Fungal Necromass Carbon Stabilizes Rhizosphere Soil Organic Carbon: Microbial Degradation Gene Insights Under Straw and Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Root Box Experiment
2.2. Soil Properties Analysis
2.3. Isolation of Soil Aggregates
2.4. Determination of MNC
2.5. Screening of C-Cycle Functional Genes
2.6. Data Analysis
3. Results
3.1. Changes in Soil Properties
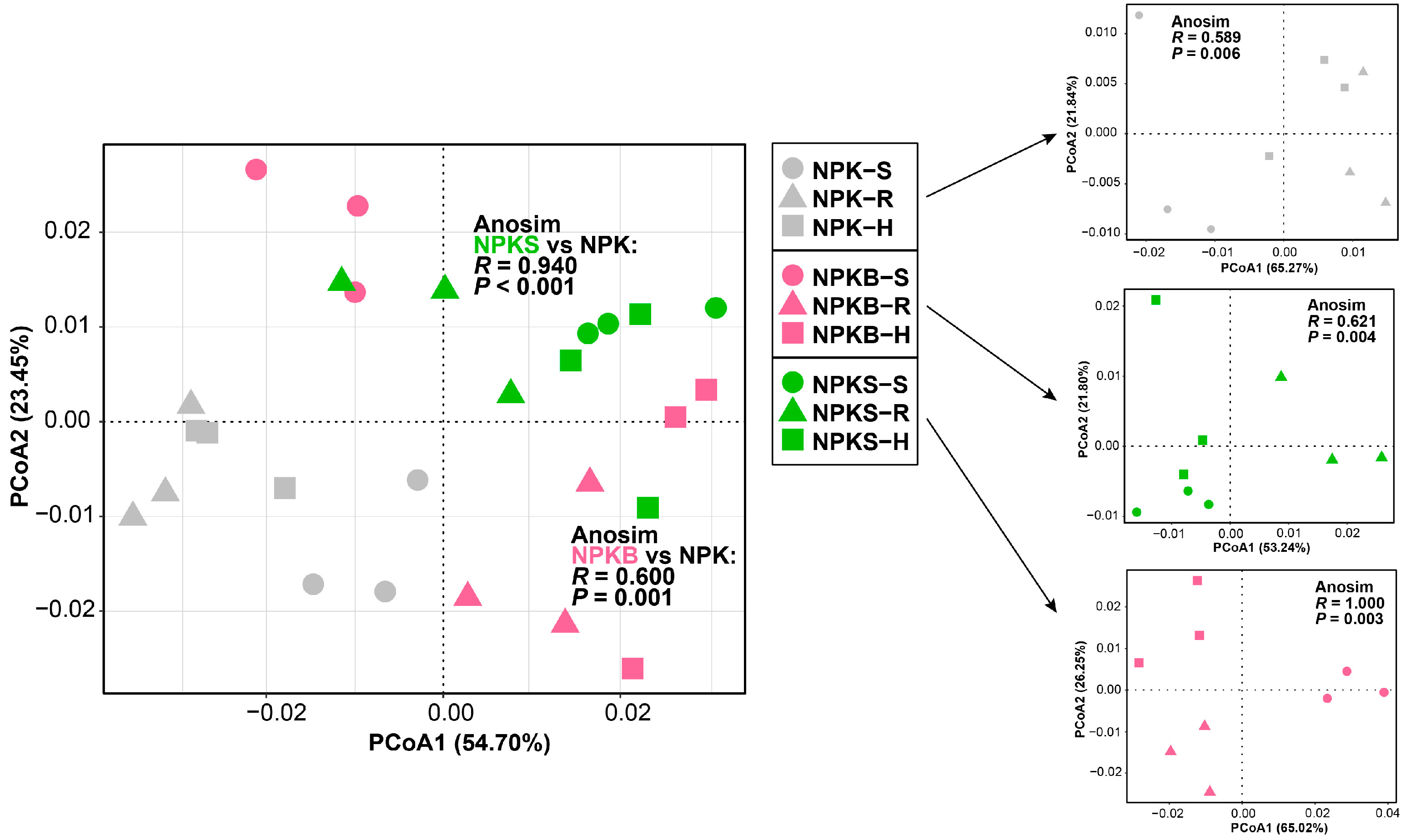
3.2. Changes in Soil Aggregate Distribution
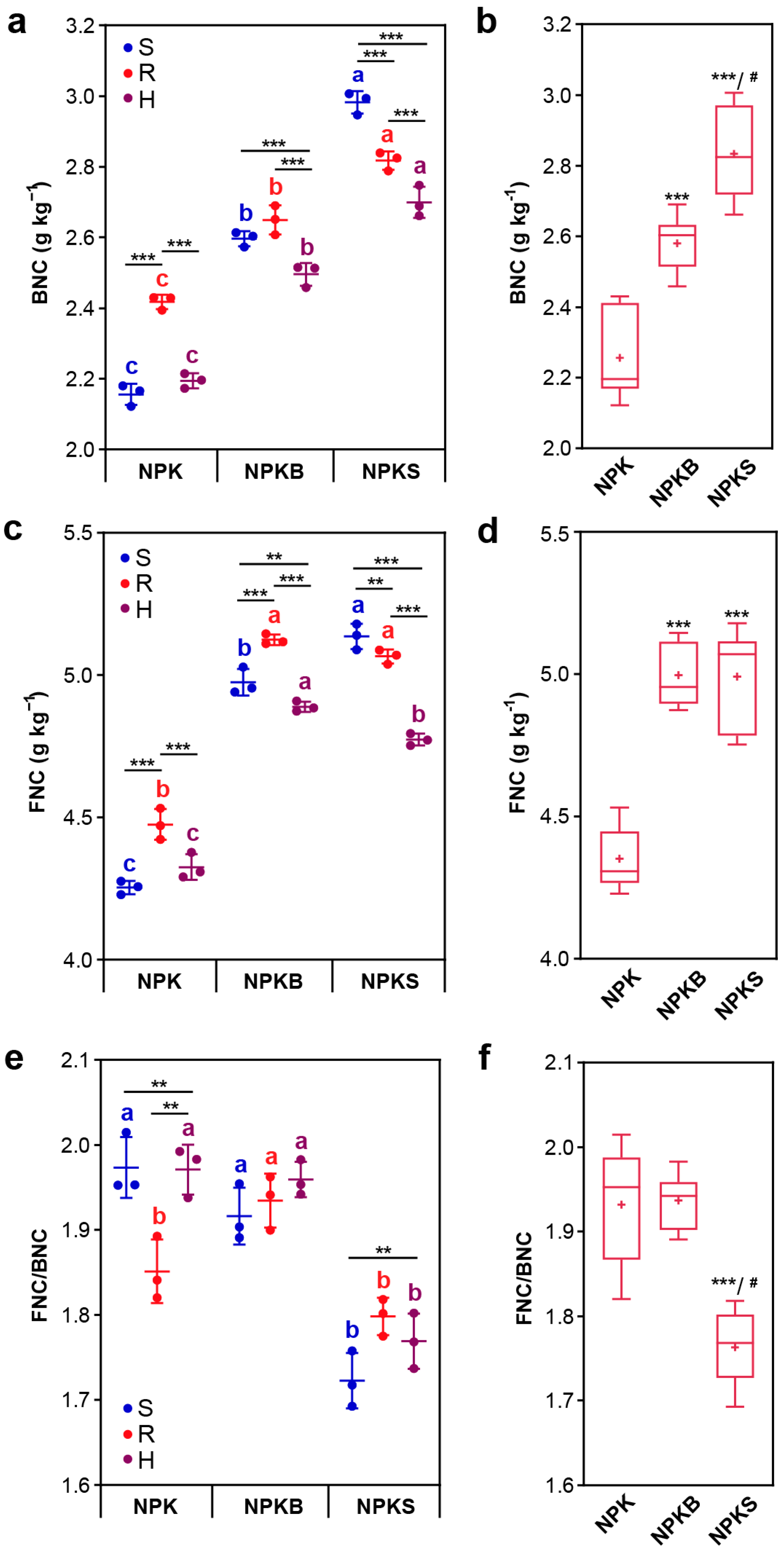
3.3. MNC and Its Contribution to SOC
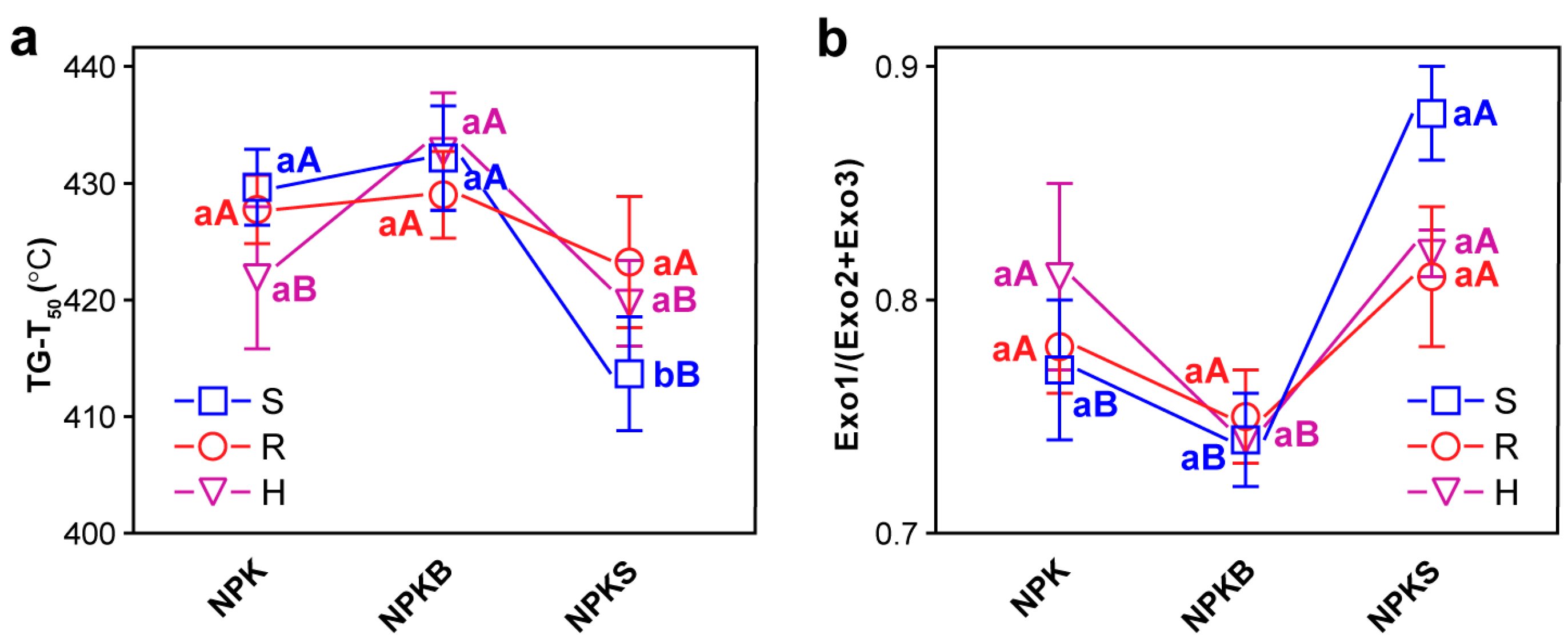
3.4. Thermal Stability of SOC
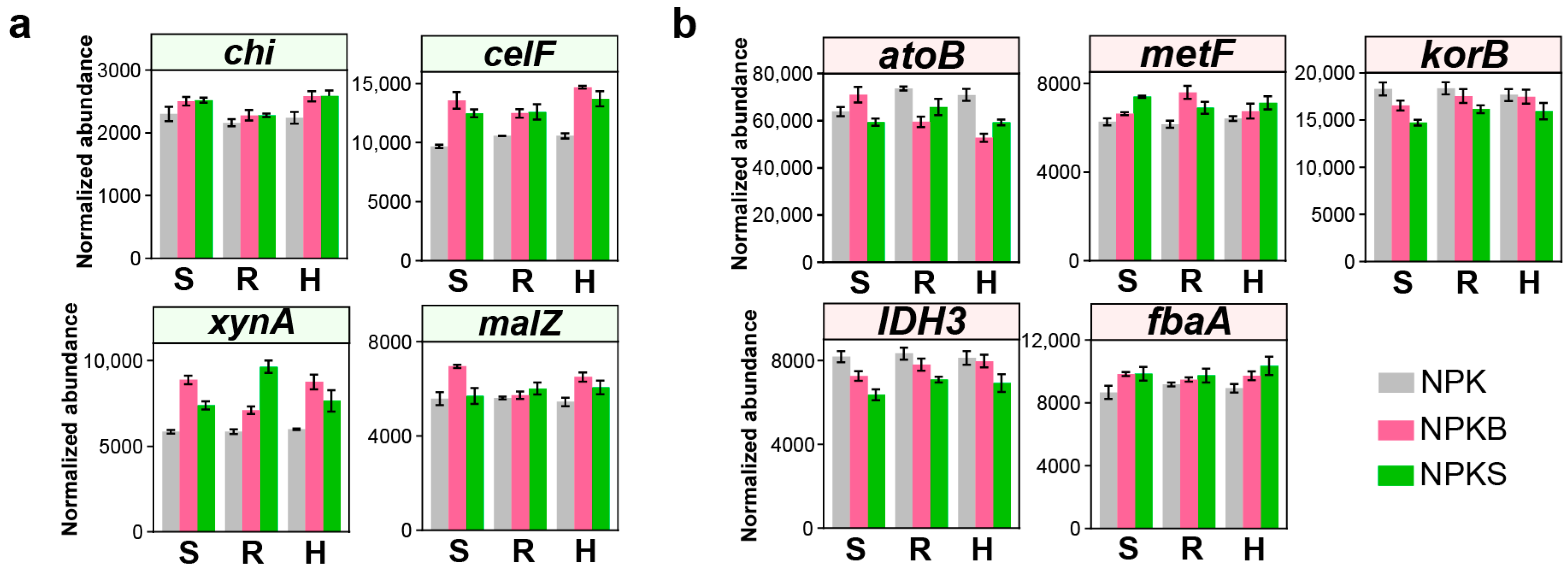
3.5. Alterations Among Genes Pertinent to C-Cycling Functions
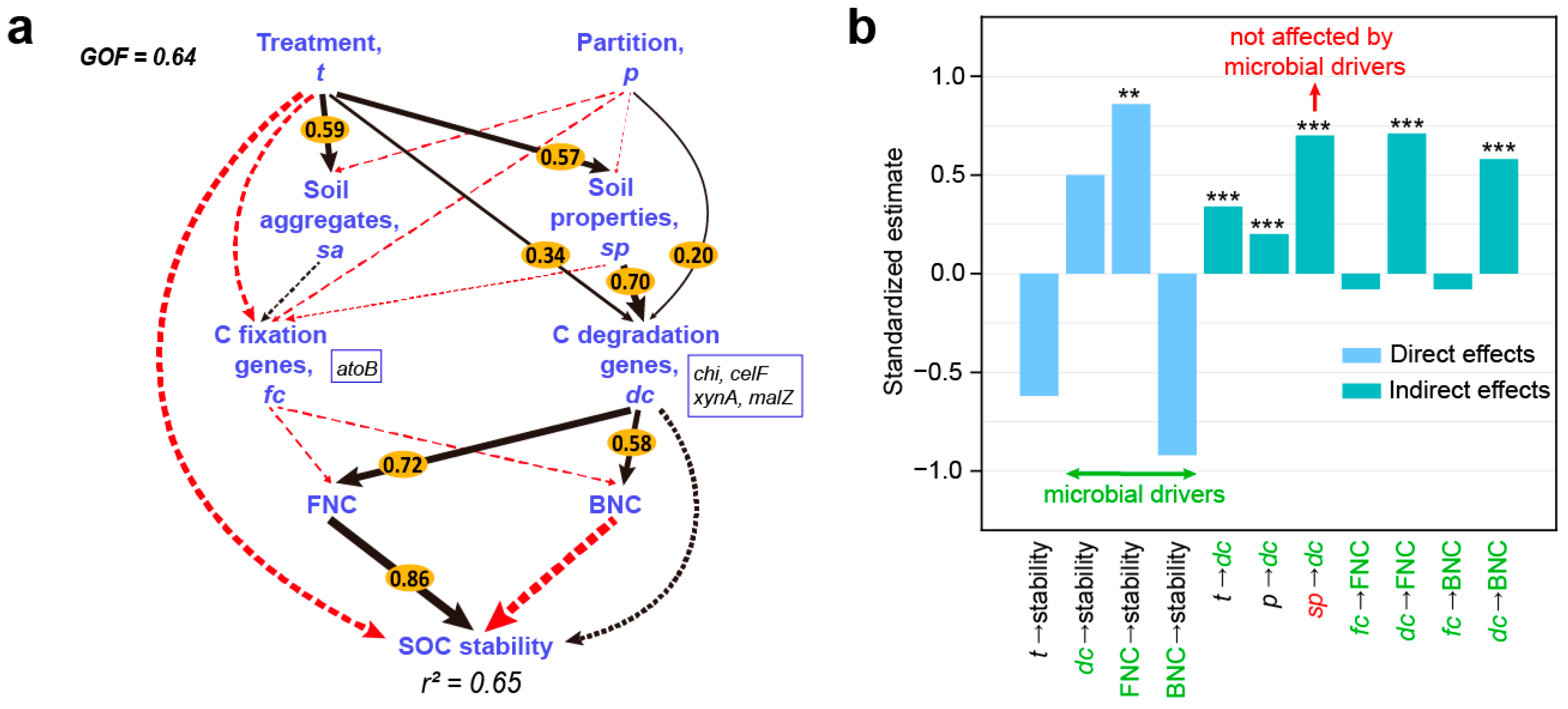
3.6. Factors Driving SOC Stability
4. Discussion
4.1. The Rhizosphere Sequestered More SOC and Was Significantly Affected by Biochar
4.2. Organic Fertilizer Addition Induces Changes in FNC and BNC in Different Soil Compartments
4.3. C Degradation Genes Drive SOC Thermal Stability via FNC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.d.R.d.; Singh, K.; et al. The Knowns, Known Unknowns and Unknowns of Sequestration of Soil Organic Carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Georgiou, K.; Jackson, R.B.; Vindušková, O.; Abramoff, R.Z.; Ahlström, A.; Feng, W.; Harden, J.W.; Pellegrini, A.F.A.; Polley, H.W.; Soong, J.L.; et al. Global Stocks and Capacity of Mineral-Associated Soil Organic Carbon. Nat. Commun. 2022, 13, 3797. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ge, T.D.; van Groenigen, K.J.; Yang, Y.H.; Wang, P.; Cheng, K.; Zhu, Z.K.; Wang, J.K.; Li, Y.; Guggenberger, G.; et al. Rice Paddy Soils Are a Quantitatively Important Carbon Store According to a Global Synthesis. Commun. Earth Environ. 2021, 2, 154. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration in China through Agricultural Intensification, and Restoration of Degraded and Desertified Ecosystems. Land Degrad. Dev. 2010, 13, 469–478. [Google Scholar] [CrossRef]
- Leng, L.J.; Xu, X.W.; Wei, L.; Fan, L.L.; Huang, H.J.; Li, J.A.; Lu, Q.; Li, J.; Zhou, W.G. Biochar Stability Assessment by Incubation and Modelling: Methods, Drawbacks and Recommendations. Sci. Total Environ. 2019, 664, 11–23. [Google Scholar] [CrossRef]
- Wang, S.C.; Lu, C.A.; Huai, S.C.; Yan, Z.H.; Wang, J.Y.; Sun, J.Y.; Raza, S. Straw Burial Depth and Manure Application Affect the Straw-C and N Sequestration: Evidence from 13C & 15N-Tracing. Soil Tillage Res. 2021, 208, 104884. [Google Scholar] [CrossRef]
