Integrated Effects of Warming Irrigation, Aeration, and Humic Acid on Yield, Quality, and GHG Emissions in Processing Tomatoes in Xinjiang
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil CO2, N2O, and CH4 Collection and Calculation
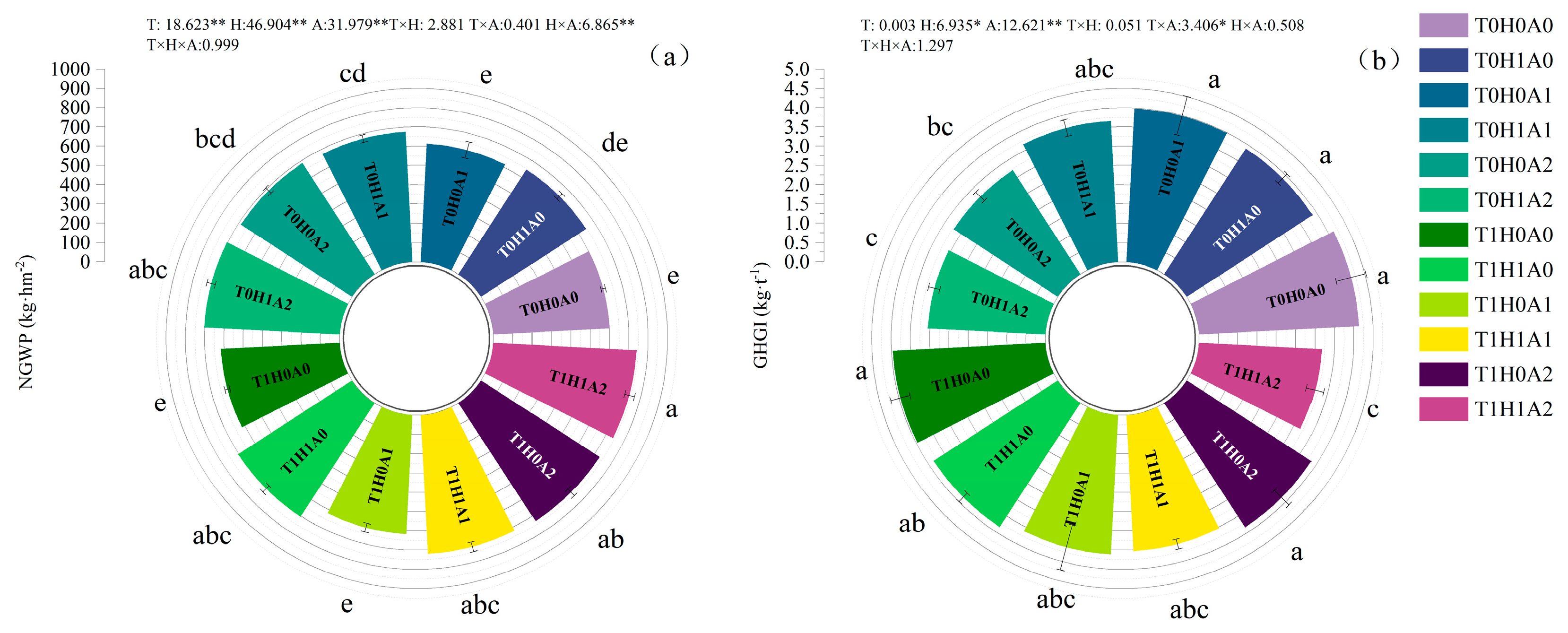
2.3. Net Global Warming Potential and Greenhouse Gas Emission Intensity
2.4. Yield and Quality Indicators
2.5. Model Construction
2.6. Data Analyses and Statistics
3. Results
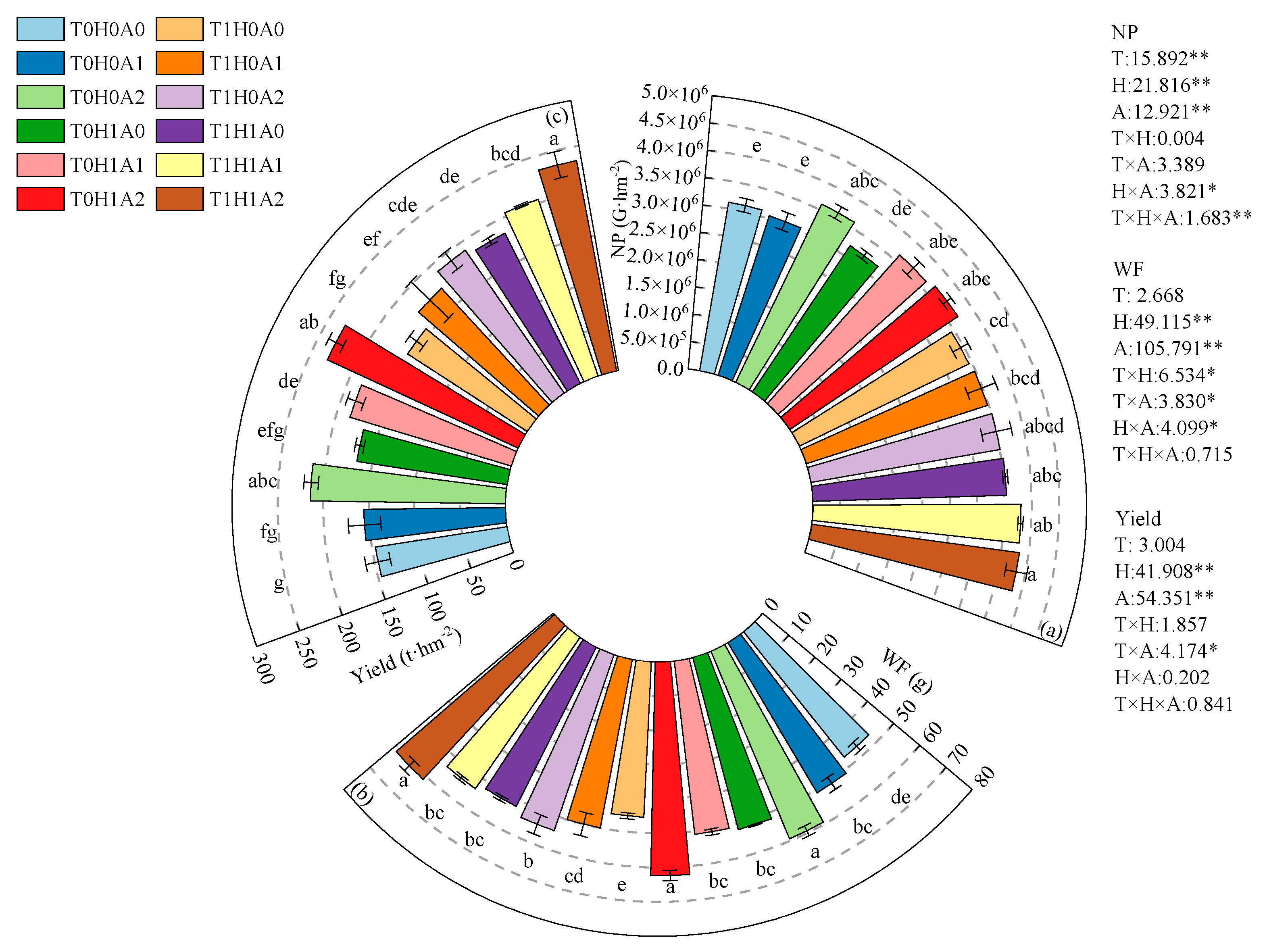
3.1. Effects of Water, Fertilizer, Air, and Heat Coupling on Yield and Quality of Processing Tomato
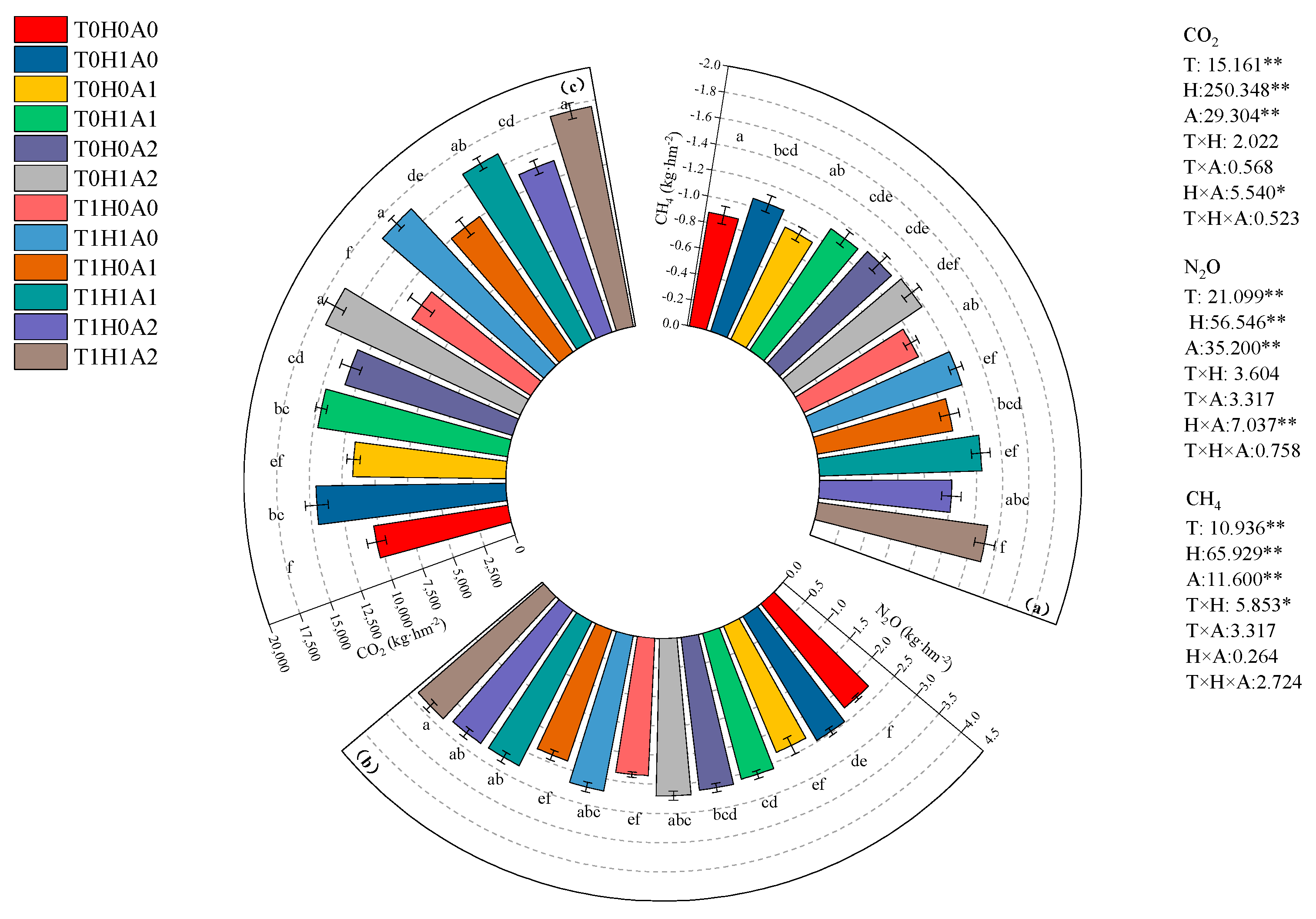
3.2. Effects of Water–Fertilizer–Gas Coupling on the Root Zone Environment of Processing Tomato Fields
3.3. Economic Benefits
3.4. Combinatorial Evaluation Based on Overall Differences
4. Discussion
4.1. The Impact of Humic Acid, Aeration, and Warming-Coupled Irrigation on Greenhouse Gas Emissions
4.2. The Impact of Humic Acid, Aerated, and Warming-Coupled Irrigation on Yield and Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NGWP | Net global warming potential |
| GHGI | Greenhouse gas emission intensity |
| NP | Number of fruits per hectare |
| VC | Vitamin C |
| WF | Weight of single fruit |
| kg·hm⁻2 | Kilograms per hectare |
| L·h⁻1 | Liters per hour |
References
- Schmidt, J.E.; Vannette, R.L.; Igwe, A.; Blundell, R.; Casteel, C.L.; Gaudin, A.C. Effects of agricultural management on rhizosphere microbial structure and function in processing tomato plants. Appl. Environ. Microbiol. 2019, 85, e01064-19. [Google Scholar] [CrossRef] [PubMed]
- Alordzinu, K.E.; Appiah, S.A.; Al Aasmi, A.; Darko, R.O.; Li, J.; Lan, Y.; Adjibolosoo, D.; Lian, C.; Wang, H.; Qiao, S. Evaluating the influence of deficit irrigation on fruit yield and quality indices of tomatoes grown in sandy loam and silty loam soils. Water 2022, 14, 1753. [Google Scholar] [CrossRef]
- Loch, R.; Grant, C.; McKenzie, D.; Raine, S. Improving Plants’ Water Use Efficiency and Potential Impacts from Soil Structure Change-Research Investment Opportunities; Land and Water Australia: Perth, Australia, 2005. [Google Scholar]
- Li, Z.; Zhang, R.; Wang, X.; Wang, J.; Zhang, C.; Tian, C. Carbon Dioxide Fluxes and Concentrations in a Cotton Field in Northwestern China: Effects of Plastic Mulching and Drip Irrigation. Pedosphere 2011, 21, 178–185. [Google Scholar] [CrossRef]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef]
