Temperature Modulates Nutrient Metabolism and Antioxidative Fluctuations in Riptortus pedestris
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Treatment
2.2. Weights of R. pedestris
2.3. Emissions of CO2 in R. pedestris
2.4. Measurement of the Content of Energy Substances
2.4.1. Sample Preparation
2.4.2. Measurement of the Total Carbohydrate Content
2.4.3. Measurement of the Glycogen Content
2.4.4. Measurement of the Fat Content
2.4.5. Measurement of the Protein Content
2.5. Measurement of the Activities of Enzymes Related to Energy Metabolism
2.6. Measurement of the Activities of Antioxidative Enzymes
2.7. Statistical Analysis
3. Results
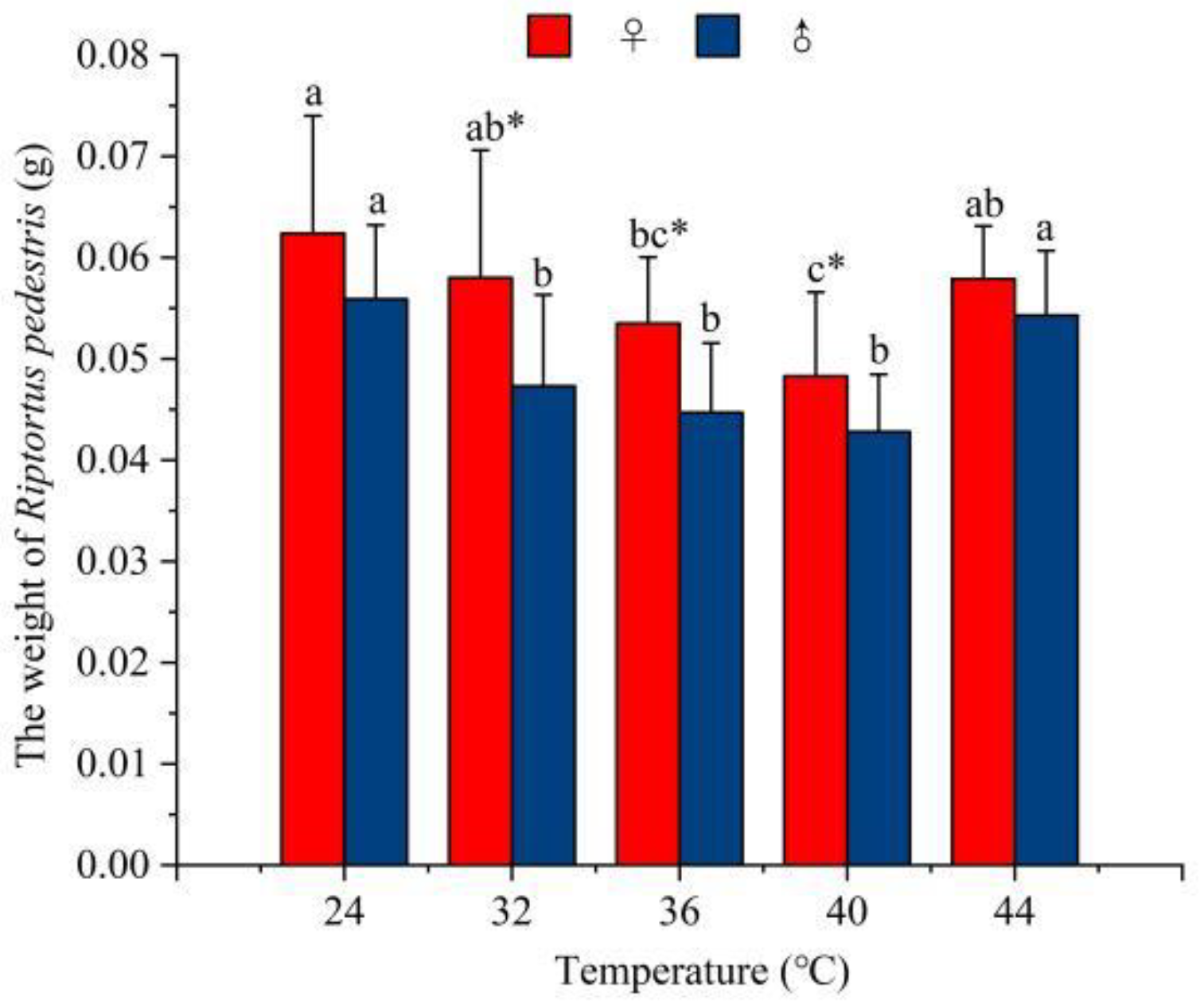
3.1. The Effects of Temperature on the Weight of R. pedestris
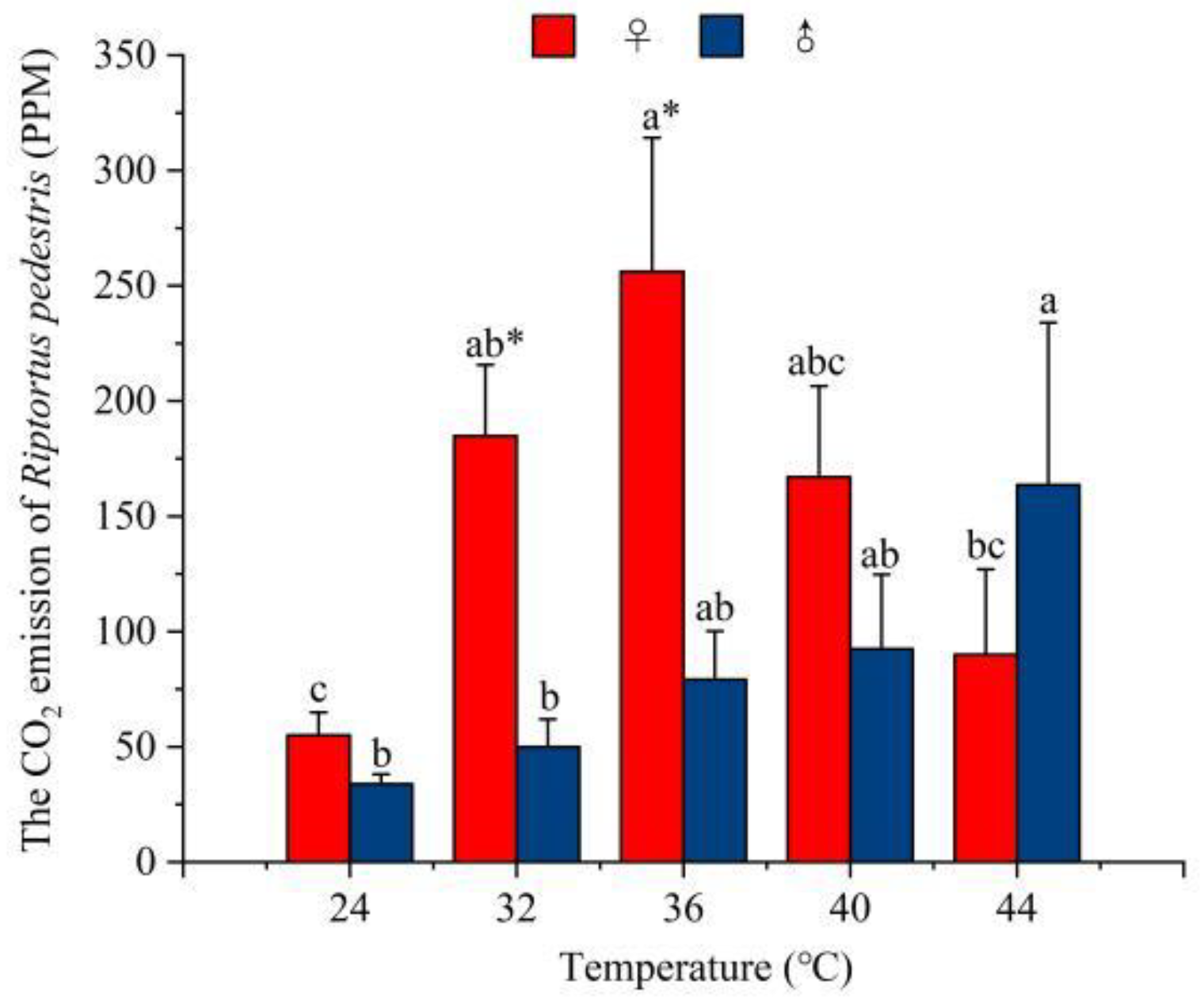
3.2. Effect of Temperature on CO2 Emissions in R. pedestris
3.3. The Effect of Temperature on the Nutrient Substance Content
3.3.1. The Effect of Temperature on the Total Carbohydrate Content
3.3.2. The Effect of Temperature on the Glycogen Content
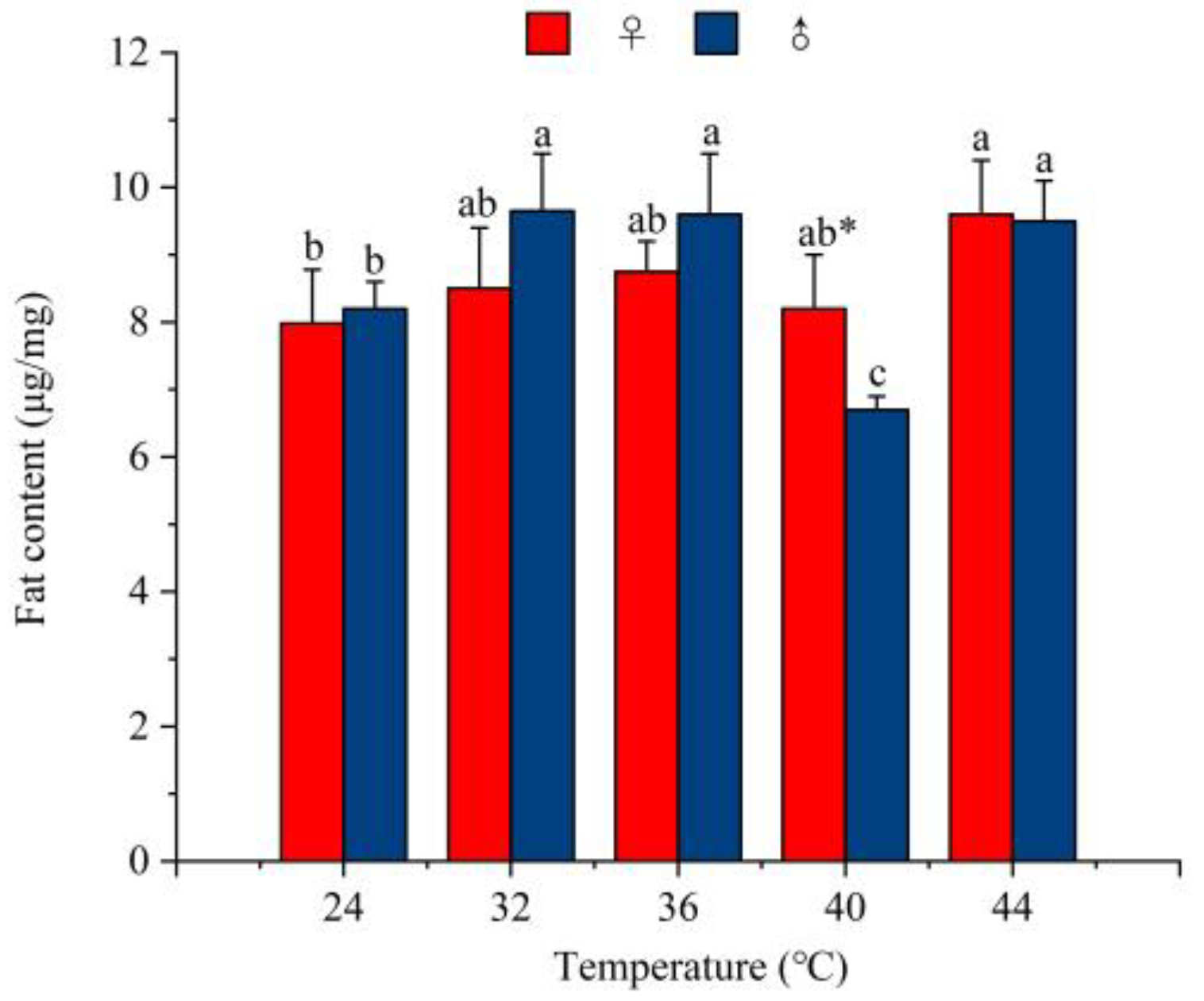
3.3.3. The Effect of Temperature on the Fat Content
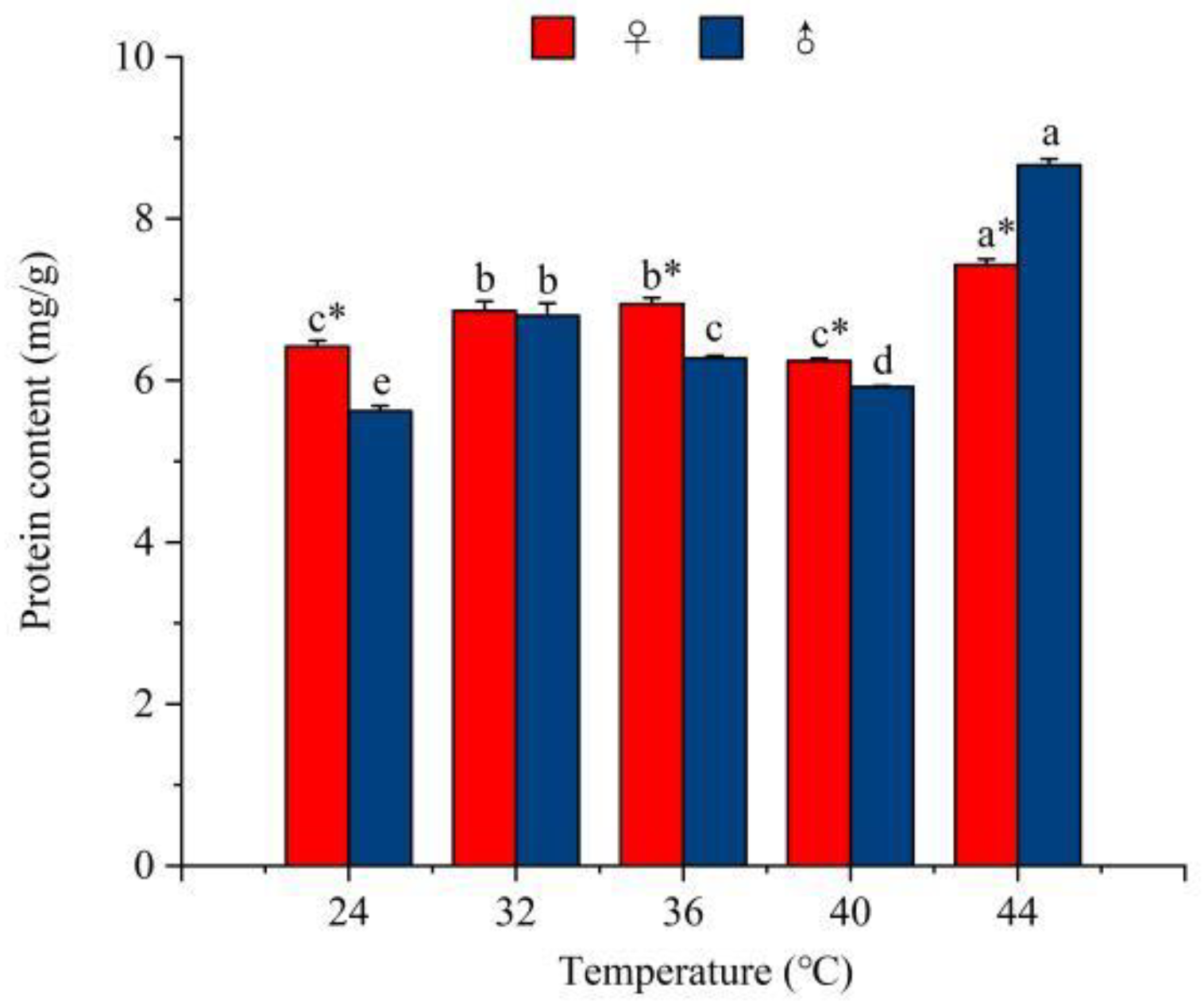
3.3.4. The Effect of Temperature on the Protein Content
3.4. The Effect of Temperature on the Activity of Nutrient Metabolic Enzymes
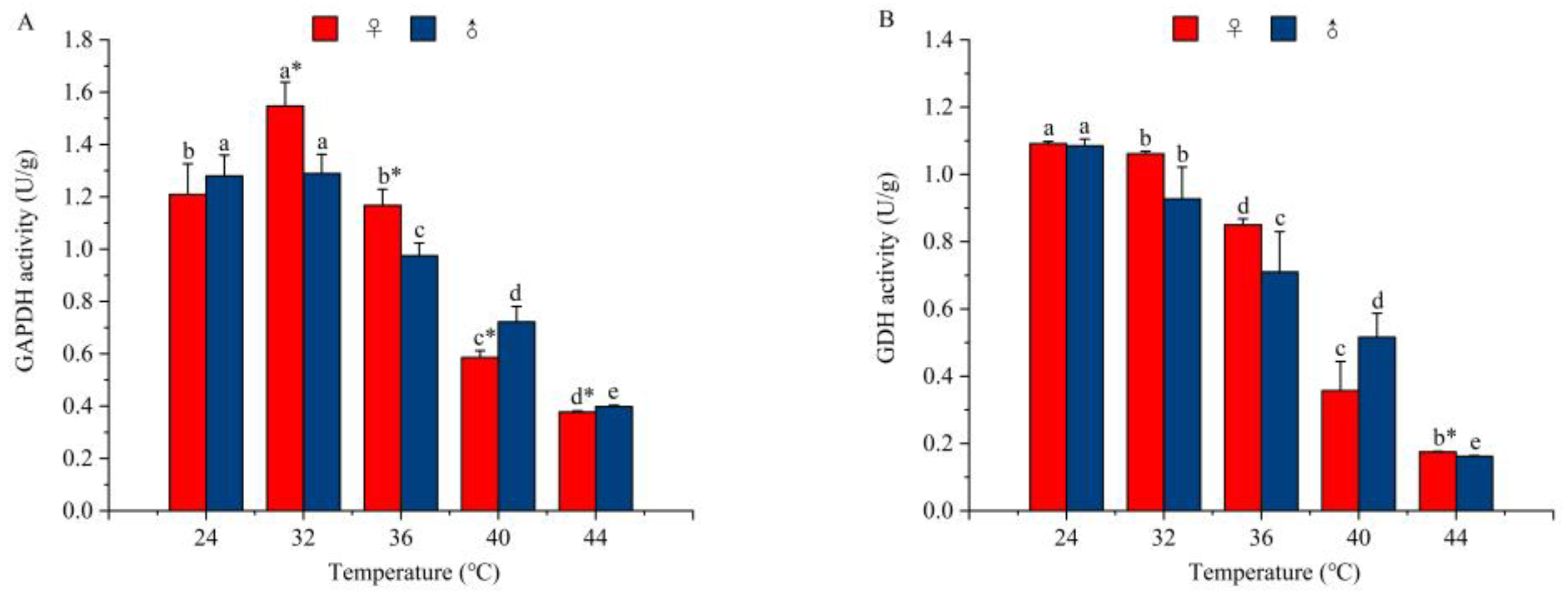
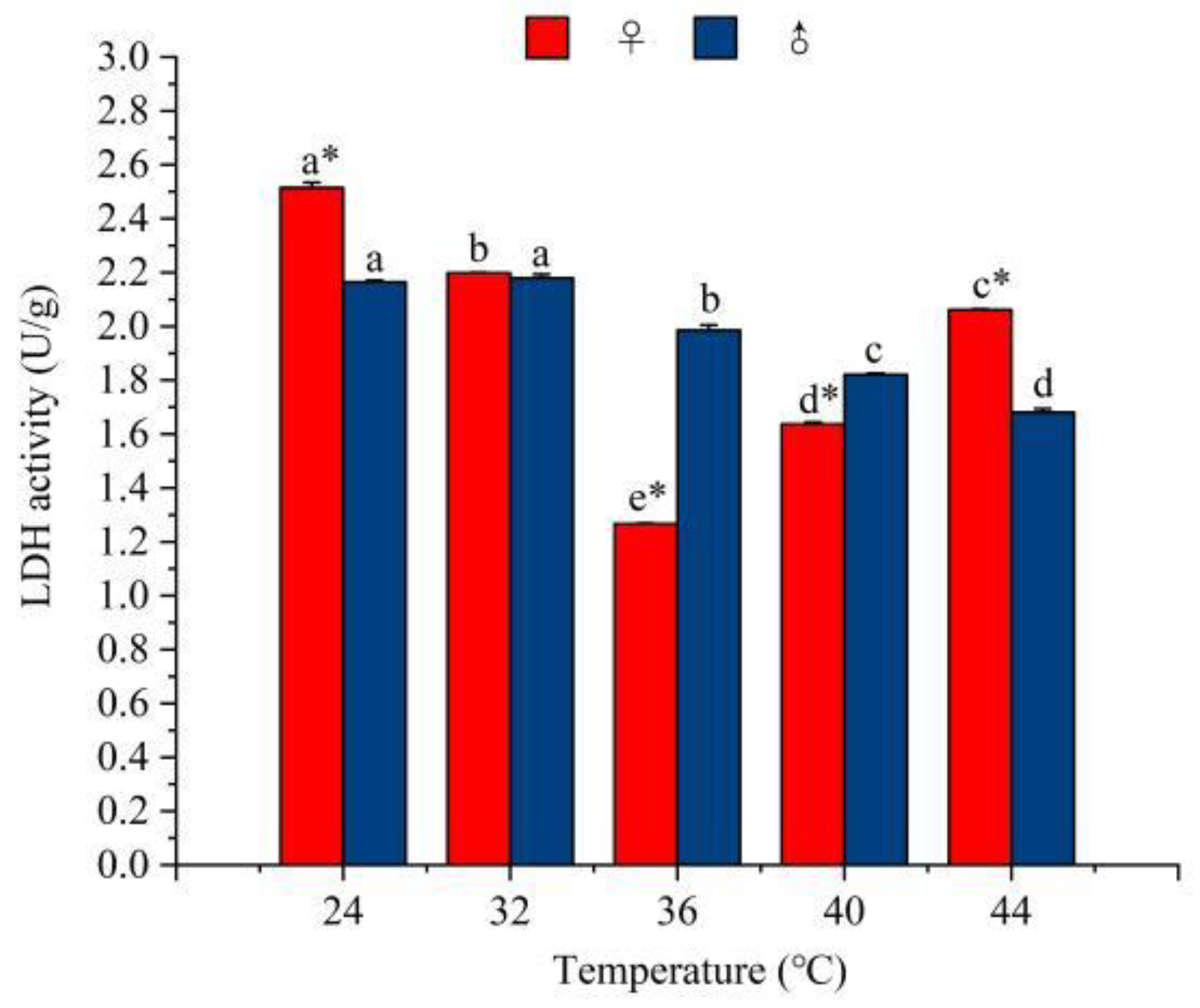
3.4.1. The Effect of Temperature on the Activity of Glycolytic Enzymes
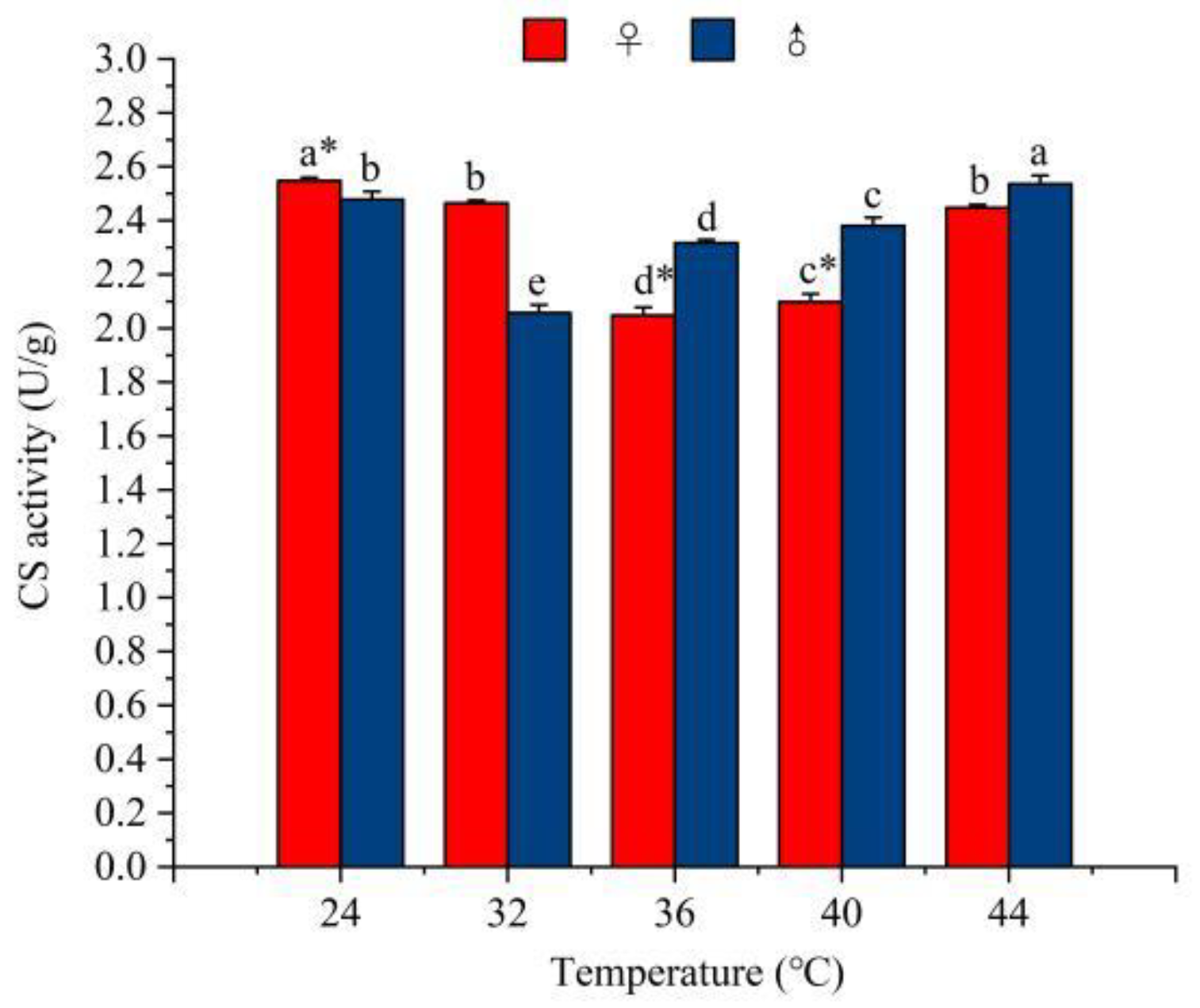
3.4.2. The Effect of Temperature on the Activity of Tricarboxylic Acid Cycle (TCA) Enzymes
3.4.3. The Effect of Temperature on the Activity of Gluconeogenic Enzymes
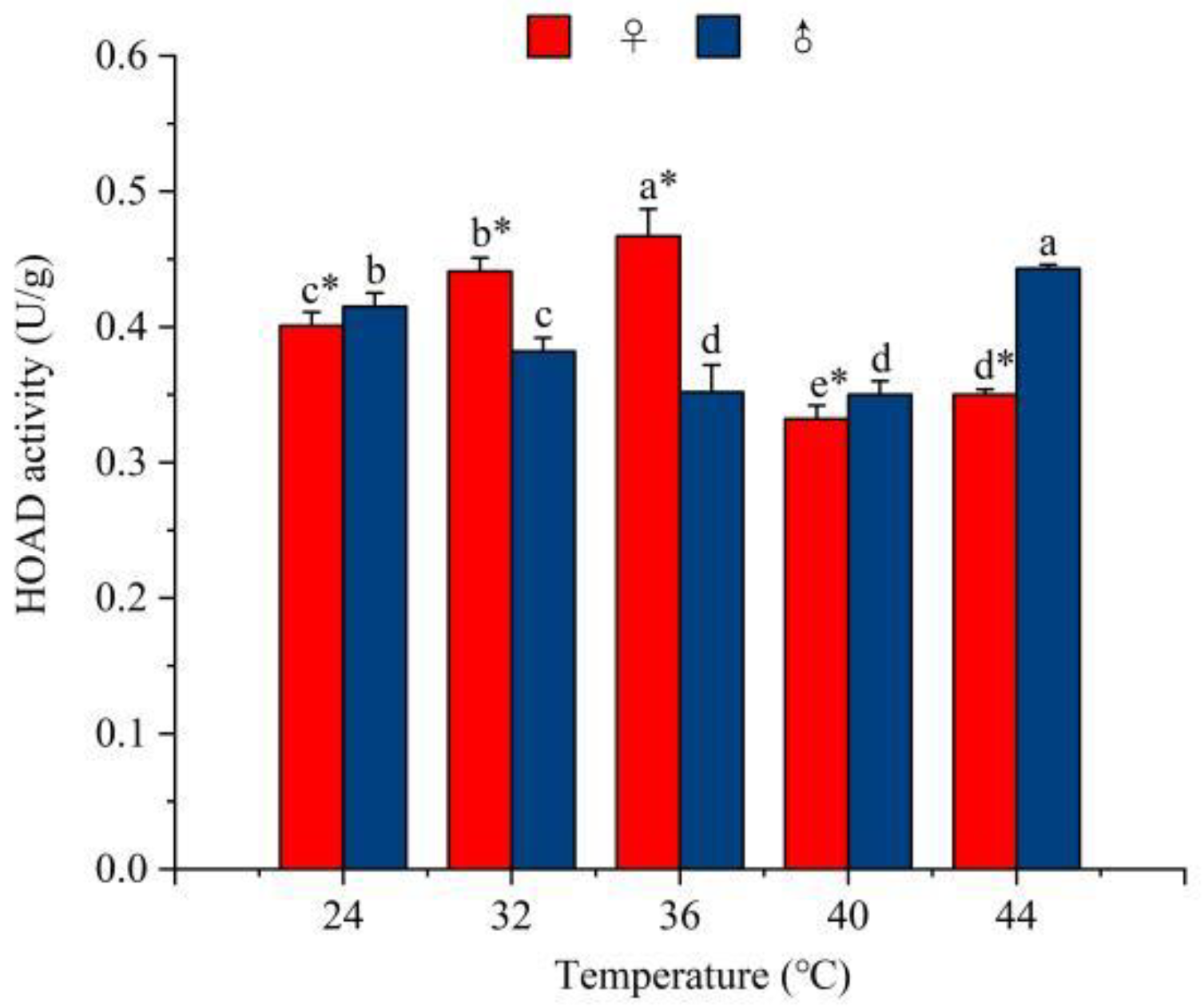
3.4.4. The Effect of Temperature on the Activity of Lipometabolism Enzymes
3.4.5. The Ratio of GAPDH to HOAD Activity in R. pedestris
3.5. The Effect of Temperature on the Activity of Antioxidative Enzymes
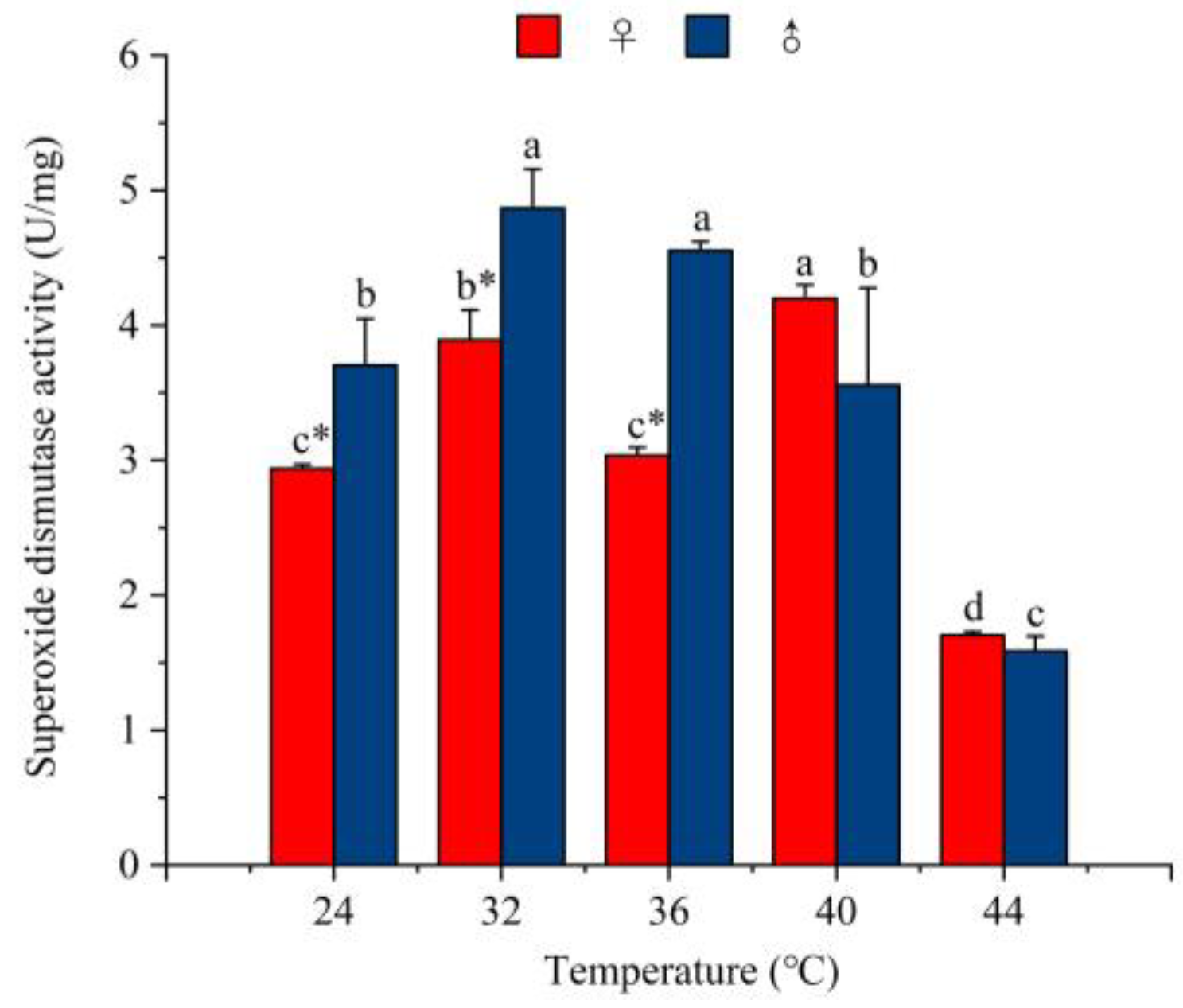
3.5.1. The Effect of Temperature on the Activity of Superoxide Dismutase Enzymes
3.5.2. The Effect of Temperature on the Activity of Peroxidase Enzymes
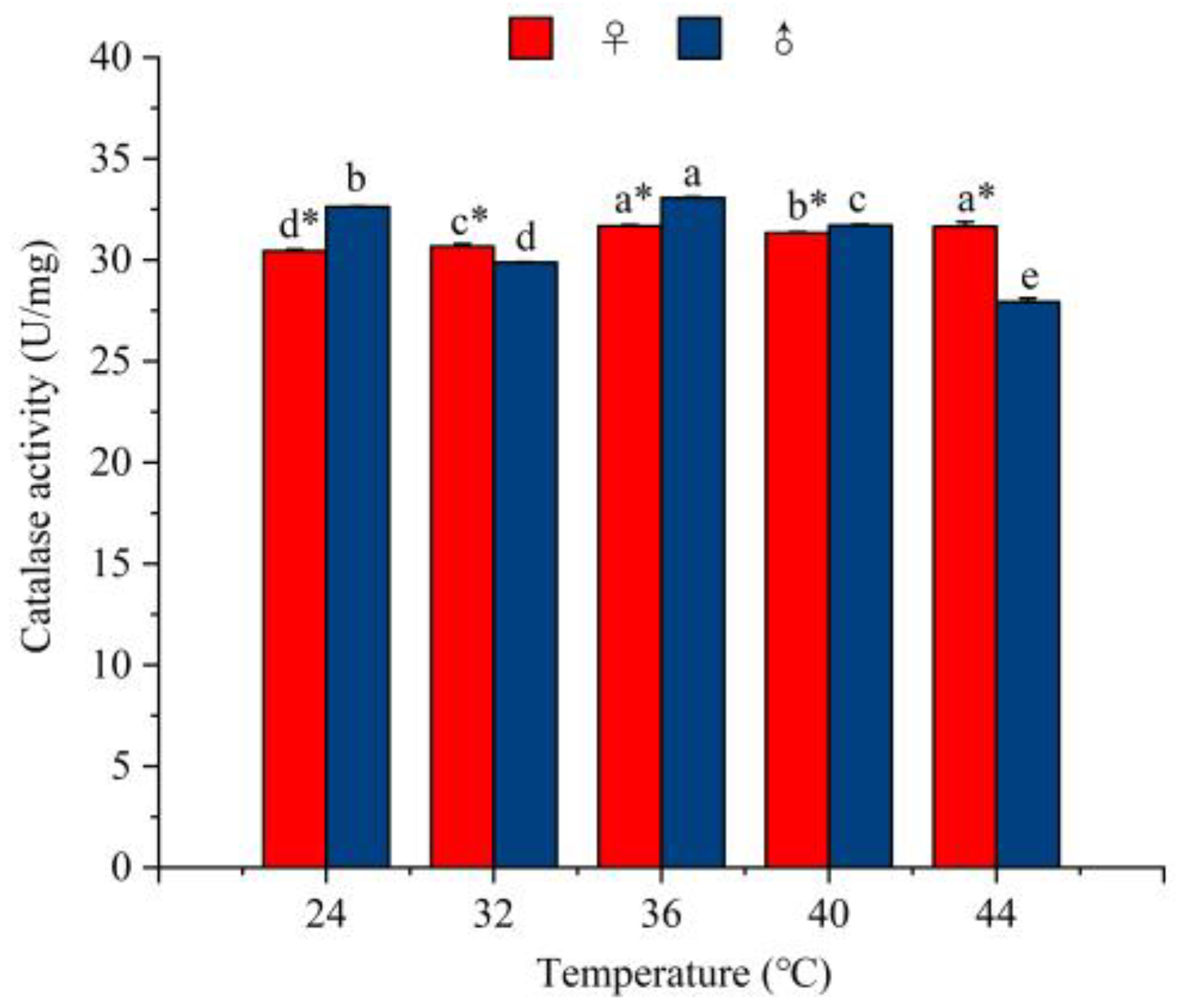
