Long-Term Irrigation Deficits Impair Microbial Diversity and Soil Quality in Arid Maize Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Management
2.2.1. Experimental Design
2.2.2. Field Management
2.2.3. Calculation of Irrigation Water Deficit Index
2.2.4. Calculation of Crop Evapotranspiration
- (1)
- Crop evapotranspiration (ETcrop)
- (2)
- Effective precipitation (Peff)
- (3)
- Irrigation volume (Wgross)
- (4)
- Irrigation Water Deficit Index (IWDI)
- (5)
- Cumulative Irrigation Water Deficit (CIWD).
- (6)
- Maximum Cumulative Irrigation Requirement (MCIR) is the theoretical maximum water deficit for each plot.
2.3. Sample Collection and Measurement
2.3.1. Collection and Analysis of Soil Samples
2.3.2. Soil Quality Assessment
2.3.3. Maize Yield
2.3.4. Bioinformatics
2.4. Data Statistical Analysis and Visualization
3. Results
3.1. Irrigation Water Deficit Index (IWDI)
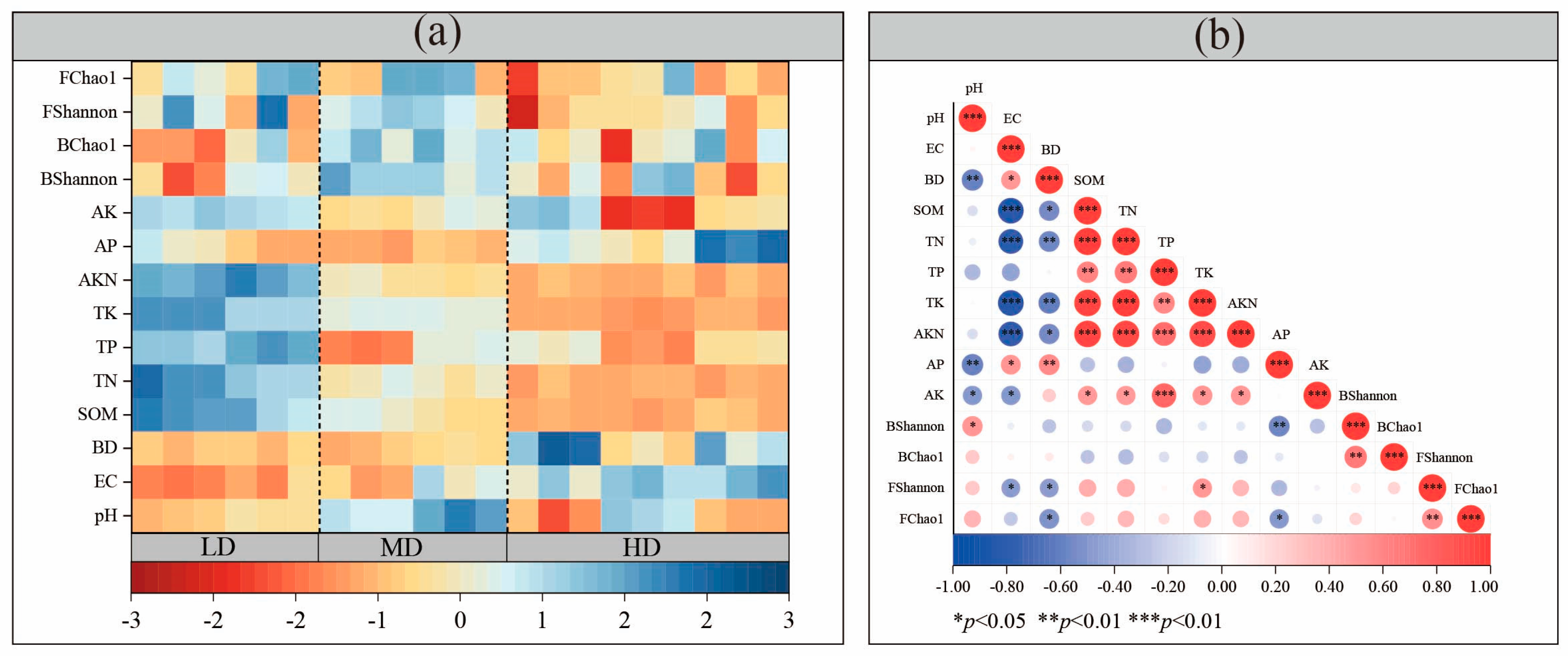
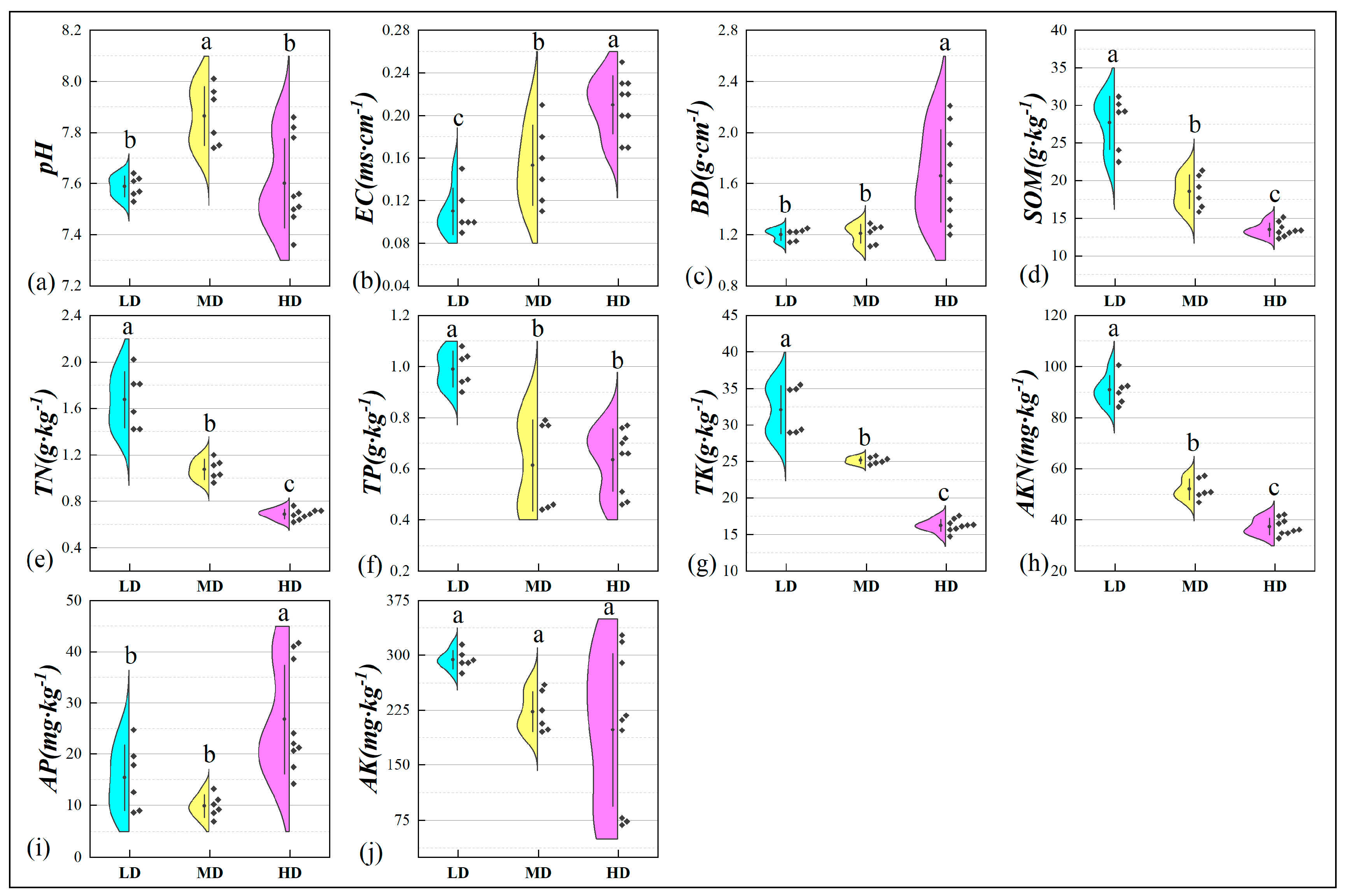
3.2. Basic Soil Properties
3.2.1. Physical Parameters
3.2.2. Chemical Parameters
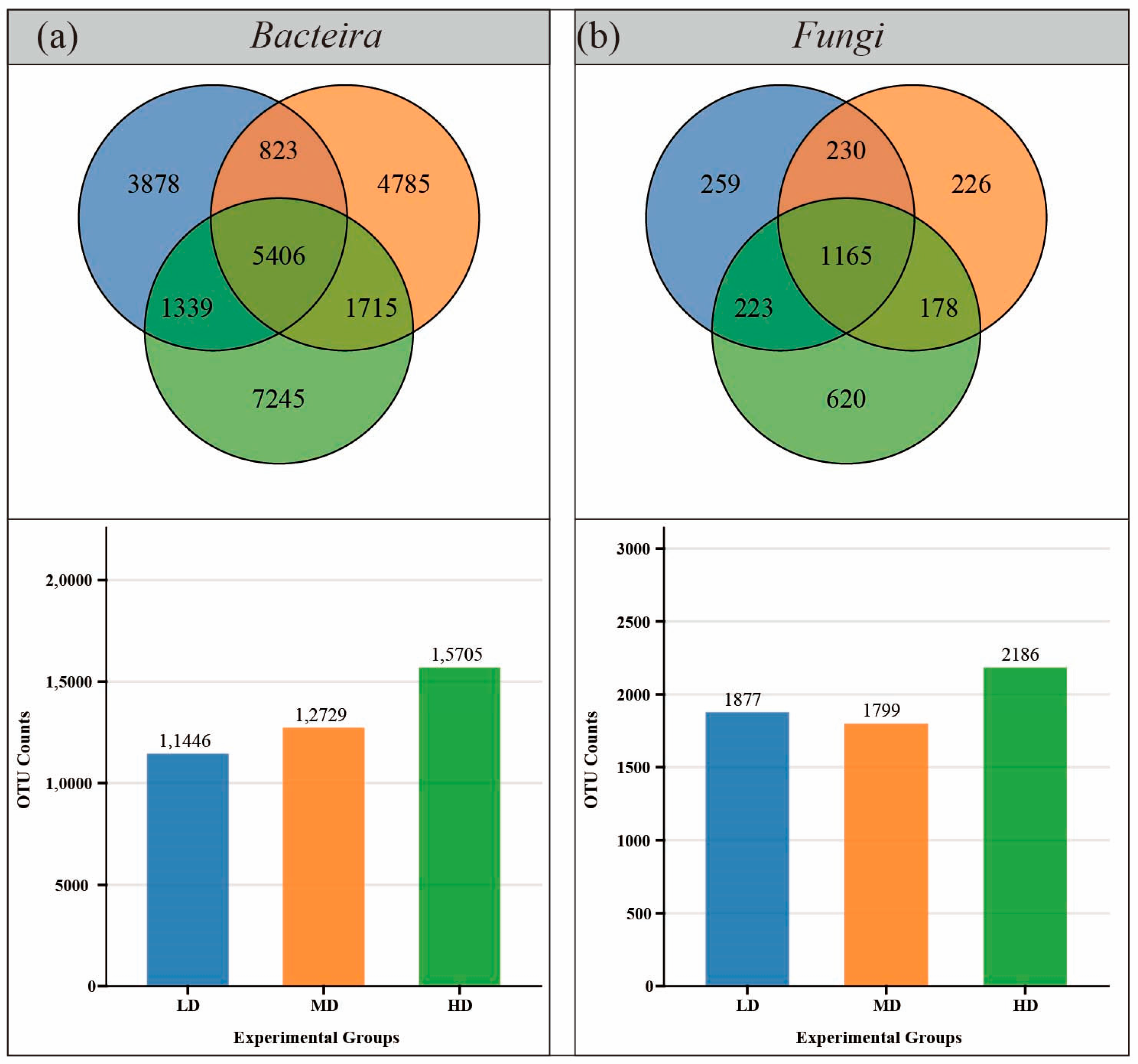
3.3. Characteristics of Soil Microbial Communities
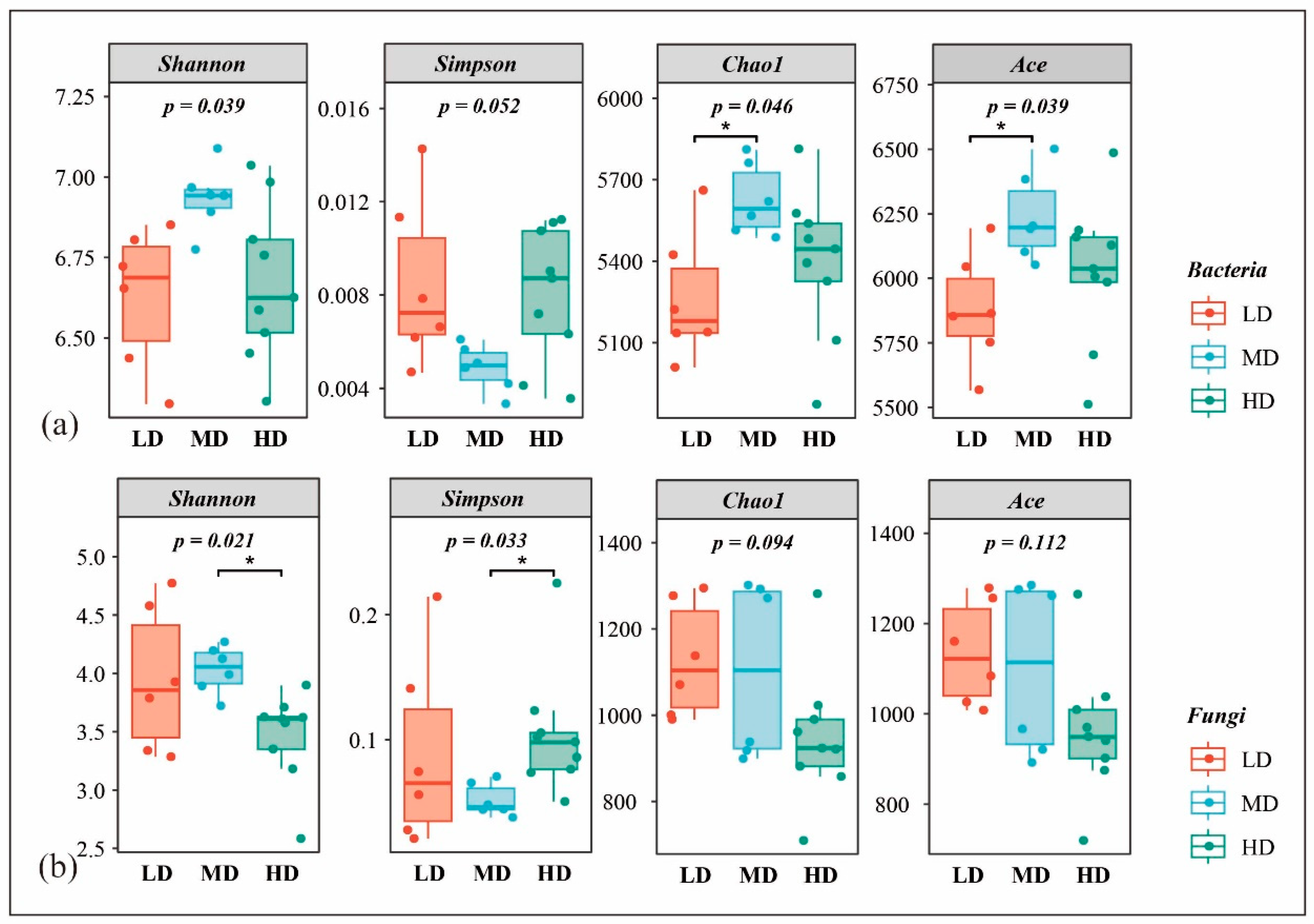
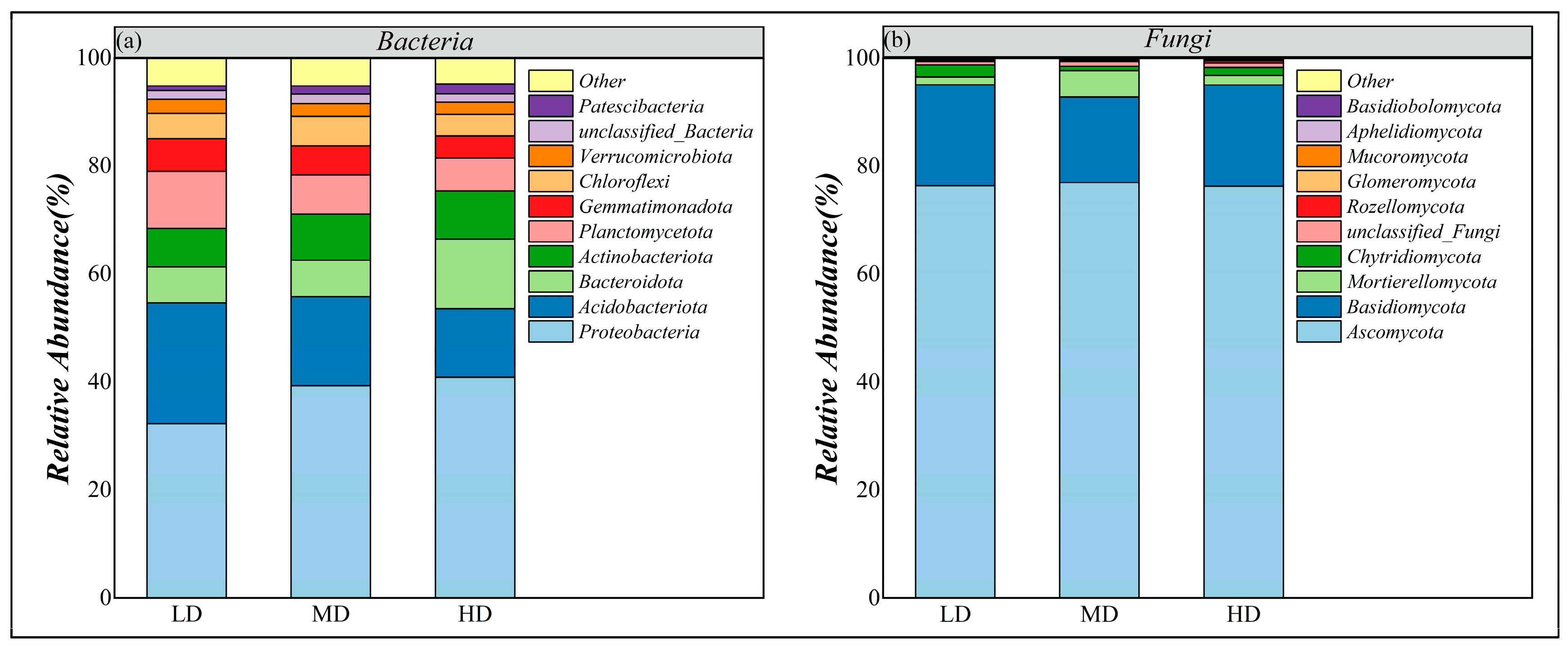
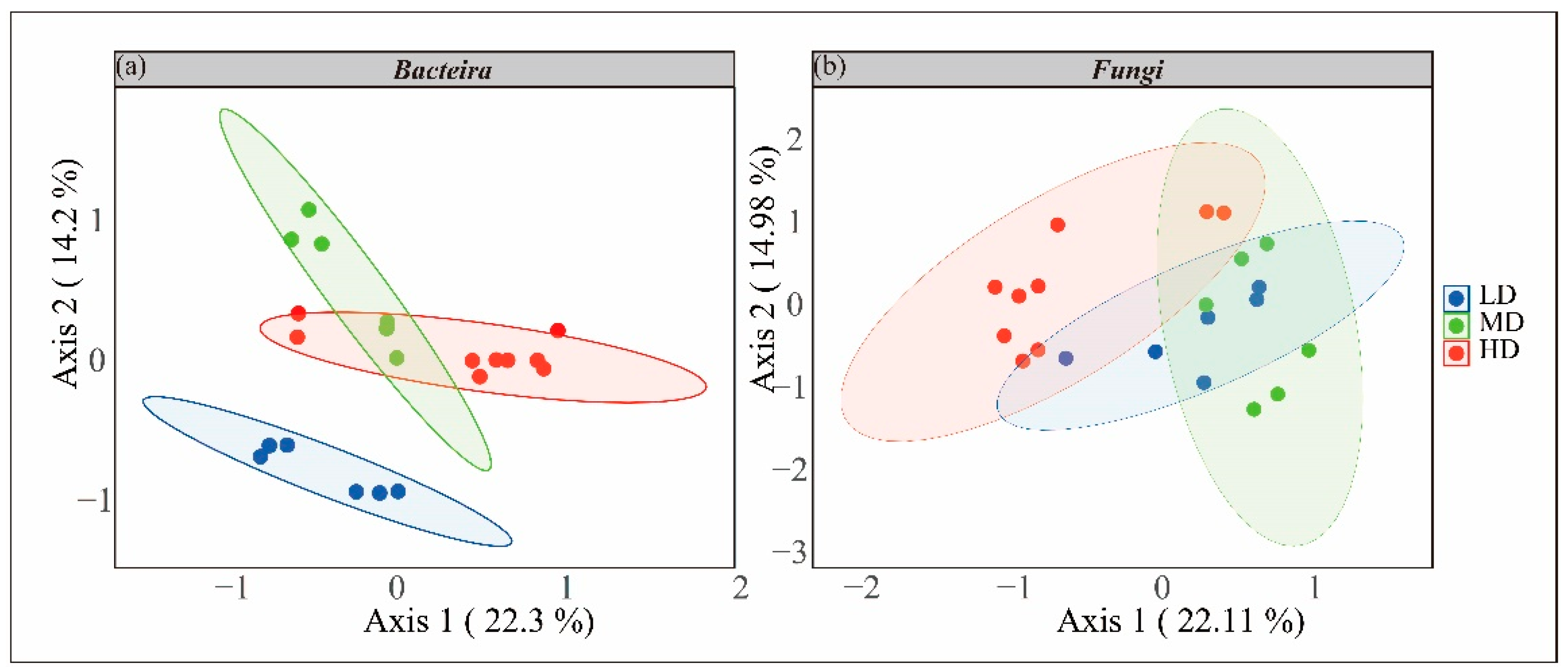
3.3.1. Species Diversity and Composition
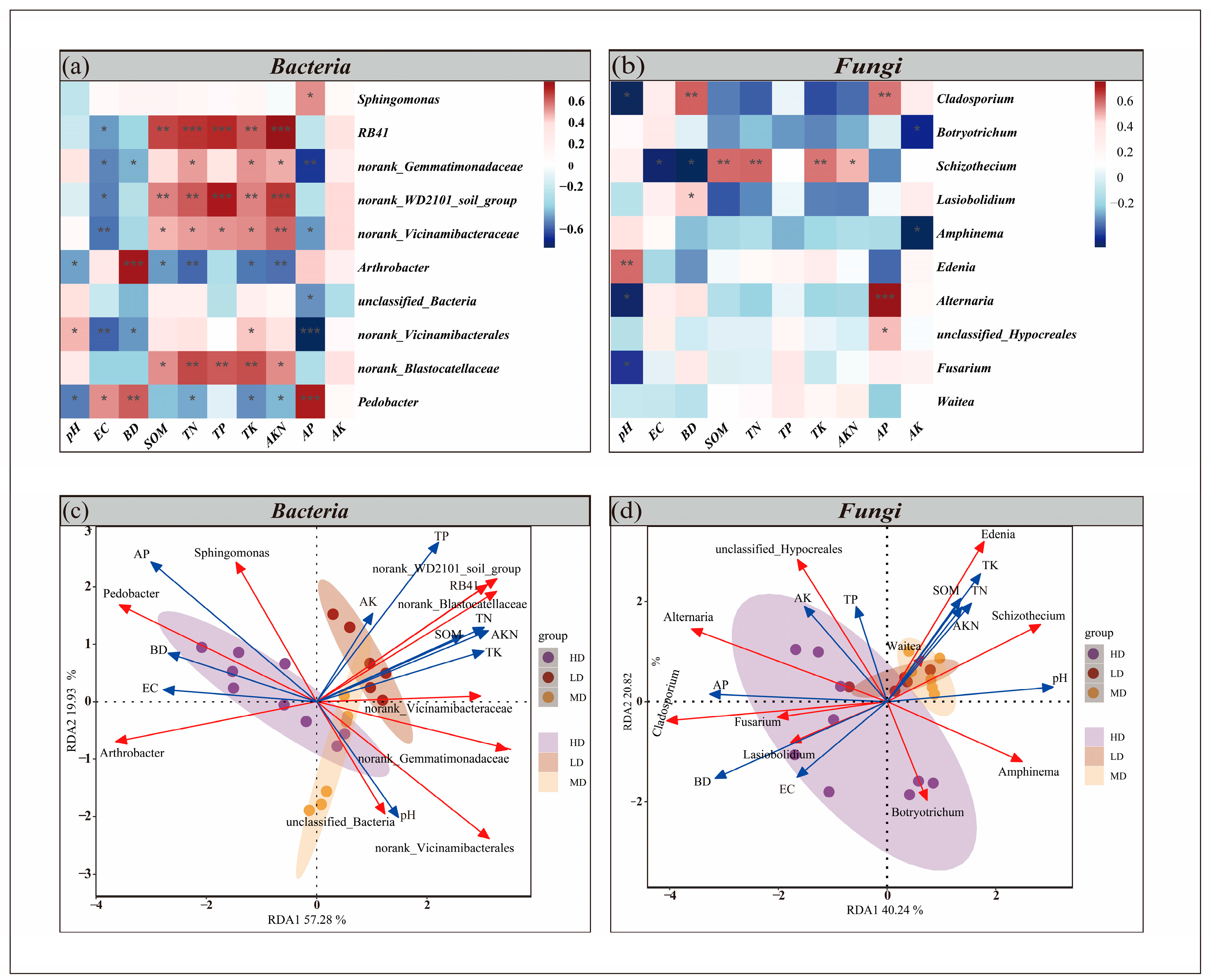
3.3.2. Inter-Species Differences
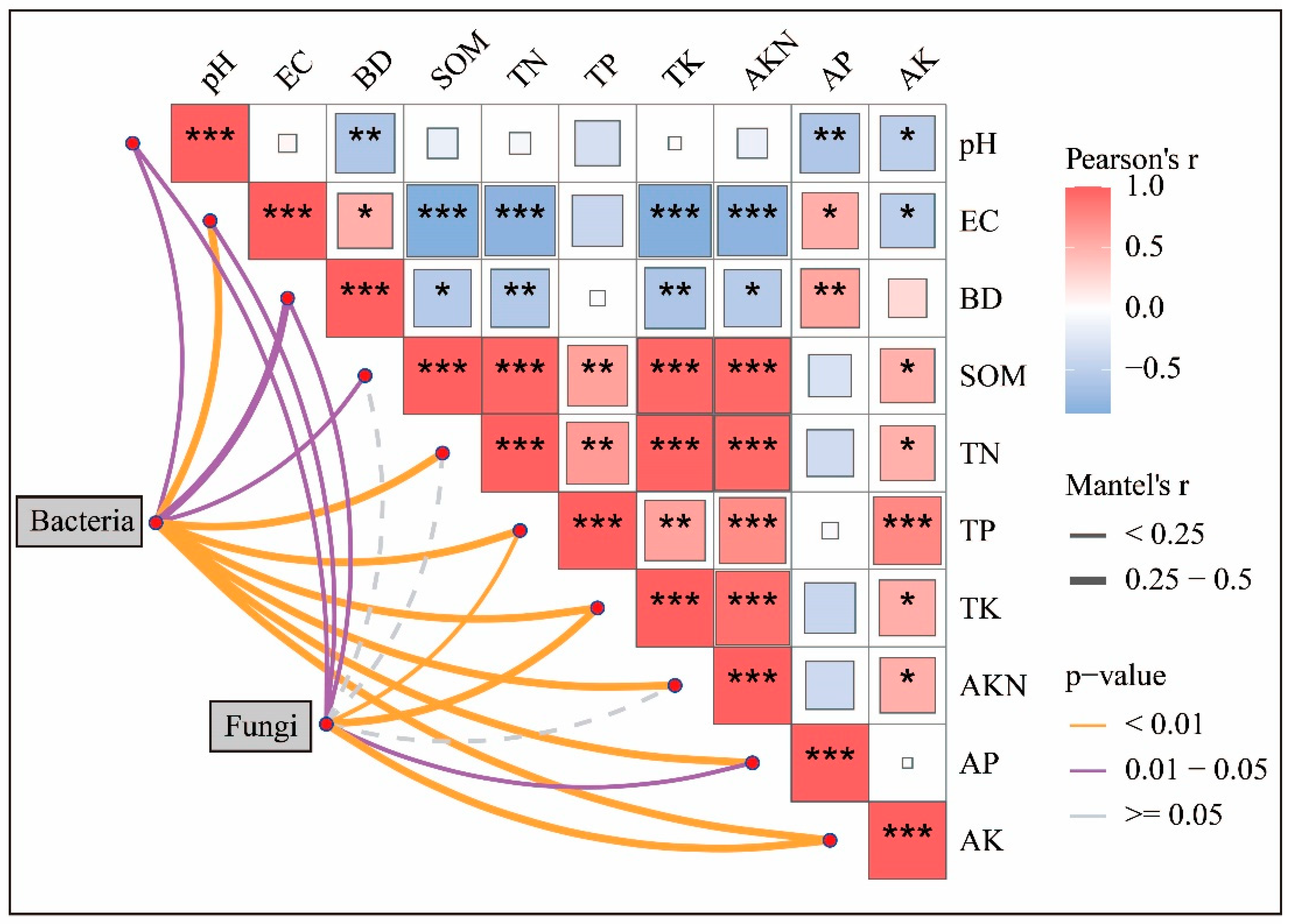
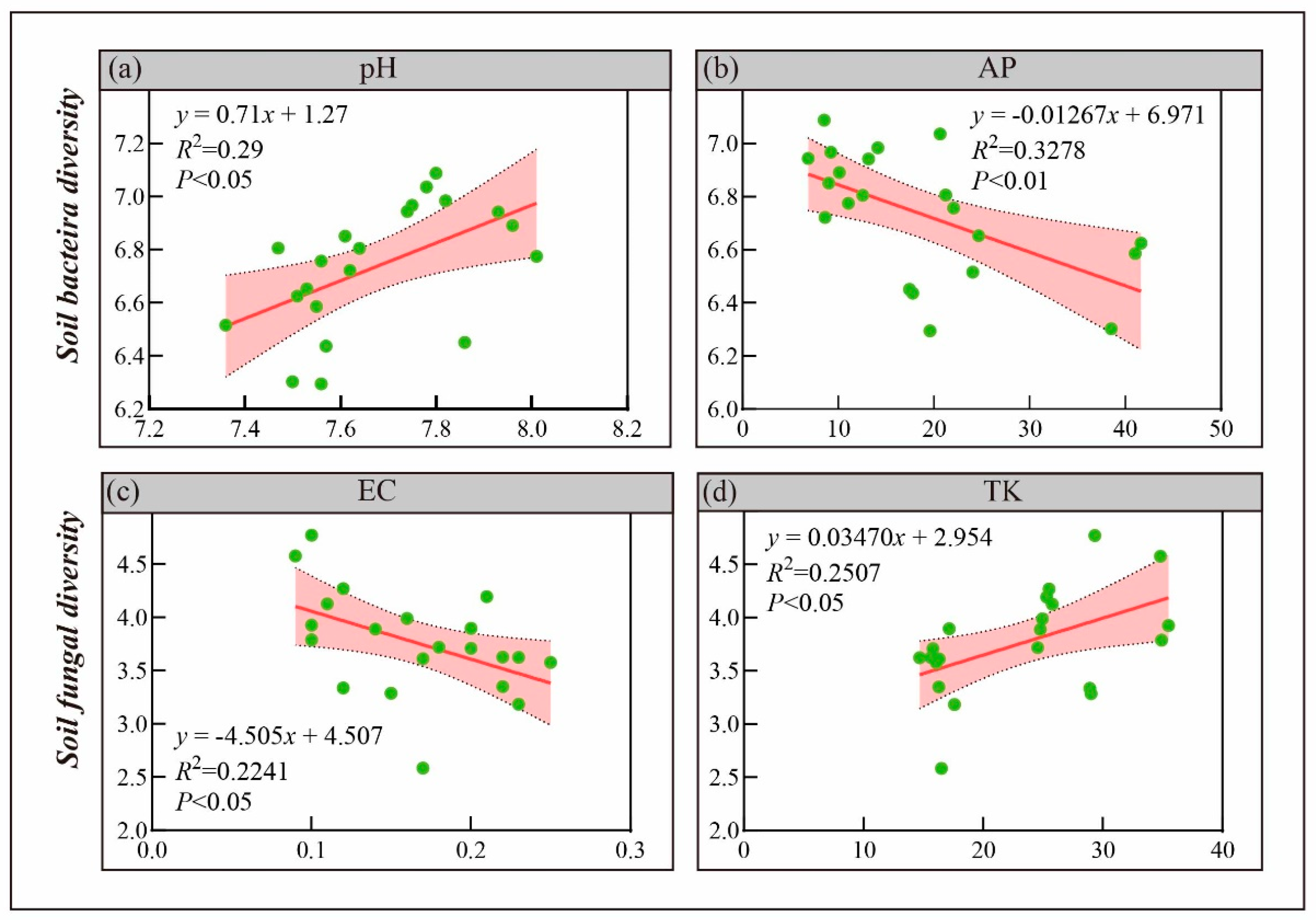
3.3.3. Relationship Between Soil Properties and Microorganisms
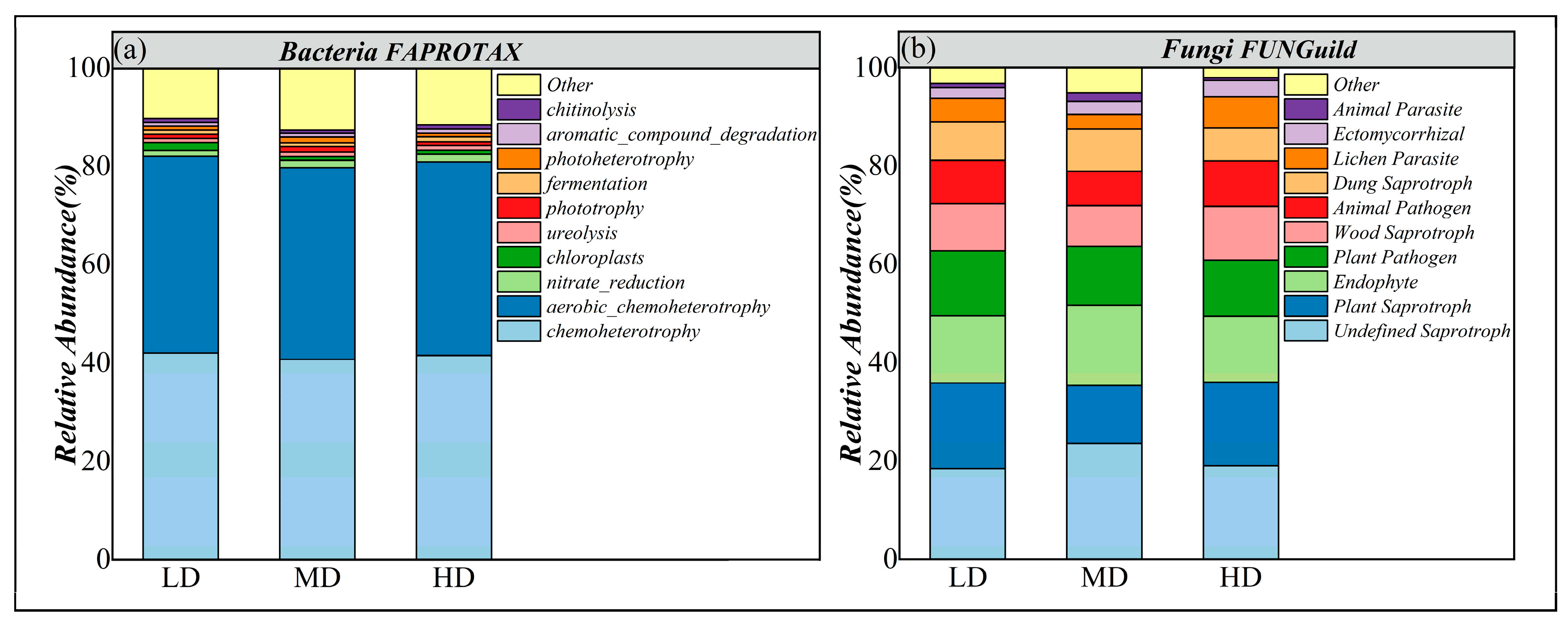
3.3.4. Functional Groups of Soil Microbial Communities
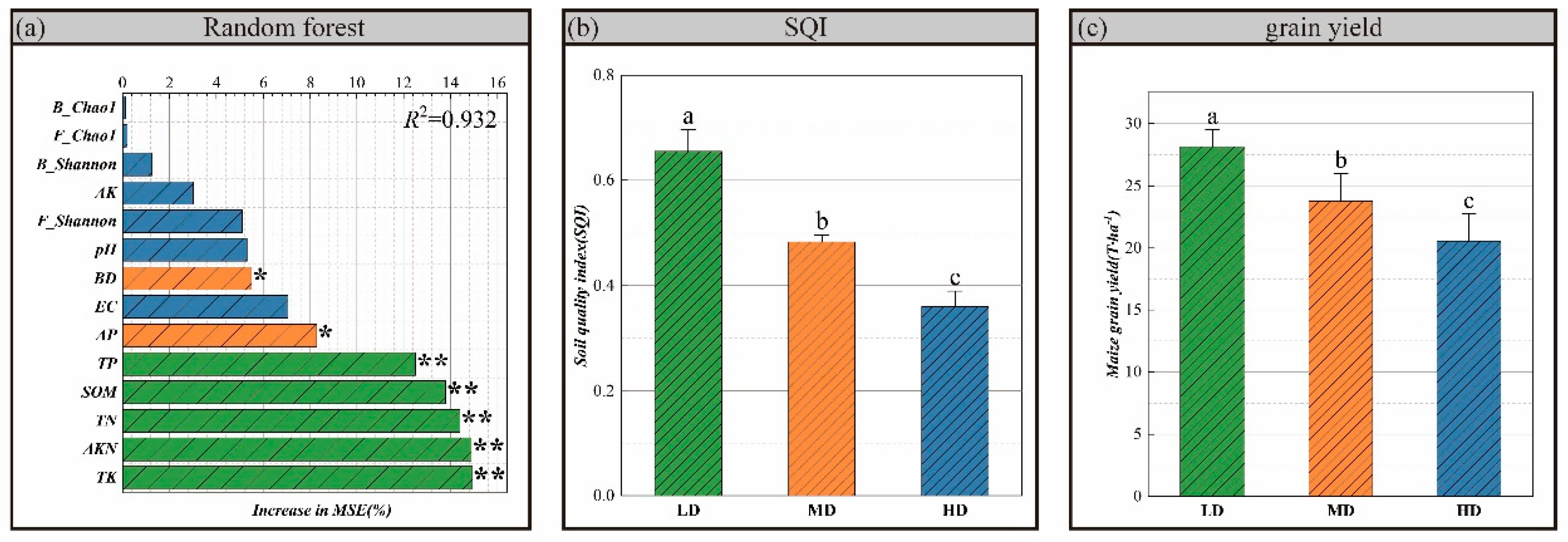
3.4. Soil Quality and Maize Yield
4. Discussion
4.1. Irrigation Water Deficit Drives Gradient Changes in Soil Physicochemical Properties
4.2. Differential Responses of Microbial Diversity to Water Stress
4.3. Water Gradient Shapes Microbial Community Restructuring
4.4. Analysis of Soil Quality–Yield Coupling Mechanisms
4.5. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Table
| Phylum | Group | ANOVO | ||
|---|---|---|---|---|
| LD | MD | HD | ||
| Proteobacteria | 32.31 b | 39.34 a | 40.89 a | ** |
| Acidobacteriota | 22.60 a | 16.53 b | 12.73 b | ** |
| Bacteroidota | 6.84 b | 6.77 b | 12.89 a | ** |
| Actinobacteriota | 7.08 a | 8.47 a | 8.82 a | ns |
| Planctomycetota | 10.24 a | 7.25 ab | 6.18 b | * |
| Gemmatimonadota | 6.12 a | 5.38 ab | 4.07 b | ** |
| Chloroflexi | 4.63 a | 5.47 a | 3.97 a | ns |
| Verrucomicrobiota | 2.59 a | 2.37 a | 2.34 a | ns |
