Use of Trans-Anethole Against Hop Flea Beetles in Field Conditions
Abstract
1. Introduction
2. Materials and Methods
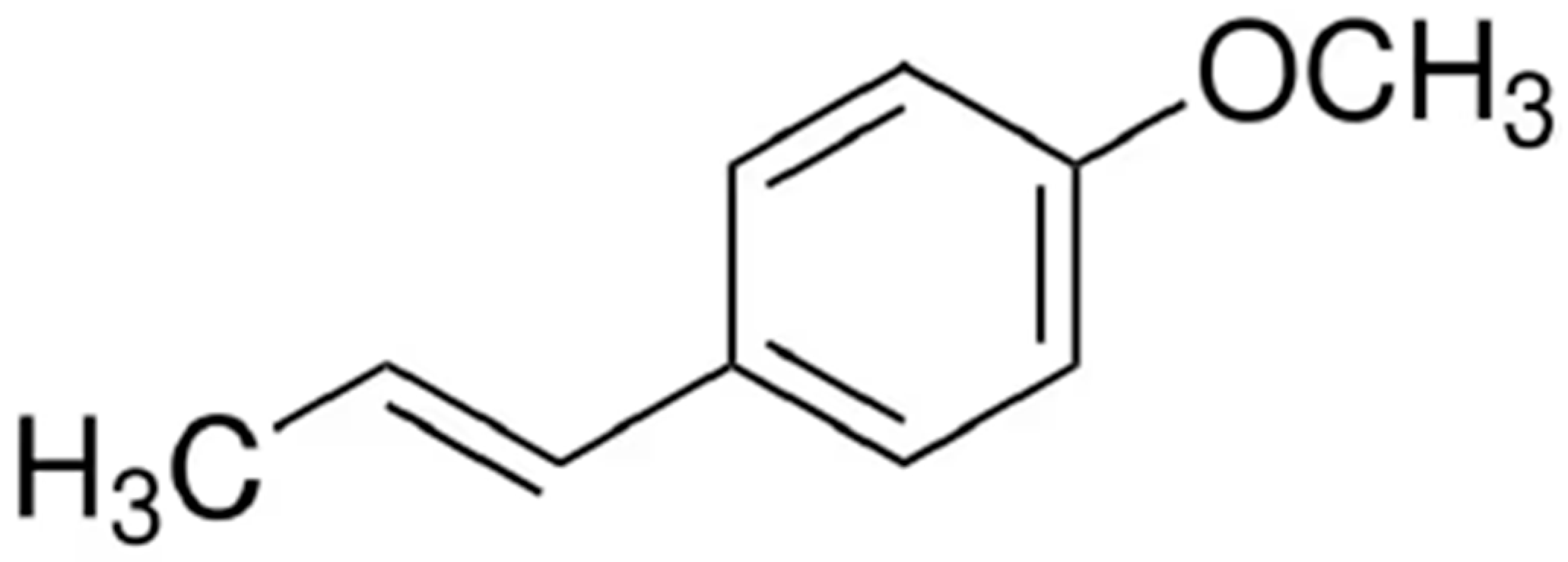
2.1. Preparation of β-CD/AT Inclusion Complex (NCH1) and AT/β-CD Physical Mixture (NCH2)
2.2. Experimental Sites
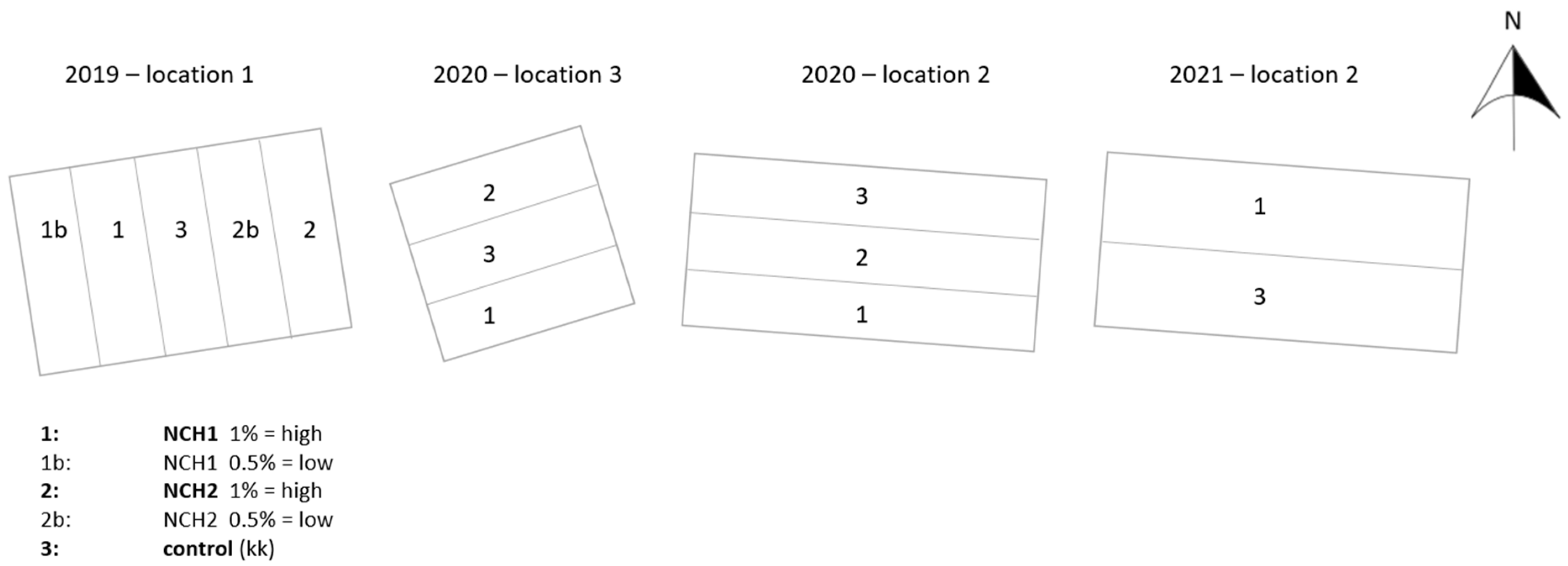
2.3. Experimental Design
2.4. Data Collection
2.5. Data Analysis
2.6. Trans-Anethole (AT) Residues Analysis
2.6.1. Sample Preparation and Optimisation of HS-SPME Parameters
2.6.2. GC/MS Analysis
2.6.3. Preparation of the Trans-Anethole Calibration Line on Hop Leaves
2.7. Monitoring of the Hop Flea Beetle in Hop Gardens in Summer
3. Results
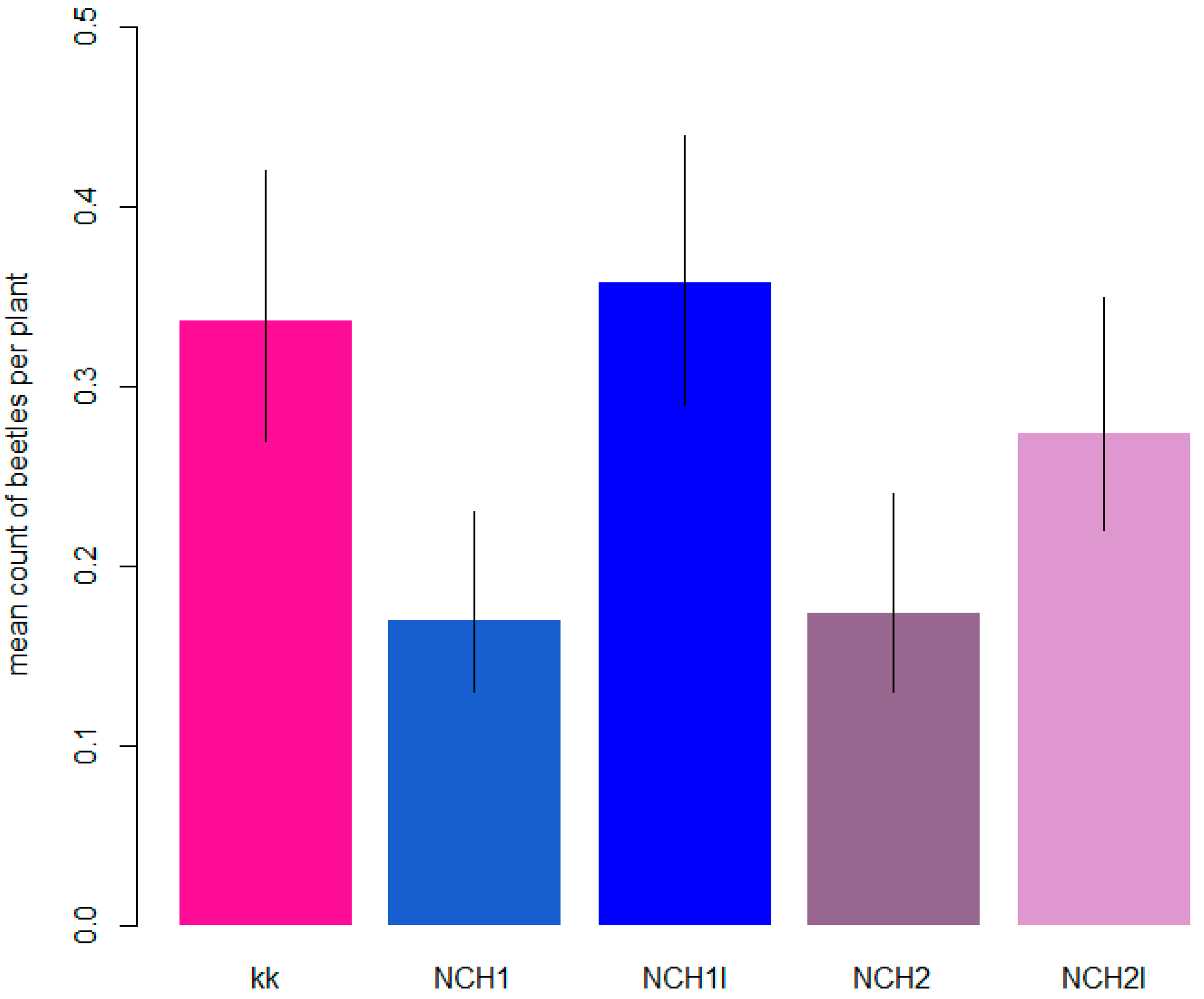
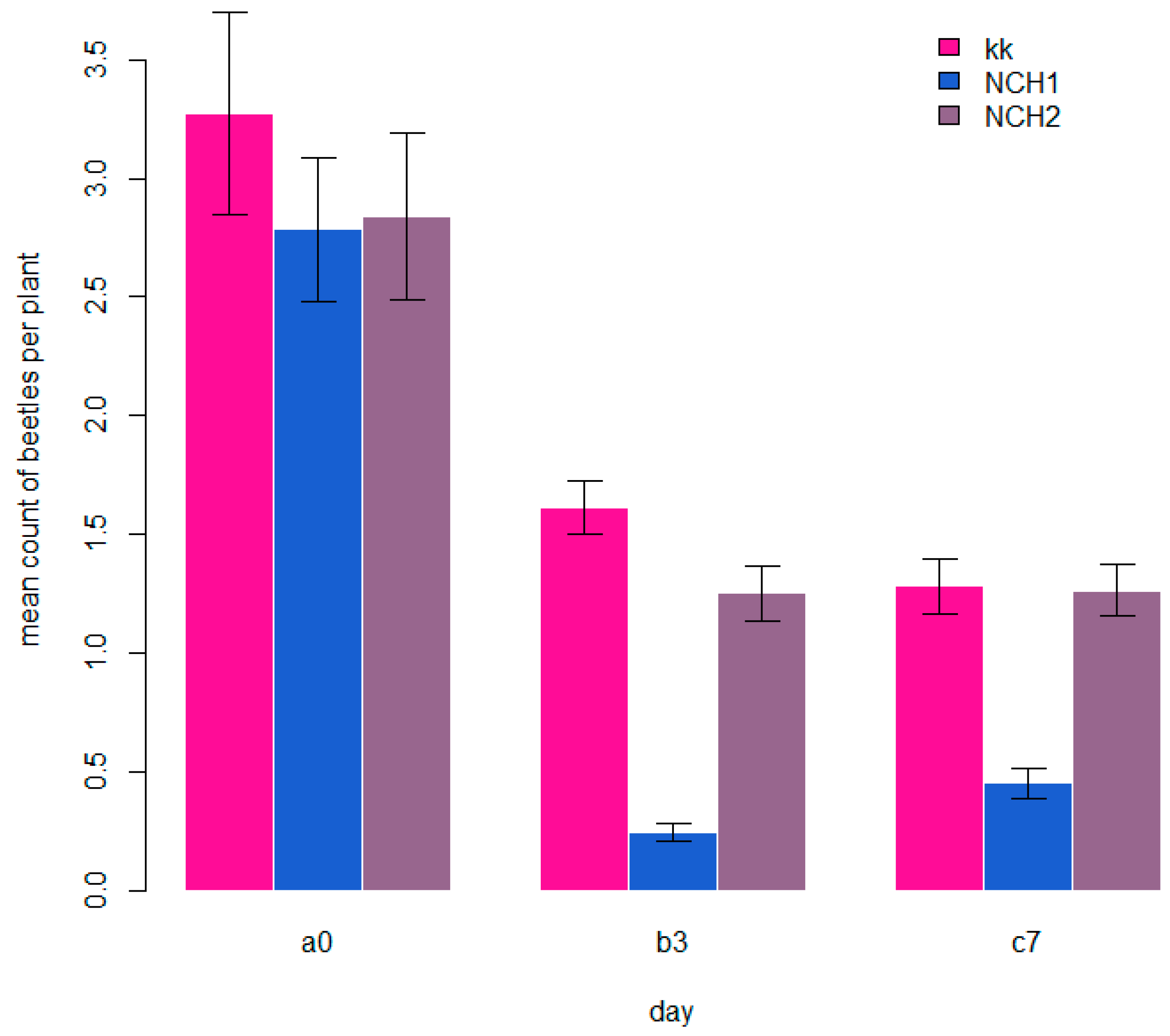
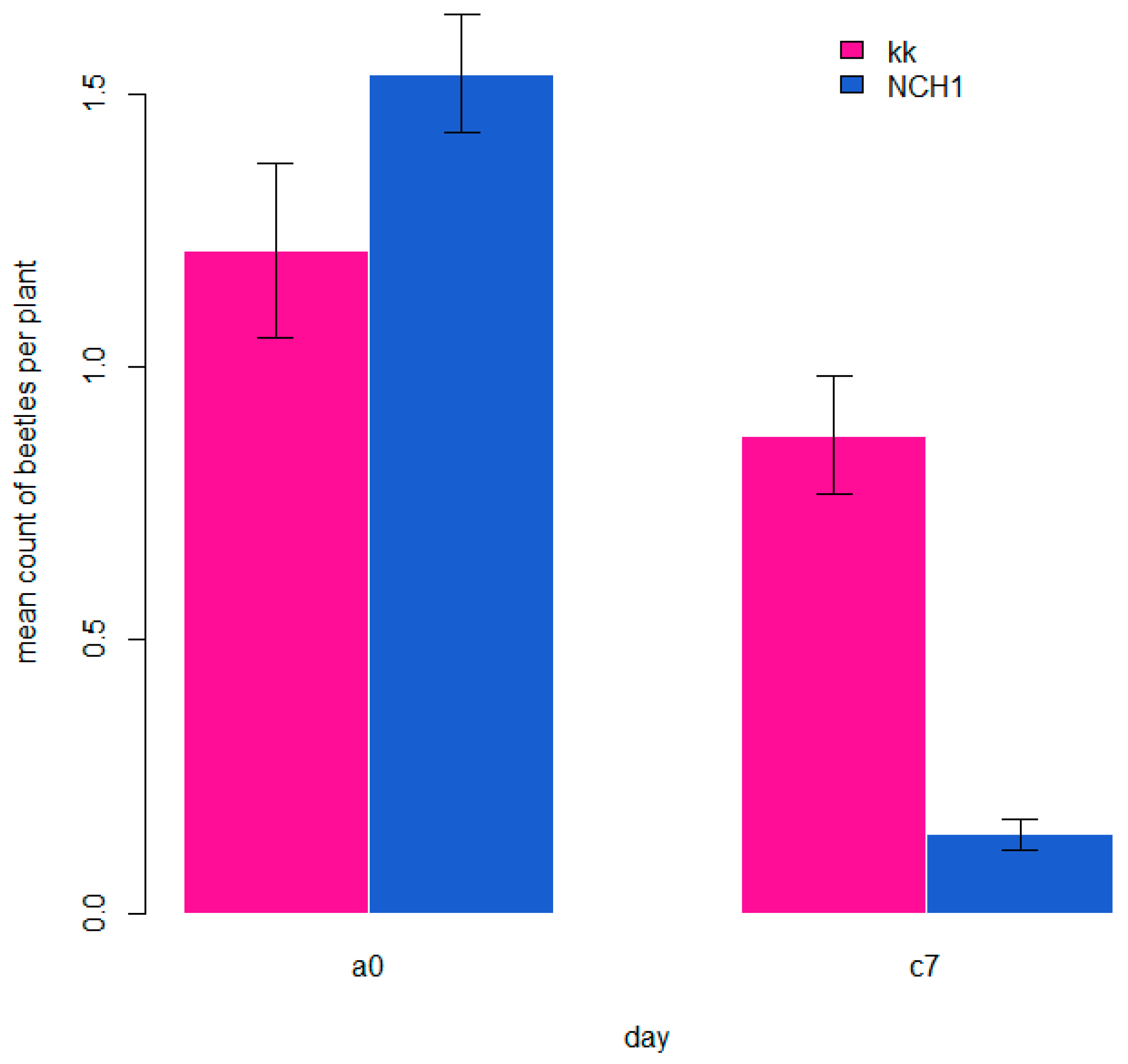
3.1. Number of Hop Flea Beetles
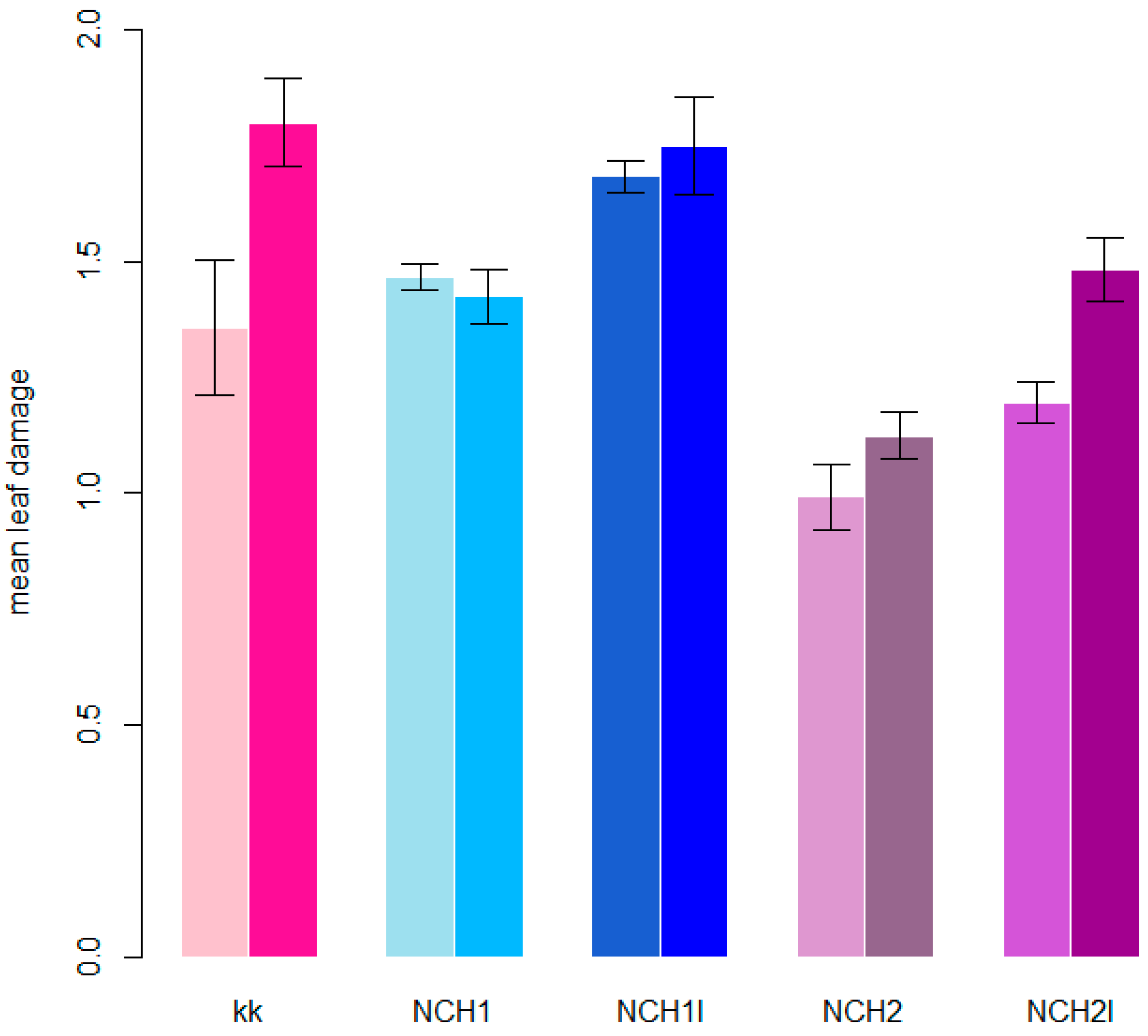
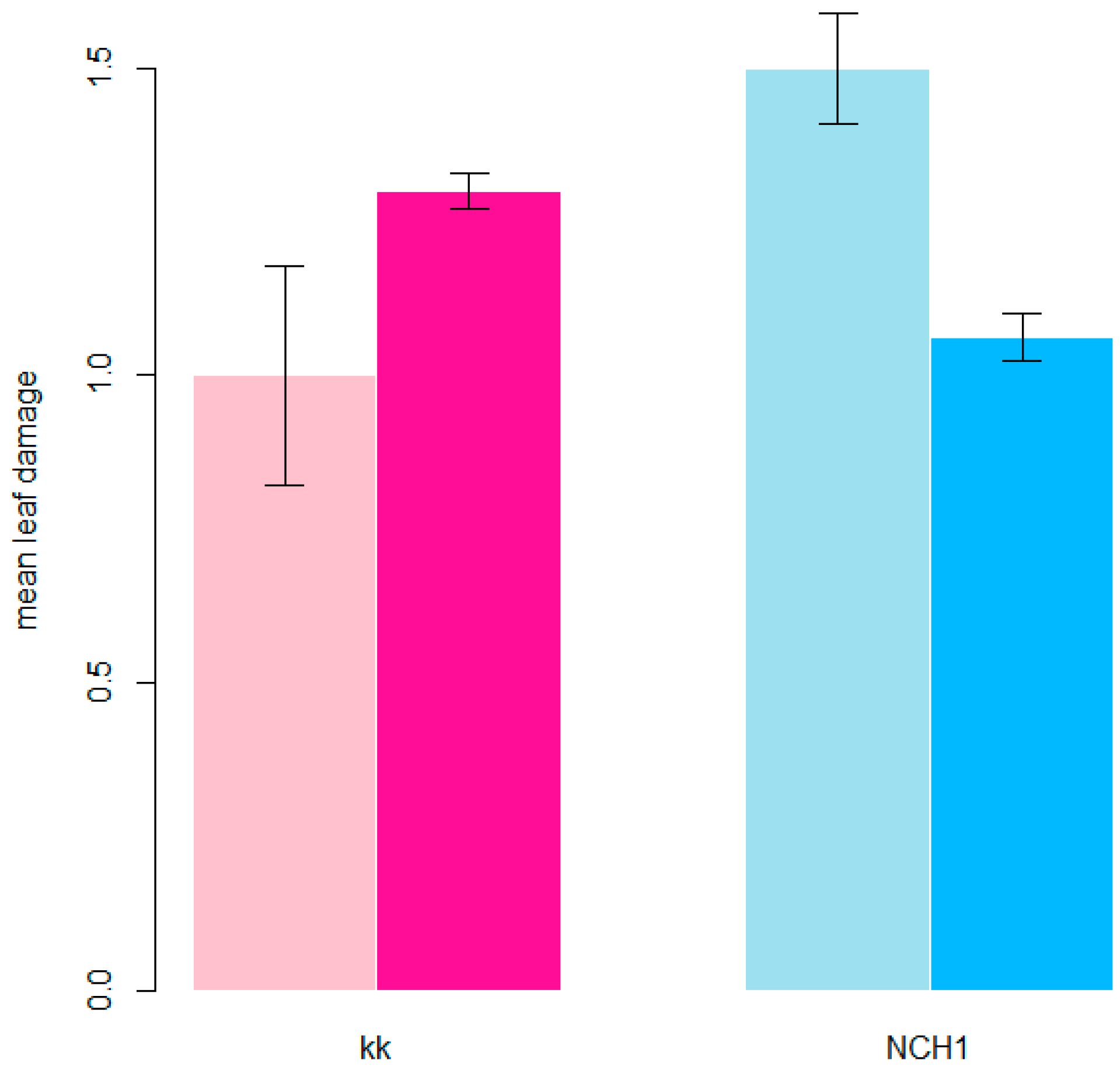
3.2. Degree of Damage of Hop Leaves
3.3. Detection of Trans-Anethole on Hop Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beranová, M.; Kubačák, F. Dějiny zemědělství v Čechách a na Moravě; Libri: Praha, Česká Republika, 2010. [Google Scholar]
- Neve, R.A. Hops. Springer: Bury St. Edmunds, UK, 1991. [Google Scholar] [CrossRef]
- Marotti, M.; Piccaglia, R.; Giovanelli, E.; Deans, S.G.; Eaglesham, E. Effects of variety and ontogenic stage on the essential oil composition and biological activity of fennel (Foeniculum vulgare Mill.). J. Essent. Oil Res. 1994, 6, 57–62. [Google Scholar] [CrossRef]
- Stefanini, M.B.; Ming, L.C.; Marques, M.O.M.; Facanali, R.; Meireles, M.A.A.; Moura, L.S.; Marchese, J.A.; Sousa, L.A. Essential oil constituents of different organs of fennel (Foeniculum vulgare var. vulgare). Rev. Bras. Pl. Med. 2006, 8, 193–198. [Google Scholar]
