Abstract
The main goal of this study was to investigate and better understand the evolution of the main non-destructive and destructive quality parameters of peach ‘Redhaven’ during ripening process. This study was conducted from 8 to 21 July 2023, during which peaches ‘Redhaven’ were harvested each second day from a commercial orchard located in Novaki Bistranjski. Maturity categories were defined according to different firmness thresholds: maturity for long-distance chain stores (H1), maturity for medium-distance chain stores (H2), maturity below the defined maximum firmness in order to preserve optimal quality traits (H3), ready to buy (H4), ready to eat (H5), and overripe (H6). The chlorophyll absorbance index was the non-destructive parameter that was mostly distinguished between maturity categories (r = 0.78 with firmness), followed by a* and h° ground colour parameters. During the first three maturity categories (H1–H3), firmness had a notably smaller correlation with titratable acidity and the ratio of total soluble solids and titratable acidity, which is not the case for a* and h° ground colour parameters, chlorophyll absorbance index, and the share of additional colour. During the last three maturity categories (H4–H6), non-destructive parameters are not reliable for maturity prediction. When ground colour parameters are measured near petiole insertion, mostly smaller segregation between maturity categories is obtained compared to when measured at the rest of the fruit. Total polyphenol and flavonoid content in peach juice notably corelated only in the last two maturity categories with L* ground colour parameter.
1. Introduction
Peaches (Prunus persica L.) are one of the most economically important, healthy, and delicious summer fruit which can be consumed fresh or as canned fruit, juice, and jelly [1,2]. In 2022, peaches and nectarines were the fifth most produced fruit crop in Europe, with a production of 3,448,491.39 t [3]. In Croatia, peaches were harvested on 610 ha in 2022, with a production of 3810 t [3]. During the peach harvest season, 39.3% of EU citizens usually consume from three to five peaches per week, while 30.5% of them consume from one to two peaches per week [4]. Determining the proper harvest window for peaches is of crucial importance from both the producer’s and consumer’s point of view, as fruit quality changes rapidly during ripening, and either side can easily be deprived. Peaches attain their quality attributes during ripening [1], and there is a close link between on-tree physiological maturity and the development of key traits responsible for their quality [5]. However, due to the fact that melting peaches ripen rapidly and soften quickly after harvest, they have a short postharvest life (usually limited to 3–4 weeks), and an inappropriate harvest window can easily lead to losses in the market chain [5,6]. Hence, in the fresh peach industry, the most limiting factors of fruit quality along the commercial chain are those dealing with the flesh mechanical properties and particularly rapid softening [7]. As a result, fruit are commonly picked at an early stage of ripening to better withstand handling and avoid bruising damages to which stone fruit are susceptible [5,8,9]. However, consumers expect a certain eating quality (flavour, aroma, texture, etc.) that peaches do not reach if they are harvested too early [5,9,10,11,12], even though they are climacteric fruit. A tree-ripe fruit guarantees consumer acceptance but is associated with high susceptibility to bruises and rapid deterioration [13]. In practice, compromises among fruit maturity, yield, and possibly higher prices for early picked fruit are often adopted [6].
Firmness, soluble solids concentration (SSC), and ground colour changes are usually the most important methods used by producers for the determination of the harvest date since the simplest parameters to appraise are also the most used [13,14]. Other than that, titratable acidity also notably changes during the ripening [6,15,16]. However, these are destructive measurements and obtaining them is a slow process, which can only be conducted on a limited number of fruits. Therefore, easily conducted, non-destructive parameters are desirable. Ground colour is traditionally used for peach maturity prediction since it changes with other important quality parameters (e.g., SSC, firmness, volatile compounds) [6,17]. However, the change in background colour is not readily perceptible to the naked eye, because the fruit surface is extensively, but irregularly, covered by blush in many cultivars [18]. Red colour is one of the historically represented components important to consumers due to its attractiveness [19], but the development of the blush colour in peaches is related to the light exposure rather than to the fruit maturation [20]. Recently, a new non-destructive index based on the difference in absorbance between two wavelengths near the chlorophyll-a absorption peak (670 and 720 nm; index of absorbance difference, IAD) was developed and has shown promising results [5]. In addition, machine learning techniques have recently been used to help automatically determine the maturity status of peaches based on sensor data in warehouses, allowing for more precise decision-making regarding the next course of action [8,14]. Although there are a considerable number of studies on peach ripening, the available literature is limited in terms of sample size, the range of maturity classes, and the variety of variables considered. Hence, reliable and detailed analysis between all maturity categories and various non-destructive and destructive traits is missing. Also, to the best of our knowledge, there is a scarcity of studies that have investigated the importance of specific variables within defined maturity classes.
‘Redhaven’ is an American peach variety that originated in 1940 from Michigan [21]. It has been cultivated since 1940 and is one of the leading peach varieties in the world, and is taken as a standard for ripening time [22]. When initially released, it was the first good freestone variety on the market, which quickly came to prominence for higher-chill regions because of its productivity, firmness, and appearance [21]. Its fruit is elongated, round, and large (160–180 g) with orange-yellow ground colour and additional intensive red colour that covers from 60–70% of the fruit surface [22]. The meat is yellow coloured [12]. It has high and regular yields, certain resistance level to frosts, and good fruit quality [22].
The main goal of this study was to investigate and better understand the evolution of the main non-destructive and destructive quality parameters of the peach ‘Redhaven’ during the ripening process. Based on the aforementioned results, the secondary goal was to further investigate the reliability of non-destructive parameters for peach maturity prediction changes between different maturity classes. This data knowledge will have potential use in packinghouses to improve automatic peach maturity classification by non-destructive sensor data. Finally, it will enable proper further peach manipulation (immediate sale to consumer, short, medium, or long storage), reduce spoilage, and assure high fruit quality and consumer satisfaction.
2. Materials and Methods
2.1. Plant Material
The study was conducted from 08 to 21 July 2023, when peaches ‘Redhaven’ were harvested every second day from a commercial orchard located in Novaki Bistranjski and afterwards analysed in the laboratory of the University of Zagreb, Faculty of Agriculture, Unit of Horticulture and Landscape Architecture, Department of Pomology. It was done to classify peaches according to their maturity status; however, due to the successive ripening of this variety, maturity classification was determined based on firmness value thresholds, which are described in detail in subchapter 2.4.1. In total, 701 peach fruits were harvested. Peaches, 18 years old and grafted on vineyard peach rootstock, were grown as a spindle bush with a spacing of 4 × 1.8 m. Standard agro- and pomotechnical measurements are conducted each year.
2.2. Non-Destructive Measurements
2.2.1. Ground and Additional Colour
The ground and additional fruit skin colour parameters were measured separately on each fruit on the most pronounced coloration place using a colorimeter (ColorTec PCM; ColorTec Associates Inc., Clinton, NJ, USA) and according to the CIE L*a*b* and CIE L*C*h° systems (Commission Internationale d’eclairage). In a three-dimensional uniform space, the L* value is defined as a vertical coordinate that defines lightness, and a* and b* values are defined as horizontal coordinates, which, if negative, indicate an intensity of green and blue colour, respectively, or if positive, an intensity of red and yellow colour, respectively [23]. According to the most widely accepted international criterion (CIELAB), when hue angle (h°) is 0°, it assigns to the semi axis +a* (redness); when 90°, it assigns to the semi axis +b* (yellowness); when 180°, it assigns to the semi axis −a* (greenness); and when 270°, it assigns to the semi axis −b* (blueness) [24]. In addition, ground colour was measured near petiole insertion (L_gc_p, a_gc_p, b_gc_p, C_gc_p, h_gc_p) and standardly on the rest of the fruit surface (L_gc, a_gc, b_gc, C_gc, h_gc). At late maturity stages, due to the prevalence of additional colouration, ground colour near petiole insertion could not be measured on all peaches (Table 1). The share of additional fruit coloration (SAC) was measured visually according to the prevalence of additional coloration on fruit skin. Scoring was done as follows: 1—0–20% of additional colouration, 2—20–40% of additional colouration, 3—40–60% of additional colouration, 4—60–80% of additional colouration, and 5—80–90% of additional colouration.

