Abstract
Trichoderma harzianum, a prominent biocontrol microorganism, often exhibits restricted colonization efficiency in nutrient-poor soil, thus reducing its biocontrol effectiveness. This study investigated the impact of vitamin C industrial fermentation byproduct (residue after evaporation, RAE), which is recognized for enhancing plant growth and stress tolerance, on the colonization ability and anti-pathogenic fungi activity of T. harzianum through in vitro and pot experiments. In vitro experiments demonstrated that RAE and its main component (2-keto-L-gulonic acid, 2KGA) significantly enhanced biomass and spore production (41.44% and 158.46% on average) of two T. harzianum strains in an oligotrophic medium (1/5 PDA). In a more nutrient-limited medium (1/10 PDA), RAE significantly increased the inhibition rates of T. harzianum S against Fusarium graminearum, Botrytis cinerea, and Alternaria alternata by 6.12–7.77%. Pot experiments further revealed that, compared with T. harzianum application alone, the combined application of RAE and T. harzianum S, (1) significantly elevated T. harzianum S abundance by 23.77% while significantly reducing B. cinerea abundance by 33.78% in rhizosphere soil; (2) significantly improved the content of soil available phosphorus (147.63%), ammonium nitrogen (60.05%), and nitrate nitrogen (32.19%); and (3) significantly improved the superoxide dismutase activity (17.39%) and fresh weight of tomato plants (130.74%). Correlation analysis revealed that there were significant positive correlations between T. harzianum S abundances/plant biomass and RAE, and significantly negative correlations between B. cinerea abundance and T. harzianum S/plant biomass/peroxidase activity. Collectively, RAE effectively promoted the growth of T. harzianum and pathogen suppression ability, while improving soil fertility and tomato biomass. This study offers novel insights into RAE’s agricultural application for plant disease control while supporting the sustainable development of vitamin C production.
1. Introduction
Biological control has been widely studied and applied in plant disease management due to its environmental friendliness, sustainability, and low risk of inducing pathogen resistance [1,2,3]. Biocontrol microorganisms primarily include Bacillus, Streptomyces, Pseudomonas, mycorrhizal fungi, Trichoderma, and others [4,5,6,7,8]. Among these, Trichoderma harzianum is one of the most extensively utilized biocontrol agents. It suppresses plant diseases through multiple mechanisms, including ecological niche competition with pathogens, nutrient competition, secretion of antimicrobial secondary metabolites, and induction of plant systemic resistance [9,10,11]. Among the antibiotics produced by Trichoderma, gliotoxin has a strong inhibitory activity against plant pathogenic fungi such as Pythium ultimum and Rhizoctonia solani Kuhn [12]. Additionally, T. harzianum can secrete phytohormones, modulate soil nutrient cycling, and promote plant growth and stress resistance [13,14]. Trichoderma-based biocontrol agents have a high level of commercialization, accounting for more than 60% of fungal biocontrol agents used for plant disease control [15]. The global market for Trichoderma-based biocontrol products has grown rapidly in recent years, with more than 250 commercial agents registered worldwide [16]. Among these, T. harzianum is the most widely used species, followed by T. viride and T. koningii [17]. The application of Trichoderma-based biocontrol agents aligns with the principles of sustainable development and green agriculture, making it a focal point of academic research and practical applications in plant disease management.
Successful colonization in soil is crucial for Trichoderma spp. to effectively promote their growth and exert their disease suppressive functions; however, their colonization efficiency is often too low in agricultural practices [18]. In field applications, the low colonization efficiency of Trichoderma biocontrol agents often results in their inability to effectively demonstrate efficacy, thereby hindering biological control methods from fully realizing their potential advantages [19]. One of the primary reasons is that the complex physicochemical properties of soil, combined with limited nutrient availability, inhibit the initial growth and reproduction of Trichoderma spp. following application [20]. Various strategies have been developed to address this issue, including screening for Trichoderma strains with high colonization efficiency, co-applying multiple biocontrol strains, and utilizing Trichoderma-based bio-organic fertilizers [21,22]. However, strain screening and improvement are time-consuming and labor-intensive, and the efficacy of co-applying multiple biocontrol strains is often limited. In contrast, Trichoderma-based bio-organic fertilizers effectively integrate the advantages of organic fertilizers and biocontrol microorganisms, not only providing essential nutrients for Trichoderma spp. but also serving as a carrier to enhance their colonization efficiency [23,24]. Therefore, seeking and identifying novel organic substances that facilitate Trichoderma spp. colonization and subsequently developing advanced Trichoderma-based bio-fertilizers represent a promising and effective approach to significantly enhance its colonization efficiency in agricultural systems.
Vitamin C (Ascorbic acid, ASA) is an essential nutrient that plays a crucial role in human health [25,26], and is industrially produced through a two-step fermentation process [27]. A major byproduct of this process is the residue after evaporation (RAE), which is generated after evaporation, concentration, and crystallization of the fermentation liquid for the extraction of 2-keto-L-gulonic acid (2KGA, a key precursor for ASA industrial production) [28]. RAE primarily comprises various low-weight-molecular organic acids, including 2KGA, oxalic acid, formic acid, acetic acid, and others [29]. Due to its high chemical oxygen demand (COD, mg/L) value (0.8 × 106 mg/L to 1.0 × 106 mg/L) and extremely low pH (<0.5), RAE poses significant challenges for environmentally sound treatment. Conventional wastewater treatment methods, such as anaerobic and aerobic processes, not only increase production costs but also risk causing secondary pollution [30]. Therefore, developing green resource utilization technologies of RAE is essential to address these challenges and promote the sustainable development of the vitamin C fermentation industry [31].
Several studies have demonstrated that the RAE exhibits great potential for agricultural applications as a novel organic resource from industrial fermentation. RAE has been shown to significantly enhance crop yield and ASA content, while positively influencing soil microbial communities [32]. Additionally, RAE improves the physicochemical properties of saline–alkali soils and increases the availability of soil nutrients [31]. Notably, 2-KGA, the primary component of RAE, actively participates in plant carbon metabolism pathways and significantly enhances the tolerance of non-heading Chinese cabbage to salt stress and Arabidopsis to cold stress [33]. Furthermore, the combined application of RAE and Trichoderma longibraciatum has been found to promote vegetation restoration and improve soil quality on mining waste dump sites [32]. Interestingly, we have observed that environments containing RAE, such as laboratories or fermentation factories, often exhibit substantial fungal growth. This observation leads us to hypothesize that RAE, as a nutrient-rich source of low-weight-molecular organic carbon, may be utilized by fungi such as T. harzianum. It may enhance the colonization efficiency of T. harzianum in the soil, thereby improving its efficacy in disease suppression.
In this study, the effects of RAE on the growth and disease-suppressive efficacy of T. harzianum were investigated through both laboratory pure culture experiments and pot trials to test our hypothesis. The study aimed to address the following key questions: (1) Can RAE or its primary component, 2KGA, be utilized by T. harzianum to enhance its growth? (2) Can RAE and 2KGA improve the colonization efficiency of T. harzianum in soil and thereby enhance its disease suppression capabilities? By addressing these questions, this study seeks to provide novel insight into the resource utilization of vitamin C fermentation waste and lay a theoretical foundation for the application of RAE in agricultural practices.
2. Materials and Methods
2.1. RAE and Fungal Strains
The RAE was obtained from Northeast Pharmaceutical Group Co., Ltd., located in Shenyang, China. The characteristics of RAE are listed in Table 1 [33].

Table 1.
The physical–chemical properties of RAE.
Botrytis cinerea was isolated from tomato plants exhibiting botrytis mold through tissue separation [34]. The ITS sequences of the isolated strains were sequenced and combined with the morphological observations to confirm the taxonomic status of the strains. The strain was stored in a refrigerator at −80 °C in the laboratory. Two T. harzianum strains (T. harzianum S, T. harzianum L.) were isolated from T. harzianum wettable powder from two companies (Kewei Baiwo Biotechnology Co., Ltd., Beijing, China; Kunming Pesticide Co., Ltd., Kunming, China). The strains of Alternaria alternata and Fusarium graminearum were provided by the Microbial Culture Preservation Center, Shenyang Institute of Applied Ecology, Chinese Academy of Sciences.
