Plant Protection Products to Control Alternaria Brown Spot Caused by Alternaria alternata in Citrus: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
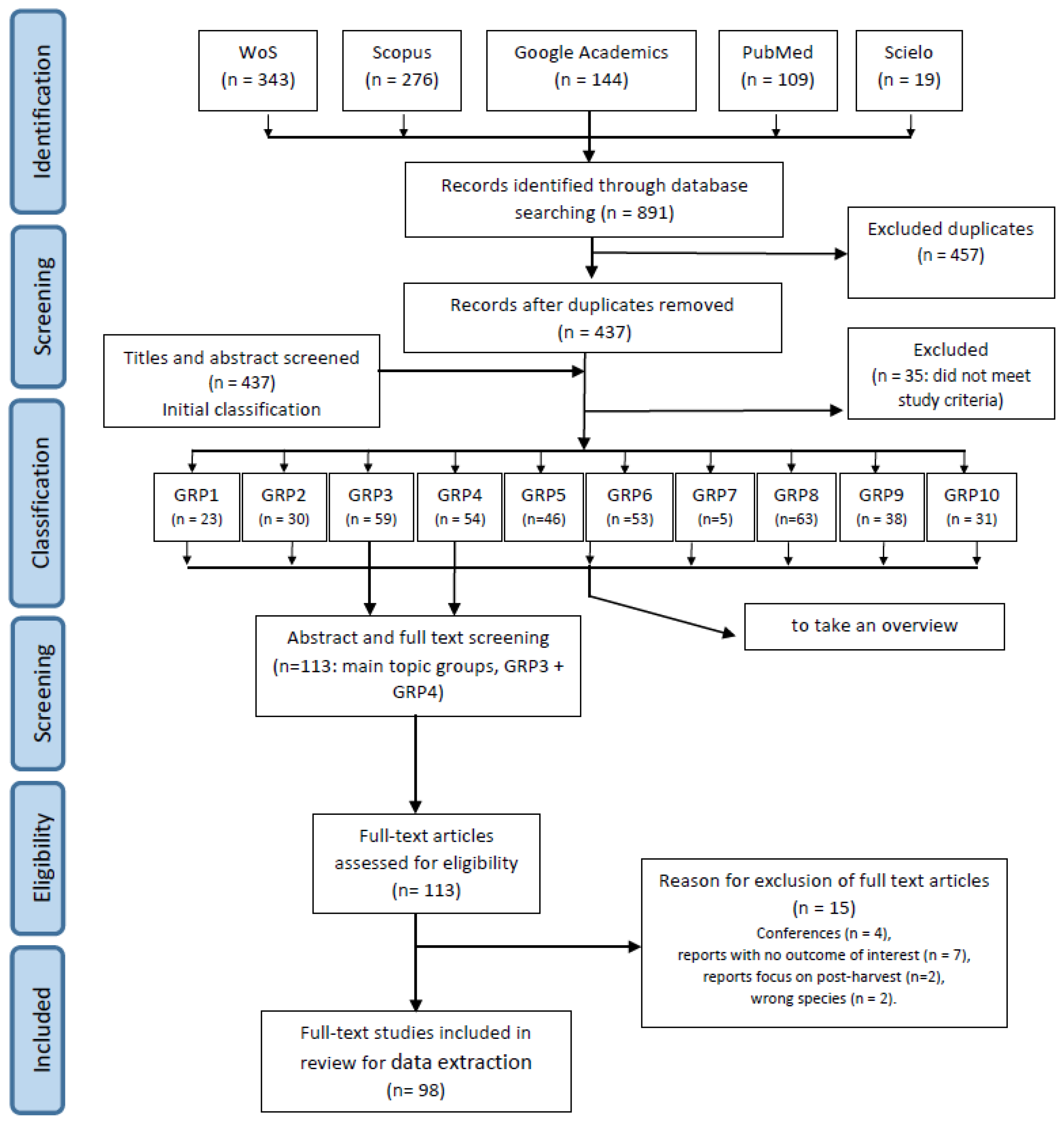
2.1. Step 1: Information Sources and Search Strategy
2.2. Step 2: Initial Classification of Records and First Data Collection
2.3. Step 3: Eligibility Criteria for Full-Text Review
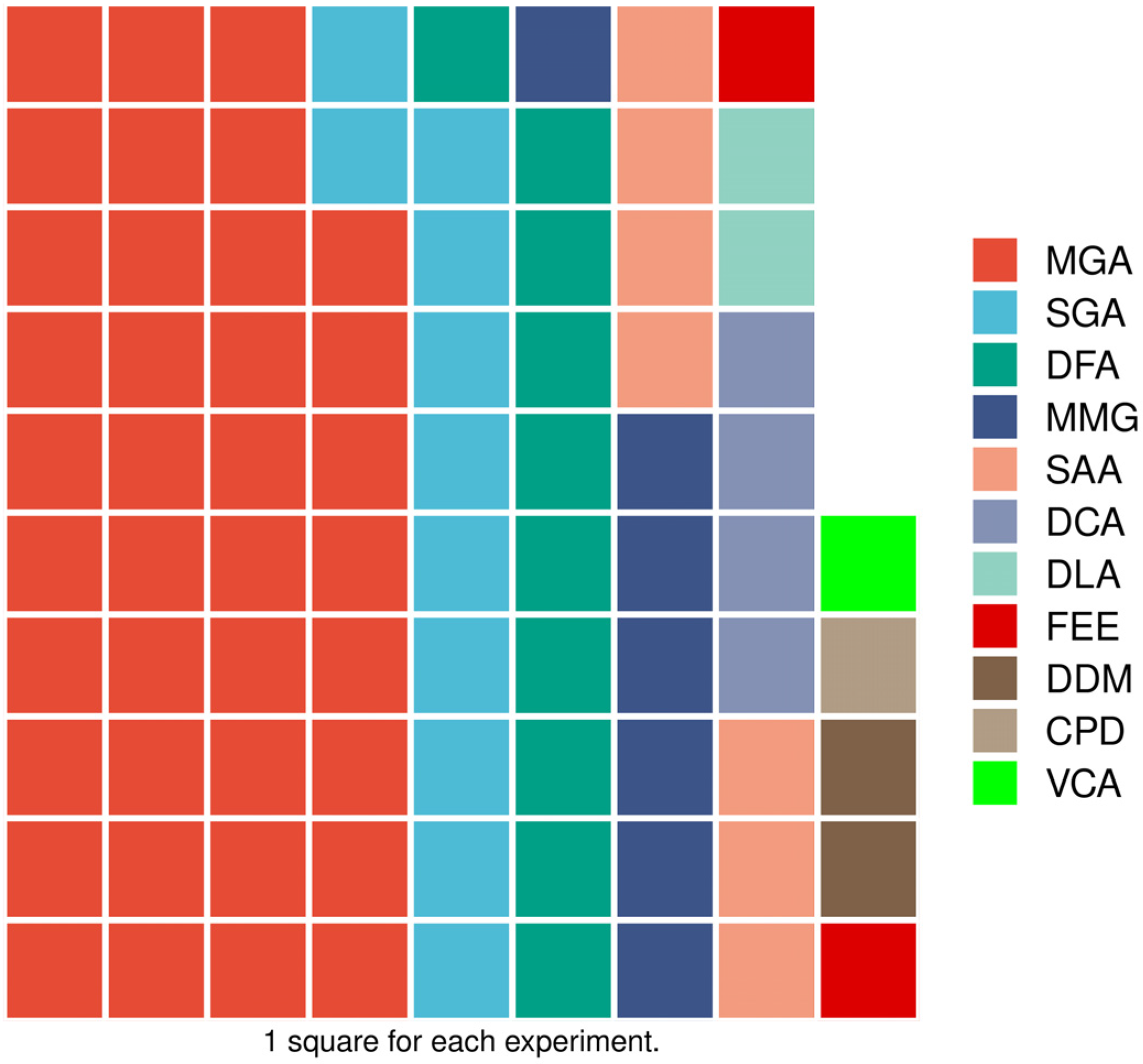
2.4. Step 4: Charting Data
- Article identifiers: authors, year of publication, country, and title.