- Gulfraz, M.; Mehmood, S.; Minhas, N.; Jabeen, N.; Kausar, R.; Jabeen, K.; Arshad, G. Composition and antimicrobial properties of essential oil of Foeniculum vulgare. Afr. J. Biotechnol. 2008, 7, 4364–4368. [Google Scholar]
- Chowdhury, J.U.; Mobarok, M.H.; Bhuiyan, M.N.I.; Nandi, N.C. Constituents of essential oils from leaves and seeds of Foeniculum vulgare Mill. cultivated in Bangladesh. Bangladesh J. Bot. 2009, 38, 181–183. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal and repellent activity of selected essential oils against of the pollen beetle, Meligethes aeneus (Fabricius) adults. Ind. Crops Prod. 2011, 34, 888–982. [Google Scholar] [CrossRef]
- Orav, A.; Raal, A.; Arak, E. Essential oil composition of Pimpinella anisum L. fruits from various European countries. Nat. Prod. Res. 2008, 22, 227–232. [Google Scholar] [CrossRef]
- Morris, J.A.; Khettry, A.; Seitz, E.W. Antimicrobial activity of aroma chemicals and essential oils. J. Am. Oil Chem. Soc. 1979, 56, 595–603. [Google Scholar] [CrossRef]
- Curtis, O.F.; Shetty, K.; Cassagnol, G.; Peleg, M. Comparison of the inhibitory and lethal effects of synthetic versions of plant metabolites (anethole, carvacrol, eugenol, and thymol) on a food spoilage yeast (Debaromyces hansenii). Food Biotechnol. 1996, 10, 55–73. [Google Scholar] [CrossRef]
- Alkan, M.; Ertürk, S. Insecticidal Efficacy and Repellency of Trans-Anethole Against Four Stored-Product Insect Pests. J. Agric. Sci. 2020, 26, 64–70. [Google Scholar] [CrossRef]
- Bertoli, A.; Conti, B.; Mazzoni, V.; Meini, L.; Pistelli, L. Volatile chemical composition and bioactivity of six essential oils against the stored food insect Sitophilus zeamais Motsch. (Coleoptera Dryophthoridae). Nat. Prod. Res. 2012, 26, 2063–2071. [Google Scholar] [CrossRef]
