Surviving the Extremes: The Synergistic Impact of Drought and Salinity on Thymus capitatus Growth, Physiology, and Biochemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Morphological Parameters
2.2.1. Shoot Parameters Determination
2.2.2. Root Parameters Determination
2.3. Physiological Parameters
2.3.1. Relative Water Content Determination
2.3.2. Pigments Determination
2.3.3. Proline Content Determination
2.3.4. Soluble Sugar Content Determination
2.4. Biochemical Parameters
2.4.1. Essential Oil Content Determination
2.4.2. Essential Oil Composition Determination
2.4.3. Total Polyphenols Determination
2.4.4. Antioxidant Capacity Determination
2.4.5. Hydrogen Peroxide (H2O2) Level Determination
2.5. Evaluation of Stress Indices
2.5.1. Stress Susceptibility Index
2.5.2. Stress Tolerance Index
2.6. Statistical Analysis
3. Results
3.1. Morphological Responses of Plants to Stress Conditions
3.1.1. Shoot Parameters
3.1.2. Root Parameters
3.2. Physiological Responses of Plants to Stress Conditions
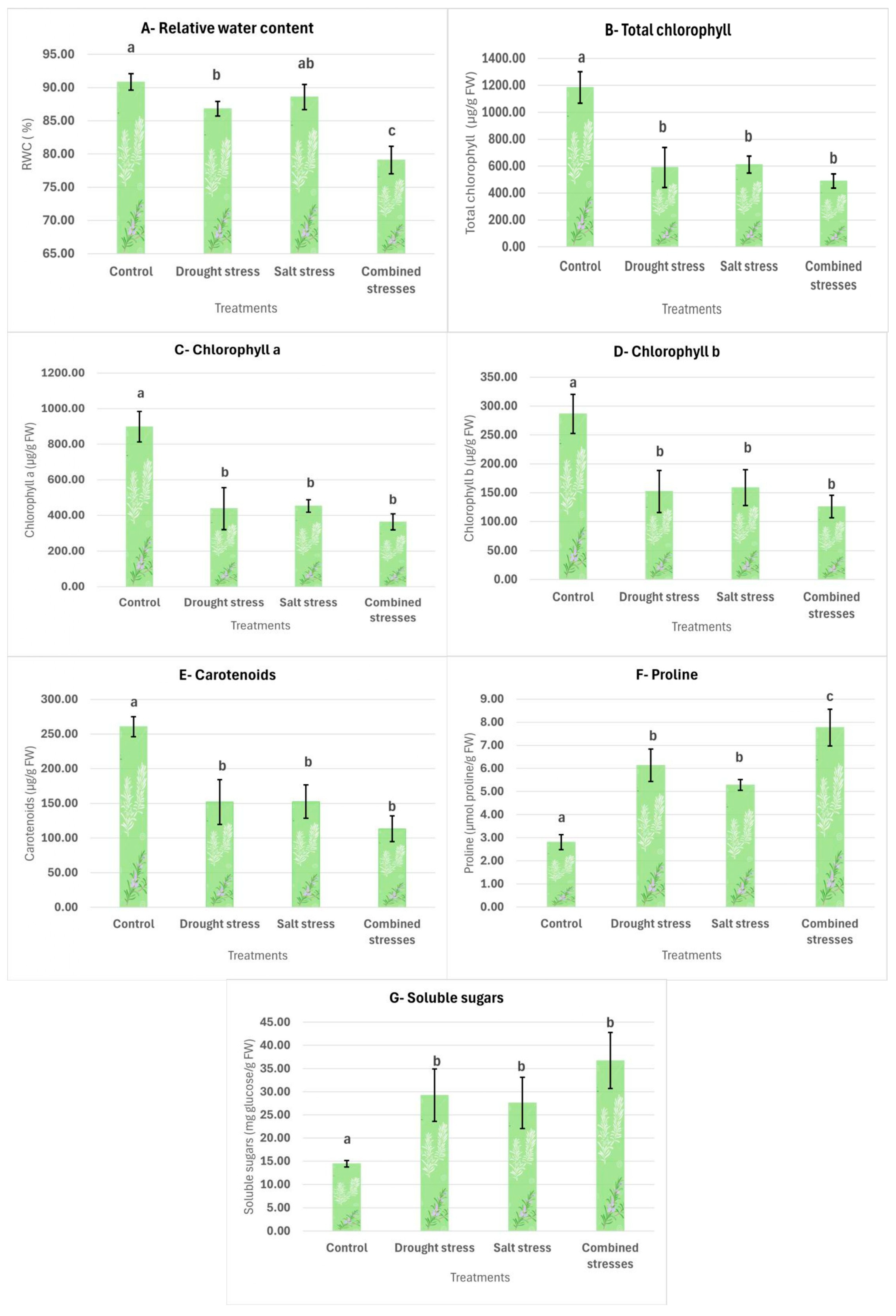
3.2.1. Relative Water Content (RWC)
3.2.2. Chlorophyll and Carotenoids
3.2.3. Proline
3.2.4. Total Soluble Sugars
3.3. Biochemical Responses of Plants to Stress Conditions
3.3.1. Essential Oil Content (EOC)
3.3.2. Essential Oil Composition
3.3.3. Total Polyphenols
3.3.4. Antioxidant Capacity
3.3.5. Hydrogen Peroxide
3.4. Stress Susceptibility and Tolerance Indexes
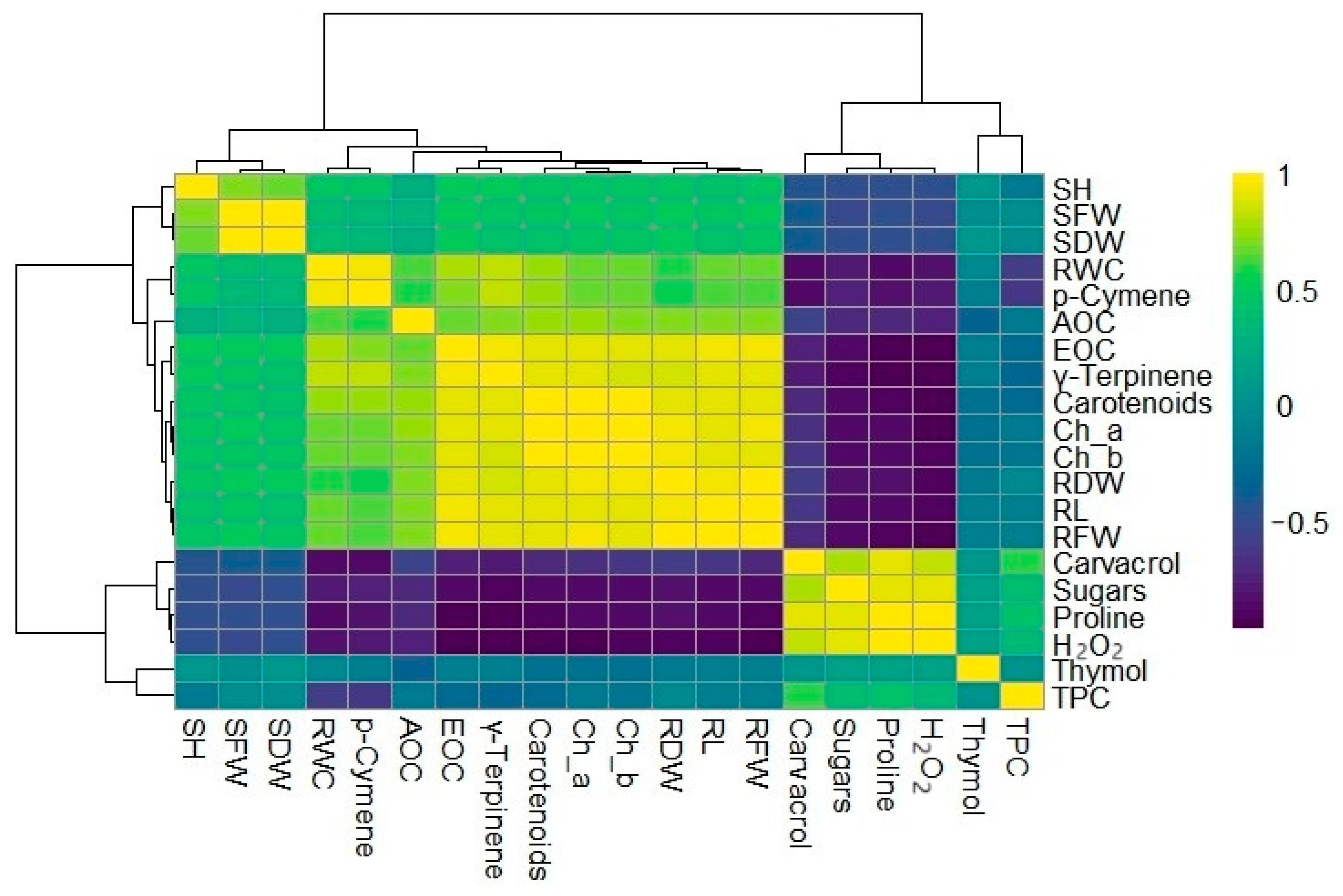
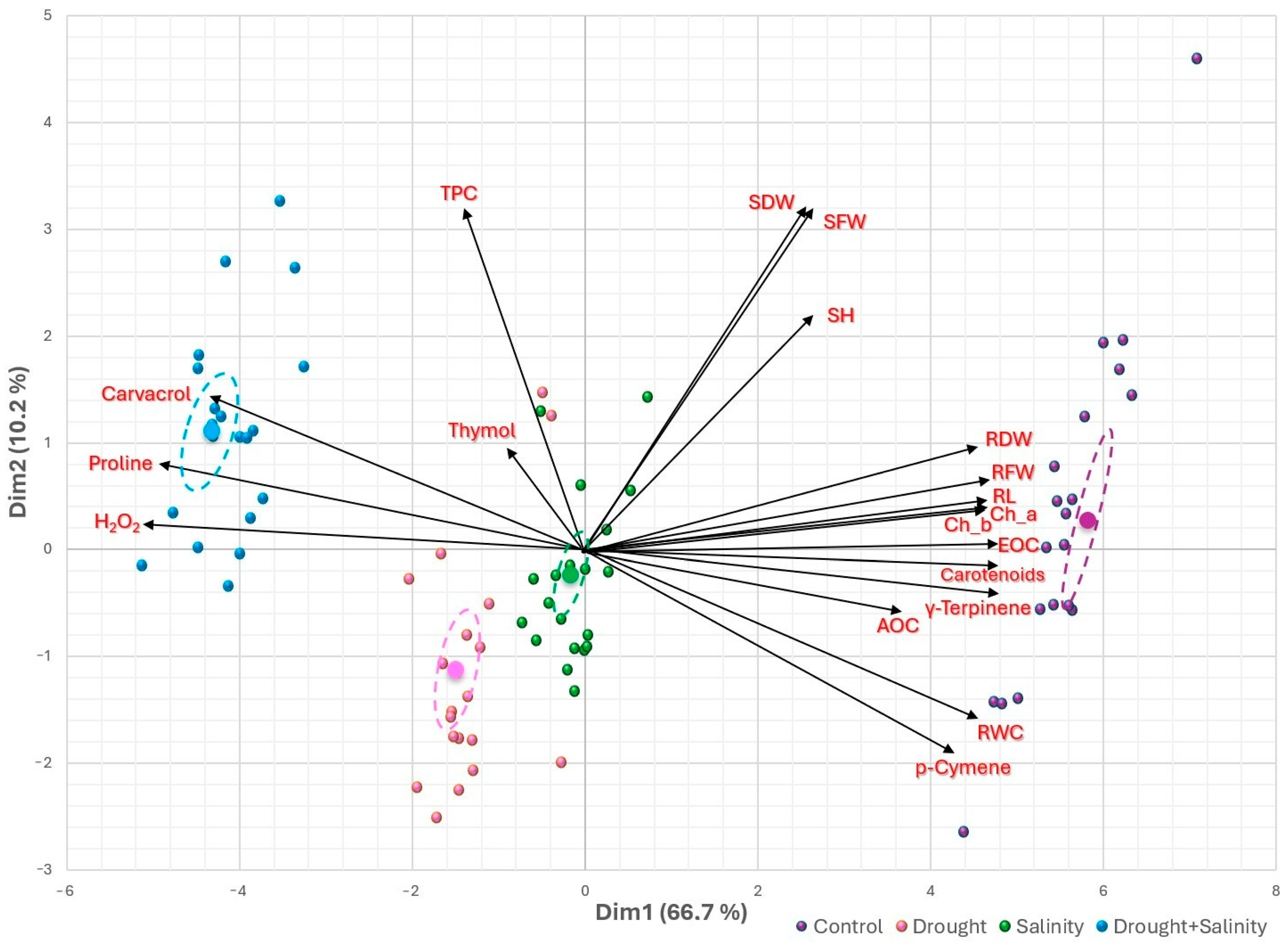
3.5. Heatmap Clusters and Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bounatirou, S.; Smiti, S.; Miguel, M.G.; Faleiro, L.; Rejeb, M.N.; Neffati, M.; Costa, M.M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Chemical Composition, Antioxidant and Antibacterial Activities of the Essential Oils Isolated from Tunisian Thymus Capitatus Hoff. et Link. Food Chem. 2007, 105, 146–155. [Google Scholar] [CrossRef]
- El Ajjouri, M.; Satrani, B.; Ghanmi, M.; Aafi, A.; Farah, A.; Rahouti, M.; Amarti, F.; Aberchane, M. Activité Antifongique Des Huiles Essentielles de Thymus Bleicherianus Pomel et Thymus capitatus (L.) Hoffm. & Link Contre Les Champignons de Pourriture Du Bois d’œuvre. Biotechnol. Agron. Soc. Environ. 2008, 12, 345–351. [Google Scholar]
