to Plant Water Content Changes and Optimal Irrigation Thresholds. Agronomy. 2025; 15(6):1477. https://doi.org/10.3390/agronomy15061477
Chicago/Turabian StyleMao, Binbin, Lulu Wang, Junhui Cheng, Bing Chen, Jiandong Wang, Kai Zhang, and Xiaowei Liu. 2025. "Study on the Response of Cotton Leaf Color to Plant Water Content Changes and Optimal Irrigation Thresholds" Agronomy 15, no. 6: 1477. https://doi.org/10.3390/agronomy15061477
APA StyleMao, B., Wang, L., Cheng, J., Chen, B., Wang, J., Zhang, K., & Liu, X. (2025). Study on the Response of Cotton Leaf Color to Plant Water Content Changes and Optimal Irrigation Thresholds. Agronomy, 15(6), 1477. https://doi.org/10.3390/agronomy15061477