- Niu, W.; Jia, Z.; Zhang, X.; Shao, H. Effects of soil rhizosphere aeration on the root growth and water absorption of tomato. CLEAN–Soil Air Water 2012, 40, 1364–1371. [Google Scholar] [CrossRef]
- Munns, R.; Sharp, R. Involvement of abscisic acid in controlling plant growth in soil of low water potential. Funct. Plant Biol. 1993, 20, 425–437. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Vo, T.S.; Tran-Nguyen, P.L.; Nguyen, M.N.; Pham, V.H.; Matsuhashi, R.; Kim, K.; Vo, T.T.B.C. A comprehensive review of aeration and wastewater treatment. Aquaculture 2024, 591, 741113. [Google Scholar] [CrossRef]
- Li, R.; Han, Q.B.; Dong, C.H.; Nan, X.; Li, H.; Sun, H.; Li, H.; Li, P.; Hu, Y.W. Effect and Mechanism of Micro–nano Aeration Treatment on a Drip Irrigation Emitter Based on Groundwater. Agron. J. 2023, 13, 2059. [Google Scholar] [CrossRef]
- Lyu, T.; Wu, S.; Mortimer, R.J.; Pan, G. Nanobubble Technology in Environmental Engineering: Revolutionization Potential and Challenges. Environ. Sci. Technol. 2019, 53, 7175–7176. [Google Scholar] [CrossRef]
- Campbell, G.S. Plants and their environment. In An Introduction to Environmental Biophysics; Springer: New York, NY, USA, 1977; pp. 115–126. [Google Scholar]
- Bassirirad, H. Kinetics of nutrient uptake by roots: Responses to global change. New Phytol. 2000, 147, 155–169. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.; Qu, Z.; Zou, D.; Sha, H.; Liu, H.; Sun, J.; Zheng, H.; Yang, L.; Zhao, H. Effects of low water temperature during reproductive growth on photosynthetic production and nitrogen accumulation in rice. Field Crops Res. 2019, 242, 107587. [Google Scholar] [CrossRef]
- Kübarsepp, L.; Laanisto, L.; Niinemets, Ü.; Talts, E.; Tosens, T. Are stomata in ferns and allies sluggish? Stomatal responses to CO2, humidity and light and their scaling with size and density. New Phytol. 2020, 225, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Drake, B.; Salisbury, F. Aftereffects of low and high temperature pretreatment on leaf resistance, transpiration, and leaf temperature in Xanthium. Plant Physiol. 1972, 50, 572–575. [Google Scholar] [CrossRef]
- Lee, S.; Chung, G.; Jang, J.; Ahn, S.; Zwiazek, J.J. Overexpression of PIP2; 5 aquaporin alleviates effects of low root temperature on cell hydraulic conductivity and growth in Arabidopsis. Plant Physiol. 2012, 159, 479–488. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, S.; Yuan, L.; Li, Y.; Zhao, L.; Wen, Y.; Xu, J.; Hu, S.; Zhao, B. Effects of humic acid–enhanced phosphate fertilizer on wheat yield, phosphorus uptake, and soil available phosphorus content. Crop Sci. 2023, 63, 956–966. [Google Scholar] [CrossRef]