- Džamić, A.M.; Nikolić, B.J.; Giweli, A.A.; Mitić-Ćulafić, D.S.; Soković, M.D.; Ristić, M.S.; Knežević-Vukčević, J.B.; Marin, P.D. Libyan Thymus Capitatus Essential Oil: Antioxidant, Antimicrobial, Cytotoxic and Colon Pathogen Adhesion-inhibition Properties. J. Appl. Microbiol. 2015, 119, 389–399. [Google Scholar] [CrossRef]
- Skoula, M.; Grayer, R.J.; Kite, G.C.; Skoula, M.; Grayer, R.J.; Kite, G.C. Surface Flavonoids in Coridothymus Capitatus and Thymbra calostachya (Lamiaceae). Biochem. Syst. Ecol. 2004, 32, 1197–1200. [Google Scholar] [CrossRef]
- Morales, R. The History Botany and Taxonomy of the Genus Thymus. In Thyme–The Genus Thymus; Stahl-Biskup, E., Saez, F., Eds.; Taylor & Francis: London, UK, 2002; pp. 11–43. [Google Scholar]
- Ben El Hadj Ali, I.; Guetat, A.; Boussaid, M. Genetic Diversity of Wild Thymus capitatus (Lamiaceae) in Tunisia Using Molecular Markers. Dendrobiology 2012, 68, 89–100. [Google Scholar]
- Tawaha, K.A.; Hudaib, M.M. Chemical Composition of the Essential Oil from Flowers, Flower Buds and Leaves of Thymus Capitatus Hoffmanns. & Link from Jordan. J. Essent. Oil Bear. Plants 2012, 15, 988–996. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Guaouguaou, F.E.; Benali, T.; Balahbib, A.; El Omari, N.; Taha, D.; El-Shazly, M.; El Menyiy, N. Ethnomedicinal Use, Phytochemistry, Pharmacology, and Food Benefits of Thymus Capitatus. J. Ethnopharmacol. 2020, 259, 112925. [Google Scholar] [CrossRef]
- Tagnaout, I.; Zerkani, H.; Hadi, N.; El Moumen, B.; El Makhoukhi, F.; Bouhrim, M.; Al-Salahi, R.; Nasr, F.A.; Mechchate, H.; Zair, T. Chemical Composition, Antioxidant and Antibacterial Activities of Thymus Broussonetii Boiss and Thymus capitatus (L.) Hoffmann and Link Essential Oils. Plants 2022, 11, 954. [Google Scholar] [CrossRef]
- Etri, K.; Pluhár, Z. Exploring Chemical Variability in the Essential Oils of the Thymus Genus. Plants 2024, 13, 1375. [Google Scholar] [CrossRef]
- Amakran, A.; Hamoudane, M.; Pagniez, F.; Lamarti, A.; Picot, C.; Figueredo, G.; Nhiri, M.; Le Pape, P. Chemical Composition, Antifungal, Antioxidant, and Hemolytic Activities of Moroccan Thymus Capitatus Essential Oil. Chem. Biodivers. 2024, 21, e202300563. [Google Scholar] [CrossRef]
- Zaïri, A.; Nouir, S.; Zarrouk, A.; Haddad, H.; Khélifa, A.; Achour, L.; Tangy, F.; Chaouachi, M.; Trabelsi, M. Chemical Composition, Fatty Acids Profile and Biological Properties of Thymus capitatus (L.) Hoffmanns, Essential Oil. Sci. Rep. 2019, 9, 20134. [Google Scholar] [CrossRef] [PubMed]
