Abstract
Late blight, caused by Phytophthora infestans, is one of the most destructive diseases in potato cultivation. Chemical fungicides are currently the primary means of controlling it, but they pose significant issues, including environmental pollution and the development of resistant strains. Plant jiaosu (PJ), derived from the direct fermentation of various plants, plays a vital part in various fields such as environmental protection, agriculture, daily chemicals, and livestock. This study illustrates that PJ, fermented from lettuce leaves, significantly inhibits the growth of P. infestans. An isolated anti-oomycete bacterium, designated X-1, has 100% homology to Bacillus subtilis in the NCBI database, shown through 16S rDNA sequence analysis. B. Subtilis X-1 effectively inhibits the hyphal growth and sporangia germination of P. infestans, induces morphological changes in the hyphae, and can promote the growth of potato. PJ exerts a synergistic effect with the chemical fungicide Infinito (the main active ingredients are fluopicolide and propamocarb hydrochloride). In summary, this study provides a novel approach for the reutilization of fruit and vegetable waste and offers an environmentally friendly and effective alternative to chemical fungicides for controlling potato late blight.
1. Introduction
Potato (Solanum tuberosum L.) is well known for its high nutritional content and unique flavor and is among the most important crops globally, ranking fourth in production after wheat (Triticum aestivum L.), rice (Oryza sativa L.), and maize (Zea mays L.) [1,2]. In accordance with the Food and Agriculture Organization (FAO) of the United Nations, global potato production in 2022 exceeded 374 million tons, and China’s planting area and total production reached 5.72 million hectares and 9.55 million tons, respectively, making China the leading producer worldwide [3]. However, diseases that occur during potato growth often cause an enormous decrease in yield and quality, among which late blight, caused by Phytophthora infestans, is the most devastating [4,5]. It is estimated that the annual global economic cost related to potato late blight damage and control measures exceeds USD 6 billion [6]. Since the first outbreak of potato late blight in Ireland in the 19th century, efforts to combat the disease have been relentless, though some attempts have been ineffective [7]. The application of chemical fungicides is one of the oldest and most effective methods for controlling late blight, and it is still widely utilized today [8]. However, the excessive and lasting usage of chemical fungicides poses several risks, including environmental and economic burdens, food safety issues, threats to human health, the emergence of resistant strains, etc. [7,9,10]. Therefore, there is an urgent need to develop alternative environmentally friendly methods for the prevention and control of potato late blight.
Biofungicides or biopesticides, produced by living organisms (fungi, bacteria, viruses, etc.) or their metabolic products (pheromones, growth regulators, sodium naphthalene acetate, etc.), are used to kill or inhibit agricultural pathogens or pests [11]. Plant jiaosu (PJ) is derived from the fermentation of fresh vegetables, fruits, mushrooms, medicinal plants, and other plant materials, with or without the addition of auxiliary materials. PJ contains various beneficial microorganisms, active vitamins, trace elements, microbial secondary metabolites, and growth-promoting factors [12,13]. Due to its complexity, PJ has multiple roles: as food, it can scavenge free radicals, enhance human immunity, aid digestion, and protect the liver; in environmental protection, it can purify water, degrade pesticide residues, and reduce heavy metal pollution; in agriculture, it can inhibit pathogenic microorganisms, improve soil fertility, regulate plant physiological metabolism, and enhance photosynthesis efficiency; in daily chemicals, it can disinfect, treat acne, whiten, and resist aging; and in livestock, it can improve reproductive rates and survival rates and enhance breeding environments [14,15,16,17].
In plant protection, PJ made from different fruits and vegetables can effectively control a variety of disease such as pear scab, decay, and bacterial spot and defeat some pathogens, like Escherichia coli, Staphylococcus aureus, and Salmonella [14,18,19,20,21,22]. On the one hand, PJ microorganisms can occupy crop surfaces preferentially, restraining harmful pathogen growth; meanwhile, on the other hand, the amino acids, ethanol, acetic acid, citric acid, and other organic acids generated in PJ solution can suppress pathogenic microorganisms’ proliferation [16]. However, research on the direct inhibition of P. infestans by PJ has not been reported.
In this study, the following work was conducted: fruit and vegetable wastes were used to prepare PJ, whose inhibitory effect on and mechanisms against P. infestans were preliminarily explored; the bacterial diversity of PJ was analyzed, and the strains isolated from PJ were tested on their anti-oomycete influence and related physiological and biochemical properties. The synergistic effects of PJ and Infinito (the most commonly used chemical fungicide for controlling potato late blight, whose main active ingredients are fluopicolide and propamocarb hydrochloride) and their growth-promoting effects on potatoes were preliminarily investigated.
2. Materials and Methods
2.1. Materials
Potatoes (Favorita) were collected from DaBao Village, WuXi County, China. Rye agar medium (Rye), nutrient agar (NA), luria–bertani (LB), and potato dextrose agar (PDA) media were prepared according to the methods of Huo and Wiegand [23,24]. The P. infestans strain T30-4 (A1 mating type) and strain 88069 (sterile mating type) were kindly provided by Professor Suomeng Dong of Nanjing Agriculture University and Professor Jiasui Zhan of Fujian Agriculture and Forestry University, China, respectively. Except where noted, the P. infestans strian was T30-4, which was incubated on rye agar medium in 90 mm Petri dishes at 20 °C in the dark. PJ was derived from the fermentation of fruit and vegetable waste (FVW) collected from local markets. Orange peels, onions peels, pepper segments, garlic peels, asparagus lettuce leaves, and lettuce leaves were selected as raw materials for PJ fermentation. Except where noted, PJ was derived from lettuce leaves. These materials were chopped into small pieces with a kitchen knife and weighed using an electronic balance. The mixture (1 part brown sugar–3 parts FVW–10 parts water), which was calculated by weight (g), was placed in fermentation tanks made by plastic containers, which were sealed and vented 1–2 times daily in the early stage (60 d); the mixture was left in a cool place until no gas was emitted (normally for 90 d at 25 °C).The pH of the PJ system (after 90 days of fermentation) was measured by a pH meter (PHS-3C, Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China). Infinito was purchased from Bayer AG, Germany, and its main active ingredients are fluopicolide and propamocarb hydrochloride.