- Target species: plant species, variety, and disease (A. a. general, ABS, or post-harvest losses).
- Substance information: group, common (commercial) name, scientific (substance) name, additional information.
- Experiment information: type (I, II: when two different types of experiments were used in the same study), concentration, and additional information (field experiments: yes/no).
- Main result: text (the results explained in the text), MIC (Minimum Inhibitory Concentration), MGI (Mycelial Growth Inhibition), EC50% (Concentration causing 50% growth inhibition), effectiveness (Type I, Type II).
- Conclusion: text.
- Interest: importance (goes from 1 to 5, and reflects the closeness to the main topic), reliability scale (goes from 1 to 3, low, medium, high; reflects the quality and reproducibility of experiments).
2.5. Step 5: Risk of Bias, Effect Measure, and Heterogeneity Among Study
2.6. Step 6: Collating, Summarizing, and Reporting the Results
3. Results
3.1. Search in Databases
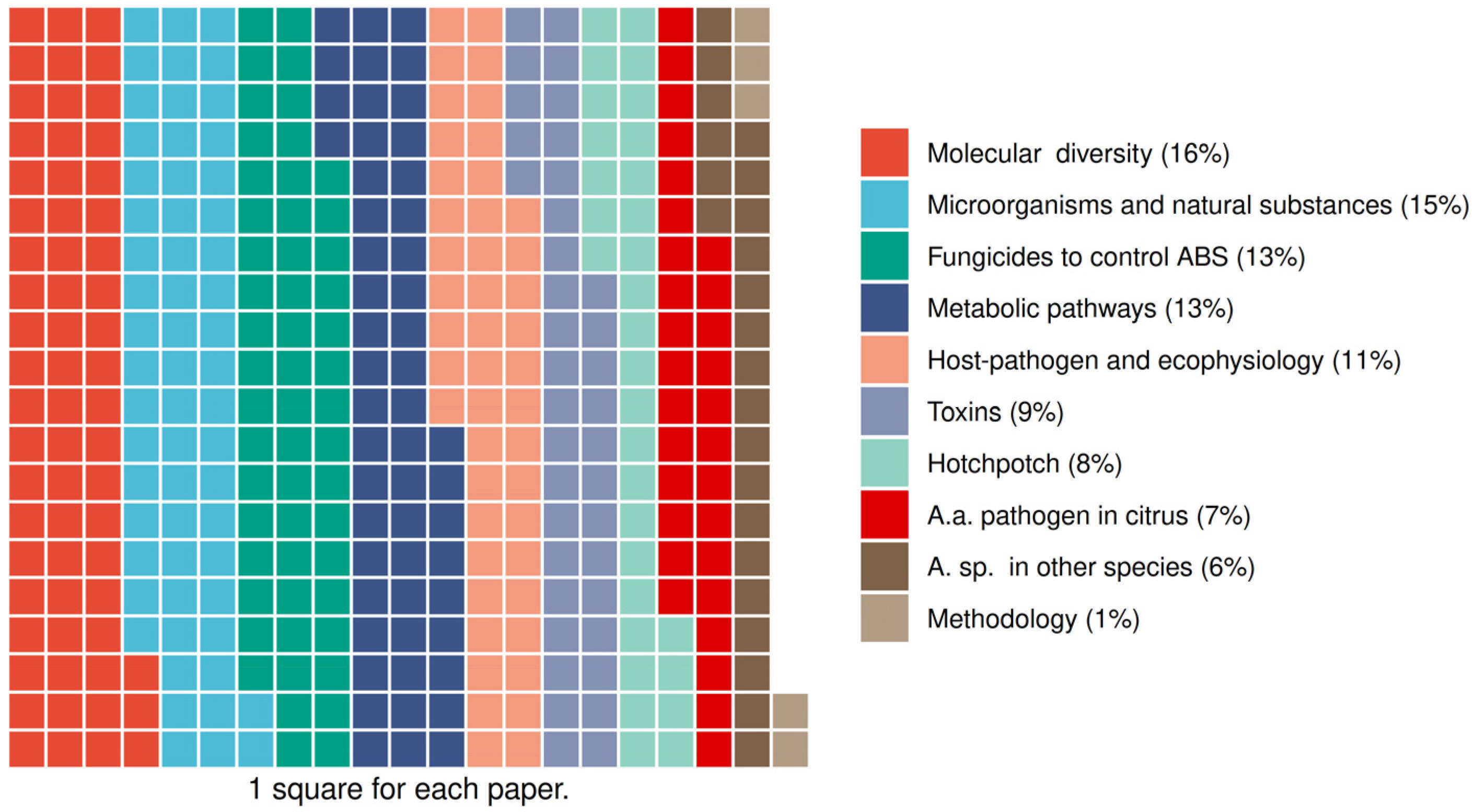
3.2. Initial Classification
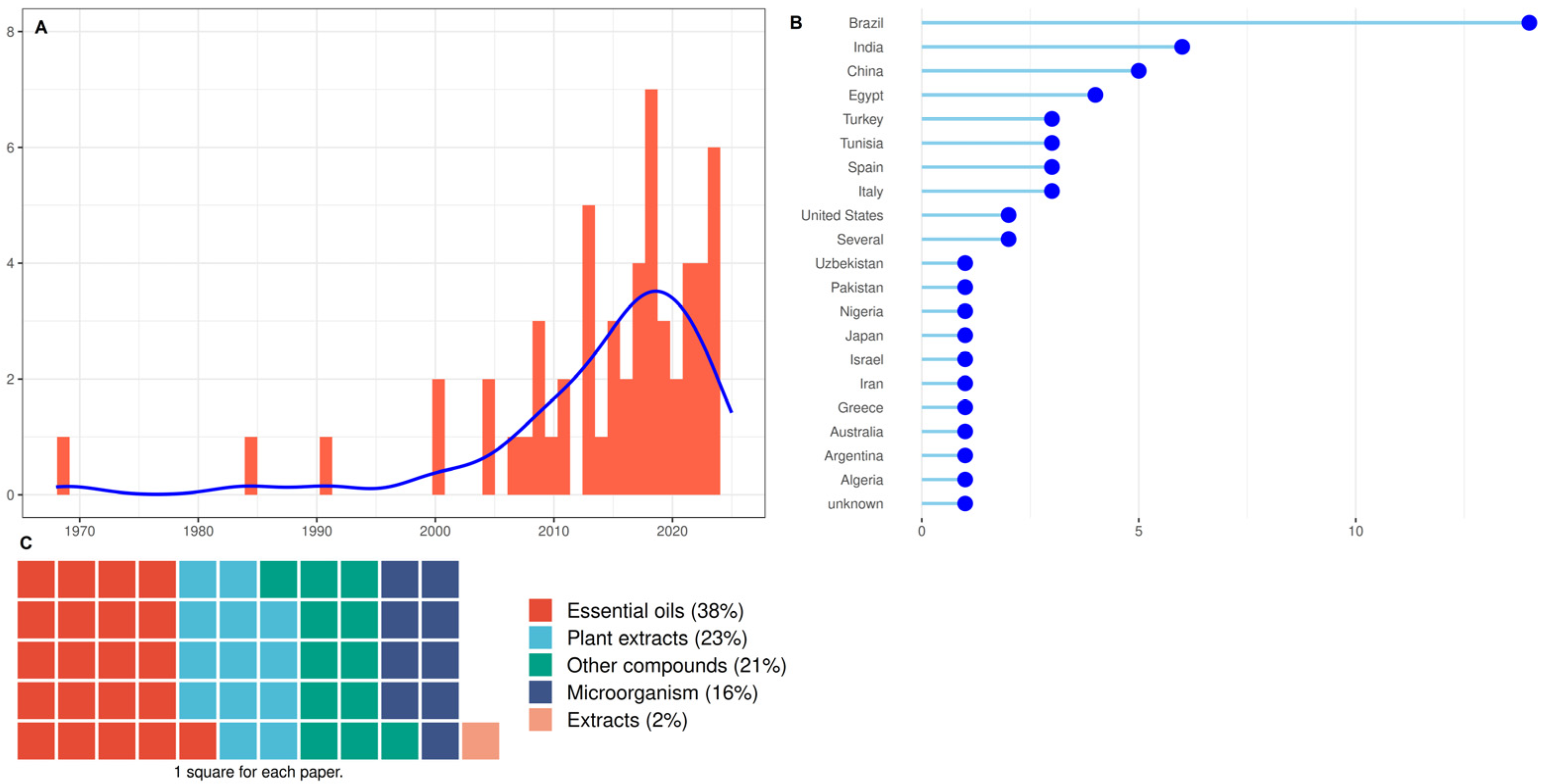
3.3. Studies Characteristics of Group 3: Microorganisms and Natural Substances
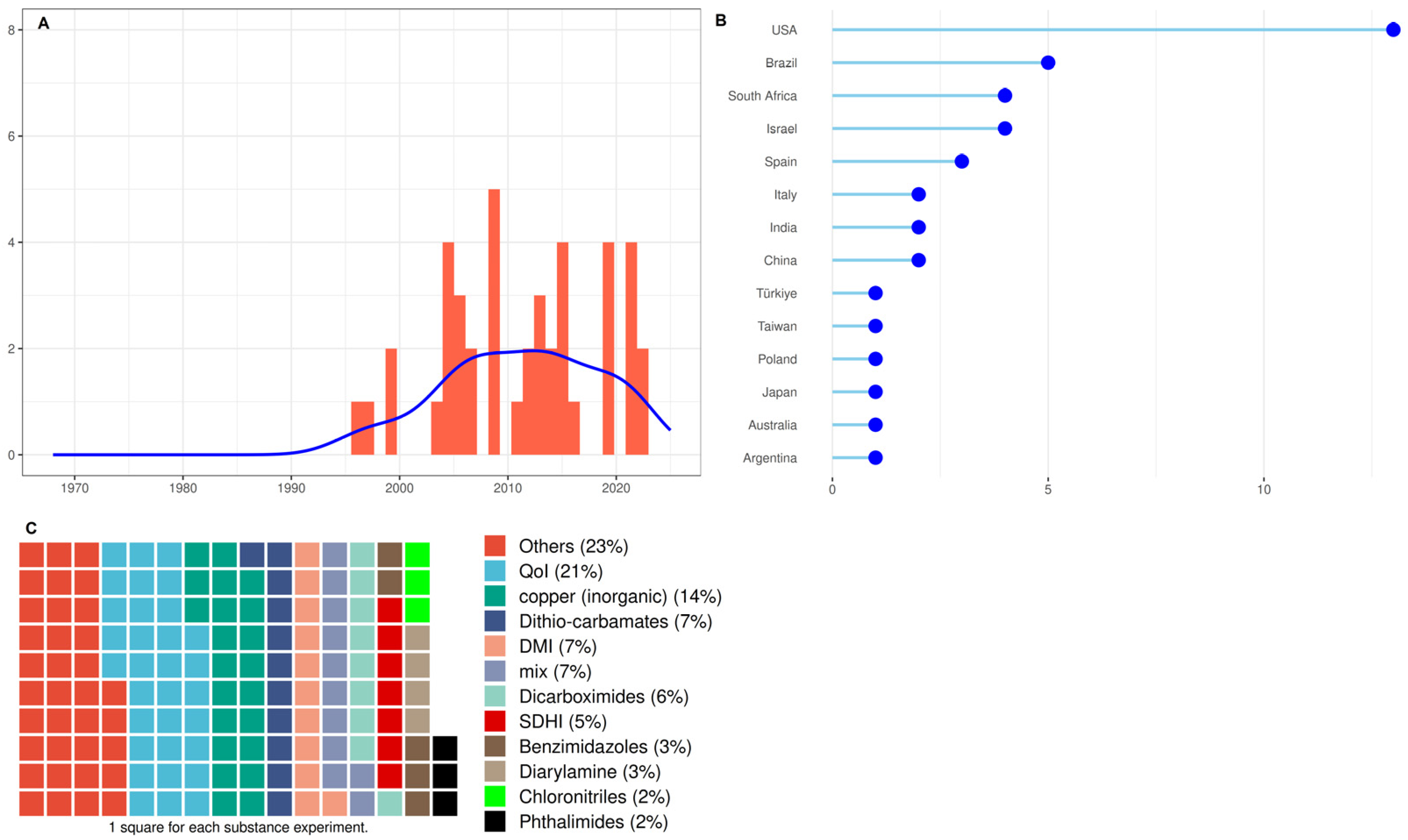
3.4. Studies Characteristics of Group 4: Fungicides to Control ABS
4. Discussion
4.1. Microorganisms and Natural Substances to Control ABS
4.1.1. Methodologies Used to Test the Antifungal Activity of Microorganisms and Natural Substances
4.1.2. Microorganisms to Control ABS
4.1.3. Natural Substances to Control ABS
- Essential Oils to Control ABS
- Plant extracts to control ABS
- Other compounds to control ABS
4.1.4. Summary of the Findings of the Biological Control and Natural Substances Group and Why These Plant Protection Products Are Rarely Used Under Field Conditions
4.2. Fungicides to Control ABS
4.2.1. Methodologies Used to Test the Antifungal Activity of Fungicides
4.2.2. Details of Fungicide Groups to Control ABS
- Copper (inorganic) group
- Dithiocarbamates group and other MSCA
- DeMethylation Inhibitors (DMIs) imidazoles and triazoles group
- Quinone outside Inhibitor (QoI) group
- Benzimidazoles, Diarylamine, and Dicarboximides group
- Succinate-dehydrogenase inhibitor (SDHI) group
- Others (not classified in previous groups)
4.2.3. Summary of the Findings of the Fungicides Group to Control ABS and Why They Are Currently Failing to Control the Disease in the Field
4.3. Breeding for Disease Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Alternaria Brown Spot |
| ACT | Alternaria Citri Toxin |
| AUDPC | Area under the disease progress curve |
| CEO | Citrus essential oil |
| DMI | DeMethylation Inhibitor |
| EC50 | Concentration causing 50% growth inhibition |
| EO | Essential oil |
| FRAC | Fungicide Resistance Action Committee |
| MBC | Methyl Benzimidazole Carbamate |
| MGA | Mycelial Growth Assay |
| MGI | Mycelial Growth Inhibition |
| MGR | Mycelial growth rate |
| MIC | Minimum Inhibitory Concentration |
| MSCA | Multi-site contact activity |
| QoI | Quinone outside Inhibitor |
| SDHI | Succinate-dehydrogenase inhibitor |
References
- Whiteside, J.O. Alternaria-Recognition, Prevention and Control of Alternaria Brown Spot on Dancy Tangerines and Minneola Tangelos. Citrus Ind. Mag. 1986, 67, 44–47. [Google Scholar]
- Pacheco, C.D.A.; Martelli, I.B.; Polydoro, D.A.; Schinor, E.H.; Pio, R.M.; Kupper, K.C.; Azevedo, F.A.D. Resistance and Susceptibility of Mandarins and Their Hybrids to Alternaria alternata. Sci. Agric. 2012, 69, 386–392. [Google Scholar] [CrossRef]
- Reis, R.F.; de Almeida, T.F.; Stuchi, E.S.; de Goes, A. Susceptibility of Citrus Species to Alternaria alternata, the Causal Agent of the Alternaria Brown Spot. Sci. Hortic. 2007, 113, 336–342. [Google Scholar] [CrossRef]
- Arlotta, C.; Ciacciulli, A.; Strano, M.C.; Cafaro, V.; Salonia, F.; Caruso, P.; Licciardello, C.; Russo, G.; Smith, M.W.; Cuenca, J.; et al. Disease Resistant Citrus Breeding Using Newly Developed High Resolution Melting and CAPS Protocols for Alternaria Brown Spot Marker Assisted Selection. Agronomy 2020, 10, 1368. [Google Scholar] [CrossRef]