- Benoutman, A.; Erbiai, E.H.; Edderdaki, F.Z.; Cherif, E.K.; Saidi, R.; Lamrani, Z.; Pintado, M.; Pinto, E.; Esteves da Silva, J.C.G.; Maouni, A. Phytochemical Composition, Antioxidant and Antifungal Activity of Thymus Capitatus, a Medicinal Plant Collected from Northern Morocco. Antibiotics 2022, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Maniki, E.; Kostoglou, D.; Paterakis, N.; Nikolaou, A.; Kourkoutas, Y.; Papachristoforou, A.; Giaouris, E. Chemical Composition, Antioxidant, and Antibiofilm Properties of Essential Oil from Thymus Capitatus Plants Organically Cultured on the Greek Island of Lemnos. Molecules 2023, 28, 1154. [Google Scholar] [CrossRef]
- Bajes, H.; Bustanji, Y.; Bustanji, Y. Phytochemical Analysis, In Vitro Assessment of Antioxidant Properties and Cytotoxic Potential of Thymus Capitatus Essential Oil. Res. J. Pharm. Technol. 2023, 16, 1100–1108. [Google Scholar] [CrossRef]
- Nehme, R.; Gini, C.; Vanbergue, E.; Even, S.; Biscarini, F.; Andrés, S.; Rault, L.; Noel, F.; Hardit, V.; Bouhallab, S.; et al. The Effects of Thymus Capitatus Essential Oil Topical Application on Milk Quality: A Systems Biology Approach. Sci. Rep. 2024, 15, 4627. [Google Scholar] [CrossRef]
- Hosseini, N.; Ghorbanpour, M.; Mostafavi, H. Habitat Potential Modelling and the Effect of Climate Change on the Current and Future Distribution of Three Thymus Species in Iran Using MaxEnt. Sci. Rep. 2024, 14, 3641. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, P. Morpho-Physiological and Biochemical Response of Plants under Drought Stress. J. Pharmacogn. Phytochem. 2020, 9, 352–357. [Google Scholar]
- Aroca, R. Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin, Heidelberg, 2013; pp. 1–466. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; Akbari, P.; Mumivand, H. Influence of Drought Stress and Foliar Application of Chitosan on Nutrient Accumulation and Phenolic Composition of Thymus Daenensis Celak. Crop Sci. 2023, 63, 921–935. [Google Scholar] [CrossRef]
- Amiri, H.; Dousty, B.; Hosseinzedeh, S.R. Water Stress-Induced Changes of Morphological, Physiological and Essential Oil Compounds in Thymus Eriocalyx from Iran. J. Essent. Oil Bear. Plants 2018, 21, 1210–1223. [Google Scholar] [CrossRef]
