Evaluation of Hybrid Sorghum Parents for Morphological, Physiological and Agronomic Traits Under Post-Flowering Drought
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Materials
2.3. Experimental Set-Up
2.4. Experimental Design and Imposition of Post-Flowering Water Stress
2.5. Data Collection
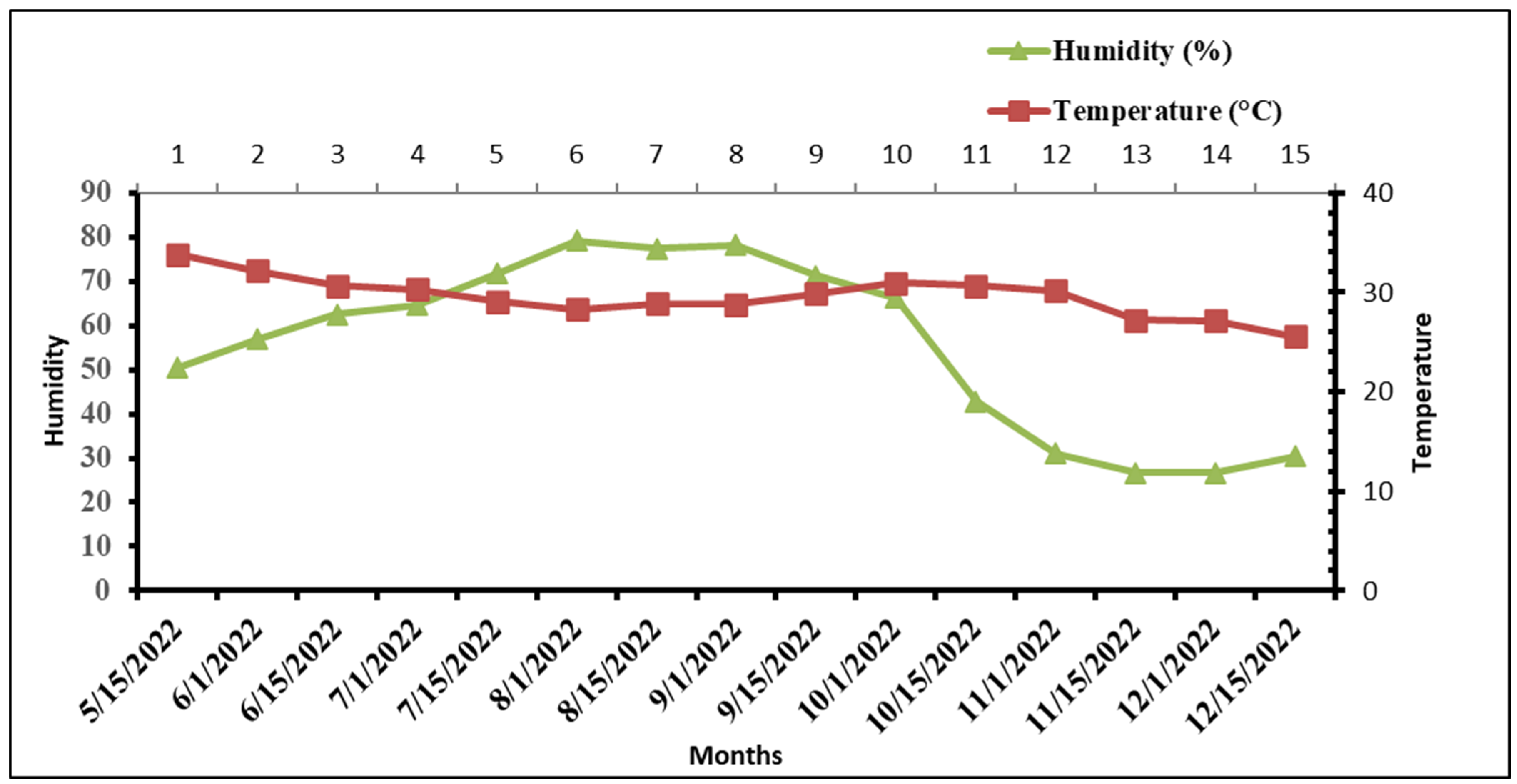
2.5.1. Climatic Data
2.5.2. Phenological Traits
2.5.3. Morphological and Physiological Parameters
2.5.4. Yield Components, Harvest Index, and Stay-Green Trait
2.5.5. Drought Tolerance Index
2.6. Statistical Analyses
3. Results
3.1. Analysis of Variance
3.2. Relationship Between Morphological, Physiological, and Agronomic Traits Under Water Stress (WS)
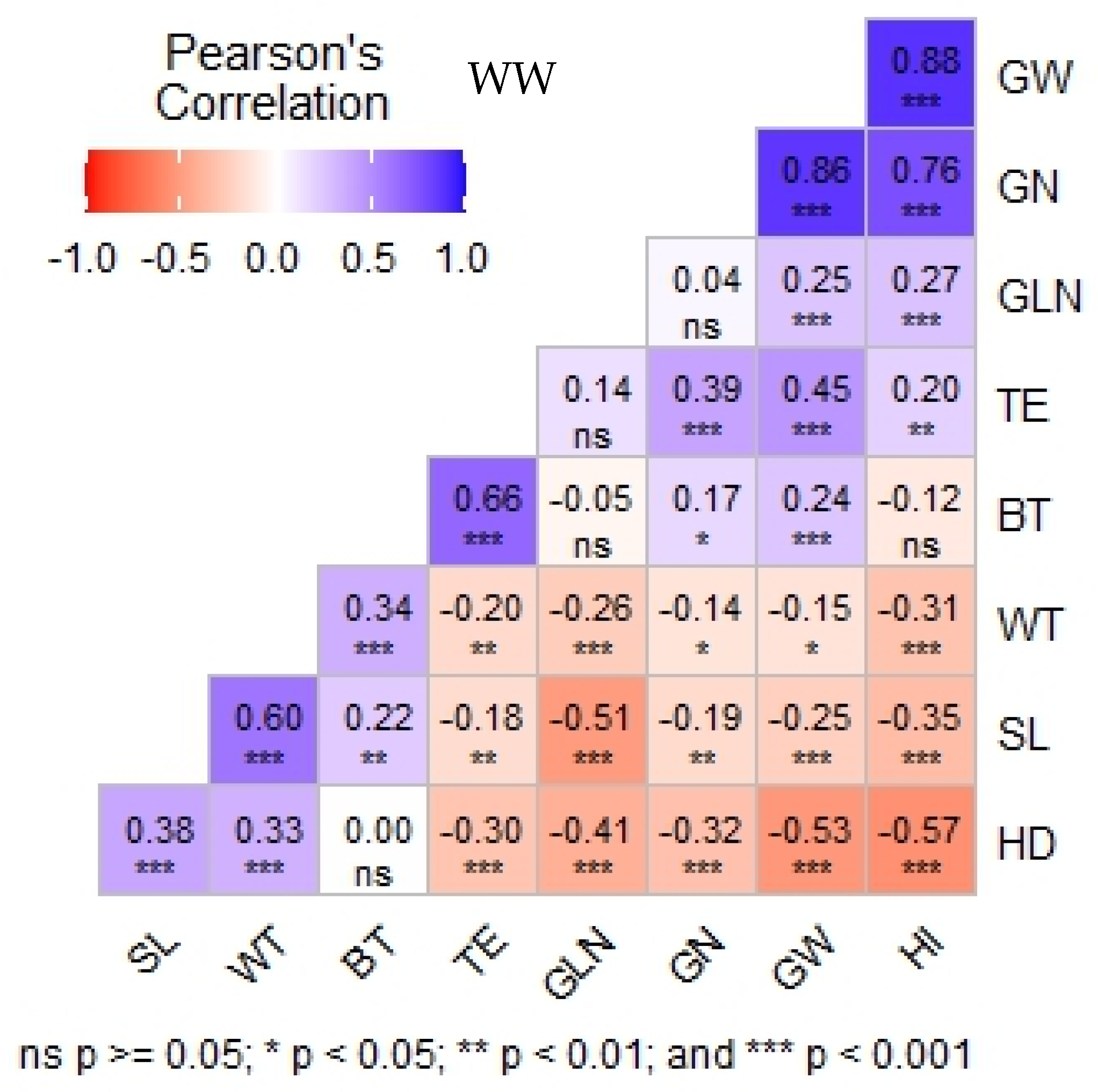
3.3. Relationship Between Morphological, Physiological, and Agronomic Traits Under Well-Watered Condition (WW)
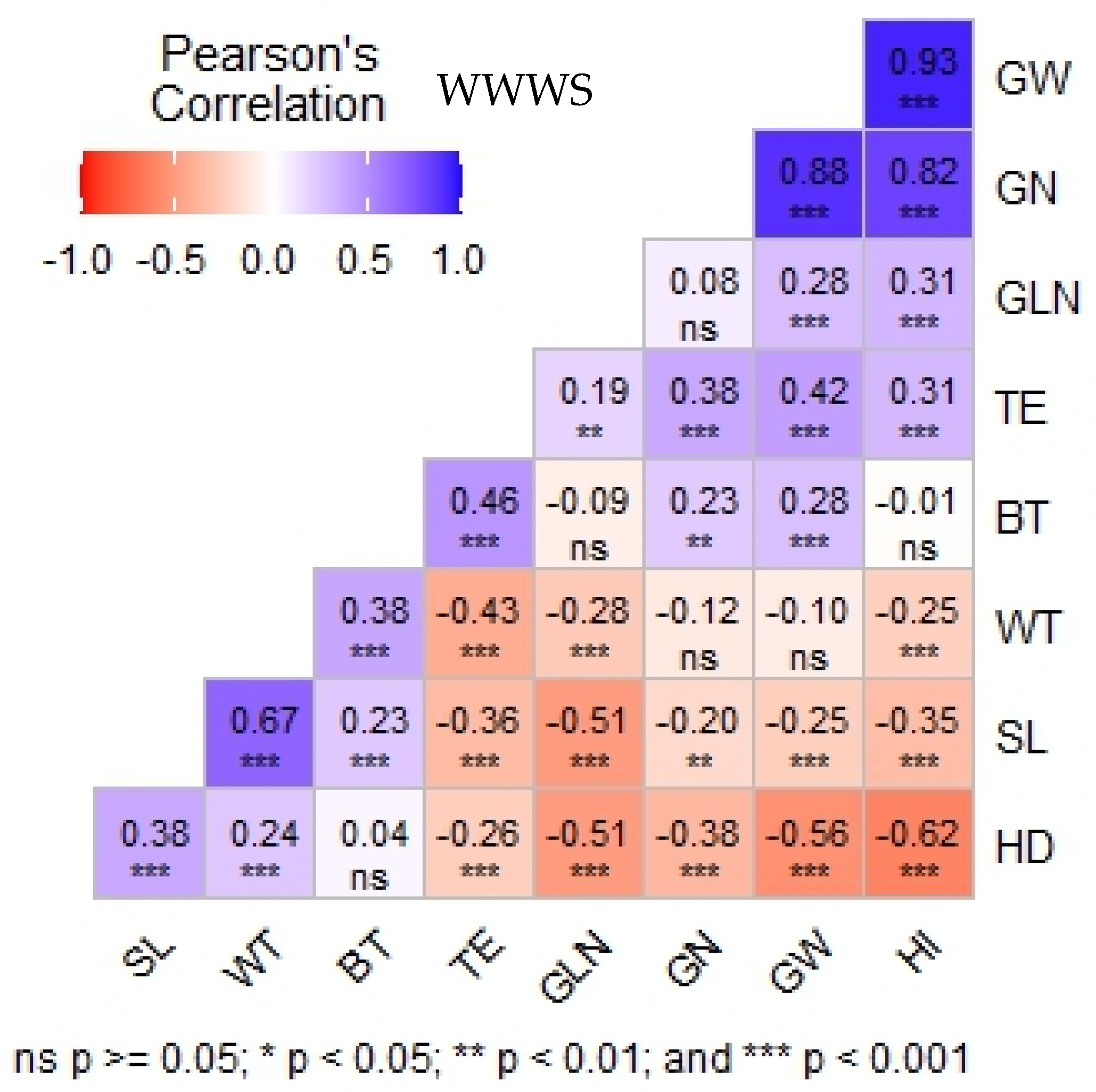
3.4. Relationship Between Traits Under Well-Watered and Water-Stressed Treatment
3.5. Grain Yield and Drought Tolerance Indices in Sorghum Hybrid Parents
3.6. Green Leaf Number (GLN) and Transpiration Efficiency in Sorghum Hybrid Parents
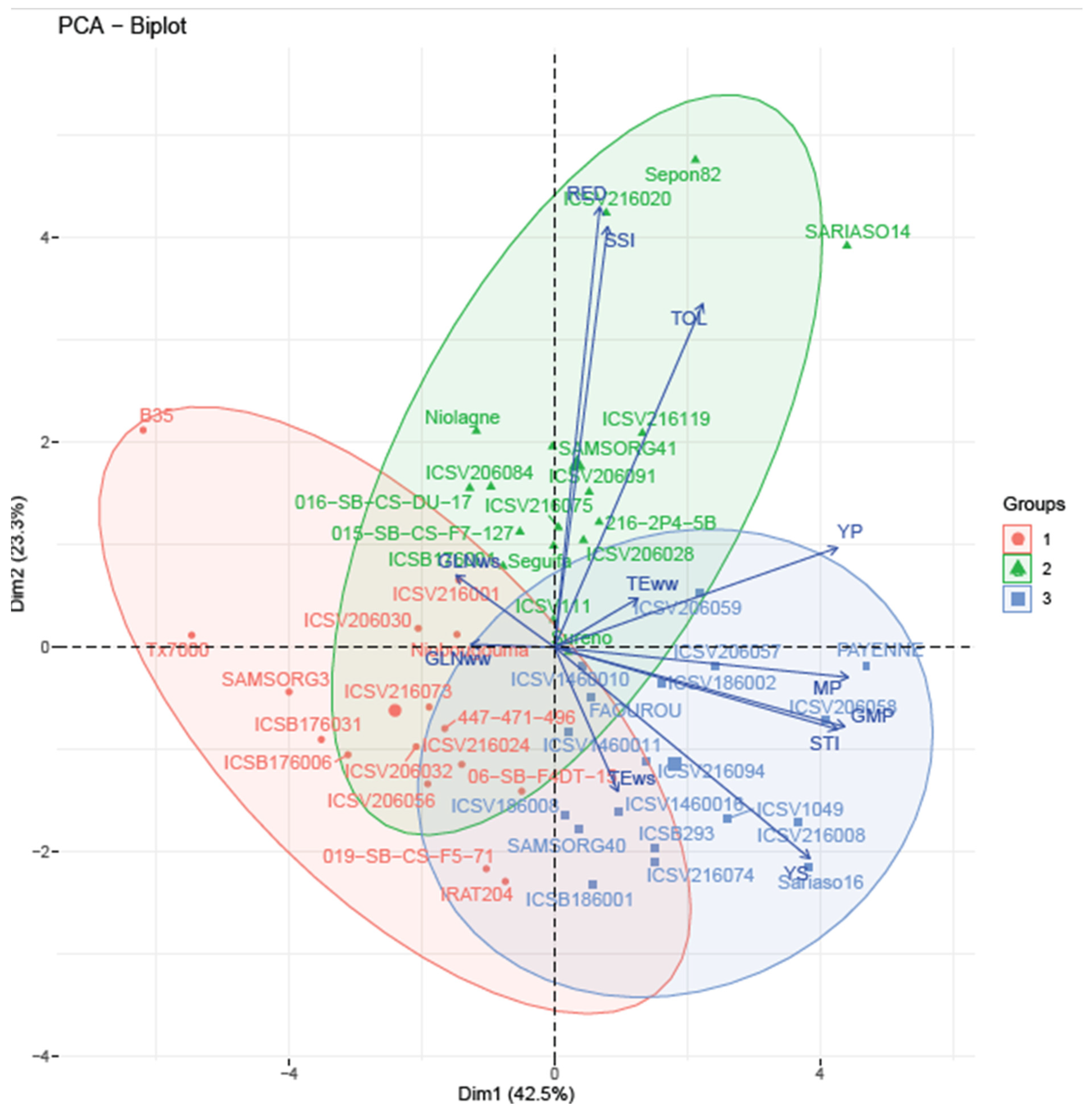
3.7. PCA-Based Clustering of Drought-Tolerant Sorghum Genotypes
4. Discussion
4.1. Correlation Analysis
4.2. Selection of Drought-Tolerant Genotypes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sissoko, S.; Diallo, A.G.; Coulibaly, M.M.; Teme, N.; Vernon, G.; Danquah, E. Genetic Analysis of VariousTraits of Hybrids Sorghum (Sorghum bicolor (L) Moench), Correlated with Drought Tolerance. J. Plant Biol. Soil Health 2017, 4, 9. [Google Scholar] [CrossRef]
- Rosenow, D.T. Breeding for pre- and post-flowering drought stress resistance in sorghum. In Proceedings of the International Conference on Genetic Improvement of Sorghum and Pearl Millet, Lubbock, TX, USA, 22–27 September 1996; pp. 400–424. [Google Scholar]
- Hammer, G.L.; Kropff, M.J.; Sinclair, T.R.; Porter, J.R. Future contributions of crop modelling—From heuristics and supporting decision making to understanding genetic regulation and aiding crop improvement. Eur. J. Agron. 2002, 18, 15–31. [Google Scholar] [CrossRef]
- Krupa, K.N.; Dalawai, N.; Shashidhar, H.E.; Harinikumar, K.M.; Manojkumar, H.B.; Bharani, S.; Turaidar, V. Mechanisms of Drought Tolerance in Sorghum: A Review. Int. J. Pure Appl. Biosci. 2017, 5, 221–237. [Google Scholar] [CrossRef]