2.2. Effect of RAE on the Growth of T. harzianum
This experiment was conducted in March 2023. Studies have demonstrated that the optimal application concentration range of RAE in both laboratory and field experiments is approximately 0.5% to 2% [31,33]. To investigate the utilization effect of T. harzianum on RAE, agar media containing different concentrations of RAE (RAE content was 2.0%, 1.0%, 0.5%, and 0.0%, v/v) were prepared (agar = 18 g/L; pH = 7.00), with the agar medium without RAE serving as the control group. T. harzianum was cultured on potato dextrose agar (PDA) medium for three days at 28 °C. Sterile hole punchers were used to cut agar blocks along the edge of the colony, which contained T. harzianum mycelia. These agar blocks were then inoculated into the center of the above medium containing varying concentrations of RAE. One agar block was placed per plate, and all plates were pre-laid flat with sterile cellophane in Petri dishes of appropriate size. All inoculated plates were incubated at 28 °C, with six plates prepared for each group. After seven days of incubation, three plates from each group were selected randomly. The mycelia growing on the cellophane were scraped off using a coating rod and weighed on an analytical balance BSA223S (Sartorius, Goettingen, Germany) to calculate the biomass of T. harzianum. The remaining three plates from each group were used to determine the number of spores. Specifically, 10 mL of phosphate-buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4·12H2O, 2 mM KH2PO4, pH = 7.00) was added to each plate. The mycelium and spores on the cellophane were scraped off with a coating rod, mixed thoroughly to form a suspension, and filtered through quantitative filter paper (diameter = 15 cm, pore size = 10–15 µm) to remove the mycelium. The resulting spore suspension was diluted in a 10-fold gradient, and the spore number was determined under a microscope (OLYMPUS, Tokyo, Japan) using a hemocytometer (Jiangsu Shitai experimental equipment Co., Ltd., Nanjing, China).
In order to investigate the promoting effect of RAE on the growth of T. harzianum, two media were prepared: an oligotrophic medium (1/5 PDA) and an oligotrophic medium supplemented with 1.0% RAE (v/v). T. harzianum was initially cultured on PDA medium for three days at 28 °C, then inoculated on the prepared medium (1/5 PDA; 1/5 PDA + 1.0% RAE, v/v) in Petri dishes. The inoculation method followed the procedure above. All plates were incubated at 28 °C, with six plates per group. After seven days of incubation, the biomass and spore count of T. harzianum were determined using the methods described earlier.
2.3. Effect of 2-Keto-L-Gulonic Acid on the Growth of T. harzianum
This experiment was conducted in March 2023. We used a basic medium (glucose 10.28 g/L, NaH2PO4 6.9 g/L, KH2PO4 2.0 g/L, (NH4)2SO4 1.4 g/L, peptone 1.0 g/L, MgSO4·7H2O 0.3 g/L, urea 0.3 g/L, agar 18 g/L, pH = 7.0), which is a synthetic medium suitable for fungal growth. To investigate whether T. harzianum can utilize 2KGA as a carbon source for growth, the carbon source (glucose) in the basic medium was substituted with 2KGA at an equimolar concentration (10.0 g/L). Additionally, two more concentration gradients were established: an extremely low concentration (1.0 g/L) and an extremely high concentration (20.0 g/L). A carbon-source-free basic medium served as the control. T. harzianum was initially cultured on PDA medium at 28 °C for three days and then inoculated onto the four prepared media. The inoculation methods followed the procedure described in Section 2.2 of Materials and Methods. Six plates per medium were incubated at 28 °C. After seven days of incubation, the biomass and spore count of T. harzianum were determined using the methods described in Section 2.2 of Materials and Methods.
2.4. Effect of RAE on Inhibitory Ability of T. harzianum Against Fungal Pathogens In Vitro
This experiment was conducted in April 2023. Firstly, the following solid media were prepared: PDA, PDA with 1.0% RAE (v/v), 1/5 PDA, 1/5 PDA with 1.0% RAE (v/v), 1/10 PDA, 1/10 PDA with 1.0% RAE (v/v). T. harzianum was initially cultured on PDA medium at 28 °C for three days, while the fungal pathogens (A. alternata, B. cinerea, and F. graminearum) were cultured on PDA medium for 5 days at 28 °C. Agar blocks containing mycelia were obtained by drilling holes along the edge of the colonies using sterile hole punchers. The agar blocks of T. harzianum and the pathogenic fungi were symmetrically placed at the center of the plates prepared with the aforementioned media. For the control group, only the pathogenic fungi agar blocks were placed. All plates were incubated at 28 °C. When the mycelium fully covered the control plate, the colony area was measured using ImageJ software (Version 1.8.0.112). The inhibition rate was calculated according to the method detailed in reference [35].
In addition, the agar blocks containing the mycelia of pathogenic fungi and the agar blocks containing the mycelia of T. harzianum were inoculated into the center of the above-configured medium, with one agar block per plate, three plates of each type of fungus for each medium, and cultured at 28 °C. The colony diameter of T. harzianum was measured after 3 days of culture, and the colony diameter of the three pathogens was measured after 5 days of culture. The growth rate of the cultured fungi (cm/h) was calculated by dividing the colony diameter by the culture time.
2.5. Pot Experiment
The pot experiment was conducted from May 2023 to July 2023.
2.5.1. Tomato Seed Germination
The tomato seeds were purchased from Shouguang Xinxinran Horticulture Co., Ltd., Shouguang, China, and the cultivar used was Fenguan F1 hybrid. The seeds were surface-sterilized by immersing them in a 5% (v/v) NaClO solution for 15 min, followed by rinsing five times with sterile water. They were then soaked in 75% (v/v) ethanol for 10 min and washed again with sterile water five times. Sterile gauze saturated with sterile water was placed inside a sterile Petri dish, and the tomato seeds were evenly distributed on the gauze. Finally, the Petri dishes were incubated in the incubator for three days to promote seed germination under the following conditions: 28 °C, 50% relative humidity, and complete darkness.
2.5.2. Seedling Raising and Transplanting
After germination, the seeds were sown in a 72-well seedling tray (4 cm × 2 cm × 4.2 cm for each well) containing sterile seedling substrate purchased from Hebei Dewoduo Biotechnology Co., Ltd. (Organic matter 340.00 g/kg, moisture content 25%, N + P2O5 + K2O 12.00 g/kg, pH 7.0). The trays were then placed in a greenhouse and maintained at a temperature of 28 ± 3 °C with a photoperiod of 16 h light/8 h dark and a relative humidity of 35%. After 28 days of incubation, the tomato seedlings were carefully removed from the trays, gently shaken to dislodge excess soil adhering to their root systems, rinsed three times with sterile water to ensure complete removal of any nutrient residue, and subsequently transplanted into larger pots (14.5 cm × 12.5 cm × 10.2 cm). These pots were pre-filled with 800 g of soil collected from the National Field Scientific Observation and Research Station of Agroecosystem (41°31′ N, 123°24′ E) in Shenyang, Liaoning Province of China. The original properties of the soil were pH 6.8, organic carbon 16.70 g/kg, ammonium nitrogen 9.50 mg/kg, nitrate nitrogen 44.60 mg/kg, available phosphorus 37.60 mg/kg, and available potassium 389.10 mg/kg.
2.5.3. Treatment
After tomato seedling transplantation, each pot was inoculated with 100 mL of B. cinerea spore solution (7.0 × 108 spores/mL). Subsequently, the pots were randomly divided into six groups, with 15 pots per group. The treatment for the six groups was as follows: (1) adding 100 mL of RAE solution (1.0%, v/v); (2) adding 100 mL of 2KGA solution (10.0 g/L); (3) adding 100 mL of T. harzianum spore solution (7.0 × 108 spores/mL); (4) adding 100 mL of a mixed solution containing RAE (1.0%, v/v) and T. harzianum spores (7.0 × 108 spores/mL); (5) adding 100 mL of a mixed solution containing 2KGA (10.0 g/L) and T. harzianum spores (7.0 × 108 spores/mL); (6) adding 100 mL of KCl solution (7.5 g/L). The pH of all solutions was adjusted to 7.00 ± 0.02 using 99.8% KOH (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). The groups using no or less KOH were supplemented with an additional equimolar concentration of KCl to ensure the same K+ concentration in all groups. Each group was treated with the above-mentioned treatment method once every 7 days, for a total of three times.
