Solving Phosphorus Fertilization-Related Drip Irrigation Emitter Clogging by Adding Mn2+
Abstract
1. Introduction
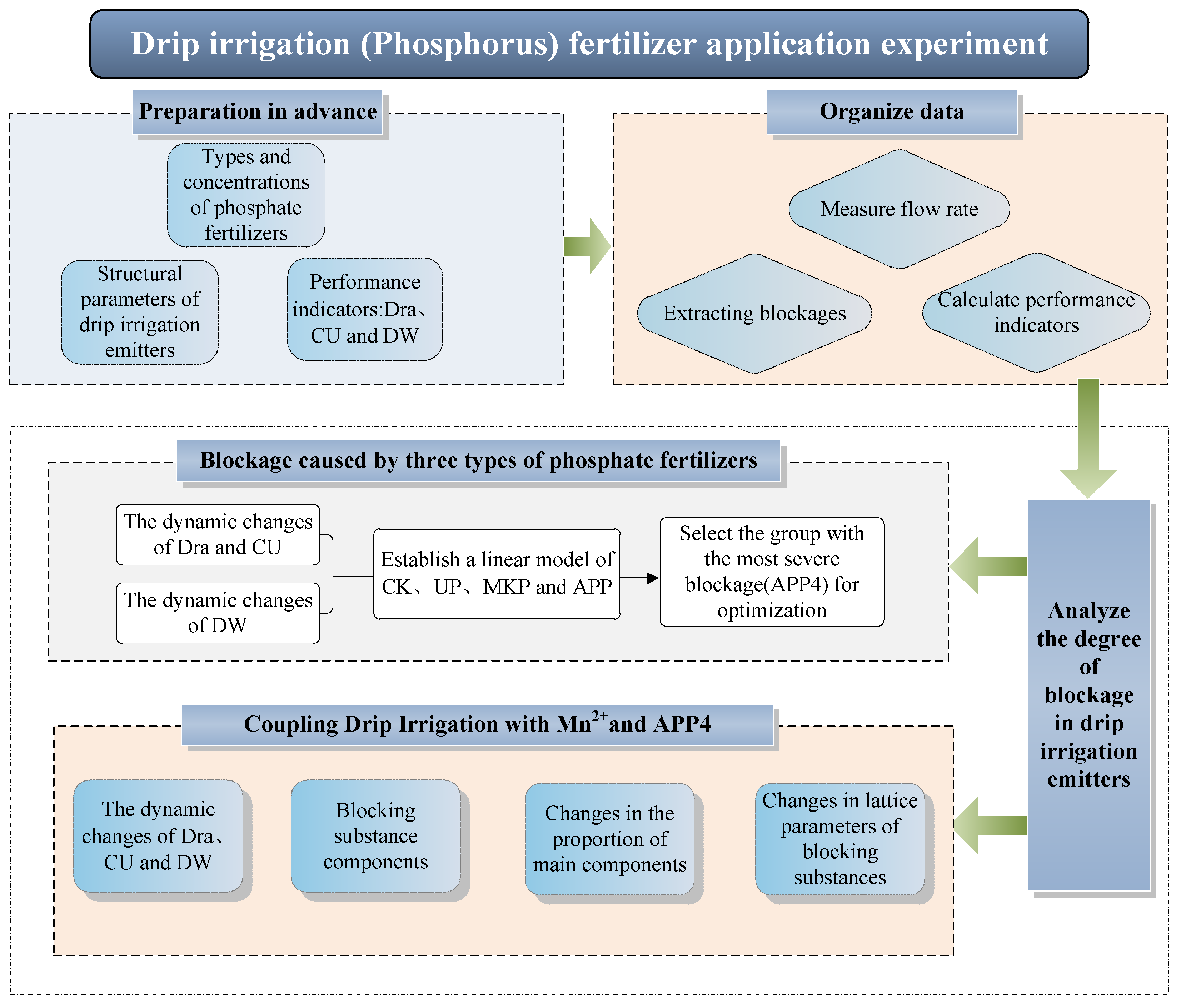
2. Experimental Materials and Methods
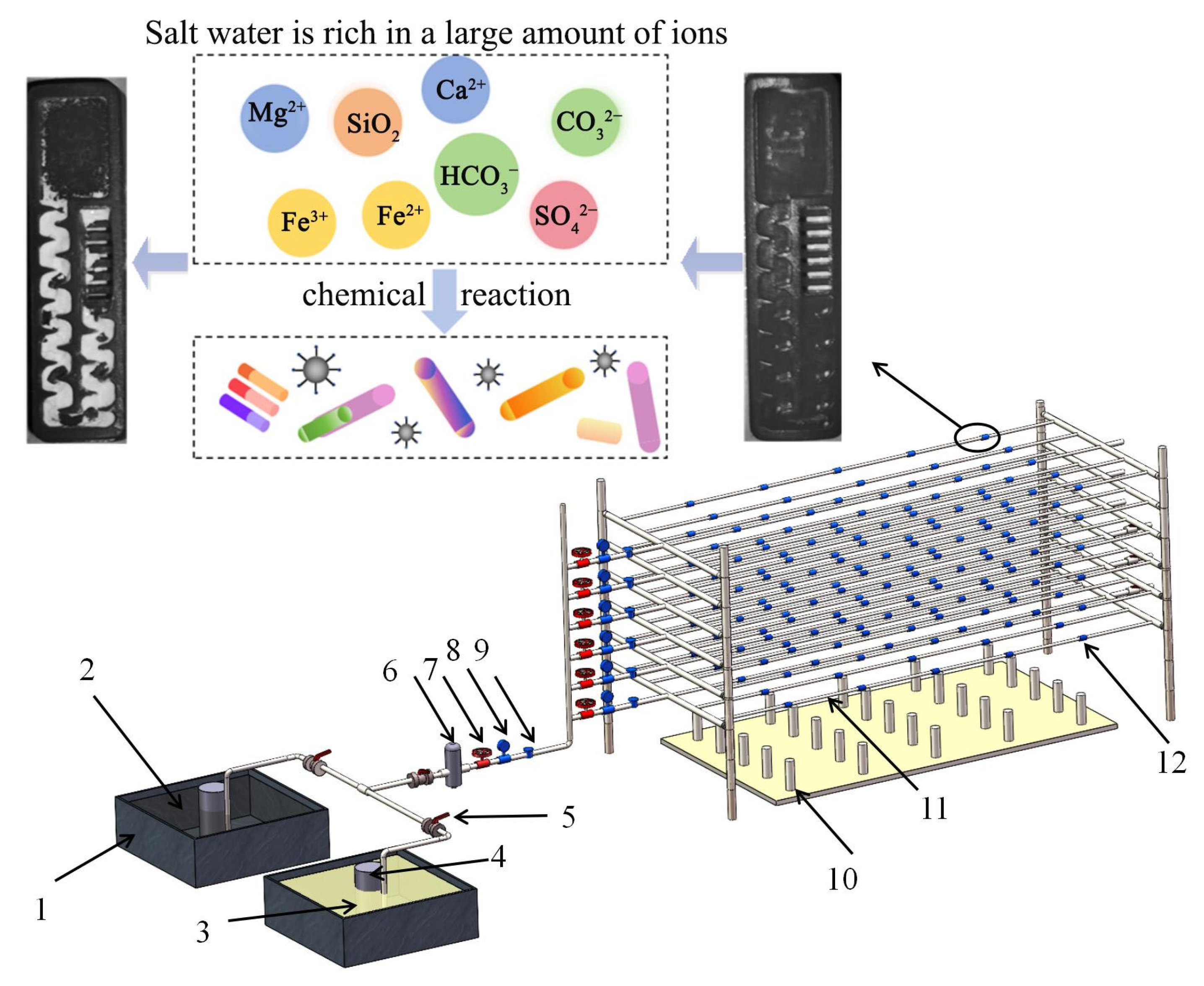
2.1. Experimental Design and Instrument Preparation
2.2. The Performance Evaluation Parameters of Drip Irrigation Emitters
2.3. Extraction and Testing of Blockages