| unclassified_Bacteria | 1.63 a | 1.76 a | 1.52 a | ns |
| Patescibacteria | 0.81 b | 1.51 a | 1.84 a | ** |
| Phylum | Group | ANOVO | ||
|---|---|---|---|---|
| LD | MD | HD | ||
| Ascomycota | 77.86 a | 77.09 a | 77.12 a | ns |
| Basidiomycota | 17.42 a | 15.40 a | 18.81 a | ns |
| Mortierellomycota | 1.00 b | 4.66 a | 1.00 b | * |
| Chytridiomycota | 1.41 a | 0.90 a | 2.06 a | ns |
| unclassified_Fungi | 0.811 a | 1.19 a | 0.50 a | ns |
| Rozellomycota | 0.38 a | 0.18 a | 0.10 a | ns |
| Glomeromycota | 0.15 a | 0.14 a | 0.12 a | ns |
| Mucoromycota | 0.08 a | 0.04 a | 0.17 a | ns |
| Aphelidiomycota | 0.05 a | 0.18 ab | 0.04 b | ** |
| Basidiobolomycota | 0.13 a | 0.11 a | 0.01 a | ns |
| Function | Group | ANOVO | ||
|---|---|---|---|---|
| LD | MD | HD | ||
| chemoheterotrophy | 42.04 a | 40.80 a | 41.59 a | ns |
| aerobic_chemoheterotrophy | 40.04 a | 38.97 a | 39.38 a | ns |
| nitrate_reduction | 1.19 a | 1.53 a | 1.53 a | ns |
| chloroplasts | 1.65 a | 0.73 a | 0.83 a | ns |
| ureolysis | 0.85 a | 0.92 a | 0.97 a | ns |
| phototrophy | 0.81 b | 1.19 a | 0.75 b | * |
| fermentation | 0.86 a | 0.72 a | 1.02 a | ns |
| photoheterotrophy | 0.79 ba | 1.17 a | 0.70 b | * |
| aromatic_compound_degradation | 0.78 a | 0.75 a | 0.84 a | ns |
| chitinolysis | 0.83 a | 0.64 a | 0.86 a | ns |
| Function | Group | ANOVO | ||
|---|---|---|---|---|
| LD | MD | HD | ||
| Undefined_Saprotroph | 18.43 a | 22.95 a | 21.55 a | ns |
| Plant_Saprotroph | 17.44 a | 12.60 a | 16.02 a | ns |
| Endophyte | 13.66 a | 15.31 a | 13.95 a | ns |
| Plant_Pathogen | 13.23 a | 12.21 a | 10.70 a | ns |
| Wood_Saprotroph | 9.55 a | 9.05 a | 9.06 a | ns |
| Animal_Pathogen | 8.89 a | 7.23 a | 7.89 a | ns |
| Dung_Saprotroph | 7.80 a | 7.84 a | 8.67 a | ns |
| Lichen_Parasite | 4.84 a | 3.27 a | 5.42 a | ns |
| Ectomycorrhizal | 2.10 a | 2.65 a | 3.34 a | ns |
| Animal_Parasite | 0.85 a | 1.69 a | 0.70 a | ns |
Appendix B. Figure
References
- Xing, Y.; Wang, X. Precision Agriculture and Water Conservation Strategies for Sustainable Crop Production in Arid Regions. Plants 2024, 13, 3184. [Google Scholar] [CrossRef] [PubMed]
- Rehaman, A.; Khan, S.; Rawat, B.; Gaira, K.S.; Asgher, M.; Semwal, P.; Tripathi, V. Mechanistic Insights into Plant Drought Tolerance: A Multi-Level Perspective. J. Crop Health 2025, 77, 53. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Quiñonez-Barzola, X.; Jaya-Montalvo, M.; Carrión-Mero, P. What Do We Know about Water Scarcity in Semi-Arid Zones? A Global Analysis and Research Trends. Water 2022, 14, 2685. [Google Scholar] [CrossRef]