- Newberne, P.; Smith, R.L.; Doull, J.; Goodman, J.I.; Munro, I.C.; Portoghese, P.S.; Wagner, B.M.; Weil, C.S.; Woods, L.A.; Adams, T.B.; et al. The FEMA GRAS assessment of trans-anethole used as a flavouring substance. Food Chem. Toxicol. 1999, 37, 789–811. [Google Scholar] [CrossRef] [PubMed]
- Döberl, M. Alticinae. In Catalogue of Palearctic Coleoptera; Löbl, C.I., Smetana, A., Eds.; Appolo Books: Stenstrup, Denmark, 2010; Volume 6, 924p. [Google Scholar]
- Douglas, H.B.; Renkema, J.; Smith, T.W.; Konstantinov, A.S.; Serres, J.M.D. Palearctic flea beetle and pest of hops and Cannabis, Psylliodes attenuata (Coleoptera, Chrysomelidae, Galerucinae), new to North America. Biodivers. Data J. 2024, 12, e120340. [Google Scholar] [CrossRef]
- Blattný, C. Nomenklatura chorob a škůdců chmele. Ochr. Rostl. 1926, 6, 134–138. [Google Scholar]
- Weihrauch, F.; Baumgartner, A.; Eisenbraun, D.; Wolf, S.; Mumm, R.; van Tol, R.W.H.M. Flea-beetle control in organic hops: Are there options? In Proceedings of the Scientific-Technical Commission, International Hop Growers’ Convention I.H.G.C., St. Stefan am Walde, Austria, 25–29 July 2017; Weihrauch, F., Ed.; Hop Research Center: Wolnzach, Germany, 2017; p. 59. [Google Scholar]
- Rak Cizej, M.; Žolnir, M. Hop flea beetle (Psylliodes attenuatus Koch) one of the most common hop pests in Slovenia. In Proceedings of the Lectures and Papers of the Sixth Slovenian Plant Protection Congress, Zreče, Slovenia, 4–6 March 2003; pp. 233–238. [Google Scholar]
- Campbell, C.A.M. New and resurgent insect pests on low trellis hops. Agric. For. Entomol. 2019, 21, 209–218. [Google Scholar] [CrossRef]
- Vostřel, J.; Klapal, I.; Kudrna, T. Metodika Ochrany Chmele Proti Dřepčíku Chmelovému (Psylliodes attenuatus Koch); Chmelařský Institut: Žatec, Slovenia, 2010. [Google Scholar]