Table 1.
Overview of the number of peach fruits analysed per variable.
2.2.2. Productivity Parameters
Fruit weight was measured using a digital analytical balance (OHAUS Adventurer AX2202, Ohaus Corporation, Parsippany, NJ, USA) with an accuracy of 0.01 g. Fruit length and width (mm) were measured at the most expanded point on two sides of the fruit using a digital caliper, Prowin HMTY0006. Fruit shape ratio (FSR), volume, and density were calculated according to following formulas:
2.2.3. IAD Index
IAD index, “absorbance difference index” between 670 nm and 720 nm, which is close to the absorption peak of chlorophyll and is related to the evolution of ethylene during ripening, was measured by DA meter 53500 (TR Turoni srl, Forlì, Italy) on peach skin on two places, where highest and lowest values were obtained.
2.3. Destructive Measurements
2.3.1. Firmness
The firmness was measured using PCE PTR-200 (PCE Instruments, Jupiter/Palm Beach, USA) fitted with an 8 mm diameter plunger and expressed in kg · cm−2. Measurements were taken at four opposite equatorial positions on each fruit at 90° after the fruit skin was removed. Consequently, the average firmness value of each fruit was calculated from the aforementioned measurements.
2.3.2. SSC and TA
The SSC was measured with a hand digital refractometer (Atago, PAL-1, Tokyo, Japan) from extracted peach juice and expressed as °Brix. TA was determined by the titration method with 0.1 mol · dm−3 NaOH and expressed as % of malic acid, as reported in [25]. The SSC and TA ratio (SSC_TA) was calculated from the corresponding values of SSC and TA for each fruit.
2.3.3. Total Polyphenolic and Flavonoid Content
Total polyphenolic content (TPC) was determined by using a modified Folin–Ciocalteu’s method [26]. A volume of 100 µL of peach juice was mixed with 7.9 mL of distilled water and 500 µL of Folin–Ciocalteu’s reagent (diluted with distilled water in a 1:2 ratio). A volume of 1.5 mL of 20% (w/v) Na2CO3 was added to the suspension. Suspension was immediately vortexed and left for 2 h to react. Gallic acid was used as a standard to produce a calibration diagram. Absorbance was measured at 765 nm, and the data were expressed as mg of gallic acid equivalents per L of peach juice.
Total flavonoid content (TFC) was determined by using the modified spectrophotometric method of Ivanova et al. [27]. A volume of 1 mL of peach juice was added to a 10 mL volumetric flask containing 4 mL of distilled water. Then, 300 µL of NaNO2 (0.5 g/L) solution was added. After 5 min, 300 µL of AlCl3 (1 g/L) solution was added and 6 min later, 2 mL of NaOH (1 mol/L) was added to the mixture. The final volume was made up to 10 mL with the addition of distilled water. The solution was mixed, and the absorbance was measured at 360 nm. Quercetin was used as a standard to produce a calibration diagram. Results were expressed as mg quercetin equivalents per L of peach juice.
2.4. Statistical Analysis
2.4.1. Dataset and Maturity Thresholds
Of 701 peaches that were harvested and analysed, 606 were included in this study. This was due to the implementation of a criterion that peaches should have at least a minimum additional colouration presence on skin. The reason was to, as much as possible, simulate conditions in packinghouses, since peaches with no additional colouration would probably not be harvested due to their unattractiveness to consumers. Moreover, a small number of peaches were excluded since they did not group in either maturity range (unripen—firmness above the highest maturity threshold).
Hence, the dataset used in this study consisted of 25 variables, with the majority of them being measured on 606 peaches, while L_gc_p, a_gc_p, b_gc_p, C_gc_p, and h_gc_p were measured on a slightly smaller number of fruits due to physiological maturity limitations (lack of ground colour near petiole insertion). TPC and TFC were also measured on a reduced sample size due to the complexity of the method (Table 1).
In this study, different firmness thresholds were used to present various maturity stages of peaches (summarised in Table 2). Firmness was selected since it is a key indicator of peach maturity prediction [2,10] and because it is responsible for the useful lifespan of the product, e.g., handling resistance and shelf-life longevity [13,19]. Shane [28], partly on the summaries developed by Carlos Crisosto, stated that the ideal peach firmness range for harvesting and packing for long-distance chain stores is from 7.26 to 5.44 kg·cm−2 and from 5.44 to 3.63 kg·cm−2 for medium ones. Hence, the firmness range for harvesting and packing for long-distance chain stores was used as the first maturity category. Peaches at harvest should have firmness no more than 4.59 kg·cm−2 in order to meet the quality standards, per Ramina et al. [6], according to Neri et al. [29]. Therefore, to address the growing dissatisfaction of consumers with the organoleptic properties of peaches harvested too early, a second maturity range was used up to the aforementioned firmness level. Furthermore, peaches can be labelled as “ready to buy” and “ready to eat”, which is a helpful tool for retailers to estimate the possible time for fruit commercialisation. Valero et al. [30] defined that “ready to buy” peaches have firmness from 1.84 to 3.57 kg·cm−2, while Crisosto et al. [31] defined that “ready to eat” peaches have firmness from 0.9 to 1.8 kg·cm−2. Hence, these two firmness ranges represent the following two categories. Peaches with firmness less than ready to eat category (<0.9 kg·cm−2) were classified as overripe. In the available literature, somewhat different peach maturity categorisations with narrower firmness ranges are also present [10,31,32,33]. However, the ranges used in this study were chosen to better integrate maturity categories, and were effective in previous studies as well [8,14,30]. Visual representation of randomly selected peaches for each maturity group is shown in Figure 1.

Table 2.
Overview of maturity categories and their defined firmness thresholds.
Figure 1.
Visual representation of randomly selected peaches for each maturity group.
2.4.2. Statistical Analysis
Data were statistically analysed using R statistical software (R version 4.3.2 (2023-10-31 ucrt)) by one-way ANOVA and Tukey’s HSD test (p ≤ 0.05). In the same software, Pearson correlation analysis was also conducted. The following packages in R were used: “agricolae”, “Hmisc”, and “corrplot”. Discriminant analysis (DA) was conducted in the XLSTAT add-on for Microsoft Office 2016. Statistical analysis was programmed not to take missing variables, as stated in Table 1.
3. Results
3.1. Segregation of Non-Destructive and Destructive Fruit Traits per Maturity Category
The maturity category had a significant effect on all morphological fruit traits (FSR, volume, weight, and density) and IAD (Table 3). Although average differences were very small (only up to 0.02 units), H1 peaches had significantly smaller FSR than H5 and H6 peaches, while H6 peaches also had significantly higher FSR than H2 and H3 peaches. H1 peaches had the significantly smallest fruit volume and weight, followed by H2 peaches. The highest average fruit volume and weight were obtained for H5 peaches, although no significant difference was obtained between them and H4 and H6 peaches. Although average differences were minimal (up to 0.03 units), H5 and H6 peaches had significantly higher fruit density than H1 and H3 peaches, while in other cases, no significant difference was recorded. The IAD trait had mostly pronounced differences between various maturity categories. All maturity categories differed significantly from each other, with the exception of the H5 and H6 categories, although the same trend of decreasing average values was observed (Table 3).

Table 3.
Segregation of morphological traits and IAD based on peach maturity category.