- de Souza, M.C.; Stuchi, E.S.; de Goes, A. Evaluation of Tangerine Hybrid Resistance to Alternaria alternata. Sci. Hortic. 2009, 123, 1–4. [Google Scholar] [CrossRef]
- Hu, J.; Liu, R.; Wang, X.; Zhou, N.; Hong, Q.; Yao, T.; Li, T.; Jiang, D.; Cao, L.; Li, H. Evaluation of Citrus Germplasm Resistance to Alternaria alternata. J. Fruit Sci. 2015, 32, 672–680. [Google Scholar]
- Solel, Z.; Kimchi, M. Susceptibility and Resistance of Citrus Genotypes to Alternaria alternata Pv. Citri. J. Phytopathol. 1997, 145, 389–391. [Google Scholar] [CrossRef]
- Llorens, E.; Scalschi, L.; Fernández-Crespo, E.; Lapeña, L.; García-Agustín, P. Hexanoic Acid Provides Long-Lasting Protection in “Fortune” Mandarin against Alternaria alternata. Physiol. Mol. Plant Pathol. 2015, 91, 38–45. [Google Scholar] [CrossRef]
- Felipini, R.B.; Brito, R.A.S.; Azevedo, F.A.; Massola, N.S. Alternaria alternata f. sp. Citri Tangerine Pathotype Induces Reactive Oxygen Species Accumulation in Susceptible Citrus Leaves. Physiol. Mol. Plant Pathol. 2023, 126, 102040. [Google Scholar]
- Kimura, N.; Tsuge, T. Gene Cluster Involved in Melanin Biosynthesis of the Filamentous Fungus Alternaria alternata. J. Bacteriol. 1993, 175, 4427–4435. [Google Scholar] [CrossRef]
- Ajiro, N.; Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Izumi, Y.; et al. Role of the Host-Selective ACT-Toxin Synthesis Gene ACTTS2 Encoding an Enoyl-Reductase in Pathogenicity of the Tangerine Pathotype of Alternaria alternata. Phytopathology 2010, 100, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Masunaka, A.; Ohtani, K.; Peever, T.L.; Timmer, L.W.; Tsuge, T.; Yamamoto, M.; Yamamoto, H.; Akimitsu, K. An Isolate of Alternaria alternata That Is Pathogenic to Both Tangerines and Rough Lemon and Produces Two Host-Selective Toxins, ACT- and ACR-Toxins. Phytopathology 2005, 95, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kohmoto, K.; Itoh, Y.; Shimomura, N.; Kondoh, Y.; Otani, H.; Kodama, M.; Nishimura, S.; Nakatsuka, S. Isolation and Biological-Activities of 2 Host-Specific Toxins from the Tangerine Pathotype of Alternaria-alternata. Phytopathology 1993, 83, 495–502. [Google Scholar] [CrossRef]
- Ma, H.J.; Zhang, B.; Gai, Y.P.; Sun, X.P.; Chung, K.R.; Li, H.Y. Cell-Wall-Degrading Enzymes Required for Virulence in the Host Selective Toxin-Producing Necrotroph Alternaria alternata of Citrus. Front. Microbiol. 2019, 10, 2514. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Chen, C.W.; Choo, C.Y.L.; Chen, Y.K.; Yago, J.I.; Chung, K.R. Proper Functions of Peroxisomes Are Vital for Pathogenesis of Citrus Brown Spot Disease Caused by Alternaria alternata. J. Fungi 2020, 6, 248. [Google Scholar] [CrossRef]
- Badal, J.; Cuenca, F.; Zornoza, C.; Jiménez, J.G.; Peris, A.; Armengol, J.; Civera, A.V.; Alfaro-Lassala, F. Conocimientos Sobre La Epidemiología de Alternaria alternata Pv. Citri y Su Utilización En El Diseño de Estrategias de Control de La Mancha Marrón de Los Cítricos. Phytoma España Rev. Prof. Sanid. Veg. 2004, 164, 112–116. [Google Scholar]
- Timmer, L.W.; Peever, T.L.; Solel, Z.; Akimitsu, K. Alternaria Diseases of Citrus-Novel Pathosystems. Phytopathol. Mediterr. 2003, 42, 99–112. [Google Scholar]
- Agostini, J.; Bushong, P.; Timmer, L. Greenhouse Evaluation of Products That Induce Host Resistance for Control of Scab, Melanose, and Alternaria Brown Spot of Citrus. Plant Dis. 2003, 87, 69–74. [Google Scholar] [CrossRef]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken Wiley 2019, 4, 1–694. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. Declaración PRISMA 2020: Una Guía Actualizada Para La Publicación de Revisiones Sistemáticas. Rev. Española Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2025. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; The R Foundation: Vienna, Austria, 2023. [Google Scholar]
- Rudis, B.; Gandy, D. Waffle: Create Waffle Chart Visualizations, R package version 1.0.2. 2023. Available online: https://CRAN.R-project.org/package=waffle (accessed on 7 March 2025).
