Altitudinal Variation in Soil Fungal Community Associated with Alpine Potentilla fruticosa Shrublands in the Eastern Qinghai–Tibet Plateau
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Experimental Design and Sample Collection
- (1)
- IV = (relative height + relative coverage + relative density)/3;
- (2)
- Shannon–Weiner index (H) = ;
- (3)
- Simpson index (D) = 1 − ;
- (4)
- Pielou index (J) = .
2.3. Soil Sampling and Analysis
2.4. DNA Extraction, PCR, and High-Throughput Sequencing
2.5. Data Analysis
3. Results
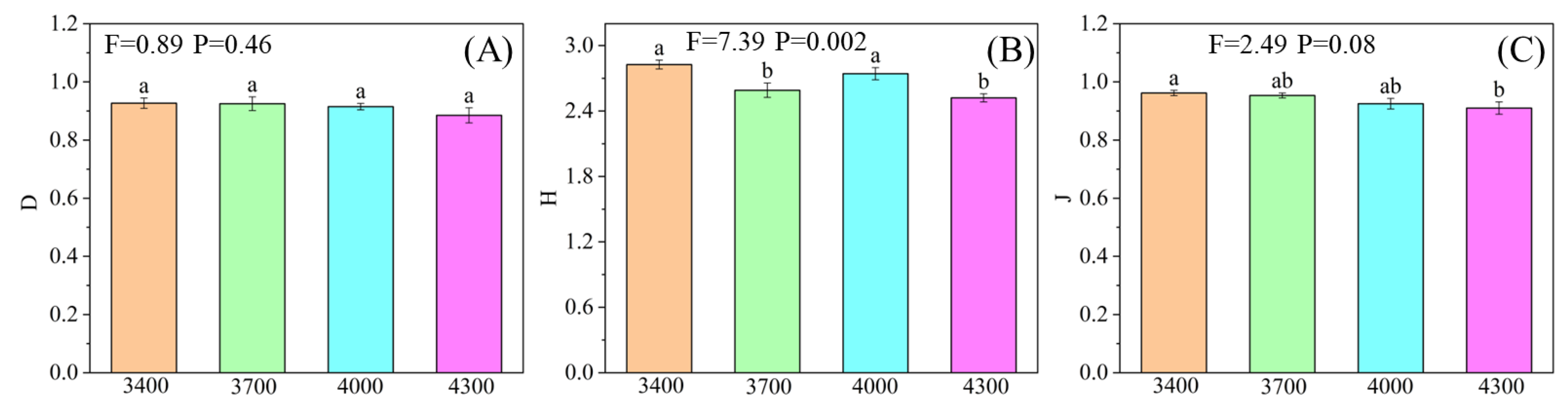
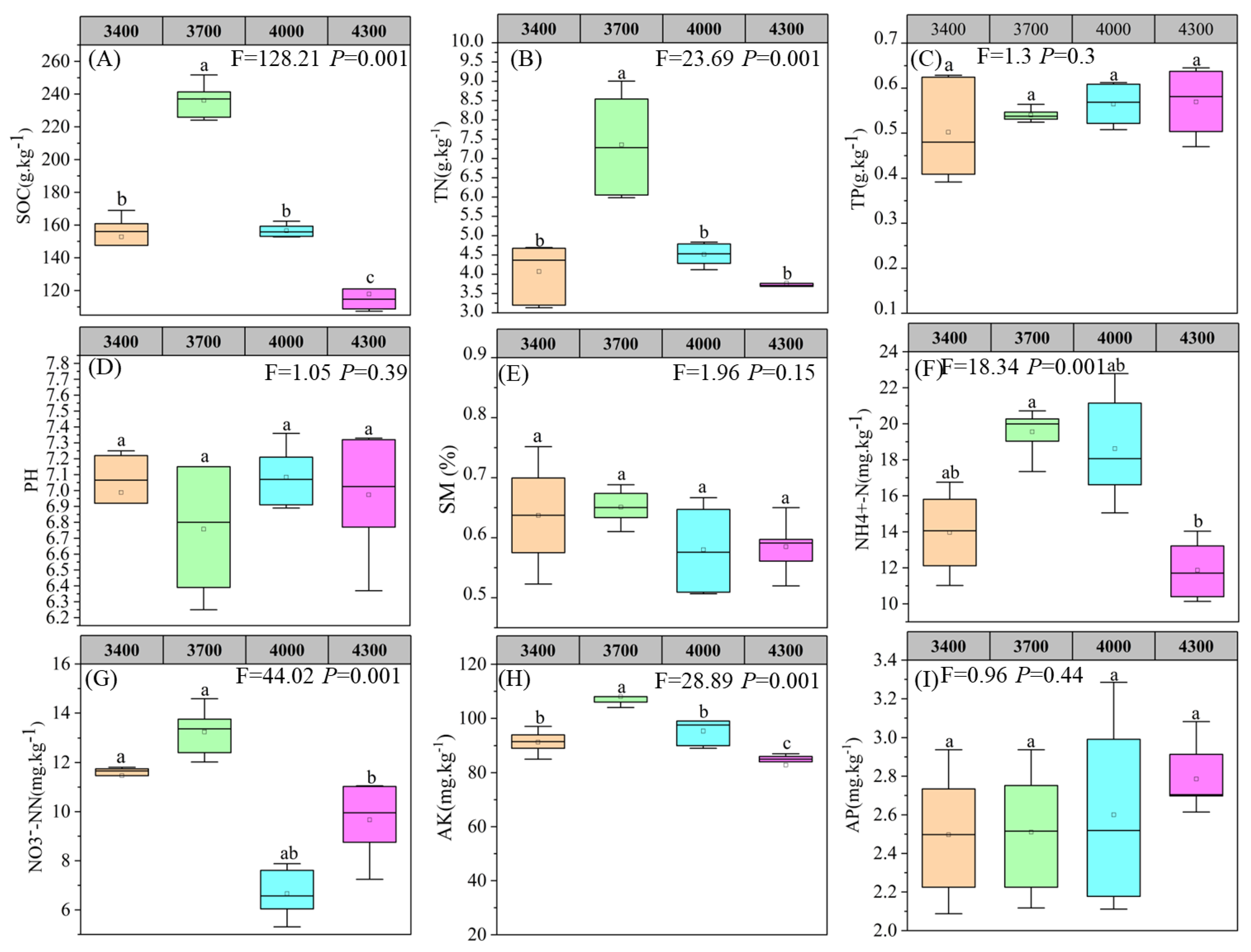
3.1. Response of Plant and Soil Properties to Different Altitudes
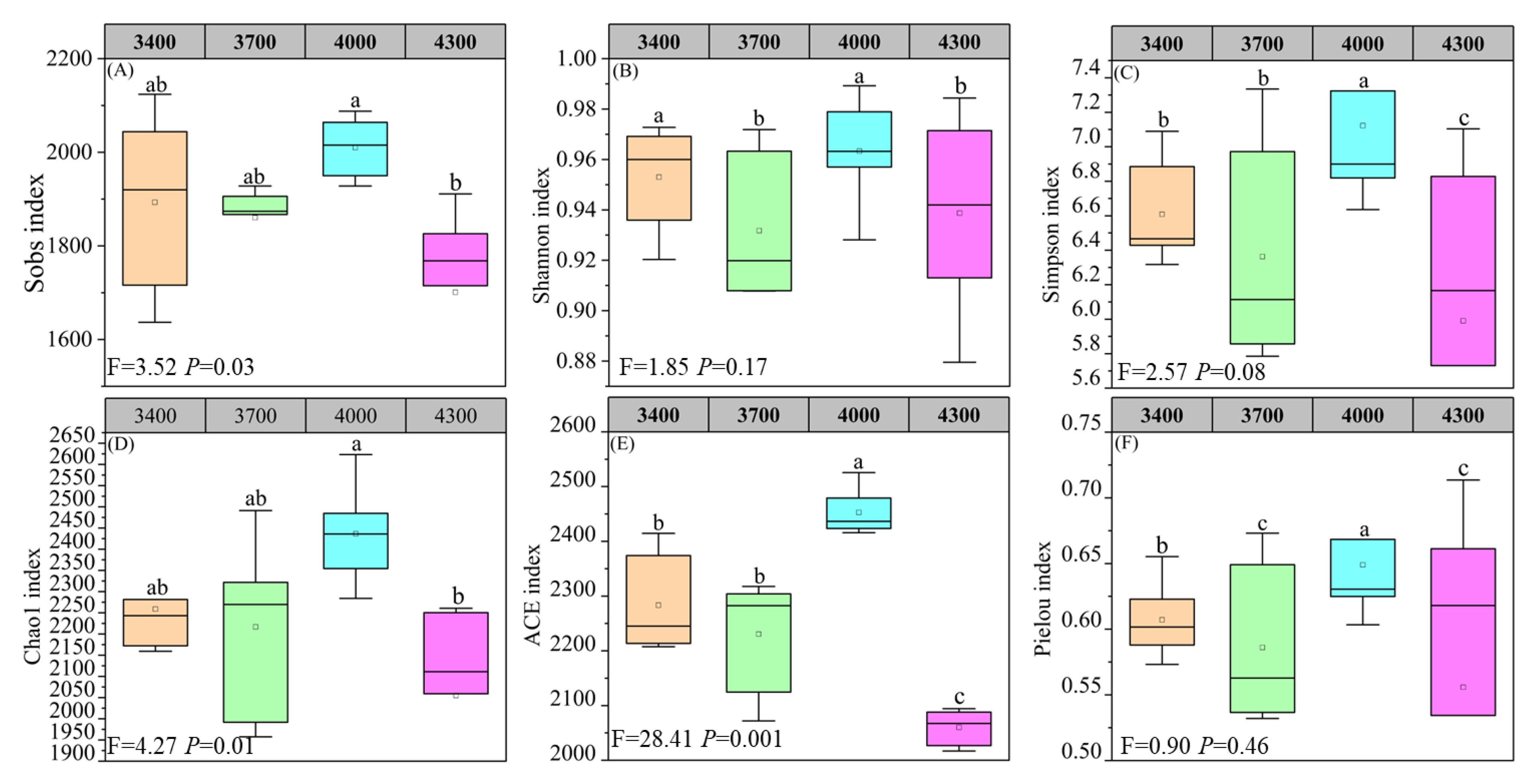
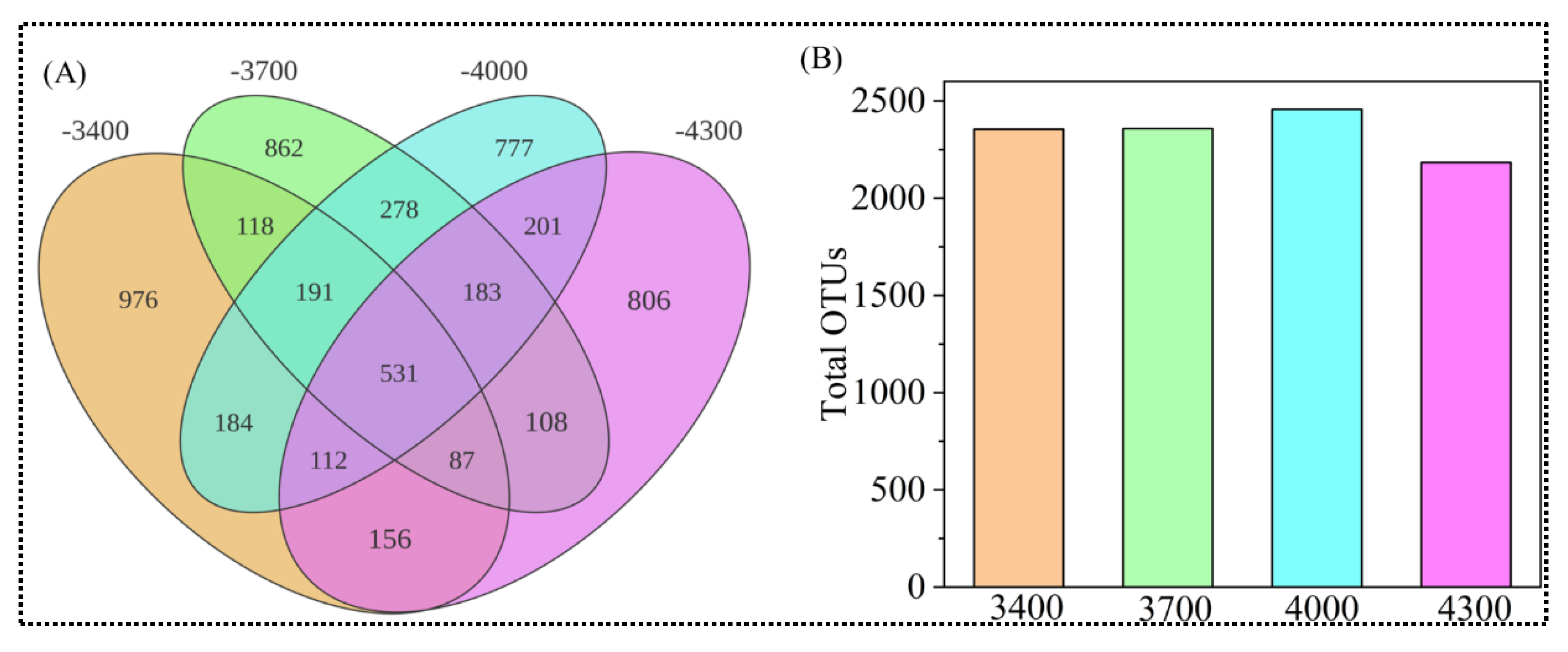
3.2. Fungal Diversity Along Altitude Gradients
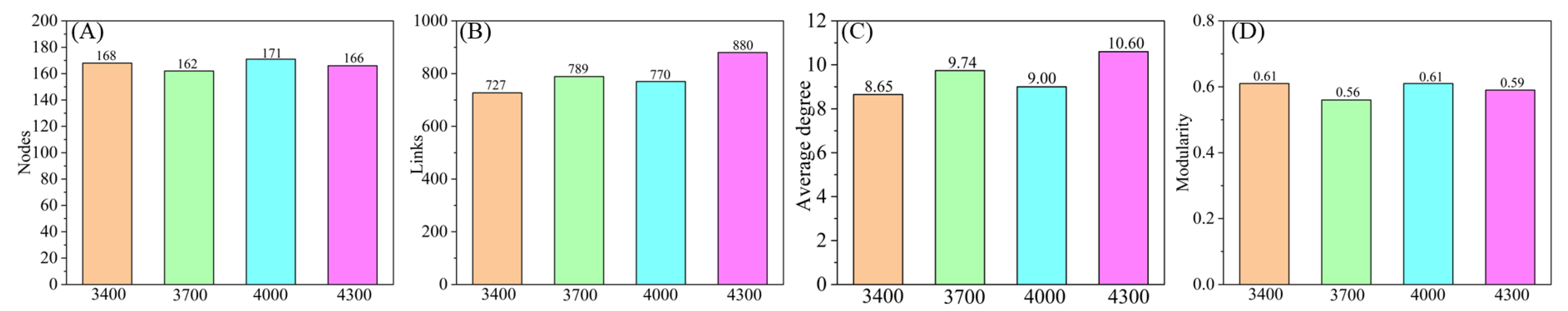
3.3. Soil Fungi Symbiotic Network
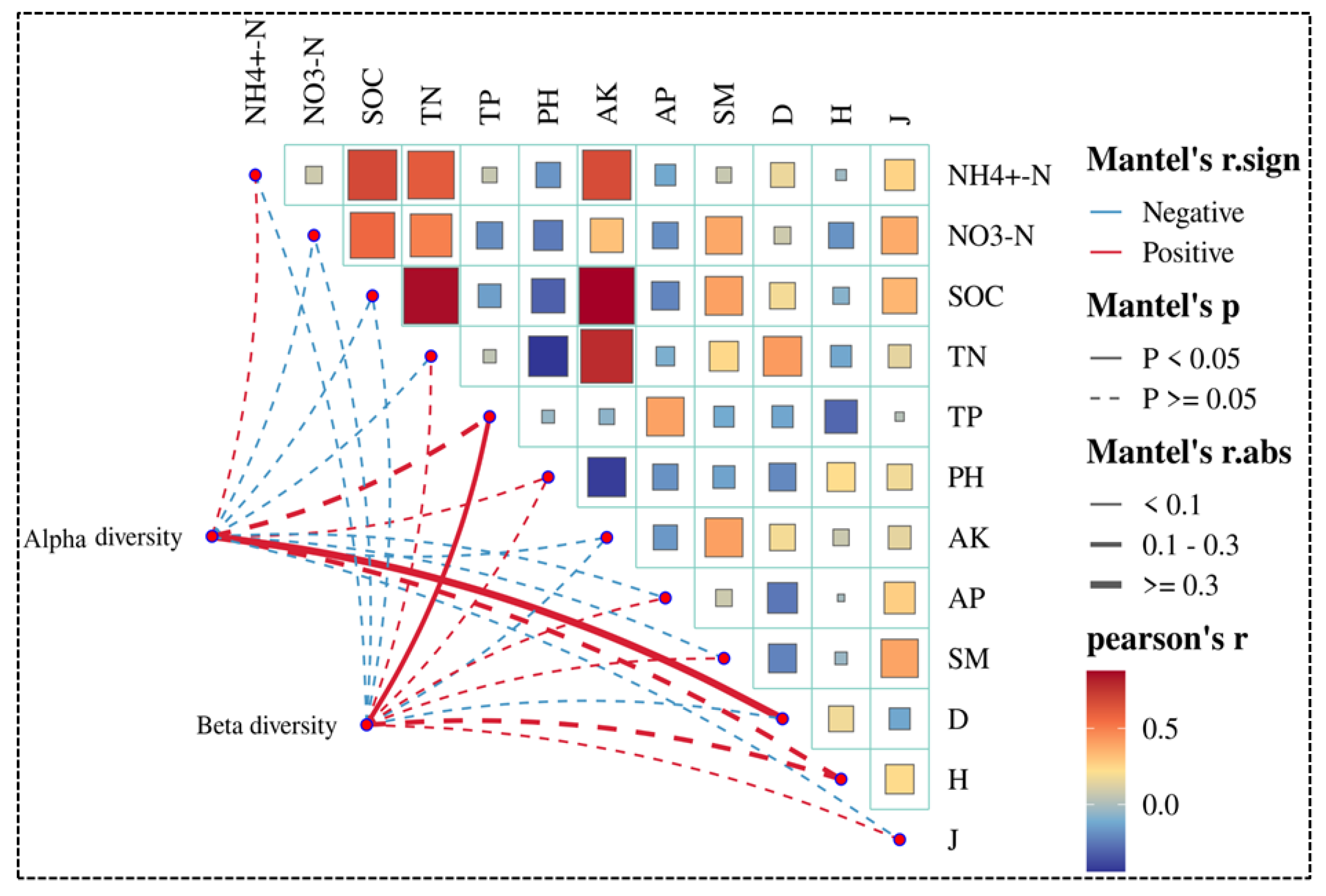
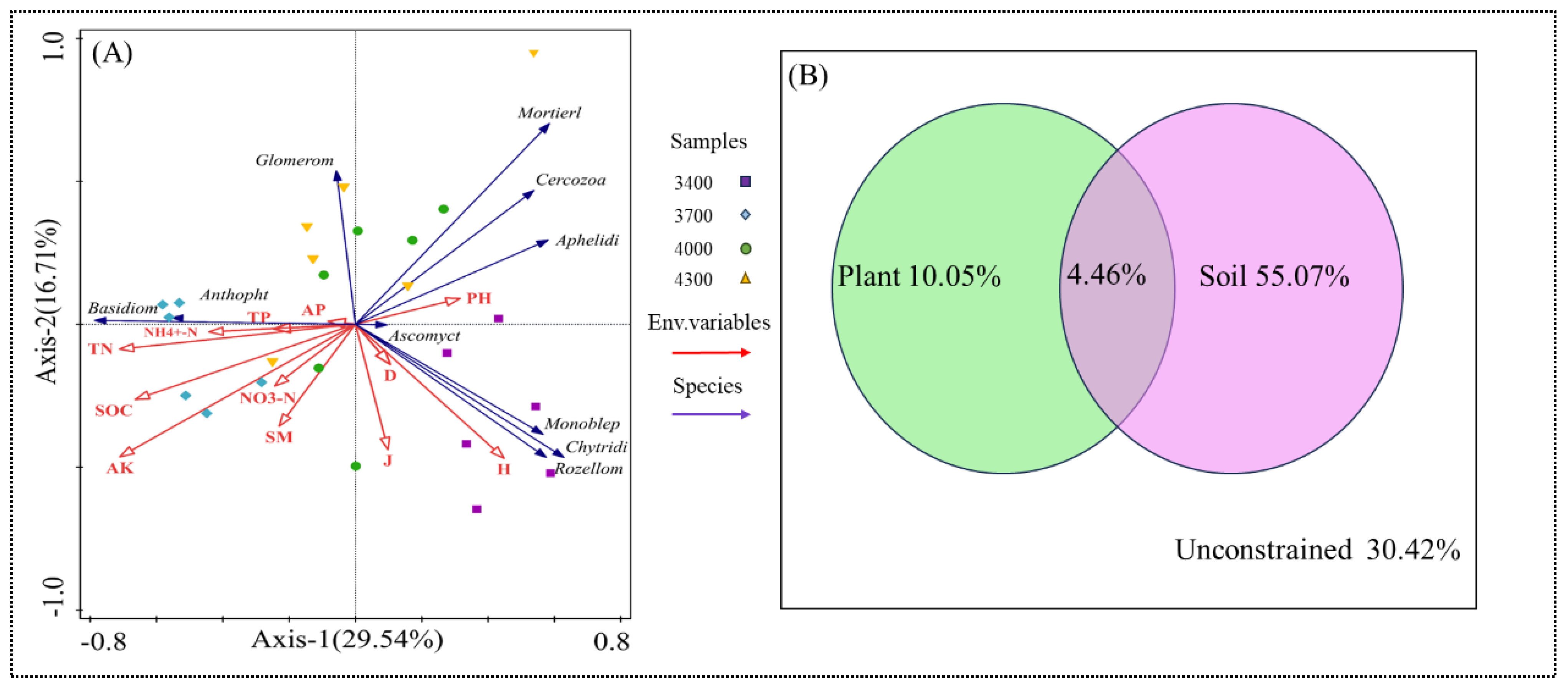
3.4. Relationships Between Soil Fungal Community Diversity and Environmental Variables
4. Discussion
4.1. Characteristics of Vegetation and Soil Factors
4.2. Effects of Altitude on the Composition of Soil Fungi Community
4.3. Effects of Altitude on Soil Fungal Diversity
4.4. Environmental Driving Factors of Soil Fungal Communities Altitude Gradient
4.5. Co-Occurrence Patterns of Soil Fungi Under Different Altitude Gradients
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Adu, B.; Wang, J.; Fan, Y. Assessing Shrub Patch Characteristics and Soil Nutrient Distribution Patterns of Four Typical Alpine Shrub Plants in the Eastern Qilian Mountains. Sustainability 2024, 16, 1547. [Google Scholar] [CrossRef]
- Nie, X.Q.; Yang, L.C.; Xiong, F.; Li, C.B.; Li, F.; Zhou, G.Y. Aboveground biomass of the alpine shrub ecosystems in Three-River Source Region of the Tibetan Plateau. J. Mt. Sci. 2018, 15, 357–363. [Google Scholar] [CrossRef]
- Zhao, J.X.; Yang, W.; Ji-Shi, A.; Ma, Y.H.; Tian, L.H.; Li, R.C.; Huang, Z.; Liu, Y.F.; Leite, P.A.M.; Ding, L.M.; et al. Shrub encroachment increases soil carbon and nitrogen stocks in alpine grassland ecosystems of the central Tibetan Plateau. Geoderma 2023, 433, 116468. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Liu, W.; Zhang, X.F.; Adamowski, J.F.; Biswas, A. Leaf stoichiometry of Potentilla fruticosa across elevations in China’s Qilian Mountains. Front. Plant Sci. 2022, 13, 814059. [Google Scholar] [CrossRef]
- Yashiro, Y.; Shizu, Y.; Hirota, M.; Shimono, A.; Ohtsuka, T. The role of shrub (Potentilla fruticosa) on ecosystem CO2 fluxes in an alpine shrub meadow. J. Plant Ecol. 2010, 3, 89–97. [Google Scholar] [CrossRef]
- Sheng, H.Y.; Cao, G.M.; Li, G.R.; Zhou, J.J.; Jiao, W.Y.; Li, J.P.; Zhang, P. Effect of grazing disturbance on plant community of alpine meadow dominated by Potentilla froticosa shrub on Qilian Mountain. Ecol. Environ. Sci. 2009, 18, 235–241. [Google Scholar]