Ground colour variables showed the significant effect of the maturity category on all ground colour parameters, with the exception of b_gc_p, where no significant difference was obtained (Table 4 and Table 5). L_gc was significantly lowest in H6 peaches, with the exception of H5, whereas a decreasing trend in average values was observed. In addition, H5 peaches had significantly lower L_gc values than H2 peaches. L_gc_p was significantly higher in H1 and 2 peaches in contrast to all other maturity categories. a_gc and a_gc_p were significantly lowest in H1 peaches and highest in H6 peaches, with the exception of H5 peaches, where no significant difference compared to H6 was observed. b_gc was significantly lowest in H1 peaches, followed by H2 peaches. C_gc was significantly lowest in H1 peaches, followed by H2 and H3 peaches. However, for C_gc_p, no significant difference was detected between maturity categories. h_gc and h_gc_p were significantly highest in H1 peaches and were followed by H2 and H3 peaches (Table 4 and Table 5).

Table 4.
Segregation of ground colour variables measured at the cheek based on peach maturity category.

Table 5.
Segregation of ground colour variables measured near petiole insertion based on peach maturity category.
Regarding additional fruit colour, ANOVA showed that the maturity category had a significant effect on all studied traits (Table 6). SAC was significantly lowest in H1 peaches, followed by H2 and H3 peaches. L_ac was significantly highest in H1 and H2 peaches, whereas in other cases, no significant difference was observed. a_ac was significantly smallest in H1 peaches, followed by H2 peaches, whereas in other cases, no significant difference was observed. b_ac was significantly lowest in H1 peaches, with the exception of H2 peaches. Also, H2 peaches had significantly smaller b_ac values than H3, H4, and H5 peaches. C_ac was significantly higher in H1 peaches than in H3, H4, and H5 peaches, and in H2 peaches than in H3 and H5 peaches. h_ac was significantly highest in H1 peaches, followed by H2 ones, whereas in other cases, no significant difference was observed (Table 6).

Table 6.
Segregation of additional colour variables based on peach maturity category.
Regarding inner fruit quality parameters, ANOVA showed that the maturity category had a significant effect on TA, SSC_TA, TPC, and TFC, whereas the maturity category did not significantly affect SSC (Table 7). H1 and H2 peaches had the significantly highest TA, and H6 peaches significantly the lowest TA. The SSC_TA ratio was significantly smallest in H1 and H2 peaches, and highest in H6 peaches. Also, between all other categories, differences were significant, with lowering TA and SSC_TA values as peaches mature. Regarding TPC traits, H5 and H6 peaches had significantly higher values than H2 and H3 peaches, as well as H4 peaches than H2 ones. H1, H2, and H3 peaches had significantly smaller TFC values than H5 peaches, while in other cases. no significant differences were observed (Table 7).

Table 7.
Segregation of inner fruit quality traits based on peach maturity category.
3.2. Correlation of Non-Destructive and Destructive Fruit Traits Within Defined Maturity Categories
When all maturity categories are taken into account (Figure 2), IAD showed the highest correlation with firmness among the non-destructive parameters (r = 0.78), followed by a_gc and h_gc (r = −0.72 and 0.69, respectively), a_gc_p (r = −0.56), SAC (r = 0.54), h_gc_p (r = 0.53), C_gc (r = −0.45), and fruit weight and volume (r = −0.44 and −0.43, respectively). Regarding destructive parameters, SSC_TA (r = −0.66), TA (r = −0.60), TPC, and TFC (r = −0.37 and −0.36, respectively) had the highest correlation with firmness. In addition to obvious notable correlations (as weight with volume), all variables that had a strong correlation with firmness were also notably correlated with each other. For example, IAD was notably correlated with a_gc, h_gc, a_gc_p, ha_gc_p, SAC, TA, and SSC_TA (r = −0.75, 0.74, −0.60, 0.58, −0.60, 0.52, −0.60, respectively).
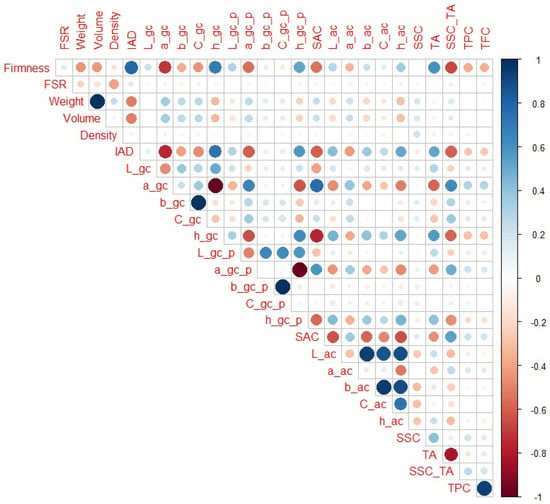
Figure 2.
Correlation matrix for all peach maturity categories (H1–H6). Legend: red or blue circles present significant positive or negative Pearson correlation coefficients (respectively), darker tone and bigger circle present higher correlation strength.
When only H1 and H2 maturity categories are observed (Figure 3), regarding non-destructive parameters, IAD (r = 0.53) had the highest correlation with firmness, followed by fruit volume and weight (r = −0.46 and −0.45, respectively), and a_gc and h_gc (r = −0.37 and 0.37, respectively). Regarding destructive parameters, no notable correlation was observed. In addition to the standard correlation between variables that were correlated with firmness, some new correlations emerged. For example, in addition to a_gc, h_gc, fruit weight, and volume (r = −0.70, 0.70, −0.48, and −0.48, respectively), IAD was also notably corelated with h_ac, SAC, a_gc_p, a_ac, h_gc_p, b_ac, SSC_TA, and L_ac (r = 0.53, −0.51, −0.45, −0.44, 0.43, 0.43, 0.42, and 0.41, respectively). Similarly, a_gc and h_gc were notably correlated with SAC (r = 0.72 and −0.72, respectively), L_ac (r = −0.47 and 0.46, respectively), a_ac (r = 0.47 and −0.47, respectively), h_ac (r = −0.47 and 0.47, respectively), b_ac (r = −0.45 and 0.45, respectively), and SSC_TA (r = 0.50 and −0.51, respectively). SSC_TA ratio was also notably correlated with SAC, L_ac, b_ac, C_ac, and h_ac (r = 0.55, −0.46, −0.44, −0.42, −0.42, respectively). It is interesting to observe that in this maturity range, some traits that had negligible or small correlation with firmness (like SSC_TA or, to some extent, TA or SAC) had much higher correlations with other variables important for non-destructive maturity prediction.
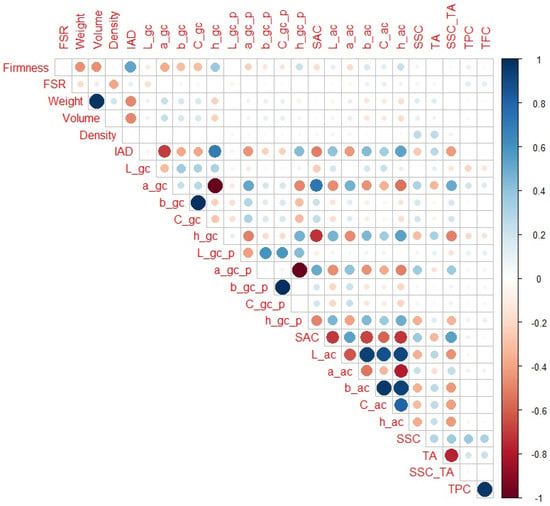
Figure 3.
Correlation matrix for H1 and H2 peach maturity categories. Legend: red or blue circles present significant positive or negative Pearson correlation coefficients (respectively), darker tone and bigger circle present higher correlation strength.