- Majumder, B.; Kuzyakov, Y. Effect of Fertilization on Decomposition of 14C Labelled Plant Residues and Their Incorporation into Soil Aggregates. Soil Tillage Res. 2010, 109, 94–102. [Google Scholar] [CrossRef]
- Melero, S.; López-Bellido, R.J.; López-Bellido, L.; Muñoz-Romero, V.; Moreno, F.; Murillo, J.M. Long-Term Effect of Tillage, Rotation and Nitrogen Fertiliser on Soil Quality in a Mediterranean Vertisol. Soil Tillage Res. 2011, 114, 97–107. [Google Scholar] [CrossRef]
- Gougoulias, C.; Joanna, M.C.; Liz, J.S. The Role of Soil Microbes in the Global Carbon Cycle: Tracking the Below-Ground Microbial Processing of Plant-Derived Carbon for Manipulating Carbon Dynamics in Agricultural Systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef]
- Cao, Y.F.; Ding, J.Z.; Li, J.; Xin, Z.M.; Ren, S.A.; Wang, T. Necromass-Derived Soil Organic Carbon and Its Drivers at the Global Scale. Soil Biol. Biochem. 2023, 181, 109025. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative Assessment of Microbial Necromass Contribution to Soil Organic Matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Camenzind, T.; Mason-Jones, K.; Mansour, I.; Rillig, M.; Lehmann, J. Formation of Necromass-Derived Soil Organic Carbon Determined by Microbial Death Pathways. Nat. Geosci. 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Yang, J.K.; Li, L.; Xu, Y.N.; Yu, Y.; Virk, A.L.; Li, F.M.; Yang, H.S.; Kan, Z.R. Effects of Straw Biochar on Microbial-Derived Carbon: A Global Meta-Analysis. J. Environ. Manag. 2024, 368, 122233. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yang, X.; Bao, Z.R.; Gao, J.; Meng, J.; Han, X.R.; Lan, Y.; Liu, Z.Q.; Chen, W.F. Responses of Microbial Necromass Carbon and Microbial Community Structure to Straw- and Straw-Derived Biochar in Brown Earth Soil of Northeast China. Front. Microbiol. 2022, 13, 967746. [Google Scholar] [CrossRef]
- Chen, Z.M.; He, L.L.; Ma, J.C.; Ma, J.W.; Ye, J.; Yu, Q.G.; Zou, P.; Sun, W.C.; Lin, H.; Wang, F.; et al. Long-Term Successive Biochar Application Increases Plant Lignin and Microbial Necromass Accumulation but Decreases Their Contributions to Soil Organic Carbon in Rice–Wheat Cropping System. GCB Bioenergy 2024, 16, e13137. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Razavi. Rhizosphere Size and Shape: Temporal Dynamics and Spatial Stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Shao, G.D.; Xu, Y.; Zhou, J.; Tian, P.; Ai, J.J.; Yang, Y.D.; Zamanian, K.; Zeng, Z.H.; Zang, H.D. Enhanced Soil Organic Carbon Stability in Rhizosphere through Manure Application. Soil Tillage Res. 2024, 244, 106223. [Google Scholar] [CrossRef]