- Li, H.P.; Song, C.G.; Zhang, F.W.; Li, Y.N. Comparison on fixed carbon amount of shrub and herb of Potentilla fruticosa shrub meadow in alpine region of Qinghai Province. J. Plant Resour. Environ. 2014, 23, 1–7. [Google Scholar]
- Jiang, Y.; Wang, P.; Xu, X.D.; Zhang, J.H. Dynamics of carbon fluxes with responses to vegetation, meteorological and terrain factors in the south-eastern Tibetan Plateau. Environ. Earth Sci. 2014, 72, 4551–4565. [Google Scholar] [CrossRef]
- Fan, Q.Y.; Yang, Y.G.; Geng, Y.Q.; Wu, Y.L.; Niu, Z.N. Biochemical composition and function of subalpine shrubland and meadow soil microbiomes in the Qilian Mountains, Qinghai–Tibetan plateau, China. PeerJ 2022, 10, e13188. [Google Scholar] [CrossRef]
- Brookes, P.C.; Pietri, J.C.A.; Wu, Y.P.; Xu, J.M.; Xu, J.; Sparks, D.L. Microorganisms indicators of soil Quality in Upland Soils. In Molecular Environmental Soil Science; Springer Dordrecht: Dordrecht, The Netherlands, 2013; pp. 413–428. [Google Scholar]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Liang, Y.M.; He, X.Y.; Chen, C.Y.; Feng, S.Z.; Liu, L.; Chen, X.B.; Zhao, Z.W.; Su, Y.R. Influence of plant communities and soil properties during natural vegetation restoration on arbuscular mycorrhizal fungal communities in a karst region. Ecol. Eng. 2015, 82, 57–65. [Google Scholar] [CrossRef]
- Kumar, M.; Prasad, R.; Kumar, V.; Tuteja, N.; Varma, A. Mycorrhizal fungi under biotic and abiotic stress. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials; Springer: Cham, Switzerland, 2017; pp. 57–69. [Google Scholar]
- Mueller, R.C.; Paula, F.S.; Mirza, B.S.; Rodrigues, J.L.; Nüsslein, K.; Bohannan, B.J. Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J. 2014, 8, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.D.; McMahon, S.; Schimel, J. Bacterial and fungal community structure in Arctic tundra tussock and shrub soils. FEMS Microbiol. Ecol. 2017, 59, 428–435. [Google Scholar] [CrossRef]
- Staddon, P.L.; Ramsey, C.B.; Ostle, N.; Ineson, P.; Fitter, A.H. Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science 2003, 300, 1138–1140. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, 867–876. [Google Scholar] [CrossRef]
- Grime, J.P. Control of species density in herbaceous vegetation. J. Environ. Manag. 1973, 1, 151–167. [Google Scholar]
- Hossain, Z.; Sugiyama, S. Geographical structure of soil microbial communities in northern Japan: Effects of distance, land use type and soil properties. Eur. J. Soil Biol. 2011, 47, 88–94. [Google Scholar] [CrossRef]
- Widden, P. Fungal communities in soils along an elevation gradient in northern England. Mycologia 1987, 79, 298–309. [Google Scholar] [CrossRef]
