Abstract
Soil amendments such as limestone and gypsum can influence microbial carbon use efficiency (CUE) by altering nutrient stoichiometry, particularly nitrogen (N) and phosphorus (P). However, their effects beyond the topsoil, especially under no-till systems, remain unclear. This study assessed microbial CUE through substrate use efficiency (SUE) following glucose addition as a factor influencing carbon (C) sequestration potential. Two experiments were conducted in tropical soil. The first evaluated the addition of 14C-glucose (G) to soil treated with lime, lime + gypsum, and a control, with or without the addition of N. The second compared limestone + gypsum and control treatments, incorporating G with N and P. Soil microbial respiration (CO2 emission) was measured after 14 and 42 days. In the surface soil (0–10 cm), CUE increased with limestone or limestone + gypsum when N was applied. In the subsoil (40–60 cm), these amendments enhanced CUE compared to untreated soil in the absence of N. Treatments with G+N+P or G+P improved CUE in the surface soil. At the same time, G+N+P increased CUE in the subsoil regardless of acidity alleviation. Differences in 14CO2 evolution indicated higher microbial CUE with acidity correction. Balanced N and P applications significantly enhanced CUE, highlighting the importance of both soil acidity correction and nutrient availability for microbial carbon processing.
1. Introduction
Soil management alters the size, structure, and activity of the soil microbial community and consequently affects biogeochemical cycling by changing the quantity and quality of plant residues entering the soil system [1]. The application of lime modifies microbial dynamics, diversity, and enzymatic activity by increasing the pH and inducing a priming effect on soil CO2 emissions [2,3,4]. Furthermore, nitrogen (N) fertilization, combined with lime and gypsum application, benefits the N and carbon (C) cycles, reduces greenhouse gas emissions, and increases soil N and C stocks in tropical weathered soils [5].
As C dynamics in the soil profile are modified, the processes regulating surface and subsurface C storage may differ [6,7]. Additionally, the microbial population may vary with soil depth due to differences in pH, substrate quality (often recalcitrant and less biodegradable), and oxygen availability. Soil pH [5] and oxygen concentration are shown to decrease with soil depth. Therefore, specialized microbial populations transform substrates added to both the arable layer and subsoil, but at different rates [6,8,9]. While lime and gypsum ameliorate soil acidity throughout the profile, their effect on C cycling via microbial biomass may be minimal, particularly in the subsoil [10]. It has been reported that greater microbial C use efficiency (CUE) may occur in subsoil microbial communities due to persistent carbon limitation [11,12]. However, the role of other nutrients on microbial CUE throughout the soil profile remains poorly understood. Microbial metabolism has been extensively studied using isotopic techniques to quantify the behaviour and fate of 13C and 14C-labeled substrates applied to soils and measure the amount of C released via microbial respiration. Of the C taken up from soil, part is incorporated in the microbial biomass for growth in anabolic processes (cell growth and maintenance), while the remainder portion is mineralized to CO2 tnrough catabolic processes associated withcell maintenance (respiration) [13]. Factors controlling the transformation of C by microbial biomasses into soil organic carbon (SOC) through immobilization [14], along with the availability of inorganic nutrients (e.g., N, P, S), are considered important regulators of C storage rates [15]. These factors are recognized as processes associated with efficient CUE by soil microbial CUE [16].
Nutrient addition changes soil C dynamics because, in the absence of other limiting factors (e.g., pH, water, oxygen availability), microbial growth can increase efficiently when nutrients are no longer limiting it [11,17,18]. Increases in soil C following nutrient addition have been attributed to enhancing net primary productivity, which induces greater rhizodeposition and an increased production of above-ground plant residues [19,20]. Cover crop species can also shape microbial community structures and networks, eventually improving soil quality and health in the arable layer by altering base saturation and nutrient availability, such as N and P, in the soil [21]. However, the microbial requirements and relative efficiency of microorganisms are often overlooked, even though microorganisms may respond to nutrient addition, especially at different soil depths, due to heterogeneity in the microbial community composition.
Soil microbes secrete enzymes to degrade complex plant and soil organic matter into smaller compounds, which are subsequently assimilated for growth and metabolism [22]. Changes in pH can alter soil enzymes through reversible ionization and deionization of functional groups at the enzyme active sites or through irreversible enzyme inactivation [23]. However, optimal pH can vary between enzymes and soils and is influenced by the origin of the enzymes and their degree of stabilization on solid surfaces [24]. As a result, soil pH serves as a key regulator of microbial processes impacting C accumulation. Raising soil pH beyond a certain threshold (~6.2) leads to C loss by stimulating microbial activity and decomposition. In contrast, in near-neutral pH soils, C loss under intensification is driven not by increased decomposition but rather to a decline in microbial biomass and growth efficiency, likely due to trade-offs between stress alleviation and resource acquisition [25]. Lime-induced changes in soil pH can affect both CUE and microbial activity; however, these effects depend on the initial pH and the extent of pH change [26].