- Venturi, V.; Keel, C. Signaling in the Rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef]
- Lange, M.; Azizi-Rad, M.; Dittmann, G.; Lange, D.F.; Orme, A.M.; Schroeter, S.A.; Simon, C.; Gleixner, G. Stability and Carbon Uptake of the Soil Microbial Community Is Determined by Differences between Rhizosphere and Bulk Soil. Soil Biol. Biochem. 2024, 189, 109280. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Nierop, and Myrna J. Simpson. Plant- or Microbial-Derived? A Review on the Molecular Composition of Stabilized Soil Organic Matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of Mineral-Associated Soil Organic Matter Formation: Integrating the Role of Plant Carbon Source, Chemistry, and Point of Entry. Glob. Change Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xiao, M.L.; Yuan, H.Z.; Liang, C.; Zhu, Z.K.; Xu, J.M.; Kuzyakov, Y.; Wu, J.S.; Ge, T.D.; Tang, C.X. Rice Rhizodeposition Promotes the Build-up of Organic Carbon in Soil via Fungal Necromass. Soil Biol. Biochem. 2021, 160, 108345. [Google Scholar] [CrossRef]
- Jia, Y.F.; Liu, Z.G.; Zhou, L.; Liu, X.J.; Ma, K.P.; Feng, X.J. Soil Organic Carbon Sourcing Variance in the Rhizosphere vs. Non-Rhizosphere of Two Mycorrhizal Tree Species. Soil Biol. Biochem. 2023, 176, 108884. [Google Scholar] [CrossRef]
- Wang, Q.T.; Ding, J.X.; Zhang, Z.L.; Liang, C.; Lambers, H.; Zhu, B.; Wang, D.G.; Wang, J.P.; Zhang, P.P.; Li, N.; et al. Rhizosphere as a Hotspot for Microbial Necromass Deposition into the Soil Carbon Pool. J. Ecol. 2024, 113, 168–179. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming Effects: Interactions between Living and Dead Organic Matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of Mechanisms and Quantification of Priming Effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Zhu, X.M.; Zhang, Z.L.; Wang, Q.T.; Peñuelas, J.; Sardans, J.; Lambers, H.; Li, N.; Liu, Q.; Yin, H.J.; Liu, Z.F. More Soil Organic Carbon Is Sequestered through the Mycelium Pathway than through the Root Pathway under Nitrogen Enrichment in an Alpine Forest. Glob. Change Biol. 2022, 28, 4947–4961. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Andrade, G.; Mihara, K.L.; Linderman, R.G.; Bethlenfalvay, G.J. Bacteria from Rhizosphere and Hyphosphere Soils of Different Arbuscular-Mycorrhizal Fungi. Plant Soil. 1997, 192, 71–79. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.C.; George, T.S.; Limpens, E.; Feng, G. Arbuscular Mycorrhizal Fungi Conducting the Hyphosphere Bacterial Orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef]