- Sun, M.; Dai, Y.; Zhang, S.; Liang, H. Risk Assessment of Extreme Drought and Extreme Wetness During Growth Stages of Major Crops in China. Sustainability 2025, 17, 2221. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L., II; Mortimore, M.; Batterbury, S.P.J.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Global Desertification: Building a Science for Dryland Development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef]
- Schimel, D.S. Drylands in the Earth System. Science 2010, 327, 418–419. [Google Scholar] [CrossRef]
- Lyu, J.; Shi, Y.; Liu, T.; Xu, X.; Liu, S.; Yang, G.; Peng, D.; Qu, Y.; Zhang, S.; Chen, C.; et al. Extreme Drought-Heatwave Events Threaten the Biodiversity and Stability of Aquatic Plankton Communities in the Yangtze River Ecosystems. Commun. Earth Environ. 2025, 6, 1–12. [Google Scholar] [CrossRef]
- Stromberger, M.; Shah, Z.; Westfall, D. Soil Microbial Communities of No-till Dryland Agroecosystems across an Evapotranspiration Gradient. Appl. Soil Ecol. 2007, 35, 94–106. [Google Scholar] [CrossRef]
- Taskin, E.; Boselli, R.; Fiorini, A.; Misci, C.; Ardenti, F.; Bandini, F.; Guzzetti, L.; Panzeri, D.; Tommasi, N.; Galimberti, A.; et al. Combined Impact of No-Till and Cover Crops with or without Short-Term Water Stress as Revealed by Physicochemical and Microbiological Indicators. Biology 2021, 10, 23. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Cheng, Z.; Yin, F.; Yang, L.; Wang, Z. Changes in Soil Bacterial and Fungal Community Characteristics in Response to Long-Term Mulched Drip Irrigation in Oasis Agroecosystems. Agric. Water Manag. 2023, 279, 108178. [Google Scholar] [CrossRef]
- Du, Y.; Lv, S.; Wang, F.; Xu, J.; Zhao, H.; Tang, L.; Wang, H.; Zhang, H. Investigation into the Temporal Impacts of Drought on Vegetation Dynamics in China during 2000 to 2022. Sci. Rep. 2025, 15, 6351. [Google Scholar] [CrossRef]
- You, Z.; Sun, X.; Sun, H.; Chen, L.; Lu, M.; Xue, J.; Ban, X.; Yan, B.; Tuo, Y.; Qin, H.; et al. Mechanisms of Meteorological Drought Propagation to Agricultural Drought in China: Insights from Causality Chain. npj Nat. Hazards 2025, 2, 1–15. [Google Scholar] [CrossRef]
- Mola, M.; Kougias, P.G.; Statiris, E.; Papadopoulou, P.; Malamis, S.; Monokrousos, N. Short-Term Effect of Reclaimed Water Irrigation on Soil Health, Plant Growth and the Composition of Soil Microbial Communities. Sci. Total Environ. 2024, 949, 175107. [Google Scholar] [CrossRef] [PubMed]