- Abd El-Maboud, M.M.; Elshalakany, W. ANTIOXIDANTS RESPONSE TO SEASONAL CHANGES IN THYMUS CAPITATUS (L.). Egypt. J. Desert Res. 2022, 72, 215–229. [Google Scholar] [CrossRef]
- Sara, K.; Hossein, A.; Masoud, S.J.; Hassan, M. Effects of Water Deficit and Chitosan Spraying on Osmotic Adjustment and Soluble Protein of Cultivars Castor Bean (Ricinus communis L.). J. Stress Physiol. Biochem. 2012, 8, 160–169. [Google Scholar]
- Cheeseman, J.M. Hydrogen Peroxide and Plant Stress: A Challenging Relationship. Plant Stress 2007, 1, 4–15. [Google Scholar]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 435515. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of Salinity Stress on the Physiological Characteristics, Phenolic Compounds and Antioxidant Activity of Thymus Vulgaris L. and Thymus Daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Wang, L.; Li, J.; Shi, Z.; Yang, L.; Yu, X. Thymol Deploys Multiple Antioxidative Systems to Suppress ROS Accumulation in Chinese Cabbage Seedlings under Saline Stress. Agronomy 2024, 14, 1059. [Google Scholar] [CrossRef]
- Khalil, S.E. Alleviating Salt Stress in Thymus Capitatus Plant Using Plant Growth-Promoting Bacteria (PGPR). Int. J. Chemtech Res. 2016, 9, 140–155. [Google Scholar]
- Reynolds, S.G. The Gravimetric Method of Soil Moisture Determination Part I A Study of Equipment, and Methodological Problems. J. Hydrol. 1970, 11, 258–273. [Google Scholar] [CrossRef]
- Orsini, F.; Cascone, P.; De Pascale, S.; Barbieri, G.; Corrado, G.; Rao, R.; Maggio, A. Systemin-Dependent Salinity Tolerance in Tomato: Evidence of Specific Convergence of Abiotic and Biotic Stress Responses. Physiol. Plant 2010, 138, 10–21. [Google Scholar] [CrossRef]
- Mackinney, G. ABSORPTION OF LIGHT BY CHLOROPHYLL SOLUTIONS. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Trevelyan, W.E.; Harrison, J.S. Studies on Yeast Metabolism. 1. Fractionation and Microdetermination of Cell Carbohydrates. Biochem. J. 1952, 50, 298. [Google Scholar] [CrossRef] [PubMed]
- Tamás, P.; Hilda, K.S.; Anita, N.; Csaba, H.; Imre, B. (Eds.) Pharmacopoea Hungarica VIII (Ph. Hg. VIII), 8th ed.; Medicina Könyvkiadó Rt.: Budapest, Hungary, 2006; Volume 2, pp. 2359–2360. [Google Scholar]
- European Pharmacopoeia (Ph. Eur.), 11th ed.; EDMQ: Strasbourg, France, 2023.