2.4. Statistical Analysis
3. Results
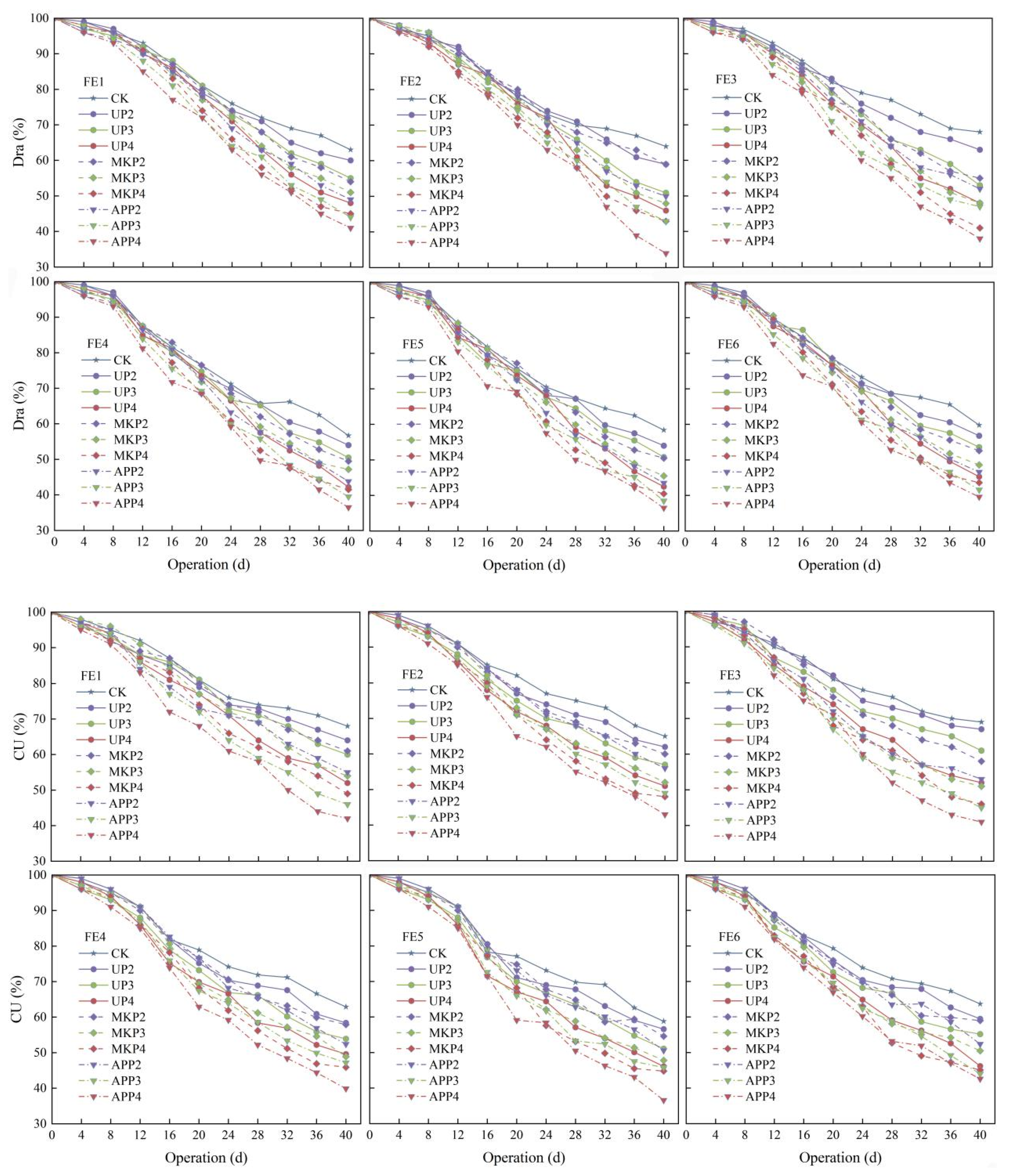
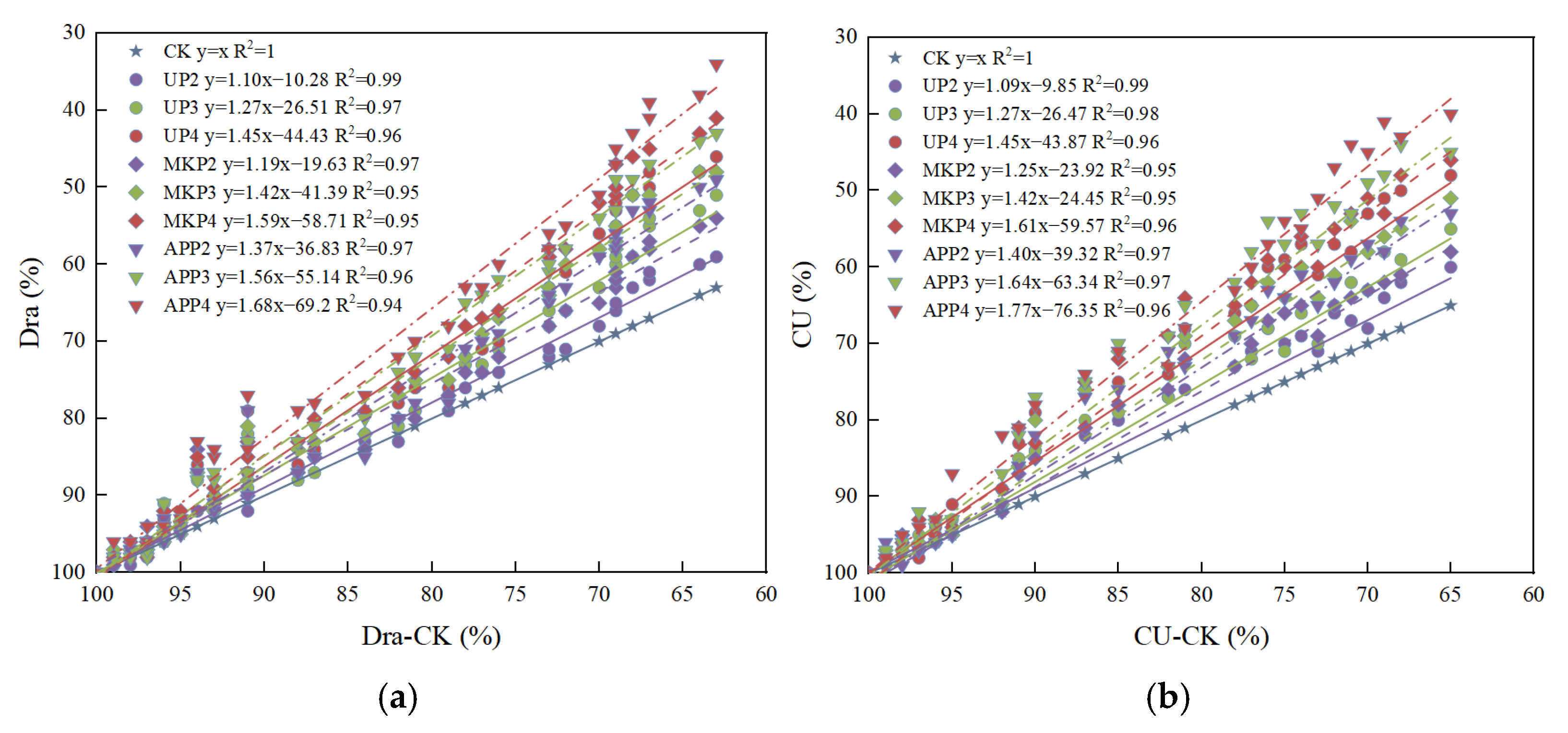
3.1. Effect of Phosphate Fertilizer on Drip Irrigation Emitter Performance
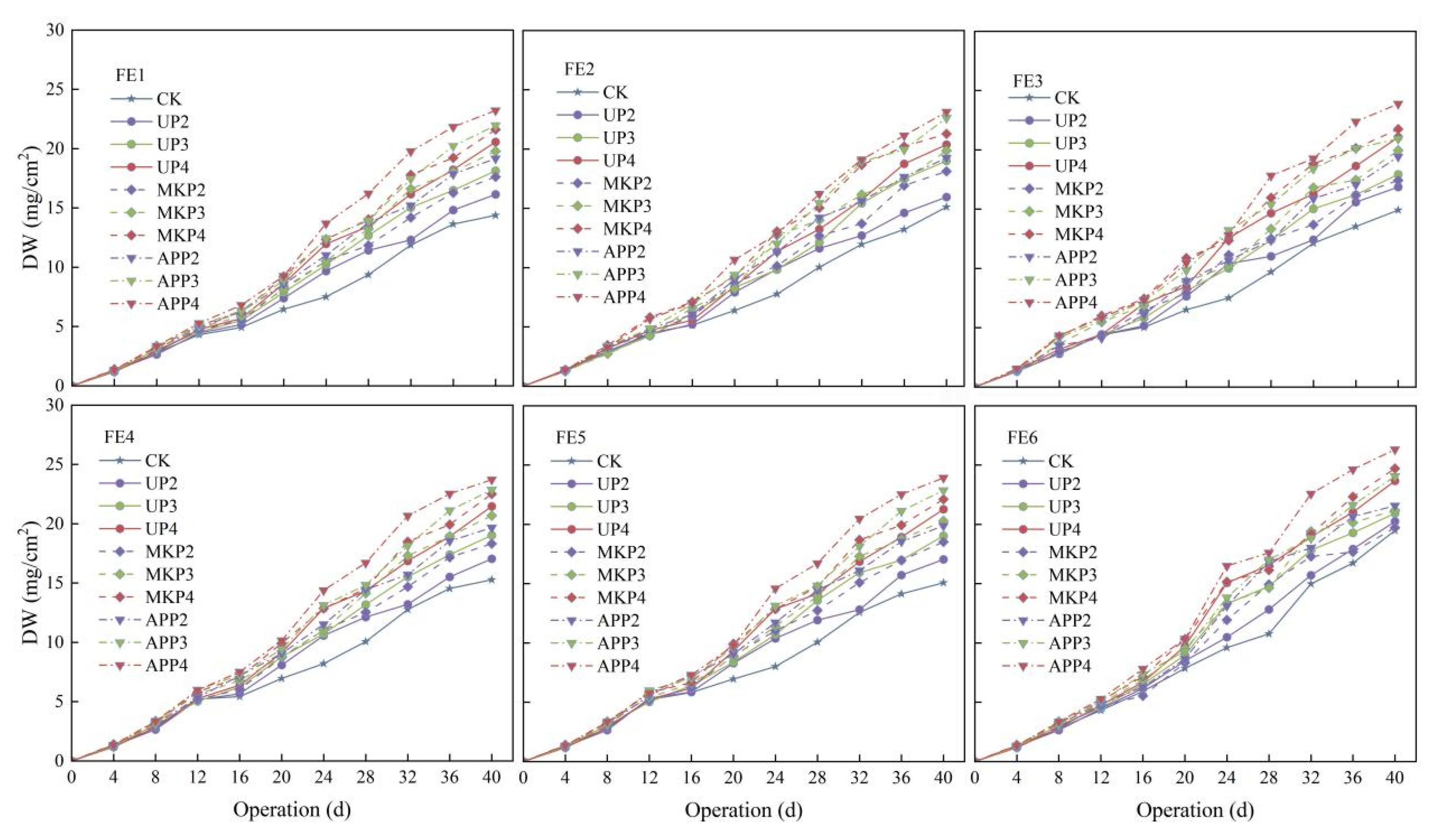
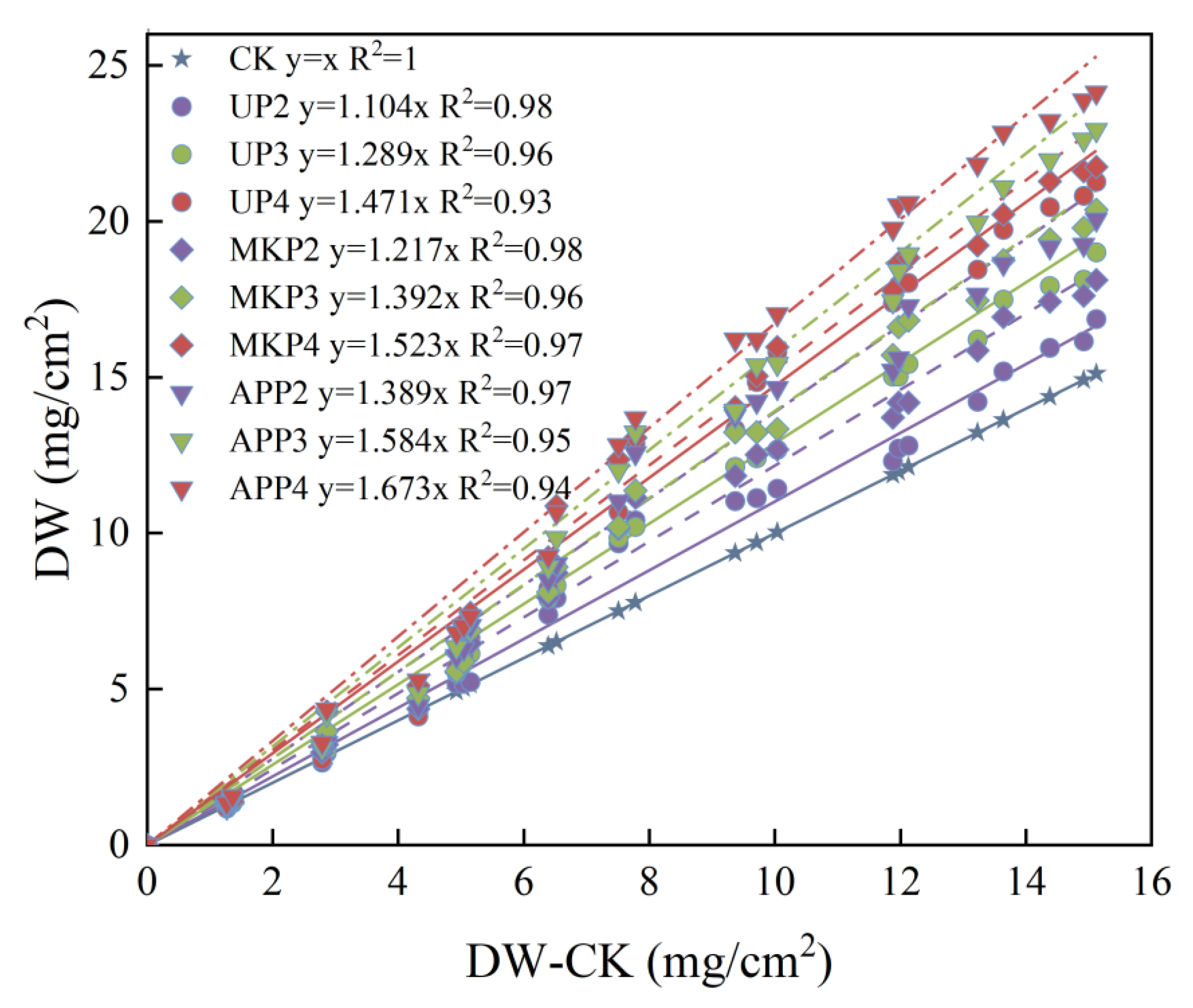
3.2. Effect of Phosphate Fertilizer on the Dry Weight of Clogging Substances
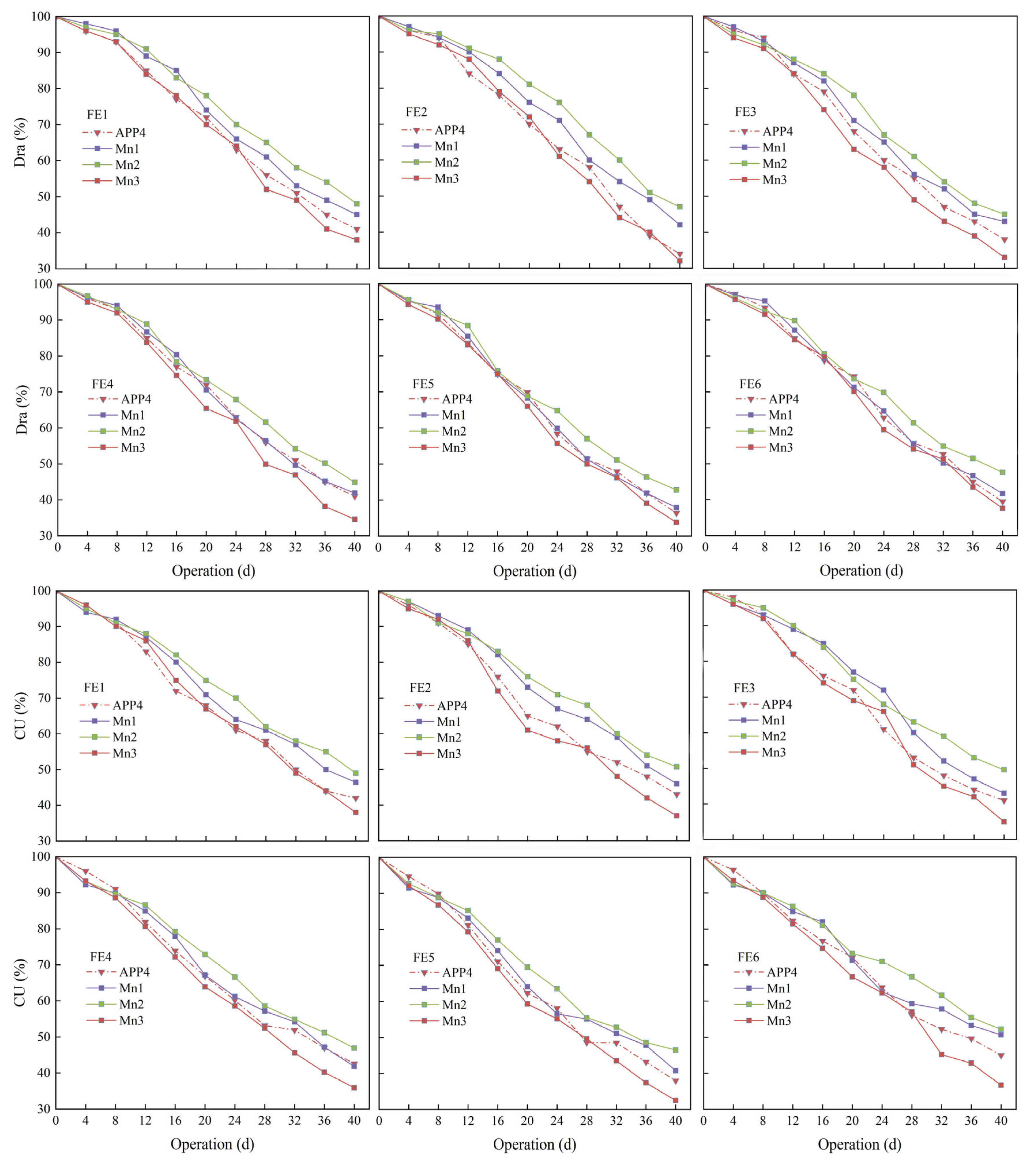
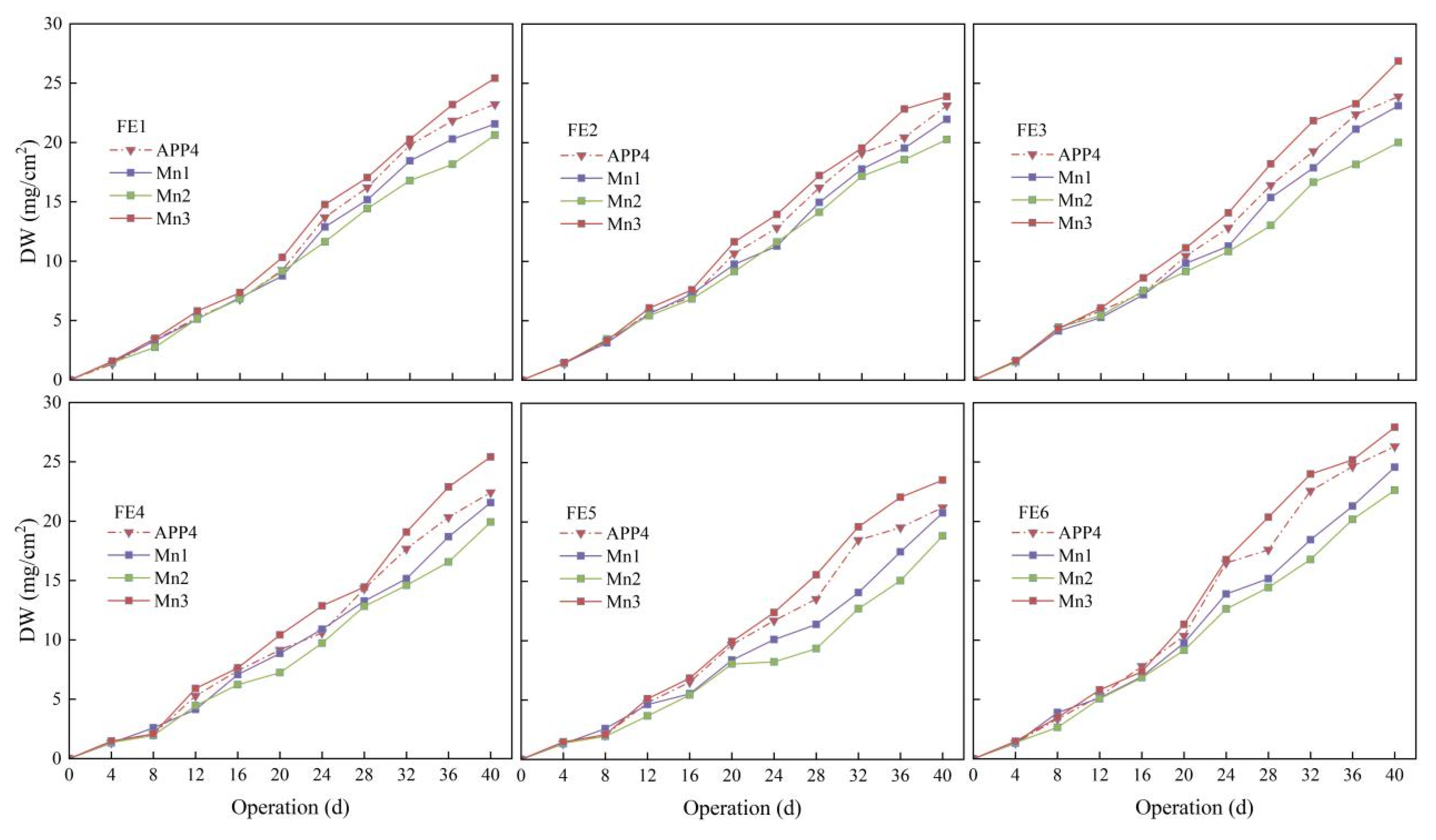