- DiDonato, N.; Chen, H.; Waggoner, D.; Hatcher, P.G. Potential origin and formation for molecular components of humic acids in soils. Geochim. Cosmochim. Acta 2016, 178, 210–222. [Google Scholar] [CrossRef]
- Suleimenov, B.; Kaisanova, G.; Suleimenova, M.; Tanirbergenov, S. Influence of Organic Humic Fertilizer “Tumat” on the Productivity of Sugar Beet. Agron. J. 2024, 14, 1100. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Fan, G.H.; Xie, S.Z.; Li, F.H. Effects of aerated brackish water irrigation on growth of Lycium barbarum seedlings. Sci. Hortic. 2023, 310, 111721. [Google Scholar] [CrossRef]
- Duxbury, J.M. The significance of agricultural sources of greenhouse gases. Fertil. Res. 1994, 38, 151–163. [Google Scholar] [CrossRef]
- Hou, H.; Chen, H.; Cai, H.; Yang, F.; Li, D.; Wang, F. CO2 and N2O emissions from Lou soils of greenhouse tomato fields under aerated irrigation. Atmos. Environ. 2016, 132, 69–76. [Google Scholar] [CrossRef]
- Ma, J.; Chen, R.; Wen, Y.; Zhang, J.; Yin, F.; Javed, T.; Zheng, J.; Wang, Z. Processing tomato (Lycopersicon esculentum Miller) yield and quality in arid regions through micro–nano aerated drip irrigation coupled with humic acid application. Agric. Water Manag. 2025, 308, 109317. [Google Scholar] [CrossRef]
- Yu, L.; Wang, M. Study on model of greenhouse gas N2O emission flux of rice field in cold region in growing season in water-saving irrigation mode. Int. J. Eng. Syst. Model. Simul. 2018, 10, 87–96. [Google Scholar]
- Wei, C.; Ren, S.; Yang, P.; Wang, Y.; He, X.; Xu, Z.; Wei, R.; Wang, S.; Chi, Y.; Zhang, M. Effects of irrigation methods and salinity on CO2 emissions from farmland soil during growth and fallow periods. Sci. Total Environ. 2021, 752, 141639. [Google Scholar] [CrossRef]
- Zhang, A.; Cheng, G.; Hussain, Q.; Zhang, M.; Feng, H.; Dyck, M.; Sun, B.; Zhao, Y.; Chen, H.; Chen, J. Contrasting effects of straw and straw–derived biochar application on net global warming potential in the Loess Plateau of China. Field Crops Res. 2017, 205, 45–54. [Google Scholar] [CrossRef]
- Lee, J.; Cho, S.; Jeong, S.; Hwang, H.; Kim, P. Different response of plastic film mulching on greenhouse gas intensity (GHGI) between chemical and organic fertilization in maize upland soil. Sci. Total Environ. 2019, 696, 133827. [Google Scholar] [CrossRef]
- Wang, C.; Gu, F.; Chen, J.; Yang, H.; Jiang, J.; Du, T.; Zhang, J. Assessing the response of yield and comprehensive fruit quality of tomato grown in greenhouse to deficit irrigation and nitrogen application strategies. Agric. Water Manag. 2015, 161, 9–19. [Google Scholar] [CrossRef]
