Verification of Seed-Priming-Induced Stress Memory by Genome-Wide Transcriptomic Analysis in Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Seed-Priming, and Drought-Stress Treatments
2.2. In Silico Genome-Wide Analyses
2.3. Individual Gene Analyses
2.4. Antioxidant Enzyme Activity Measurements
2.4.1. Estimation of the Peroxidase (POX) Activity
2.4.2. Estimation of the Catalase (CAT) Activity
2.4.3. Estimation of the Glutathione Reductase (GR) Activity
2.5. Statistical Analysis
3. Results
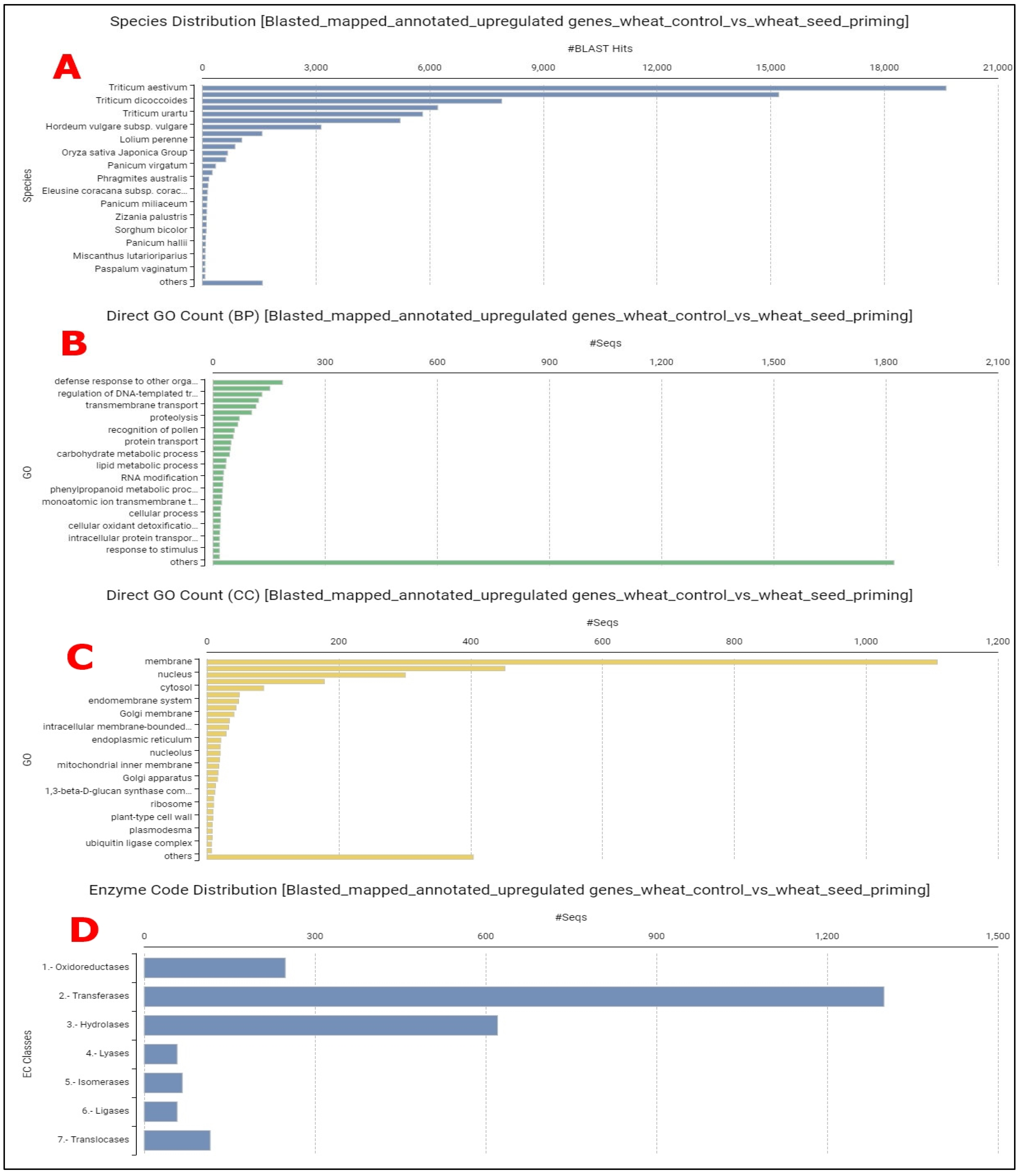
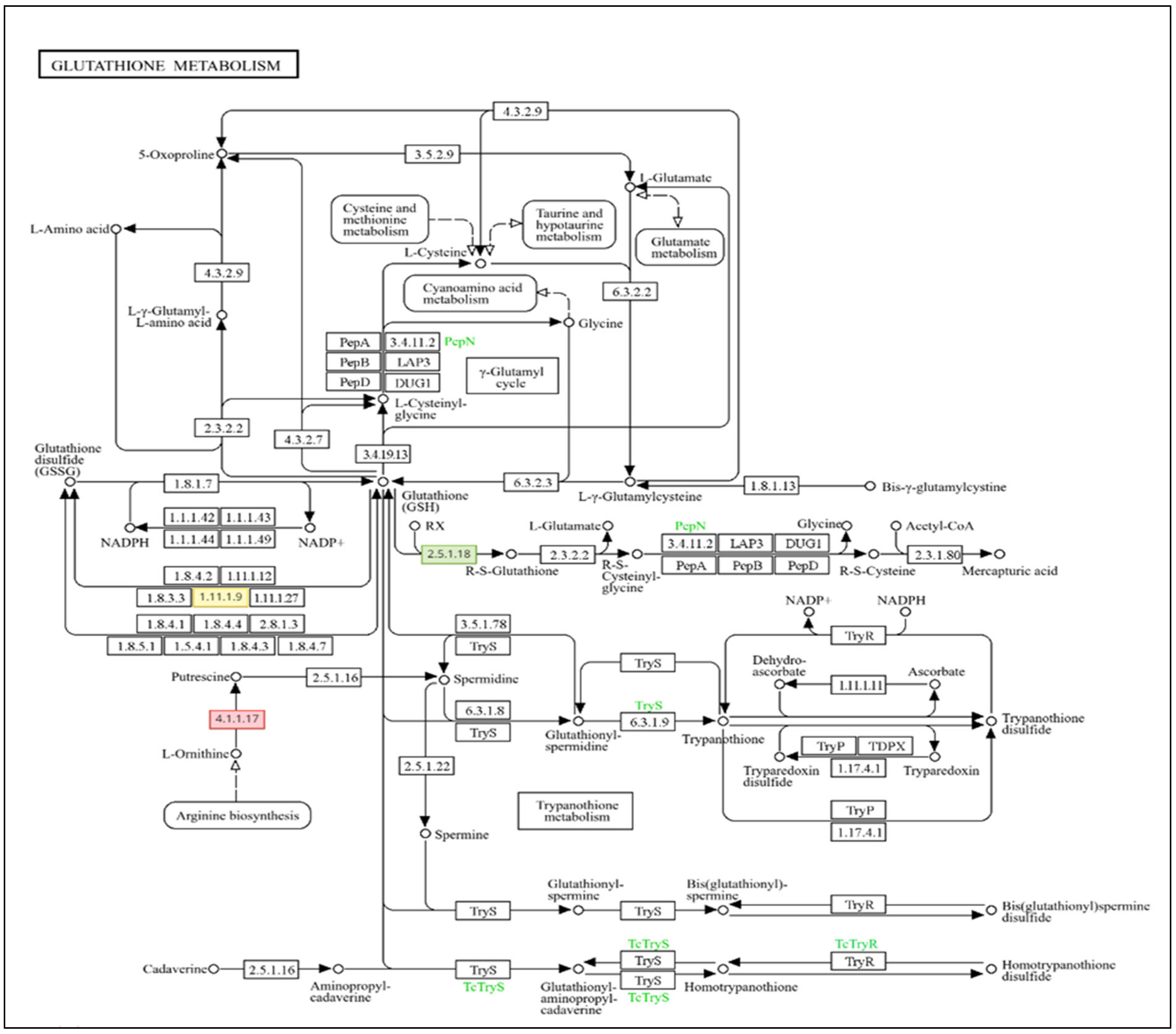
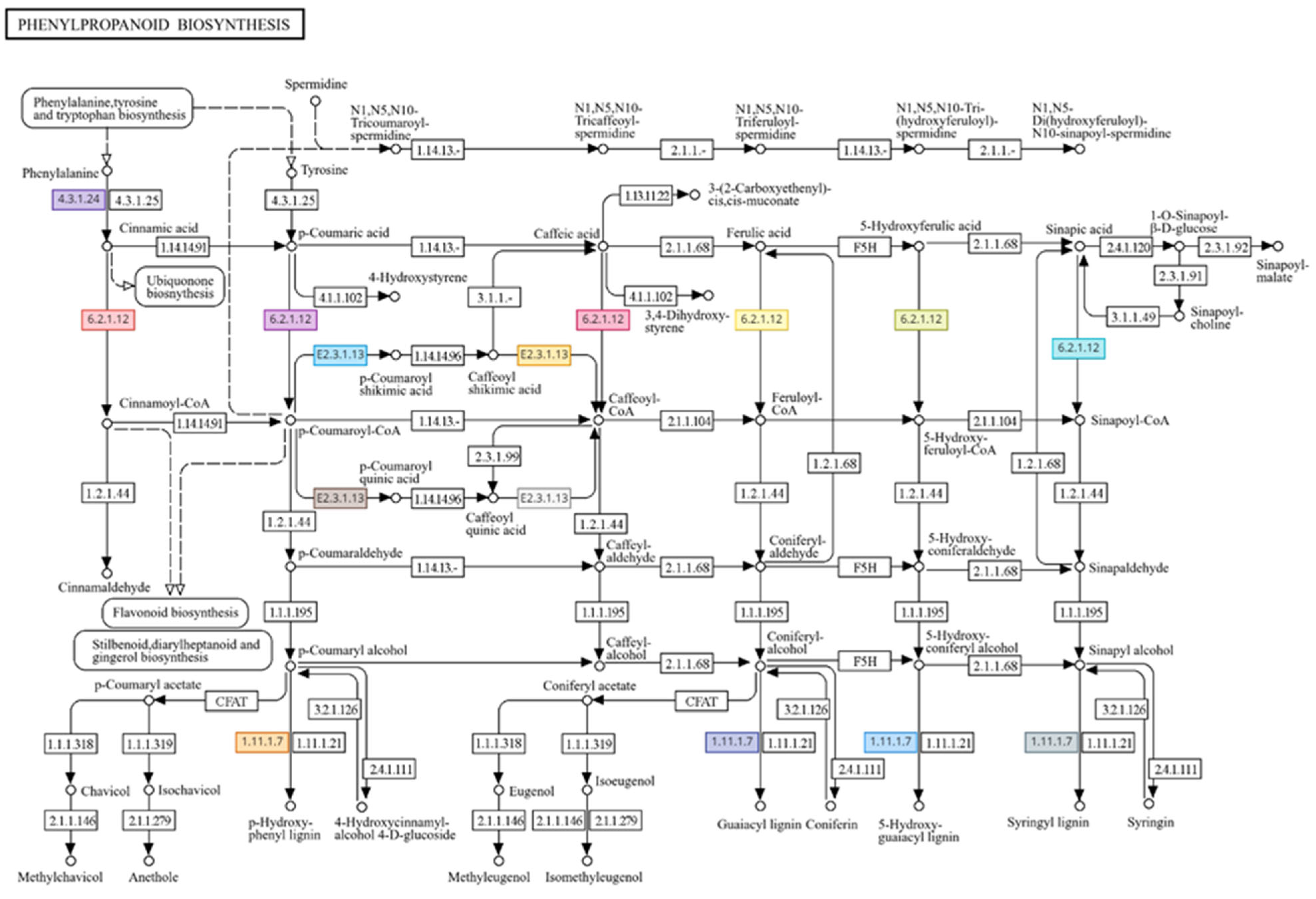
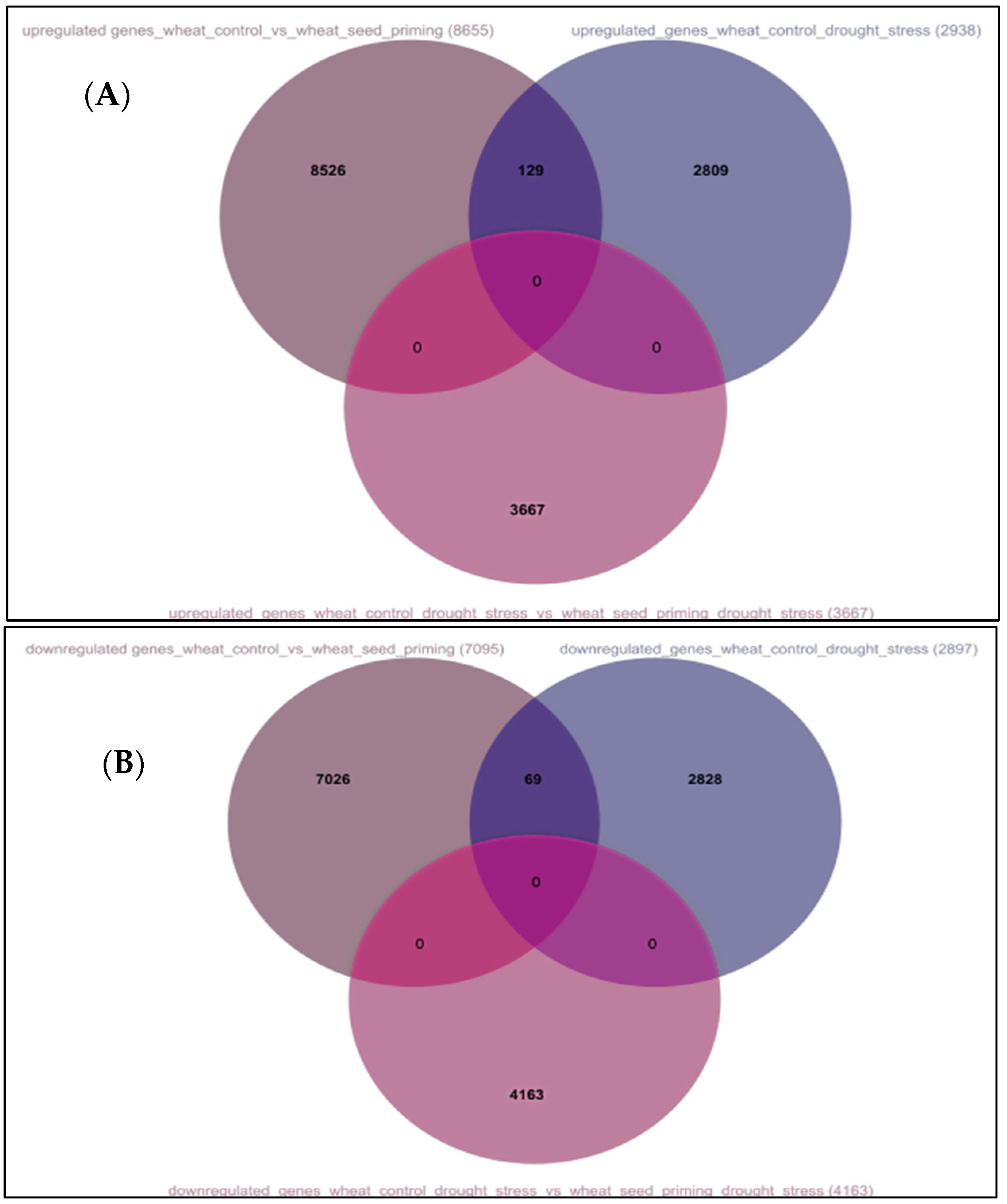
3.1. Genome-Wide Transcriptomic Analyses
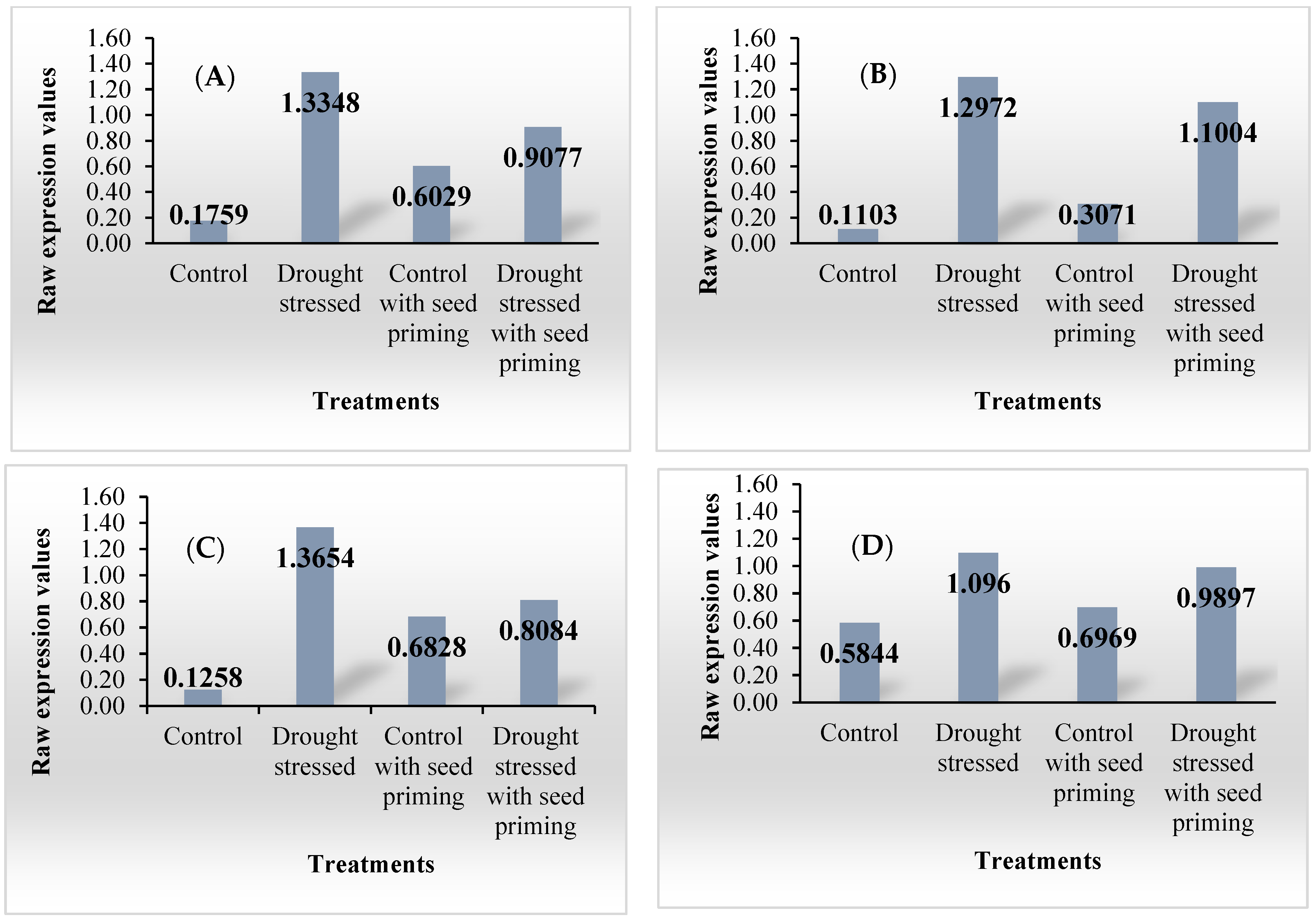
3.2. Individual Gene Analyses
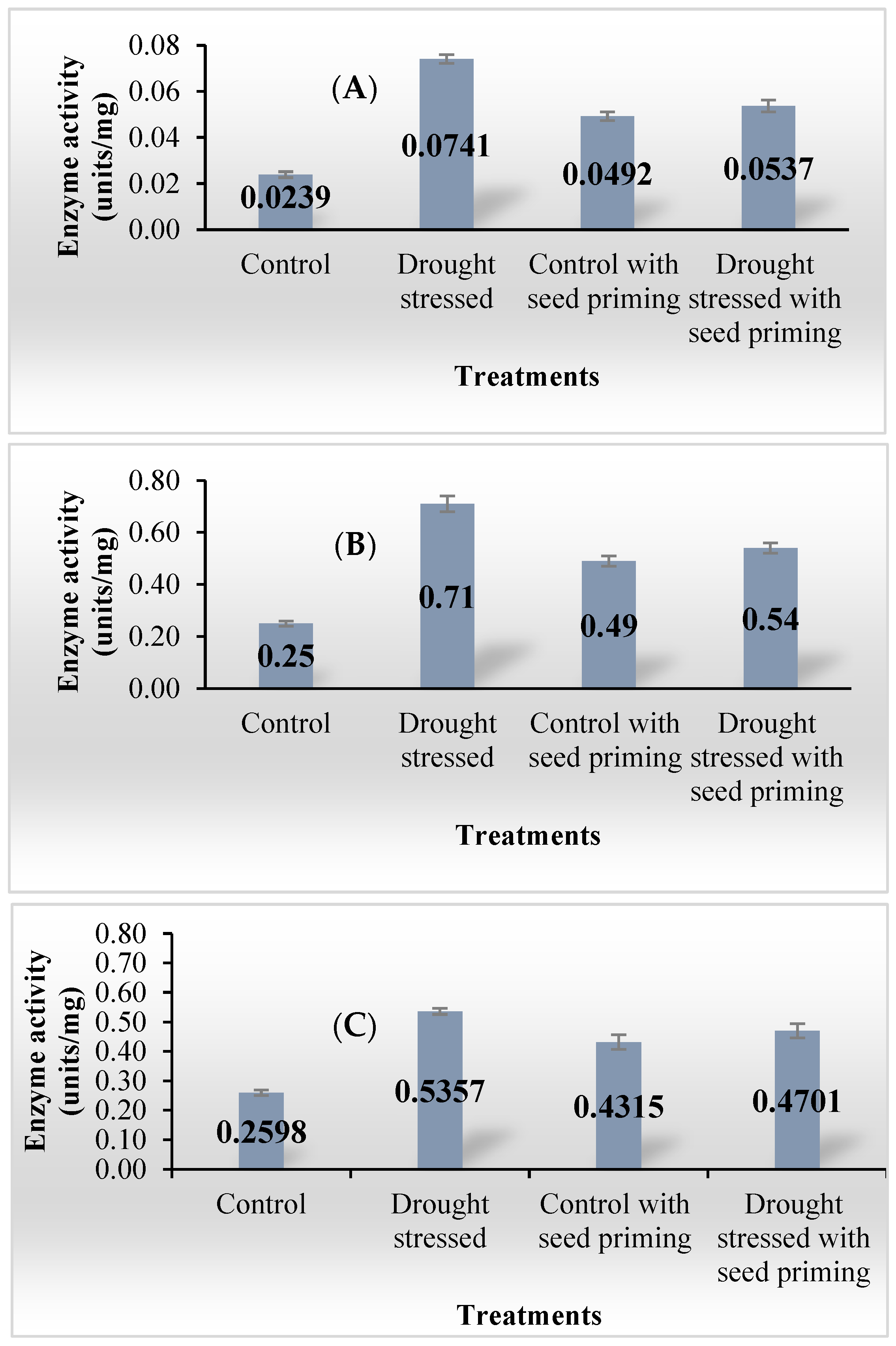