- Vadez, V.; Krishnamurthy, L.; Hash, C.T.; Upadhyaya, H.D.; Borrell, A.K. Yield, transpiration efficiency, and water-use variations and their interrelationships in the sorghum reference collection. Crop Pasture Sci. 2011, 62, 645–655. [Google Scholar] [CrossRef]
- Assefa, Y.; Staggenborg, S.A.; Prasad, V.P. Grain Sorghum Water Requirement and Responses to Drought Stress: A Review. Crop Manag. 2010, 9, 1–11. [Google Scholar] [CrossRef]
- Das, I.K.; Rakshit, S. Millets, Their Importance, and Production Constraints; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Dwivedi, S.L.; Vetriventhan, M.; Krishnamurthy, L.; Singh, S.K. Post-flowering drought tolerance using managed stress trials, adjustment to flowering, and mini core collection in sorghum. Crop Sci. 2017, 57, 310–321. [Google Scholar] [CrossRef]
- de Souza, A.A.; de Carvalho, A.J.; Bastos, E.A.; Portugal, A.F.; Torres, L.G.; Batista, P.S.C.; Julio, M.P.M.; Julio, B.H.M.; de Menezes, C.B. Grain Sorghum Grown Under Drought Stress at Pre- and Post-flowering in Semiarid Environment. J. Agric. Sci. 2020, 12, 97. [Google Scholar] [CrossRef]
- Mwamahonje, A.; Eleblu, J.S.Y.; Ofori, K.; Feyissa, T.; Deshpande, S.; Tongoona, P. Evaluation of traits performance contributing to drought tolerance in sorghum. Agronomy 2021, 11, 1698. [Google Scholar] [CrossRef]
- Kholová, J.; Murugesan, T.; Kaliamoorthy, S.; Malayee, S.; Baddam, R.; Hammer, G.L.; McLean, G.; Deshpande, S.; Hash, C.T.; Craufurd, P.Q.; et al. Modelling the effect of plant water use traits on yield and stay-green expression in sorghum. Funct. Plant Biol. 2014, 41, 1019–1034. [Google Scholar] [CrossRef]
- Xin, Z.; Aiken, R.; Burke, J. Genetic diversity of transpiration efficiency in sorghum. Field Crops Res. 2009, 111, 74–80. [Google Scholar] [CrossRef]
- Vadez, V.; Kholova, J.; Yadav, R.S.; Hash, C.T. Small temporal differences in water uptake among varieties of pearl millet (Pennisetum glaucum (L.) R. Br.) are critical for grain yield under terminal drought. Plant Soil 2013, 371, 447–462. [Google Scholar] [CrossRef]
- Abdou, H.; Falalou, H.; Oumarou, H.; Achirou, B.F.; Yacoubou, B. Lysimetre Et Performance Agronomique Au Champ Physiological response of cowpea [Vigna unguiculata (L.) Walp.] genotypes to water deficit under lysimeter conditions and agronomical performance in field conditions. IOSR J. Agric. Vet. Sci. 2019, 12, 22–33. [Google Scholar]