Some authors suggest that, under nutrient-limited conditions, microorganisms decompose native soil organic matter (SOM) to meet their nutrient demand. Following nutrient addition, this “mining” behavior diminishes, potentially increasing SOC stocks [27,28]. Therefore, we hypothesize the following: (i) The application of gypsum and lime to acidic soils creates favorable conditions for soil microorganisms to transform the C added to the soil and increase SOC; (ii) Carbon not used for growth due to nutrient limitation is redirected to overflow respiration; thus, nutrient addition should enhance the amount of C immobilized in the microbial biomass; and (iii) consequently, CUE would be increased in amended soils. This study aimed to evaluate microbial CUE by assessing CO2 evolution from microbial respiration in the arable layer and subsoil as affected by acidity alleviation, with or without N and P addition.
2. Materials and Methods
2.1. Soil
Soil samples were collected from a non-tilled medium-term field experiment—the cash crops were planted over the cover crop desiccated residues, with soil preparation—in Botucatu, SP, Brazil (782 m asl; 22°49′50.70″ S; 48°25′38.79″ W). The soil was a well-structured Typic Hapludox [29], with 343 g kg−1 (34.3%) of clay, 109 (10.9%) g kg−1 of silt, and 547 g kg−1 (54.7%) of sand. The climate is tropical with dry winters and warm, rainy summers. The annual average temperature is 20.7 °C and the annual average rainfall is 1568 mm. The experiment had soybeans (Glycine max L.) grown in the spring/summer, double-cropped system with maize (Zea mays L.) and intercropped with Guinea grass (Megathyrsus maximus (Jacq.) B.K. Simon & S.W.L. Jacobs cv. Tanzania) during the fall/winter season for three consecutive years. Lime and gypsum were applied at 2.9 and 2.1 t ha−1 before the first soybean crop and again the next year. Nitrogen as ammonium sulphate was applied annually to maize intercropped with Guinea grass at either 0 or 240 kg ha−1. Before the experiment, the area was fallow with spontaneous vegetation, mainly grasses. Soil samples were collected randomly down to a 60cm soil depth to determine soil chemical attributes [30] (Table 1). The soil samples for the 14C experiment were collected at two depths, 0–10 cm and 40–60 cm. The field experimental design was a 3 × 2 factorial in complete randomized blocks with four replicates, where the treatments were limestone, limestone + gypsum, and control without acidity alleviation, combined with zero and 240 kg ha−1 of N.

Table 1.
Chemical characterization of soil samples taken at different depths down the soil profile prior to starting the laboratory experiments.
2.2. Experiment 1—Microbial C Use Efficiency
To evaluate the dynamics by which the topsoil (0–10 cm) and subsoil (40–60 cm) microbial community metabolized C, 14C-uniformly labelled D-glucose was used as a substrate. This was chosen because it represents a very common C source incorporated into acidic soils within plant residues (e.g., as a polymer within cell walls and as a free sugar in the cytosol and vacuole) and is classified as a readily used C source by almost all soil microbes [31].
The water content of the soil samples was measured gravimetrically (24 h at 105 °C) for subsequent experimentation. Soil samples (5 g) were placed in 50 mL sterile polypropylene tubes, pre-incubated, rewetted to reach 65% water holding capacity, and kept at 25 °C for 72 h to restore microbial activity [32]. After incubation, 0.25 mL of a 10 mM solution containing 14C-labeled D-glucose with a specific activity of 1.8 kBq mL−1 (Sigma Aldrich Company Ltd., St. Louis, MO, USA) was added to each sample and spread homogeneously over the soil. This concentration was chosen to reflect typical concentrations that exist in the soil when plant cell lysis occurs [33]. A polypropylene vial containing 1 mL of 1.0 M NaOH was placed above the soil immediately after the addition of the labelled D-glucose to capture the evolved 14CO2. The tubes were then sealed and incubated at 23 °C, and the CO2 traps containing NaOH were changed after 1, 3, 6, 9, and 24 h and then 2, 3, 4, 5, 6, 7, 8, and 14 days after the addition of 14C. Immediately after changing the NaOH traps, the NaOH solution containing 14CO2 released from the soil was mixed with 4 mL of scintillation solution (Optiphase HiSafe 3®, Perkin Elmer Inc., Waltham, MA, USA), and the amount of 14CO2 was determined using a Wallac liquid scintillation counter (Wallac EG&G, Milton Keynes, UK) with automated quench correction.
To determine how much 14C-glucose remained in the soil at the end of the 14-day incubation period, the 14C-glucose adsorbed to the solid phase or in the soil solution was extracted with 0.5 M K2SO4 at 1:5 w/v [13,34]. After extraction, 1 mL of the supernatant was mixed with 4 mL of scintillation solution as described previously, and 14C was determined using a liquid scintillation counter.
The microbial immobilization of 14C-glucose (14Cim) at the end of the incubation period was estimated using the following equations:
where 14Ctot is the total 14C-glucose added to the soil at time zero (t) = 0, 14CK2SO4 is the 14C recovered in the 0.5 M K2SO4 extract, and 14CO2 is the 14C recovered as 14CO2.