- Leyva, A.; Quintana, A.; Sánchez, M.; Rodríguez, E.N.; Cremata, J.; Sánchez, J.C. Rapid and sensitive anthrone–sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef]
- Guo, Y.; Yi, P. Whole diversity-based reasoning for objective combined evaluation. Chin. J. Manag. Sci. 2006, 14, 60–64. [Google Scholar]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob. Chang. Biol. 2004, 10, 1756–1766. [Google Scholar] [CrossRef]
- Ben-Noah, I.; Friedman, S. Aeration of clayey soils by injecting air through subsurface drippers: Lysimetric and field experiments. Agric. Water Manag. 2016, 176, 222–233. [Google Scholar] [CrossRef]
- Galambos, N.; Compant, S.; Moretto, M.; Sicher, C.; Puopolo, G.; Wäckers, F.; Sessitsch, A.; Pertot, I.; Perazzolli, M. Humic acid enhances the growth of tomato promoted by endophytic bacterial strains through the activation of hormone-, growth-, and transcription-related processes. Front. Plant Sci. 2020, 11, 582267. [Google Scholar]
- Soong, J.L.; Marañon-Jimenez, S.; Cotrufo, M.F.; Boeckx, P.; Bodé, S.; Guenet, B.; Peñuelas, J.; Richter, A.; Stahl, C.; Verbruggen, E. Soil microbial CNP and respiration responses to organic matter and nutrient additions: Evidence from a tropical soil incubation. Soil Biol. Biochem. 2018, 122, 141–149. [Google Scholar]
- Qian, Z.; Zhuang, S.; Gao, J.; Tang, L.; Harindintwali, J.D.; Wang, F. Aeration increases soil bacterial diversity and nutrient transformation under mulching-induced hypoxic conditions. Sci. Total Environ. 2022, 817, 153017. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gu, X.; Wang, J.; Niu, W. Yield and gas exchange of greenhouse tomato at different nitrogen levels under aerated irrigation. Sci. Total Environ. 2019, 668, 1156–1164. [Google Scholar]
- Singh, J.S.; Gupta, S. Plant decomposition and soil respiration in terrestrial ecosystems. Bot. Rev. 1977, 43, 449–528. [Google Scholar] [CrossRef]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors that influence nitrous oxide emissions from agricultural soils as well as their representation in simulation models: A review. Agron. J. 2021, 11, 770. [Google Scholar] [CrossRef]
- Ndzelu, B.S.; Dou, S.; Zhang, X.; Zhang, Y.; Ma, R.; Liu, X. Tillage effects on humus composition and humic acid structural characteristics in soil aggregate-size fractions. Soil Tillage Res. 2021, 213, 105090. [Google Scholar] [CrossRef]