- Etri, K.; Gosztola, B.; Végvári, G.; Ficzek, G.; Radácsi, P.; Simon, G.; Pluhaŕ, Z. Unravelling the Impact of Drought and Salt Stresses on Thymus Pannonicus: Morpho-Physiological and Biochemical Insights. Plant Stress 2024, 13, 100557. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of Hydrogen Peroxide in Plant Extracts Using Titanium(IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought Resistance in Spring Wheat Cultivars. I. Grain Yield Responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fernandez, G.C. Effective Selection Criteria for Assessing Plant Stress Tolerance. In Adaptation of Food Crops to Temperature and Water Stress: Proceedings of an International Symposium; Kuo, C.G., Ed.; AVRDC: Shanhua, Taiwan, 13–18 August 1992; pp. 257–270. [Google Scholar]
- Xylia, P.; Chrysargyris, A.; Tomou, E.M.; Goumenos, C.; Skaltsa, H.; Tzortzakis, N. Quality Characteristics and Essential Oil Properties of Thymus Capitatus, Mentha Piperita, and Sideritis Cypria Dried under Different Conditions. Plants 2024, 13, 3150. [Google Scholar] [CrossRef]
- Tamma, N.; Benchikha, N.; Messaoudi, M.; Caruso, G.; Bin Emran, T.; Atoki, A.V.; Adeniyi, A.I. Chemical Composition and Biological Properties of Thymus Capitatus Plants from Algerian High Plains: A Comparative and Analytical Study. Open Chem. 2024, 22, 20230192. [Google Scholar] [CrossRef]
- Saoulajan, C.; Boujida, N.; El Mihyaoui, A.; El Baakili, A.; Alshahrani, M.M.; Lee, L.H.; Bouyahya, A. Phytochemistry, Pharmacological Investigations, Industrial Applications, and Encapsulation of Thymbra Capitata L., a Review. Trends Food Sci. Technol. 2022, 129, 463–491. [Google Scholar] [CrossRef]
- Anwar, F.; Mahrye, I.; Khan, R.; Qadir, R.; Saadi, S.; Gruczynska-Sekowska, E.; Saari, N.; Hossain Brishti, F. Exploring the Biochemical and Nutra-Pharmaceutical Prospects of Some Thymus Species–A Review. Chem. Biodivers. 2024, 21, e202400500. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Eom, S.H. Growth and Abscisic Acid Changes of Creeping Thyme in the Exposure of NaCl and Drought. Korean J. Med. Crop Sci. 2009, 17, 328–334. [Google Scholar]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of Drought Stress on Biomass, Essential Oil Content, Nutritional Parameters, and Costs of Production in Six Lamiaceae Species. Water 2019, 11, 573. [Google Scholar] [CrossRef]
- Jangpromma, N.; Thammasirirak, S.; Jaisil, P.; Songsri, P. Effects of Drought and Recovery from Drought Stress on above Ground and Root Growth, and Water Use Efficiency in Sugarcane (“Saccharum officinarum” L.). Aust. J. Crop Sci. 2012, 6, 1298–1304. [Google Scholar]
- Abd Elbar, O.H.; Farag, R.E.; Shehata, S.A. Effect of Putrescine Application on Some Growth, Biochemical and Anatomical Characteristics of Thymus vulgaris L. under Drought Stress. Ann. Agric. Sci. 2019, 64, 129–137. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of Plant Response to Salt and Drought Stress and Their Alteration by Rhizobacteria. Plant Soil 2016, 410, 335–356. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Sustain. Agric. 2009, 29, 153–188. [Google Scholar] [CrossRef]
- Litvin, A.G.; Van Iersel, M.W.; Malladi, A. Drought Stress Reduces Stem Elongation and Alters Gibberellin-Related Gene Expression during Vegetative Growth of Tomato. J. Am. Soc. Hortic. Sci. 2016, 141, 591–597. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 152207. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Shahbaz, M.; Ali, Q. Drought-Induced Modulation in Growth and Mineral Nutrients in Canola (Brassica napus L.). Pak. J. Bot. 2013, 45, 93–98. [Google Scholar]
- Nazari, M.; Ghasemi-Soloklui, A.A.; Kordrostami, M.; Abdel Latef, A.A.H. Deciphering the Response of Medicinal Plants to Abiotic Stressors: A Focus on Drought and Salinity. Plant Stress 2023, 10, 100255. [Google Scholar] [CrossRef]
- Johal, N.; Goyal, P. Drought and Salinity Stress: An Overlapping Osmotic Resistance. In Salinity and Drought Tolerance in Plants Physiological Perspective; Springer: Singapore, 2023; pp. 87–96. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and Its Actions during the Drought Stress in Plants. Physiol. Plant 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?—A Systematic Review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef]