- Schweiger, O.; Maelfait, J.P.; van Wingerden, W.; Hendrickx, F.; Billeter, R.; Speelmans, M.; Augenstein, I.; Aukema, B.; Aviron, S.; Bailey, D.; et al. Quantifying the impact of environmental factors on arthropod communities in agricultural landscapes across organizational levels and spatial scales. J. Appl. Ecol. 2005, 42, 1129–1139. [Google Scholar] [CrossRef]
- Holý, K.; Procházka, P.; Štranc, J.; Štranc, D.; Štranc, P. Integrovaná Ochrana Chmele. Certifikovaná Metodika, 1st ed.; Výzkumný Ústav Rostlinné Výroby, v.v.i.: Praha, Česká Republika, 2017; p. 98. [Google Scholar]
- Dmitriev, G.V. Results obtained in studying Psylliodes attenuata Koch, under conditions prevailing in the right bank area of the Kuybyshev district. Plant Prot. 1935, 5, 91–106. [Google Scholar]
- Minář, P. Nařízení Ústředního Kontrolního a Zkušebního Ústavu Zemědělského o Povolení Přípravku na Ochranu Rostlin Pro omezené a Kontrolované Použití. ÚKZÚZ. Available online: https://www.syngenta.cz/sites/g/files/kgtney1451/files/media/document/2023/04/04/actara_narizeni-ukzuz-pro-omezene-a-kontrolovane-pouziti-pripravku-actara-25-wg.pdf (accessed on 23 March 2021).
- Weihrauch, F. Developing methods pf controlling the hop flea beetle, Psylliodes attenuatus, in organic hop farming: Completion of the project. In Annual Report 2018, Special Crop: Hops; Institute for Crop Science and Plant Breeding, Ed.; Bayerische Landesanstalt für Landwirtschaft: Freising-Weihenstephan, Germany, 2019; pp. 107–111. [Google Scholar]
- Weihrauch, F.; Baumgartner, A.; Laupheimer, S.; Mühlbauer, M. Hop-flea beetle revisited: In search for attractants. In Proceedings of the Scientific-Technical Commission, International Hop Growers’ Convention I.H.G.C., Bischoffsheim, Alsace, France, 7–11 July 2019; Weihrauch, F., Ed.; Hop Research Center: Wolnzach, Germany, 2019; p. 70. [Google Scholar]