14Cim = 14Ctot − 14CK2SO4 − 14CO2
Microbial substrate carbon use efficiency (CUE) is defined as the amount of C in the microbial biomass relative to the C in microbial biomass plus the C respired as CO2. CUE was determined through substrate use efficacy (SUE).
CUE = Cim/(Cim + 14CO2)
2.3. Experiment 2—Stoichiometric Control of C Respiration
In this study, only samples from the field treatments with lime + gypsum and the control (without acidity correction) without N were used. Soil samples (5 g) were placed in sterile 50 mL polypropylene tubes and moistened to 65% of their water retention capacity. The tubes were capped and pre-incubated in the dark at 22 °C for 72 h [34]. After pre-incubation, as in the first experiment, 0.25 mL of a 10 mM solution containing 14C-labeled D-glucose with a specific activity of 1.8 kBq mL−1 (Sigma Aldrich Company Ltd., St. Lois, MO, USA) was added. N and P were added to the soil at a ratio of 75:8.75:1 (C:N:P) based on published values for soil microbial communities [17,35,36,37,38]. Based on the amount of 14C-glucose, N and P rates were calculated to obtain a ratio of 75:8 N and 75:1 P, then added into the solution, as NH4NO3 and KH2PO4. After adding the glucose + nutrient solutions, 1 mL of 1 M NaOH was placed into the vials and placed in the tubes, which were capped to capture the respired 14CO2, as previously described in experiment 1. The NaOH traps to capture 14CO2 respired from the soil were changed after 1, 6, and 24 h, and then weekly for 6 weeks (42 days in total). In each sample, the NaOH trap was replaced, 4 mL of Optiphase ‘HiSafe’ 3 scintillation fluid (Perkin Elmer, Waltham, MA, USA) was added to the NaOH traps, and 14C activity was determined by liquid scintillation counting (LSC; Tri-Carb 3110 TR scintillation counter, Perkin Elmer Inc.). At the end of the incubation period (42 days), the amount of 14C-glucose adsorbed in the solid phase, or the soil solution, was extracted with a 0.5 M K2SO4 solution at 1:5 (w/v) [34]. Again, the cumulative amount of 14CO2 in the NaOH traps was calculated as described in the first experiment, the 14C-glucose (14Cim) was estimated through Equation (1), and the CUE was calculated using Equation (2).
2.4. Statistics
The data obtained were subjected to homogeneity of variance and normality tests (Levene and Shapiro–Wilk, respectively). Once the assumptions were met, ANOVA was performed considering a 3 × 2 factorial in the first experiment and a 2 × 2 factorial in the second experiment, both with four replications. When the F-test was significant, means were compared using the modified Fisher’s protected least significant difference (LSD, p < 0.05) using SAS software, version 9.2.
3. Results
In the surface layer, from 0 to 10 cm, the alleviation of soil acidity led to higher CUE only when residual N was present (Figure 1A), regardless of the association with gypsum. However, in the subsoil layer (40–60 cm), limestone increased CUE compared to the control without residual N, regardless of its association with gypsum. When comparing soils with and without residual N, higher CUE was observed in the subsoil in the control (without soil pH correction) and with limestone + gypsum in soil with residual N, which was not observed when limestone was applied alone (Figure 1B).
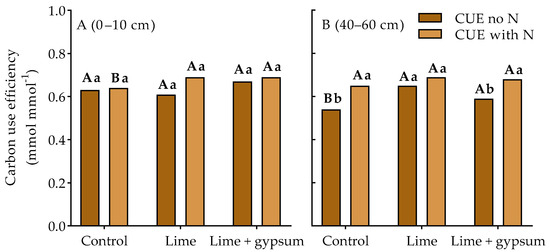
Figure 1.
Effect of soil acidity alleviation–lime (2.9 t ha−1), gypsum (2.1 t ha−1)–on microbial C use efficiency (CUE) in response to the addition of a glucose-C substrate. Mean percentage of initial C after 14 days for each treatment in the topsoil (Panel (A), 0–10 cm) and subsoil (Panel (B), 40–60 cm)) Values represent means (n = 4). Lowercase letters compare soil with (240 kg ha−1) and without (0 kg ha−1) residual N in the cropping system for each soil amendment, and capital letters compare soil amendments for N application in the cropping system (p < 0.05, LSD test).
It was observed that soil acidity alleviation with lime and lime plus gypsum did not affect the evolution of 14CO2 from the soil without residual N in the surface layer 0–10 cm (Figure 2A). However, in the presence of residual N, the cumulative 14CO2 evolution was higher in the control and limestone treatments. In the subsoil (40–60 cm), no difference was observed in the 14CO2 accumulated evolution with N (Figure 2B). In contrast, under acidic conditions without N, it was greater. Furthermore, still in the subsoil, the highest evolution of 14CO2 was observed in the control (without acidity correction) when the soil had no residual N (Figure 2B). Similarly, when soil acidity was corrected with limestone + gypsum, the soil with residual N showed lower 14CO2 evolution than the soil without N.