- Ngetich, K.F.; Diels, J.; Shisanya, C.A.; Mugwe, J.N.; Mucheru-muna, M.; Mugendi, D.N. Effects of Selected Soil and Water Conservation Techniques on Runoff, Sediment Yield and Maize Productivity under Sub-Humid and Semi-Arid Conditions in Kenya. Catena 2014, 121, 288–296. [Google Scholar] [CrossRef]
- Uwizeyimana, D.; Mureithi, S.M.; Karuku, G.; Kironchi, G. Effect of Water Conservation Measures on Soil Moisture and Maize Yield under Drought Prone Agro-Ecological Zones in Rwanda. Int. Soil Water Conserv. Res. 2018, 6, 214–221. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Kim, D.-G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought Effects on Soil Carbon and Nitrogen Dynamics in Global Natural Ecosystems. Earth-Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil Salinity and Drought Tolerance: An Evaluation of Plant Growth, Productivity, Microbial Diversity, and Amelioration Strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Peralta Ogorek, L.L.; Gao, Y.; Farrar, E.; Pandey, B.K. Soil Compaction Sensing Mechanisms and Root Responses. Trends Plant Sci. 2024, 30, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple Elements of Soil Biodiversity Drive Ecosystem Functions across Biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Gu, H.; Liu, Z.; Yao, Q.; Jiao, F.; Liu, J.; Jin, J.; Liu, X.; Wang, G. Distinct Effects of Abundant and Rare Microbial Communities on Ecosystem Multifunctionality across the Soil Profiles in Agricultural Isohumosols. Soil. Ecol. Lett. 2025, 7, 240289. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The Interplay between Microbial Communities and Soil Properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Loiko, N.; Islam, M.N. Plant–Soil Microbial Interaction: Differential Adaptations of Beneficial vs. Pathogenic Bacterial and Fungal Communities to Climate-Induced Drought. Agronomy 2024, 14, 1949. [Google Scholar] [CrossRef]
- Oram, N.J.; Brennan, F.; Praeg, N.; Bardgett, R.D.; Illmer, P.; Ingrisch, J.; Bahn, M. Plant Community Composition and Traits Modulate the Impacts of Drought Intensity on Soil Microbial Community Composition and Function. Soil Biol. Biochem. 2025, 200, 109644. [Google Scholar] [CrossRef]
- Liu, K.; Deng, F.; Zeng, F.; Chen, Z.-H.; Qin, Y.; Chen, G. Plant Growth-Promoting Rhizobacteria Improve Drought Tolerance of Crops: A Review. Plant Growth Regul. 2025, 105, 567–581. [Google Scholar] [CrossRef]