- Mumm, R.; van Tol, R.W.H.M.; Weihrauch, F. Elucidation of the role of volatile compounds in the chemical communication of the hop flea beetle Psylliodes attenuatus. In Proceedings of the Scientific-Technical Commission, International Hop Growers’ Convention I.H.G.C., St. Stefan am Walde, Austria, 25–29 July 2017; Weihrauch, F., Ed.; Hop research Center: Wolnzach, Germany, 2017; pp. 60–64. [Google Scholar]
- Kumar, S. Biopesticides: A Need for Food and Environmental Safety. J. Biofertil. Biopestici. 2012, 3, e107. [Google Scholar] [CrossRef]
- Dively, G.P.; Patton, T.; Barranco, L.; Kulhanek, K. Comparative Efficacy of Common Active Ingredients in Organic Insecticides Against Difficult to Control Insect Pests. Insects 2020, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Bhosale, J.D.; Kumar, N.; Mandal, T.K.; Bendre, R.S.; Lavekar, G.S.; Dabur, R. Natural products—Antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.H. Toxicity and antifeedant activities of cinnamaldehyde against the grain storage insects, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J. Stored Prod. Res. 1998, 34, 11–17. [Google Scholar] [CrossRef]
- Sighamony, S.; Anees, I.; Chandrakala, T.S.; Osmani, Z. Natural products as repellents for Tribolium castaneum Herbst. Int. Pest Control 1984, 26, 156–157. [Google Scholar]
- Ho, S.H.; Koh, L.; Ma, Y.; Huang, Y.; Sim, K.Y. The oil of garlic, Allium sativum L. (Amaryllidaceae), as a potential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol. Technol. 1996, 9, 41–48. [Google Scholar] [CrossRef]
- Ho, S.H.; Ma, Y.; Huang, Y. Anethole, a potential insecticide from Illicium verum Hook F., against two stored product insects. Int. Pest Control 1997, 39, 50–51. [Google Scholar]