- Falalou, H.; Sani, M.A.; Hassane, Y.B.; Falke, B.A.; Upadhyaya, D.H. Abiotic Stresses Tolerance and Nutrients Contents in Groundnut, Pearl Millet and Sorghum Mini Core Germplasm for Food and Nutrition Security. Indian J. Plant Genet. Resour. 2017, 30, 201. [Google Scholar] [CrossRef]
- Vadez, V.; Ratnakumar, P. High transpiration efficiency increases pod yield under intermittent drought in dry and hot atmospheric conditions but less so under wetter and cooler conditions in groundnut (Arachis hypogaea (L.)). Field Crops Res. 2016, 193, 16–23. [Google Scholar] [CrossRef]
- Ratnakumar, P.; Vadez, V.; Nigam, S.N.; Krishnamurthy, L. Assessment of transpiration efficiency in peanut (Arachis hypogaea L.) under drought using a lysimetric system. Plant Biol. 2009, 11 (Suppl. S1), 124–130. [Google Scholar] [CrossRef]
- Affortit, P.; Effa-Effa, B.; Ndoye, M.S.; Moukouanga, D.; Luchaire, N.; Cabrera-Bosquet, L.; Perálvarez, M.; Pilloni, R.; Welcker, C.; Champion, A.; et al. Physiological and genetic control of transpiration efficiency in African rice, Oryza glaberrima Steud. J. Exp. Bot. 2022, 73, 5279–5293. [Google Scholar] [CrossRef]
- Belko, N.; Zaman-Allah, M.; Cisse, N.; Diop, N.N.; Zombre, G.; Ehlers, J.D.; Vadez, V. Lower soil moisture threshold for transpiration decline under water deficit correlates with lower canopy conductance and higher transpiration efficiency in drought-tolerant cowpea. Funct. Plant Biol. 2012, 39, 306–322. [Google Scholar] [CrossRef]
- Grégoire, L.; Kholova, J.; Srivastava, R.; Hash, C.T.; Vigouroux, Y.; Vadez, V. Transpiration efficiency variations in the pearl millet reference collection PMiGAP. PLoS ONE 2024, 19, e0299686. [Google Scholar] [CrossRef]
- Xin, Z.; Franks, C.; Payton, P.; Burke, J.J. A simple method to determine transpiration efficiency in sorghum. Field Crops Res. 2008, 107, 180–183. [Google Scholar] [CrossRef]
- Rajcan, I.; Tollenaar, M. Source: Sink ratio and leaf senescence in maize: I. Dry matter accumulation and partitioning during grain filling. Field Crops Res. 1999, 60, 245–253. [Google Scholar] [CrossRef]
- Borrell, A.K.; Tao, Y.; Mcintyre, C.L. Physiological Basis, QTL, and MAS of the Stay-Green Drought Resistance Trait in Grain sorghum; CIMMYT: El Batan, Mexico, 1999; Volume 240, pp. 142–146. [Google Scholar]
- Maduraimuthu, D.; Alagarswamy, S.; Prabhakaran, J.; Karuppasami, K.M.; Venugopal, P.B.; Koothan, V.; Natarajan, S.; Dhashnamurthi, V.; Veerasamy, R.; Rathinavelu, S.; et al. Drought Tolerance of Mungbean Is Improved by Foliar Spray of Nanoceria. Agronomy 2023, 13, 201. [Google Scholar] [CrossRef]