3.5.3. The Effect of Temperature on the Activity of Catalase Enzymes
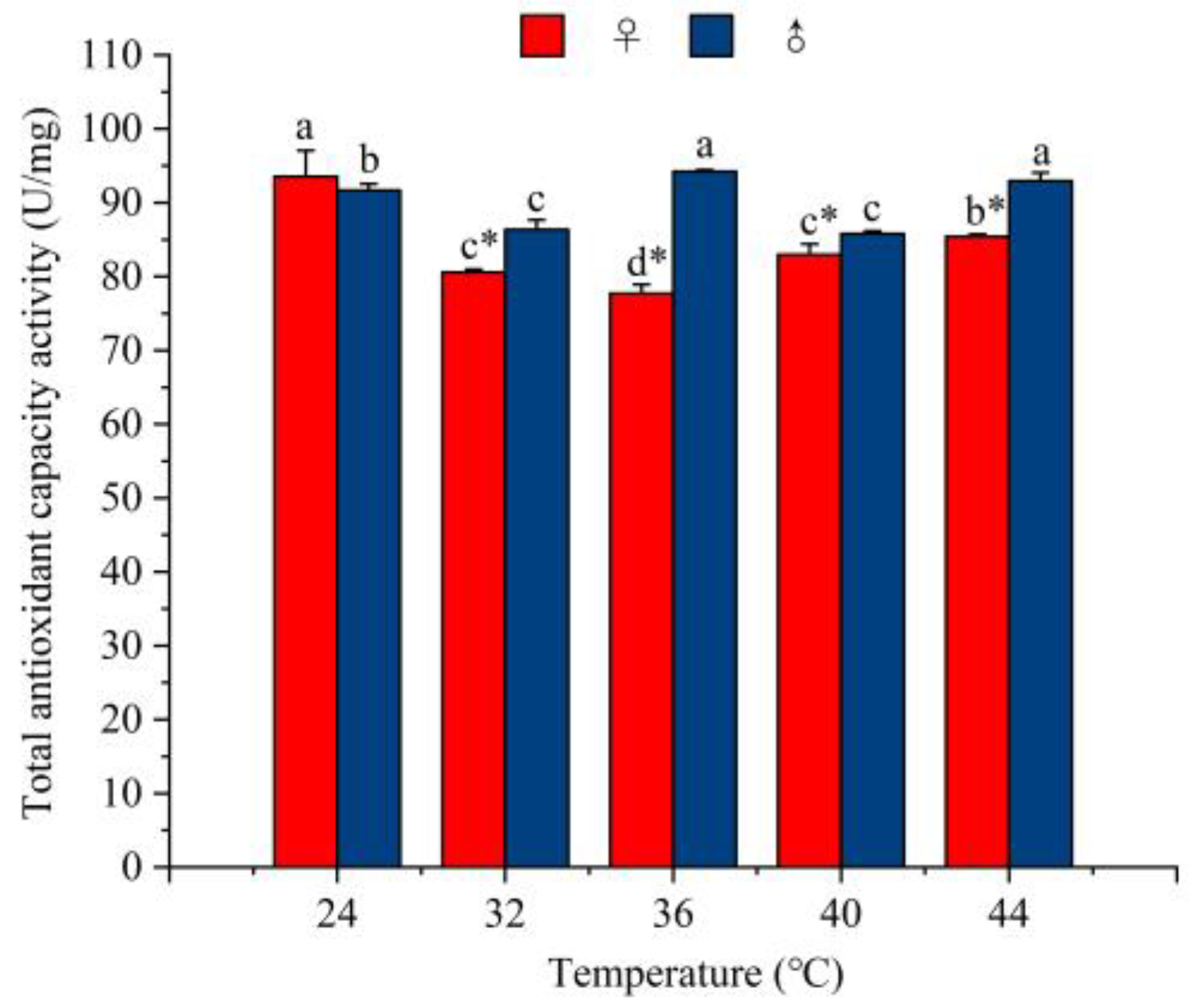
3.5.4. The Effect of Temperature on the Total Antioxidative Enzyme Capacity
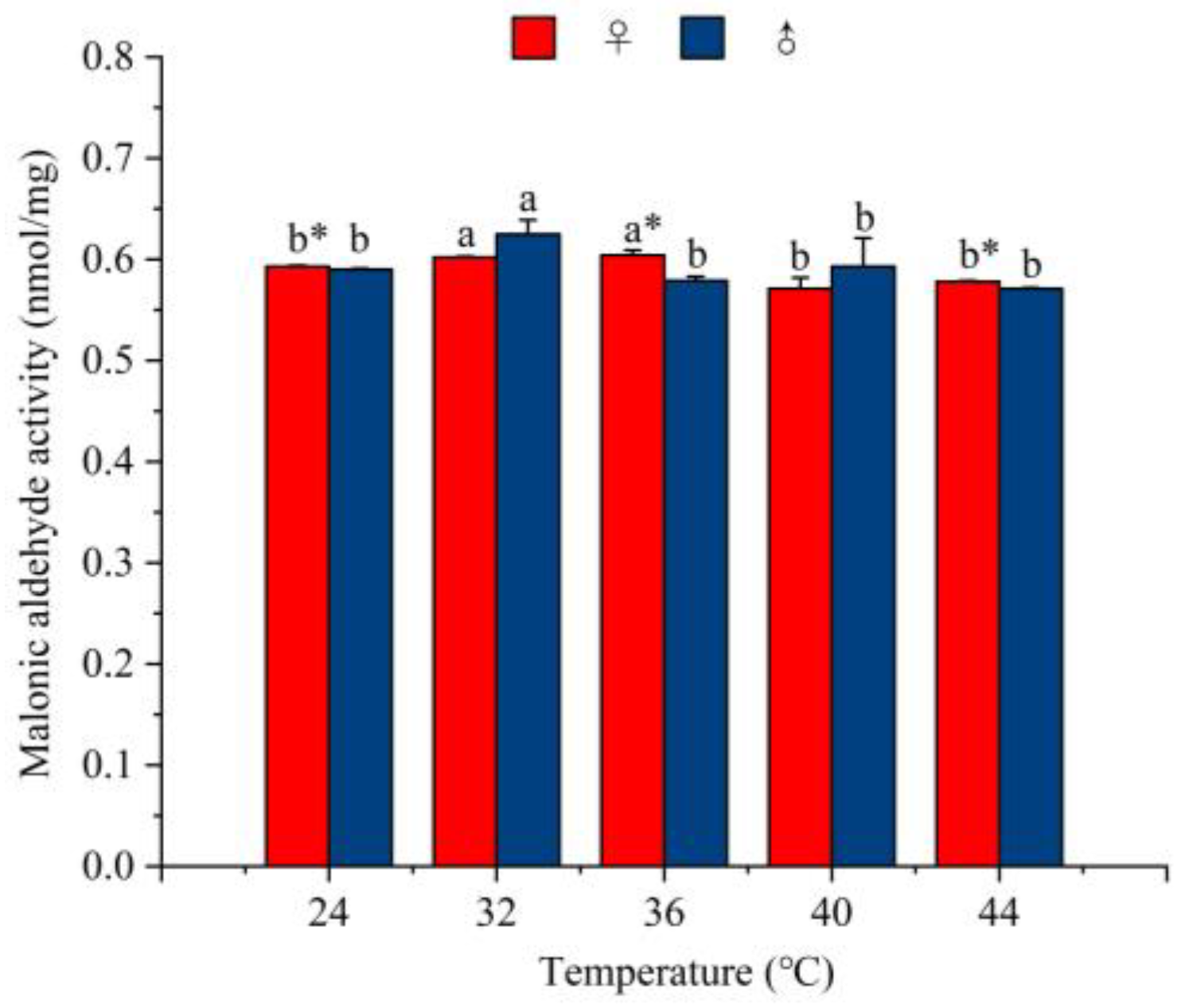
3.5.5. The Effect of Temperature on the Activity of Malondialdehyde Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chloé, L. Recent advances in insect thermoregulation. J. Exp. Biol. 2023, 226, jeb245751. [Google Scholar] [CrossRef]
- Heinrich, E.; Bradley, T. Temperature-dependent variation in gas exchange patterns and spiracular control in Rhodnius prolixus. J. Exp. Biol. 2014, 217, 2752–2760. [Google Scholar] [CrossRef]
- Cairns, S.C. Growth energetics in relation to temperature of the larvae of Rhopaea verreauxi (Coleoptera: Scarabaeidae). Oecologia 1982, 54, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, K.J.; Harrison, J.F. Development of respiratory function in the American locust Schistocerca americana. II. Within-instar effects. J. Exp. Biol. 2004, 207, 509–517. [Google Scholar] [CrossRef]
- Rostgaard, S.; Jacobsen, D. Respiration rate of stream insects measured in situ along a large altitude range. Hydrobiologia 2005, 549, 79–98. [Google Scholar] [CrossRef]
- Vogt, J.; Appel, A. Metabolic costs of spontaneous movement in the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Sociobiology 2000, 35, 89–98. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Ma, C.S. Sporadic short temperature events cannot be neglected in predicting impacts of climate change on small insects. J. Insect Physiol. 2019, 112, 48–56. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, W.; Song, S.; Wang, Z.; Zhu, X.; Gao, S.; He, W.; Xu, J. The impact of high-temperature stress on the growth and development of Tuta absoluta (Meyrick). Insects 2024, 15, 423. [Google Scholar] [CrossRef]
- Cui, X.H.; Wan, F.H.; Xie, M.; Liu, T.X. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J. Insect Sci. 2018, 8, 24. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, R.; Zhou, W.; Pan, Y.; Tian, H.; Yin, Z.; Chen, W. Fruit fly in a challenging environment: Impact of shortterm temperature stress on the survival, development, reproduction, and trehalose metabolism of Bactrocera dorsalis (Diptera: Tephritidae). Insects 2022, 13, 753. [Google Scholar] [CrossRef]