- Kool, D.M.; Dolfing, J.; Wrage, N.; Van Groenigen, J.W. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 2011, 43, 174–178. [Google Scholar] [CrossRef]
- Atkin, O.K.; Edwards, E.J.; Loveys, B.R. Response of root respiration to changes in temperature and its relevance to global warming. New Phytol. 2000, 147, 141–154. [Google Scholar] [CrossRef]
- Braker, G.; Schwarz, J.; Conrad, R. Influence of temperature on the composition and activity of denitrifying soil communities. FEMS Microbiol. Ecol. 2010, 73, 134–148. [Google Scholar]
- Li, S.; Yue, A.; Moore, S.S.; Ye, F.; Wu, J.; Hong, Y.; Wang, Y. Temperature-related N2O emission and emission potential of freshwater sediment. Processes 2022, 10, 2728. [Google Scholar] [CrossRef]
- Conrad, R. Methane production in soil environments—Anaerobic biogeochemistry and microbial life between flooding and desiccation. Microorganisms 2020, 8, 881. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.; Allen, D.; Livesley, S.; Richards, G. Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: A review. Plant Soil 2008, 309, 43–76. [Google Scholar] [CrossRef]
- Mehmood, F.; Wang, G.; Gao, Y.; Liang, Y.; Zain, M.; Rahman, S.U.; Duan, A. Impacts of irrigation managements on soil CO2 emission and soil CH4 uptake of winter wheat field in the North China plain. Water 2021, 13, 2052. [Google Scholar] [CrossRef]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Tringovska, I. The effects of humic and bio-fertilizers on growth and yield of greenhouse tomatoes. In Proceedings of the V Balkan Symposium on Vegetables and Potatoes 960, Tirana, Albania, 30 September 2012; pp. 443–449. [Google Scholar]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Maffia, A.; Oliva, M.; Marra, F.; Mallamaci, C.; Nardi, S.; Muscolo, A. Humic Substances: Bridging Ecology and Agriculture for a Greener Future. Agron. J 2025, 15, 410. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Su, N.; Midmore, D.J. Oxygation unlocks yield potentials of crops in oxygen-limited soil environments. Adv. Agron. 2005, 88, 313–377. [Google Scholar]
- Chen, X.; Dhungel, J.; Bhattarai, S.P.; Torabi, M.; Pendergast, L.; Midmore, D.J. Impact of oxygation on soil respiration, yield and water use efficiency of three crop species. J. Plant Ecol. 2011, 4, 236–248. [Google Scholar] [CrossRef]
- Bhattarai, S.; McHugh, A.; Lotz, G.; Midmore, D. The response of cotton to subsurface drip and furrow irrigation in a vertisol. Exp. Agric. 2006, 42, 29–49. [Google Scholar] [CrossRef]