- Bayranvand, M.; Akbarinia, M.; Jouzani, G.S.; Gharechahi, J.; Kooch, Y.; Baldrian, P. Composition of soil bacterial and fungal communities in relation to vegetation composition and soil characteristics along an altitudinal gradient. FEMS Microbiol. Ecol. 2021, 97, fiaa201. [Google Scholar] [CrossRef]
- Cui, Y.X.; Bing, H.J.; Fang, L.C.; Wu, Y.H.; Yu, J.L.; Shen, G.T.; Jiang, M.; Wang, X.; Zhang, X.C. Diversity patterns of the rhizosphere and bulk soil microbial communities along an altitudinal gradient in an alpine ecosystem of the eastern Tibetan Plateau. Geoderma 2019, 338, 118–127. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient in alpine forest soils: What are the driving factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Nakano, T.; Hattori, M.; Nara, K. The mid-domain effect in ectomycorrhizal fungi: Range overlap along an elevation gradient on Mount Fuji, Japan. ISME J. 2014, 8, 1739–1746. [Google Scholar] [CrossRef]
- Sheng, Y.Y.; Cong, W.; Yang, L.S.; Liu, Q.; Zhang, Y.G. Forest soil fungal community elevational distribution pattern and their ecological assembly processes. Front. Microbiol. 2019, 10, 2226. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, X.; Shi, S.L.; Liu, H.F.; Liu, G.H. A hollow bacterial diversity pattern with elevation in Wolong Nature Reserve, Western Sichuan Plateau. J. Soils Sediments 2016, 16, 2365–2374. [Google Scholar] [CrossRef]
- Han, D.X.; Wang, N.; Sun, X.; Hu, Y.B.; Feng, F.J. Biogeographical distribution of bacterial communities in Changbai Mountain, Northeast China. Microbiologyopen 2018, 7, e00529. [Google Scholar] [CrossRef]
- Luo, Z.M.; Liu, J.X.; Zhao, P.Y.; Jia, T.; Li, C.; Chai, B.F. Biogeographic patterns and assembly mechanisms of bacterial communities differ between habitat generalists and specialists across elevational gradients. Front. Microbiol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Si, G.C.; Wang, J.; Luo, T.X.; Zhang, G.X. Bacterial community in alpine grasslands along an altitudinal gradient on the Tibetan Plateau. FEMS Microbiol. Ecol. 2014, 87, 121–132. [Google Scholar] [CrossRef]
- Dai, L.C.; Guo, X.W.; Ke, X.; Du, Y.G.; Zhang, F.W.; Li, Y.K.; Li, Q.; Lin, L.; Cao, G.M.; Peng, C.J.; et al. The response of Potentilla fruticosa communities to degradation succession in Qinghai-Tibet Plateau. Ecol. Environ. Sci. 2019, 28, 732–740. [Google Scholar]
- Wang, C.E.; Huang, M.; Wang, W.Y.; Li, Z.H.; Zhang, T.; Ma, L.; Bai, Y.F.; Wang, Y.L.; Shi, J.J.; Long, R.J.; et al. Variation characteristics of plant community diversity and above-ground biomass in alpine degraded slopes along altitude gradients in the headwaters region of three-river on Tibetan plateau. Acta Ecol. Sin. 2022, 42, 3640–3655. [Google Scholar]