- Yang, T.; Lu, X.; Wang, Y.; Xie, Y.; Ma, J.; Cheng, X.; Xia, E.; Wan, X.; Zhang, Z. HAK/KUP/KT Family Potassium Transporter Genes Are Involved in Potassium Deficiency and Stress Responses in Tea Plants (Camellia sinensis L.): Expression and Functional Analysis. BMC Genom. 2020, 21, 556. [Google Scholar] [CrossRef]
- Liang, L.; Guo, L.; Zhai, Y.; Hou, Z.; Wu, W.; Zhang, X.; Wu, Y.; Liu, X.; Guo, S.; Gao, G.; et al. Genome-Wide Characterization of SOS1 Gene Family in Potato (Solanum tuberosum) and Expression Analyses under Salt and Hormone Stress. Front. Plant Sci. 2023, 14, 1201730. [Google Scholar] [CrossRef]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and Salt-Stress Signaling in Plants: Shedding Light on SOS Pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Esmail, S.E.A.; El-Attar, A.B.; Othman, E.Z.; El-Bahbohy, R.M. Prospective Practice for Compound Stress Tolerance in Thyme Plants Using Nanoparticles and Biochar for Photosynthesis and Biochemical Ingredient Stability. Agronomy 2022, 12, 1069. [Google Scholar] [CrossRef]
- Ashrafi, M.; Azimi-Moqadam, M.R.; Mohsenifard, E.; Shekari, F.; Jafary, H.; Moradi, P.; Pucci, M.; Abate, G.; Mastinu, A. Physiological and Molecular Aspects of Two Thymus Species Differently Sensitive to Drought Stress. BioTech. 2022, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Ghorbanpour, M.; Brestic, M. Exogenous Putrescine Changes Redox Regulations and Essential Oil Constituents in Field-Grown Thymus vulgaris L. under Well-Watered and Drought Stress Conditions. Ind. Crops Prod. 2018, 122, 119–132. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Pirzad, A. Biochemical Responses of Mycorrhizal-Inoculated Lamiaceae (Lavender, Rosemary and Thyme) Plants to Drought: A Field Study. Soil. Sci. Plant Nutr. 2021, 67, 41–49. [Google Scholar] [CrossRef]
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and Physiological Plant Responses to Drought Stress in Thymus Citriodorus. Int. J. Agron. 2016, 2016, 4165750. [Google Scholar] [CrossRef]
- Killi, D.; Raschi, A.; Bussotti, F. Lipid Peroxidation and Chlorophyll Fluorescence of Photosystem II Performance during Drought and Heat Stress Is Associated with the Antioxidant Capacities of C3 Sunflower and C4 Maize Varieties. Int. J. Mol. Sci. 2020, 21, 4846. [Google Scholar] [CrossRef]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I.; Mano, J. Lipid Peroxidation-Derived Reactive Carbonyl Species (RCS): Their Interaction with ROS and Cellular Redox during Environmental Stresses. Environ. Exp. Bot. 2019, 165, 139–149. [Google Scholar] [CrossRef]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; Ahmad, S. Oxidative Stress and Antioxidant Defense in Plants Under Drought Conditions. In Plant Abiotic Stress Tolerance Agronomic, Molecular and Biotechnological Approaches; Springer: Cham, Switzerland, 2019; pp. 207–219. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of Proline Function in Higher Plants under Extreme Temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef]
- Atteya, A.K.G.; El-Serafy, R.S.; El-Zabalawy, K.M.; Elhakem, A.; Genaidy, E.A.E. Exogenously Supplemented Proline and Phenylalanine Improve Growth, Productivity, and Oil Composition of Salted Moringa by Up-Regulating Osmoprotectants and Stimulating Antioxidant Machinery. Plants 2022, 11, 1553. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. ROS-Activated Ion Channels in Plants: Biophysical Characteristics, Physiological Functions and Molecular Nature. Int. J. Mol. Sci. 2018, 19, 1263. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; Lin, X.; Sun, C. Hydrogen Peroxide Alleviates Salinity-Induced Damage through Enhancing Proline Accumulation in Wheat Seedlings. Plant Cell Rep. 2020, 39, 567–575. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Soluble Sugars in Reactive Oxygen Species Balance and Responses to Oxidative Stress in Plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, Y.K. Proline as a Key Player in Heat Stress Tolerance: Insights from Maize. Discov. Agric. 2024, 2, 121. [Google Scholar] [CrossRef]