2.2. Anti-Oomycete Effect of PJ on P. infestans
The PJ underwent treatments in the following groups: the original group (without any treatment, OJ), filtration group (filtration through a 0.22 μm microporous membrane to remove microorganisms, FJ), and sterilization group (autoclaving at 121 °C for 20 min, SJ). First, a preliminary experiment was conducted, where the concentrations of the three treated PJ solutions were adjusted to 0, 0.1, 1, 5, 10, 20, 40, 60, 80, and 100 mL/L to assess their inhibitory effects on P. infestans. Based on these observations, a scientifically reasonable concentration gradient was established. Then, PJ was then added to create Rye with concentrations as follows: 0, 0.01, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, and 10 mL/L in the original group, 0, 0.1, 0.5, 1, 5, 10, 25, and 50 mL/L in the filtration group, and the same as those in the filtration group in the sterilization group. P. infestans blocks with a diameter of 7 mm were inoculated in triplicate. The cultures were incubated at 20 °C in darkness, and after 10 d, the diameters were measured using a ruler using the cross method that follows a standardized protocol: two perpendicular reference lines are drawn on the agar plate, ensuring that the colony to be measured is centered at the intersection; the colony diameter is measured along both perpendicular directions; and the average value is taken to minimize measurement error. The inhibition rates were calculated by the following formula:
where Fck and Ft represent the colony diameter of the P. infestans in the control and treatment groups, respectively, and F0 is 7 mm.
The half-maximal inhibitory concentration (IC50) of PJ in P. infestans was determined using IBM SPSS Statistics 27.0 (IBM Corp., Armonk, NY, USA) based on the inhibition rates. The contribution rate of different components of PJ to the inhibition of P. infestans was calculated by the following formula:
where OCR represents the contribution rate of other components (including heat-sensitive substances and other materials), IRs denotes the inhibition rate of the FJ treatment, and IR indicates the inhibition rate of the OJ treatment, with all concentrations at 0.1 mL/L. MCR signifies the contribution rate of the microbial components. Similarly, the contribution rate of heat-sensitive substances within the OCR can be determined.
2.3. Bacterial Diversity Sequencing
Given that PJ is a non-homogeneous system, samples were pretreated by centrifugation. The PJ was thoroughly mixed, sampled, and subjected to three biologically replicated procedures. The samples were centrifuged at 13,000× g for 10 min, the supernatant was discarded, and the precipitate was frozen in liquid nitrogen for 10 min before being stored at −80 °C. The samples were then sent to Shanghai Majorbio Bio-pharm Technology Co., Ltd., for diversity sequencing. Total microbial genomic DNA was extracted using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s instructions. The hypervariable regions V3-V4 of the bacterial 16S rDNA gene were amplified with the primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) using a T100 Thermal Cycler PCR thermocycler (BIO-RAD, Hercules, CA, USA). The PCR product was extracted from 2% agarose gel and purified using the PCR Clean-Up Kit (YuHua, Shanghai, China) according to manufacturer’s instructions and quantified using Qubit 4.0 (Thermo Fisher Scientific, Waltham, MA, USA). Purified amplicons were pooled in equimolar amounts and paired-end-sequenced on an Illumina Nextseq2000 platform (Illumina, San Diego, San Diego, CA, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The obtained data were denoised using DADA2 software to obtain ASVs. These ASVs were annotated using Qiime2 (v2022.2, https://qiime2.org, accessed on 12 April 2024) software with the Silva138/16s_bacteria species annotation database to determine the bacterial species present in the PJ. Based on the community abundance data in the samples, a Circos sample–species relationship diagram was constructed to identify the microorganisms with higher relative abundance at the phylum and genus levels, i.e., the dominant species in the PJ.
2.4. Isolation, Purification, and Screening of Anti-Oomycete Strains in PJ
About 20 μL of PJ was spread on LB and PDA plates (three plates each). The plates were incubated at appropriate temperatures (LB incubated at 37 °C, PDA at 28 °C) and observed daily for colony growth. Once colonies appeared, bacteria were screened and purified using the streak plate method, while fungi were screened and purified using the punch method. PJ isolates were continuously transferred to new plates and further purified until pure single colonies were obtained. The anti-oomycete activity of PJ isolates against P. infestans was determined using the confrontation culture method. A mycelial block of P. infestans T30-4 was placed at the center of rye agar medium. PJ isolates were inoculated equidistantly around the T30-4 mycelial block (at the vertices of an equilateral triangle, 2.5 cm from the center), with a single T30-4 mycelial block serving as the negative control. After incubation at 20 °C for 5 d, the P. infestans colony diameter was observed.
2.5. Tolerance Analysis of Selected Strains
The selected anti-oomycete strains were exposed to four different stress conditions. For salt stress tests, the candidate strains were inoculated on LB agar plates containing different concentrations of NaCl calculated by weight: 0, 0.01, 0.05, 0.1, and 0.3%. A pH stress test was performed in which the pH of the LB agar plates was adjusted to 5, 7, 9, and 11 by using 0.1 M HCl/NaOH. In UV stress tests, LB agar plates inoculated with candidate strains were exposed to UV light for 5, 10, 15, 20, and 40 min. In temperature stress tests, candidate isolates were cultivated at 4 °C, 20 °C, and 28 °C for 12 h. After inoculating the candidate strains, all plates—except those in the temperature treatment groups—were incubated inverted at 37 °C in a dark environment for 5 days. The colony diameter and inhibition rate were determined in accordance with Section 2.2.
2.6. Identification of Candidate Anti-Oomycete Bacteria
Morphological identification was conducted by observing the relevant characteristics of individual colonies, including color, shape, edge condition, surface texture, and viscosity. The collection of physiological and biochemical identification followed the protocols outlined in Bergey’s Manual of Determinative Bacteriology and the Manual of Systematic Bacteriology [25,26].
For molecular identification via 16S rDNA analysis, total genomic DNA of the selected bacteria was extracted using a bacterial genomic DNA extraction kit (Omega BioTek, Inc., Norcross, GA, USA). The amplified 16S rDNA fragments based on the universal primers 27F/1492R were sequenced by Qingke Biotechnology (Qingke, Chongqing, China)). Nucleotide sequence homology searches and multiple sequence alignment were performed and a phylogenetic tree was constructed using the NCBI, Clustal X, and the neighbor-joining method using MEGA11.0 software(MEGA Software, Philadelphia, PA, USA), respectively.
2.7. Effects of Selected Strains on Mycelial Growth and Sporangia Germination of P. infestans
Anti-oomycete effect on mycelial growth: The confrontation culture method was used to study the anti-oomycete effect of selected strains on the mycelial growth of P. infestans T30-4 and 88069 strains. A mycelial block (10 d old, T30-4 and 88069) was placed in the center of rye agar medium, and the selected strains were inoculated equidistantly around the block. Only P. infestans was cultured as the control. After 7 d of dark incubation at 20 °C, the final colony diameters were measured using the cross method to calculate inhibition rates. The phenotype of P. infestans mycelia with or without the treatment of selected strains were observed under the OptiPlex 3050 inverted fluorescence phase-contrast microscope (LEICA DFC450 C, Deep Space Exploration Center).