- Nie, X.Q.; Zhou, G.Y.; Du, Y.G.; Ren, L.N.; Chen, Y.Z.; Wang, D.; Li, X.L.; Li, C.B. Grazing intensity affects soil organic carbon stock and its chemical compositions in Potentilla fruticosa shrublands on the Tibetan Plateau. J. Soil Sci. Plant Nutr. 2023, 23, 5887–5898. [Google Scholar] [CrossRef]
- Zhao, W.; Yin, Y.L.; Song, J.Q.; Li, S.X. Mixed sowing improves plant and soil bacterial community restoration in the degraded alpine meadow. Plant Soil 2024, 499, 379–392. [Google Scholar] [CrossRef]
- Zhou, X.B.; Wang, X.L.; Wang, Y.L.; Ma, Y.; Liu, Y.; Ma, Y.S. Vegetation restoration has an implication for fungal diversity and composition in a degraded temperate desert type rangeland of China. Ecol. Eng. 2024, 207, 107348. [Google Scholar] [CrossRef]
- Wu, X.; Yang, J.; Ruan, H.; Wang, S.; Yang, Y.; Naeem, I.; Wang, L.; Liu, L.; Wang, D. The diversity and co-occurrence network of soil bacterial and fungal communities and their implications for a new indicator of grassland degradation. Ecol. Indic. 2021, 129, 107989. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Cao, J.X.; Shi, S.L.; Pan, H.; Chen, Z.; Shang, H. Restoration Efficacy of Picea likiangensis var. rubescens Rehder & EH Wilson Plantations on the Soil Microbial Community Structure and Function in a Subalpine Area. Microorganisms 2021, 9, 1145. [Google Scholar]
- Jamil, A.; Yang, J.Y.; Su, D.F.; Tong, J.Y.; Chen, S.Y.; Luo, Z.W.; Shen, X.M.; Wei, S.J.; Cui, X.L. Rhizospheric soil fungal community patterns of Duchesnea indica in response to altitude gradient in Yunnan, southwest China. Can. J. Microbiol. 2020, 66, 359–367. [Google Scholar] [CrossRef]
- Chick, M.P.; Nitschke, C.R.; Cohn, J.S.; Penman, T.D.; York, A. Factors influencing above-ground and soil seed bank vegetation diversity at different scales in a quasi-Mediterranean ecosystem. J. Veg. Sci. 2018, 29, 684–694. [Google Scholar] [CrossRef]
- Shen, C.C.; Gunina, A.; Luo, Y.; Wang, J.J.; He, J.Z.; Kuzyakov, Y.; Hemp, A.; Classen, A.T.; Ge, Y. Contrasting patterns and drivers of soil bacterial and fungal diversity across a mountain gradient. Environ. Microbiol. 2020, 22, 3287–3301. [Google Scholar] [CrossRef]
- Busch, V.; Klaus, V.H.; Penone, C.; Schäfer, D.; Boch, S.; Prati, D.; Müller, J.; Socher, S.A.; Niinemets, Ü.; Peñuelas, J.; et al. Nutrient stoichiometry and land use rather than species richness determine plant functional diversity. Ecol. Evol. 2018, 8, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.R.; Yang, W.Q.; Li, Q.Y.; Qiao, Q.L.; Zhao, S.; Zhang, Y.C.; Yu, Y.H.; Zhang, S.X.; Li, X.L.; Kou, J.C. Microbial Community Response to Alpine Meadow Degradation and Its Impact on Soil Nutrient Cycling. Agronomy 2024, 14, 195. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, I.; Hussain, S.; Ali, H.; Ali, M. Effect of altitude on forest soil properties at Northern Karakoram. Eurasian Soil Sci. 2019, 52, 1159–1169. [Google Scholar]