- Renard, C.; Boudouresque, E.; Bationd, G.S.A. Dans Une Zone Protegée Du Sahel (Sadoré, Niger). 1989. Available online: https://www.researchgate.net/publication/32975711 (accessed on 17 April 2025).
- Saidouet, A.K.; Ichaou, A. Gestion durable des sols au Niger: Contraintes, défis, opportunités et priorités. Nat. Faune 2016, 30, 31–34. [Google Scholar]
- Golabadi, M.; Arzani, A.; Maibody, S.A.M.M. Assessment of drought tolerance in segregating populations in durum wheat. Afr. J. Agric. Res. 2006, 1, 162–171. [Google Scholar]
- Subhani, G.M.; Ahmad, J.; Anwar, J.; Hussain, M.; Mahmood, A. Identification of drought tolerant genotypes of barley (Hordeum vulgare L.) through stress tolerance indices. J. Anim. Plant Sci. 2015, 25, 686–692. [Google Scholar]
- Hossain, A.B.; Sears, A.G.; Cox, T.S.; Paulsen, G. Desiccation tolerance and its relationship to assimilate partitioning in winter wheat. Crop Sci. 1990, 30, 622–627. [Google Scholar] [CrossRef]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environment. Crop Sci. 1981, 26, 943–946. [Google Scholar] [CrossRef]
- Choukan, R.; Taherkhani, T.; Ghanadha, M.R.; Khodarahmi, M. Evaluation of drought tolerance in grain maize inbred lines using drought tolerance indices. Iran. J. Agric. Sci 2006, 8, 79–89. [Google Scholar]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fernandez, G.C. Effective selection criteria for assessing stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress Tolerance, Asian Vegetable Research and Development Centre, Shanhua, Taiwan, 13–18 August 1992; pp. 257–270. [Google Scholar]
- Dembele, J.S.B.; Gano, B.; Vaksmann, M.; Kouressy, M.; Dembele, L.L.; Doumbia, M.; Teme, N.; Diouf, D.; Audebert, A. Response of eight sorghum varieties to plant density and nitrogen fertilization in the Sudano-Sahelian zone in Mali. Afr. J. Agric. Res. 2020, 16, 1401–1410. [Google Scholar] [CrossRef]
- Sarkar, B.; Varalaxmi, Y.; Vanaja, M.; RaviKumar, N.; Prabhakar, M.; Yadav, S.K.; Maheswari, M.; Singh, V.K. Mapping of QTLs for morphophysiological and yield traits under water-deficit stress and well-watered conditions in maize. Front. Plant Sci. 2023, 14, 1124619. [Google Scholar] [CrossRef]
- Chiulele, R.M.; Mwangi, G.; Tongoona, P.; Ehlers, J.D.; Ndeve, A.D. Assessment of cowpea genotypes for variability to drought tolerance. In Proceedings of the 10th African Crop Science Conference Proceedings, Maputo, Mozambique, 10–13 October 2011; pp. 531–537. [Google Scholar]