- Bian, Q.; Dong, Z.; Zhao, Y.; Feng, Y.; Fu, Y.; Wang, Z.; Zhu, J. Phosphorus Supply Under Micro–nano Bubble Water Drip Irrigation Enhances Maize Yield and Phosphorus Use Efficiency. Plants 2024, 13, 3046. [Google Scholar] [CrossRef] [PubMed]
- Apostol, K.G.; Jacobs, D.F.; Wilson, B.C.; Salifu, K.F.; Dumroese, R.K. Growth, gas exchange, and root respiration of Quercus rubra seedlings exposed to low root zone temperatures in solution culture. For. Ecol. Manag. 2007, 253, 89–96. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Lee, S. Root-zone CO2 and root-zone temperature effects on photosynthesis and nitrogen metabolism of aeroponically grown lettuce (Lactuca sativa L.) in the tropics. Photosynthetica 2013, 51, 330–340. [Google Scholar] [CrossRef]
- Cassel, D.; Nielsen, D.; Biggar, J. Soil-water movement in response to imposed temperature gradients. Soil Sci. Soc. Am. J. 1969, 33, 493–500. [Google Scholar] [CrossRef]
- Nambiar, E.S. Interplay between nutrients, water, root growth and productivity in young plantations. For. Ecol. Manag. 1990, 30, 213–232. [Google Scholar] [CrossRef]

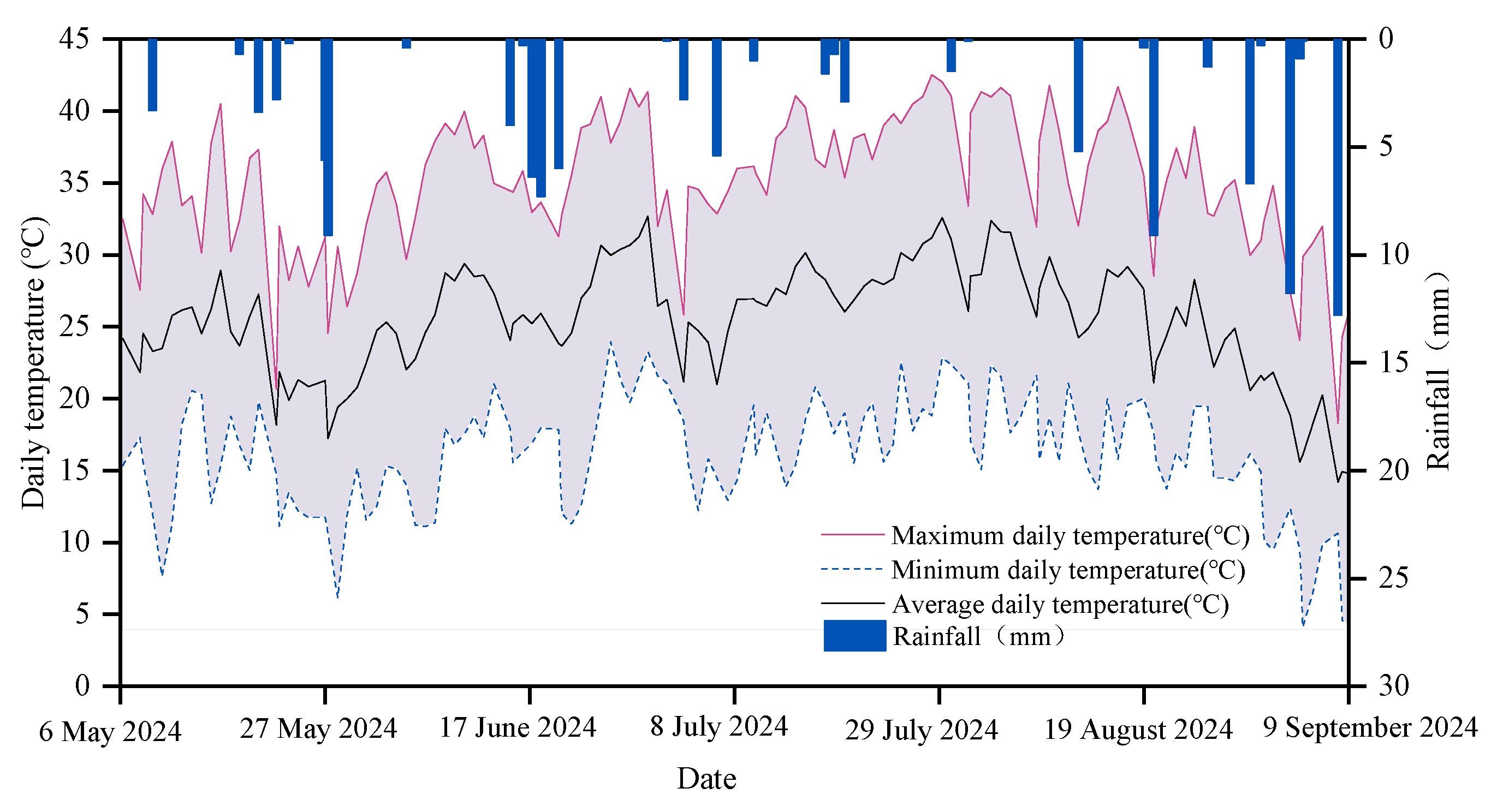



| Reproductive Period | Date | Duration of Crop Fertility (d) | Irrigation Amount (m3·hm−2) | KH2PO4 (K2O: 33.8%) (P2O5: 51.0%) kg·hm−2 | CH4N2O (N: 46.4%) kg·hm−2 | Frequency of Irrigation and Fertilization | Date for Irrigation and Fertilization |
|---|---|---|---|---|---|---|---|
| Seedling stage | 6 May–8 June 2024– | 33 | 562.5 | 23.5 | 30 | 1 | 8 June 2024 |
| Blossoming and fruiting stage | 9–27 June 2024 | 18 | 1125 | 47 | 60 | 2 | 17 June 2024, 27 June 2024 |
| Full fruit stage | 28 June–2 August 2024 | 35 | 2250 | 94 | 120 | 4 | 6 July 2024, 12 July 2024, 19 July 2024, 26 July 2024, |
| Red ripening stage | 3–18 August 2024 | 15 | 562.5 | 23.5 | 30 | 1 | 15 August 2024 |
| Full life span | 6 May 2024–18 August 2024 | 111 | 4500 | 188 | 240 | 8 |
| Treatment | Temperature of Irrigation Water (°C) | Humic Acid Addition (%) | Dissolved Oxygen Content (mg·L−1) |
|---|---|---|---|
| T0H0A0 | 10–15 | 0 | 5 |
| T0H0A1 | 9 | ||
| T0H0A2 | 18 | ||
| T0H1A0 | 0.5 | 5 | |
| T0H1A1 | 9 | ||
| T0H1A2 | 18 | ||
| T1H0A0 | 20–25 | 0 | 5 |
| T1H0A1 | 9 | ||
| T1H0A2 | 18 | ||
| T1H1A0 | 0.5 | 5 | |
| T1H1A1 | 9 | ||
| T1H1A2 | 18 |
| Treatment | Input (CNY·hm−2) | Outputs (CNY·hm−2) | Net Profit (CNY·hm−2) | ||||
|---|---|---|---|---|---|---|---|
| Irrigation System | Fertilizer | Water and Electricity | Other | Total | |||
| T0H0A0 | 24,153 | 4936 | 171 | 7476 | 36,736 | 81,863 ± 7667 g | 45,126 ± 7667 c |