3.3. Enzyme Activity Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
- -
- Number of reads for control sequences: 56,067,452
- -
- Number of reads for drought-stressed sequences: 61,000,344
- -
- Number of reads for seed-primed sequences: 62,857,128
- -
- Number of reads for seed-primed and drought-stressed sequences: 61,610,194
- -
- Length of the raw reads: 151 bp
- (1)
- Repository name: Wheat treated by ZnO nanoparticles or by seed-priming conditioner; Data identification number: PRJNA1142041; Direct URL to data: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1142041 (accessed on 30 July 2024).
- (2)
- Repository name: Wheat_1_R1 and Wheat_1_R2; Data identification number: SRR30042189; Direct URL to data: https://www.ncbi.nlm.nih.gov/sra/?term=SRR30042189 (accessed on 30 July 2024).
- (3)
- Repository name: Wheat_5_R1 and Wheat_5_R2; Data identification number: SRR30042187; Direct URL to data: https://www.ncbi.nlm.nih.gov/sra/?term=SRR30042187 (accessed on 30 July 2024).
- (4)
- Repository name: Wheat_17_R1 and Wheat_17_R2; Data identification number: SRR30042184; Direct URL to data: https://www.ncbi.nlm.nih.gov/sra/?term=SRR30042184 (accessed on 30 July 2024).