- Amelework, B.A.; Shimelis, H.A.; Tongoona, P.; Mengistu, F.; Laing, M.D.; Ayele, D.G. Sorghum production systems and constraints, and coping strategies under drought-prone agro-ecologies of Ethiopia. S. Afr. J. Plant Soil 2016, 33, 207–217. [Google Scholar] [CrossRef]
- Mofokeng, A.M.; Shimelis, H.A.; Laing, M.D. Agromorphological diversity of South African sorghum genotypes assessed through quantitative and qualitative phenotypic traits. S. Afr. J. Plant Soil 2017, 34, 361–370. [Google Scholar] [CrossRef]
- Sissoko, S. Marker Assisted Selection for Post-Flowering Drought Tolerance in Sorghum bicolor [L.] Moench. Ph.D. Thesis, University of Ghana, Legon, Accra, Ghana, 2015. [Google Scholar]
- Enyew, M.; Carlsson, A.S.; Geleta, M.; Tesfaye, K.; Hammenhag, C.; Seyoum, A.; Feyissa, T. Novel sources of drought tolerance in sorghum landraces revealed via the analyses of genotype-by-environment interactions. Front. Plant Sci. 2022, 13, 1062984. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, W.D. Genetics; Mc Graw-Hill Inc.: New York, NY, USA, 1975; p. 281. [Google Scholar]
- Menezes, C.B.D.; Ticona-Benavente, C.A.; Tardin, F.D.; Cardoso, M.J.; Bastos, E.A.; Nogueira, D.W.; Portugal, A.F.; Santos, C.V.; Schaffert, R.E. Selection indices to identify drought-tolerant grain sorghum cultivars. Genet. Mol. Res. 2014, 13, 9817–9827. [Google Scholar] [CrossRef]
- Ladoui, K.K.; Mefti, M.; Benkherbache, N. Sélection de génotypes d’orge (Hordeum vulgare L.) tolérants au stress hydrique par les indices de tolérance à la sécherèsse. Agrobiologia 2020, 10, 1805–1812. [Google Scholar]
- Teklay, A.; Gurja, B.; Taye, T.; Gemechu, K. Selection efficiency of yield based drought tolerance indices to identify superior sorghum [Sorghum bicolor (L.) Moench] near isogenic lines (NILs) under two-contrasting environments. Afr. J. Agric. Res. 2020, 15, 379–392. [Google Scholar] [CrossRef]

| Trait | WS | WW | G*E | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | GEN | H2 | CV (%) | Mean | GEN | H2 | CV (%) | ||
| GLN | 3.03 | 26.479 *** | 0.88 | 58.60 | 6.79 | 26.44 *** | 0,79 | 34.69 | 7.1 *** |
| SL | 192.26 | 11,899 *** | 0.9 | 17.90 | 197.41 | 12,861 *** | 0.94 | 15.71 | 1101 ns |
| HD | 143.40 | 4461 *** | 0.98 | 6.25 | 143.57 | 4807 *** | 0.97 | 8.04 | 131.4 ns |
| GW | 10.49 | 171.49 *** | 0.83 | 50.88 | 19.53 | 370 *** | 0.85 | 38.73 | 54.9 ns |
| GN | 498.53 | 31,525 *** | 0.78 | 51.74 | 863.16 | 562839 *** | 0.77 | 41.86 | 119,644 ns |