| T0H0A1 | 26,633 | 4936 | 171 | 7476 | 39,216 | 86,991 ± 10,021 fg | 47,774 ± 10,022 c |
| T0H0A2 | 64,856 | 4936 | 171 | 7476 | 77,439 | 119,779 ± 4459 abc | 42,339 ± 4459 c |
| T0H1A0 | 24,153 | 4939 | 171 | 7476 | 36,740 | 93,329 ± 2712 efg | 56,589 ± 2712 bc |
| T0H1A1 | 26,633 | 4939 | 171 | 7476 | 39,220 | 103,466 ± 5236 de | 64,246 ± 5236 ab |
| T0H1A2 | 64,856 | 4939 | 171 | 7476 | 77,443 | 129,116 ± 4947 ab | 51,673 ± 4948 bc |
| T1H0A0 | 24,153 | 4936 | 2662 | 7476 | 39,227 | 87,375 ± 5219 fg | 48,148 ± 5220 c |
| T1H0A1 | 26,633 | 4936 | 2662 | 7476 | 41,707 | 97,386 ± 16,064 ef | 55,679 ± 16,064 bc |
| T1H0A2 | 64,856 | 4936 | 2662 | 7476 | 79,930 | 106,572 ± 6465 cde | 26,642 ± 6465 d |
| T1H1A0 | 24,153 | 4939 | 2662 | 7476 | 39,231 | 103,028 ± 2438 de | 63,798 ± 2438 ab |
| T1H1A1 | 26,633 | 4939 | 2662 | 7476 | 41,711 | 114,281 ± 959 bcd | 72,570 ± 959 a |
| T1H1A2 | 64,856 | 4939 | 2662 | 7476 | 79,934 | 131,184 ± 8135 a | 51,250 ± 8135 bc |
| Three-way ANOVA | |||||||
| T (irrigation water temperature) | 3.004 | 0.502 | |||||
| H (humic acid application) | 41.908 ** | 41.888 ** | |||||
| A (dissolved oxygen content) | 54.351 ** | 16.741 ** | |||||
| T × H | 1.857 | 1.857 | |||||
| T × A | 4.174 * | 4.174 * | |||||
| H × A | 0.202 | 0.202 | |||||
| T × H × A | 0.841 | 0.841 | |||||
| Treatment | TOPSIS | RSR | PCA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D+ | D− | C | Raking | RSR | Raking | PCA1 | PCA2 | PCA3 | Score | Raking | |
| T0H0A0 | 0.913 | 0.339 | 0.271 | 12 | 0.201 | 12 | −1.523 | −0.488 | 0.077 | −1.328 | 12 |
| T0H0A1 | 0.807 | 0.345 | 0.299 | 10 | 0.340 | 10 | −1.107 | −0.521 | 0.272 | −0.971 | 11 |
| T0H0A2 | 0.470 | 0.599 | 0.560 | 4 | 0.605 | 4 | 0.453 | −0.949 | 1.687 | 0.368 | 4 |
| T0H1A0 | 0.684 | 0.376 | 0.354 | 9 | 0.388 | 9 | −0.578 | 0.219 | −0.117 | −0.468 | 9 |
| T0H1A1 | 0.548 | 0.488 | 0.471 | 5 | 0.557 | 5 | 0.025 | 1.149 | −0.143 | 0.136 | 6 |
| T0H1A2 | 0.313 | 0.795 | 0.718 | 2 | 0.744 | 2 | 1.246 | −0.407 | 1.412 | 1.077 | 2 |
| T1H0A0 | 0.822 | 0.349 | 0.298 | 11 | 0.283 | 11 | −1.136 | 0.072 | −0.043 | −0.949 | 10 |
| T1H0A1 | 0.613 | 0.464 | 0.431 | 8 | 0.431 | 8 | −0.42 | 0.478 | 0.736 | −0.263 | 8 |
| T1H0A2 | 0.574 | 0.508 | 0.470 | 6 | 0.514 | 6 | 0.397 | −1.931 | −2.017 | 0.02 | 7 |
| T1H1A0 | 0.567 | 0.477 | 0.457 | 7 | 0.473 | 7 | 0.13 | 0.997 | −1.049 | 0.161 | 5 |
| T1H1A1 | 0.451 | 0.642 | 0.587 | 3 | 0.663 | 3 | 0.725 | 1.727 | −0.452 | 0.77 | 3 |
| T1H1A2 | 0.343 | 0.908 | 0.726 | 1 | 0.873 | 1 | 1.788 | −0.345 | −0.362 | 1.446 | 1 |