2.5.4. Sampling and Biomass Statistics
Plant leaves and soil samples (rhizosphere soil and bulk soil) were collected on the seventh day after the last treatment; 3~4 leaves (excluding heart leaves) were collected from the top of tomato plants to measure the activity of antioxidant enzymes. Each plant was gently uprooted and vigorously shaken to remove loosely attached soil from the root surface. The dislodged soil was collected as the bulk soil for subsequent analysis of available nutrients. The remaining roots with tightly adhered soil were then placed into a sterile centrifuge tube containing 30 mL of sterile PBS solution. The mixture was shaken at 200 rpm for 5 min to detach the tightly bound soil from the root surface. Subsequently, the roots were removed, and the suspension was centrifuged at 8000 rpm for 5 min. The supernatant was completely discarded to obtain the rhizosphere soil, which was immediately frozen at −80 °C in a refrigerator for subsequent DNA extraction. Plant biomass and plant height were measured according to the method described in reference [36].
2.6. DNA Extraction and Quantitative Polymerase Chain Reaction (qPCR) Assay
Soil DNA was extracted using the XPure Soil DNA Extraction Kit (Chengdu Xinbaiji Biotechnology Co., Ltd., Chengdu, China) according to the manufacturer’s protocol. The quality and concentration of the extracted DNA were determined by 1.0% agarose gel electrophoresis and a TGem Plus full-wavelength spectrophotometer OSE-260-05/06 (Tiangen Biochemical Technology Co., Ltd., Beijing, China). Primers ITS1-S/ITS1-R (ACA ACT CCC AAA CCC AAT GTG A/CGT TGT TGA AAG TTT TGA TTC ATT T) and ITS-R/ITS-F (TCG AGC GTC ATT TCA ACC CTC AAG/TTG GCA GAA GCA CAC CGG AAC) were used for quantifying the abundance of T. harzianum and B. cinerea in soils [37,38]. The qPCR reaction was performed on a Rotor-Gene 3000 (CORBETT RESEARCH, Sydney, Australia) using a SYBR Green Pro Taq HS premixed qPCR kit (Hunan Aikerui Biological Engineering Co., Ltd., Changsha, China), with a total volume of 20 µL: 10 µL of 2 × SYBR Green Pro Taq HS Premix, 0.4 µL each for forward and reverse primers (10 µmol each), 2 µL of DNA template, and 7.2 µL of ddH2O. Standard curves were generated using 10-fold serial dilutions of a plasmid containing the ITS genes from T. harzianum and B. cinerea, with each sample analyzed in triplicate (see Supplementary Materials Figures S1 and S2 for standard curves). The thermal cycling conditions consisted of an initial denaturation step at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s, with a final extension at 72 °C for 5 min. Each sample was analyzed in three replicates, and the results were expressed as log10 values (target copy number per gram of soil).
2.7. Antioxidant Enzyme Activity Determination
Leaf samples (0.2 g) were added to 2 mL of PBS (20 mM, pH 7.2 ± 0.1), containing 0.1 mM EDTA-Na2, 0.5 mM ASA, and 0.1% (w/v) polyethylpyridone (PVP). The samples were then ground uniformly over an ice bath using a mortar. The homogenate for each sample was centrifuged at 7200× g for 10 min at 4 °C, and the supernatant was collected as the crude enzyme extract to determine the activities of several antioxidant enzymes. The activities of superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), and catalase (CAT) were assayed according to the methods described in references [32,39,40].
2.8. Soil Available Nutrient Determination
Soil ammonium nitrogen, soil available phosphorus, and soil available potassium were determined using the indophenol blue colorimetric method after KCl extraction, the NaHCO3 extraction method, and the NH4OAc extraction with flame spectrophotometry, respectively [41]. Soil nitrate nitrogen was measured according to the method detailed in reference [42].
2.9. Data Analysis
IBM SPSS 20.0 software program was used to conduct one-way analysis of variance (one-way ANOVA) and two-tailed Student’s t-test. One-way ANOVA was adjusted by Tukey’s corrections for multiple comparisons. The histogram is plotted using chiplot https://www.chiplot.online (accessed on 5 August 2023). The Spearman method was used for correlation analysis, which was calculated and visualized using chiplot. The significance level of all statistical analyses in this study was p < 0.05.
3. Results
3.1. RAE Significantly Promoted the Growth of T. harzianum
In the pure culture experiment, RAE was utilized by T. harzianum, promoting its growth (Figure 1a–c). When the RAE content was 1.0% (v/v), two strains (T. harzianum S and T. harzianum L.) exhibited optimal growth, with significantly higher biomass (Figure 1a, n = 3, F = 23.12, F = 14.12, p < 0.05) and spore yields (Figure 1b, n = 3, F = 18.57, F = 12.66, p < 0.05) compared to the other two concentrations (0.5% and 2.0%). Specifically, under the 1.0% RAE cultured condition, the spore yield of T. harzianum L. increased by 269% and 111%, respectively, compared to the 0.5% and 2.0% RAE conditions, while the spore yield of T. harzianum S increased by 155% and 146%, respectively. In terms of biomass, the 1.0% RAE condition resulted in a 109% and 36% increase for T. harzianum L. and a 210% and 161% increase for T. harzianum S, compared to the 0.5% and 2.0% RAE conditions, respectively.
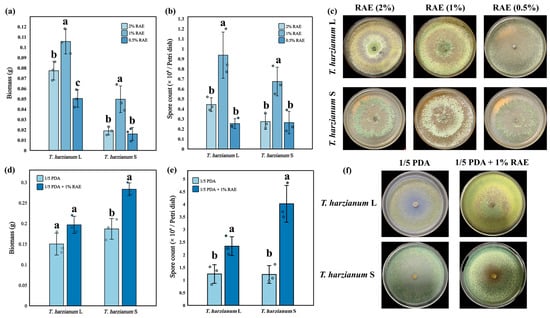
Figure 1.
Effects of RAE on the growth of T. harzianum strains. (a,b) The effects of different concentrations of RAE on the biomass and spore production of two T. harzianum strains (mean ± standard deviation, n = 3). Different lowercase letters indicated significant differences among respective groups based on one-way ANOVA tests for multiple comparisons by Tukey’s corrections (p < 0.05). (c) Phenotypes of two T. harzianum strains under different concentrations of RAE. (d,e) Effects of RAE on biomass and spore production of T. harzianum under oligotrophic culture (mean ± standard deviation, n = 3). Different lowercase letters indicate a significant difference between two groups (p < 0.05, Student’s t test). (f) The effect of RAE on the phenotype of T. harzianum under oligotrophic culture. Spore count in the figure refers to the number of spores produced in each Petri dish, and the biomass in the figure refers to the weight of mycelial produced in each Petri dish.
In the oligotrophic state (1/5 PDA), compared with the control group, the addition of 1.0% RAE significantly enhanced the spore production of both T. harzianum strains (T. harzianum S and T. harzianum L.) (Figure 1e, n = 3, F = 1.014, F = 4.289, p < 0.05) and the biomass of T. harzianum S (Figure 1d, n = 3, F = 2.714, p < 0.05). Specifically, the biomass of T. harzianum L. and T. harzianum S increased by 31% and 51% in the 1.0% RAE addition group, respectively, compared to the control group (Figure 1d). The spore yields of T. harzianum L. and T. harzianum S increased by 88% and 228% in 1% RAE addition groups, respectively, compared to the control group (Figure 1e).
3.2. Effects of 2-Keto-L-Gulonic Acid on the Growth of T. harzianum
Compared with the control group (no carbon source), the 2KGA with high concentration (20.0, 10.0 g/L) significantly increased the biomass (Figure 2c, n = 3, F = 102.8, F = 42.66, p < 0.05) and spore production (Figure 2b, n = 3, F = 36, F = 41.69, p < 0.05) of both T. harzianum strains (T. harzianum S and T. harzianum L.). When the concentration of KGA in the medium was 10.0 g/L, the spore yield of T. harzianum L. and T. harzianum S was 0.84 × 108 and 1.48 × 108 spores/Petri dish, respectively, representing increases of 502% and 319% compared to the control group. Additionally, at the same concentration of KGA (10.0 g/L), the biomass of T. harzianum L. and that of T. harzianum S were 0.15 g and 0.12 g, respectively, corresponding to an increase of 646% and 228% compared to the control group (Figure 2c). Notably, at the 2KGA concentration of 10.0 g/L, the spore yield and biomass of both T. harzianum strains were significantly higher than those in groups containing other concentrations of 2KGA (Figure 2b,c). This may be attributed to the fact that when the concentration of 2KGA in the culture medium is 10.0 g/L, its carbon–nitrogen ratio and osmotic pressure become more favorable for the growth of T. harzianum.