- Chen, H.; Solangi, G.S.; Guo, J.; Wan, F.H.; Zhou, Z.S. Antioxidant responses of ragweed leaf beetle Ophraella communa (Coleoptera: Chrysomelidae) exposed to thermal stress. Front. Physiol. 2018, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Chen, X.; Wang, K.; Chen, J.; Zhou, J.; Yi, W.; Lyu, M.; Ye, Z.; Bu, W. Shared phylogeographic patterns and environmental responses of co-distributed soybean pests: Insights from comparative phylogeographic studies of Riptortus pedestris and Riptortus linearis in the subtropics of East Asia. Mol. Phylogenet. Evol. 2024, 195, 108055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Wang, Z.; Ding, W.; Xu, K.; Li, L.; Wang, Y.; Li, J.; Yang, M.; Liu, X.; et al. Modelling the current and future potential distribution of the bean bug Riptortus pedestris with increasingly serious damage to soybean. Pest Manag. Sci. 2022, 78, 4340–4352. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, L.; Hu, Y.L.; Tian, X.Y.; Wang, Y.Y.; Wang, Z.J.; Zhao, Y.J.; Li, J.B.; Shi, S.S. Laboratory evaluation of leguminous plants for the development and reproduction of the bean bug Riptortus pedestris (Hemiptera: Alydidae). Entomol. Sci. 2022, 25, e12525. [Google Scholar] [CrossRef]
- Li, K.; Zhang, X.X.; Guo, J.Q.; Penn, H.; Wu, T.T.; Li, L.; Jiang, H.; Chang, L.D.; Wu, C.X.; Han, T.F. Feeding of Riptortus pedestris on soybean plants, the primary cause of soybean staygreen syndrome in the Huang-Huai-Hai river basin. Crop. J. 2019, 7, 360–367. [Google Scholar] [CrossRef]
- Ji, P.; Yuan, X.; Ma, F.; Xu, Q.B. Drivers of long-term changes in summer compound hot extremes in China: Climate change, urbanization, and vegetation greening. Atmos. Res. 2024, 310, 107632. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Hu, Y.; Chen, J.; Zhang, J.; Shi, S. Field cage assessment of feeding damage by Riptortus pedestris on soybeans in China. Insects 2021, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.Q.; Huang, Y.; Guo, C.Y.; Hu, B.; Zhang, H.H.; Li, Y.J.; Chen, J.P.; Wei, Z.Y.; Sun, Z.T. A salivary secretory protein from Riptortus pedestris facilitates pest infestation and soybean staygreen syndrome. Mol. Plant Pathol. 2023, 24, 560–569. [Google Scholar] [CrossRef]