- Li, W.; Meng, R.; Liu, Y.; Chen, S.; Jiang, J.; Wang, L.; Zhao, S.; Wang, Z.; Fang, W.; Chen, F.; et al. Heterografted Chrysanthemums Enhance Salt Stress Tolerance by Integrating Reactive Oxygen Species, Soluble Sugar, and Proline. Hortic. Res. 2022, 9, uhac073. [Google Scholar] [CrossRef]
- Shafi, A.; Zahoor, I.; Mushtaq, U. Proline Accumulation and Oxidative Stress: Diverse Roles and Mechanism of Tolerance and Adaptation Under Salinity Stress. Salt Stress Microbes Plant Interact. Mech. Mol. Approaches 2019, 2, 269–300. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Salama, K.H.A. Proline and Abiotic Stresses: Responses and Adaptation. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives II: Mechanisms of Adaptation and Stress Amelioration; Springer: Singapore, 2020; pp. 357–397. [Google Scholar] [CrossRef]
- Suprasanna, P.; Nikalje, G.C.; Rai, A.N. Osmolyte Accumulation and Implications in Plant Abiotic Stress Tolerance. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: New Delhi, India, 2016; pp. 1–12. [Google Scholar] [CrossRef]
- Wu, S.; Tian, J.; Ren, T.; Wang, Y. Osmotic Adjustment and Antioxidant System Regulated by Nitrogen Deposition Improve Photosynthetic and Growth Performance and Alleviate Oxidative Damage in Dwarf Bamboo Under Drought Stress. Front. Plant Sci. 2022, 13, 819071. [Google Scholar] [CrossRef]
- Wang, Q.; Du, B.; Bai, Y.; Chen, Y.; Li, F.; Du, J.; Wu, X.; Yan, L.; Bai, Y.; Chai, G. Saline-Alkali Stress Affects the Accumulation of Proanthocyanidins and Sesquiterpenoids via the MYB5-ANR/TPS31 Cascades in the Rose Petals. Hortic. Res. 2024, 11, uhae243. [Google Scholar] [CrossRef]
- Alavi-Samani, S.M.; Ghasemi Pirbalouti, A.; Ataei Kachouei, M.; Hamedi, B. The Influence of Reduced Irrigation on Herbage, Essential Oil Yield and Quality of Thymus Vulgaris and Thymus Daenensis. J. Med. Herbs 2013, 4, 109–113. [Google Scholar]
- Emami Bistgani, Z.; Siadat, S.A.; Bakhshandeh, A.; Ghasemi Pirbalouti, A.; Hashemi, M. Interactive Effects of Drought Stress and Chitosan Application on Physiological Characteristics and Essential Oil Yield of Thymus Daenensis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Tammar, S.; Salem, N.; Bettaieb Rebey, I.; Sriti, J.; Hammami, M.; Khammassi, S.; Marzouk, B.; Ksouri, R.; Msaada, K. Regional Effect on Essential Oil Composition and Antimicrobial Activity of Thymus Capitatus L. J. Essent. Oil Res. 2019, 31, 129–137. [Google Scholar] [CrossRef]
- Németh-Zámbori, É.; Szabó, K.; Pluhár, Z.; Radácsi, P.; Inotai, K. Changes in Biomass and Essential Oil Profile of Four Lamiaceae Species Due to Different Soil Water Levels. J. Essent. Oil Res. 2016, 28, 391–399. [Google Scholar] [CrossRef]
- Mkaddem, M.G.; Romdhane, M.; Ibrahim, H.; Ennajar, M.; Lebrihi, A.; Mathieu, F.; Bouajila, J. Essential Oil of Thymus Capitatus Hoff. et Link. from Matmata, Tunisia: Gas Chromatography-Mass Spectrometry Analysis and Antimicrobial and Antioxidant Activities. J. Med. Food 2010, 13, 1500–1504. [Google Scholar] [CrossRef]
- Laftouhi, A.; Eloutassi, N.; Ech-Chihbi, E.; Rais, Z.; Abdellaoui, A.; Taleb, A.; Beniken, M.; Nafidi, H.A.; Salamatullah, A.M.; Bourhia, M.; et al. The Impact of Environmental Stress on the Secondary Metabolites and the Chemical Compositions of the Essential Oils from Some Medicinal Plants Used as Food Supplements. Sustainability 2023, 15, 7842. [Google Scholar] [CrossRef]
- Razavizadeh, R.; Mohagheghiyan, N. An Investigation of Changes in Antioxidant Enzymes Activities and Secondary Metabolites of Thyme (Thymus Vulgaris) Seedlings under in Vitro Salt Stress. J. Plant Biol. Sci. 2015, 7, 41–58. [Google Scholar]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules: Role and Regulation Under Stressful Environments; Woodhead Publishing: Daryaganj, New Delhi, 2019; pp. 157–168. [Google Scholar] [CrossRef]
- Khosh-Khui, M.; Ashiri, F.; Saharkhiz, M. Effects of Irrigation Regimes on Antioxidant Activity and Total Phenolic Content of Thyme (Thymus vulgaris L.). Med. Aromat Plants 2012, 1, 1–4. [Google Scholar] [CrossRef]