When only H1, H2, and H3 maturity categories are observed (Figure 4), highest correlation with firmness of non-destructive parameters had IAD (r = 0.76), followed by a_gc and h_gc (r = −0.57 and 0.56, respectively), fruit volume and weight (r = −0.48 and −0.47, respectively), a_gc_p, h_ac and h_gc_p (r = −0.43, 0.43 and 0.42, respectively). Regarding destructive parameters, no notable correlation was observed. In addition to obvious notable correlations (as weight with volume), all variables that had a strong correlation with firmness were also notably correlated with each other. For example, IAD was notably correlated with a_gc, h_gc, h_ac, SAC, a_gc_p, h_gc_p, fruit volume, L_ac, fruit weight (r = −0.74, 0.73, 0.59, −0.55, −0.54, 0.52, −0.51, 0.49, −0.49, respectively), etc. Similarly, a_gc and h_gc were notably correlated with SAC (r = 0.72 and −0.72, respectively), h_ac (r = −0.58 and 0.58, respectively), SSC_TA (r = 0.52 and −0.51, respectively), b_ac (r = −0.47 and 0.48, respectively), L_ac (r = −0.50 and 0.50, respectively), a_ac (r = 0.44 and −0.44, respectively), and TA (r = −0.43 and 0.42, respectively). SSC_TA ratio was also notably correlated with SAC, L_ac, b_ac, h_ac, and C_ac (r = 0.51, −0.45, −0.43, −0.42, and −0.40, respectively). It is again interesting to observe here that some traits that had small correlation with firmness (like SSC_TA or, to some extent, TA or SAC) had much higher correlation with other variables important for non-destructive maturity prediction. In addition, a_gc and h_gc had much higher correlation with additional fruit colour parameters than with firmness.
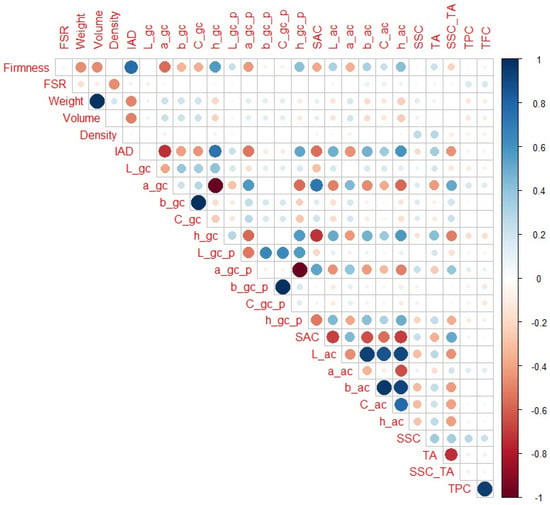
Figure 4.
Correlation matrix for H1–H3 peach maturity categories. Legend: red or blue circles present significant positive or negative Pearson correlation coefficients (respectively), darker tone and bigger circle present higher correlation strength.
When only H2, H3, and H4 maturity categories are observed (Figure 5), IAD (r = 0.67) had the highest correlation with firmness among the non-destructive parameters, followed by a_gc and h_gc (r = −0.62 and 0.61, respectively), fruit volume and weight (r = −0.48 and −0.47, respectively), SAC (r = −0.49), and fruit weight and volume (r = −0.45, for both variables). Regarding destructive parameters, SSC_TA (r = −0.46) and TA (r = 0.43) had the highest correlation with firmness. It should also be noted that in this maturity range, TFC and TPC had the highest correlation with firmness (r = −0.35 and −0.34, respectively). In addition to obvious notable correlations (as weight with volume), all variables that had a strong correlation with firmness were also notably correlated with each other. Again, a_gc and h_gc had higher correlation with additional colour parameters than firmness or IAD. For example, h_gc more notably correlated with SAC, h_ac, and L_ac (r = −0.73, 0.51, and 0.46, respectively), while IAD (r = −0.51, 0.48, and 0.41, respectively) and firmness (r = −0.49, 0.35, and 0.30, respectively) to a somewhat lower extent. In this maturity range, although again some traits that had small correlation with firmness (like SSC_TA or TA) had higher values with a_gc or h_gc, the differences are much lower.
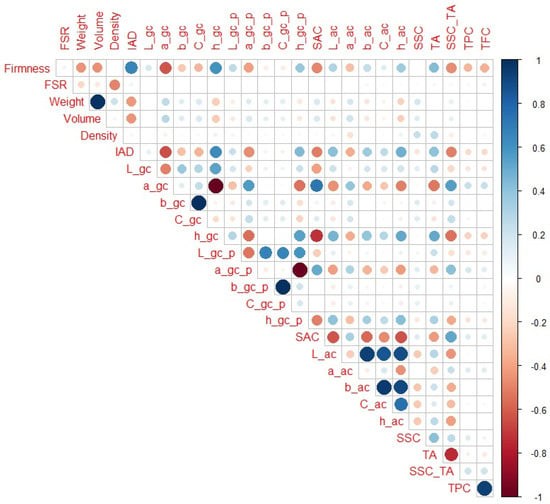
Figure 5.
Correlation matrix for H2–H4 peach maturity categories. Legend: red or blue circles present significant positive or negative Pearson correlation coefficients (respectively), darker tone and bigger circle present higher correlation strength.
When only H4, H5, and H6 maturity categories are observed (Figure 6), IAD (r = 0.39) had the highest correlation with firmness among the non-destructive parameters, followed by a_gc and h_gc (r = −0.35 and 0.31, respectively). Much higher correlation coefficients were obtained for destructive parameters, whereas the highest were for SSC_TA, followed by TA (r = −0.65 and 0.54, respectively). In addition to the obvious notable correlations (as weight with volume), L_gc, a_gc, and h_gc were notably correlated with SAC (r = −0.51, 0.56 and −0.58, respectively), which was not the case for IAD or firmness. In contrast to previous maturity ranges, here, firmness had higher correlations with SSC_TA and TA than a_gc or h_gc.
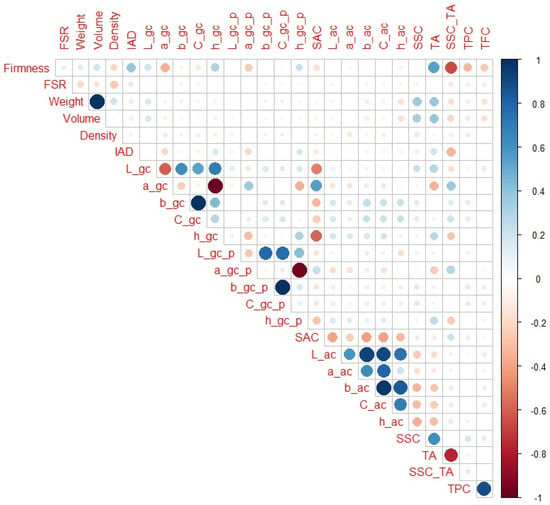
Figure 6.
Correlation matrix for H4–H6 peach maturity categories. Legend: red or blue circles present significant positive or negative Pearson correlation coefficients (respectively), darker tone and bigger circle present higher correlation strength.
When only H5 and H6 maturity categories are observed (Figure 7), IAD (r = 0.43) had the highest correlation with firmness among the non-destructive parameters, and SSC_TA and TA (r = −0.54 and 0.0.42, respectively) had the highest correlation with firmness among the destructive parameters. In addition to obvious notable correlations (as weight with volume), L_gc, a_gc, and h_gc were notably correlated with SAC (r = −0.46, 0.62 and −0.64, respectively), which was not the case for IAD or firmness. In contrast to the previous maturity ranges, firmness and IAD had higher correlations with SSC_TA than a_gc and h_gc (r = 0.31 and −0.18, respectively). Additionally, firmness had a higher correlation with TA than IAD, a_gc, or h_gc (r = 0.19, −0.23, and 0.14, respectively). It should also be noted that L_gc showed notable correlation with TPC and TFC (r = 0.45 and 0.53, respectively), which was not the case in other maturity ranges. In addition, TA and SSC_TA had notable correlations with TFC (r = 0.53 and −0.43, respectively).