| Evaluation Model | Rank Average Value | Median | Kendall’s W Coefficient | χ2 | p |
|---|---|---|---|---|---|
| TOPSIS evaluation value | 1.917 | 0.463 | 0.799 | 7.167 | 0.028 * |
| RSR evaluation value | 2.583 | 0.493 | |||
| PCA evaluation value | 1.5 | −0.08 |
| Treatment | Combined Weight Vector 1 | Combined Weight Vector 2 | Combined Weight Vector 3 | TOPSIS Score | RSR Score | PCA Score | Evaluation Value | Ranking |
|---|---|---|---|---|---|---|---|---|
| T1H1A2 | 0.3309 | 0.3402 | 0.3289 | 0.726 | 0.873 | 1.446 | 1.013 | 1 |
| T0H1A2 | 0.3309 | 0.3402 | 0.3289 | 0.718 | 0.744 | 1.077 | 0.845 | 2 |
| T1H1A1 | 0.3309 | 0.3402 | 0.3289 | 0.587 | 0.663 | 0.770 | 0.673 | 3 |
| T0H0A2 | 0.3309 | 0.3402 | 0.3289 | 0.560 | 0.605 | 0.368 | 0.512 | 4 |
| T0H1A1 | 0.3309 | 0.3402 | 0.3289 | 0.471 | 0.557 | 0.136 | 0.390 | 5 |
| T1H1A0 | 0.3309 | 0.3402 | 0.3289 | 0.457 | 0.473 | 0.161 | 0.365 | 6 |
| T1H0A2 | 0.3309 | 0.3402 | 0.3289 | 0.470 | 0.514 | 0.020 | 0.337 | 7 |
| T1H0A1 | 0.3309 | 0.3402 | 0.3289 | 0.431 | 0.431 | −0.263 | 0.203 | 8 |
| T0H1A0 | 0.3309 | 0.3402 | 0.3289 | 0.354 | 0.388 | −0.468 | 0.095 | 9 |
| T0H0A1 | 0.3309 | 0.3402 | 0.3289 | 0.299 | 0.340 | −0.971 | −0.105 | 10 |
| T1H0A0 | 0.3309 | 0.3402 | 0.3289 | 0.298 | 0.283 | −0.949 | −0.117 | 11 |
| T0H0A0 | 0.3309 | 0.3402 | 0.3289 | 0.271 | 0.201 | −1.328 | −0.279 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Lu, Y.; Song, L.; Wang, J.; Zhu, Y.; Ma, J.; Zheng, J. Integrated Effects of Warming Irrigation, Aeration, and Humic Acid on Yield, Quality, and GHG Emissions in Processing Tomatoes in Xinjiang. Agronomy 2025, 15, 1353. https://doi.org/10.3390/agronomy15061353
Wang C, Lu Y, Song L, Wang J, Zhu Y, Ma J, Zheng J. Integrated Effects of Warming Irrigation, Aeration, and Humic Acid on Yield, Quality, and GHG Emissions in Processing Tomatoes in Xinjiang. Agronomy. 2025; 15(6):1353. https://doi.org/10.3390/agronomy15061353
Chicago/Turabian StyleWang, Chubo, Yuhang Lu, Libing Song, Jingcheng Wang, Yan Zhu, Jiaying Ma, and Jiliang Zheng. 2025. "Integrated Effects of Warming Irrigation, Aeration, and Humic Acid on Yield, Quality, and GHG Emissions in Processing Tomatoes in Xinjiang" Agronomy 15, no. 6: 1353. https://doi.org/10.3390/agronomy15061353
APA StyleWang, C., Lu, Y., Song, L., Wang, J., Zhu, Y., Ma, J., & Zheng, J. (2025). Integrated Effects of Warming Irrigation, Aeration, and Humic Acid on Yield, Quality, and GHG Emissions in Processing Tomatoes in Xinjiang. Agronomy, 15(6), 1353. https://doi.org/10.3390/agronomy15061353