- Tian, X.Y.; Gao, Y.; Ali, M.Y.; Li, X.H.; Hu, Y.L.; Li, W.B.; Wang, Z.J.; Shi, S.S.; Zhang, J.P. Impact of temperature on age-stage, two-sex life table analysis of a Chinese population of bean bug, Riptortus pedestris (Hemiptera: Alydidae). Agriculture 2022, 12, 1505. [Google Scholar] [CrossRef]
- Ahn, J.; Choi, K. Effects of temperature on the development, fecundity, and life table parameters of Riptortus pedestris (Hemiptera: Alydidae). Appl. Entomol. Zool. 2019, 54, 63–74. [Google Scholar] [CrossRef]
- Ahn, J.; Choi, K. Population parameters and growth of Riptortus pedestris (Fabricius) (Hemiptera: Alydidae) under fluctuating temperature. Insects 2022, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Ding, D.; Ma, C.; Guo, W.; Kang, L. Locust density shapes energy metabolism and oxidative stress resulting in divergence of flight traits. Proc. Natl. Acad. Sci. USA 2022, 119, e2115753118. [Google Scholar] [CrossRef] [PubMed]
- Lighton, J.R.B.; Halsey, L.G. Flow-through respirometry applied to chamber systems: Pros and cons, hints and tips. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2011, 158, 265–275. [Google Scholar] [CrossRef]
- Kibar, H. CO2 respiration rates of einkorn wheat at different temperature and moisture contents. Turk. J. Agric. For. 2021, 45, 179–190. [Google Scholar] [CrossRef]
- Colinet, H.; Vernon, P.; Hance, T. Does thermal-related plasticity in size and fat reserves influence supercooling abilities and cold-tolerance in Aphidius colemani (Hymenoptera: Aphidiinae) mummies? J. Therm. Biol. 2007, 32, 374–382. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, T.; Yu, H.; Xu, J.; Zhang, J.Z.; Fu, D.Y.; Ye, H. The transcription of flight energy metabolism enzymes declined with aging while enzyme activity increased in the long-distance migratory moth, Spodoptera frugiperda. Insects 2022, 13, 936. [Google Scholar] [CrossRef]
- Gunn, A.; Gatehouse, A.G. The development of enzymes involved in flight muscle metabolism in Spodoptera exempta and Mythimna separata. Comp. Biochem. Physiol. 1988, 91, 315–324. [Google Scholar] [CrossRef]