Effect on sporangia germination: Sporangia of T30-4 were obtained by washing plates covered with T30-4 mycelia with sterile water. On the basis of 200 µL of T30-4 sporangia suspension, 100 µL of a suspension of the selected strain, X-1, was added, with the same volume of LB liquid medium added as a control. The sporangia were incubated at 20 °C for 18 h, and sporangia germination was observed under the OptiPlex 3050 inverted fluorescence phase-contrast microscope (LEICA DFC450 C, Deep Space Exploration Center).
2.8. Localization of Anti-Oomycete Substances in Selected Strains
X-1 was inoculated into prepared LB medium at a 1% ratio (30 mL). The shaking incubator was set to 37 °C and 180 rpm. After 1 d of incubation, the bacterial culture of the X-1 strain was obtained. A total of 14 mL of the bacterial culture was centrifuged at 10,000 rpm for 20 min, and the supernatant and bacterial cells were collected separately. The bacterial cells underwent two treatments: (1) A total of 5 mL of PBS solution was added to the bacterial pellet. The cells were resuspended by pipetting and centrifuged, and this was repeated three times. The final pellet was completely resuspended in PBS solution and placed in an ice bath. Cells were disrupted using an ultrasonic cell disruptor (SCIENTZ-ⅡD, Ningbo Xinzhi Biotechnology Co., Ltd., Ningbo, Zhejiang, China) for 30 min (5 s on, 5 s off). The disrupted cells were centrifuged, and the supernatant was collected to obtain the cell lysate. (2) The bacterial pellet was resuspended in sterile water and centrifuged, to obtain the sterile water cell suspension. The supernatant obtained was filtered using a 0.22 μm microporous membrane to obtain the cell-free culture filtrate.
The anti-oomycete activity was observed by the plate confrontation method. A total of 20 µL of the bacterial culture, cell lysate, and sterile water cell suspension were added to three wells, equidistantly to the T30-4 strain mycelium disk, respectively. The control consisted of a rye agar plate (90 mm) inoculated only with the pathogen. In addition, 5% (v/v) cell-free culture filtrate was added to rye agar plates, and a 7 mm mycelium disk of T30-4 was inoculated in the center to observe the inhibitory effect of extracellular anti-oomycete substances on the pathogen. Negative controls included rye agar plates with 5% LB and plain rye agar plates. After 7 d of incubation at 20 °C, colony diameters were measured using the cross method, and the inhibition rate was calculated as described in Section 2.2.
2.9. Fungicide Tolerance of X-1
LB medium was prepared with Infinito at one and ten times the field application concentration (1 and 10 mL/L). After the plates cooled and solidified, the candidate strains were put on them. The plates were incubated at 37 °C for 12 h, and colony growth was observed.
To test the inhibitory performance of X-1 after fungicide treatment, Infinito was added to LB liquid medium to final concentrations of 1 and 10 mL/L, and X-1 inoculum was added. The cultures were incubated at 37 °C with shaking at 180 r/min for 12 h. The anti-oomycete ability of fungicide-treated X-1 was tested by the plate confrontation method: a 7 mm T30-4 mycelial block (10 d old) was placed in the center of a rye agar plate (90 mm), and X-1 cultures treated with different Infinito concentrations were inoculated equidistantly (2.5 cm from the center). Only P. infestans was cultured as a control. Plates were incubated at 20 °C for 7 d, and the final colony diameters of the pathogen were measured using the cross method.
2.10. Combined Effect of PJ and Fungticide
Rye agar medium was supplemented with PJ original solution and Infinito to final concentrations of 1 mL/L of Infinito, 0.2 mL/L of PJ original solution, and a combination of 0.2 mL/L of PJ stock solution and 1 mL/L of Infinito. The anti-oomycete activity was tested using the plate confrontation method: a 7 mm T30-4 mycelial block (10 d old) was placed in the center of the plate. Only P. infestans was cultured as a control. The plates were incubated at 20 °C for 7 d, and final colony diameters were measured using the cross method. To evaluate the combined effect of drugs, the Q value was calculated using the following formula derived from the Jin equation [27]:
where represents the colony diameter under the combined action of PJ and Infinito, while represent the colony diameters under the individual treatments of PJ and Infinito, respectively. A Q value greater than 1 indicates a synergistic effect; a Q value between 0.85 and 1 suggests an additive effect; and a Q value less than 0.85 indicates an antagonistic effect.
2.11. Growth Promotion Experiment with PJ and Anti-Oomycete Effect of PJ Derived from FVW on P. infestans
Potato seed tubers were grown in nutrient-rich soil which was evenly mixed with PJ and an equal volume of tap water (control). All plants were grown under identical conditions (25 °C, 12 h light/12 h dark cycle) for 45 d, after which the main stem length above the soil was measured.
Five kinds of PJ, produced by five types of FVWs (onion skins, vegetable leaves, pepper segments, orange peels, garlic peels) that stock solution was added to, created a medium which was used to create solid rye media with concentrations of 0, 0.1, 0.2, 0.4, 0.8, 1.0, and 10 mL/L. P. infestans blocks with a diameter of 7 mm were inoculated in triplicate. The cultures were incubated at 20 °C in darkness, and after 10 d, diameters were measured using the cross method to calculate inhibition rates and IC50.
2.12. Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics 27.0 software (IBM Corp., Armonk, NY, USA) for t-tests or Duncan’s multiple-range tests. Standard deviations were calculated and graphs were plotted using Origin 2021 software (OriginLab Corporation, Northampton, MA, USA). Data were presented as the mean ± SD, with whiskers representing standard deviation
3. Results
3.1. Anti-Oomycete Effect of PJ and Localization of Anti-Oomycete Substances
PJ represents a complex system comprising various microorganisms and organic and inorganic substances. To ascertain the anti-oomycete effect of PJ (fermented from lettuce (Lactuca sativa L. var. ramosa Hort.) leaves) and identify the specific agents responsible for these effects, co-culture experiments were carried out with P. infestans using three treatment groups: the original group, the sterilized group, and the filtrate group. The growth of P. infestans on the plates after 10 d of cultivation is depicted in Figure 1A–C. As the concentration of PJ increased, the inhibition rates of all three treatments progressively rose. At PJ concentrations of 1, 25, and 5 mL/L for the original, sterilized, and filtrate groups, respectively, the growth of P. infestans hyphae was almost completely inhibited (Figure 1D–F). The IC50 values indicated that the sterilized group had the highest IC50 at 5.19 mL/L, meaning that it required the highest concentration to achieve 50% inhibition. The filtrate group followed with an IC50 of 0.92 mL/L, while the original group had the lowest IC50 at 0.28 mL/L, suggesting that heat-sensitive compounds and microorganisms in PJ play a major role in anti-oomycete activity (Figure S1). Notably, redundancy analysis revealed that the anti-oomycete contribution of microorganisms was 77.92%, indicating that microorganisms in PJ play the most crucial role in resisting P. infestans. In addition, the pH value of the PJ was 3.98, suggesting that acidity may be one factor in the inhibitory effect of PJ.
