Effects of Low Green Light Combined with Different Red and Far-Red Light Ratios on the Growth and Secondary Metabolites of Cilantro (Coriandrum sativum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Germination and Plant Seedlings
2.2. Growth Conditions and Seedling
2.3. Nutrient Solution
2.4. Light Treatments
2.5. Measurements of Physical Characteristics
2.6. Measurements of Pigments
2.7. Measurement of Total Phenolics Content
2.8. Nitrates and Vitamin C
2.9. Energy Yield and Photon Yield
2.10. Statistical Analysis
2.11. Clustering and Graphical Representation
2.12. Surface Response Curve
3. Results
3.1. Cilantro Growth Under R:Fr + G
3.2. Pigment Content Under R:Fr + G
3.3. Nitrates and Phenolics Content Under R:Fr + G
4. Discussion
4.1. Light Quality for Cilantro Growth—Exploring R:Fr Ratios Under High Red Light
4.2. Light Quality for Cilantro Secondary Metabolite—Exploring R:Fr Ratios Under High Red Light
4.3. Spectral Quantification Indices
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAS | Days after sowing |
| BPFD | Biological photon flux density |
| LED | Light-emitting diode |
| GAE | Gallic acid equivalents |
| RGBFR | Red, green, blue, and far-red |
| PFAL | Plant factory with artificial lighting |
| EY | Energy yield |
| PY | Photon yield |
References
- Stutte, G.W. Controlled environment production of medicinal and aromatic plants. In Medicinal and Aromatic Crops: Production, Phytochemistry, and Utilization, 1st ed.; American Chemical Society: Richland, MS, USA, 2016; Volume 1218, pp. 49–63. [Google Scholar] [CrossRef]