- (5)
- Repository name: Wheat_21_R1 and Wheat_21_R2; Data identification number: SRR30042188; Direct URL to data: https://www.ncbi.nlm.nih.gov/sra/?term=SRR30042188 (accessed on 30 July 2024).
Conflicts of Interest
References
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F. Effect of Seed Priming on Horticultural Crops. Sci. Hortic. 2021, 286, 110197. [Google Scholar] [CrossRef]
- Thakur, M.; Tiwari, S.; Kataria, S.; Anand, A. Recent Advances in Seed Priming Strategies for Enhancing Planting Value of Vegetable Seeds. Sci. Hortic. 2022, 305, 111355. [Google Scholar] [CrossRef]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 1–10. ISBN 9789811386251. [Google Scholar]
- Zammali, I.; Dabbous, A.; Youssef, S.; Ben Hamed, K. Effects of Chemical Priming on the Germination of the Ornamental Halophyte Lobularia Maritima under NaCl Salinity. Seeds 2022, 1, 99–109. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.; Wahid, A.; Basra, S.; Siddique, K. Seed Priming in Field Crops: Potential Benefits, Adoption and Challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Alhammad, B.A.; Ahmad, A.; Seleiman, M.F.; Tola, E. Seed Priming with Nanoparticles and 24-Epibrassinolide Improved Seed Germination and Enzymatic Performance of Zea mays L. in Salt-Stressed Soil. Plants 2023, 12, 690. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, B.; Saqib, M. Chapter 15—Seed Priming to Enhance Salt and Drought Stress Tolerance in Plants: Advances and Prospects. In Climate Change and Crop Stress; Shanker, A.K., Shanker, C., Anand, A., Maheswari, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 441–464. ISBN 978-0-12-816091-6. [Google Scholar]
- Szalai, G.; Pál, M.; Árendás, T.; Janda, T. Priming Seed with Salicylic Acid Increases Grain Yield and Modifies Polyamine Levels in Maize. Cereal Res. Commun. 2016, 44, 537–548. [Google Scholar] [CrossRef]
- Alam, M.U.; Fujita, M.; Nahar, K.; Rahman, A.; Anee, T.I.; Masud, A.A.C.; Amin, A.K.M.R.; Hasanuzzaman, M. Seed Priming Upregulates Antioxidant Defense and Glyoxalase Systems to Conferring Simulated Drought Tolerance in Wheat Seedlings. Plant Stress 2022, 6, 100120. [Google Scholar] [CrossRef]
- Louis, N.; Dhankher, O.P.; Puthur, J.T. Seed Priming Can Enhance and Retain Stress Tolerance in Ensuing Generations by Inducing Epigenetic Changes and Trans-Generational Memory. Physiol. Plant 2023, 175, e13881. [Google Scholar] [CrossRef]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef]
- Aswathi, K.P.R.; Kalaji, H.M.; Puthur, J.T. Seed Priming of Plants Aiding in Drought Stress Tolerance and Faster Recovery: A Review. Plant Growth Regul. 2022, 97, 235–253. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming Crops for the Future: Rewiring Stress Memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Md, P.; Gupta, N.K.; Mehta, B.K.; Kumar, P.; Pandey, S.; et al. Drought Stress Responses and Inducing Tolerance by Seed Priming Approach in Plants. Plant Stress 2022, 4, 100066. [Google Scholar] [CrossRef]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple Exposures to Drought ‘Train’ Transcriptional Responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467. [Google Scholar] [CrossRef]
- Pastor, V.; Luna, E.; Mauch-Mani, B.; Ton, J.; Flors, V. Plants Do Not Forget. Environ. Exp. Bot. 2013, 94, 46–56. [Google Scholar] [CrossRef]
- Lukić, N.; Kukavica, B.; Davidović-Plavšić, B.; Hasanagić, D.; Walter, J. Plant Stress Memory Is Linked to High Levels of Anti-Oxidative Enzymes over Several Weeks. Environ. Exp. Bot. 2020, 178, 104166. [Google Scholar] [CrossRef]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A.; Shahid, M. Terminal Drought and Seed Priming Improves Drought Tolerance in Wheat. Physiol. Mol. Biol. Plants 2018, 24, 845–856. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Sattar, A.; Ijaz, M.; Sher, A.; Ul-Allah, S. Mitigating the Adverse Effects of Drought Stress through Seed Priming and Seed Quality on Wheat (Triticum aestivum L.) Productivity. Pak. J. Agric. Sci. 2018, 55, 313–319. [Google Scholar] [CrossRef]
- Zanganeh, R.; Jamei, R.; Rahmani, F. Impacts of Seed Priming with Salicylic Acid and Sodium Hydrosulfide on Possible Metabolic Pathway of Two Amino Acids in Maize Plant under Lead Stress. Mol. Biol. Res. Commun. 2018, 7, 83–88. [Google Scholar] [CrossRef]