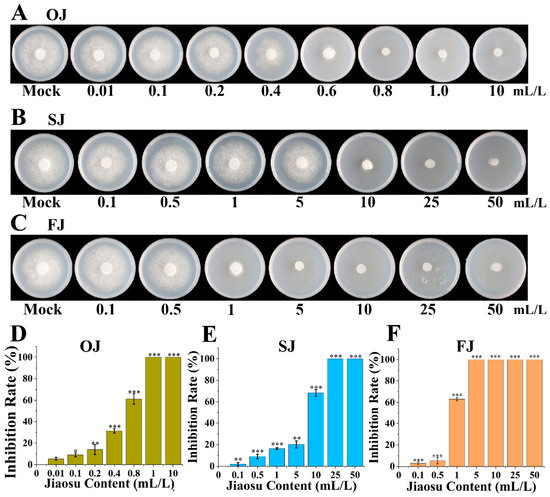
Figure 1.
Anti-oomycete effect of PJ on P. infestans and localization of anti-oomycete substances in PJ. (A–C) Growth of P. infestans on OJ, SJ, and FJ; (D–F) inhibition rate of OJ, SJ, and FJ (t-test: * p < 0.05; ** p < 0.01; *** p < 0.001); OJ: original jiaosu; SJ: sterilized jiaosu; FJ: filtered jiaosu.
3.2. Bacterial Diversity Analysis of PJ
After processing the data from bacterial diversity sequencing, a total of 794,507 optimized sequences of the 16S rDNA V4 region were obtained from the three samples, with an average sequence length of 428 bp, consistent with the expected length for the 16S rDNA V4 region. A total of 227 amplicon sequence variants (ASVs) were generated and annotated using the silva138/16s bacteria database, identifying 17 phyla, 31 classes, 69 orders, 98 families, 125 genera, and 145 species, which suggested rich bacterial diversity in the PJ (Figure 2A). The community bar chart and Circos plots illustrating the relationship between samples and species show that, at the phylum level, the dominant phyla with relative abundance > 4% were primarily from Firmicutes and Proteobacteria. At the genus level, focusing on those with relative abundance > 1%, Lactobacillus was the most abundant, followed by Acetobacter and Bacillus (Figure 2B,C). This indicates that Lactobacillus, Acetobacter, and Bacillus were the dominant species in the PJ, potentially playing significant roles in fermentation and anti-oomycete activity.
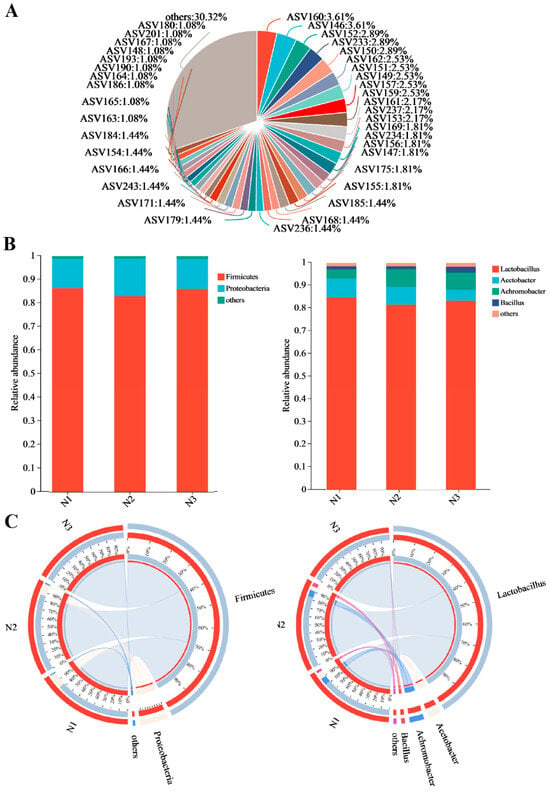
Figure 2.
Bacterial community composition in plant jiaosu (PJ). (A) The abundance of each ASV in the PJ; (B) the composition and proportions of different microorganisms within the PJ at both the phylum and genus levels; (C) the relationship between samples and species and the major dominant microorganisms in the PJ at the phylum and genus levels. (Note: N represents PJ.)
3.3. Isolation, Purification, Screening, and Identification of Anti-Oomycete Strains from PJ
PDA, LB, and NA media were used for isolating the microorganisms in the PJ. Four bacteria (X-1, X-2, X-3, P-4) and two fungi (P-5, P-6) with fast growth were selected for anti-oomycete experiments (Figure 3A). Three strains demonstrated significant anti-oomycete effects against P. infestans, among which X-1 and P-4 completely inhibited its growth on plates within 7 d (Figure 3B). In the pH, NaCl, temperature, and UV stress experiments, the strain X-1 exhibited better growth than P-4 (Table S1); herein, X-1 was chosen for further experiments. The strain X-1 had milky white, sticky, wet, smooth, and opaque colonies with folds on the surface (Figure 3A) and was identified as a Gram-positive bacterium (Table S2). Physiological and biochemical identification results showed that X-1 could produce amylase and gelatinase, and the catalase test was positive, indicating its need for oxygen (Table S2). The 16S rDNA sequence of the strain X-1 was obtained and compared against the NCBI database using BLASTN, showing that the strain X-1 had 100% homology with Bacillus subtilis. Further, based on the maximum-likelihood method, MEGA11.0 software was employed to construct the phylogenetic tree, with Bootstrap values set at 1000, which implied that X-1 is most closely related to B. subtilis CR-502, DSM 10, etc. (Figure 3C).