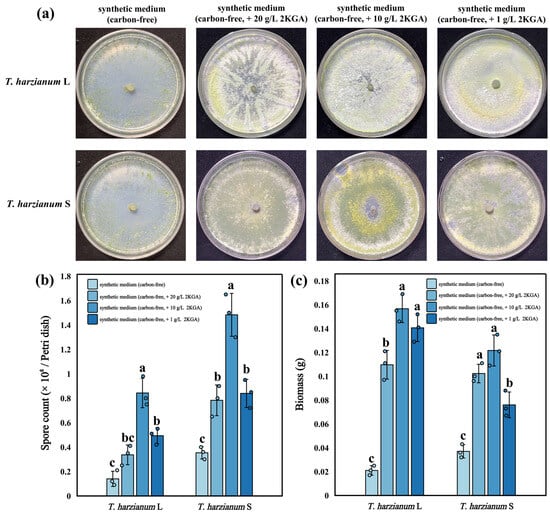
Figure 2.
Effect of 2KGA on the growth of T. harzianum. (a) Phenotypes of two T. harzianum strains under different synthetic media with different concentrations of 2KGA. (b,c) Effects of different concentrations of 2KGA on the spore production and biomass of two T. harzianum strains (mean ± standard deviation, n = 3). Different lowercase letters indicated significant differences among respective groups based on one-way ANOVA tests for multiple comparisons by Tukey’s corrections (p < 0.05). Spore count in the figure refers to the number of spores produced in each Petri dish, and the biomass in the figure refers to the weight of mycelial produced in each Petri dish.
3.3. RAE Significantly Enhanced the Inhibitory Ability of T. harzianum S Against Pathogenic Fungi In Vitro
We investigated the impact of RAE on the inhibitory effect of the T. harzianum strain on pathogenic fungi through a plate confrontation experiment. The two strains (T. harzianum L. and T. harzianum S) demonstrated a significant inhibitory effect against several common pathogenic fungi, and the average inhibitory effect of T. harzianum S was better than that of T. harzianum L. (Figure S3). Therefore, we selected T. harzianum S as the target strain and further investigated the effect of RAE on its antifungal activity through a plate confrontation experiment.
The experimental results showed that the growth rate of T. harzianum S was significantly higher than that of three pathogenic fungi (F. graminearum, B. cinerea, A. alternata). Moreover, with the decrease in nutrient concentration in the medium, the growth rate of T. harzianum S and three pathogenic fungi decreased. However, compared to pathogenic fungi, T. harzianum S exhibited a greater sensitivity to reductions in nutrient concentration; specifically, the growth rate of T. harzianum S was 0.162 cm/h and 0.145 cm/h under 1/5 PDA and 1/10 PDA, respectively, which was significantly lower than that observed under PDA medium. Additionally, we found that the addition of RAE in oligotrophic medium (1/5 PDA, 1/10 PDA) could significantly increase the growth rate of T. harzianum S (Figure 3a, n = 3, F = 2.652, F = 5.278, p < 0.05). Specifically, compared with 1/10 PDA and 1/5 PDA, the growth rate of T. harzianum S was increased by 23% and 14% after adding RAE to 1/10 PDA and 1/5 PDA, and the growth rate of T. harzianum S after RAE addition showed no significant difference with that in PDA medium (Figure 3a). This indicates that RAE effectively alleviated the reduction in the growth rate of T. harzianum S caused by decreased nutrient concentration.
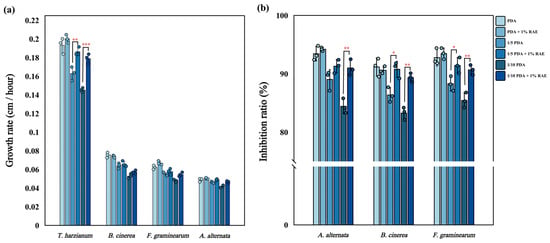
Figure 3.
(a) Effect of RAE on the growth rate of T. harzianum S and several pathogenic fungi (mean ± standard deviation, n = 3). (b) Effect of RAE on the inhibitory ability of T. harzianum S on pathogenic fungi in vitro (mean ± standard deviation, n = 3). An asterisk indicates a significant difference between the two groups (Student’s t-test), * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
The decrease in nutrient concentration in the medium also had a significant effect on the inhibitory ability of T. harzianum S. With the decrease in nutrient concentration in the medium, the inhibition rate of T. harzianum S against the three pathogenic fungi decreased significantly. However, the addition of RAE in oligotrophic medium (1/5 PDA, 1/10 PDA) could significantly enhance the inhibitory ability of T. harzianum S against B. cinerea (Figure 3b, n = 3, F = 1.202, F = 2.030, p < 0.05) and F. graminearum (Figure 3b, n = 3, F = 1.161, F = 2.355, p < 0.05). When RAE was added to 1/5 PDA medium and 1/10 PDA medium, the inhibition rate of T. harzianum S against F. graminearum increased by 3% and 6%, respectively, and the inhibition rate against B. cinerea increased by 5% and 7%, respectively. The addition of RAE in 1/10 PDA could significantly enhance the inhibitory ability of T. harzianum S against A. alternata (Figure 3b, n = 3, F = 1.179, p < 0.05); compared with 1/10 PDA, the inhibition rate increased by 7%. In general, after adding RAE to oligotrophic medium (1/5 PDA, 1/10 PDA), there was no significant difference in the inhibition rate of T. harzianum S against pathogenic fungi compared with PDA medium. It can be seen that after adding RAE to 1/10 PDA medium, the improvement effect on the inhibition rate is significantly greater than that of 1/5 PDA medium (Figure 3b). This indicates that RAE can significantly enhance the inhibitory effect of T. harzianum S on pathogenic fungi in oligotrophic medium. Although the physical and chemical properties of soil are more complex than those of oligotrophic media, both represent nutritionally limited environments for microbial growth. Consequently, the addition of RAE could potentially enhance the inhibitory effect of T. harzianum on pathogenic fungi in soil significantly.
3.4. Combined Application of RAE and T. harzianum S Significantly Promoted Tomato Growth
The pot experiment results demonstrated that the addition of RAE significantly promoted the growth of tomato plants, with markedly increased plant height (Figure 4b, n = 8, F = 37.85, p < 0.05) and biomass (Figure 4a, n = 8, F = 15.43, p < 0.05) compared to the control group (Figure 5). The addition of T. harzianum S also exhibited a promoting effect on tomato growth, though this effect was not statistically significant (p > 0.05). Compared with the T. harzianum S addition group, the RAE addition group significantly increased the biomass and plant height of tomato plants. Moreover, the combined application of RAE and T. harzianum S (RAE + T. harzianum S group) resulted in significantly greater plant height and biomass, increasing by 102% and 130%, respectively, compared to the T. harzianum S group. While these values were higher than those observed in the RAE group, no statistically significant difference was detected between the RAE and the control groups (Figure 4). Notably, the addition of 2KGA alone did not promote tomato growth, suggesting that the growth-promoting effects of RAE may not be primarily attributable to its primary component, 2KGA.
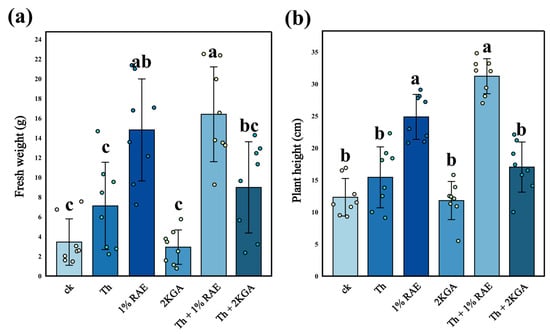
Figure 4.
Biomass (a) and height (b) of tomato plants (mean ± standard deviation, n = 8). Different lowercase letters indicated significant differences among respective groups based on one-way ANOVA tests for multiple comparisons by Tukey’s corrections (p < 0.05). CK: KCl application group; Th: T. harzianum S application group; 1% RAE: 1% RAE application group; 2KGA: 2-keto-L-gulonic acid application group; Th + 1% RAE: the combined application of T. harzianum S and 1% RAE group; Th + 2KGA: the combined application of T. harzianum S and 2-keto-L-gulonic acid group.

Figure 5.
Growth of tomato plants under different treatments.