- Russo, S.E.; Legge, R.; Weber, K.A.; Brodie, E.L.; Goldfarb, K.C.; Benson, A.K.; Tan, S. Bacterial community structure of contrasting soils underlying Bornean rain forests: Inferences from microarray and next-generation sequencing methods. Soil Biol. Biochem. 2012, 55, 48–59. [Google Scholar] [CrossRef]
- Xie, L.L.; Li, W.T.; Pang, X.Y.; Liu, Q.H.; Yin, C.Y. Soil properties and root traits are important factors driving rhizosphere soil bacterial and fungal community variations in alpine Rhododendron nitidulum shrub ecosystems along an altitudinal gradient. Sci. Total Environ. 2023, 864, 161048. [Google Scholar] [CrossRef]
- Zhao, H.B.; Zhang, X.F.; Liu, H.Y.; Bai, X.; Nie, J.M.; Han, G.D.; Han, B. Reduced moisture caused by short-term grazing prohibition results in a significant decrease of fungi abundance. J. Arid Environ. 2024, 221, 105138. [Google Scholar] [CrossRef]
- Li, Q.; He, G.X.; Wen, T.; Zhang, D.G.; Liu, X.N. Distribution pattern of soil fungi community diversity in alpine meadow in Qilian Mountains of eastern Qinghai-Tibetan Plateau. Ecol. Indic. 2022, 141, 109054. [Google Scholar] [CrossRef]
- Sui, X.; Li, M.S.; Frey, B.; Dai, G.H.; Yang, L.B.; Li, M.H. Effect of elevation on composition and diversity of fungi in the rhizosphere of a population of Deyeuxia angustifolia on Changbai Mountain, northeastern China. Front. Microbiol. 2023, 14, 1087475. [Google Scholar] [CrossRef]
- Yang, H.; Lu, G.; Jiang, H.; Shi, D.N.; Liu, Z. Diversity and distribution of soil micro-fungi along an elevation gradient on the north slope of Changbai Mountain. J. For. Res. 2017, 28, 831–839. [Google Scholar] [CrossRef]
- Bennett, R.J.; Turgeon, B.G. Fungal Sex: The Ascomycota. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Li, J.B.; Shen, Z.H.; Li, C.N.; Kou, Y.P.; Wang, Y.S.; Tu, B.; Zhang, S.H.; Li, X.Z. Stair-step pattern of soil bacterial diversity mainly driven by pH and vegetation types along the elevational gradients of Gongga Mountain, China. Front. Microbiol. 2018, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.J.; Zhou, Z.H.; Guo, Y.X.; Yang, G.H.; Zhao, F.Z.; Wei, G.H.; Han, X.H.; Feng, L.; Feng, Y.Z.; Ren, G.X. Contrasting patterns of microbial community and enzyme activity between rhizosphere and bulk soil along an elevation gradient. Catena 2021, 196, 104921. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; Fornasier, F.; Paletto, A.; Ceotto, E.; De Meo, I. Ascomycota and Basidiomycota fungal phyla as indicators of land use efficiency for soil organic carbon accrual with woody plantations. Ecol. Indic. 2024, 160, 111796. [Google Scholar] [CrossRef]