- Hu, D.; Zhou, X.; Ma, G.; Pan, J.; Ma, H.; Chai, Y.; Li, Y.; Yue, M. Increased Soil Bacteria-Fungus Interactions Promote Soil Nutrient Availability, Plant Growth, and Coexistence. Sci. Total Environ. 2024, 955, 176919. [Google Scholar] [CrossRef]
- Kang, H.; Xue, Y.; Cui, Y.; Moorhead, D.L.; Lambers, H.; Wang, D. Nutrient Limitation Mediates Soil Microbial Community Structure and Stability in Forest Restoration. Sci. Total Environ. 2024, 935, 173266. [Google Scholar] [CrossRef]
- Li, S. The Effects of Different Irrigation Methods on Corn Growth and Soil Quality. Trop. Agric. Eng. 2024, 48, 77–79. [Google Scholar]
- Xing, Z.; Ma, M.; Wei, Y.; Zhang, X.; Yu, Z.; Yi, P. A New Agricultural Drought Index Considering the Irrigation Water Demand and Water Supply Availability. Nat. Hazards 2020, 104, 2409–2429. [Google Scholar] [CrossRef]
- Ding, Y.; Peng, S. Spatiotemporal Trends and Attribution of Drought across China from 1901–2100. Sustainability 2020, 12, 477. [Google Scholar] [CrossRef]
- Peng, S.; Ding, Y.; Liu, W.; Li, Z. 1 Km Monthly Temperature and Precipitation Dataset for China from 1901 to 2017. Earth Syst. Sci. Data 2019, 11, 1931–1946. [Google Scholar] [CrossRef]
- Peng, S.; Gang, C.; Cao, Y.; Chen, Y. Assessment of Climate Change Trends over the Loess Plateau in China from 1901 to 2100. Int. J. Climatol. 2018, 38, 2250–2264. [Google Scholar] [CrossRef]
- Peng, S.; Ding, Y.; Wen, Z.; Chen, Y.; Cao, Y.; Ren, J. Spatiotemporal Change and Trend Analysis of Potential Evapotranspiration over the Loess Plateau of China during 2011–2100. Agric. For. Meteorol. 2017, 233, 183–194. [Google Scholar] [CrossRef]
- Pereira, L.S.; Paredes, P.; Hunsaker, D.J.; López-Urrea, R.; Mohammadi Shad, Z. Standard Single and Basal Crop Coefficients for Field Crops. Updates and Advances to the FAO56 Crop Water Requirements Method. Agric. Water Manag. 2021, 243, 106466. [Google Scholar] [CrossRef]
- Rallo, G.; Paço, T.A.; Paredes, P.; Puig-Sirera, À.; Massai, R.; Provenzano, G.; Pereira, L.S. Updated Single and Dual Crop Coefficients for Tree and Vine Fruit Crops. Agric. Water Manag. 2021, 250, 106645. [Google Scholar] [CrossRef]
- Boundi, A.; Yacine, Z.A.; Lahyane, S.A.; Elhabty, M.; Mouradi, A.; Saaf, M. Efficiency of Usual Irrigation Systems and Water Productivity for Crops in Mediterranean and Semi-Arid Climates with Reduce Hydric Requirements. Review. Arab. J. Chem. Environ. Res. 2017, 4, 107–126. [Google Scholar]