- Gonzalez, M.S.; Lima, B.G.; Oliveira, A.F.R.; Nunesa, D.D.; Fernandes, C.P.; Santos, M.G.; Tietbohl, L.A.C.; Mello, C.B.; Rocha, L.; Feder, D. Effects of essential oil from leaves of Eugenia sulcata on the development of agricultural pest insects. Rev. Bras. Farmacogn. 2014, 24, 413–418. [Google Scholar] [CrossRef]
- Shaaya, E.; Ravid, U.; Paster, N.; Juven, B.; Zisman, U.; Pissarev, V. Fumigant toxicity of essential oils against four major storedproduct insects. J. Chem. Ecol. 1991, 17, 499–504. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Hamraoui, A.; Holeman, M.; Theron, E.; Pinel, R. Insecticidal efect of essential oils from mediterranean plants upon Acanthoscelides obtecus Say (Coleoptera: Bruchidae), a pest of kidney bean (Phaseolus vulgaris L.). J. Chem. Ecol. 1993, 19, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Hedges, A.R. Industrial applications of cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yu, T.; Yuan, L.; Rao, G.; Li, D.; Mu, C. Preparation, physicochemical characterization and release behavior of the inclusion complex of trans-anethole and β-cyclodextrin. Food Res. Int. 2015, 74, 55–62. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Krofta, K.; Ježek, J.; Klapal, I.; Křivánek, J.; Pokorný, J.; Pulkrábek, J.; Vostřel, J. Integrovaný Systém Pěstování Chmele; Metodika pro praxi 02/2012; Chmel. Inst.: Žatec, Česká Republika, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 7 April 2025).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Package Version 1.10.6. 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 7 April 2025).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models; R Package Version 0.4.7. 2024. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 7 April 2025).