3.5. Combined Application of RAE and T. harzianum S Significantly Reduced the Abundance of B. cinerea in Soil
The results of quantitative PCR (qPCR) showed that the abundance of T. harzianum S in the rhizosphere soil of the combined addition group (RAE + T. harzianum S) was significantly higher than that observed in the single group (T. harzianum S) (Figure 6a, n = 15, F = 112.5, p < 0.05). This indicates that RAE substantially enhanced the colonization of T. harzianum S in tomato rhizosphere soil. The abundance of T. harzianum S in the rhizosphere soil of the combined group (2KGA + T. harzianum S) was numerically higher than that in the single addition group (T. harzianum S), but this difference was not statistically significant (p > 0.05). Additionally, compared with the control group, the abundance of T. harzianum S in the rhizosphere soil of tomatoes significantly increased in the group treated with RAE alone (Figure 6a). However, compared with the well-controlled conditions of pot experiments, the field environment is considerably more complex and dynamic. Consequently, for the further field application of RAE and T. harzianum, it is essential to investigate more efficient application strategies.
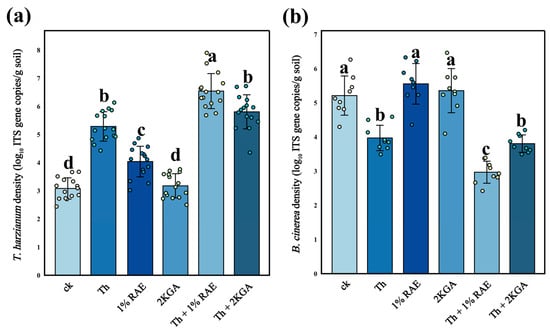
Figure 6.
Copy numbers of ITS genes in T. harzianum (a) and B. cinerea (b) among rhizosphere soil of different treatments (mean ± standard deviation, n = 15). Different lowercase letters indicated significant differences among respective groups based on one-way ANOVA tests for multiple comparisons by Tukey’s corrections (p < 0.05). CK: KCl application group; Th: T. harzianum S application group; 1% RAE: 1% RAE application group; 2KGA: 2-keto-L-gulonic acid application group; Th + 1% RAE: the combined application of T. harzianum S and 1% RAE group; Th + 2KGA: the combined application of T. harzianum S and 2-keto-L-gulonic acid group.
The addition of T. harzianum S significantly reduced the abundance of the pathogenic fungus B. cinerea in soil compared with the control (Figure 6b, n = 9, F = 41.87, p < 0.05). When RAE was applied in combination with T. harzianum S, the abundance of B. cinerea in soil decreased significantly by 25% relative to the group treated with T. harzianum S alone. Notably, although 2KGA can serve as a carbon source for T. harzianum S in vitro, it did not significantly enhance the inhibitory activity of T. harzianum S against B. cinerea (Figure 6b). In both the 2KGA group and the RAE group, no significant reduction was observed in the abundance of B. cinerea, which is consistent with the conclusion of our pure culture experiment (RAE and 2KGA had no significant effect on the growth of B. cinerea). This suggests that the inhibitory effects of RAE and 2KGA on B. cinerea may be primarily attributed to enhancing the anti-pathogenic fungi capacity of T. harzianum.
3.6. Effects of RAE and T. harzianum S on the Contents of Soil Available Nutrients
Compared with the control group, RAE addition alone or the combined addition of RAE and T. harzianum S significantly increased the contents of soil available phosphorus (by 243% and 343%, respectively) (Figure 7a, n = 6, F = 45.41, p < 0.05; Table 2) and ammonium nitrogen (by 107% and 95%, respectively) (Figure 7c, n = 6, F = 18.83, p < 0.05; Table 2). Furthermore, the soil available phosphorus content in the RAE group was significantly higher than that in any other group. Notably, the combined addition group (RAE + T. harzianum S) exhibited a significantly lower concentration of available phosphorus compared to the single addition group (RAE) (Figure 7a). The combined addition of RAE and T. harzianum S significantly increased the soil nitrate nitrogen content by 36% compared with the control group, whereas RAE addition alone did not result in a significant increase in soil nitrate nitrogen content (Figure 7b, n = 6, F = 7.180, p < 0.05; Table 2). In contrast, neither 2KGA nor T. harzianum S alone had any significant effect on the four indices (available phosphorus, ammonium nitrogen, nitrate nitrogen, available potassium) compared to the control group. No statistically significant differences in available potassium content were observed among the six groups (Figure 7d, n = 6, F = 3.452, p > 0.05).
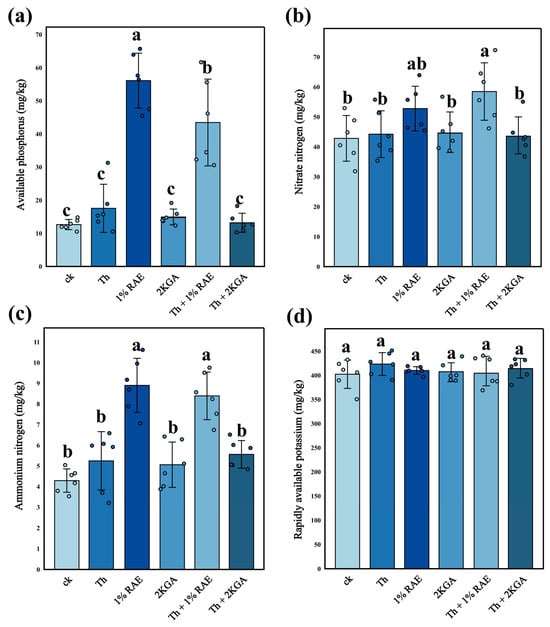
Figure 7.
Nutrient content of potting soil under different treatments (mean ± standard deviation, n = 6). (a) The available phosphorus content of the soil; (b) The nitrate nitrogen content of the soil; (c) The ammonium nitrogen content of the soil; (d) The rapidly available potassium content of the soil. Different lowercase letters indicated significant differences among the six groups based on one-way ANOVA tests for multiple comparisons by Tukey’s corrections (p < 0.05). CK: KCl application group; Th: T. harzianum S application group; 1% RAE: 1% RAE application group; 2KGA: 2-keto-L-gulonic acid application group; Th + 1% RAE: the combined application of T. harzianum S and 1% RAE group; Th + 2KGA: the combined application of T. harzianum S and 2-keto-L-gulonic acid group.

Table 2.
The increment rates of several available nutrient contents in the treatment group compared with the control group.
3.7. Effects of RAE and T. harzianum S on the Activities of Antioxidant Enzymes in Tomato Plants
Compared with the control group, the addition of T. harzianum S significantly increased the peroxidase (POD) activity of tomato plants, whereas RAE or 2KGA added alone did not significantly affect POD activity. However, when RAE or 2KGA was combined with T. harzianum S, POD activity was significantly enhanced (Figure 8a, n = 6, F = 15.62, p < 0.05). Compared to the control group, superoxide dismutase (SOD) activity in tomato plants was significantly elevated in both the RAE group and the combined group of T. harzianum S and RAE (Figure 8d, n = 6, F = 20.12, p < 0.05). The addition of RAE significantly increased catalase (CAT) activity, while the combination of T. harzianum S and RAE also led to an increase in CAT activity, though this effect was not statistically significant (Figure 8c, n = 6, F = 4.554, p > 0.05). No statistically significant differences in ascorbate peroxidase (APX) activity were observed among the six groups (Figure 8b, n = 6, F = 0.8327, p > 0.05). Overall, T. harzianum S had a more pronounced effect on POD activity in tomato plants, while RAE exhibited a greater influence on SOD and CAT activities. The combined application of RAE and T. harzianum S resulted in the highest levels of POD, APX, and SOD activities among the six groups.
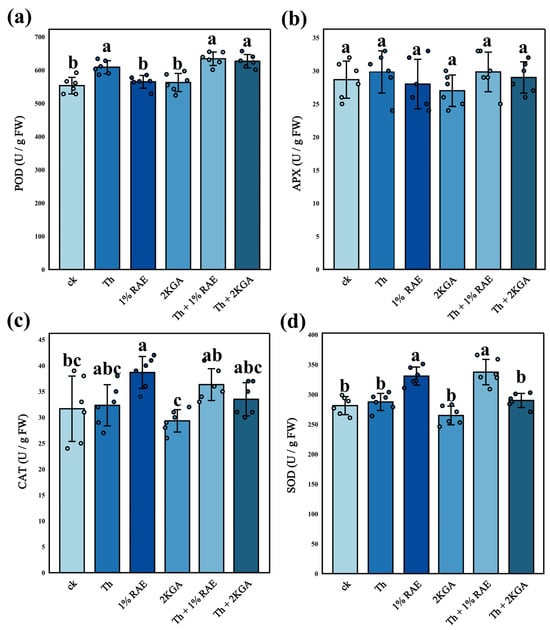
Figure 8.