- Wang, C.; Wang, G.; Wang, Y.; Rafique, R.; Ma, L.; Hu, L.; Luo, Y. Urea Addition and Litter Manipulation Alter Plant Community and Soil Microbial Community Composition in a Kobresia humilis Meadow. Eur. J. Soil Biol. 2015, 70, 7–14. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and Assessing Soil Quality. In Defining Soil Quality for a Sustainable Environment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1994; pp. 1–21. ISBN 978-0-89118-930-5. [Google Scholar]
- Shukla, M.K.; Lal, R.; Ebinger, M. Determining Soil Quality Indicators by Factor Analysis. Soil Tillage Res. 2006, 87, 194–204. [Google Scholar] [CrossRef]
- Chao, A. Non-Parametric Estimation of the Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Chao, A.; Yang, M.C.K. Stopping Rules and Estimation for Recapture Debugging with Unequal Failure Rates. Biometrika 1993, 80, 193–201. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, Y.; Mao, Q.; Zhong, B.; Chen, W.; Mo, J.; Wang, F.; Lu, X. Consistent Effects of Nitrogen Addition on Soil Microbial Communities across Three Successional Stages in Tropical Forest Ecosystems. Catena 2023, 227, 107116. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling Function and Taxonomy in the Global Ocean Microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Hagage, M.; Abdulaziz, A.M.; Elbeih, S.F.; Hewaidy, A.G.A. Monitoring Soil Salinization and Waterlogging in the Northeastern Nile Delta Linked to Shallow Saline Groundwater and Irrigation Water Quality. Sci. Rep. 2024, 14, 27838. [Google Scholar] [CrossRef]
- Kramer, I.; Peleg, N.; Mau, Y. Climate Change Shifts Risk of Soil Salinity and Land Degradation in Water-Scarce Regions. Agric. Water Manag. 2025, 307, 109223. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, W.; Xie, Q.; Ran, E. Effects of Soil Compaction Stress Combined with Drought on Soil Pore Structure, Root System Development, and Maize Growth in Early Stage. Plants 2024, 13, 3185. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Xue, L.; Razavi, B.; Zamanian, K.; Zhao, X. The Microbial Mechanism of Maize Residue Decomposition under Different Temperature and Moisture Regimes in a Solonchak. Sci. Rep. 2025, 15, 2215. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Chen, A.; Li, Y.; Xue, M.; Feng, S. Effects of Exogenous Organic Matter on Soil Nutrient Dynamics and Its Role in Replacing Chemical Fertilizers for Vegetable Yield and Quality. Horticulturae 2024, 10, 1355. [Google Scholar] [CrossRef]