3.3. Effect of Mn2+ on Drip Irrigation with Fertilizer Application
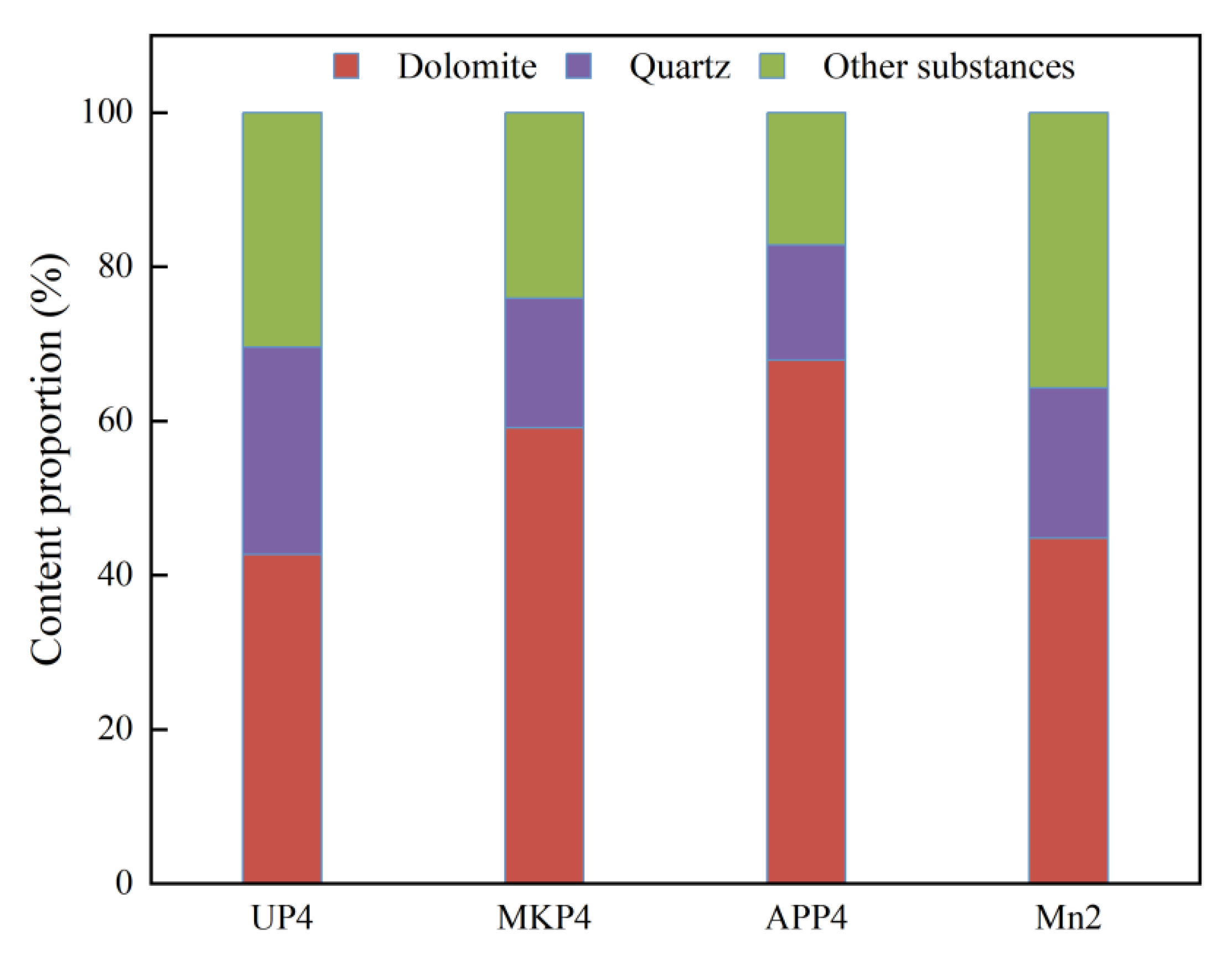
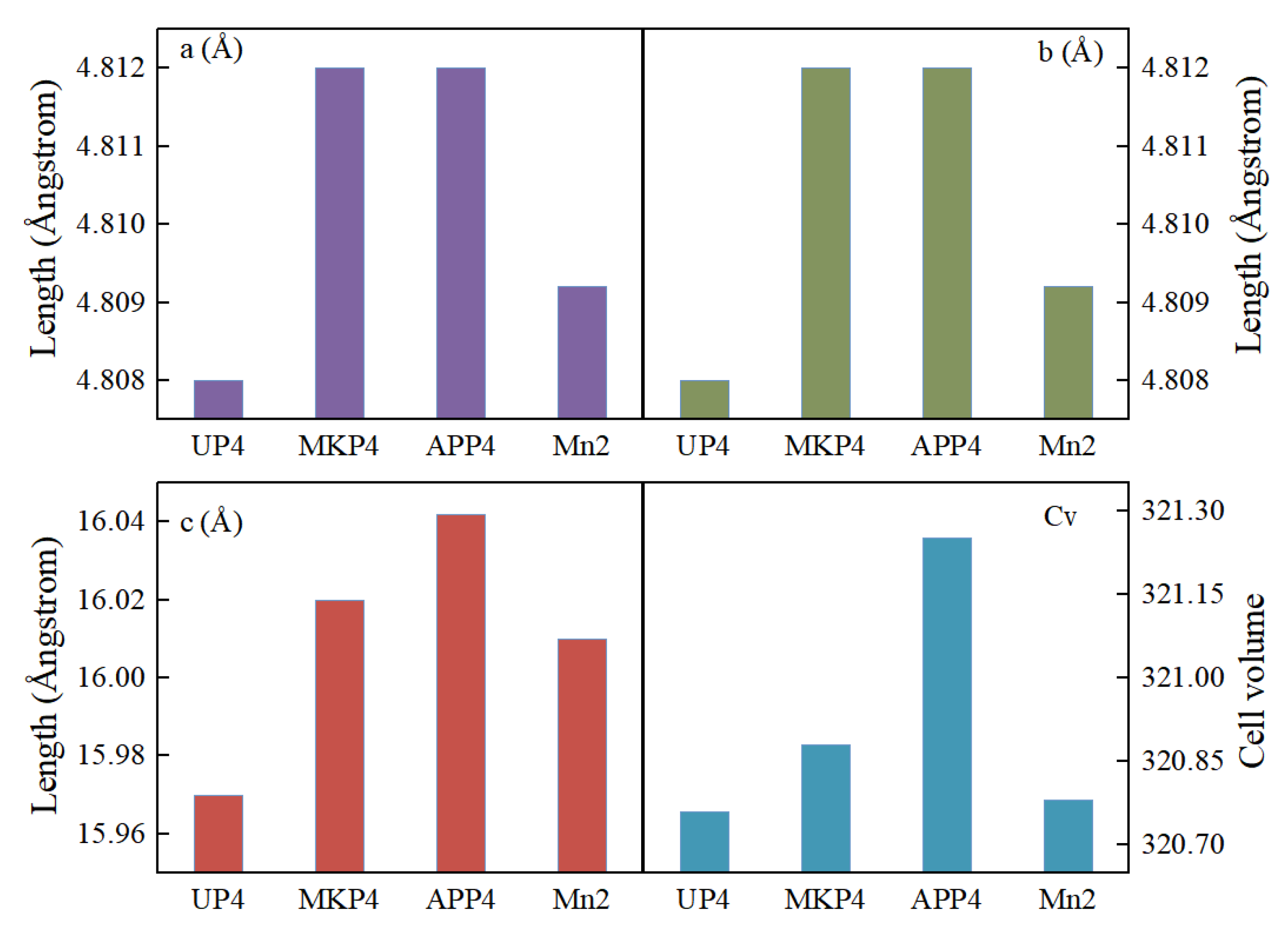
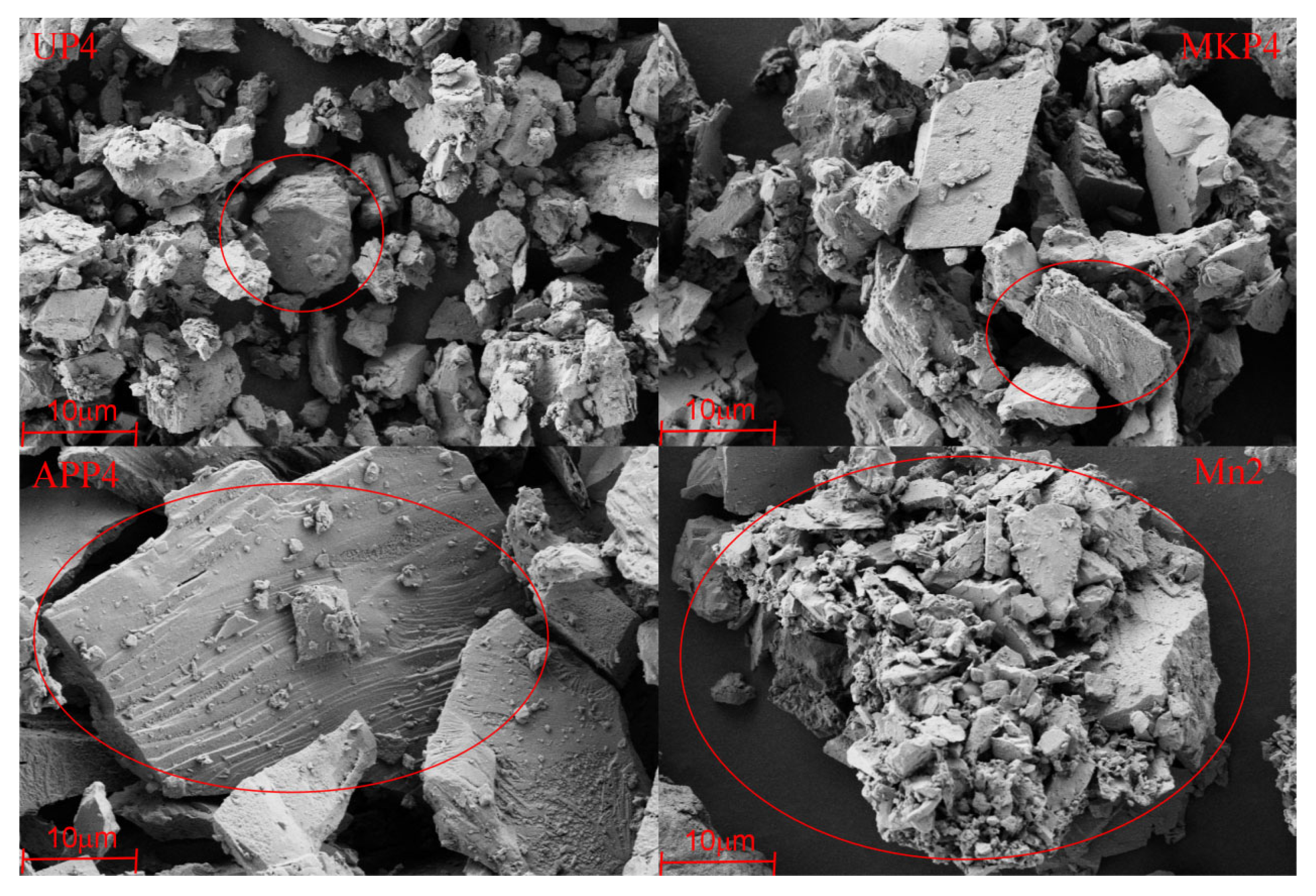
3.4. The Mineral Composition, Lattice Parameters, and Morphology of Blocking Substances
4. Discussion
5. The Preventive Strategies for Drip Irrigation of Phosphate Fertilizer
- (1)
- Types of phosphate fertilizers. We suggest the use of UP for drip irrigation. The blockage under UP treatment after fertilization was relatively light and the performance of drip irrigation emitter would not significantly decrease under low concentration conditions. The crops only absorbed phosphorus in the form of H2PO4 [41]. UP fertilization could lower the pH value of water and soil. The conversion of phosphate to H2PO4 form was promoted and the effectiveness and utilization efficiency of phosphorus were improved [42]. In addition, drip irrigation technology is mainly used in saline alkali areas [43]. UP could be used as a soil amendment to reduce soil salinity and alkalinity. It is not advisable to use UP in acidic soil. Future research should explore the availability of phosphate fertilizers in different soils.