- Anpo, M.; Fukuda, H.; Wada, T. Introduction: Artificial light-type plant factories—Outline and a vision for the future. In Plant Factory Using Artificial Light, 1st ed.; Anpo, M., Fukuda, H., Wada, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. xxiii–xxviii. [Google Scholar] [CrossRef]
- Anpo, M.; Fukuda, H.; Wada, T. Chapter 3.1 Optimization of growth environment and development of technology to control the concentration of functional components. In Plant Factory Using Artificial Light, 1st ed.; Anpo, M., Fukuda, H., Wada, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 121–130. [Google Scholar] [CrossRef]
- Fukuda, N. Chapter 2.2—Plant growth and physiological responses to light conditions. In Plant Factory Using Artificial Light, 1st ed.; Anpo, M., Fukuda, H., Wada, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 71–77. [Google Scholar] [CrossRef]
- Sena, S.; Kumari, S.; Kumar, V.; Husen, A. Light emitting diode (led) lights for the improvement of plant performance and production: A comprehensive review. Curr. Res. Biotechnol. 2024, 7, 100184. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef] [PubMed]
- Mottus, M.; Sulev, M.; Baret, F.; Lopez-Lozano, R.; Reinart, A. Photosynthetically active radiation: Measurement and modelling. In Encyclopedia of Sustainability Science and Technology, 1st ed.; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 7902–7932. [Google Scholar] [CrossRef]
- Yu, L.; Bu, L.; Li, D.; Zhu, K.; Zhang, Y.; Wu, S.; Chang, L.; Ding, X.; Jiang, Y. Effects of far-red light and ultraviolet light-a on growth, photosynthesis, transcriptome, and metabolome of mint (Mentha haplocalyx briq.). Plants 2024, 13, 3495. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.H.; Huang, Q.C.; Wang, H.J.; Pan, H. Comparative analysis on pigment content of sun plants and shade plants. Hubei Agric. Sci. 2010, 49, 1126–1129. [Google Scholar]