- Canarini, A.; Fuchslueger, L.; Schnecker, J.; Metze, D.; Nelson, D.B.; Kahmen, A.; Watzka, M.; Pötsch, E.M.; Schaumberger, A.; Bahn, M.; et al. Soil Fungi Remain Active and Invest in Storage Compounds during Drought Independent of Future Climate Conditions. Nat. Commun. 2024, 15, 10410. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-Growth-Promoting Rhizobacteria: Drought Stress Alleviators to Ameliorate Crop Production in Drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Parasar, B.J.; Kashyap, S.; Sharma, I.; Marme, S.D.; Das, P.; Agarwala, N. Microbe Mediated Alleviation of Drought and Heat Stress in Plants- Current Understanding and Future Prospects. Discov. Plants 2024, 1, 26. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Zhang, Y.; Liao, J.; Guan, K.; Zhai, J.; Meng, P.; Tang, X.; Dong, T.; Song, Y. Root Hair Developmental Regulators Orchestrate Drought Triggered Microbiome Changes and the Interaction with Beneficial Rhizobiaceae. Nat. Commun. 2024, 15, 10068. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Liu, Y.; Tang, X.; Li, Y.; Sun, T.; Yan, G.; Yin, C. Drought Stress Increases the Complexity of the Bacterial Network in the Rhizosphere and Endosphere of Rice (Oryza sativa L.). Agronomy 2024, 14, 1662. [Google Scholar] [CrossRef]
- Dai, Z.; Zang, H.; Chen, J.; Fu, Y.; Wang, X.; Liu, H.; Shen, C.; Wang, J.; Kuzyakov, Y.; Becker, J.N.; et al. Metagenomic Insights into Soil Microbial Communities Involved in Carbon Cycling along an Elevation Climosequences. Environ. Microbiol. 2021, 23, 4631–4645. [Google Scholar] [CrossRef]
- Ding, J.-L.; Jiang, X.; Ma, M.-C.; Guan, D.-W.; Zhao, B.-S.; Wei, D.; Cao, F.-M.; Li, L.; Li, J. Structure of soil fungal communities under long-term inorganic and organic fertilization in black soil of Northeast China. zwyyyflxb 2017, 23, 914–923. [Google Scholar] [CrossRef]
- Gao, M.; Hu, W.; Li, M.; Wang, S.; Chu, L. Network Analysis Was Effective in Establishing the Soil Quality Index and Differentiated among Changes in Land-Use Type. Soil Tillage Res. 2025, 246, 106352. [Google Scholar] [CrossRef]
- Sarkar, S.; Dhar, A.; Dey, S.; Chatterjee, S.K.; Mukherjee, S.; Chakraborty, A.; Chatterjee, G.; Ravisankar, N.; Mainuddin, M. Natural and Organic Input-Based Integrated Nutrient-Management Practices Enhance the Productivity and Soil Quality Index of Rice–Mustard–Green Gram Cropping System. Land 2024, 13, 1933. [Google Scholar] [CrossRef]
- Araujo, F.F.; Salvador, G.L.O.; Lupatini, G.C.; Pereira, A.P.D.A.; Costa, R.M.; De Aviz, R.O.; De Alcantara Neto, F.; Mendes, L.W.; Araujo, A.S.F. Exploring the Diversity and Composition of Soil Microbial Communities in Different Soybean-Maize Management Systems. Microbiol. Res. 2023, 274, 127435. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, S.; Wang, J.; Chen, M.; Wang, K.; Liu, C.; Shangguan, Z. Assessing the Change in Soil Water Deficit Characteristics from Grassland to Forestland on the Loess Plateau. Ecol. Indic. 2024, 167, 112616. [Google Scholar] [CrossRef]