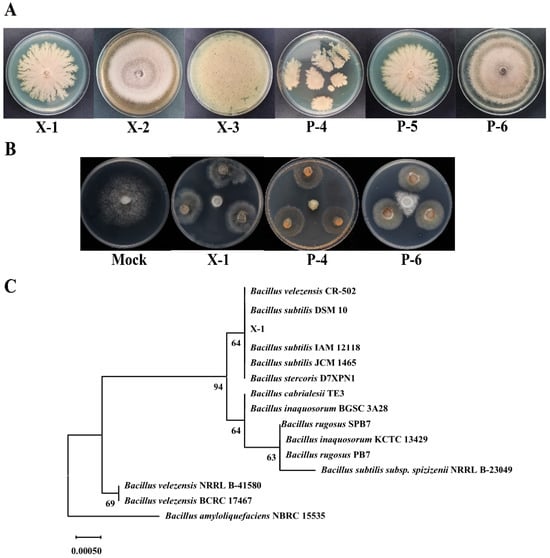
Figure 3.
The isolation, purification, screening, and identification of anti-oomycete strains in the plant jiaosu (PJ). (A) The isolation and purification of microorganisms; (B) the anti-oomycete activity of X-1, P-4, and P-6 in P. infestans; (C) the phylogenetic tree of the strain X-1 with the strains downloaded from NCBI. (The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to produce the phylogenetic tree. The evolutionary distances were computed using the p-distance method.)
3.4. Effect of B. subtilis X-1 on Mycelial Growth and Sporangia Germination of P. infestans
Using the confrontation culture method, we found that B. subtilis X-1 exhibited good anti-oomycete activity in P. infestans T30-4 (A1 mating type) and 88069 (sterile mating type), with 73.81% and 82.30% inhibition rates, respectively (Figure 4A–D). Further observation of plates incubated at 20 °C for several months found that P. infestans remained inhibited, indicating the long-lasting anti-oomycete effect of B. subtilis X-1. Microscopic observations revealed that the hyphae of the pathogen treated with X-1 were curved, with uneven widths and bulging formations, whereas the hyphae in the control group exhibited normal growth (Figure 4E) When sporangia were statically incubated in darkness at 20 °C for 18 h, most sporangia in the control group reached completed germination, while the sporangia germination rate significantly decreased to 12.31% under the treatment with B. subtilis X-1. Moreover, the germination speed and germ tube growth of the germinated sporangia were also inhibited when P. infestans was cultured with X-1 (Figure 4F,G). The anti-oomycete effect of B. subtilis X-1 against P. infestans was most pronounced when the bacterial culture was used, achieving an inhibition rate of 73.30%, followed by cell lysate (71.64%) and the bacterial suspension in sterile water (70.32%), while the cell-free supernatant resulted in the lowest rate (16.58%), indicating that anti-oomycete substances were present both inside and outside the cells of B. subtilis X-1, but they were primarily intracellular.
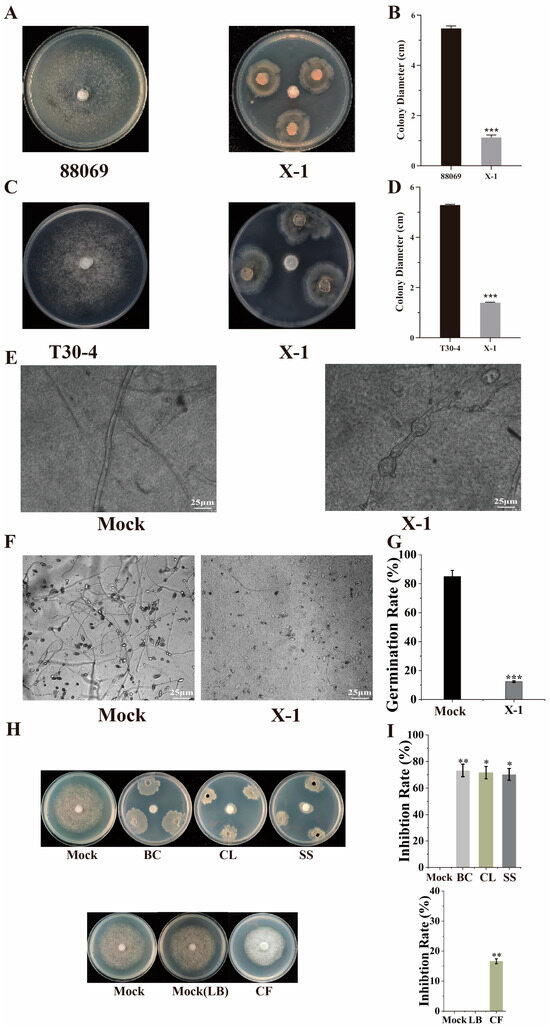
Figure 4.
Effect of B. subtilis X-1 on P. infestans mycelial growth and sporangia germination. (A,B) Colony morphology and diameter of strain T30-4 under X-1 confrontation; (C,D) colony morphology and diameter of strain 88069 under X-1 confrontation; (E) hyphal phenotype of T30-4 treated with X-1 under microscope; (F,G) sporangia germination and inhibition rate of P. infestans with or without X-1 treatment; (H,I) colony diameter and inhibition rate of strain T30-4 treated with X-1 after different treatments. BC: bacterial culture; CL: cell lysate; SS: sterile water cell suspension; CF: cell-free supernatant (t-test: * p < 0.05; ** p < 0.01; *** p < 0.001).
3.5. Evaluation of PJ Application Potential
The fungicide Infinito is one of the most commonly used chemical fungicides for controlling potato late blight. B. subtilis X-1 demonstrated good growth on the rye agar plate with ten times the field application concentration of Infinito (Table S3), indicating its tolerance to this fungicide. To further assess the impact of Infinito on X-1’s ability to inhibit the growth of P. infestans, co-cultivation experiments were conducted using X-1 with LB liquid medium containing the fungicide Infinito. Regardless of whether X-1 was co-cultured with the field application concentration or ten times the field application concentration of Infinito, its anti-oomycete efficacy was inferior to that of X-1 applied alone (Figure 5A,B). In summary, although Infinito does not affect the growth of X-1, it attenuates the anti-oomycete activity of X-1. Therefore, the combined use of Infinito and X-1 is not recommended in production settings.