- Morales, M.; R Development Core Team; Murdoch, D. Sciplot: Scientific Graphing Functions for Factorial Designs; R Package Version 1.2-0. 2020. Available online: https://CRAN.R-project.org/package=sciplot (accessed on 23 May 2025).
- Richter, M.T.; Eyres, G.T.; Silcock, P.; Bremer, P.J. Comparison of four extraction methods for analysis of volatile hop-derived aroma compounds in beer. J. Sep. Sci. 2017, 40, 4366–4376. [Google Scholar] [CrossRef] [PubMed]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Optimisation of a complete method for the analysis of volatiles involved in the flavour stability of beer by solid-phase microextraction in combination with gas chromatography and mass spectrometry. J. Chrom. A. 2008, 1190, 342–349. [Google Scholar] [CrossRef]
- Goncalves, J.; Figueira, J.; Rodrigues, F.; Camara, J.S. Headspace solid-phase microextraction combined with mass spectrometry as a powerfull analytical tool for profiling the terpenoid metabolomic pattern of hop-essential oil derived from Saaz variety. J. Sep. Sci. 2012, 35, 2282–2296. [Google Scholar] [CrossRef]
- Piepho, H.P. An algorithm for a letter-based representation of all pairwise comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]
- Boháč, J. Biologie Ochrany Přírody Pro Agroekology; JČU: České Budějovice, Czech Republic, 2013; p. 110. [Google Scholar]
- Klas, M.; Klas, M.; Klas, Š. Příspěvek k částečné mechanizaci zavádění chmele. Chmelařství 2019, 92, 46–51. [Google Scholar]
- Rak Cizej, M.; Milevoj, L. Nutrition Habits of Hop Flea Beetles (Psylliodes attenuatus Koch). In Proceedings of the Zbornik Predavanj in Referatov 8. Slovenskega Posvetovanja o Varstvu Rastlin, Radenci, Slovenija, 6–7 March 2007; pp. 173–178. Available online: https://api.semanticscholar.org/CorpusID:85867624 (accessed on 7 April 2025).
- Honsová, H. Technické konopí u nás opomíjené. Úroda 2022, 7, 50–52. [Google Scholar]
- Chang, C.L.; Cho, I.K.; Li, Q.X. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactocera dorsalis, and Bactocera cucurbitae. J. Econ. Entomol. 2009, 102, 203–209. [Google Scholar] [CrossRef]
- Rak Cizej, M.; Poličnik, F. Control of hop flea beetle (Psylliodes attenuatus KOCH) on hops (Humulus lupulus L.) using alternative preparations. Hop Bull. 2022, 29, 97–108. [Google Scholar]
- Suchánková, M.; Šefrová, H. Škodlivost dřepčíka chmelového v různě starých chmelnicích. Rostlinolékař 2023, 5, 14–16. [Google Scholar]