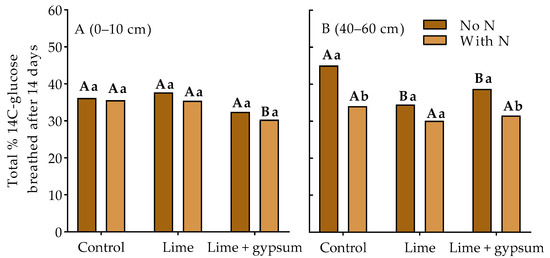
Figure 2.
Effect of soil acidity alleviation–lime (2.9 t ha−1), gypsum (2.1 t ha−1) and N addition on cumulative 14CO2 release in microbial respiration after incubating the soil microbial community with 14C-glucose for 14 d, in the topsoil (Panel (A), 0–10 cm) and subsoil (Panel (B), 40–60 cm)). Values represent means (n = 4). Lowercase letters compare soil with (240 kg ha−1) and without (0 kg ha−1) residual N in the cropping system for each soil amendment, and capital letters compare soil amendments in each N rate (p < 0.05, LSD test).
Evaluating the addition of nutrients with glucose in the surface layer (Figure 3A), we observed that applying G+N+P and G+P resulted in higher CUE compared with adding only G to the soil. However, it was no different from applying G+N (Figure 3A). In the subsoil (Figure 3B), the treatment without added nutrients resulted in the lowest CUE, regardless of soil acidity alleviation. Similar to the surface layer, the addition of G+N+P resulted in higher CUE in the subsoil, regardless of acidity alleviation, as did the addition of G+N. This suggests that, in the subsoil, N is a limiting factor for substrate transformation. Furthermore, when G+N was added, a higher CUE was observed in the amended soil compared with the acidic soil. The addition of P separately was not efficient in increasing CUE, regardless of soil acidity correction, and CUE was lower than with the addition of G+N and G+N+P.
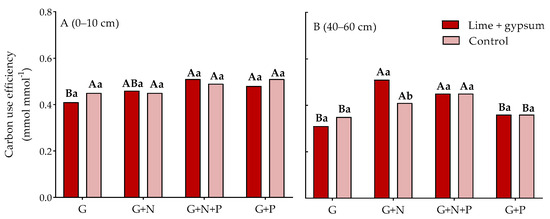
Figure 3.
Microbial carbon use efficiency (CUE) in response to glucose addition (10 mM 14C-labeled D-Glucose) in the topsoil (Panel (A), 0–10 cm) and subsoil (Panel (B), 40–60 cm) either in the presence or absence of N and P and with and without lime and gypsum application. Values represent means (n = 4). G+N ratio of 78:1; G+P ratio of 75:1; G+N+P ratio of 75:8.75:1; lime (2.9 t ha−1); gypsum (2.1 t ha−1); control (0 t ha−1 of lime and gypsum). Capital letters compare the addition of glucose (G), nitrogen (N), and phosphorus (P) for each soil improver, and lowercase letters compare the soil improvers for each nutrient added in the cropping system (p < 0.05, LSD test).
The evolution of 14CO2 in the surface layer was not affected by the addition of nutrients to the acidic soil (Figure 4A). However, in the amended soil, there was a lower 14CO2 evolution with the addition of G+N+P compared with G alone in the subsoil (Figure 4B) because of the greater CUE (Figure 3A). Furthermore, in the subsoil, the addition of G+N+P also resulted in a reduced 14CO2 evolution compared with the addition of G+P, regardless of soil acidity (Figure 4B). A lower 14CO2 evolution was also observed after the addition of G+N compared with G alone. Notably, the addition of G+N resulted in even lower 14CO2 evolution in the subsoil when the pH was increased compared with treatments without acidity correction (Figure 4B).
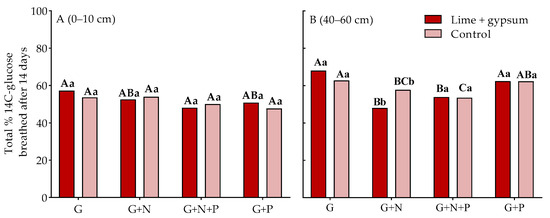
Figure 4.
Total amount of CO2 produced from the added glucose by the soil microbial community after 42 d in either the topsoil (Panel (A), 0–10 cm) or subsoil (Panel (B), 40–60 cm) either in the presence or absence of N and P and with or without lime and gypsum application. G+N ratio of 78:1; G+P ratio of 75:1; G+N+P ratio of 75:8.75:1; lime (2.9 t ha−1); gypsum (2.1 t ha−1); control (0 t ha−1 of lime and gypsum). Capital letters compare the addition of glucose (G), nitrogen (N), and phosphorus (P) for each soil amendment, and lowercase letters compare the soil amendments for each nutrient added (n = 4; p < 0.05, LSD test).
4. Discussion
Although mineral surfaces play an important role in chemically protecting soil C [39,40], SOM transformations by microorganisms have been increasingly recognized as dominant drivers of C stock formation and long-term stabilization [10,41,42]. This is because the transformation of organic matter (i.e., substrate added to the soil) can both increase or contribute to C sequestration rates in the soil [43] and enhance CO2 emitted into the atmosphere [13].