- Fernández, C.W.; Langley, J.A.; Chapman, S.; McCormack, M.L.; Koide, R.T. The Decomposition of Ectomycorrhizal Fungal Necromass. Soil Biol. Biochem. 2016, 93, 38–49. [Google Scholar] [CrossRef]
- See, C.R.; Keller, A.B.; Hobbie, S.E.; Kennedy, P.G.; Weber, P.K.; Pett-Ridge, J. Hyphae Move Matter and Microbes to Mineral Microsites: Integrating the Hyphosphere into Conceptual Models of Soil Organic Matter Stabilization. Glob. Change Biol. 2022, 28, 2527–2540. [Google Scholar] [CrossRef] [PubMed]
- Gorka, S.; Dietrich, M.; Mayerhofer, W.; Gabriel, R.; Kaiser, C. Rapid Transfer of Plant Photosynthates to Soil Bacteria via Ectomycorrhizal Hyphae and Its Interaction with Nitrogen Availability. Front. Microbiol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; La Rosa, A.F.; Mason, K.E.; Whitaker, J.; McNamara, N.P.; Grant, H.K.; Ostle, N.J. Sticky Dead Microbes: Rapid Abiotic Retention of Microbial Necromass in Soil. Soil Biol. Biochem. 2020, 149, 107929. [Google Scholar] [CrossRef]
- Ding, X.L.; Liang, C.; Zhang, B.; Yuan, Y.R.; Han, X.Z. Higher Rates of Manure Application Lead to Greater Accumulation of Both Fungal and Bacterial Residues in Macroaggregates of a Clay Soil. Soil Biol. Biochem. 2015, 84, 137–146. [Google Scholar] [CrossRef]
- See, C.R.; Fernandez, C.W.; Conley, A.M.; DeLancey, L.C.; Heckman, K.A.; Kennedy, P.G.; Hobbie, S.E. Distinct Carbon Fractions Drive a Generalisable Two-Pool Model of Fungal Necromass Decomposition. Funct. Ecol. 2021, 35, 796–806. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The Macromolecular Organic Composition of Plant and Microbial Residues as Inputs to Soil Organic Matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Deveautour, C.; Chieppa, J.; Nielsen, U.N.; Boer, M.M.; Mitchell, C.; Horn, S.; Power, S.A.; Guillen, A.; Bennett, A.E.; Powell, J.R. Biogeography of arbuscular mycorrhizal fungal spore traits along an aridity gradient, and responses to experimental rainfall manipulation. Fungal Ecol. 2020, 46, 100899. [Google Scholar] [CrossRef]