| HI | 0.08127501 | 0.010210 *** | 0.77 | 59.70 | 0.1305423 | 0.01727 *** | 0.77 | 47.90 | 0.00425 ** |
| BT | 136.66 | 3861 ** | 0.37 | 35.84 | 157.83 | 5099 *** | 0.39 | 35.19 | 3320 * |
| TE | 2.81 | 2.07 *** | 0.5 | 36.40 | 2.35 | 1.045 *** | 0.36 | 34.71 | 1.135 * |
| WT | 44.95 | 220 *** | 0.68 | 18.63 | 56.74 | 111.9 *** | 0.64 | 11.23 | 48.6 ns |
| GEN | YS | YP | STI | TOL | MP | GMP | RED | SSI | GLNws | GLNww | TEws | TEww |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sepon82 | 14.25 | 47.05 | 1.76 | 32.80 | 30.65 | 25.90 | 2.30 | 1.52 | 7.00 | 10.33 | 2.05 | 3.09 |
| PAYENNE | 27.77 | 45.26 | 3.30 | 17.48 | 36.52 | 35.45 | 0.63 | 0.84 | 3.25 | 7.00 | 3.56 | 5.06 |
| ICSV 206058 | 28.07 | 43.48 | 3.20 | 15.41 | 35.78 | 34.94 | 0.55 | 0.77 | 1.33 | 6.00 | 3.19 | 3.05 |
| ICSV 216008 | 30.58 | 41.81 | 3.35 | 11.23 | 36.20 | 35.76 | 0.37 | 0.58 | 3.00 | 9.00 | 3.31 | 1.97 |
| SARIASO14 | 28.49 | 40.89 | 3.05 | 12.40 | 34.69 | 34.13 | 0.44 | 0.66 | 3.50 | 8.00 | 2.63 | 3.32 |
| Sariaso 16 | 31.70 | 40.69 | 3.38 | 8.99 | 36.20 | 35.92 | 0.28 | 0.48 | 4.67 | 10.00 | 4.20 | 3.21 |
| ICSV 216020 | 12.31 | 40.11 | 1.29 | 27.79 | 26.21 | 22.22 | 2.26 | 1.51 | 6.25 | 10.25 | 2.82 | 2.62 |
| ICSV 206059 | 19.56 | 39.01 | 2.00 | 19.45 | 29.29 | 27.62 | 0.99 | 1.08 | 0.00 | 6.33 | 5.07 | 2.23 |
| ICSV 206057 | 24.13 | 38.43 | 2.43 | 14.31 | 31.28 | 30.45 | 0.59 | 0.81 | 1.00 | 7.67 | 2.09 | 2.95 |
| ICSV 1049 | 27.86 | 37.56 | 2.74 | 9.70 | 32.71 | 32.35 | 0.35 | 0.56 | 4.50 | 9.00 | 3.94 | 2.52 |
| ICSV 216119 | 16.16 | 37.47 | 1.59 | 21.31 | 26.81 | 24.61 | 1.32 | 1.24 | 2.75 | 5.67 | 2.84 | 3.02 |
| ICSV 186002 | 21.67 | 35.27 | 2.00 | 13.60 | 28.47 | 27.65 | 0.63 | 0.84 | 6.00 | 9.00 | 4.78 | 3.42 |
| 216-2P4-5B | 16.83 | 34.87 | 1.54 | 18.04 | 25.85 | 24.23 | 1.07 | 1.12 | 2.75 | 6.75 | 3.08 | 1.76 |
| ICSV 216094 | 24.13 | 34.05 | 2.15 | 9.92 | 29.09 | 28.66 | 0.41 | 0.63 | 2.33 | 5.75 | 2.56 | 1.56 |
| SAMSORG 41 | 14.08 | 33.25 | 1.23 | 19.16 | 23.67 | 21.64 | 1.36 | 1.25 | 8.50 | 11.25 | 4.53 | 2.89 |
| ICSV 206091 | 15.73 | 32.99 | 1.36 | 17.26 | 24.36 | 22.78 | 1.10 | 1.14 | 0.00 | 4.25 | 1.98 | 2.51 |
| ICSV 206028 | 15.91 | 32.77 | 1.37 | 16.86 | 24.34 | 22.83 | 1.06 | 1.12 | 0.75 | 5.25 | 3.46 | 1.85 |
| ICSB 293 | 24.42 | 32.26 | 2.07 | 7.84 | 28.34 | 28.07 | 0.32 | 0.53 | 4.50 | 7.25 | 5.63 | 2.78 |
| ICSV 216075 | 16.27 | 32.11 | 1.37 | 15.83 | 24.19 | 22.86 | 0.97 | 1.07 | 6.33 | 8.67 | 2.97 | 2.69 |