- Gangana Gowdra, V.M.; Lalitha, B.S.; Halli, H.M.; Senthamil, E.; Negi, P.; Jayadeva, H.M.; Basavaraj, P.S.; Harisha, C.B.; Boraiah, K.M.; Adavi, S.B.; et al. Root Growth, Yield and Stress Tolerance of Soybean to Transient Waterlogging under Different Climatic Regimes. Sci. Rep. 2025, 15, 6968. [Google Scholar] [CrossRef]
- He, H.; Xu, M.; Li, W.; Chen, L.; Chen, Y.; Moorhead, D.L.; Brangarí, A.C.; Liu, J.; Cui, Y.; Zeng, Y.; et al. Linking Soil Depth to Aridity Effects on Soil Microbial Community Composition, Diversity and Resource Limitation. Catena 2023, 232, 107393. [Google Scholar] [CrossRef]
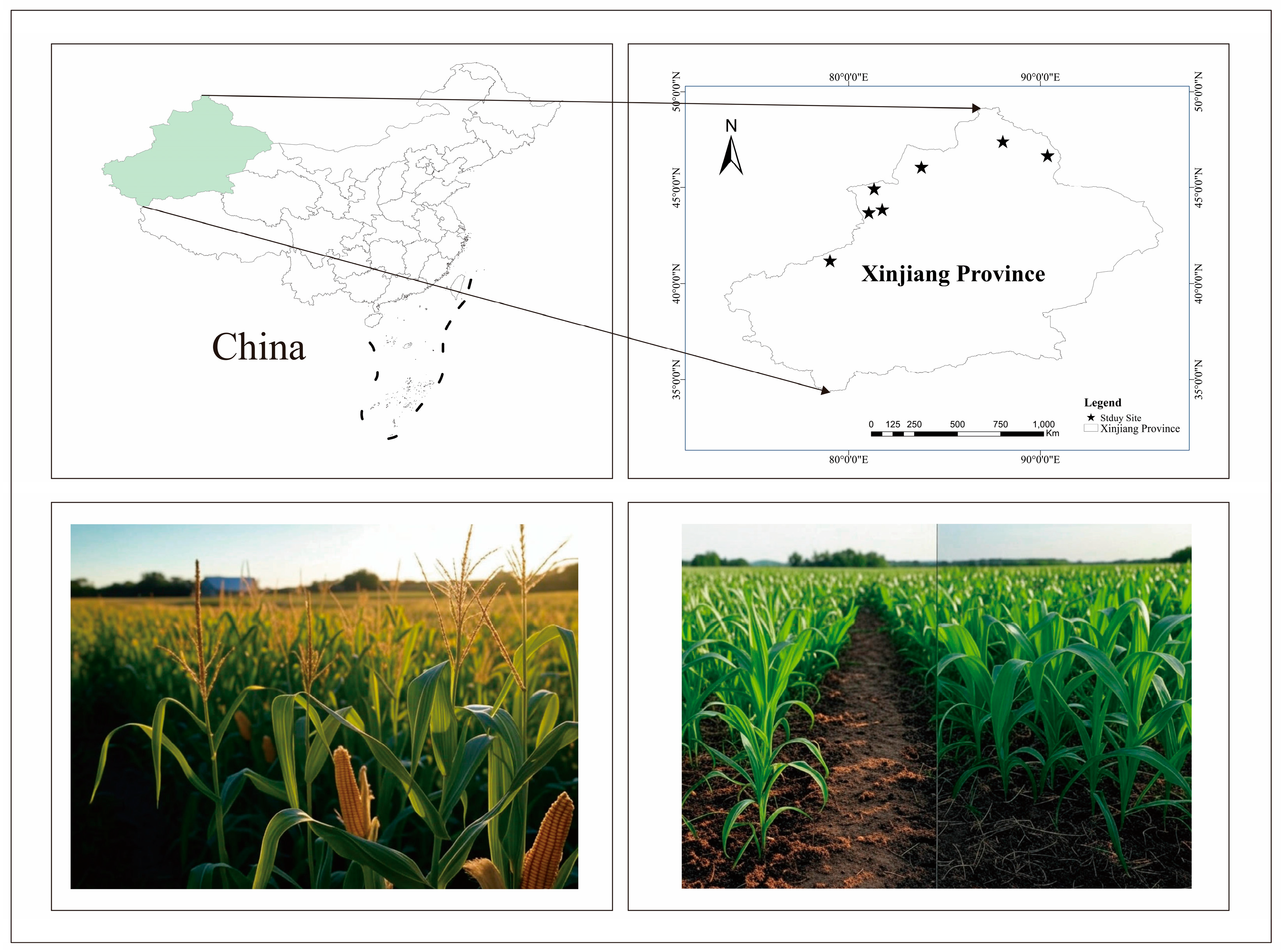
| Site | Lat and Lon | Total P (mm) | Total ETcrop (mm) | Total Wgross (mm) | IWDI | Group |
|---|---|---|---|---|---|---|
| Wenquan | 44°57′ N, 81°20′ E | 212.07 | 604.41 | 430 | 16.37% | LD |
| Yining | 43°52′ N, 81°45′ E | 157.54 | 663.33 | 400 | 22.30% | LD |
| Tuoli | 46°04′ N, 83°48′ E | 148.90 | 579.57 | 350 | 30.54% | MD |
| Chaxian | 43°42′ N, 81°03′ E | 159.67 | 667.48 | 350 | 38.10% | MD |
| Qinghe | 46°41′ N, 90°23′ E | 112.63 | 545.21 | 260 | 48.36% | HD |
| Wushi | 41°12′ N, 79°02′ E | 130.04 | 644.58 | 300 | 47.49% | HD |
| Altai | 47°25′ N, 88°03′ E | 108.86 | 592.24 | 280 | 50.00% | HD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, D.; Sun, R.; Huo, Z.; Chen, J.; Dong, S.; Dong, H. Long-Term Irrigation Deficits Impair Microbial Diversity and Soil Quality in Arid Maize Fields. Agronomy 2025, 15, 1355. https://doi.org/10.3390/agronomy15061355
Zhong D, Sun R, Huo Z, Chen J, Dong S, Dong H. Long-Term Irrigation Deficits Impair Microbial Diversity and Soil Quality in Arid Maize Fields. Agronomy. 2025; 15(6):1355. https://doi.org/10.3390/agronomy15061355
Chicago/Turabian StyleZhong, Dongdong, Renhua Sun, Zhen Huo, Jian Chen, Shengtianzi Dong, and Hegan Dong. 2025. "Long-Term Irrigation Deficits Impair Microbial Diversity and Soil Quality in Arid Maize Fields" Agronomy 15, no. 6: 1355. https://doi.org/10.3390/agronomy15061355
APA StyleZhong, D., Sun, R., Huo, Z., Chen, J., Dong, S., & Dong, H. (2025). Long-Term Irrigation Deficits Impair Microbial Diversity and Soil Quality in Arid Maize Fields. Agronomy, 15(6), 1355. https://doi.org/10.3390/agronomy15061355