In our experiment, a higher CUE (estimated through SUE) was observed following soil acidity alleviation, which corresponded to a lower 14CO2 release from soil. This effect can be explained by reduced Al activity and higher pH. It is known that, when the microbial community is exposed to high levels of Al3+ and low pH, mineralization of labile C is higher, and a higher CO2 emission rate is observed, resulting in a lower CUE [44]. The increased CUE observed with soil acidity alleviation likely reflects both direct and indirect improvements in microbial metabolism. While reduced Al toxicity directly benefits microbial growth, the higher pH enhances nutrient availability and enzyme functioning, creating a more energetically efficient environment for microbial carbon processing [26,45,46]. This multi-mechanism improvement explains why our results showed stronger CUE responses than those from previous single-factor studies. For example, ref. [10] reported that CUE was not affected by soil acidity alleviation. In the present study, a higher CUE and, consequently, a lower evolution of 14CO2 originating from 14C-glucose were observed in soils with corrected acidity, suggesting that soil acidity alleviation can help mitigate the emission of CO2 from the soil into the atmosphere.
The CUE for 14C-glucose averaged 0.71, consistent with values similar to those reported in other studies [10,18,44]. With residual N, CUE was not higher in the subsoil when soil acidity was corrected, likely due to co-limitation by other nutrients such P [47]. Nutrient shortage in soil without residual N also decreased subsoil glucose-derived C at the end of two weeks, both in soil with corrected acidity and in the control treatment, resulting in lower CUE. Some studies have shown that the onset of SOM decomposition may accelerate after nutrient addition, while others report negative or no effects [48], which may explain the lack of response to residual N when lime was applied alone.
Evidence from this study suggests that, although C-glucose can be transported into the cell, it was not immediately partitioned into respiration when added together with G+N+P. Potentially, this C was allocated to internal storage or other C-rich structures [49,50,51]. The increased CUE with G+N+P treatment, particularly in subsoil, suggests that microbial communities may be operating well below their metabolic potential when nutrient availability is limited. This indicates that strategic nutrient and lime management could unlock significant additional C storage capacity in agricultural soils by enhancing microbial CUE, not merely by increasing C inputs. The C cycle is tightly coupled with the N cycle, and N is considered one of the most limiting elements for biomass production. Nitrogen inputs, whether from fertilization (mineral, organic) or biological N fixation, have been associated with enhanced C sequestration in agricultural soils [52]. According to [53], the introduction of plant species with high N concentration into the crop rotation can increase soil C inputs. With the addition of G+P, the P available in soil potentially increases, creating a state of limitation for other nutrients such as N in the subsoil, which probably inhibited CUE and decomposition of C derived from C-glucose. However, the addition of G+N+P overcame this limitation and resulted in increased CUE. Nutrient restriction in agricultural soils, particularly N and P, are well known constraints on the C cycle [54], and these constraints are especially pronounced in highly weathered soils [55].
Stoichiometric balance can be considered an alternative to increase soil organic C, and the addition of nutrients can promote SOM formation through the anabolic processes of microbial decomposition and the transformation of substrates derived from microorganisms. Conversely, under nutrient limitation, microorganisms may decompose SOM to acquire nutrients, a process known as microbial mining, which may decrease following nutrient addition, thereby enhancing SOM stocks [56]. However, the increase in soil C still depends on the interaction of stabilization mechanisms such as aggregation, organo-mineral associations, or humification [57]. When the nutritional requirements to build microbial biomass are met (i.e., nutrient stoichiometry), decomposing C is more likely to be incorporated into microbial biomass rather than respired as CO2 [56,58,59]. This was evidenced in our study by the higher CUE observed with G+N+P additions, particularly in the subsoil, suggesting that balanced nutrient stoichiometry is crucial for efficient C processing. The contrasting responses between surface and subsoil further suggest that stoichiometric requirements may vary with depth, possibly due to distinct microbial community compositions and resource availabilities.
For microorganisms, communities, and ecosystems, respiration can vary with local conditions because nutrient supply and other environmental factors affect the responses of microorganisms and eventually CO2 respiration [60,61]. Alternatively, the growth limitation of soil microbiota caused by the limited availability of an essential element may affect the rate of CO2 related to soil microbial growth, decreasing CUE [18]. The regulation of microbial communities by different controlling factors and nutrient limitations at depth has been supported by several studies investigating C dynamics in subsoils [11,62].
The lack effect in this study with the addition of glucose alone, a labile source of C, suggests that mineral concentrations in tropical soils do not provide optimal C:N:P ratios to meet the needs of the microbial community for SOM decomposition. This fact may reflect a more active and abundant microbial community, leading to a greater use of C for respiration [63]. The CUE of microbial communities varies with environmental conditions, substrate availability, stoichiometry, physiological state, and community composition [64]. For example, yield may be higher in rich-nutrient soils under no-till than in conventional cropping systems on acidic soils. This example underscores that both environmental constraints and nutrient supply must be considered when interpreting CUE patterns in agricultural ecosystems.