- (2)
- The concentration of Phosphate fertilizer. The results of this study found a positive correlation between the degree of blockage and concentration at concentrations of 0.2~0.4 g/L. The optimal concentration of phosphorus fertilizer required for crops varies [44]. Low concentration phosphorus fertilizer would prolong the total drip irrigation time, which is not conducive to crop growth in the optimal season. The selection of phosphate fertilizer concentration should take into account both clogging issues and crop growth conditions.
- (3)
- Types of drip irrigation emitters. The channel structure was a direct factor that affected the anticlogging performance of drip irrigation emitters and did not change with external factors [45]. This study used a labyrinth drip irrigation emitter. Many scholars had optimized flow channel structure, such as inverted labyrinth flow channel [46] and stellate water-retaining labyrinth channels [47]. Xu et al. created a pit drip irrigation emitter and leaf vein drip irrigation emitter based on plant bionics [48,49]. These scholars demonstrated through CFD and sediment experiments that these drip irrigation emitters could reduce sand sedimentation. It is still unclear whether they could alleviate chemical blockages. The phosphorus fertilizer drip irrigation experiments could be combined with these new drip irrigation emitters.
- (4)
- Carbonate control. The commonly used method for removing carbonates is to add acid regularly. This method is prone to damaging soils, crops, and increased precipitation of silicates [50]. Regarding environmental issues, previous studies have shown that Merus rings [51], nano bubble generators [52], and electrochemical reactors [53] could be used. Finally, we propose the use of Mn2+ to reduce carbonate precipitation. This method is easy to operate and promotes crop growth. Other ions that may be crucial for alleviating carbonate stress but have not been widely studied include Fe2+, Fe3+, and Cu2+.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UP | Urea phosphate |
| PDP | Potassium dihydrogen phosphate |
| APP | Ammonium polyphosphate |
| Dra | The system discharge variation ratio |
| CU | The Christiansen coefficient of uniformity |
| DW | The dry weight of clogging substances |
| XRD | X-ray diffractometer |
| SEM | Scanning electron microscopy |
| APP4 | Irrigation water with ammonium polyphosphate concentration of 0.4 g/L |
| Mn1 | Irrigation water mixed with 0.4 g/L ammonium polyphosphate and 1 mg/L Mn2+ |
| Mn2 | Irrigation water mixed with 0.4 g/L ammonium polyphosphate and 2 mg/L Mn2+ |
| Mn3 | Irrigation water mixed with 0.4 g/L ammonium polyphosphate and 3 mg/L Mn2+ |
| Cv | Crystal lattice volume |
References
- Yadav, R.; Meena, S.; Patel, S.; Patel, K.; Akhtar, M.S.; Yadav, B.; Panwar, J. Bioavailability of soil P for plant nutrition. In Farming for Food and Water Security; Springer: Berlin/Heidelberg, Germany, 2012; pp. 177–200. [Google Scholar] [CrossRef]
- Kokulan, V.; Schneider, K.; Macrae, M.L.; Wilson, H. Struvite application to field corn decreases the risk of environmental phosphorus loss while maintaining crop yield. Agriculture. Ecosyst. Environ. 2024, 366, 108936. [Google Scholar] [CrossRef]
- Schneider, K.D.; Thiessen Martens, J.R.; Zvomuya, F.; Reid, D.K.; Fraser, T.D.; Lynch, D.H.; Wilson, H.F. Options for improved phosphorus cycling and use in agriculture at the field and regional scales. J. Environ. Qual. 2019, 48, 1247–1264. [Google Scholar] [CrossRef] [PubMed]
- Grzebisz, W.; Niewiadomska, A.; Potarzycki, J.; Andrzejewska, A. Phosphorus HotSpots in Crop Plants Production on the Farm—Mitigating Critical Factors. Agronomy 2024, 14, 200. [Google Scholar] [CrossRef]
- Peng, X.; Chen, D.; Zhou, Z.; Zhen, J.; Xu, C.; Hu, X.; Wang, Y. Optimizing drip fertigation management to simultaneously improve the yield, water productivity and fertilizer agronomic utilization efficiency of grapes in different precipitation year patterns. Agric. Water Manag. 2024, 295, 108749. [Google Scholar] [CrossRef]
- Çetin, Ö.; Akalp, E. Efficient use of water and fertilizers in irrigated agriculture: Drip irrigation and fertigation. Acta Hortic. Regiotect. 2019, 22, 97–102. [Google Scholar] [CrossRef]
- Xu, T.; Bao, S.; Li, Z.; Yu, Q.; Zheng, E. The Study of Structural Optimization on Hydraulic Performance and Anti-Clogging Performance of Labyrinth Drip Irrigation Emitters. Agronomy 2023, 13, 2496. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, W.; Jing, B. Optimizing brackish water and nitrogen application regimes for soil salinity, yield, fertilizer and water productivity of a mulched drip irrigated cotton cropping system. Field Crops Res. 2023, 302, 109097. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Chen, X.; Xue, S.; Feng, J.; Muhammad, T.; Zhou, B. Dynamic effects of chemical precipitates on drip irrigation system clogging using water with high sediment and salt loads. Agric. Water Manag. 2019, 213, 833–842. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Zhang, J.; Xu, F.; Li, Y. Effects of fertilizer types on biofilm growth in the drip irrigation system using the reclaimed water. Irrig. Sci. 2021, 39, 725–734. [Google Scholar] [CrossRef]
- Wang, Y.; Puig-Bargués, J.; Ma, C.; Xiao, Y.; Maitusong, M.; Li, Y. Influence and selection of nitrogen and phosphorus compound fertilizers on emitter clogging using brackish water in drip irrigation systems. Agric. Water Manag. 2024, 291, 108644. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Y.; Xiao, Y.; Liu, Z. Different operation patterns on mineral components of emitters clogging substances in drip phosphorus fertigation system. Irrig. Sci. 2019, 37, 691–707. [Google Scholar] [CrossRef]
- Muhammad, T.; Zhou, B.; Liu, Z.; Chen, X.; Li, Y. Effects of phosphorus-fertigation on emitter clogging in drip irrigation system with saline water. Agric. Water Manag. 2021, 243, 106392. [Google Scholar] [CrossRef]
- Barrow, N.; Debnath, A.; Sen, A. Measurement of the effects of pH on phosphate availability. Plant Soil 2020, 454, 217–224. [Google Scholar] [CrossRef]