- Kelly, N.; Runkle, E.S. End-of-production ultraviolet a and blue light similarly increase lettuce coloration and phytochemical concentrations. Hort. Sci. 2023, 58, 525–531. [Google Scholar] [CrossRef]
- Chung, H.Y.; Chang, M.Y.; Wu, C.C.; Fang, W. Quantitative evaluation of electric light recipes for red leaf lettuce cultivation in plant factories. Horttechnology 2018, 28, 755–763. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Q.; Ji, G.; Wang, J.; Ding, Y.; Wang, S. Effects of light intensity and photoperiod on morphological development and photosynthetic characteristics of coriander. Horticulturae 2024, 10, 215. [Google Scholar] [CrossRef]
- Takano, M.; Inagaki, N.; Xie, X.; Kiyota, S.; Baba-Kasai, A.; Tanabata, T.; Shinomura, T. Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 14705–14710. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.P.; Kitayama, M.; Lu, N.; Takagaki, M. Improving secondary metabolite accumulation, mineral content, and growth of coriander (Coriandrum sativum L.) by regulating light quality in a plant factory. J. Hortic. Sci. Biotechnol. 2019, 95, 356–363. [Google Scholar] [CrossRef]
- Akter, N.; Cammarisano, L.; Taylor, G.; Naznin, M.; Verdonk, J.; Ahamed, S. Impact of light spectral combinations on morphology, yield, and quality of indoor-grown cilantro. Front. Sustain. Food Syst. 2024, 8, 1499954. [Google Scholar] [CrossRef]