- Pinto, K.M.S.; de Melo, P.A.F.R.; Nascimento, L.C.D.; Cortez, M.I.G.M.; Aires, A.A.d.C.; Mondego, J.M.; Lima, R.P.; da Silva, E.C.; Mesquita, M.L.R.; de Lemos, R.N.S. Biological Potential of Extracts of Caatinga Plants in the Control of Alternaria alternata f. sp. Citri in Citrus. J. Agric. Sci. 2018, 10, 116–125. [Google Scholar] [CrossRef]
- Soylu, E.M.; Kose, F. Antifungal Activities of Essential Oils Against Citrus Black Rot Disease Agent Alternaria alternata. J. Essent. Oil Bear. Plants 2015, 18, 894–903. [Google Scholar] [CrossRef]
- Carvalho, D.D.C.; Alves, E.; Camargos, R.B.; Oliveira, D.F.; Scolforo, J.R.S.; de Carvalhod, D.A.; Batista, T.R.S. Plant Extracts to Control Alternaria alternata in Murcott Tangor Fruits. Rev. Iberoam. Micol. 2011, 28, 173–178. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, F.A.; Devite, F.T.; Bastianel, M.; Schinor, E.H.; Da Conceição, P.M. Citrus Essential Oils as an Alternative Method of Control of the Fungus Alternaria alternata (Fr.: Fr.) Keissler. arXiv 2023. [Google Scholar] [CrossRef]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal Activity of Essential Oils against Filamentous Fungi Determined by Broth Microdilution and Vapour Contact Methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Fokkema, N.J. Fungal Antagonisms in the Phyllosphere. Ann. Appl. Biol. 1978, 89, 115–119. [Google Scholar] [CrossRef]
- Dilantha Fernando, W.G.; Linderman, R.G. Inhibition of Phytophthora Vignae and Stem and Root Rot of Cowpea by Soil Bacteria. Biol. Agric. Hortic. 1995, 12, 1–14. [Google Scholar] [CrossRef]
- Tozlu, E.; Kotan, M.S.; Tekiner, N.; Dikbas, N.; Kotan, R. Biological Control of Postharvest Spoilage in Fresh Mandarins (Citrus Reticulata Blanco) Fruits Using Bacteria During Storage. Erwerbs-Obstbau 2019, 61, 157–164. [Google Scholar] [CrossRef]
- Perina, F.J.; Lage de Andrade, C.C.; Moreira, S.I.; Nery, E.M.; Ogoshi, C.; Alves, E. Cinnamomun Zeylanicum Oil and Trans-Cinnamaldehyde against Alternaria Brown Spot in Tangerine: Direct Effects and Induced Resistance. Phytoparasitica 2019, 47, 575–589. [Google Scholar] [CrossRef]
- Camargos, R.B.; Perina, F.J.; Carvalho, D.D.C.; Alves, E.; Mascarello, A.; Chiaradia-Delatorre, L.D.; Yunes, R.A.; Nunes, R.J.; Oliveira, D.F. Chalcones to Control Alternaria alternata in Murcott Tangor Fruits. Biosci. J. 2016, 32, 1512–1521. [Google Scholar] [CrossRef]
- Demartelaere, A.C.F.; do Nascimento, L.C.; Abraão, P.C.; Gomes, R.S.S.; Marinho, C.O.; Nunes, M.C. Alternatives in the Control of Alternaria Brown Spot in ‘Dancy’ Tangerine. Summa Phytopathol. 2018, 44, 164–169. [Google Scholar] [CrossRef]
- Lamine, M.; Hamdi, Z.; Zemni, H.; Rahali, F.Z.; Melki, I.; Mliki, A.; Gargouri, M. From Residue to Resource: The Recovery of High-Added Values Compounds through an Integral Green Valorization of Citrus Residual Biomass. Sustain. Chem. Pharm. 2024, 37, 101379. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Abdelgaleil, S.A.M. Composition and Antimicrobial Activity of Essential Oils Isolated from Egyptian Plants against Plant Pathogenic Bacteria and Fungi. Ind. Crops Prod. 2014, 52, 776–782. [Google Scholar] [CrossRef]
- Perina, F.J.; Amaral, D.C.; Fernandes, R.S.; Labory, C.R.G.; Teixeira, G.A.; Alves, E. Thymus Vulgaris Essential Oil and Thymol against Alternaria alternata (Fr.) Keissler: Effects on Growth, Viability, Early Infection and Cellular Mode of Action. Pest Manag. Sci. 2015, 71, 1371–1378. [Google Scholar] [CrossRef]
- Papoutsis, K.; Vuong, Q.V.; Tesoriero, L.; Pristijono, P.; Stathopoulos, C.E.; Gkountina, S.; Lidbetter, F.; Bowyer, M.C.; Scarlett, C.J.; Golding, J.B. Microwave Irradiation Enhances the in Vitro Antifungal Activity of Citrus By-Product Aqueous Extracts against Alternaria alternata. Int. J. Food Sci. Technol. 2018, 53, 1510–1517. [Google Scholar] [CrossRef]
- Saks, Y.; Barkaigolan, R. Aloe Vera Gel Activity Against Plant-Pathogenic Fungi. Postharvest Biol. Technol. 1995, 6, 159–165. [Google Scholar] [CrossRef]