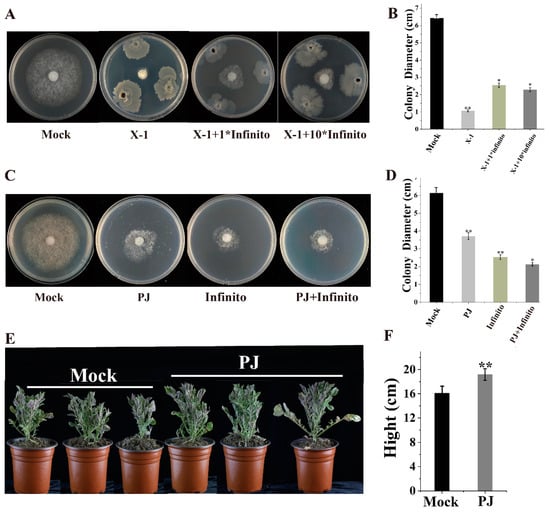
Figure 5.
Application potential of X-1 and plant jiaosu (PJ). (A,B) Growth and colony diameter of P. infestans under treatment with X-1 and Infinito; (C,D) growth and colony diameter of P. infestans after PJ and Infinito treatments; (E,F) growth status and height of potato plants with PJ treatment (t-test: * p < 0.05; ** p < 0.01).
The combined anti-oomycete effect of PJ and Infinito was also explored in in vitro experiments (Figure 5C,D). On the plates only treated with PJ (0.2 mL/L) and Infinito (1 mL/L), the mycelial diameters of P. infestans were 3.72 and 2.53 cm, respectively. When combining them, the mycelial diameter of P. infestans decreased to 2.13 cm. The Q value calculated using the Jin formula was 0.87 (greater than 0.85), indicating an additive effect and potential for combined application.
Forty-five days after planting, potato plants treated with PJ demonstrated a significant increase in growth, with an average plant height of 19.2 cm compared to 16.1 cm in the control group (Figure 5E,F). Additionally, plants treated with the vegetable PJ exhibited 34.1% and 21.2% increases in leaf area and leaf number compared to the control group, respectively (Table S4). These results indicate that the PJ significantly promoted the growth and development of the potato plants.
3.6. Anti-Oomycete Effect of PJ Derived from Fruit and Vegetable Waste (FVW) on P. infestans
Lastly, the anti-oomycete effects of PJ, fermented from different raw materials (orange (Citrus reticulata Blanco), onion (Allium cepa L.), chili pepper (Capsicum annuum L.), garlic (Allium sativum L.), and asparagus lettuce (Lactuca sativa var. angustata)), on P. infestans were investigated. After 10 d of culturing, all five groups exhibited significant inhibitory effects on P. infestans, displaying dose-dependent relationships. The IC50 values for the five treatments were 0.43, 0.21, 0.39, 0.52, and 0.31, respectively, and among the five treatments, PJ derived from asparagus lettuce leaves had the best anti-oomycete effect (Figure 6A,B). Simultaneously, anti-oomycete assays were conducted over a six-month period, all of which demonstrated consistent and effective inhibitory activity, implying that the antagonistic effect of the PJ on P. infestans was stable and enduring. Therefore, utilizing FVW to ferment PJ not only promotes its reuse but also provides an environmentally friendly choice for the prevention and control of potato late blight. Considering factors such as cost, the ease of acquisition, and inhibitory efficacy, the PJ made from asparagus lettuce leaves and lettuce leaves has significant application potential.
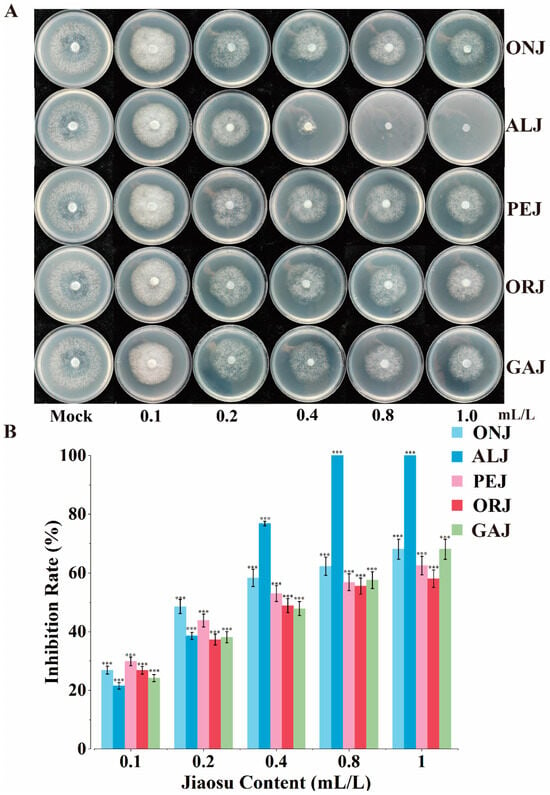
Figure 6.
Inhibition of Phytophthora infestans by plant jiaosu (PJ) derived from fruit and vegetable waste. (A) Growth of P. infestans on plates with different PJ (ONJ: PJ derived from onion peels; ALJ: PJ derived from asparagus lettuce leaves; PEJ: PJ derived from pepper segments; ORJ: PJ derived from orange peels; GAJ: PJ derived from garlic peels); (B) inhibition rate of different forms of PJ (ONJ, ALJ, PEJ, ORJ, GAJ) (t-test: *** p < 0.001).
4. Discussion
Fruit and vegetable waste (FVW), generated during the harvesting, processing, and consumption of fruits and vegetables, amounts to over 100 million tons annually, accounting for 25% to 30% of total fruit and vegetable production, which poses a significant environmental burden [28,29]. However, since the raw materials for PJ are primarily fruits, vegetables, and natural plants [30,31,32], it is possible to recycle FVW to produce PJ. Additionally, relevant research indicates that PJ exhibits notable inhibitory effects on certain fungal diseases such as Botrytis cinerea and root rot [33,34]. Our research also demonstrates that PJ effectively inhibits P. infestans, the pathogen of potato late blight, in a dose-dependent manner. Furthermore, PJ, fermented from five different types of FVW, has good inhibitory effects on P. infestans, suggesting that the raw materials of PJ for potato late blight are widely sourced, low-cost, and easy to obtain. Compared with conventional biopesticides (e.g., biocontrol agents), PJ demonstrates superior application potential through its dual advantages of environmental protection and resource utilization via FVW, warranting further exploration. In a word, this study makes PJ a promising candidate for controlling potato late blight.