- Bae, J.; Park, S.; Oh, M. Supplemental irradiation with far-red light-emitting diodes improves growth and phenolic contents in Crepidiastrum denticulatum in a plant factory with artificial lighting. Hortic. Environ. Biotechnol. 2017, 58, 357–366. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Somsong, P.; Weeplian, T.; Kiravittaya, S.; Tayjasanant, T. Effects of far-red and UV-A lighting on phytochemicals and antioxidant activity in late growth stage of coriander (Coriandrum sativum L.). Acta Hortic. 2025, 1423, 235–242. [Google Scholar] [CrossRef]
- Vogelmann, T.C.; Han, T. Measurement of gradients of absorbed light in spinach leaves from chlorophyll fluorescence profiles. Plant Cell Environ. 2000, 23, 1303–1311. [Google Scholar] [CrossRef]
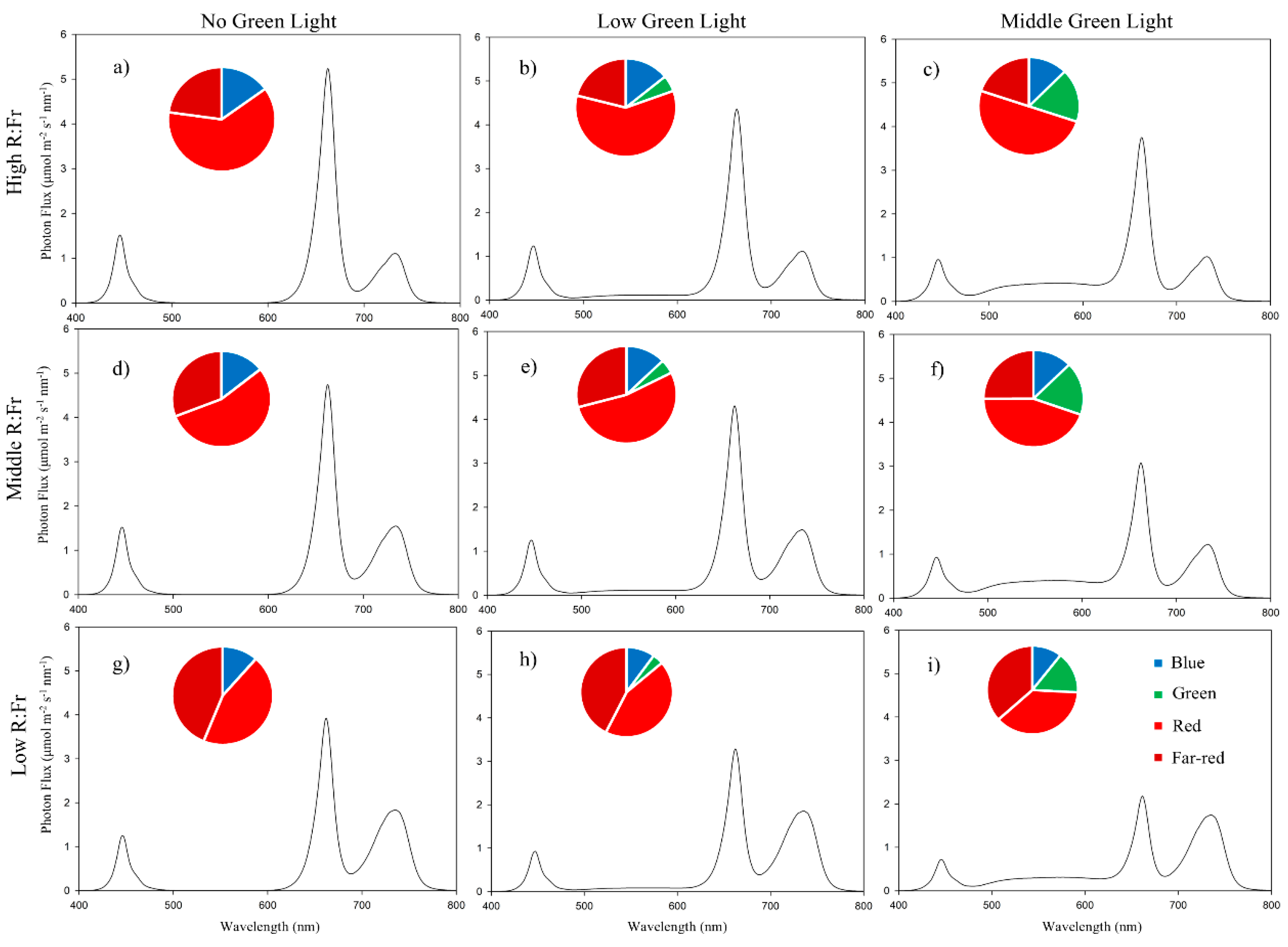


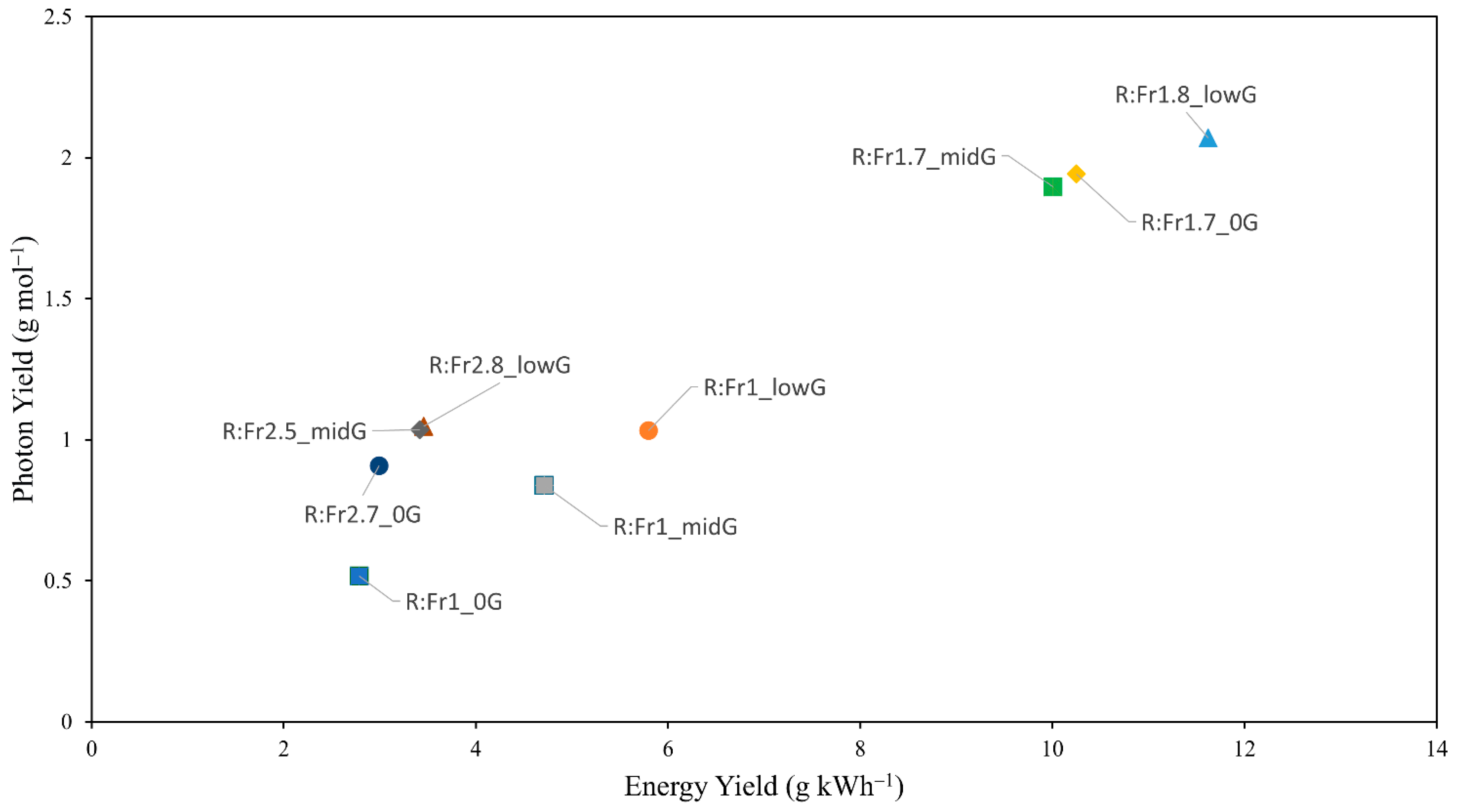
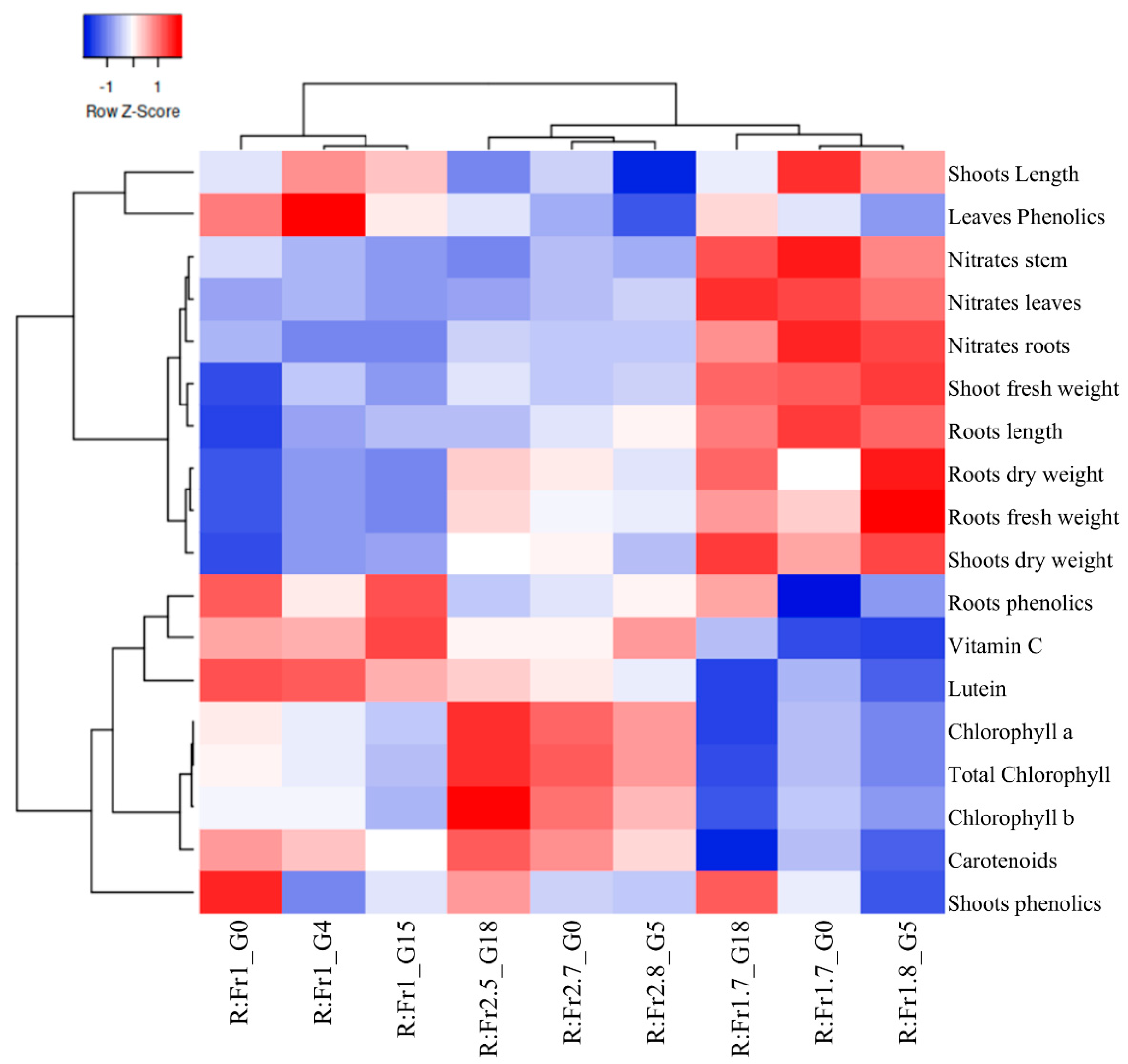
| Treatment | Percentage Contribution of Each Spectral to the Biological Photon Flux Density (BPFD = 200 µmol m−2 s−1) | R:Fr y | |||
|---|---|---|---|---|---|
| Red 600–700 nm (%) | Green 500–600 nm (%) | Blue 400–500 nm (%) | Far-Red 700–800 nm (%) | ||
| R:Fr2.7_0G | 62 z | 0 | 15 | 23 | 2.7 |
| R:Fr2.8_lowG | 60 | 5 | 14 | 21 | 2.8 |
| R:Fr2.5_midG | 50 | 18 | 12 | 20 | 2.5 |
| R:Fr1.7_0G | 55 | 0 | 14 | 31 | 1.7 |
| R:Fr1.8_lowG | 53 | 5 | 13 | 29 | 1.8 |
| R:Fr1.7_midG | 44 | 18 | 13 | 25 | 1.7 |
| R:Fr1_0G | 45 | 0 | 11 | 44 | 1 |
| R:Fr1_lowG | 44 | 4 | 10 | 42 | 1 |
| R:Fr1_midG | 37 | 15 | 11 | 37 | 1 |
| Treatment | Shoot FW (g) | Shoot DW (g) | Root FW (g) | Root DW (g) |
|---|---|---|---|---|
| R:Fr2.7_0G | 33.2 ± 18.0 bc | 4.2 ± 1.8 abc | 12.9 ± 4.5 a | 1.6 ± 0.2 abcd |
| R:Fr2.8_lowG | 35.1 ± 17.4 b | 3.1 ± 1.7 bc | 12.8 ± 1.9 a | 1.5 ± 0.1 bcd |
| R:Fr2.5_midG | 37.6 ± 19.2 b | 4.2 ± 2.8 abc | 15.4 ± 4.5 a | 1.7 ± 0.3 abc |
| R:Fr1.7_0G | 62.3 ± 11.5 a | 5.5 ± 0.3 ab | 15.9 ± 3.2 b | 1.6 ± 0.1 abcd |