- Zebe, E.C.; McShan, W.H. Lactic and alpha-glycerophosphate dehydrogenases in insects. J. Gen. Physiol. 1957, 40, 779–790. [Google Scholar] [CrossRef]
- O’Brien, D.M.; Suarez, R.K. Fuel use in hawkmoth (Amphion floridensis) flight muscle: Enzyme activities and flux rates. J. Exp. Zool. 2001, 290, 108–114. [Google Scholar] [CrossRef]
- Schimpf, N.G.; Matthews, P.G.D.; White, C.R. Cockroaches that exchange respiratory gases discontinuously survive food and water restriction. Evolution 2012, 66, 597–604. [Google Scholar] [CrossRef]
- Stavrianakis, G.; Sentas, E.; Zafeirelli, S.; Tscheulin, T.; Kizos, T. Utilizing Olive Fly Ecology Towards Sustainable Pest Management. Biology 2025, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Beenakkers, A.M. Carbohydrate and fat as a fuel for insect flight: A comparative study. J. Insect Physiol. 1969, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, J.F. Some errors in respirometry of aquatic breathers: How to avoid and correct for them. Fish Physiol. Biochem. 1989, 6, 49–59. [Google Scholar] [CrossRef]
- Malte, C.L.; Nørgaard, S.; Wang, T. Closed system respirometry may underestimate tissue gas exchange and bias the respiratory exchange ratio (RER). Comp. Biochem. Physiol. A 2016, 192, 17–27. [Google Scholar] [CrossRef]
- Lighton, J.R.B.; Ottesen, E.A. To DGC or not to DGC: Oxygen guarding in the termite Zootermopsis nevadensis (Isoptera: Termopsidae). J. Exp. Biol. 2005, 208, 4671–4678. [Google Scholar] [CrossRef] [PubMed]
- Slăma, K. A new look at discontinuous respiration in pupae of Hyalophora cecropia (Lepidoptera: Saturniidae): Haemocoelic pressure, extracardiac pulsations and O2 consumption. Eur. J. Endocrinol. 2010, 107, 487–507. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Liu, Z.; Torson, A.S. Energy consumption and cold hardiness of diapausing fall webworm pupae. Insects 2022, 13, 853. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Insect cold hardiness: Metabolic, gene, and protein adaptation. Can. J. Zool. 2012, 90, 456–475. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Metabolic rate depression in animals: Transcriptional and translational controls. Biol. Rev. Camb. Philos. Soc. 2007, 79, 207–233. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, Q.; Bilal, M.; Wu, Y.; Gong, Z.; Wu, R.; Miao, J. Comparative transcriptome analysis reveals different responses in three developmental stages of Mythimna loreyi to cold stress. Insects 2024, 15, 554. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Yang, W.; Wu, Q.; Xiao, W.; Zhu, Y.; Wei, Q.; Dong, Z.; Zhang, G.; Lu, C.; et al. The Bombyx mori singed gene is involved in the high-temperature resistance of silkworms. Insects 2024, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J.; Amanda, J.B.; Tom, T.; Joseph, L.T.; David, J.H. Metabolic rate does not decrease with starvation in Gryllus bimaculatus when changing fuel use is taken into account. Physiol. Entomol. 2011, 36, 84–89. [Google Scholar] [CrossRef]
- Wilmsen, S.M.; Dzialowski, E. Substrate use and temperature effects in flight muscle mitochondria from an endothermic insect, the hawkmoth Manduca sexta. Comp. Biochem. Phys. A 2023, 281, 111439. [Google Scholar] [CrossRef]
- Kim, K.N.; Sin, U.C.; Yun, C.N.; Song, H.S.; Huang, Z.J.; Huang, Q.Y.; Lei, C.L. Influence of green light illumination on several enzymes involved in energy metabolism in the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2019, 22, 487–492. [Google Scholar] [CrossRef]
- Zera, A.J.; Sall, J.; Otto, K. Biochemical aspects of flight and flightlessness in Gryllus: Flight fuels, enzyme activities and electrophoretic profiles of flight muscles from flight-capable and flightless morphs. J. Insect Physiol. 1999, 45, 275–285. [Google Scholar] [CrossRef]
- Madern, D. Molecular evolution within the L-malate and L-Lactate dehydrogenase super-family. J. Mol. Evol. 2002, 54, 825–840. [Google Scholar] [CrossRef]
- Yuan, F.; DiClemente, D. Hybrid voltage-controlled oscillator with low phase noise and large frequency tuning range. Analog. Integr. Circ. Signal Process. 2015, 82, 471–478. [Google Scholar] [CrossRef]
- Basson, C.H.; Terblanche, J.S. Metabolic responses of Glossina pallidipes (Diptera Glossinidae) puparia exposed to oxygen and temperature variation implications for population dynamics and subterranean life. J. Insect Physiol. 2010, 56, 1789–1797. [Google Scholar] [CrossRef]