- Zhang, E.H.; Liu, P.P.; He, P.; Jian, Y.; Xu, Y.T.; Chen, C.X.; Lu, Y.Z.; Lan, X.Z.; Suo Lang, S.M. Physiochemical properties and microbial community structure in rhizosphere soil of dracocephalum tanguticum. J. Agric. Sci. Technol. 2024, 26, 201–213. [Google Scholar]
- Zhao, J.Y.; Xie, X.; Jiang, Y.Y.; Li, J.X.; Fu, Q.; Qiu, Y.B.; Fu, X.H.; Yao, Z.Y.; Dai, Z.M.; Qiu, Y.P.; et al. Effects of simulated warming on soil microbial community diversity and composition across diverse ecosystems. Sci. Total Environ. 2024, 911, 168793. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, T.; Zhang, K.; Shen, C.; Chu, H. Fungal communities along a small-scale elevational gradient in an alpine tundra are determined by soil carbon nitrogen ratios. Front. Microbiol. 2018, 9, 1815. [Google Scholar] [CrossRef]
- Lu, N.N.; Xu, X.L.; Wang, P.; Zhang, P.; Ji, B.M.; Wang, X.J. Succession in arbuscular mycorrhizal fungi can be attributed to a chronosequence of Cunninghamia lanceolata. Sci. Rep. 2019, 9, 18057. [Google Scholar] [CrossRef]
- Paluch, E.C.; Thomsen, M.A.; Volk, T.J. Effects of resident soil fungi and land use history outweigh those of commercial mycorrhizal inocula: Testing a restoration strategy in unsterilized soil. Restor. Ecol. 2013, 21, 380–389. [Google Scholar] [CrossRef]
- Wang, J.T.; Zheng, Y.M.; Hu, H.W.; Zhang, L.M.; Li, J.; He, J.Z. Soil pH determines the alpha diversity but not beta diversity of soil fungal community along altitude in a typical Tibetan forest ecosystem. J. Soils Sediments 2015, 15, 1224–1232. [Google Scholar] [CrossRef]
- Zhao, W.; Yin, Y.L.; Li, S.X.; Dong, Y.L.; Su, S.F. Changes in soil fungal community composition and functional groups during the succession of Alpine grassland. Plant Soil 2023, 484, 201–216. [Google Scholar] [CrossRef]
- Williams, M.A.; Jangid, K.; Shanmugam, S.G.; Whitman, W.B. Bacterial communities in soil mimic patterns of vegetative succession and ecosystem climax but are resilient to change between seasons. Soil Biol. Biochem. 2013, 57, 749–757. [Google Scholar] [CrossRef]
- Wang, K.B.; Zhang, Y.W.; Tang, Z.S.; Shangguan, Z.P.; Chang, F.; Jia, F.A.; Chen, Y.P.; He, X.H.; Shi, W.Y.; Deng, L. Effects of grassland afforestation on structure and function of soil bacterial and fungal communities. Sci. Total Environ. 2019, 676, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hu, Y.T.; Zhang, S.S.; Noll, I.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jia, R.L.; Yu, L.Y. Diversity and distribution of soil fungal communities associated with biological soil crusts in the southeastern Tengger Desert (China) as revealed by 454 pyrosequencing. Fungal Ecol. 2016, 23, 156–163. [Google Scholar] [CrossRef]
- Ushio, M.; Wagai, R.; Balser, T.C.; Kitayama, K. Variations in the soil microbial community composition of a tropical montane forest ecosystem: Does tree species matter? Soil Biol. Biochem. 2008, 40, 2699–2702. [Google Scholar] [CrossRef]