| R:Fr1.8_lowG | 66.4 ± 7.4 a | 6.9 ± 0.5 a | 24.4 ± 6.9 a | 2.1 ± 0.3 a |
| R:Fr1.7_midG | 60.8 ± 7.5 a | 7.0 ± 0.3 a | 18.1 ± 4.3 b | 1.9 ± 0.1 ab |
| R:Fr1_0G | 16.5 ± 5.0 c | 1.3 ± 0.0 c | 5.8 ± 1.1 e | 1.2 ± 0.0 d |
| R:Fr1_lowG | 33.1 ± 15.7 bc | 2.5 ± 0.2 cb | 8.8 ± 3.0 de | 1.3 ± 0.0 cd |
| R:Fr1_midG | 26.9 ± 10.1 bc | 2.6 ± 0.2 cb | 7.9 ± 1.4 e | 1.3 ± 0.0 cd |
| R:Fr | *** | *** | *** | *** |
| Green light % | NS | NS | NS | NS |
| R:Fr × Green light % | NS | NS | NS | NS |
| Treatment | Lutein (mg/g) | Carotenoids (mg/g) | Vitamin C (mg/g) | Total Chlorophyll (mg/g) |
|---|---|---|---|---|
| R:Fr2.7_0G | 0.34 ± 0.02 abc | 0.52 ± 0.04 ab | 1.50 ± 0.08 bc | 1.59 ± 0.10 a |
| R:Fr2.8_lowG | 0.32 ± 0.01 bcd | 0.47 ± 0.02 ab | 1.79 ± 0.06 ab | 1.45 ± 0.06 ab |
| R:Fr2.5_midG | 0.36 ± 0.02 abc | 0.55 ± 0.05 a | 1.51 ± 0.25 bc | 1.7 ± 0.17 a |
| R:Fr1.7_0G | 0.28 ± 0.07 cde | 0.39 ± 0.09 bcd | 0.86 ± 0.02 d | 1.04 ± 0.26 cd |
| R:Fr1.8_lowG | 0.22 ± 0.03 de | 0.32 ± 0.04 cd | 0.83 ± 0.047 d | 0.90 ± 0.13 cd |
| R:Fr1.7_midG | 0.20 ± 0.03 e | 0.28 ± 0.05 d | 1.27 ± 0.07 c | 0.75 ± 0.16 d |
| R:Fr1_0G | 0.44 ± 0.03 a | 0.51 ± 0.04 ab | 1.78 ± 0.08 ab | 1.24 ± 0.10 bc |
| R:Fr1_lowG | 0.43 ± 0.10 ab | 0.48 ± 0.09 ab | 1.74 ± 0.19 b | 1.17 ± 0.20 bc |
| R:Fr1_midG | 0.38 ± 0.05 abc | 0.44 ± 0.06 abc | 2.06 ± 0.18 a | 1.05 ± 0.18 cd |
| R:Fr | *** | *** | *** | *** |
| Green light % | NS | * | *** | NS |
| R:Fr × Green light % | NS | NS | *** | * |
| Treatment | Nitrates Concentration (mg/kg) | Phenolics Concentration (mg GAE z/g FW) | ||||
|---|---|---|---|---|---|---|
| Leaves | Stem | Roots | Leaves | Stem | Roots | |