- Geister, T.L.; Lorenz, M.W.; Meyering-Vos, M.; Hoffmann, K.H.; Fischer, K. Effects of temperature on reproductive output, egg provisioning, juvenile hormone and vitellogenin titres in the butterfly Bicyclus anynana. J. Insect Physiol. 2008, 54, 1253–1260. [Google Scholar] [CrossRef]
- Lu, Y.; Bai, Q.; Zheng, X. Expression and enzyme activity of catalase in Chilo suppressalis (Lepidoptera: Crambidae) is responsive to environmental stresses. J. Econ. Entomol. 2017, 110, 1803–1812. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, W.; Li, N.; Zhang, F.; Liu, T.X. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chang, X.Q.; Hoffmann, A.; Zhang, S.; Ma, C.S. Impact of hot events at different developmental stages of a moth: The closer to adult stage, the less reproductive output. Sci. Rep. 2015, 5, 10436. [Google Scholar] [CrossRef]
- Lighton, J.R.B.; Joos, B. Discontinuous gas exchange in a tracheate arthropod, the pseudoscorpion Garypus californicus: Occurrence, characteristics and temperature dependence. J. Insect Sci. 2002, 2, 23. [Google Scholar] [CrossRef][Green Version]
- Duncan, F.D.; Byrne, M.J. Discontinuous gas exchange in dung beetles: Patterns and ecological implications. Oecologia 2000, 122, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Medison, R.G.; Zhang, S.; Ma, P.; Shi, C. Impacts of non-lethal high-temperature stress on the development and reproductive organs of Bradysia odoriphaga. Insects 2022, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Heinen, R.; Gols, R.; Thakur, M.P. Climate change-mediated temperature extremes and insects: From outbreaks to breakdowns. Glob. Change Biol. 2020, 26, 6685–6701. [Google Scholar] [CrossRef]
- Zeng, B.; Lian, Y.; Jia, J.; Liu, Y.; Wang, A.; Yang, H.; Li, J.; Yang, S.; Peng, S.; Zhou, S. Multigenerational effects of short-term high temperature on the development and reproduction of the Zeugodacus cucurbitae (Coquillett, 1899). Agriculture 2022, 12, 954. [Google Scholar] [CrossRef]