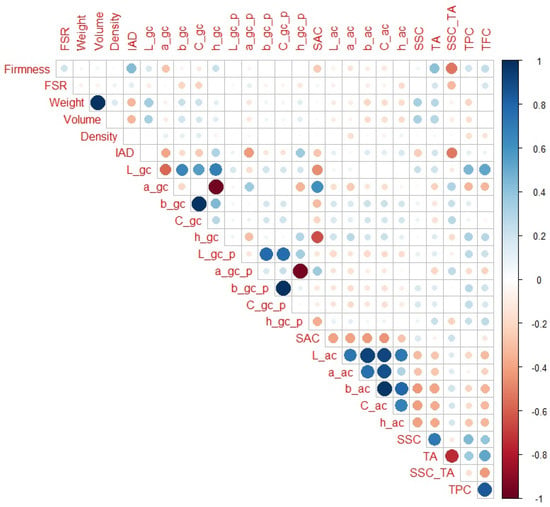
Figure 7.
Correlation matrix for H5 and H6 peach maturity categories. Legend: red or blue circles present significant positive or negative Pearson correlation coefficients (respectively), darker tone and bigger circle present higher correlation strength.
3.3. Discriminant Analysis
DA (Figure 8 and Figure 9) revealed that the first component (F1) with an eigenvalue of 2.62 explained 73.07% of total variability, while second component (F2) with an eigenvalue of 0.70 explained 19.50% of total variability, cumulatively accounting for 92.58% of total variability. Standardized canonical discriminant function coefficients showed that F1 was mostly influenced by IAD, a_gc, h_ac, L_gc_p, while F2 was mostly influenced by b_gc, C_gc, b_gc_p, C_gc_p, volume, weight, SSC_TA, a_gc_p, and h_gc_p.
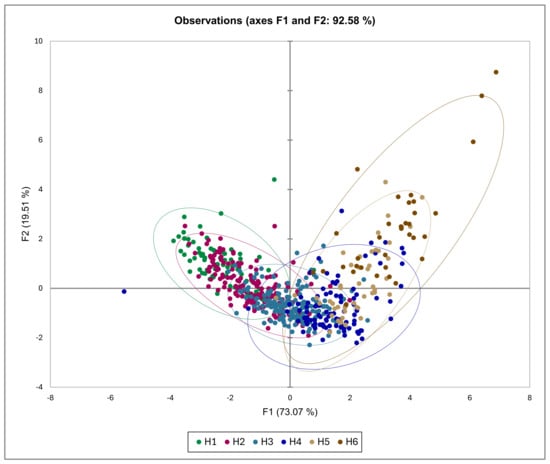
Figure 8.
Canonical Plot for a discriminant analysis in relation to PC1 and PC2 with respect to peach maturity category.
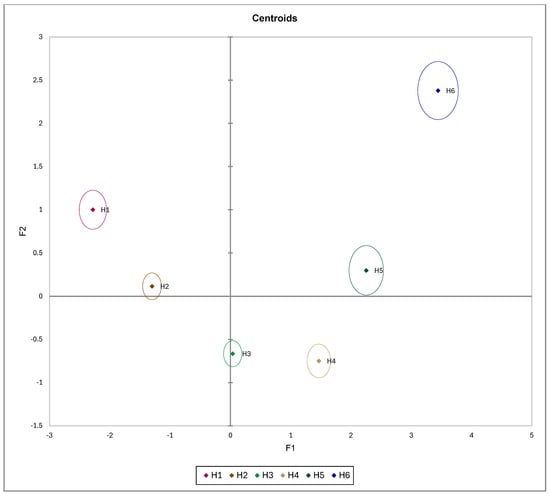
Figure 9.
Centroids of a discriminant analysis in relation to PC1 and PC2 with respect to peach maturity category.
4. Discussion
4.1. Segregation of Non-Destructive and Destructive Fruit Traits per Maturity Category
Fruit weight and volume are one of the most important attributes for the producer due to their direct link to revenue. It is evident that fruit weight and volume tend to increase up to the H4 maturity category and then decrease. Hence, in this case, the grower’s profit is directly linked to a properly detected harvest window, with fruit weight differences being between the H1 and H4 maturity categories, being on average even 61%. These results are generally in accordance with the available literature, where peaches usually gain weight or volume during ripening [1,2,7,34,35,36,37,38]. There is difficulty in comparing data from the final maturity stages, as to the best of our knowledge, only a minority of studies include them. In two studies [34,36], it was reported that peach weight did not decrease between the last two maturity stages, which is contrary to our results. However, for the ‘Mihong’ peach in the second year of the study, a drop in fruit weight between the last two maturity stages was reported [2]. Additionally, Pandova et al. [39] reported that, on average, ‘Evmolpiya’ peaches harvested in the fourth harvest week had higher fruit weight than those harvested in the fifth or sixth harvest week.
Although FSR and fruit density showed small variations in average values between maturity categories, significant differences were observed in certain cases. Generally, as peaches mature, these values decrease as the fruit becomes flatter. Similarly, Selli et al. [37] reported a decrease in the FSR ratio during ripening, resulting in a more globular fruit shape, but in their case, changes were much more expressed than in this study.
The IAD index showed the most prominent segregation of peaches between different maturity categories, with no significant difference observed only between the last two categories. It is evident that as peaches mature, IAD values decrease, with strong differences observed between maturity categories. Gasic et al. [40] segregated 13 peach cultivars in three maturity classes according to IAD values and reported significant differences in firmness values. Reig et al. [41] reported that during maturation, IAD decreased for each studied peach cultivar.
The colour is an important characteristic of agricultural produce, as consumers often judge the maturity of the product by its external colour and overall appearance [39]. Ground colour parameters were measured in two different areas, and it is evident that when measured near the petiole insertion, smaller segregation between maturity categories is observed, with the exception of the L_gc_p variable. The L_gc variable mostly decreased in the H6 (overripen) stage, while the L_gc_p variable was at the crossing between H2 and H3 maturity stages. The a_gc and a_gc_p variables increased as peaches matured, with somewhat smaller differences from the H4 to H6 stages. The b_gc variable tends to increase as peaches mature, but with small alternation from the H4–H6 stages. The same overall trend based on average values is evident for the b_gc_p variable. The C_gc variable differed from C_gc_p in the level of significance between maturity categories and a more clear and pronounced increase trend during maturation in favour of the C_gc variable. The most pronounced differences for C_gc variable were from the H1–H4 stages. The h_gc and h_gc_p variables had the same overall trend of decreasing values as peaches mature, with clearer differences in favour of the h_gc variable from the H4–H6 stages. It is evident that higher h_gc variable differences exist from the H1–4 stages, while they tend to decrease afterwards. In accordance with our results, Robertson et al. [36] observed the same trend for L*, a*, and h° variables, while the trend for b* was opposite. In the stated study, the L* variable mostly decreased between the last two maturity categories, similar to the L_gc variable in this study. Reduction in the h° variable during the ripening of three different peach cultivars was reported by Shinya et al. [7], in agreement with the results obtained in this study. However, the chroma variable (C_gc in this study) was only in accordance with the study conducted on peach ‘Kakamas’ [7]. Orazem et al. [42] reported a similar trend, as seen in this study, regarding the evolution of L* and a* ground colour parameters during the maturation of the peach ‘Redhaven’ grafted on six different rootstocks. Of all the ground colour variables, b_gc showed the least relevance to maturation due to its small changes during ripening, a finding also noted in two other studies [8,43].