| ICSV 216074 | 26.15 | 32.04 | 2.20 | 5.89 | 29.10 | 28.95 | 0.23 | 0.40 | 0.00 | 8.33 | 3.05 | 2.54 |
| FAOUROU | 20.79 | 32.04 | 1.75 | 11.25 | 26.41 | 25.81 | 0.54 | 0.76 | 8.00 | 9.33 | 3.99 | 2.64 |
| ICSV 1460016 | 24.86 | 31.60 | 2.06 | 6.74 | 28.23 | 28.03 | 0.27 | 0.46 | 6.00 | 8.50 | 2.95 | 2.57 |
| ICSV111 | 17.84 | 31.06 | 1.45 | 13.22 | 24.45 | 23.54 | 0.74 | 0.92 | 7.00 | 10.00 | 3.74 | 2.42 |
| Seguifa | 16.20 | 30.79 | 1.31 | 14.59 | 23.50 | 22.33 | 0.90 | 1.03 | 5.33 | 9.50 | 3.04 | 3.38 |
| Sureno | 18.95 | 30.78 | 1.53 | 11.83 | 24.87 | 24.15 | 0.62 | 0.84 | 7.00 | 11.00 | 3.76 | 3.29 |
| ICSV 1460011 | 21.02 | 29.92 | 1.65 | 8.90 | 25.47 | 25.08 | 0.42 | 0.65 | 5.00 | 6.00 | 2.59 | 1.88 |
| ICSV 1460010 | 19.07 | 29.87 | 1.49 | 10.80 | 24.47 | 23.87 | 0.57 | 0.79 | 3.75 | 9.00 | 3.29 | 4.00 |
| ICSB 176001 | 16.25 | 29.33 | 1.25 | 13.08 | 22.79 | 21.84 | 0.80 | 0.97 | 9.50 | 11.00 | 2.72 | 2.41 |
| 015-SB-CS-F7-127 | 14.63 | 28.97 | 1.11 | 14.34 | 21.80 | 20.59 | 0.98 | 1.08 | 0.00 | 8.00 | 2.09 | 2.20 |
| ICSB 186001 | 25.72 | 28.89 | 1.95 | 3.17 | 27.30 | 27.26 | 0.12 | 0.24 | 4.67 | 7.75 | 2.50 | 2.39 |
| SAMSORG 40 | 23.68 | 28.78 | 1.79 | 5.10 | 26.23 | 26.11 | 0.22 | 0.39 | 5.33 | 8.75 | 2.89 | 2.79 |
| ICSV 186008 | 21.72 | 28.23 | 1.61 | 6.52 | 24.98 | 24.76 | 0.30 | 0.50 | 4.67 | 8.50 | 4.23 | 2.48 |
| Niolagne | 12.68 | 28.02 | 0.93 | 15.34 | 20.35 | 18.85 | 1.21 | 1.19 | 7.33 | 8.25 | 1.62 | 2.81 |
| 016-SB-CS-DU-17 | 13.10 | 27.23 | 0.94 | 14.13 | 20.17 | 18.89 | 1.08 | 1.13 | 5.33 | 8.75 | 2.06 | 2.20 |
| ICSV 206084 | 12.35 | 26.97 | 0.87 | 14.61 | 19.66 | 18.25 | 1.18 | 1.18 | 4.50 | 6.25 | 3.40 | 2.82 |
| 06-SB-F4DT-15 | 20.90 | 26.57 | 1.46 | 5.67 | 23.73 | 23.56 | 0.27 | 0.46 | 5.00 | 8.25 | 2.58 | 2.10 |
| ICSB 176003 | 16.08 | 25.98 | 1.10 | 9.89 | 21.03 | 20.44 | 0.62 | 0.83 | - | - | 3.31 | 3.63 |
| ICSV 216001 | 13.94 | 25.43 | 0.93 | 11.49 | 19.69 | 18.83 | 0.82 | 0.98 | 1.00 | 15.25 | 2.64 | 2.67 |
| ICSV 206030 | 14.89 | 24.18 | 0.94 | 9.28 | 19.54 | 18.98 | 0.62 | 0.83 | 11.25 | 15.50 | 3.29 | 2.44 |
| IRAT204 | 20.06 | 23.16 | 1.22 | 3.10 | 21.61 | 21.56 | 0.15 | 0.29 | 4.50 | 6.25 | 4.71 | 2.68 |
| ICSV 216073 | 16.40 | 22.77 | 0.98 | 6.37 | 19.59 | 19.32 | 0.39 | 0.61 | 9.50 | 11.00 | 2.70 | 2.55 |
| Niobougouma | 13.52 | 22.42 | 0.79 | 8.91 | 17.97 | 17.41 | 0.66 | 0.86 | 2.75 | 3.50 | 3.02 | 2.67 |
| 447(471)496 | 15.97 | 22.39 | 0.94 | 6.43 | 19.18 | 18.91 | 0.40 | 0.62 | 6.33 | 9.50 | 3.59 | 2.65 |