Due to the high complexity of PJ, its bacteriostatic capacity is determined by multiple factors, among which microorganisms and organic acids in PJ are key bacteriostat components, which can restrain the reproduction of harmful microorganisms and enhance crop resistance to diseases [33,35,36]. In our study, the effect of raw PJ solution was significantly better than that of sterilized, filtered, and pH-matched control groups, with microorganisms contributing 77.92% of the anti-oomycete effect, and this is consistent with previous studies [34,37], in which PJ has been widely used to protect crops from diseases [38,39,40,41]. To identify the dominant species within the PJ, bacterial diversity analysis was conducted. The Circos diagram shows that the genera with the highest relative abundance were Lactobacillus, Acetobacter, and Bacillus. These genera are likely the key microorganisms contributing to the anti-oomycete activity of the PJ. Previous studies have shown that PJ derived from various substrates, such as blueberries (Vaccinium spp.), grapes (Vitis vinifera L.), and cabbages (Brassica rapa var. glabra Regel), also predominantly contains these genera [37,42]. Additionally, it has been reported that Lactobacillus can inhibit the growth of S. aureus, Fusarium oxysporum, and E. coli, thereby enhancing the effectiveness of PJ in agricultural disease control [43,44,45]. Gil et al. reported that Acetobacter could produce organic substances and also exhibit anti-oomycete properties [46], while Bacillus can synergize with organic acids and other bacterial metabolites to enhance the antifungal activity of PJ [34]. To further study the microorganisms with anti-oomycete activity among the core microorganisms, previous studies on the selection criteria for antagonistic bacteria have primarily focused on the extent of anti-oomycete activity and resistance to various stress factors [47,48,49]. Considering that the practical application environment is often neutral, in addition to these criteria, the growth rate in a neutral environment is also a critical factor, and isolated B.subtilis X-1 stands out for its high growth speed, strong anti-oomycete effect, and good tolerance for stresses (salt, pH, temperature, and UV). It is reported that B. subtilis is a well-known antagonist that antagonizes various plant pathogens, such as Alternaria alternata, Phytophthora capsica, Fusarium moniliforme Sheld, and Phaeoisariopsis personata [50,51], through producing spores or antibiotic substances [52,53,54,55,56]. There are also Bacillus subtilis H17-16, EG21, and WL-2, which have been proven to inhibit the growth of P. infestans [5,57,58].
Multiple broad-spectrum fungicides like mancozeb and chlorothalonil, as well as mixtures such as fluopicolide–propamocab, are widely used [59], and among them, Infinito is highly favored for its protective and curative properties, stability, and low toxicity. However, chemical fungicides often have a single target, and the use of a single fungicide often leads to pathogen resistance [60]. There have been numerous studies on the combined use of biological control and chemical control, such as the formulation of chemical fungicides with biocontrol agents [42,61]. When studying the formulation of biocontrol agents, the primary consideration is the impact of chemical fungicides on the growth of biocontrol bacteria. Consistent with previous research [62,63,64], the biocontrol agent X-1 exhibited good tolerance to the fungicide Infinito. However, Infinito reduced the anti-oomycete activity of X-1, indicating that their combined application may be less suitable. The observed phenomenon may be mediated by Infinito’s disruption of anti-oomycete substances produced by X-1, requiring further investigation to elucidate the precise mechanism. Since there have been no reports of combining PJ with Infinito, our experiments demonstrated that PJ exhibited good anti-oomycete ability when used in combination with Infinito. The Q value calculated using Jin’s formula [27] was 0.87 (greater than 0.85), indicating an additive effect, making it a promising product. Furthermore, PJ has been reported to promote plant growth [15,65], similarly to in this study. In conclusion, recycling FVW to produce PJ is undoubtedly an excellent new method of turning waste into usable products for controlling potato late blight.
5. Conclusions
This study finds that PJ significantly inhibits the growth of P. infestans in a dose-dependent manner and the microorganisms within PJ play a crucial role in the anti-oomycete effect. We identified an isolated strain, X-1, which showed a great inhibition effect against P. infestans, and the additive effect of PJ with Infinito and its ability to promote the growth of potato plants suggest that PJ has great, promising application potential. Furthermore, PJ fermented from five different FVWs also exerted significant inhibitory effects on P. infestans. Additionally, the use of fruit and vegetable waste for producing PJ could provide economic benefits and help protect the environment.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15040946/s1. Figure S1: The IC50 values of OJ, SJ, and FJ; Table S1: Stress tolerance of candidate strains; Table S2: Physiological and biochemical identification of candidate strains X-1 and P-4; Table S3: Fungicides’ tolerance of X-1; Table S4: Leaf number and area.
Author Contributions
Conceptualization, P.X., P.D. and Z.Z.; Methodology, P.X., Y.L. (Yuxuan Liu), A.H., Z.Z., Y.L. (Yi Liu) and P.D.; Software, Y.H., S.L. and L.S.; Formal analysis, P.X., Y.H., A.H., Z.Z. and X.Z. (Xinze Zhang); Investigation, A.H., Y.L. (Yuxuan Liu), P.X. and Y.H.; Resources, Y.Z., D.S. and P.D.; Data curation, P.X., X.O. and X.Z. (Xinze Zhang); Writing—original draft, P.X., Y.L. (Yuxuan Liu), S.L. and Y.L. (Yi Liu); Writing—review & editing, P.X., Y.L. (Yi Liu), Y.H., X.O., C.L. and P.D.; Visualization, P.X., Y.H., Y.L. (Yuxuan Liu), Y.L. (Yi Liu) and X.Z. (Xiaotian Zhang); Supervision, C.L., L.Y., L.S., X.Z. (Xiaotian Zhang), D.S., Y.Z. and P.D.; Project administration, D.S., Y.Z. and P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chongqing Technology Innovation and Application Development Project, grant number CSTB2022TIAD-ZXX0053, the Scientific Research Project of Chongqing Ecological Environment Bureau, grant number CQEE2022-STHBZZ118, the Fundamental Research Funds for the Central Universities, grant number 2024CDJXY016, and the Open Project Program of Panxi Crops Re-search and Utilization Key Laboratory of Sichuan Province, grant number SZKF202302.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PJ | Plant jiaosu |
| FAO | Food and Agriculture Organization |
| Mock | Control group |
| OJ | Original jiaosu |
| SJ | Sterilized jiaosu |
| FJ | Filtered jiaosu |
| ONJ | PJ derived from onion peels |
| ALJ | PJ derived from asparagus lettuce leaves |
| PEJ | PJ derived from pepper segments |
| ORJ | PJ derived from orange peels |
| GRJ | PJ derived from garlic peels |
| LB | Luria–bertani |
| PDA | Potato dextrose agar |
| NA | Nutrient agar |
References
- Wu, Z.H.; Ma, Q.; Sun, Z.N.; Cui, H.C.; Liu, H.R. Biocontrol mechanism of Myxococcus fulvus B25-I-3 against Phytophthora infestans and its control efficiency on potato late blight. Folia Microbiol. 2021, 66, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Kondhare, K.R.; Natarajan, B.; Banerjee, A.K. Molecular signals that govern tuber development in potato. Int. J. Dev. Biol. 2020, 64, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Available online: http://www.fao.org/faostat/ (accessed on 4 November 2024).