Activity of antioxidant enzymes in tomato plants (mean ± standard deviation, n = 6). (a) The peroxidase activity of tomato plants; (b) The ascorbate peroxidase activity of tomato plants; (c) The catalase activity of tomato plants; (d) The superoxide dismutase activity of tomato plants. Different lowercase letters indicated significant differences among respective groups based on one-way ANOVA tests for multiple comparisons by Tukey’s corrections (p < 0.05). FW indicates fresh weight. U indicates the unit of enzyme activity. SOD: superoxide dismutase; POD: peroxidase; APX: ascorbate peroxidase; CAT: catalase. CK: KCl application group; Th: T. harzianum S application group; 1% RAE: 1% RAE application group; 2KGA: 2-keto-L-gulonic acid application group; Th + 1% RAE: the combined application of T. harzianum S and 1% RAE group; Th + 2KGA: the combined application of T. harzianum S and 2-keto-L-gulonic acid group.
3.8. Correlation Analysis of Plant and Soil Indexes
The results of correlation analysis showed that the abundances of T. harzianum S, plant biomass, plant height, soil available phosphorus content, soil ammonium nitrogen content, soil nitrate nitrogen content, POD activity of leaves, CAT activity of leaves, and SOD activity of leaves were all significantly positively correlated with RAE. These results suggest that RAE may play a critical role in enhancing soil nutrient availability, promoting the growth of plants and T. harzianum S, and increasing antioxidant enzyme activities. Furthermore, the abundance of T. harzianum S in the rhizosphere soil was significantly positively correlated with plant biomass, plant height, POD activity, and SOD activity, indicating its potential to stimulate plant growth and improve antioxidant enzyme activities. In contrast, the abundance of B. cinerea in the rhizosphere soil exhibited significant negative correlations with the abundance of T. harzianum S, plant biomass, plant height, POD activity, and APX activity, suggesting that B. cinerea might be suppressed by the rapid growth of T. harzianum S and the associated increases in plant biomass and antioxidant enzyme activities (Figure 9).
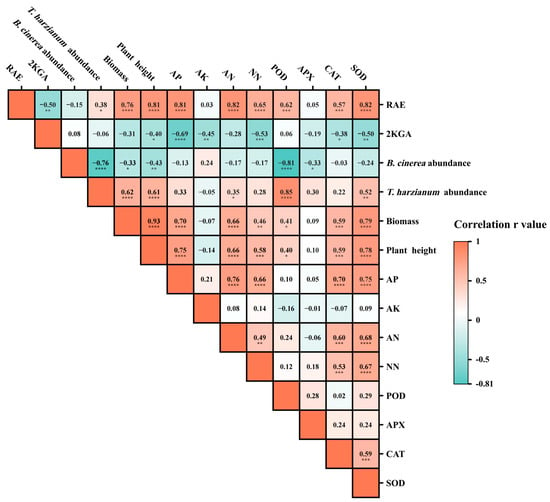
Figure 9.
Correlation analysis of plant and soil indexes. AP: available phosphorus; AK: available potassium; AN: ammonium nitrogen; NN: nitrate nitrogen. SOD: superoxide dismutase; POD: peroxidase; APX: ascorbate peroxidase; CAT: catalase. Correlation analysis based on Spearman’s method, r-value, and the coefficient of correlation. Asterisks (*) in the figure indicate statistically significant correlation, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, **** indicates p < 0.0001.
4. Discussion
Trichoderma is a group of fungi with strong vitality that can survive in a variety of environments and can use a variety of organic and inorganic nutrient sources to grow and reproduce [43]. Among the variety of carbon sources, glucose, starch, sorbinose, sucrose, and mannose are more easily utilized by Trichoderma [44]. The results of this study showed that RAE and 2KGA could be utilized by T. harzianum, and significantly promoted the growth of T. harzianum in an oligotrophic state (Figure 1, Figure 2 and Figure 3). This could be due to the substantial presence of organic small molecular carbon sources in RAE, such as 2-keto-L-gulonic acid, formic acid, oxalic acid, acetic acid, etc., which can be rapidly utilized by T. harzianum, thereby promoting its growth. Existing studies have confirmed that small molecular carbon in RAE can activate nutrients in the plant rhizosphere, potentially enhancing both plant and root growth [31,41]. This activation may also stimulate the release of root exudates, which T. harzianum can utilize to further foster its growth [45]. Additionally, the application of RAE can significantly influence the growth and metabolic functions of beneficial soil bacteria, which may also provide organic nutrients that support the growth of T. harzianum in the soil [43]. However, RAE is a complex mixture, and its specific composition has not been fully elucidated. Consequently, the primary components that promote fungal growth remain unidentified. The further utilization of HPLC or LC-MS to analyze the composition of RAE is essential for deeper exploration.
Numerous studies have demonstrated that the inhibitory ability of T. harzianum on pathogenic fungi primarily relies on its rapid growth rate [46,47]. Specifically, when T. harzianum coexists with pathogenic fungi in the same environment, its superior growth rate enables it to effectively compete for nutrients and ecological niches [48]. This competitive advantage subsequently inhibits the proliferation of pathogenic fungi. The results of this study showed that the addition of RAE significantly enhanced the inhibitory ability of T. harzianum against pathogenic fungi in an oligotrophic state (Figure 4b). This may be attributed to the substantial increase in the growth rate of T. harzianum induced by RAE. Although pathogenic fungi can also utilize RAE for their growth, the impact of RAE on their growth rate was markedly lower compared to that observed in T. harzianum. After adding RAE to the oligotrophic medium, the growth rate of T. harzianum increased by 0.028 cm/h, which was 5.53 times the increase in the growth rate of the three pathogenic fungi (Figure 4a). T. harzianum has strong ecological adaptability [49]. In comparison to pathogenic fungi, T. harzianum exhibits a greater preference and affinity for RAE (Figure 4a). This difference may enable RAE and T. harzianum to more effectively inhibit pathogenic fungi in soil and other nutrient-poor environments.
The results of the pot experiment indicated that the combination of RAE with T. harzianum significantly reduced the number of pathogenic fungi (B. cinerea) in the soil compared to the application of T. harzianum alone. This finding suggests that RAE, as a new type of biological control enhancer, significantly enhances the disease-inhibitory ability of T. harzianum. Based on existing research, we speculated that this phenomenon may be due to the fact that in a nutrient-poor environment (such as soil), compared with pathogenic fungi, T. harzianum can use RAE as an effective carbon source for its rapid growth. The proliferation of T. harzianum may subsequently inhibit the growth of pathogenic fungi. This study presents an innovative approach for enhancing the efficacy of biological control. In contrast to traditional intensification methods (e.g., screening Trichoderma strains with high colonization efficiency or co-applying multiple biocontrol strains), the proposed approach demonstrates superior cost-effectiveness and operational efficiency. Beyond effectively reducing disease incidence, this method provides additional agronomic benefits, including (a) enhanced crop growth performance, (b) improved soil nutrient availability, and (c) optimized soil microbial community structure and diversity. Furthermore, correlation analysis results showed that the biomass and plant height of tomato plants and the activities of some key antioxidant enzymes (POD, APX) were significantly negatively correlated with the abundance of pathogenic fungi (B. cinerea). We propose that RAE may also suppress the pathogen’s proliferation in the soil by enhancing soil nutrient availability, promoting plant growth, and improving plant resistance to disease. The bacterial community in soil is significantly associated with plant disease [50]. Numerous studies have demonstrated that RAE has significant effects on the structure, composition, and function of soil bacterial communities [31,41]. Thus, the combination of RAE and T. harzianum may also inhibit soil pathogenic fungi by regulating the abundance and function of soil beneficial bacteria. Further exploration is needed through amplicon sequencing and metagenomic sequencing.