| R:Fr2.7_0G | 3840 ± 403 b | 8040 ± 610 b | 4280 ± 216 c | 60.38 ± 9.73 bc | 13.50 ± 2.56 abc | 6.94 ± 1.85 abc |
| R:Fr2.8_lowG | 4700 ± 0 b | 7600 ± 391 b | 4280 ± 383 c | 52.48 ± 9.89 c | 13.32 ± 0.99 abc | 8.07 ± 1.67 ab |
| R:Fr2.5_midG | 3025 ± 95 b | 6366 ± 57 b | 4360 ± 194 c | 66.56 ± 15.89 bc | 17.50 ± 3.63 abc | 6.10 ± 1.01 abc |
| R:Fr1.7_0G | 11600 ± 1019 a | 16300 ± 503 a | 12880 ± 1584 a | 66.55 ± 4.52 bc | 14.35 ± 5.19 abc | 0.16 ± 0.05 c |
| R:Fr1.8_lowG | 10200 ± 516 a | 13500 ± 382 a | 11920 ± 1035 a | 59.24 ± 7.64 bc | 10.24 ± 1.59 bc | 4.56 ± 1.53 bc |
| R:Fr1.7_midG | 12000 ± 1939 a | 14880 ± 521 a | 9520 ± 715 b | 72.13 ± 14.40 bc | 19.14 ± 7.14 ab | 10.76 ± 2.45 ab |
| R:Fr1_0G | 3028 ± 961 b | 9040 ± 723 b | 3616 ± 1037 cd | 80.08 ± 8.78 ab | 20.36 ± 1.91 a | 12.68 ± 6.39 a |
| R:Fr1_lowG | 3520 ± 851 b | 7900 ± 1205 b | 2090 ± 377 d | 99.07 ± 22.67 a | 11.4 ± 2.76 bc | 8.37 ± 2.02 ab |
| R:Fr1_midG | 2708 ± 1526 b | 6900 ± 3940 b | 2008 ± 857 d | 70.20 ± 5.78 bc | 14.05 ± 5.42 abc | 12.93 ± 0.70 a |
| R:Fr | *** | *** | *** | *** | NS | *** |
| Green light % | NS | * | *** | NS | ** | * |
| R:Fr × Green light % | * | NS | *** | ** | * | ** |
| Treatment | Total Nitrates Yield (g/m2) | Total Phenolics Yield (g GAE z/m2) | ||
|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | |
| R:Fr2.7_0G | 3.18 ± 1.72 b | 0.74 ± 0.26 c | 19.48 ± 10.55 cde | 1.42 ± 0.50 bcd |
| R:Fr2.8_lowG | 3.03 ± 1.67 bc | 0.73 ± 0.11 cd | 18.3 ± 9.08 de | 1.64 ± 0.25 b |
| R:Fr2.5_midG | 3.60 ± 1.84 b | 0.89 ± 0.26 bc | 25.05 ± 12.80 cd | 1.49± 0.44 bcd |
| R:Fr1.7_0G | 5.96 ± 1.11 a | 0.91 ± 0.18 bc | 39.93 ± 7.42 ab | 0.04 ± 0.01 e |