- Rak Cizej, M.; Milevoj, L. Hop flea beetle (Psylliodes attenuatus KOCH) in Slovenia. In Proceedings of the Scientific Commission, International Hop Growers’ Convention, Tettnang, Germany, 24–28 June 2007; Seigner, E., Ed.; Hop Research Center: Wolnzach, Germany, 2017; pp. 91–94. [Google Scholar]
- Marrone, P.G. Pesticidal natural products—Status and future potencial. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxicity of essential oils on selected weeds: Potential hazard on food crops. Plants 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]

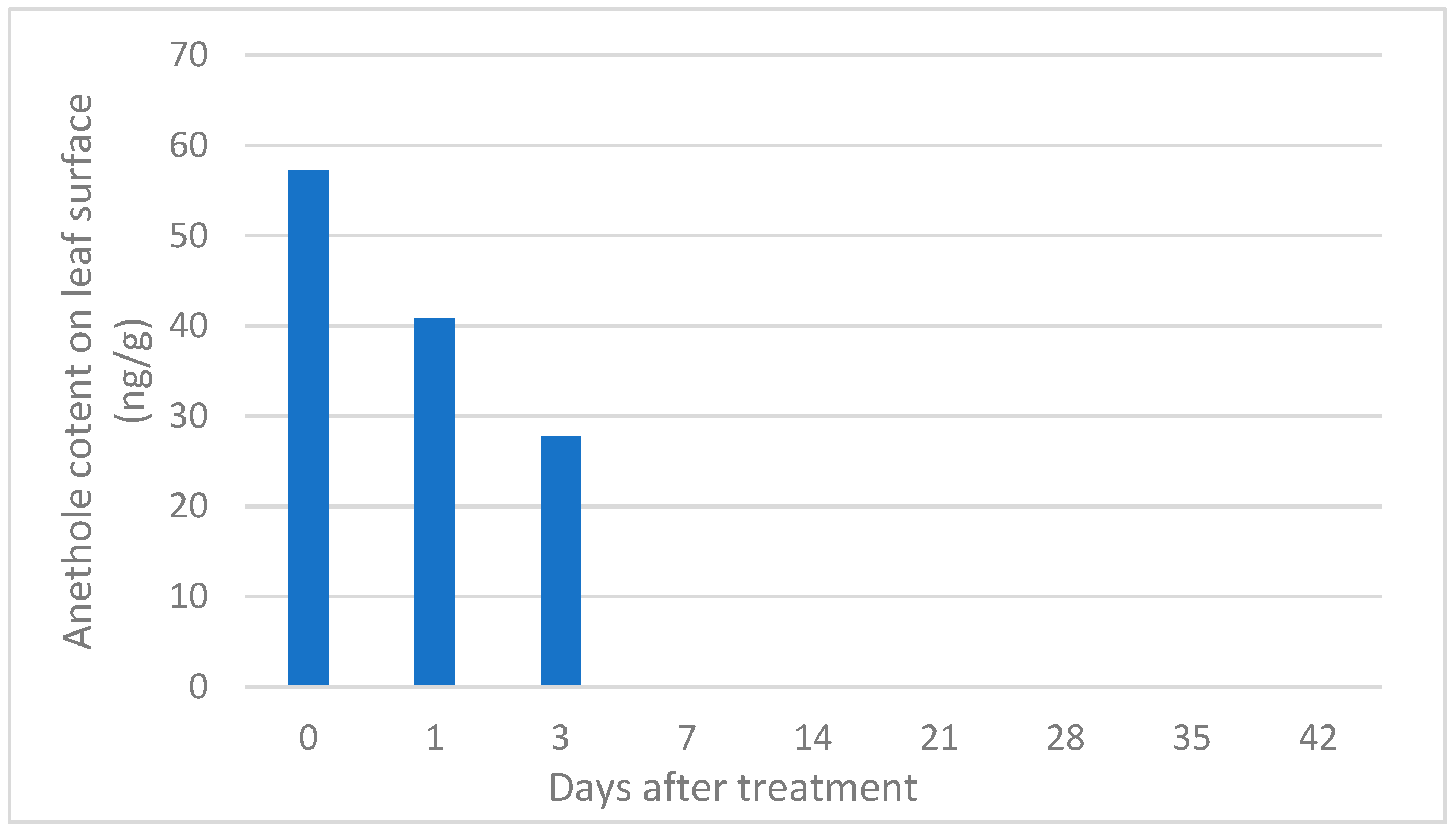
| Location | Altitude | Soil Type | Area (ha) | Cultivar | Sampling Years |
|---|---|---|---|---|---|
| 1 | 400 m | rendzina, pararendzina | 1.7 | Saazer | 2019 |
| 2 | 212 m | chernozem | 2.2 | Saazer/Premiant | 2020, 2021 |
| 3 | 287 m | rendzina, pararendzina | 1.6 | Saazer | 2020 |
| Location-Year | Temperetures (°C) | Precipitation (mm) | Duration of the Trial (Day/Month) |
|---|---|---|---|
| 1–2019 | 12.4/272 | 70.9 | 7/5–28/5 |
| 2–2020 | 11.9/262.4 | 59.6 | 21/4–13/5 |
| 2–2021 | 12.5/274.8 | 65.6 | 10/5–1/6 |
| 3–2020 | 12.1/265.4 | 46.2 | 25/4–18/5 |
| Year | Location | kk | NCH1l | NCH1 | NCH2l | NCH2 | Days |
|---|---|---|---|---|---|---|---|
| 2019 | 1 | 0.34 (0.27–0.42) b | 0.36 (0.29–0.44) b | 0.17 (0.13–0.23) a | 0.28 (0.22–0.35) ab | 0.18 (0.13–0.24) a | grouped |
| 2020 | 2/3 grouped | 2.82 (2.24–3.56) e | x | 2.69 (2.16–3.35) e | x | 2.74 (2.20–3.40) e | 0 |
| 2020 | 2/3 grouped | 1.61 (1.39–1.87) d | x | 0.25 (0.19–0.33) a | x | 1.24 (1.06–1.47) c | 3 |
| 2020 | 2/3 grouped | 1.20 (1.02–1.42) c | x | 0.42 (0.32–0.54) b | x | 1.19 (1.00–1.40) c | 7 |
| Kk | x | NCH1 | |||||
| 2021 | 2 | 1.21 (0.97–1.52) bc | x | 1.54 (1.33–1.78) c | x | x | 0 |
| 2021 | 2 | 0.88 (0.68–1.13) b | x | 0.14 (0.09–0.22) a | x | x | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovaříková, K.; Pavela, R.; Krofta, K.; Vostřel, J. Use of Trans-Anethole Against Hop Flea Beetles in Field Conditions. Agronomy 2025, 15, 1311. https://doi.org/10.3390/agronomy15061311
Kovaříková K, Pavela R, Krofta K, Vostřel J. Use of Trans-Anethole Against Hop Flea Beetles in Field Conditions. Agronomy. 2025; 15(6):1311. https://doi.org/10.3390/agronomy15061311
Chicago/Turabian StyleKovaříková, Kateřina, Roman Pavela, Karel Krofta, and Josef Vostřel. 2025. "Use of Trans-Anethole Against Hop Flea Beetles in Field Conditions" Agronomy 15, no. 6: 1311. https://doi.org/10.3390/agronomy15061311
APA StyleKovaříková, K., Pavela, R., Krofta, K., & Vostřel, J. (2025). Use of Trans-Anethole Against Hop Flea Beetles in Field Conditions. Agronomy, 15(6), 1311. https://doi.org/10.3390/agronomy15061311