During peach ripening, it is evident that SAC increases up to the H4 category and a_gc up to the H3 category, while L_ac, b_ac, C_ac, and h_ac decrease up to the H3 category. Colour is one of the more important indicators of peach grade, with the amount of overcolour (pink or red colour) determining the peach grade level [17]. However, in the UNECE standard concerning the marketing and commercial quality control of peaches and nectarines [44], it is defined that Class 1 can have slight defects in colouring, Class 2 can have defects in colouring, while Extra Class needs to be uniform in colouring. Similarly, in the USA, U.S. No. 1 Grade has no colour requirement, U.S. Extra No. 1 Grade requires that 50% of the peaches must have at least 25% of the surface with a blush, pink, or red colour, and U.S. Fancy grade requires that 90% of the peaches must have at least 33% of the surface with a blush, pink, or red colour [45]. In this study, U.S. Extra No. 1 Grade is achieved around the H3–H4 maturity category. Similarly, on the ‘Redhaven’ variety, but grafted on six different rootstocks, ref. [42] reported an overall reduction in L* and a* additional colour values during maturation.
Soluble solid concentration is a good predictor for the peach sugar content [39]. Iglesias and Echeverría [46], according to Clareton [47], state that soluble solids below 10% are generally unacceptable to consumers. Since no significant difference was reported between different maturity categories, all peaches had satisfactory levels of SSC for consumers. However, these results are contrary to the results of the majority of other studies where peaches gain SSC levels as they mature [7,34,35,36,37,38,39]. However, Ropelewska et al. [1] noted the absence of significant difference in SSC levels between three maturity stages for the peach ‘Royal Glory’, although the contrary was reported for ‘Redhaven’. Generally, during maturation, peaches tend to reduce acidity levels [6,15,16,35,37,38]. Ropelewska et al. [1] reported the same decrease in acidity levels during maturation for peach ‘Royal Glory’, while for peach ‘Redhaven’, no trend was observed. Kader [48] proposed that the maximum titratable acidity for peaches for acceptable flavour quality should be no more than 0.6%. In this study, H5 peaches had acidity levels beneath the aforementioned threshold, which is logical since this is the ready to eat phase, while higher maturity class peaches need to mature off the tree. As in this study, Gasic et al. [40] and Selli et al. [37] reported an increase in SSC_TA levels during the maturation of various peach cultivars, while Gasic et al. [49] for half of them and Guizani et al. [38] for four of five studied cultivars. With regard to TA and SSC_TA variables, in this study, significant differences were not observed only between the first two maturity groups.
Orazem et al. [42] reported that neochlorogenic and chlorogenic acid (the most abundant phenolics in the flesh tissue) were generally highest at 94 days after full bloom (DAFB), decreased by 100 DAFB and then increased again at 107 DAFB in the flesh of ‘Redhaven’ peaches grafted on different rootstocks. Gasic et al. [40] reported that in the flesh tissue of thirteen peach cultivars, TPC was significantly lowest in the most pronounced maturity class during the first year of the study, while in the second year, both TPC and TFC were significantly highest in the most pronounced maturity class. In the first year of study, no significant difference was obtained for TFC. Liu et al. [35] reported that in five peach cultivars, TPC and TFC values significantly drop with each next maturity stage. Andreotti et al. [50] reported that TPC in the peel and pulp of peach ‘Stark Red Gold’ continuously declined during fruit development and ripening. According to Dabbou et al. [51], flesh antioxidant activity tends to increase during ripening in different peach varieties. Guizani et al. [38] reported a general decrease in total phenol content in the mesocarp of the four studied peach cultivars during ripening, while in the fifth cultivar, it increased. TFC showed various patterns during ripening depending on the studied cultivar. As evident, there is variability in the results regarding the behaviour of biochemicals in peach fruits during ripening. It should also be noted that in this study, TPC and TFC were determined in filtered peach juice, differently from the available literature data.
4.2. Correlation of Non-Destructive and Destructive Fruit Traits Within Defined Maturity Categories
Correlation matrix for all peach maturity groups, and per defined groups, showed interesting results. As indicated in the first part of the study, IAD showed the highest correlation coefficients with firmness among the non-destructive parameters in all maturity groups and was followed by the a_gc parameter (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). Moreover, IAD followed by a_gc and h_gc had the highest correlation with firmness when all peach maturity groups were considered, and within the H1–H2, H1–H3, and H2–H4 maturity groups (Figure 2, Figure 3, Figure 4 and Figure 5). Within maturity groups H4–H6 and H5–H6 (Figure 6 and Figure 7), SSC_TA had the highest correlation with firmness, followed by TA for the first and IAD for the second maturity group. Zhang et al. [18] reported a robust positive correlation between IAD and firmness in all six investigated peach cultivars, which is in accordance with the results of this study. Robertson et al. [36] reported that peach ‘Majestic’ a* ground colour value, weight, SSC_TA, SSC, and TA had correlations of −0.87, −0.87, −0.82, −0.73, and 0.59, respectively, with firmness. Pandova et al. [39] reported that weight and SSC of the peach ‘Laskava’ had correlations of 0.48 and 0.21, respectively, while weight and SSC of the peach ‘Evmolpiya’ had correlations of 0.34 and 0.03, respectively. SSC and fruit weight results from this study are in line with Pandova et al. [39], and not with Robertson et al. [36], who obtained much higher coefficients, especially for SSC. However, other mentioned parameters are generally in accordance with Pandova et al. [39], with an emphasis on the fact that in this study, SSC_TA had a higher correlation with firmness than TA alone. Zhang et al. [18] reported that peaches with lower IAD values exhibited higher SSC and SSC_TA ratios, and lower TA values, but with no significant linear relationships between IAD and SSC in any cultivar. A similar observation was made in this study, with the exception that IAD and SSC had a significant, though very small, correlation. Correlation coefficients for SSC_TA were generally similar between studies.
The special significance of this study is that it observes correlations between various peach quality attributes within defined maturity ranges. This approach showed that during the first maturity ranges (H1–H3), some parameters that had small correlation with firmness (like SSC_TA or, to some extent, TA or SAC), had much higher correlations with a_gc, h_gc, or IAD. In the following maturity categories (H4–H6), SSC_TA and TA had higher correlation with firmness than the aforementioned parameters. In addition, a_gc, h_gc, and SAC had higher correlations with SSC_TA from the H1–H4 maturity range to a small extent, while the opposite was observed for the remaining maturity ranges. Again similarly, TA was higher correlated with a_gc and h_gc during all maturity categories, with the exception of H5–H6, where correlation was higher with IAD than with h_gc. This observation was interesting since IAD had an overall higher correlation with firmness than a_gc, h_gc, and SAC. It is also interesting to observe that although in the majority of maturity ranges there were no notable correlations with TPC or TFC, during the last two maturity stages (H5–H6), L_gc, as well as TA and SSC_TA, showed a notable correlation with TFC. Firmness also had the highest correlation with TPC and TFC in the maturity range H2–H4, which is also higher in later maturity categories than in the earliest one.
4.3. Discriminant Analysis
According to DA (Figure 8 and Figure 9), it is evident that the H1 and H2 peaches distinguish between H5 and H6 peaches according to the F1 axis, meaning that they mostly differ between each other with respect to IAD, a_gc, h_ac, and L_gc_p. The F2 axis showed no clear observation, only that H1 and H6 peaches partly differ from H3 and H4 peaches. Separation along the F1 axis is logical, as previous statistical analysis showed that IAD was the parameter that most clearly distinguished between various maturity groups.