5. Conclusions
The results of this study provide important new insights into the effects of nutrient addition and substrate transformation in soil following acidity alleviation. Our findings demonstrate that soil acidity alleviation, when combined with balanced nutrient additions (N+P), significantly enhances microbial CUE, particularly in subsoils. The depth-dependent responses to nutrient addition highlight the distinct nutrient limitations and microbial strategies operating at different soil depths. While surface soil was responsive to N and P, subsoil microbial communities were primarily limited by N availability. Applying nutrients such as N and P enhances microbial C transformation, potentially increasing C stabilization and CUE in acidity-corrected soil and suggesting that mineral nutrient constraints limit the soil microbial functioning community. Therefore, integrated management approaches addressing both soil acidity and stoichiometric balance are crucial for maximizing soil C stabilization potential in weathered soils. These findings have important implications for developing more effective strategies for enhancing soil C sequestration in agricultural systems, particularly in weathered tropical soils.
Author Contributions
K.M.B.: Software, writing, review, and editing. M.d.S.: Writing—original draft, validation, software, methodology, formal analysis, data curation, investigation. D.L.J.: Conceptualization, methodology, supervision, visualization, writing—review and editing. C.A.R.: Conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, visualization, writing—review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
São Paulo Research Foundation—FAPESP—grant 2015/50305/8 and National Council for Scientific and Technological Development (CNPq), INCT–Low Carbon Agriculture, grant 406635/2022.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Christensen, B.T. Matching measurable soil organic matter fractions with conceptual pools in simulation models of carbon turnover: Revision of model structure. In Evaluation of Soil Organic Matter Models Using Existing Long-Term Datasets; Powlson, D.S., Smith, P., Smith, J.U., Eds.; Global Environmental Change; Springer: Berlin/Heidelberg, Germany, 1996; Volume 38, pp. 143–160. [Google Scholar]
- Zelles, L.; Stepper, I.; Zsolnay, A. The effect of liming on microbial activity in spruce (Picea abies L.) forests. Biol. Fertil. Soils 1990, 9, 78–82. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Tabatabai, M.A. Enzyme activities in a limed agricultural soil. Biol. Fertil. Soils 2000, 31, 85–91. [Google Scholar] [CrossRef]
- Lal, R. Challenges and opportunities in soil organic matter research. Eur. J. Soil Sci. 2009, 60, 158–169. [Google Scholar] [CrossRef]
- Barcelos, J.P.Q.; Souza, M.; Nascimento, C.A.C.; Rosolem, C.A. Soil acidity amelioration improves N and C cycles in the short term in a system with soybean followed by maize-guinea grass intercropping. Geoderma 2022, 421, 115909. [Google Scholar] [CrossRef]
- Salome, C.; Nunan, N.; Pouteau, V.; Lerch, T.Z.; Chenu, C. Carbon dynamics in topsoil and subsoil may be controlled by different regulatory mechanisms. Glob. Change Biol. 2010, 16, 416–426. [Google Scholar] [CrossRef]
- Sanaullah, M.; Chabbi, A.; Leifeld, J.; Bardoux, G.; Billou, D.; Rumpel, C. Decomposition and stabilization of root litter in top- and subsoil horizons: What is the difference? Plant Soil 2011, 338, 127–141. [Google Scholar] [CrossRef]
- Cook, F.J.; Knight, J.H. Where does oxygen extinction occur in a soil profile? In Proceedings of the 21st International Congress on Modelling and Simulation, Gold Coast, Australia, 29 November–4 December 2015; Available online: https://www.mssanz.org.au/modsim2015/J8/cook.pdf (accessed on 10 April 2025).