| R:Fr1.8_lowG | 6.35 ± 0.71 a | 1.40 ± 0.40 a | 36.55 ± 4.08 ab | 1.76 ± 0.50 b |
| R:Fr1.7_midG | 5.82 ± 0.72 a | 1.04 ± 0.25 b | 43.99 ± 5.45 a | 3.00 ± 0.74 a |
| R:Fr1_0G | 1.59 ± 0.48 c | 0.34 ± 0.06 e | 13.20 ± 3.99 e | 1.17 ± 0.23 cd |
| R:Fr1_lowG | 3.17 ± 1.50 b | 0.51 ± 0.18 de | 28.99 ± 13.74 bc | 1.17 ± 0.41 cd |
| R:Fr1_midG | 2.43 ± 0.97 bc | 0.46 ± 0.09 e | 17.96 ± 6.74 de | 1.63 ± 0.31 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda Sotelo, M.M.; Tu, Y.-K.; Chang, P.P.-C.; Fang, W.; Chung, H.-Y. Effects of Low Green Light Combined with Different Red and Far-Red Light Ratios on the Growth and Secondary Metabolites of Cilantro (Coriandrum sativum L.). Agronomy 2025, 15, 1363. https://doi.org/10.3390/agronomy15061363
Miranda Sotelo MM, Tu Y-K, Chang PP-C, Fang W, Chung H-Y. Effects of Low Green Light Combined with Different Red and Far-Red Light Ratios on the Growth and Secondary Metabolites of Cilantro (Coriandrum sativum L.). Agronomy. 2025; 15(6):1363. https://doi.org/10.3390/agronomy15061363
Chicago/Turabian StyleMiranda Sotelo, Manuel Mayam, Yuan-Kai Tu, Pearl Pei-Chun Chang, Wei Fang, and Hsing-Ying Chung. 2025. "Effects of Low Green Light Combined with Different Red and Far-Red Light Ratios on the Growth and Secondary Metabolites of Cilantro (Coriandrum sativum L.)" Agronomy 15, no. 6: 1363. https://doi.org/10.3390/agronomy15061363
APA StyleMiranda Sotelo, M. M., Tu, Y.-K., Chang, P. P.-C., Fang, W., & Chung, H.-Y. (2025). Effects of Low Green Light Combined with Different Red and Far-Red Light Ratios on the Growth and Secondary Metabolites of Cilantro (Coriandrum sativum L.). Agronomy, 15(6), 1363. https://doi.org/10.3390/agronomy15061363