- Gryta, M. Polyphosphates used for membrane scaling inhibition during water desalination by membrane distillation. Desalination 2012, 285, 170–176. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, C.; Li, M.; Zhangzhong, L.; Song, P.; Li, Y. Interaction and adaptation of phosphorus fertilizer and calcium ion in drip irrigation systems: The perspective of emitter clogging. Agric. Water Manag. 2023, 282, 108269. [Google Scholar] [CrossRef]
- Mills, J.V.; Barnhart, H.A.; DePaolo, D.J.; Lammers, L.N. New insights into Mn2+ and Mg2+ inhibition of calcite growth. Geochim. Cosmochim. Acta 2022, 334, 338–367. [Google Scholar] [CrossRef]
- Rashed, M.H.; Hoque, T.S.; Jahangir, M.M.R.; Hashem, M.A. Manganese as a micronutrient in agriculture: Crop requirement and management. J. Environ. Sci. Nat. Resour. 2019, 12, 225–242. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Li, N.; Chang, L.; Cui, N. Influence of flushing velocity and flushing frequency on the service life of labyrinth-channel emitters. Water 2018, 10, 1630. [Google Scholar] [CrossRef]
- Xiao, Y.; Puig-Bargués, J.; Zhou, B.; Li, Q.; Li, Y. Increasing phosphorus availability by reducing clogging in drip fertigation systems. J. Clean. Prod. 2020, 262, 121319. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, H.; Puig-Bargués, J.; Li, Y. Using an anti-clogging relative index (CRI) to assess emitters rapidly for drip irrigation systems with multiple low-quality water sources. Agric. Water Manag. 2019, 221, 270–278. [Google Scholar] [CrossRef]
- Li, Q.; Song, P.; Zhou, B.; Xiao, Y.; Muhammad, T.; Liu, Z.; Li, Y. Mechanism of intermittent fluctuated water pressure on emitter clogging substances formation in drip irrigation system utilizing high sediment water. Agric. Water Manag. 2019, 215, 16–24. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, L.; Wu, P.; Liu, Y.; Cai, Y.; Zhou, W. Clogging formation and an anti-clogging method in subsurface irrigation system with porous ceramic emitter. Agric. Water Manag. 2021, 250, 106770. [Google Scholar] [CrossRef]
- Lombi, E.; McLaughlin, M.J.; Johnston, C.; Armstrong, R.D.; Holloway, R.E. Mobility, solubility and lability of fluid and granular forms of P fertiliser in calcareous and non-calcareous soils under laboratory conditions. Plant Soil 2005, 269, 25–34. [Google Scholar] [CrossRef]
- Lun, F.; Liu, J.; Ciais, P.; Nesme, T.; Chang, J.; Wang, R.; Obersteiner, M. Global and regional phosphorus budgets in agricultural systems and their implications for phosphorus-use efficiency. Earth Syst. Sci. Data 2018, 10, 1–18. [Google Scholar] [CrossRef]
- Ma, C.; Xiao, Y.; Puig-Bargués, J.; Shukla, M.K.; Tang, X.; Hou, P.; Li, Y. Using phosphate fertilizer to reduce emitter clogging of drip fertigation systems with high salinity water. J. Environ. Manag. 2020, 263, 110366. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO3). Appl. Microbiol. Biotechnol. 2017, 101, 3131–3142. [Google Scholar] [CrossRef]
- Kosev, K.; Petrova, N.; Georgieva, I.; Titorenkova, R.; Nikolova, R. Crystalline adducts of urea with magnesium iodide. J. Mol. Struct. 2021, 1224, 129009. [Google Scholar] [CrossRef]
- Maher, K.; Johnson, N.C.; Jackson, A.; Lammers, L.N.; Torchinsky, A.B.; Weaver, K.L.; Brown, G.E., Jr. A spatially resolved surface kinetic model for forsterite dissolution. Geochim. Cosmochim. Acta 2016, 174, 313–334. [Google Scholar] [CrossRef]
- Sazali, R.A.; Sorbie, K.S.; Boak, L.S. The Effect of pH on Silicate Scaling. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Budapest, Hungary, 3–5 June 2015. [Google Scholar] [CrossRef]
- Crundwell, F.K. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part II Application of a new theory to silicates, aluminosilicates and quartz. Hydrometallurgy 2014, 149, 265–275. [Google Scholar] [CrossRef]
- Zheng, Z.; Gao, Q.; Jiang, J. High hydrogen uptake capacity of mesoporous nitrogen-doped carbons activated using potassium hydroxide. Carbon 2010, 48, 2968–2973. [Google Scholar] [CrossRef]
- Shi, K.; Lu, T.; Zheng, W.; Zhang, X.; Zhangzhong, L. A review of the category, mechanism, and controlling methods of chemical clogging in drip irrigation system. Agriculture 2022, 12, 202. [Google Scholar] [CrossRef]
- Kulakovskaya, T.V.; Vagabov, V.M.; Kulaev, I.S. Inorganic polyphosphate in industry, agriculture and medicine: Modern state and outlook. Process Biochem. 2012, 47, 1–10. [Google Scholar] [CrossRef]
- Shen, Y.; Puig-Bargués, J.; Li, M.; Xiao, Y.; Li, Q.; Li, Y. Physical, chemical and biological emitter clogging behaviors in drip irrigation systems using high-sediment loaded water. Agric. Water Manag. 2022, 270, 107738. [Google Scholar] [CrossRef]
- Habermann, D.; Neuser, R.D.; Richter, D.K. Quantitative High Resolution Spectral Analysis of Mn2+ in Sedimentary Calcite. In Cathodoluminescence in Geosciences; Springer: Berlin/Heidelberg, Germany, 2000; pp. 331–358. [Google Scholar]
- Ali, S.; Iqbal, Y.; Shah, K.H.; Fahad, M. Synthesis and kinetic modeling of manganese carbonate precipitated from manganese sulfate solution. Chem. Eng. Commun. 2022, 209, 96–107. [Google Scholar] [CrossRef]
- Han, Z.; Li, J.; Zhao, Y.; Chen, Q.; Gao, X.; Hu, K.; Han, C. Dissolved Mn2+ promotes microbially-catalyzed protodolomite precipitation in brackish oxidized water. Chem. Geol. 2024, 650, 121986. [Google Scholar] [CrossRef]
- Dromgoole, E.L.; Walter, L.M. Inhibition of calcite growth rates by Mn2+ in CaCl2 solutions at 10, 25, and 50 °C. Geochim. Cosmochim. Acta 1990, 54, 2991–3000. [Google Scholar] [CrossRef]
- Hu, R.; Fan, Z.; Liu, Z.; Huang, T.; Wen, G. Experimental study on the effects of different factors on the crystallization rate and products of Mn–Ca co-crystallization. Environ. Sci. Pollut. Res. 2023, 30, 13521–13531. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Bargaz, A.; Elhaissoufi, W.; Khourchi, S.; Benmrid, B.; Borden, K.A.; Rchiad, Z. Benefits of phosphate solubilizing bacteria on belowground crop performance for improved crop acquisition of phosphorus. Microbiol. Res. 2021, 252, 126842. [Google Scholar] [CrossRef]
- Yahaya, S.M.; Mahmud, A.A.; Abdullahi, M.; Haruna, A. Recent advances in the chemistry of nitrogen, phosphorus and potassium as fertilizers in soil: A review. Pedosphere 2023, 33, 385–406. [Google Scholar] [CrossRef]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of long-term chemical fertilizers and organic manures on soil fertility—A review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar]