- Lighton, J.R.B.; Fielden, L.J. Gas exchange in wind spiders (Arachnida, Solphugidae): Independent evolution of convergent control strategies in solphugids and insects. J. Insect Physiol. 1996, 42, 347–357. [Google Scholar] [CrossRef]
- Matthews, P.G.D.; White, C.R. Discontinuous gas exchange, water loss, and metabolism in Protaetia cretica (Cetoniinae, Scarabaeidae). Physiol. Biochem. Zool. 2012, 85, 174–182. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Zhao, J.; Sheng, Y.; Xiao, Y.F.; Zhang, Y.J.; Bai, L.X.; Tan, Y.; Xiao, L.B.; Xu, G.C. Identification of heat shock cognate protein 70 gene (Alhsc70) of Apolygus lucorum and its expression in response to different temperature and pesticide stresses. Insect Sci. 2016, 23, 37–49. [Google Scholar] [CrossRef]
- Gruntenko, N.E.; Bownes, M.; Terashima, J.; Sukhanova, M.Z.; Raushenbach, I.Y. Heat stress affects oogenesis differently in wild-type Drosophila virilis and a mutant with altered juvenile hormone and 20-hydroxyecdysone levels. Insect Mol. Biol. 2003, 12, 393–404. [Google Scholar] [CrossRef] [PubMed]



| Sex | Temperature (°C) | ||||
|---|---|---|---|---|---|
| 24 | 32 | 36 | 40 | 44 | |
| ♀ | 3.01 | 3.51 | 2.50 | 1.77 | 1.08 |
| ♂ | 3.08 | 3.37 | 2.77 | 2.06 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Niu, Y.; Cui, X.; Cui, Y.; Chen, S.; Shi, S.; Gao, Y. Temperature Modulates Nutrient Metabolism and Antioxidative Fluctuations in Riptortus pedestris. Agronomy 2025, 15, 1300. https://doi.org/10.3390/agronomy15061300
Li X, Niu Y, Cui X, Cui Y, Chen S, Shi S, Gao Y. Temperature Modulates Nutrient Metabolism and Antioxidative Fluctuations in Riptortus pedestris. Agronomy. 2025; 15(6):1300. https://doi.org/10.3390/agronomy15061300
Chicago/Turabian StyleLi, Xiaofeng, Yulong Niu, Xin Cui, Yue Cui, Simeng Chen, Shusen Shi, and Yu Gao. 2025. "Temperature Modulates Nutrient Metabolism and Antioxidative Fluctuations in Riptortus pedestris" Agronomy 15, no. 6: 1300. https://doi.org/10.3390/agronomy15061300
APA StyleLi, X., Niu, Y., Cui, X., Cui, Y., Chen, S., Shi, S., & Gao, Y. (2025). Temperature Modulates Nutrient Metabolism and Antioxidative Fluctuations in Riptortus pedestris. Agronomy, 15(6), 1300. https://doi.org/10.3390/agronomy15061300