- Kulbat, K.; Leszczyńska, J. Antioxidants as a Defensive Shield in Thyme (Thymus vulgaris L.) Grown on the Soil Contaminated with Heavy Metals. Biotechnol. Food Sci. 2016, 80, 109–117. [Google Scholar] [CrossRef]
- Thakur, K.; Garg, N. Oxidative Stress and Antioxidant Enzymes in Cereals Under Abiotic Stress. In Sustainable Remedies for Abiotic Stress in Cereals; Springer: Singapore, 2022; pp. 51–82. [Google Scholar] [CrossRef]



| Component | Origin | pH | N (m/m%) | P2O5 (m/m%) | K2O (m/m%) | Dry Matter Content (m/m%) | Organic Matter Content (m/m%) |
|---|---|---|---|---|---|---|---|
| Peat moss | Kekkilä Professional, Estonia | 5.90 ± 0.50 | 0.30 | 0.10 | 0.10 | 70.00 | |
| Potting soil | AGRO CS, Slovakia | 5.50 ± 0.50 | 0.10 | 0.01 | 0.03 | 30.00 | 75.00 |
| pH | Electric Conductivity (EC) | Sodium Ions (Na+) | Chloride Ions (Cl−) | Sodium Chloride (NaCl) |
|---|---|---|---|---|
| 7.33 | 494 µS/cm | 20.31 mg/L | 40.23 mg/L | 60.54 mg/L~1.037 mM |
| Treatments | Shoots Height (cm) | Number of Shoots (Pcs/Plant) | Weight of Fresh Shoots (g/Plant) | Weight of Dry Shoots (g/Plant) |
|---|---|---|---|---|
| Control | 27.50 ± 5.52 a | 29.25 ± 2.49 a | 12.69 ± 6.09 a | 2.61 ± 1.16 a |
| Drought Stress | 22.25 ± 4.17 bc | 17.00 ± 3.85 bc | 7.94 ± 3.31 b | 1.52 ± 0.67 b |
| Salinity Stress | 25.50 ± 3.71 ab | 19.50 ± 4.37 b | 9.18 ± 3.05 b | 2.09 ± 0.68 ab |
| Combined Stresses | 20.70 ± 3.84 c | 14.25 ± 3.53 c | 6.53 ± 2.86 b | 1.44 ± 0.52 b |
| Treatments | Length of the Roots (cm) | Weight of Fresh Roots (g) | Weight of Dry Roots (g) |
|---|---|---|---|
| Control | 39.53 ± 1.25 a | 4.36 ± 0.45 a | 0.74 ± 0.10 a |
| Drought Stress | 23.98 ± 1.84 b | 1.65 ± 0.40 b | 0.23 ± 0.05 b |
| Salinity Stress | 26.75 ± 4.34 b | 2.03 ± 0.75 b | 0.29 ± 0.10 b |
| Combined Stresses | 23.00 ± 2.94 b | 1.33 ± 0.25 b | 0.18 ± 0.08 b |
| Essential Oil Compounds | RT | LRI | Relative Percentages of Compounds (%) | |||
|---|---|---|---|---|---|---|
| Control | Drought Stress | Salt Stress | Combined Stresses | |||
| α-Pinene | 5.56 | 938 | 0.49 ± 0.05 a | 0.29 ± 0.15 b | 0.26 ± 0.07 bc | 0.11 ± 0.08 c |
| ß-Myrcene | 6.99 | 995 | 1.52 ± 0.02 a | 1.20 ± 0.01 a | 1.20 ± 0.11 a | 0.77 ± 0.23 b |
| α-Terpinene | 7.79 | 1018 | 1.65 ± 0.07 a | 1.24 ± 0.02 bc | 1.34 ± 0.10 ab | 0.90 ± 0.25 c |
| p-Cymene | 8.09 | 1026 | 5.25 ± 0.11 a | 4.94 ± 0.15 a | 4.93 ± 0.11 a | 3.89 ± 0.64 b |
| γ-Terpinene | 9.20 | 1056 | 5.86 ± 0.35 a | 4.22 ± 0.04 bc | 4.61 ± 0.11 b | 3.34 ± 0.69 c |
| Linalool | 10.76 | 1097 | 1.07 ± 0.04 a | 1.62 ± 0.02 b | 1.70 ± 0.03 b | 1.50 ± 0.25 b |
| Borneol | 13.43 | 1162 | 0.38 ± 0.02 a | 0.55 ± 0.03 c | 0.61 ± 0.01 c | 0.48 ± 0.01 b |
| Thymol | 18.81 | 1290 | 0.16 ± 0.02 | 0.16 ± 0.02 | 0.19 ± 0.01 | 0.18 ± 0.01 |
| Carvacrol | 19.20 | 1300 | 78.86 ± 0.65 a | 80.73 ± 0.58 ab | 79.59 ± 0.48 b | 82.58 ± 1.43 b |
| ß-Caryophyllene | 23.68 | 1420 | 2.10 ± 0.04 a | 2.56 ± 0.12 ab | 2.43 ± 0.33 a | 3.10 ± 0.32 b |
| Total identified (%) | 97.33 | 97.51 | 96.85 | 96.85 | ||
| Treatments | SSI | STI |
|---|---|---|
| Drought Stress | 0.987 ± 0.689 ab | 0.626 ± 0.261 ab |
| Salinity Stress | 0.730 ± 0.636 a | 0.723 ± 0.241 a |
| Combined Stresses | 1.282 ± 0.597 b | 0.514 ± 0.226 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etri, K.; Gosztola, B.; Pluhár, Z. Surviving the Extremes: The Synergistic Impact of Drought and Salinity on Thymus capitatus Growth, Physiology, and Biochemistry. Agronomy 2025, 15, 1449. https://doi.org/10.3390/agronomy15061449
Etri K, Gosztola B, Pluhár Z. Surviving the Extremes: The Synergistic Impact of Drought and Salinity on Thymus capitatus Growth, Physiology, and Biochemistry. Agronomy. 2025; 15(6):1449. https://doi.org/10.3390/agronomy15061449
Chicago/Turabian StyleEtri, Karim, Beáta Gosztola, and Zsuzsanna Pluhár. 2025. "Surviving the Extremes: The Synergistic Impact of Drought and Salinity on Thymus capitatus Growth, Physiology, and Biochemistry" Agronomy 15, no. 6: 1449. https://doi.org/10.3390/agronomy15061449
APA StyleEtri, K., Gosztola, B., & Pluhár, Z. (2025). Surviving the Extremes: The Synergistic Impact of Drought and Salinity on Thymus capitatus Growth, Physiology, and Biochemistry. Agronomy, 15(6), 1449. https://doi.org/10.3390/agronomy15061449