- Li, Y.H.; Xiao, M.L.; Wei, L.; Liu, Q.; Zhu, Z.K.; Yuan, H.Z.; Wu, J.S.; Yuan, J.; Wu, X.H.; Kuzyakov, Y.; et al. Bacterial necromass determines the response of mineral-associated organic matter to elevated CO2. Biol. Fertil. Soils 2024, 60, 327–340. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Peltre, C.; Fernández, J.M.; Craine, J.M.; Plante, A.F. Relationships between biological and thermal indices of soil organic matter stability differ with soil organic carbon level. Soil Sci. Soc. Am. J. 2013, 77, 2020–2028. [Google Scholar] [CrossRef]
- Gong, K.Y.; Yang, F.; Long, H.; Gu, J.; Zhang, G.L. Vertical variations of thermal stability of soil organic matter in black soils under different pedogenetic modes. Acta Pedol. Sin. 2024, 61, 662–673. [Google Scholar] [CrossRef]
- Capel, E.L.; de la Rosa Arranz, J.M.; González-Vila, F.J.; González-Perez, J.A.; Manning, D.A.C. Elucidation of different forms of organic carbon in marine sediments from the Atlantic coast of Spain using thermal analysis coupled to isotope-ratio and quadrupole mass spectrometry. Org. Geochem. 2006, 37, 1983–1994. [Google Scholar] [CrossRef]
- Plante, A.F.; Fernández, J.M.; Leifeld, J. Application of thermal analysis techniques in soil science. Geoderma 2009, 153, 1–10. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K.; Doran, J.W. Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Zhang, X.D.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Tang, Z.X.; Feng, J.N.; Chen, L.L.; Chen, Z.B.; Shao, X.D.; Xia, T.Y. Coupling amendment of microbial and compound fertilizers increases fungal necromass carbon and soil organic carbon by regulating microbial activity in flue-cured tobacco-planted field. Eur. J. Soil Biol. 2023, 117, 103518. [Google Scholar] [CrossRef]
- Wang, Q.T.; Zhang, Y.; Zhang, P.P.; Li, N.; Wang, R.H.; Zhang, X.J.; Yin, H.J. Nitrogen deposition induces a greater soil C sequestration in the rhizosphere than bulk soil in an alpine forest. Sci. Total Environ. 2023, 875, 162701. [Google Scholar] [CrossRef]
- Hu, X.J.; Gu, H.D.; Liu, J.J.; Wei, D.; Zhu, P.; Cui, X.A.; Zhou, B.K.; Chen, X.L.; Jin, J.; Liu, X.B.; et al. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 2022, 418, 115846. [Google Scholar] [CrossRef]
- Kalu, S.; Seppänen, A.; Mganga, K.Z.; Sietiö, O.M.; Glaser, B.; Karhu, K. Biochar reduced the mineralization of native and added soil organic carbon: Evidence of negative priming and enhanced microbial carbon use efficiency. Biochar 2024, 6, 7. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C.M. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Xiong, Z.Q.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Liu, L.; Liu, D.M.; Ding, X.D.; Chen, M.M.; Zhang, S.R. Straw incorporation and nitrogen fertilization enhance soil carbon sequestration by altering soil aggregate and microbial community composition in saline-alkali soil. Plant Soil 2024, 498, 341–356. [Google Scholar] [CrossRef]