T. harzianum, as a common biocontrol microorganism, exerts its inhibitory effect on plant disease through the production of inhibitory secondary metabolites [51]. This study found that the aseptic fermentation supernatant of T. harzianum had no significant inhibitory effect on A. alternata and Corynespora cassiicola, and the addition of RAE had no significant effect on the inhibitory effect (see Supplementary Material Figure S4). This may be because the fermentation conditions of T. harzianum are not suitable, or the metabolites of T. harzianum may have an inhibitory effect on other pathogenic fungi. Therefore, it is essential to systematically investigate the effects of various carbon sources, nitrogen sources, pH levels, and temperatures on the fermentation products of T. harzianum to optimize their fermentation conditions. At the same time, it is also highly necessary to broaden the inhibitory spectrum of T. harzianum. Furthermore, transcriptome sequencing and metabolome sequencing techniques can also be employed to investigate the impact of RAE on the overall metabolite profile of T. harzianum.
Previous studies have revealed that the application of RAE into soil can activate soil nutrients and promote the transformation of nutrients from an unusable state to an available state [31]. In addition, it is well known that soil extracellular enzymes play an important role in the soil nutrient conversion cycle [52]. Previous research results also show that the addition of RAE significantly increases the activity of some extracellular enzymes in soil, such as phosphatase and urease. The improvement of this enzyme activity greatly increased the availability of nutrients in soil [33]. This study also found that the addition of RAE can significantly increase the content of soil available nutrients (ammonium nitrogen and available phosphorus), which is consistent with the results of several studies [29]. In summary, our findings indicate that RAE exerts a significant positive systematic effect on soil nutrient availability, highlighting its considerable potential as a resourceful organic amendment for agricultural applications.
Antioxidant enzymes in plants play a crucial role in effectively eliminating harmful reactive oxygen species generated within the plant, and are of great significance in the process of plant resistance to various biological and abiotic stresses [53,54]. Previous studies have demonstrated that RAE can significantly enhance the activity of certain antioxidant enzymes in plants [29]. By increasing the activity of these enzymes, RAE can significantly enhance the resistance of non-heading Chinese cabbage to salt stress and the resistance of Arabidopsis thaliana to cold stress [32]. Consistent with the findings of earlier research, this study reveals that RAE can markedly elevate the activity of antioxidant enzymes (CAT, SOD) in tomato, thereby aiding its resistance to plant diseases. In addition, the overall impact of RAE on plant metabolism has been elucidated through the application of plant metabolome detection technology, specifically concerning its effects on the metabolites of Perilla, Portulaca, and Arabidopsis. The findings indicate that RAE significantly influences both carbon metabolism and the metabolism of secondary metabolites [31]. The effect of RAE on tomato disease resistance metabolites under biological stress also needs to be further explored using plant metabolome detection technology (LC-MS) to clarify the specific molecular mechanism.
In general, the reasonable use of RAE can not only alleviate the competitive pressure of the vitamin C fermentation factory but also meet the requirements of sustainable green development. Our research shows that, as the bulk waste of vitamin C fermentation, RAE can function as a “prebiotic” and play an important role in bolstering the disease resistance capabilities of biocontrol fungi (T. harzianum). In this study, RAE was utilized for the first time to enhance the growth of beneficial fungi and to improve the efficacy of biocontrol fungi in disease suppression. This approach not only expands the avenues for RAE resource utilization but also demonstrates its significant potential for agricultural applications.
5. Conclusions
In this study, we examined the effects of vitamin C industrial byproduct (RAE) on the growth and anti-phytopathogenic fungi capacity of T. harzianum under controlled indoor conditions. In vitro experiments demonstrated that RAE and its primary component 2KGA, served as effective carbon sources, promoting T. harzianum biomass accumulation under oligotrophic conditions while enhancing its antagonistic capacity against phytopathogenic fungi. Pot experiments further revealed that RAE application significantly improved soil nutrient dynamics (e.g., available phosphorus, ammonium/nitrate nitrogen), promoted T. harzianum rhizosphere colonization, and effectively suppressed pathogens, thereby enhancing tomato growth. However, the application efficacy of RAE under field conditions requires further validation through rigorous testing. These findings introduce a new approach for utilizing industrial byproducts in sustainable agriculture, addressing both waste management challenges in vitamin C production and limitations in biological control for crop protection. Future research should focus on the molecular mechanisms of RAE-T. harzianum interactions, as well as the verification of field-scale disease suppression effects on different soil types (such as red soil and brown soil). This study lays a foundation for the development of next-generation biofertilizers that integrate waste valorization with enhanced biological control strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15061298/s1; Figure S1: The standard curve of the ITS gene of T. harzianum; Figure S2: The standard curve of the ITS gene of Botrytis cinerea; Figure S3: The inhibition rates of two strains of T. harzianum (T. harzianum S and T. harzianum L.) against pathogenic fungi; Figure S4: Inhibitory effect of supernatant of aseptic fermentation of T. harzianum S against Alternaria alternata and Corynespora cassiicola.
Author Contributions
Conceptualization, W.Y. and H.X.; methodology, W.S.; software, W.S. and H.S.; formal analysis, W.S. and M.G.; investigation, W.S., M.G. and W.Y.; resources, W.Y. and H.X.; data curation, W.S. and M.G.; writing—original draft preparation, W.S.; writing—review and editing, W.Y. and H.X.; visualization, W.S. and H.S.; supervision, H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number 2023YFD1501200; the Key R&D Program of Liaoning Province, grant number 2024JH2/102400011; the Science and Technology Plan Project of Liaoning Province, grant number 2023JH2/101700358; the Science and Technology Plan Project of Shenyang, grant number 24-216-2-02; the Natural Science Foundation Project of Liaoning Province, grant number 2024-BSBA-56; and the Central Government Guides Local Funds for Science and Technology Development, grant number 2022ZY0104.
Data Availability Statement
All data presented in this study are contained within the article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RAE | residue after evaporation |
| 2KGA | 2-keto-L-gulonic acid |
| PDA | potato dextrose agar |
| ASA | ascorbic acid |
| COD | chemical oxygen demand |
| PBS | phosphate-buffered saline |
| qPCR | quantitative polymerase chain reaction |
| ANOVA | analysis of variance |
| ITS | internal transcriptional spacer |
| HPLC | high-performance liquid chromatography |
| LC-MS | liquid chromatography–mass spectrometry |
References
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 10054. [Google Scholar] [CrossRef]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Ketta, H.A.; Hewedy, O.A.E.-R. Biological control of Phaseolus vulgaris and Pisum sativum root rot disease using Trichoderma species. Egypt. J. Biol. Pest Control 2021, 31, 456. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 352. [Google Scholar] [CrossRef]
- Pacios-Michelena, S.; Aguilar González, C.N.; Alvarez-Perez, O.B.; Rodriguez-Herrera, R.; Chávez-González, M.; Arredondo Valdés, R.; Ascacio Valdés, J.A.; Govea Salas, M.; Ilyina, A. Application of Streptomyces Antimicrobial Compounds for the Control of Phytopathogens. Front. Sustain. Food Syst. 2021, 5, 351–365. [Google Scholar] [CrossRef]
- Weng, W.; Yan, J.; Zhou, M.; Yao, X.; Gao, A.; Ma, C.; Cheng, J.; Ruan, J. Roles of Arbuscular mycorrhizal Fungi as a Biocontrol Agent in the Control of Plant Diseases. Microorganisms 2022, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Leylaie, S.; Zafari, D. Antiproliferative and Antimicrobial Activities of Secondary Metabolites and Phylogenetic Study of Endophytic Trichoderma Species From Vinca Plants. Front. Microbiol. 2018, 9, 456–461. [Google Scholar] [CrossRef]