- Affes, T.G.; Lasram, S.; Hammami, M.; Yeddes, W.; Wannes, W.A.; Khammassi, S.; Ben Hmida, N.L.; Nasraoui, B.; Tounsi, M.S. In Vitro Antifungal Potential of Peel Essential Oils from Different Citrus Species on Alternaria alternata. Trends Phytochem. Res. 2022, 6, 214–223. [Google Scholar] [CrossRef]
- Demartelaere, A.C.F.; Nascimento, L.C.; Almeida, L.C.; Vargas, C.S.; Porcino, M.M.; Clemente, P.A. Using Caesalpinia Ferrea Extract the Management of Alternaria Brown Spot in Tangerine Seedlings “Dancy”. Rev. Bras. Plantas Med. 2021, 19, 58–66. [Google Scholar]
- Pinto, K.M.S.; de Melo, P.A.F.R.; Mondego, J.M.; do Nascimento, L.C.; Cortez, M.I.M.M.; de Carvalho Aires, A.A.; dos Anjos Neto, A.P.; de Medeiros, R.L.S.; Araujo, J.R.G.; da Silva, H.F. Plant Extracts Enhancers of Defense Response in Ponkan Mandarin Seedlings against Alternaria Alternate f. Spp. Citri Infection. Afr. J. Agric. Res. 2018, 13, 650–656. [Google Scholar] [CrossRef]
- Cirvilleri, G.; Bonaccorsi, A.; Scuderi, G.; Scortichini, M. Potential Biological Control Activity and Genetic Diversity of Pseudomonas Syringae Pv. Syringae Strains. J. Phytopathol. 2006, 154, 654–666. [Google Scholar] [CrossRef]
- Khalil, M.S.A.; El-Gamal, N.G.; El-Mougy, N.S.; Abdel-Kader, M.M. Occurrence of Citrus Brown and Black Spot Diseases and Their Control Using Pre-Harvest Approaches. Biosci. J. 2022, 38, e38098. [Google Scholar] [CrossRef]
- Wilson, C.; Chalutz, E.; Wilson, C.L.; McLaughlin, R.J.; McLaughin, R.J. Biological Control of Post-Harvest Rot on Fruits by Applying New Strains of Pichia Guilliermondii (Anamorph Candida Guilliermondii). Patent Number US7530381-N, 4 December 1990. [Google Scholar]
- Zhang, R.; Yu, J.; Yin, X.; Ren, X.; Kong, Q. Biocontrol of Postharvest Decay on Cherry Tomatoes by Recombinant Strain GS115/CEC and Its Possible Mechanism. Food Biotechnol. 2018, 32, 163–177. [Google Scholar] [CrossRef]
- Riera, N.; Handique, U.; Zhang, Y.Z.; Dewdney, M.M.; Wang, N.A. Characterization of Antimicrobial-Producing Beneficial Bacteria Isolated from Huanglongbing Escape Citrus Trees. Front. Microbiol. 2017, 8, 2415. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Herrmann-Andrade, A.M.; Calabrese, C.D.; Bello, F.; Vazquez, D.; Musumeci, M.A. Effectiveness of Trichoderma Strains Isolated from the Rhizosphere of Citrus Tree to Control Alternaria alternata, Colletotrichum Gloeosporioides and Penicillium Digitatum A21 Resistant to Pyrimethanil in Post-Harvest Oranges (Citrus sinensis L. (Osbeck)). J. Appl. Microbiol. 2020, 129, 712–727. [Google Scholar] [CrossRef]
- Scuderi, G.; Bonaccorsi, A.; Panebianco, S.; Vitale, A.; Polizzi, G.; Cirvilleri, G. Some Strains of Burkholderia Gladioli Are Potential Candidates for Postharvest Biocontrol of Fungal Rots in Citrus and Apple Fruits. J. Plant Pathol. 2009, 91, 207–213. [Google Scholar]
- Turaeva, B.I.; Soliev, A.B.; Karimov, H.K.; Azimova, N.S.Q.; Kutlieva, G.J.; Khamidova, K.M.; Zuxritdinova, N.Y. Disease Causing Phytopathogenic Micromycetes in Citrus in Uzbekistan. Pak. J. Phytopathol. 2021, 33, 383–393. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, J.; Tu, C.; Li, J.; Lei, C.; Guo, Q.; Zhang, Z.; Qin, W. In Vitro Antibacterial Activity of 34 Plant Essential Oils against Alternaria alternata. In E3S Web of Conferences; EDP Sciences: Les Ulis Cedex, France, 2019; Volume 136. [Google Scholar]
- Aslam, M.F.; Irshad, G.; Naz, F.; Khan, M.A. Evaluation of the Antifungal Activity of Essential Oils against Alternaria alternata Causing Fruit Rot of Eriobotrya Japonica. Turk. J. Biochem. 2022, 47, 511–521. [Google Scholar] [CrossRef]
- Ajayi-Moses, O.B.; Ogidi, C.O.; Akinyele, B.J. Bioactivity of Citrus Essential Oils (CEOs) against Microorganisms Associated with Spoilage of Some Fruits. Chem. Biol. Technol. Agric. 2019, 6, 22. [Google Scholar] [CrossRef]
- Shukla, A.C.; Shahi, S.K.; Anupam Dikshit, A.D. Epicarp of Citrus Sinensis: A Potential Source of Natural Pesticide. Indian Phytopathol. 2000, 53, 468–471. [Google Scholar]
- Raina, P.K. In Vitro Fungitoxicity of Citrus Sinensis Essential Oil to Compost-Based Weed Fungi of Agaricus Bisporus. Mushroom Res. 2004, 13, 82–83. [Google Scholar]