- Xu, P.D.; Wang, Q.; Duan, C.J.; Huang, G.Y.; Dong, K.H.; Wang, C.H. Biochar addition promotes soil organic carbon sequestration dominantly contributed by macro-aggregates in agricultural ecosystems of China. J. Environ. Manag. 2024, 359, 121042. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Sun, C.X.; Wang, S.Q.; Xie, H.T.; Jiang, N.; Chen, Z.H.; Wei, K.; Bao, X.L.; Song, X.Y.; Bai, Z. Stover and biochar can improve soil microbial necromass carbon, and enzymatic transformation at the genetic level. GCB Bioenergy 2022, 14, 1082–1096. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.C.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Liu, W.X.; Yang, S.; Yang, L.; Peng, Z.Y.; Deng, M.F.; Xu, S.; Zhang, B.B.; Ahirwal, J.; Liu, L.L. Plant carbon inputs through shoot, root, and mycorrhizal pathways affect soil organic carbon turnover differently. Soil Biol. Biochem. 2021, 160, 108322. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Zhu, B.; Cheng, W. Root effects on soil organic carbon: A double-edged sword. New Phytol. 2021, 230, 60–65. [Google Scholar] [CrossRef]
- Potthoff, M.; Dyckmans, J.; Flessa, H.; Beese, F.; Joergensen, R.G. Decomposition of maize residues after manipulation of colonization and its contribution to the soil microbial biomass. Biol. Fertil. Soils 2008, 44, 891–895. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xie, H.T.; Wang, F.P.; Sun, C.X.; Zhang, X.D. Effects of biochar incorporation on soil viable and necromass carbon in the Luvisol soil. Soil Use Manage 2021, 38, 318–330. [Google Scholar] [CrossRef]
- He, H.B.; Zhang, W.; Zhang, X.D.; Xie, H.T.; Zhuang, J. Temporal responses of soil microorganisms to substrate addition as indicated by amino sugar differentiation. Soil Biol. Biochem. 2011, 43, 1155–1161. [Google Scholar] [CrossRef]
- Paterson, E.; Osler, G.; Dawson, L.A.; Gebbing, T.; Sim, A.; Ord, B. Labile and recalcitrant plant fractions are utilised by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol. Biochem. 2008, 40, 1103–1113. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Song, W.C.; Wang, J.; Hou, L. Effects of frequency and amount of stover mulching on soil nitrogen and the microbial functional guilds of the endosphere and rhizosphere. Front. Microbiol. 2022, 13, 976154. [Google Scholar] [CrossRef] [PubMed]