- Kang, E.Z.; Li, Y.; Zhang, X.D.; Yan, Z.Q.; Wu, H.D.; Li, M.; Yan, L.; Zhang, K.R.; Wang, J.Z.; Kang, X.M. Soil pH and nutrients shape the vertical distribution of microbial communities in an alpine wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Sun, Y.M.; Chen, X.L.; Cai, J.W.; Li, Y.Z.; Zhou, Y.H.; Zhang, H.X.; Zheng, K.H. Altitudinal Effects on Soil Microbial Diversity and Composition in Moso Bamboo Forests of Wuyi Mountain. Plants 2024, 13, 2471. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Sinaj, S.; Davis, M.R.; Sherlock, R.R.; Frossard, E. Effects of plant species on phosphorus availability in a range of grassland soils. Plant Soil 2003, 256, 115–130. [Google Scholar] [CrossRef]
- Yoder, C.K.; Nowak, R.S. Phosphorus acquisition by Bromus madritensis ssp. rubens from soil interspaces shared with Mojave Desert shrubs. Funct. Ecol. 2000, 14, 685–692. [Google Scholar] [CrossRef]
- Gao, X.L.; Li, X.G.; Zhao, L.; Kuzyakov, Y. Regulation of soil phosphorus cycling in grasslands by shrubs. Soil Biol. Biochem. 2019, 133, 1–11. [Google Scholar] [CrossRef]
- He, J.L.; Li, X.G. Potentilla fruticosa has a greater capacity to translocate phosphorus from the lower to upper soils than herbaceous grasses in an alpine meadow. Agric. Ecosyst. Environ. 2016, 228, 19–29. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Teste, F.P.; Veneklaas, E.J.; Dixon, K.W.; Lambers, H. Complementary plant nutrient-acquisition strategies promote growth of neighbour species. Funct. Ecol. 2014, 28, 819–828. [Google Scholar] [CrossRef]
- Zhong, Z.K.; Wang, X.; Zhang, X.Y.; Zhang, W.; Xu, Y.D.; Ren, C.J.; Han, X.H.; Yang, G.H. Edaphic factors but not plant characteristics mainly alter soil microbial properties along a restoration chronosequence of Pinus tabulaeformis stands on Mt. Ziwuling, China. For. Ecol. Manag. 2019, 453, 117625. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.F.; He, Z.L.; Luo, F.; Zhou, J.Z. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Cazelles, K.; Gravel, D. Co-occurrence is not evidence of ecological interactions. Ecol. Lett. 2020, 23, 1050–1063. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wang, J.Y.; Chen, X.; Meng, Z.X.; Xu, R.; Duoji, D.; Zhang, J.H.; He, J.; Wang, Z.A.; Chen, J.; et al. Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Duan, Y.L.; Lian, J.; Wang, L.L.; Wang, X.Y.; Luo, Y.Q.; Wang, W.F.; Wu, F.S.; Zhao, J.H.; Ding, Y.; Ma, J.; et al. Variation in soil microbial communities along an elevational gradient in alpine meadows of the Qilian Mountains, China. Front. Microbiol. 2021, 12, 684386. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- Zhu, B.J.; Li, C.N.; Wang, J.M.; Li, J.B.; Li, X.Z. Elevation rather than season determines the assembly and co-occurrence patterns of soil bacterial communities in forest ecosystems of Mount Gongga. Appl. Microbiol. Biotechnol. 2020, 104, 7589–7602. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Yuan, M.H.; Duan, W.H.; Xia, J.Y.; Zhang, X.S.; Zhao, Y.F.; Wang, J.W. Effect of soil microbial community on ecosystem multifunctionality in an alpine grassland. Catena 2025, 249, 108714. [Google Scholar] [CrossRef]