- Feng, J.; Li, Y.; Wang, W.; Xue, S. Effect of optimization forms of flow path on emitter hydraulic and anti-clogging performance in drip irrigation system. Irrig. Sci. 2018, 36, 37–47. [Google Scholar] [CrossRef]
- Ma, R.; Wei, Z.; Chen, X.; Ma, S. Barbed labyrinth channel optimization based on constrained multi-objective particle swarm algorithm. J. Drain. Irrig. Mach. Eng. 2018, 36, 1330–1336. [Google Scholar]
- Li, Y.; Feng, X.; Han, X.; Sun, Y.; Li, H. Machine Learning Approach to Predict Flow Regime Index of a Stellate Water-Retaining Labyrinth Channel Emitter. Agronomy 2023, 13, 1063. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L. Influence and Analysis of Structure Design and Optimization On the Performance of a Pit Drip Irrigation Emitter. Irrig. Drain. 2020, 69, 633–645. [Google Scholar] [CrossRef]
- Li, Z.; Bao, S.; Cheng, Q.; Yu, Q.; Xu, T. The Structural Optimization of Leaf Vein Drip Irrigation Emitter on Hydraulic Performance, Energy Entropy and Anti-Clogging Ability. Agronomy 2024, 14, 1102. [Google Scholar] [CrossRef]
- De Kreij, C.; Van der Burg, A.M.M.; Runia, W.T. Drip irrigation emitter clogging in Dutch greenhouses as affected by methane and organic acids. Agric. Water Manag. 2003, 60, 73–85. [Google Scholar] [CrossRef]
- Barati, K.; Mostafazadeh-Fard, B.; Sheikhbahaei, A.A. Molecular oscillation technology: New phenomenon to reduce emitter clogging in trickle irrigation. J. Irrig. Drain. Eng. 2014, 140, 04014034. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, S.C.; Wang, X.; Muhammad, T.; Song, P.; Zhou, B.; Li, Y. Mitigation of biofouling in agricultural water distribution systems with nanobubbles. Environ. Int. 2020, 141, 105787. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, N.; Li, Y.; Song, P.; Liu, Z. Pilot electrochemical prevention of reclaimed water irrigation clogging: Function interactions and microbial metabolism. J. Clean. Prod. 2022, 336, 130436. [Google Scholar] [CrossRef]

| pH | Total Suspended Solids (mg/L) | Electrical Conductivity (ms/cm) | Ca2+ (mg/L) | Mg2+ (mg/L) | Work Pressure (MPa) | Water Temperature (°C) |
|---|---|---|---|---|---|---|
| 7.6 ± 0.5 | 43.6 ± 7.2 | 754 ± 14 | 41.7 ± 6.3 | 34.8 ± 4.7 | 0.1 | 16 ± 3 |
| CK | UP2 | UP3 | UP4 | PDP2 | PDP3 | PDP4 | APP2 | APP3 | APP4 |
|---|---|---|---|---|---|---|---|---|---|
| 7.6 ± 0.3 | 6.3 ± 0.3 | 5.1 ± 0.3 | 3.7 ± 0.3 | 7.2 ± 0.3 | 6.9 ± 0.3 | 6.7 ± 0.3 | 7.6 ± 0.3 | 7.6 ± 0.3 | 7.6 ± 0.3 |
| Experiment Number | Fertilizer | Chemical Composition | Fertilizer Concentration/(g/L) |
|---|---|---|---|
| Ck | - | - | - |
| UP2 | Urea phosphate | CO(NH2)2·H3PO4 | 0.2 |
| UP3 | Urea phosphate | CO(NH2)2·H3PO4 | 0.3 |
| UP4 | Urea phosphate | CO(NH2)2·H3PO4 | 0.4 |
| PDP2 | Potassium dihydrogen phosphate | KH2PO4 | 0.2 |
| PDP3 | Potassium dihydrogen phosphate | KH2PO4 | 0.3 |
| PDP4 | Potassium dihydrogen phosphate | KH2PO4 | 0.4 |
| APP2 | Ammonium polyphosphate | (NH4)n+2PnO3n+1 | 0.2 |
| APP3 | Ammonium polyphosphate | (NH4)n+2PnO3n+1 | 0.3 |
| APP4 | Ammonium polyphosphate | (NH4)n+2PnO3n+1 | 0.4 |
| Label | Initial Flow (L/h) | Flow Path Length (mm) | Flow Path Width (mm) | Flow Path Depth (mm) | Flow Index | Structural Style |
|---|---|---|---|---|---|---|
| FE1 | 2.55 | 26 | 1 | 0.7 | 0.52 |  |
| FE2 | 2.47 | 22 | 0.6 | 0.7 | 0.56 |  |
| FE3 | 2.70 | 13 | 0.8 | 0.6 | 0.55 |  |
| FE4 | 2.63 | 23 | 0.8 | 0.7 | 0.54 |  |
| FE5 | 2.59 | 24 | 0.7 | 0.8 | 0.55 |  |
| FE6 | 2.71 | 26 | 0.8 | 0.8 | 0.56 |  |
| Clogging Parameters | Statistical Parameters | UP2 | UP3 | UP4 | PDP2 | PDP3 | PDP4 | APP2 | APP3 | APP4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Dra | t-value | 0.66 | 1.97 * | 2.37 * | 1.77 * | 2.01 * | 2.47 * | 2.34 * | 2.49 * | 2.81 * |
| standard deviation | 12.68 | 15.78 | 18.11 | 14.76 | 17.15 | 19.21 | 15.84 | 17.49 | 20.24 | |
| Mean value difference | 2.93 | 10.14 | 13.20 | 8.60 | 10.80 | 14.33 | 11.92 | 13.53 | 16.97 | |
| CU | t-value | 0.59 | 1.17 | 1.98 * | 0.76 | 1.32 * | 1.99 * | 1.42 * | 2.28 * | 2.52 * |
| standard deviation | 11.82 | 12.92 | 15.83 | 12.53 | 13.95 | 16.05 | 14.59 | 17.42 | 19.16 | |
| Mean value difference | 2.45 | 5.13 | 9.87 | 3.26 | 6.07 | 9.72 | 6.73 | 12.13 | 14.48 |
| Clogging Parameters | Statistical Parameters | UP2 | UP3 | UP4 | PDP2 | PDP3 | PDP4 | APP2 | APP3 | APP4 |
|---|---|---|---|---|---|---|---|---|---|---|
| DW | t-value | −0.75 | −1.21 * | −1.71 * | −1.16 * | −1.52 * | −1.94 * | −1.60 * | −1.92 * | −2.18 * |
| standard deviation | 5.45 | 6.33 | 7.41 | 5.98 | 6.75 | 7.43 | 6.84 | 7.71 | 8.35 | |
| Mean value difference | −1.37 | −2.41 | −3.81 | −2.23 | −3.18 | −4.34 | −3.37 | −4.42 | −5.34 |
| Experiment Number | Sediment Concentration (g/L) | The Group with the Most Severe Blockage | Mn2+ Concentration /(mg/L) |
|---|---|---|---|
| APP4 | 2 | APP4 | 0 |
| Mn1 | 2 | APP4 | 1 |
| Mn2 | 2 | APP4 | 2 |
| Mn3 | 2 | APP4 | 3 |
| Phosphate Fertilizer Type | Phosphate Fertilizer Type | Dra Mean Value Difference | CU Mean Value Difference | DW Mean Value Difference |
|---|---|---|---|---|
| APP4 | Mn1 | −5.68 * | −3.15 * | 1.19 |
| Mn2 | −9.13 * | −7.56 * | 3.11 * | |
| Mn3 | 3.27 | 5.23 * | −1.98 * | |
| Mn1 | APP4 | 5.68 * | 3.15 * | −1.19 |
| Mn2 | −3.27 | −4.62 * | 1.92 * | |
| Mn3 | 9.13 * | 8.48 * | −3.18 * | |
| Mn2 | APP4 | 9.13 * | 7.56 * | −3.11 * |
| Mn1 | 3.27 | 4.62 * | −1.91 * | |
| Mn3 | 12.28 * | 13.11 * | −5.09 * | |
| Mn3 | APP4 | −3.27 | −5.23 * | 1.98 * |
| Mn1 | −9.13 * | −8.48 * | 3.18 * | |
| Mn2 | −12.28 * | −13.11 * | 5.09 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Bao, S.; Yu, Q.; Gao, Y. Solving Phosphorus Fertilization-Related Drip Irrigation Emitter Clogging by Adding Mn2+. Agronomy 2025, 15, 127. https://doi.org/10.3390/agronomy15010127
Xu T, Bao S, Yu Q, Gao Y. Solving Phosphorus Fertilization-Related Drip Irrigation Emitter Clogging by Adding Mn2+. Agronomy. 2025; 15(1):127. https://doi.org/10.3390/agronomy15010127
Chicago/Turabian StyleXu, Tianyu, Sanlin Bao, Qiuyue Yu, and Yu Gao. 2025. "Solving Phosphorus Fertilization-Related Drip Irrigation Emitter Clogging by Adding Mn2+" Agronomy 15, no. 1: 127. https://doi.org/10.3390/agronomy15010127
APA StyleXu, T., Bao, S., Yu, Q., & Gao, Y. (2025). Solving Phosphorus Fertilization-Related Drip Irrigation Emitter Clogging by Adding Mn2+. Agronomy, 15(1), 127. https://doi.org/10.3390/agronomy15010127