- Singh, P.; Shukla, R.; Prakash, B.; Kumar, A.; Singh, S.; Mishra, P.K.; Dubey, N.K. Chemical Profile, Antifungal, Antiaflatoxigenic and Antioxidant Activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck Essential Oils and Their Cyclic Monoterpene, DL-Limonene. Food Chem. Toxicol. 2010, 48, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, F.Z.; Allem, R. Antifungal Properties of Leaf Essential Oils of Citrus against Alternaria alternata and Penicillium sp in Vitro. Phytotherapie 2017, 15, 263–266. [Google Scholar] [CrossRef]
- de Souza Gomes, M.; das Graças Cardoso, M.; de Souza, P.E.; Machado, S.M.F.; Silva, L.F.; Teixeira, M.L.; de Andrade, J.; de Miranda, C.A.S.F.; Andrade, M.A. Multivariate Analysis of the Essential Oil Components of the Genus Citrus and Their Antifungal Activity. Científica 2013, 41, 111–121. [Google Scholar]
- de Lima, C.B.; Assumpcao Rentschler, L.L.; Bueno, J.T.; Boaventura, A.C. Plant Extracts and Essential Oils on the Control of Alternaria alternata, Alternaria Dauci and on the Germination and Emergence of Carrot Seeds (Daucus carota L.). Cienc. Rural. 2016, 46, 764–770. [Google Scholar] [CrossRef]
- Shafique, S.; Shafique, S.; Ahmed, A. Ecofriendly Response of Citrus Peels to Alternaria Leaf Spots of Tomato: Exclusive Role of Peel Phenolics. Int. J. Agric. Biol. 2013, 15, 1236–1242. [Google Scholar]
- Triaca, T.; Cavião, H.C.; Pansera, M.R.; Venturin, L.; Sartori, V.C. Detection of Antifungal Activity of Plant Extracts on Alternaria Citrus. Summa Phytopathol. 2018, 44, 185–188. [Google Scholar] [CrossRef]
- Campos, V.A.; Perina, F.J.; Alves, E.; Sartorelli, J.; Moura, A.M.; Oliveira, D.F. Anadenanthera Colubrina (Vell.) Brenan Produces Steroidal Substances That Are Active against Alternaria alternata (Fr.) Keissler and That May Bind to Oxysterol-Binding Proteins. Pest Manag. Sci. 2014, 70, 1815–1822. [Google Scholar] [CrossRef]
- Hernández, A.; Ruiz-Moyano, S.; Galván, A.I.; Merchán, A.V.; Pérez Nevado, F.; Aranda, E.; Serradilla, M.J.; Córdoba, M.D.G.; Martín, A. Anti-Fungal Activity of Phenolic Sweet Orange Peel Extract for Controlling Fungi Responsible for Post-Harvest Fruit Decay. Fungal Biol. 2021, 125, 143–152. [Google Scholar] [CrossRef]
- Llorens, E.; Camanes, G.; Lapena, L.; Garcia-Agustin, P. Priming by Hexanoic Acid Induce Activation of Mevalonic and Linolenic Pathways and Promotes the Emission of Plant Volatiles. Front. Plant Sci. 2016, 7, 495. [Google Scholar] [CrossRef]
- Abdel-Ghafar, R.Y.; Sehim, A.E.; Hamza, Z.K.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Evaluation of the Antimicrobial, Antioxidant, and Cytotoxicity Against MCF-7 Breast Cell Lines of Biosynthesized Vanadium Nanoparticles. BioNanoScience 2022, 12, 1097–1105. [Google Scholar] [CrossRef]
- Shende, S.S.; Gade, A.K.; Minkina, T.M.; Ingle, P.U.; Rajput, V.D.; Sushkova, S.N.; Mandzhieva, S.S.; Rai, M.; Wong, M.H. Exploring Sustainable Management by Using Green Nano-Silver to Combat Three Post-Harvest Pathogenic Fungi in Crops. Discov. Nano 2024, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Tatano, S.; Gomi, K.; Ohtani, K.; Fukumoto, T.; Akimitsu, K. Chloroplast-Localized Nonspecific Lipid Transfer Protein with Anti-Fungal Activity from Rough Lemon. Physiol. Mol. Plant Pathol. 2008, 72, 134–140. [Google Scholar] [CrossRef]
- Li, Y.; Fan, L.; Tang, X.-M.; Yang, D.-M.; Hu, J.-H.; Wu, Y.-Z.; Zhan, S.; Yang, D.-C. Synthesis and Antibacterial Activity of C-7 Haloacyl Cephalosporins. Yaoxue Xuebao 2021, 56, 1965–1975. [Google Scholar] [CrossRef]
- Arslan, D.; Tuccitto, N.; Auditore, A.; Licciardello, A.; Marletta, G.; Riolo, M.; La Spada, F.; Conti Taguali, S.; Calpe, J.; Meca, G.; et al. Chitosan-Based Films Grafted with Citrus Waste-Derived Antifungal Agents: An Innovative and Sustainable Approach to Enhance Post-Harvest Preservation of Citrus Fruit. Int. J. Biol. Macromol. 2024, 264, 130514. [Google Scholar] [CrossRef]
- Fungicide Classification According to Mode of Action 2024. In Fungicide Resistance Action Committee FRAC Code List 2024; Fungicide Resistance Action Committee: Lausanne, Switzerland, 2024.