- Rovira, P.; Vallejo, V.R. Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: An acid hydrolysis approach. Geoderma 2002, 107, 109–141. [Google Scholar] [CrossRef]
- Barcelos, J.P.Q.; Mariano, E.; Jones, D.L.; Rosolem, C.A. Topsoil and subsoil C and N turnover are affected by superficial lime and gypsum application in the short-term. Soil Biol. Biochem. 2021, 163, 108456. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Blagodatsky, S.A.; Anderson, T.-H.; Kuzyakov, Y. Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl. Soil Ecol. 2007, 37, 95–105. [Google Scholar] [CrossRef]
- Glanville, H.C.; Hill, P.W.; Schnepf, A.; Oburger, E.; Jones, D.L. Combined use of empirical data and mathematical modelling to better estimate the microbial turnover of isotopically labelled carbon substrates in soil. Soil Biol. Biochem. 2016, 94, 154–168. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Liang, G.; Du, Z.; Zhou, J.; Zhu, C.; Huang, K.; Zhou, X.; Luo, Y.; Xia, J. Global variation of soil microbial carbon-use efficiency in relation to growth temperature and substrate supply. Sci. Rep. 2019, 9, 5621. [Google Scholar] [CrossRef] [PubMed]
- Creamer, R.E.; Stone, D.; Berry, P.; Kuiper, I. Measuring respiration profiles of soil microbial communities across Europe using MicroResp™ method. Appl. Soil Ecol. 2016, 97, 36–43. [Google Scholar] [CrossRef]
- Creamer, R.E.; Schulte, R.P.O.; Stone, D.; Gal, A.; Krogh, P.H.; Lo Papa, G.; Murray, P.J.; Peres, G.; Foerster, B.; Rutgers, M.; et al. Measuring basal soil respiration across Europe: Do incubation temperature and incubation period matter? Ecol. Indic. 2014, 36, 409–418. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Emmett, B.A.; Gundersen, P.; Kjonaas, O.J.; Koopmans, C.J.; Schleppi, P.; Tietema, A.; Wright, R.F. Nitrogen deposition makes a minor contribution to carbon sequestration in temperate forests. Nature 1998, 398, 145–148. [Google Scholar] [CrossRef]
- Magnani, F.; Mencuccini, M.; Borghetti, M.; Berbigier, P.; Berninger, F.; Delzon, S.; Grelle, A.; Hari, P.; Jarvis, P.G.; Kolari, P.; et al. The human footprint in the carbon cycle of temperate and boreal forests. Nature 2007, 447, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Leite, H.M.F.; Calonego, J.C.; Rosolem, C.A.; Mendes, L.W.; Moraes, L.N.; Grotto, R.M.T.; Araujo, F.F.; Pereira, A.P.A.; Melo, V.M.M.; Araujo, A.S.F. Cover crops shape the soil bacterial community in a tropical soil under no-till. Appl. Soil Ecol. 2021, 168, 104166. [Google Scholar] [CrossRef]
- Allison, S.D. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol. Lett. 2005, 8, 626–635. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Johanson, J.B. Effect of pH on enzyme stability in soils. Soil Biol. Biochem. 1982, 14, 433–437. [Google Scholar] [CrossRef]
- Turner, B.L. Variation in pH optima of hydrolytic enzyme activities in tropical rain forest soils. Appl. Environ. Microbiol. 2010, 76, 6485–6493. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Manson, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.; Damatîca, C.; Bolscher, T.; Chenu, C.; Elsgaard, L.; Tebbe, C.C.; Skadell, L.; Poeplau, C. Liming effects on microbial carbon use efficiency and its potential consequences for soil organic stocks. Soil Biol. Biochem. 2024, 191, 109342. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef]
- Billings, S.A.; Ziegler, S.E. Altered patterns of soil carbon substrate usage and heterotrophic respiration in a pine forest with elevated CO2 and N fertilization. Glob. Change Biol. 2008, 14, 1025–1036. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Keys to Soil Taxonomy, 12th ed.; Soil Survey Staff: Washington, DC, USA, 2014.
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; IAC: Campinas, Brazil, 2001. [Google Scholar]
- Gunina, A.; Kuzyakov, Y. Sugars in soil and sweets for microorganisms: Review of origin, content, composition and fate. Soil Biol. Biochem. 2015, 90, 87–100. [Google Scholar] [CrossRef]
- Haney, R.L.; Franzluebbers, A.J.; Porter, E.B.; Hons, F.M.; Zuberer, D.A. Soil carbon and nitrogen mineralization: Influence of drying temperature. Soil Sci. Soc. Am. J. 2004, 68, 489–492. [Google Scholar] [CrossRef]
- Hill, P.W.; Farrar, J.F.; Jones, D.L. Decoupling of microbial glucose uptake and mineralization in soil. Soil Biol. Biochem. 2008, 40, 616–624. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Banerjee, M.R.; Chapman, S.J. The significance of microbial biomass sulphur in soil. Biol. Fertil. Soils 1996, 22, 116–125. [Google Scholar] [CrossRef]
- He, Z.L.; Wu, J.; O’Donnell, A.G.; Syers, J.K. Seasonal responses in microbial biomass carbon, phosphorus and sulphur in soils under pasture. Biol. Fertil. Soils 1997, 24, 421–428. [Google Scholar] [CrossRef]
- Chowdhury, M.A.H.; Kouno, K.; Ando, T. Correlation among microbial biomass S, soil properties, and other biomass nutrients. Soil Sci. Plant Nutr. 1999, 45, 175–186. [Google Scholar] [CrossRef]