- Rozmoš, M.; Bukovská, P.; Hršelová, H.; Kotianová, M.; Dudáš, M.; Gančarčíková, K.; Jansa, J. Organic nitrogen utilisation by an arbuscular mycorrhizal fungus is mediated by specific soil bacteria and a protist. ISME J. 2022, 16, 676–685. [Google Scholar] [CrossRef]
- Hou, Z.N.; Wang, R.H.; Chang, S.; Zheng, Y.; Ma, T.T.; Xu, S.Q.; Zhang, X.J.; Shi, X.; Lu, J.; Luo, D.Q.; et al. The Contribution of Microbial Necromass to Soil Organic Carbon and Influencing Factors Along a Variation of Habitats in Alpine Ecosystems. Sci. Total Environ. 2024, 921, 171126. [Google Scholar] [CrossRef]
- Ni, X.; Liao, S.; Tan, S.; Peng, Y.; Wang, D.; Yue, K.; Wu, F.; Yang, Y. The vertical distribution and control of microbial necromass carbon in forest soils. Glob. Ecol. Biogeogr. 2020, 29, 1829–1839. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Liang, A.J.; Li, Y.Y.; Song, Q.Y.; Li, X.Y.; Li, D.P.; Hou, N. Insight into the soil aggregate-mediated restoration mechanism of degraded black soil via biochar addition: Emphasizing the driving role of core microbial communities and nutrient cycling. Environ. Res. 2023, 228, 115895. [Google Scholar] [CrossRef]
- Kozjek, K.; Manoharan, L.; Urich, T.; Ahrén, D.; Hedlund, K. Microbial gene activity in straw residue amendments reveals carbon sequestration mechanisms in agricultural soils. Soil Biol. Biochem. 2023, 179, 108994. [Google Scholar] [CrossRef]



| Index | Soil | Straw | Biochar |
|---|---|---|---|
| pH | 5.13 | \ | \ |
| Total carbon (g kg−1) | 15.00 | 303.00 | 454.00 |
| Total nitrogen (g kg−1) | 1.90 | 9.80 | 12.90 |
| Total phosphorus (g kg−1) | \ | 1.72 | 3.03 |
| Total potassium (g kg−1) | \ | 11.43 | 17.31 |
| Available nitrogen (mg kg−1) | 164.50 | \ | \ |
| Available phosphorus (mg kg−1) | 8.82 | \ | \ |
| Available potassium (mg kg−1) | 339.73 | \ | \ |
| Treatments | Partitions | SOC (g kg−1) | TN (g kg−1) | SOC/TN | pH | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| NPK | S | 15.30 ± 0.30 aC | 1.83 ± 0.12 aB | 8.09 ± 0.31 aB | 5.33 ± 0.07 bA | 154.58 ± 3.59 aB | 9.98 ± 0.62 aA | 379.92 ± 12.13 aC |
| R | 15.60 ± 0.26 aC | 1.70 ± 0.00 aC | 8.08 ± 0.15 aA | 5.66 ± 0.16 aA | 133.58 ± 7.15 bA | 8.55 ± 0.65 bB | 213.14 ± 3.96 bB | |
| H | 15.13 ± 0.40 aC | 1.77 ± 0.06 aA | 7.69 ± 0.41 aB | 5.47 ± 0.20 abA | 156.22 ± 6.65 aB | 9.55 ± 0.67 abA | 362.75 ± 13.06 aC | |
| NPKB | S | 21.73 ± 0.19 bA | 2.03 ± 0.06 aA | 8.62 ± 0.24 aA | 5.38 ± 0.08 bA | 164.92 ± 8.70 aB | 9.62 ± 0.33 abA | 718.9 ± 45.99 aA |