- Bortolin, G.S.; Teixeira, S.B.; Mesquita Pinheiro, R.; Ávila, G.E.; Carlos, F.S.; Silva Pedroso, C.E.; Deuner, S. Seed Priming with Salicylic Acid Minimizes Oxidative Effects of Aluminum on Trifolium Seedlings. J. Soil Sci. Plant Nutr. 2020, 20, 2502–2511. [Google Scholar] [CrossRef]
- Ghafoor, M.F.; Ali, Q.; Malik, A. Effects of Salicylic Acid Priming for Salt Stress Tolerance in Wheat. Biol. Clin. Sci. Res. J. 2020, e024. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Shahbaz, M.; Zulfiqar, U.; Saman, R.U.; Rehman, A.; Naz, N.; Akram, M.; Haider, F.U. Enhancing Wheat Growth and Yield through Salicylic Acid-Mediated Regulation of Gas Exchange, Antioxidant Defense, and Osmoprotection under Salt Stress. Stresses 2023, 3, 372–386. [Google Scholar] [CrossRef]
- Imakumbili, M.L.E.; Semu, E.; Semoka, J.M.R.; Abass, A.; Mkamilo, G. Managing Cassava Growth on Nutrient Poor Soils under Different Water Stress Conditions. Heliyon 2021, 7, e07331. [Google Scholar] [CrossRef]
- van Dijk, E.L.; Jaszczyszyn, Y.; Thermes, C. Library Preparation Methods for Next-Generation Sequencing: Tone down the Bias. Exp. Cell Res. 2014, 322, 12–20. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- OmicsBox. Bioinformatics Made Easy; BioBam Bioinformatics: Valencia, Spain, 2019. [Google Scholar]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-Throughput Functional Annotation and Data Mining with the Blast2GO Suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Forslund, K.; Cook, H.; Heller, D.; Walter, M.C.; Rattei, T.; Mende, D.R.; Sunagawa, S.; Kuhn, M.; et al. eggNOG 4.5: A Hierarchical Orthology Framework with Improved Functional Annotations for Eukaryotic, Prokaryotic and Viral Sequences. Nucleic Acids Res. 2016, 44, D286–D293. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, S.; Furió-Tarí, P.; Turrà, D.; Pietro, A.D.; Nueda, M.J.; Ferrer, A.; Conesa, A. Data Quality Aware Analysis of Differential Expression in RNA-Seq with NOISeq R/Bioc Package. Nucleic Acids Res. 2015, 43, e140. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, S.; García-Alcalde, F.; Dopazo, J.; Ferrer, A.; Conesa, A. Differential Expression in RNA-Seq: A Matter of Depth. Genome Res. 2011, 21, 2213–2223. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Al-Shahrour, F.; Díaz-Uriarte, R.; Dopazo, J. FatiGO: A Web Tool for Finding Significant Associations of Gene Ontology Terms with Groups of Genes. Bioinformatics 2004, 20, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Venisse, J.-S.; Gullner, G.; Brisset, M.-N. Evidence for the Involvement of an Oxidative Stress in the Initiation of Infection of Pear by Erwinia Amylovora 1. Plant Physiol. 2001, 125, 2164–2172. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. [136] Assay of Catalases and Peroxidases. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1955. [Google Scholar]
- Bonnichsen, R.K.; Chance, B.; Theorell, H. Catalase Activity. Acta Chem. Scand. 1947, 1, 685–709. [Google Scholar] [CrossRef]
- Maehly, A.C. The Assay of Catalases and Peroxidases. In Methods of Biochemical Analysis; Wiley: Hoboken, NJ, USA, 1954; pp. 357–424. [Google Scholar]
- Jangam, A.K.; Thali, P. WASP-Web Agri Stat Package; ICAR Research Complex for Goa; Ela: Old Goa, India, 2004. [Google Scholar]
- Li, Y.; Ye, Z.; Xiang, J.; Li, S.; Zheng, Z.; Li, Y.; Fang, Y.; Zhang, X.; Chen, X.; Xue, D. Purine Nucleotide Metabolism Response to Drought Stress in Rice. Plant Growth Regul. 2025, 105, 821–832. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Huang, W.; Song, X.; Niu, J. Transcriptomics and Metabolomics Reveal Purine and Phenylpropanoid Metabolism Response to Drought Stress in Dendrobium Sinense, an Endemic Orchid Species in Hainan Island. Front. Genet. 2021, 12, 692702. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Giori, G.S.D. B Group Vitamins: Current Uses and Perspectives; BoD—Books on Demand: Hamburg, Germany, 2018; ISBN 978-1-78923-989-8. [Google Scholar]
- Yusof, Z.N.B. Thiamine and Its Role in Protection Against Stress in Plants (Enhancement in Thiamine Content for Nutritional Quality Improvement). In Nutritional Quality Improvement in Plants; Jaiwal, P.K., Chhillar, A.K., Chaudhary, D., Jaiwal, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 177–186. ISBN 978-3-319-95354-0. [Google Scholar]