- Chowdhury, M.A.H.; Kouno, K.; Ando, T. Critical sulphur concentration and sulphur requirement of microbial biomass in a glucose and cellulose-amended regosol. Biol. Fertil. Soils 2000, 32, 310–317. [Google Scholar] [CrossRef]
- Kramer, C.; Trumbore, S.; Fröberg, M.; Dozal, L.M.C.; Zhang, D.C.; Xu, X.M.; Santos, G.M.; Hanson, P.J. Recent (4 year old) leaf litter is not a major source of microbial carbon in a temperate forest mineral soil. Soil Biol. Biochem. 2010, 42, 1028–1037. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Chapter One—Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar] [CrossRef]
- Jenkinson, D.S. Determination of microbial biomass carbon and nitrogen in soil. In Advances in Nitrogen Cycling in Agricultural Systems; Wilson, J.R., Ed.; CAB International: Wallingford, UK, 1998; pp. 368–386. [Google Scholar]
- Fischer, H.; Ingwersen, J.; Kuzyakov, Y. Microbial uptake of low-molecular-weight organic substances out competes sorption in soil. Eur. J. Soil Sci. 2010, 61, 504–513. [Google Scholar] [CrossRef]
- Mota Neto, L.V.; Barros, J.V.S.; Costa, V.E.; Galdos, M.V.; Santos, A.R.P.; Rosolem, C.A. Insights on soil carbon cycling in intercropped maize-forage systems as affected by nitrogen. Geoderma 2024, 449, 116998. [Google Scholar] [CrossRef]
- Malik, A.A.; Thomson, B.C.; Whiteley, A.S.; Bailey, M.; Griffiths, R.I. Bacterial physiological adaptations to contrasting edaphic conditions identified using landscape scale metagenomics. mBio 2017, 8, e0079. [Google Scholar] [CrossRef]
- Puissant, J.; Jones, B.; Goodall, T.; Mang, D.; Blaud, A.; Gweon, H.S.; Malik, A.; Jones, D.L.; Clark, I.M.; Hirsch, P.R. The pH optimum of soil exoenzymes adapt to long term changes in soil pH. Soil Biol. Biochem. 2019, 138, 107601. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Soil organic matter priming: The pH effects. Glob. Change Biol. 2024, 30, e17349. [Google Scholar] [CrossRef] [PubMed]
- Commichau, F.M.; Forchhammer, K.; Stülke, J. Regulatory links between carbon and nitrogen metabolism. Curr. Opin. Microbiol. 2006, 9, 167–172. [Google Scholar] [CrossRef]
- Conde, E.; Cardenas, M.; Ponce-Mendoza, A.; Luna-Guido, M.L.; Cruz-Mondragón, C.; Dendooven, L. The impacts of inorganic nitrogen application on mineralization of 14C-labelled maize and glucose, and on priming effect in saline alkaline soil. Soil Biol. Biochem. 2005, 37, 681–691. [Google Scholar] [CrossRef]
- Russell, J.B. The energy spilling reactions of bacteria and other organisms. J. Mol. Microbiol. Biotechnol. 2007, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- De Goffau, M.C.; Yang, X.M.; Van Dijl, J.M.; Harmsen, H.J.M. Bacterial pleomorphism and competition in a relative humidity gradient. Environ. Microbiol. 2009, 11, 809–822. [Google Scholar] [CrossRef]
- Lebre, P.H.; De Maayer, P.; Cowan, D.A. Xerotolerant bacteria: Surviving through a dry spell. Nat. Rev. Microbiol. 2017, 15, 285–296. [Google Scholar] [CrossRef]
- Santi, A.; Amado, T.J.C.; Costa, J.A.A. Adubação nitrogenada na aveia preta: Influência na produção de matéria seca e ciclagem de nutrientes sob sistema plantio direto. Rev. Bras Cienc. Solo 2003, 27, 1075–1083. [Google Scholar] [CrossRef]
- Raphael, J.P.; Calonego, J.C.; Milori, D.M.B.; Rosolem, C.A. Soil organic matter in crop rotations under no-till. Soil Tillage Res. 2016, 155, 45–53. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Harpole, W.S.; Ngai, J.T.; Cleland, E.E.; Seabloom, E.W.; Borer, E.T.; Bracken, M.E.S.; Elser, J.J.; Gruner, D.S.; Hillebrand, H.; Shurin, J.B.; et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 2011, 14, 852–862. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, C.A.; Kirkegaard, J.A.; Richardson, A.E.; Wade, L.J.; Blanchard, C.; Batten, G. Stable soil organic matter: A comparison of C: N:P: S ratios in Australian and other world soils. Geoderma 2011, 163, 197–208. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; Ruiter, P.C. Carbon sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Ågren, G.I.; Wetterstedt, J.Å.M. What determines the temperature response of soil organic matter decomposition? Soil Biol. Biochem. 2007, 39, 1794–1798. [Google Scholar] [CrossRef]
- Wagai, R.; Kishimoto-Mo, A.W.; Yonemura, S.; Shirato, Y.; Hiradate, S.; Yagasaki, Y. Linking temperature sensitivity of soil organic matter decomposition to its molecular structure, accessibility, and microbial physiology. Glob. Change Biol. 2013, 19, 1114–1125. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.; Wang, D.; Wang, M.; Liao, C.; Yang, X.; Liu, F. Decoupled linkage between soil carbon and nitrogen mineralization among soil depths in a subtropical mixed forest. Soil Biol. Biochem. 2017, 109, 135–144. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Manzoni, S.; Pineiro, G.; Jackson, R.B.; Jobbagy, E.G.; Kim, J.H.; Porporato, A. Analytical models of soil and litter decomposition: Solutions for mass loss and time-dependent rates. Soil Biol. Biochem. 2012, 50, 66–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).