5. Conclusions
This study enabled a better understanding of the ripening process of peach ‘Redhaven’ in the ecological conditions of central Croatia. The most important findings include:
- IAD was the non-destructive parameter that was mostly distinguished between maturity categories, followed by a_gc and h_gc
- During the first three maturity categories (H1–H3), firmness had a notably smaller correlation with SSC, TA, and SSC_TA, which is not the case for a_gc, h_gc, IAD, and SAC. From H4, firmness has a higher correlation with TA and SSC_TA than the aforementioned parameters
- TA and SSC_TA were mostly, to a smaller extent, better correlated with a_gc and h_gc than with IAD, depending on the maturity category
- When ground colour parameters are measured near petiole insertion, smaller segregation between maturity categories is obtained than when measured at the rest of the fruit, with the only exception of the L_gc_p variable
- Fruit mass and volume had the highest correlation with firmness from the H1 to H4 maturity category, afterwards becoming negligible
- During the last three maturity categories (H4–H6), SSC_TA and TA had the highest correlation with firmness, while IAD was notably less correlated than in previous categories. Hence, non-destructive parameters within the last maturity categories are not reliable
- TPC and TFC did not achieve notable correlation with any other variable, with the exception of the H5–H6 maturity categories, when were moderately correlated with the L* ground colour parameter
Author Contributions
Conceptualisation, M.V.; methodology, M.V., T.J., D.L., M.M. and M.B.B.; resources, M.M., M.V.; data curation, M.V., D.L. and S.J.; writing—original draft preparation, M.V.; writing—review and editing, M.V., T.J., D.L., M.M., M.B.B. and S.J.; supervision, T.J., M.M. and M.B.B.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Rijeka, grant number uniri-iskusni-drustv-23-143.
Data Availability Statement
The datasets analysed during the current study are not publicly available due to further research.
Acknowledgments
We would also like to thank Nikola Tomljenović for help with laboratory analysis work during late-night hours.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| a_gc | a* ground colour parameter measured on the rest of the fruit surface |
| a_gc_p | a* ground colour parameter measured near petiole insertion |
| b_gc | b* ground colour parameter measured on the rest of the fruit surface |
| b_gc_p | b* ground colour parameter measured near petiole insertion |
| C_gc | C ground colour parameter measured on the rest of the fruit surface |
| C_gc_p | C ground colour parameter measured near petiole insertion |
| h_gc | h° ground colour parameter measured on the rest of the fruit surface |
| h_gc_p | h° ground colour parameter measured near petiole insertion |
| H1 | maturity category harvesting for long-distance chain stores |
| H2 | maturity category harvesting for medium-distance chain stores |
| H3 | maturity category below maximum defined firmness for preserving optimal quality traits |
| H4 | maturity category ready to buy |
| H5 | maturity category ready to eat |
| H6 | maturity category overripe |
| IAD | absorbance difference index between 670 nm and 720 nm |
| L_gc | L* ground colour parameter measured on the rest of the fruit surface |
| L_gc_p | L* ground colour parameter measured near petiole insertion |
| SAC | share of additional fruit colouration |
| SSC | soluble solids concentration |
| SSC_TA | ratio between soluble solids concentration and titratable acidity |
| TA | titratable acidity |
| TFC | total flavonoid content |
| TPC | total polyphenolic content |
References
- Ropelewska, E.; Rutkowski, K.P. The Classification of Peaches at Different Ripening Stages Using Machine Learning Models Based on Texture Parameters of Flesh Images. Agriculture 2023, 13, 498. [Google Scholar] [CrossRef]
- Jayasooriya, L.S.H.; Shin, M.H.; Wijethunga, W.M.U.D.; Lee, S.K.; Cho, J.G.; Jang, S.H.; Kim, J.G. Selection of a Proper Maturity Index for the Mechanical Harvesting of ‘Mihong’ Peach Fruit. Horticulturae 2023, 9, 730. [Google Scholar] [CrossRef]
- FAOSTAT (2024) FAOSTAT Database. Food and Agriculture Organization of the United Nations. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 June 2024).
- Konopacka, D.; Jesionkowska, K.; Kruczyńska, D.; Stehr, R.; Schoorl, F.; Buehler, A.; Egger, S.; Codarin, S.; Hilaire, C.; Höller, I.; et al. Apple and Peach Consumption Habits across European Countries. Appetite 2010, 55, 478–483. [Google Scholar] [CrossRef]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A New Index Based on Vis Spectroscopy to Characterize the Progression of Ripening in Peach Fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- Ramina, A.; Tonutti, P.; McGlasson, W.; McGlasson, B. Ripening, Nutrition and Postharvest Physiology. In The Peach, Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CAB International: Wallingford, UK, 2008; pp. 550–574. [Google Scholar]
- Shinya, P.; Contador, L.; Predieri, S.; Rubio, P.; Infante, R. Peach Ripening: Segregation at Harvest and Postharvest Flesh Softening. Postharvest Biol. Technol. 2013, 86, 472–478. [Google Scholar] [CrossRef]
- Ljubobratović, D.; Vuković, M.; Brkić Bakarić, M.; Jemrić, T.; Matetić, M. Utilization of Explainable Machine Learning Algorithms for Determination of Important Features in ‘Suncrest’ Peach Maturity Prediction. Electronics 2021, 10, 3115. [Google Scholar] [CrossRef]
- Crisosto, C.; Slaughter, D.; Garner, D.; Boyd, J. Stone Fruit Critical Bruising Tresholds. J. Am. Pomol. Soc. 2001, 55, 76–81. [Google Scholar]
- Crisosto, C.H. How Do We Increase Peach Consumption? Acta Hortic. 2002, 592, 601–605. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and Orchard Bases of Peach Fruit Quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Ninkovski, I. Breskva i Nektarina: Savremeni Način Gajenje; Nolit: Beograd, Serbia, 1988. [Google Scholar]
- Infante, R. Harvest Maturity Indicators in the Stone Fruit Industry. Stewart Postharvest Rev. 2012, 8, 1. [Google Scholar] [CrossRef]
- Ljubobratović, D.; Vuković, M.; Brkić Bakarić, M.; Jemrić, T.; Matetić, M. Assessment of Various Machine Learning Models for Peach Maturity Prediction Using Non-Destructive Sensor Data. Sensors 2022, 22, 5791. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Yun, S.K.; Jun, J.H.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H. Assessment of Organic Acid and Sugar Composition in Apricot, Plumcot, Plum, and Peach during Fruit Development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, L.; Jiang, X.; Cherono, S.; Liu, J.J.; Ogutu, C.; Ntini, C.; Zhang, X.; Han, Y. Assessment of Organic Acid Accumulation and Its Related Genes in Peach. Food Chem. 2021, 334, 127567. [Google Scholar] [CrossRef]
- Miller, B.K.; Delwiche, M.J. Color Vision System for Peach Grading. Trans. Am. Soc. Agric. Eng. 1989, 32, 1484–1490. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Y.; Xu, F.; Wang, H.; Chen, M.; Shao, X. Changes in the Chlorophyll Absorbance Index (IAD) Are Related to Peach Fruit Maturity. N. Z. J. Crop Hortic. Sci. 2020, 48, 34–46. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Costa, G. Preharvest Factors Affecting Peach Quality. In The Peach: Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CAB International: Wallingford, UK, 2008; pp. 536–549. ISBN 9781845933869. [Google Scholar]
- Nunes, M.C.d.N. Color Atlas of Postharvest Quality of Fruits and Vegetables; Blackwell Publishing: Hoboken, NJ, USA, 2008; ISBN 9780813817521. [Google Scholar]
- Okie, W.R.; Bacon, T.; Bassi, D. Fresh Market Cultivar Development. In The Peach: Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CAB International: Wallingford, UK, 2008; pp. 139–174. [Google Scholar]
- Milatović, D. Koštičave Voćke; Naučno voćarsko društvo Srbije: Čačak, Serbia, 2023. [Google Scholar]
- AN 1005.00 Measuring Color Using Hunter L, a, b Versus CIE 1976 L*a*b*. Available online: https://support.hunterlab.com/hc/en-us/articles/204137825-Measuring-Color-using-Hunter-L-a-b-versus-CIE-1976-L-a-b-AN-1005b (accessed on 15 June 2021).