- Huang ChiaoWen, H.C.; Wu ChaoJung, W.C.; Yang HongRen, Y.H.; Lai SuYu, L.S.; Ni HuiFang, N.H. Physiological Characteristics, Pathogenicity and Fungicide Screening of Citrus Alternaria Brown Spot Disease Caused by Alternaria alternata. J. Taiwan Agric. Res. 2018, 67, 387–400. [Google Scholar]
- Sharma, R.N.; Gaur, R.B. Management of Post-Harvest Core Rot, Alternaria alternata in Kinnow, Citrus Deliciosa Fruits. Indian J. Plant Prot. 2009, 37, 207–210. [Google Scholar]
- Colturato, A.B.; Paulossi, T.; Venâncio, W.S.; Furtado, E.L. Efficiency and Cost of Chemical Control of Alternaria Brown Spot; [Eficiência e Custo Do Controle Químico Da Mancha de Alternaria Em Tangor Murcote]. Summa Phytopathol. 2009, 35, 210–215. [Google Scholar] [CrossRef]
- Dinesh Singh, D.S.; Thakur, A.K.; Bhagwat, V.R. Effect of Fungicides and Calcium Nitrate on Fruit Drop of Kinnow. Bioved 2005, 16, 47–50. [Google Scholar]
- Garganese, F.; Sanzani, S.M.; Di Rella, D.; Schena, L.; Ippolito, A. Pre- and Postharvest Application of Alternative Means to Control Alternaria Brown Spot of Citrus. Crop Prot. 2019, 121, 73–79. [Google Scholar] [CrossRef]
- Peres, N.A.; Timmer, L.W. Evaluation of the Alter-Rater Model for Spray Timing for Control of Alternaria Brown Spot on Murcott Tangor in Brazil. Crop Prot. 2006, 25, 454–460. [Google Scholar] [CrossRef]
- van Zyl, J.G.; Fourie, P.H.; Schutte, G.C. Spray Deposition Assessment and Benchmarks for Control of Alternaria Brown Spot on Mandarin Leaves with Copper Oxychloride. Crop Prot. 2013, 46, 80–87. [Google Scholar] [CrossRef]
- Vicent, A.; Armengol, J.; García-Jiménez, J. Rain Fastness and Persistence of Fungicides for Control of Alternaria Brown Spot of Citrus. Plant Dis. 2007, 91, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Vicent, A.; Armengol, J.; García-Jiménez, J. Protectant Activity of Reduced Concentration Copper Sprays against Alternaria Brown Spot on “Fortune” Mandarin Fruit in Spain. Crop Prot. 2009, 28, 1–6. [Google Scholar] [CrossRef]
- Camiletti, B.X.; Lichtemberg, P.S.F.; Paredes, J.A.; Carraro, T.A.; Velascos, J.; Michailides, T.J. Characterization, Pathogenicity, and Fungicide Sensitivity of Alternaria Isolates Associated with Preharvest Fruit Drop in California Citrus. Fungal Biol. 2022, 126, 277–289. [Google Scholar] [CrossRef]
- Miles, A.K.; Willingham, S.L.; Cooke, A.W. Field Evaluation of a Plant Activator, Captan, Chlorothalonil, Copper Hydroxide, Iprodione, Mancozeb and Strobilurins for the Control of Citrus Brown Spot of Mandarin. Australas. Plant Pathol. 2005, 34, 63–71. [Google Scholar] [CrossRef]
- Mondal, S.N.; da Silva, A.G.; Dewdney, M.M. Resistance to Strobilurin Fungicides in a Population of Alternaria alternata Causing Alternaria Brown Spot of Citrus. Phytopathology 2009, 99, S88. [Google Scholar]
- Solel, Z.; Oren, Y.; Kimchi, M. Control of Alternaria Brown Spot of Minneola Tangelo with Fungicides. Crop Prot. 1997, 16, 659–664. [Google Scholar] [CrossRef]
- Oren, Y.; Solel, Z.; Kimki, M.; Sadovski, A. Controlling Alternaria alternata in the Citrus Varieties ‘Minneola’and ‘Nova’. Phytoparasitica 1999, 27, 152–153. [Google Scholar]
- Reis, R.F.; de Goes, A.; Mondal, S.N.; Shilts, T.; Brentu, F.C.; Timmer, L.W. Effect of Lesion Age, Humidity, and Fungicide Application on Sporulation of Alternaria alternata, the Cause of Brown Spot of Tangerine. Plant Dis. 2006, 90, 1051–1054. [Google Scholar] [CrossRef]
- Nouhra, G.; Poloni, N.M.; Pereira, F.D.; de Goes, A. Efficiency of Trifloxystrobin and Tebuconazole, in a Commercial Formulation, Associated with Protective Fungicides to Control Alternaria Brown Spot on “Murcott” Tangors. Summa Phytopathol. 2021, 47, 122–125. [Google Scholar] [CrossRef]
- Mondal, S.; Bhatia, A.; Shilts, T.; Timmer, L. Baseline Sensitivities of Fungal Pathogens of Fruit and Foliage of Citrus to Azoxystrobin, Pyraclostrobin, and Fenbuconazole. Plant Dis. 2005, 89, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Jamiołkowska, A. Laboratory Effect of Azoxystrobin (Amistar 250 SC) and Grapefruit Extract (Biosept 33 SL) on Growth of Fungi Colonizing Zucchini Plants; [Laboratoryjna Ocena Wpływu Azoksystrobiny i Ekstraktu z Grejpfruta Na Wzrost Grzybów Wystepujacych Na Cukinii]. Acta Sci. Pol. Hortorum Cultus 2011, 10, 245–257. [Google Scholar]
- Chitolina, G.M.; Silva-Junior, G.J.; Feichtenberger, E.; Pereira, R.G.; Amorim, L. Distribution of Alternaria alternata Isolates with Resistance to Quinone Outside Inhibitor (QoI) Fungicides in Brazilian Orchards of Tangerines and Their Hybrids. Crop Prot. 2021, 141, 105493. [Google Scholar] [CrossRef]
- Chitolina, G.M.; Silva-Junior, G.J.; Feichtenberger, E.; Pereira, R.G.; Amorim, L. First Report on Quinone Outside Inhibitor Resistance of Alternaria alternata Causing Alternaria Brown Spot in Tangerines in São Paulo, Brazil. Plant Health Prog. 2019, 20, 94. [Google Scholar] [CrossRef]
- Nicoletta, K.R.; Vega, B.; Dewdney, M.M. Distribution of Azoxystrobin Resistance in Nonpathogenic Alternaria alternata Isolates of Citrus. Phytopathology 2015, 105, 101. [Google Scholar]
- Vega, B.; Dewdney, M.M. Fla State Hort Soc Geographical Distribution of Strobilurin Resistance of Alternaria alternata, Causal Agent of Alternaria Brown Spot in Florida Citrus Groves. Proc. Fla. State Hortic. Soc. 2012, 125, 33–35. [Google Scholar]
- Vega, B.; Dewdney, M.M. Distribution of Qol Resistance in Populations of Tangerine-Infecting Alternaria alternata in Florida. Plant Dis. 2014, 98, 67–76. [Google Scholar] [CrossRef]
- Yogev, E.; Sadowsky, A.; Solel, Z.; Oren, Y.; Orbach, Y. The Performance of Potassium Phosphite for Controlling Alternaria Brown Spot of Citrus Fruit. J. Plant Dis. Prot. 2006, 113, 207–213. [Google Scholar] [CrossRef]
- Mondal, S.N.; Vicent, A.; Reis, R.F.; Timmer, L.W. Efficacy of Pre- and Postinoculation Application of Fungicides to Expanding Young Citrus Leaves for Control of Melanose, Scab, and Alternarial Brown Spot. Plant Dis. 2007, 91, 1600–1606. [Google Scholar] [CrossRef]
- He, M.; Fu, Y.; Ruan, R.; Li, H. Sensitivity Assay of Alternaria alternata from Citrus in China to Four New Fungicides. J. Zhejiang Univ. (Agric. Life Sci.) 2016, 42, 535–542. [Google Scholar]
- Highland, B.H.; Timmer, L.W. The Use of Serenade Biofungicide to Control Foliar Fungal Diseases of Florida Citrus. Proc. Fla. State Hortic. Soc. 2004, 117, 127–130. [Google Scholar]
- Erkiliç, A.; Canihoş, Y.; Biçici, M.; Kurt, Ş. Iprodione Resistance of Alternaria alternata f.sp. Citri Minneola Tangelo Isolates in Turkey. Turk. J. Agric. For. 1999, 23, 1051–1056. [Google Scholar]
- Solel, Z.; Timmer, L.W.; Kimchi, M. Iprodione Resistance of Alternaria alternata Pv. Citri from Minneola Tangelo in Israel and Florida. Plant Dis. 1996, 80, 291–293. [Google Scholar] [CrossRef]
- Byron, V. Sensitivity of Alternaria alternata from Citrus to Boscalid and Polymorphism in Iron-Sulfur and in Anchored Membrane Subunits of Succinate Dehydrogenase. Plant Dis. 2015, 99, 231–239. [Google Scholar]