| 019-SB-CS-F5-71 | 19.96 | 21.88 | 1.15 | 1.92 | 20.92 | 20.90 | 0.10 | 0.19 | 0.50 | 4.75 | 2.21 | 2.53 |
| ICSV 206032 | 15.73 | 21.77 | 0.90 | 6.03 | 18.75 | 18.51 | 0.38 | 0.60 | 11.50 | 12.50 | 4.83 | 2.52 |
| ICSV 216024 | 16.47 | 21.63 | 0.93 | 5.15 | 19.05 | 18.87 | 0.31 | 0.52 | 0.75 | 5.00 | 2.63 | 2.47 |
| ICSV 206056 | 15.90 | 19.70 | 0.82 | 3.80 | 17.80 | 17.70 | 0.24 | 0.42 | 1.25 | 7.75 | 2.52 | 2.78 |
| ICSB 176006 | 12.03 | 15.20 | 0.48 | 3.16 | 13.62 | 13.52 | 0.26 | 0.45 | 3.00 | 10.75 | 3.01 | 3.38 |
| SAMSORG 3 | 10.06 | 13.79 | 0.36 | 3.73 | 11.93 | 11.78 | 0.37 | 0.59 | 8.00 | 8.00 | 2.23 | 2.01 |
| ICSB 176031 | 9.63 | 13.17 | 0.33 | 3.54 | 11.40 | 11.26 | 0.37 | 0.58 | 1.25 | 10.33 | 4.00 | 3.06 |
| Tx7000 | 4.12 | 6.34 | 0.07 | 2.22 | 5.23 | 5.11 | 0.54 | 0.76 | 5.00 | 7.33 | 2.25 | 2.19 |
| B 35 | 1.33 | 3.32 | 0.01 | 2.00 | 2.33 | 2.10 | 1.51 | 1.31 | 10.50 | 10.00 | 2.74 | 2.66 |
| Overall Mean | 9.95 | 20 | 0.68 | 10.06 | 14.97 | 13.81 | 1.74 | 1.16 | 4.59 | 8.47 | 3.17 | 2.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touré, K.; Jumbo, M.B.; Sissoko, S.; Nebie, B.; Falalou, H.; Diancoumba, M.; Abdou, H.; Dembele, J.S.B.; Gano, B.; Sodio, B. Evaluation of Hybrid Sorghum Parents for Morphological, Physiological and Agronomic Traits Under Post-Flowering Drought. Agronomy 2025, 15, 1399. https://doi.org/10.3390/agronomy15061399
Touré K, Jumbo MB, Sissoko S, Nebie B, Falalou H, Diancoumba M, Abdou H, Dembele JSB, Gano B, Sodio B. Evaluation of Hybrid Sorghum Parents for Morphological, Physiological and Agronomic Traits Under Post-Flowering Drought. Agronomy. 2025; 15(6):1399. https://doi.org/10.3390/agronomy15061399
Chicago/Turabian StyleTouré, Kadiatou, MacDonald Bright Jumbo, Sory Sissoko, Baloua Nebie, Hamidou Falalou, Madina Diancoumba, Harou Abdou, Joseph Sékou B. Dembele, Boubacar Gano, and Bernard Sodio. 2025. "Evaluation of Hybrid Sorghum Parents for Morphological, Physiological and Agronomic Traits Under Post-Flowering Drought" Agronomy 15, no. 6: 1399. https://doi.org/10.3390/agronomy15061399
APA StyleTouré, K., Jumbo, M. B., Sissoko, S., Nebie, B., Falalou, H., Diancoumba, M., Abdou, H., Dembele, J. S. B., Gano, B., & Sodio, B. (2025). Evaluation of Hybrid Sorghum Parents for Morphological, Physiological and Agronomic Traits Under Post-Flowering Drought. Agronomy, 15(6), 1399. https://doi.org/10.3390/agronomy15061399