| R | 23.35 ± 0.45 aA | 2.07 ± 0.06 aA | 8.65 ± 0.54 aA | 5.75 ± 0.03 aA | 145.48 ± 7.81 bA | 8.92 ± 0.47 bB | 576.09 ± 16.13 bA | |
| H | 21.95 ± 0.05 bA | 2.07 ± 0.12 aA | 8.67 ± 0.64 aA | 5.43 ± 0.10 bA | 172.92 ± 3.08 aB | 10.15 ± 0.43 aA | 723.26 ± 16.31 aA | |
| NPKS | S | 17.30 ± 0.44 aB | 1.97 ± 0.06 a AB | 7.86 ± 0.27 aB | 5.16 ± 0.08 bB | 200.88 ± 7.82 aA | 10.58 ± 0.52 aA | 553.01 ± 37.98 aB |
| R | 17.33 ± 0.21 aB | 1.90 ± 0.00 aB | 8.18 ± 0.71 aA | 5.52 ± 0.14 aA | 146.18 ± 5.25 bA | 10.65 ± 0.29 aA | 562.37 ± 12.96 aA | |
| H | 17.23 ± 0.12 aB | 1.90 ± 0.00 aA | 7.89 ± 0.29 aA | 5.23 ± 0.04 bA | 200.32 ± 4.50 aA | 10.28 ± 0.56 aA | 510.6 ± 37.67 aB |
| Treatments | Partitions | Exo 1 (%) | Exo 2 (%) | Exo 3 (%) |
|---|---|---|---|---|
| NPK | S | 43.53 ± 1.05 aB | 48.38 ± 0.74 aA | 8.09 ± 0.31 aB |
| R | 43.92 ± 0.70 aA | 48.00 ± 0.56 aA | 8.08 ± 0.15 aA | |
| H | 44.79 ± 1.37 aA | 47.52 ± 1.01 aA | 7.69 ± 0.41 aB | |
| NPKB | S | 42.37 ± 0.74 aB | 49.01 ± 0.64 aA | 8.62 ± 0.24 aA |
| R | 43.00 ± 0.53 aA | 48.35 ± 1.05 aA | 8.65 ± 0.54 aA | |
| H | 42.66 ± 0.72 aB | 48.67 ± 1.15 aA | 8.67 ± 0.64 aA | |
| NPKS | S | 46.81 ± 0.83 aA | 45.33 ± 1.06 bB | 7.86 ± 0.27 aB |
| R | 44.99 ± 1.06 bB | 46.82 ± 0.37 aB | 8.18 ± 0.71 aA | |
| H | 45.19 ± 0.38 bA | 46.91 ± 0.17 aB | 7.89 ± 0.29 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Wu, D.; Chen, J.; Luan, H.; Zhou, C.; Zhao, X.; Wu, J.; Rong, Q. Fungal Necromass Carbon Stabilizes Rhizosphere Soil Organic Carbon: Microbial Degradation Gene Insights Under Straw and Biochar. Agronomy 2025, 15, 1303. https://doi.org/10.3390/agronomy15061303
Jiang H, Wu D, Chen J, Luan H, Zhou C, Zhao X, Wu J, Rong Q. Fungal Necromass Carbon Stabilizes Rhizosphere Soil Organic Carbon: Microbial Degradation Gene Insights Under Straw and Biochar. Agronomy. 2025; 15(6):1303. https://doi.org/10.3390/agronomy15061303
Chicago/Turabian StyleJiang, Haiyan, Duoji Wu, Jie Chen, Haoan Luan, Chunhuo Zhou, Xiaomin Zhao, Jianfu Wu, and Qinlei Rong. 2025. "Fungal Necromass Carbon Stabilizes Rhizosphere Soil Organic Carbon: Microbial Degradation Gene Insights Under Straw and Biochar" Agronomy 15, no. 6: 1303. https://doi.org/10.3390/agronomy15061303
APA StyleJiang, H., Wu, D., Chen, J., Luan, H., Zhou, C., Zhao, X., Wu, J., & Rong, Q. (2025). Fungal Necromass Carbon Stabilizes Rhizosphere Soil Organic Carbon: Microbial Degradation Gene Insights Under Straw and Biochar. Agronomy, 15(6), 1303. https://doi.org/10.3390/agronomy15061303