- Nishimura, S.; Tatano, S.; Miyamoto, Y.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Tada, Y.; Ichimura, K.; Akimitsu, K. A Zinc-Binding Citrus Protein Metallothionein Can Act as a Plant Defense Factor by Controlling Host-Selective ACR-Toxin Production. Plant Mol. Biol. 2013, 81, 1–11. [Google Scholar] [CrossRef]
- Asanzi, N.M.; Taylor, N.J.; Vahrmeijer, J.T. Can Silicon Be Used to Prevent Alternaria alternata in Citrus Trees? SA Fruit J. 2014, 13, 48–51. [Google Scholar]
- Mvondo-She, M.A.; Gatabazi, A.; Laing, M.D.; Ndhlala, A.R. A Review on the Role of Silicon Treatment in Biotic Stress Mitigation and Citrus Production. Agron. Basel 2021, 11, 2198. [Google Scholar] [CrossRef]
- Alayon Luaces, P.; Chabbal, M.D.; Piccoli, A.B.; Yfran Elvira, M.M.; Gaiad, J.E.; Gimenez, L.I. Combination of Treatments with Fungicides and Calcium Nitrate for the Control of Brown Spot (Alternaria alternata) and Its Effect on the Production of “Murcott” Tangor. RIA Rev. Investig. Agropecu. 2022, 48, 10–15. [Google Scholar]
- Vicent, A.; Badal, J.; Asensi, M.; Sanz, N.; Armengol, J.; García-Jiménez, J. Laboratory Evaluation of Citrus Cultivars Susceptibility and Influence of Fruit Size on Fortune Mandarin to Infection by Alternaria alternata Pv. Citri. Eur. J. Plant Pathol. 2004, 110, 245–251. [Google Scholar] [CrossRef]
- Llorens, E.; Fernández-Crespo, E.; Vicedo, B.; Lapeña, L.; García-Agustín, P. Enhancement of the Citrus Immune System Provides Effective Resistance against Alternaria Brown Spot Disease. J. Plant Physiol. 2013, 170, 146–154. [Google Scholar] [CrossRef]
- Timmer, L.W.; Darhower, H.M.; Bhatia, A. The Alter-Rater, a New Weather-Based Model for Timing Fungicide Sprays for Alternaria Control; University of Florida Cooperative Extension Service: Gainesville, FL, USA, 2001. [Google Scholar]
- Doria, M.S.; Guedes, M.S.; Silva, E.M.D.; de Oliveira, T.M.; Pirovani, C.P.; Kupper, K.C.; Bastianel, M.; Micheli, F. Comparative Proteomics of Two Citrus Varieties in Response to Infection by the Fungus Alternaria alternata. Int. J. Biol. Macromol. 2019, 136, 410–423. [Google Scholar] [CrossRef]
- Ohtani, K.; Yamamoto, H.; Akimitsu, K. Sensitivity to Alternaria alternata Toxin in Citrus Because of Altered Mitochondrial RNA Processing. Proc. Natl. Acad. Sci. USA 2002, 99, 2439–2444. [Google Scholar] [CrossRef]
| Database | Specific Search String | Published | Doc Type | Lang | n |
|---|---|---|---|---|---|
| WoS | (TI = (Alternaria alternata AND citrus)) OR AB = (Alternaria alternata AND citrus) OR AK = (Alternaria alternata AND citrus) OR KP = (Alternaria alternata AND citrus) | all years | all | Auto | 343 |
| Scopus | TITLE-ABS-KEY (Alternaria AND alternata AND citrus) | all years | all | all | 276 |
| Google Scholar | allintitle: Alternaria alternata citrus | all years | all | all | 144 |
| PubMed | (Alternaria alternata [Title/Abstract]) AND citrus [Title/Abstract] | all years | all | all | 109 |
| SciELO | All indexes: (Alternaria alternata) AND (citrus) | all years | all | all | 19 |
| Group | FRAC Code | Substance | Resist | Lab Effectiveness | Field Effectiveness | Refs | References |
|---|---|---|---|---|---|---|---|
| Copper (inorganic) | MSCA | Copper oxychloride | No | low to medium | low to high | 9 | [72,73,74,75,76,77,78,79,80] |
| Copper hydroxide | No | low to medium | medium | 5 | [72,79,81,82,83] | ||
| Bordeaux mixture | No | ineffective | not tested | 3 | [72,79,80] | ||
| Cuprous oxide | No | high | not tested | 3 | [72,79,80] | ||
| Tribasic copper sulfate | No | low | not tested | 1 | [72] | ||
| Dithiocarbamates | MSCA | Mancozeb | No | high | medium to high | 8 | [72,73,74,75,77,79,82,84] |
| Propineb | No | high | medium to high | 3 | [73,74,85] | ||
| Maneb | No | not tested | ineffective | 1 | [84] | ||
| Ferbam | No | ineffective | not tested | 1 | [86] | ||
| Metiram | No | not tested | medium | 1 | [84] | ||
| Chloronitriles | MSCA | Chlorothalonil | No | not tested | ineffective | 2 | [82,84] |
| Phthalimides | MSCA | Captan | No | not tested | contradictory | 2 | [82,84] |
| DeMethylation Inhibitors | DMIs | Prochloraz | high | low | 2 | [73,84] | |
| Tebuconazole | medium to high | low | 3 | [81,84,87] | |||
| Difenoconazole | not tested | low | 2 | [74,79] | |||
| Pyrifenox | low to high | not tested | 1 | [85] | |||
| Quinone outside Inhibitors | QoIs | Azoxystrobin | Yes | contradictory | medium | 12 | [72,74,81,82,85,88,89,90,91,92,93,94] |
| Pyraclostrobin | Yes | high | high | 12 | [18,74,76,82,83,88,90,91,93,94,95,96] | ||
| Trifloxystrobin | Yes | low | contradictory | 3 | [74,81,82] | ||
| Methoxycrylate | Yes | not tested | medium | 1 | [82] | ||
| Famoxadone | Yes | not tested | not tested | 1 | [96] | ||
| Benzimidazole | MBC | Carbendazim | Yes | low | not tested | 2 | [72,75] |
| Thiophanate methyl | Yes | low | not tested | 2 | [72,73] | ||
| Diarylamine | Fluazinam | No | high | contradictory | 4 | [72,84,97,98] | |
| Dicarboximides | Iprodione | Yes | high | high | 6 | [74,82,84,95,99,100] | |
| Procymidone | Yes | not tested | low | 1 | [84] | ||
| Succinate-dehydrogenase inhibitors | SDHIs | Fluopyram | Yes | high | not tested | 1 | [81] |
| Flutolanil | Yes | high | not tested | 1 | [97] | ||
| Thifluzamide | Yes | high | not tested | 1 | [97] | ||
| Boscalid | Yes | medium to low | not tested | 3 | [95,97,101] | ||
| Others | Natamycin | No | high | not tested | 1 | [74] | |
| Metallothionein | No | high | not tested | 1 | [102] | ||
| Silicon | No | medium to low | not tested | 2 | [103,104] | ||
| Calcium nitrate | No | not tested | medium to low | 2 | [75,105] | ||
| Chitosan | No | not tested | medium to low | 1 | [76] | ||
| Salicyl-hydroxamic acid | No | medium to low | not tested | 1 | [83] | ||
| Acibenzolar | No | medium to low | not tested | 2 | [18,82] | ||
| Potassium Phosphite | No | ineffective | medium to high | 1 | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garmendia, A.; Ferriol, M.; Beltrán, R.; García-Breijo, F.; Raigón, M.D.; Parra, M.D.C.; Merle, H. Plant Protection Products to Control Alternaria Brown Spot Caused by Alternaria alternata in Citrus: A Systematic Review. Agronomy 2025, 15, 1343. https://doi.org/10.3390/agronomy15061343
Garmendia A, Ferriol M, Beltrán R, García-Breijo F, Raigón MD, Parra MDC, Merle H. Plant Protection Products to Control Alternaria Brown Spot Caused by Alternaria alternata in Citrus: A Systematic Review. Agronomy. 2025; 15(6):1343. https://doi.org/10.3390/agronomy15061343
Chicago/Turabian StyleGarmendia, Alfonso, María Ferriol, Roberto Beltrán, Francisco García-Breijo, María Dolores Raigón, María Del Carmen Parra, and Hugo Merle. 2025. "Plant Protection Products to Control Alternaria Brown Spot Caused by Alternaria alternata in Citrus: A Systematic Review" Agronomy 15, no. 6: 1343. https://doi.org/10.3390/agronomy15061343
APA StyleGarmendia, A., Ferriol, M., Beltrán, R., García-Breijo, F., Raigón, M. D., Parra, M. D. C., & Merle, H. (2025). Plant Protection Products to Control Alternaria Brown Spot Caused by Alternaria alternata in Citrus: A Systematic Review. Agronomy, 15(6), 1343. https://doi.org/10.3390/agronomy15061343