- Oszust, K.; Cybulska, J.; Frąc, M. How Do Trichoderma Genus Fungi Win a Nutritional Competition Battle against Soft Fruit Pathogens? A Report on Niche Overlap Nutritional Potentiates. Int. J. Mol. Sci. 2020, 21, 4325. [Google Scholar] [CrossRef]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In Vitro Antibacterial, Antifungal, Nematocidal and Growth Promoting Activities of Trichoderma hamatum FB10 and Its Secondary Metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, A.; Harel, M.; Horwitz, B.A.; Chet, I.; Mukherjee, P.K. Trichoderma Mitogen-Activated Protein Kinase Signaling Is Involved in Induction of Plant Systemic Resistance. Appl. Environ. Microbiol. 2005, 71, 6241–6246. [Google Scholar] [CrossRef]
- Ma, Y.; Zuohereguli, K.; Zhang, L.; Kang, Y.; Shi, L.; Xu, H.; Ruan, Y.; Wen, T.; Mei, X.; Dong, C.; et al. Soil Microbial Mechanisms to Improve Pear Seedling Growth by Applying Bacillus and Trichoderma-Amended Biofertilizers. Plant Cell Environ. 2025, 5, 672. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2022, 21, 312–326. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, M.U.; Raza, M.; Raza, W.; Qamar, M.I. Trichoderma as potential biocontrol agent, its exploitation in agriculture a review. Plant Prot. 2018, 02, 109–135. [Google Scholar]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montesinos, B.; Santos, M.; Moreno-Gavíra, A.; Marín-Rodulfo, T.; Gea, F.J.; Diánez, F. Biological Control of Fungal Diseases by Trichoderma aggressivum f. europaeum and Its Compatibility with Fungicides. J. Fungi 2021, 7, 598. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch. Phytopathol. Plant Prot. 2017, 50, 629–648. [Google Scholar] [CrossRef]
- Wu, K.; Yuan, S.; Wang, L.; Shi, J.; Zhao, J.; Shen, B.; Shen, Q. Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial communities. Biol. Fertil. Soils 2014, 50, 961–971. [Google Scholar] [CrossRef]
- Ding, C.; Shen, Q.; Zhang, R.; Chen, W. Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant Soil 2013, 366, 453–466. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, N.; Li, Y.; Zhu, C.; Qu, B.; Liu, H.; Li, R.; Bai, Y.; Shen, Q.; Falcao Salles, J. Bio-organic soil amendment promotes the suppression of Ralstonia solanacearum by inducing changes in the functionality and composition of rhizosphere bacterial communities. New Phytol. 2022, 235, 1558–1574. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, X.; Yin, S.; Shen, Q.; Ran, W.; Xu, Y. Efficacy of Bacillus-fortified organic fertiliser in controlling bacterial wilt of tomato in the field. Appl. Soil Ecol. 2011, 48, 152–159. [Google Scholar] [CrossRef]
- Bedhiafi, T.; Inchakalody, V.P.; Fernandes, Q.; Mestiri, S.; Billa, N.; Uddin, S.; Merhi, M.; Dermime, S. The potential role of vitamin C in empowering cancer immunotherapy. Biomed. Pharmacother. 2022, 146, 112553. [Google Scholar] [CrossRef]
- Milani, G.P.; Macchi, M.; Guz-Mark, A. Vitamin C in the Treatment of COVID-19. Nutrients 2021, 13, 1172. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Q.; Guo, R.; Lyu, S. Two-step fermentation of vitamin C with mixed bacteria based on omics: A review. Microbiol. China 2023, 50, 2191–2203. [Google Scholar] [CrossRef]
- Yang, W.; Han, L.; Mandlaa, M.; Zhang, H.; Zhang, Z.; Xu, H. A plate method for rapid screening of Ketogulonicigenium vulgare mutants for enhanced 2-keto-l-gulonic acid production. Braz. J. Microbiol. 2017, 48, 397–402. [Google Scholar] [CrossRef]
- Gao, M.; Han, X.; Yang, W.; Sun, H.; Zhang, L.; Xu, H. A strategy for improving saline-alkali soil properties and cotton stress tolerance using vitamin C industrial wastes: A “prebiotics-probiotics” interaction between organic acids and Bacillus endophyticus. Ind. Crops Prod. 2024, 220, 119187. [Google Scholar] [CrossRef]
- See, X.Z.; Yeo, W.S.; Saptoro, A. A comprehensive review and recent advances of vitamin C: Overview, functions, sources, applications, market survey and processes. Chem. Eng. Res. Des. 2024, 206, 108–129. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, Z.; Yang, W.; Sun, H.; Xu, H. Application of Organic Waste Derived from Vitamin C Industry Increases Yield and Bioactive Constituents of Medicinal Food Plant Purslane (Portulaca oleracea L.). Horticulturae 2024, 10, 683. [Google Scholar] [CrossRef]
- Gao, M.; Sun, H.; Shi, M.; Wu, Q.; Ji, D.; Wang, B.; Zhang, L.; Liu, Y.; Han, L.; Ruan, X.; et al. 2-Keto-L-Gulonic Acid Improved the Salt Stress Resistance of Non-heading Chinese Cabbage by Increasing L-Ascorbic Acid Accumulation. Front. Plant Sci. 2021, 12, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Gao, M.; Yang, W.; Sun, H.; Kong, T.; Xu, H. Combined application of organic wastes and Trichoderma longibraciatum to promote vegetation restoration and soil quality on mining waste dump sites. Plant Soil 2024, 7, 354. [Google Scholar] [CrossRef]
- Khalil, A.M.A.; Hassan, S.E.-D.; Alsharif, S.M.; Eid, A.M.; Ewais, E.E.-D.; Azab, E.; Gobouri, A.A.; Elkelish, A.; Fouda, A. Isolation and Characterization of Fungal Endophytes Isolated from Medicinal Plant Ephedra pachyclada as Plant Growth-Promoting. Biomolecules 2021, 11, 140. [Google Scholar] [CrossRef]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N.; Vishwanathaswamy, D.K.; Chunduri, S. Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. SpringerPlus 2014, 3, 543. [Google Scholar] [CrossRef]
- Wang, M.; Ge, A.-H.; Ma, X.; Wang, X.; Xie, Q.; Wang, L.; Song, X.; Jiang, M.; Yang, W.; Murray, J.D.; et al. Dynamic root microbiome sustains soybean productivity under unbalanced fertilization. Nat. Commun. 2024, 15, 555. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Pang, G.; Cai, F.; Zhang, J.; Shen, Z.; Li, R.; Shen, Q. Two-step genomic sequence comparison strategy to design Trichoderma strain-specific primers for quantitative PCR. AMB Express 2019, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.B.; Walsh, K.; Boonham, N.; O’Neill, T.; Pearson, S.; Barker, I. Development of real-time PCR (TaqMan®) assays for the detection and quantification of Botrytis cinerea in planta. Plant Physiol. Biochem. 2005, 43, 890–899. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, X.; Li, Y.; Peng, F.; Wang, L. Regulation of POD activity by pelargonidin during vegetative growth in radish (Raphanus sativus L.). Sci. Hortic. 2014, 174, 105–111. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, S.; Liang, X.; Wang, X.; Xie, H.; Wang, D.; Gai, Z.; Wang, N.; Xiang, P.; Han, D.; et al. Foliar spraying of exogenous uniconazole (S3307) at the flowering stage as an effective method to resist low-temperature stress on mung bean [Vigna radiata (L.) Wilczek]. Sci. Rep. 2023, 13, 567. [Google Scholar] [CrossRef]
- Wang, B.; Sun, H.; Yang, W.; Gao, M.; Zhong, X.; Zhang, L.; Chen, Z.; Xu, H. Potential utilization of vitamin C industrial effluents in agriculture: Soil fertility and bacterial community composition. Sci. Total Environ. 2022, 851, 158253. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Asis, A.; Shahriar, S.A.; Naher, L.; Saallah, S.; Fatihah, H.N.N.; Kumar, V.; Siddiquee, S. Identification patterns of Trichoderma strains using morphological characteristics, phylogenetic analyses and lignocellulolytic activities. Mol. Biol. Rep. 2021, 48, 3285–3301. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Fox, G.; Muhammadali, H.; Hollywood, K.A.; Xu, Y.; Goodacre, R.; de Vries, F.T. Comparing root exudate collection techniques: An improved hybrid method. Soil Biol. Biochem. 2021, 161, 108391. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Kumar, D. Trichoderma: A beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt. J. Biol. Pest Control 2020, 30, 133. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Gu, S.; Wei, Z.; Shao, Z.; Friman, V.-P.; Cao, K.; Yang, T.; Kramer, J.; Wang, X.; Li, M.; Mei, X.; et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 2020, 5, 1002–1010. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Keswani, C.; Mishra, S.; Sarma, B.K.; Singh, S.P.; Singh, H.B. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Appl. Microbiol. Biotechnol. 2014, 98, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Wan, X.; Tan, J.; Lu, S.; Lin, C.; Hu, Y.; Guo, Z. Increased tolerance to oxidative stress in transgenic tobacco expressing a wheat oxalate oxidase gene via induction of antioxidant enzymes is mediated by H2O2. Physiol. Plant. 2009, 136, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Bio/Technol. 2011, 11, 55–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).