- Carreño, J.; Martínez, A.; Almela, L.; Fernández-López, J.A. Proposal of an Index for the Objective Evaluation of the Colour of Red Table Grapes. Food Res. Int. 1995, 28, 373–377. [Google Scholar] [CrossRef]
- Skendrović Babojelić, M.; Fruk, G. Priručnik Iz Voćarstva: Građa, Svojstva i Analize Voćnih Plodova; Hrvatska sveučilišna naklada; Sveučilište u Zagrebu Agronomski fakultet: Zagreb, Croatia, 2016; ISBN 978-953-169-318-9. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the Polyphenol Contents in Macedonian Grapes and Wines by Standardized Spectrophotometric Methods. J. Serbian Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Shane, B.; Monitoring Peach and Nectarine Ripening. Michigan State University Extension, USA. Available online: https://www.canr.msu.edu/news/monitoring_peach_and_nectarine_ripening (accessed on 10 November 2023).
- Neri, F.; Brigati, S. Sensory and Objective Evaluation of Peaches. In Proceedings of the Cost 94: The Postharvest Treatment of Fruit and Vegetables, Bled, Slovenia, 19–21 April 1994; De Jager, A., Jhonson, A., Hohn, E., Eds.; European Comission: Brussels, Belgium, 1994; pp. 107–115. [Google Scholar]
- Valero, C.; Crisosto, C.H.; Slaughter, D. Relationship between Nondestructive Firmness Measurements and Commercially Important Ripening Fruit Stages for Peaches, Nectarines and Plums. Postharvest Biol. Technol. 2007, 44, 248–253. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Valero, D. Harvesting and Postharvest Handling of Peaches for the Fresh Market. In The Peach: Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CAB International: Wallingford, UK, 2008; pp. 575–596. ISBN 9781845933869. [Google Scholar]
- Crisosto, C.H.; Kader, A. Peach Postharvest Quality Maintenance Guidelines; Department of Pomology University of California Davis: Davis, CA, USA, 2000. [Google Scholar]
- Crisosto, C.H. Peach quality and postharvest technology. Acta Hortic. 2006, 713, 479–488. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, X.; Mu, Q.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.; Yu, X. Differences in Total Phenolics, Antioxidant Activity and Metabolic Characteristics in Peach Fruits at Different Stages of Ripening. LWT 2023, 178. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Evaluation of Physiochemical and Antioxidant Activity Changes during Fruit On-Tree Ripening for the Potential Values of Unripe Peaches. Sci. Hortic. 2015, 193, 32–39. [Google Scholar] [CrossRef]
- Robertson, J.A.; Meredith, F.I.; Forbus, W.R. Changes in Quality Characteristics during Peach (Cv. ‘Majestic’) Maturation. J. Food Qual. 1991, 14, 197–207. [Google Scholar] [CrossRef]
- Selli, R.; Sansavini, S. Sugar, Acid and Pectin Content in Relation to Ripening and Quality of Peach and Nectarine Fruits. Acta Hortic. 1995, 379, 345–358. [Google Scholar] [CrossRef]
- Guizani, M.; Maatallah, S.; Dabbou, S.; Serrano, M.; Hajlaoui, H.; Helal, A.N.; Kilani-Jaziri, S. Physiological Behaviors and Fruit Quality Changes in Five Peach Cultivars during Three Ripening Stages in a Semi-Arid Climate. Acta Physiol. Plant 2019, 41, 154. [Google Scholar] [CrossRef]
- Pandova, S.; Mihaylova, D.; Popova, A.; Savchovska, S.; Zhivondov, A. Dynamic Biometric Data, Total Soluble Solids, Ash Content, Firmness, and Color Characteristics of Two Peach Varieties. Agric. Sci. Technol. 2023, 15, 76–83. [Google Scholar] [CrossRef]
- Gasic, K.; Abdelghafar, A.; Reighard, G.; Windham, J.; Ognjanov, M. Fruit Maturity Affects Fruit Quality and Bioactive Compound Accumulation in Peach. Acta Hortic. 2016, 1119, 197–202. [Google Scholar] [CrossRef]
- Reig, G.; Alegre, S.; Iglesias, I.; Echeverría, G.; Gatius, F. Fruit Quality, Colour Development and Index of Absorbance Difference (IAD) of Different Nectarine Cultivars at Different Harvest Dates. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Postharvest Technology in the Global Market, Lisbon, Portugal, 22 August 2010; Cantwell, M.I., Almeida, D.P.F., Eds.; Acta Horticulturae: Lisbon, Portugal, 2012; pp. 1117–1125. [Google Scholar]
- Orazem, P.; Mikulic-Petkovsek, M.; Stampar, F.; Hudina, M. Changes during the Last Ripening Stage in Pomological and Biochemical Parameters of the “Redhaven” Peach Cultivar Grafted on Different Rootstocks. Sci. Hortic. 2013, 160, 326–334. [Google Scholar] [CrossRef]
- Shewfelt, R.L.; Myers, S.C.; Resurreccion, A.V.A. Effect of Physiologycal Maturity at Harvest on Peach Quality during Low Temperature Storage. J. Food Qual. 1987, 10, 9–20. [Google Scholar] [CrossRef]
- UNITED NATIONS UNECE. Standard FFV-26 Concerning the Marketing and Commercial Quality Control of Peaches and Nectarines; 2023 Edition; UNITED NATIONS UNECE: New York, NY, USA; Geneva, Switzerland, 2023. [Google Scholar]
- Peaches- Color Requirements. Available online: https://www.ipt.us.com/produce-inspection-resources/inspectors-blog/produce-defects-and-grade-standard-changes/peaches-color-requirements (accessed on 2 September 2024).
- Iglesias, I.; Echeverría, G. Differential Effect of Cultivar and Harvest Date on Nectarine Colour, Quality and Consumer Acceptance. Sci. Hortic. 2009, 120, 41–50. [Google Scholar] [CrossRef]
- Clareton, M. Peach and Nectarine Production in France: Trends, Consumption and Perspectives. In Proceedings of the Prunus Breeders Meeting, Pelotas, RS, Brazil, 28 November–2 December 2000; Embrapa Clima Temperado: Pelotas, RS, Brazil; pp. 83–91. [Google Scholar]
- Kader, A.A. Fruit Maturity, Ripening, and Quality Relationships. Acta Hortic. 1999, 485, 203–208. [Google Scholar] [CrossRef]
- Gasic, K.; Reighard, G.L.; Windham, J.; Ognjanov, M. Relationship between Fruit Maturity at Harvest and Fruit Quality in Peach. Acta Hortic. 2015, 1084, 643–648. [Google Scholar] [CrossRef]
- Andreotti, C.; Ravaglia, D.; Ragaini, A.; Costa, G. Phenolic Compounds in Peach (Prunus Persica) Cultivars at Harvest and during Fruit Maturation. Ann. Appl. Biol. 2008, 153, 11–23. [Google Scholar] [CrossRef]
- Dabbou, S.; Maatallah, S.; Castagna, A.; Guizani, M.; Sghaeir, W.; Hajlaoui, H.; Ranieri, A. Carotenoids, Phenolic Profile, Mineral Content and Antioxidant Properties in Flesh and Peel of Prunus Persica Fruits during Two Maturation Stages. Plant Foods Hum. Nutr. 2017, 72, 103–110. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).