- Chen, S.; Zhao, C.-B.; Ren, R.-M.; Jiang, J.-H. Salicylic Acid Had the Potential to Enhance Tolerance in Horticultural Crops against Abiotic Stress. Front. Plant Sci. 2023, 14, 1141918. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fu, K.; Guo, W.; Zhang, X.; Li, C.; Li, C. Starch and Sugar Metabolism Response to Post-Anthesis Drought Stress During Critical Periods of Elite Wheat (Triticum aestivum L.) Endosperm Development. J. Plant Growth Regul. 2023, 42, 5476–5494. [Google Scholar] [CrossRef]
- Zeng, R.; Chen, T.; Wang, X.; Cao, J.; Li, X.; Xu, X.; Chen, L.; Xia, Q.; Dong, Y.; Huang, L.; et al. Physiological and Expressional Regulation on Photosynthesis, Starch and Sucrose Metabolism Response to Waterlogging Stress in Peanut. Front. Plant Sci. 2021, 12, 601771. [Google Scholar] [CrossRef]
- Akula, R.; and Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Chapter 8—Environmental Stress and Secondary Metabolites in Plants: An Overview. In Plant Metabolites and Regulation Under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 153–167. ISBN 978-0-12-812689-9. [Google Scholar]
- Liu, M.; Lu, S. Plastoquinone and Ubiquinone in Plants: Biosynthesis, Physiological Function and Metabolic Engineering. Front. Plant Sci. 2016, 7, 1898. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E.; Iglesias, A.A. Nucleotide-Sugar Metabolism in Plants: The Legacy of Luis F. Leloir. J. Exp. Bot. 2021, 72, 4053–4067. [Google Scholar] [CrossRef]
- Bar-Peled, M.; O’Neill, M.A. Plant Nucleotide Sugar Formation, Interconversion, and Salvage by Sugar Recycling. Annu. Rev. Plant Biol. 2011, 62, 127–155. [Google Scholar] [CrossRef]
- da Fonseca-Pereira, P.; Monteiro-Batista, R.d.C.; Araújo, W.L.; Nunes-Nesi, A. Harnessing Enzyme Cofactors and Plant Metabolism: An Essential Partnership. Plant J. 2023, 114, 1014–1036. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione Metabolism in Plants under Stress: Beyond Reactive Oxygen Species Detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Sun, S.; Yan, Y.; Jing, X.; Shi, Q. Glutathione Metabolism and Its Function in Higher Plants Adapting to Stress. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 181–205. ISBN 978-3-319-75088-0. [Google Scholar]
- Hameed, A.; Sharma, I.; Kumar, A.; Azooz, M.; Ahmad Lone, H.; Ahmad, P. Chapter 6—Glutathione Metabolism in Plants under Environmental Stress. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 183–200. ISBN 978-0-12-799963-0. [Google Scholar]
- Li, C.; Jiang, Y.; Xu, C.; Mei, X. Editorial: Contribution of Phenylpropanoid Metabolism to Plant Development and Stress Responses. Front. Plant Sci. 2024, 15, 1456913. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Zhang, X.; Busatto, N. Editorial: Phenylpropanoid Biosynthesis in Plants. Front. Plant Sci. 2023, 14, 1230664. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, M.C.; Ozgur, R.; Uzilday, B. Reactive Oxygen Species: Connecting Eustress, Hormesis, and Allostasis in Plants. Plant Stress 2023, 8, 100164. [Google Scholar] [CrossRef]
- Villagómez-Aranda, A.L.; Feregrino-Pérez, A.A.; García-Ortega, L.F.; González-Chavira, M.M.; Torres-Pacheco, I.; Guevara-González, R.G. Activating Stress Memory: Eustressors as Potential Tools for Plant Breeding. Plant Cell Rep. 2022, 41, 1481–1498. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decsi, K.; Ahmed, M.; Abdul-Hamid, D.; Rizk, R.; Tóth, Z. Verification of Seed-Priming-Induced Stress Memory by Genome-Wide Transcriptomic Analysis in Wheat (Triticum aestivum L.). Agronomy 2025, 15, 1365. https://doi.org/10.3390/agronomy15061365
Decsi K, Ahmed M, Abdul-Hamid D, Rizk R, Tóth Z. Verification of Seed-Priming-Induced Stress Memory by Genome-Wide Transcriptomic Analysis in Wheat (Triticum aestivum L.). Agronomy. 2025; 15(6):1365. https://doi.org/10.3390/agronomy15061365
Chicago/Turabian StyleDecsi, Kincső, Mostafa Ahmed, Donia Abdul-Hamid, Roquia Rizk, and Zoltán Tóth. 2025. "Verification of Seed-Priming-Induced Stress Memory by Genome-Wide Transcriptomic Analysis in Wheat (Triticum aestivum L.)" Agronomy 15, no. 6: 1365. https://doi.org/10.3390/agronomy15061365
APA StyleDecsi, K., Ahmed, M., Abdul-Hamid, D., Rizk, R., & Tóth, Z. (2025). Verification of Seed-Priming-Induced Stress Memory by Genome-Wide Transcriptomic Analysis in Wheat (Triticum aestivum L.). Agronomy, 15(6), 1365. https://doi.org/10.3390/agronomy15061365