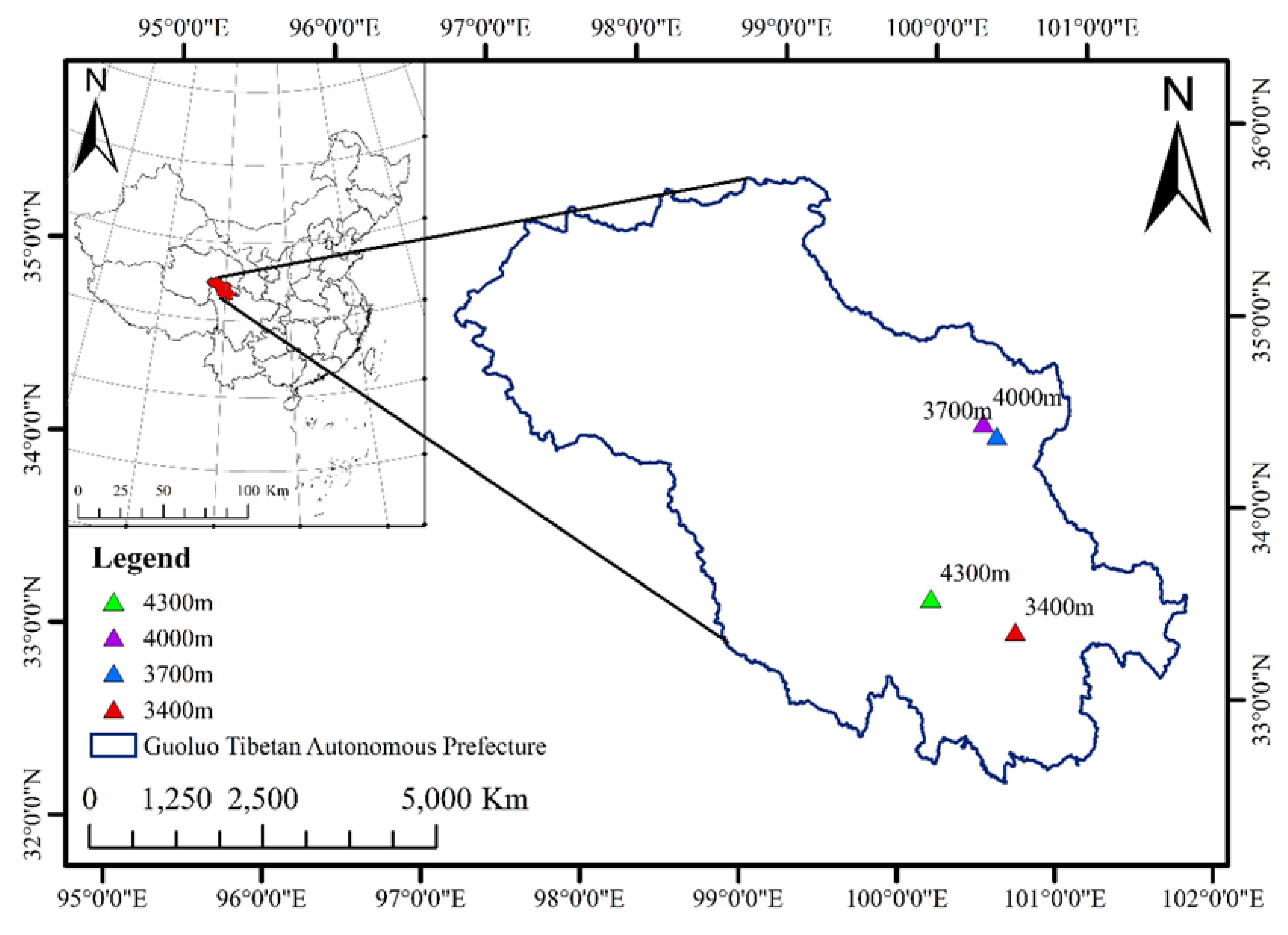

| Altitude | Location | Latitude and Longitude | Dominant Species | Total Coverage |
|---|---|---|---|---|
| 3400 | Banma | 100°4′81″ 33°18′51″ | Potentilla fruticosa, Kobresia capillifolia, Spiraea alpina, Leontopodium nanum, Oxytropis ochrocephala | 85–90% |
| 3700 | Maqin | 100°30′37″ 34°19′51″ | Potentilla fruticos, Salix oritrepha, Kobresia humilis, Polygonum viviparum, Poa orinosa, Pedicularis kansuensis | 80–85% |
| 4000 | Dari | 100°25′9″ 34°23′47″ | Potentilla fruticosa, Spiraea alpina, Helictotrichon tibeticum, Poa orinosa, Anaphalis lactea | 65–75% |
| 4300 | Jiuzhi | 100°8′6″ 33°28′9″ | Potentilla fruticosa, Leontopodium nanum, Poa orinosa, Stipa aliena, Aster flaccidus | 60–70% |
| Soil Chemical Properties | Importance Ranking | Explains (%) | Contribution (%) | Pseudo-F | p |
|---|---|---|---|---|---|
| AK | 1 | 19.6 | 29.8 | 5.4 | 0.002 |
| H | 2 | 11.0 | 16.8 | 3.3 | 0.012 |
| AP | 3 | 6.6 | 10.0 | 2.1 | 0.040 |
| TN | 4 | 5.2 | 7.90 | 1.7 | 0.094 |
| J | 5 | 4.2 | 6.50 | 1.4 | 0.186 |
| D | 6 | 4.6 | 6.90 | 1.6 | 0.110 |
| NH4+-N | 7 | 3.5 | 5.40 | 1.3 | 0.238 |
| TP | 8 | 3.9 | 5.90 | 1.4 | 0.200 |
| pH | 9 | 3 | 4.5 | 1.1 | 0.392 |
| SOC | 10 | 1.5 | 2.4 | 0.5 | 0.752 |
| SM | 11 | 1.5 | 2.3 | 0.5 | 0.794 |
| NO3−-N | 12 | 1 | 1.5 | 0.3 | 0.912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Ma, Y.; Wang, Y.; Ma, Y.; Liu, Y. Altitudinal Variation in Soil Fungal Community Associated with Alpine Potentilla fruticosa Shrublands in the Eastern Qinghai–Tibet Plateau. Agronomy 2025, 15, 1345. https://doi.org/10.3390/agronomy15061345
Xie L, Ma Y, Wang Y, Ma Y, Liu Y. Altitudinal Variation in Soil Fungal Community Associated with Alpine Potentilla fruticosa Shrublands in the Eastern Qinghai–Tibet Plateau. Agronomy. 2025; 15(6):1345. https://doi.org/10.3390/agronomy15061345
Chicago/Turabian StyleXie, Lele, Yushou Ma, Yanlong Wang, Yuan Ma, and Yu Liu. 2025. "Altitudinal Variation in Soil Fungal Community Associated with Alpine Potentilla fruticosa Shrublands in the Eastern Qinghai–Tibet Plateau" Agronomy 15, no. 6: 1345. https://doi.org/10.3390/agronomy15061345
APA StyleXie, L., Ma, Y., Wang, Y., Ma, Y., & Liu, Y. (2025). Altitudinal Variation in Soil Fungal Community Associated with Alpine Potentilla fruticosa Shrublands in the Eastern Qinghai–Tibet Plateau. Agronomy, 15(6), 1345. https://doi.org/10.3390/agronomy15061345