- Wang, Y.; Pruitt, R.N.; Nürnberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Sorokan, A.; Benkovskaya, G.; Burkhanova, G.; Blagova, D.; Maksimov, I. Endophytic strain Bacillus subtilis 26DCryChS producing Cry1Ia toxin from Bacillus thuringiensis promotes multifaceted potato defense against Phytophthora infestans (Mont.) de Bary and pest Leptinotarsa decemlineata say. Plants 2020, 9, 1115. [Google Scholar] [CrossRef]
- Fry, W.E.; Birch, P.R.; Judelson, H.S.; Grünwald, N.J.; Danies, G.; Everts, K.L.; Gevens, A.J.; Gugino, B.K.; Johnson, D.A.; Johnson, S.B.; et al. Five reasons to consider Phytophthora infestans a reemerging pathogen. Phytopathology 2015, 105, 966–981. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Ukladov, E.O.; Golubeva, T.S. Phytophthora infestans: An overview of methods and attempts to combat late blight. J. Fungi 2021, 7, 1071. [Google Scholar] [CrossRef]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Effective control of two genotypes of Phytophthora infestans in the field by three oxathiapiprolin fungicidal mixtures. PLoS ONE 2021, 16, e0258280. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Coffey, A.; Guo, J.; Waters, D.M.; Arendt, E.K. Ecofriendly control of potato late blight causative agent and the potential role of lactic acid bacteria: A review. Appl. Microbiol. Biotechnol. 2012, 96, 37–48. [Google Scholar] [CrossRef]
- Deahl, K.L.; Cooke, L.R.; Black, L.L.; Wang, T.C.; Perez, F.M.; Moravec, B.C.; Quinn, M.; Jones, R.W. Population changes in Phytophthora infestans in Taiwan associated with the appearance of resistance to metalaxyl. Pest Manag. Sci. 2002, 58, 951–958. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.Q.; Feng, J.T.; Wu, H.; Han, L.R. Review on research and development of botanical pesticides. Chin. J. Biol. Control 2015, 31, 685–698. [Google Scholar] [CrossRef]
- Mao, J.W.; Wu, Y.F.; Fang, S. Research progress of microbial enzymes. Bull. Ferment. Sci. Technol. 2010, 39, 42–44. [Google Scholar] [CrossRef]
- Dai, J.; Sha, R.Y.; Wang, Z.Z.; Cui, Y.L.; Fang, S.; Mao, J.W. Edible plant Jiaosu: Manufacturing, bioactive compounds, potential health benefits, and safety aspects. J. Sci. Food Agric. 2020, 100, 5313–5323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.B.; Cai, Y.F.; Liu, X.P.; Hu, Y.G.; Wang, X.F. Ecological effects of plant Jiaosu for agriculture: A review. J. China Agric. Univ. 2020, 25, 25–35. [Google Scholar] [CrossRef]
- Yin, X.J.; Wu, J.; Su, S.P.; Zheng, F.L. Advances in the function and application of fruit and vegetable microbial enzyme. Mod. Chem. Res. 2022, 22, 71–73. [Google Scholar]
- Wang, X.F. Control of potato diseases by plant nutrition immunity. Mod. Agric. 2018, 40, 2–4. [Google Scholar]
- Liang, H.X.; Lian, Y.W.; Sun, W.B.; Zhang, Y.M.; Sun, Z.Y. Effect and mechanism of plant enzymes on potato. Sci-Tech Innov. Brands 2016, 10, 75–77. [Google Scholar]
- Hui, W.; Ma, X.M.; Zhang, Y. Bioactivity and inhibiton effects of greengage ferment. Sci. Technol. Food Ind. 2018, 39, 39–43. [Google Scholar] [CrossRef]
- Jiang, Z. Study on Mechanism of Fermentation, Metabolic Process and Bioactivities of Microbial Natural-Ferments During Fermentation. Master’s Thesis, Zhejiang Sci-Tech University, Hangzhou, China, 2013. [Google Scholar]
- Liu, Y.N.; Cui, N.N.; Zhang, J.; Yu, F.M.; Zhang, L.B.; Yao, Y.C. Effect of aromatic plant-derived nutrient solution on inhibition of harmful bacteria and nutrition for pear plants. Sci. Agric. Sin. 2011, 44, 3981–3990. [Google Scholar]
- Dong, Y.J.; Chen, J.; Yang, X.P.; Han, Y.D.; Wu, Q. Evaluation of antibacterial test of fermented Chinese herbal preparations (Bencaojiaosu) in vitro. Mod. J. Anim. Husb. Vet. Med. 2020, 11, 6–8. [Google Scholar]
- Liu, F.; Wang, F.; Wang, C.; Yin, S.G.; Li, L.H.; Wang, Z.W. Effects of polo microbial enzymes fertilizer application on potato. J. Northeast. Agric. Sci. 2011, 36, 31–32. [Google Scholar] [CrossRef]
- Huo, C.; Cao, J.F.; Yin, R.J.; Yang, M.W.; Zhao, Z.J. First report of Phytophthora infestans causing late blight on pepino (solanum muricatum) in China. Plant Dis. 2023, 107, 3321. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Buchanan, R.E. Berger Bacterial Identification Manual; Science Press: Beijing, China, 1984. [Google Scholar]
- Dong, X.C.M. Common Bacterial System Identification Manua; Science Press: Beijing, China, 2001. [Google Scholar]
- Jin, Z.J. About the evaluation of drug combination. Acta Pharmacol. Sin. 2004, 25, 146–147. [Google Scholar] [PubMed]
- Yang, X.Q.; Zhao, L.N. Discussion on harmless treatment and reuse of vegetable waste. Agric. Technol. 2019, 39, 23–24. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, S.; Yin, J.; Feng, H.; Wang, M.; Chen, T. Resource potential and global warming potential of fruit and vegetable waste in China based on different treatment strategies. Waste Manag. 2022, 140, 225–232. [Google Scholar] [CrossRef]
- Simsek, S.; El, S.N.; Kancabas Kilinc, A.; Karakaya, S. Vegetable and fermented vegetable juices containing germinated seeds and sprouts of lentil and cowpea. Food Chem. 2014, 156, 289–295. [Google Scholar] [CrossRef]
- Zulkawi, N.; Ng, K.H.; Zamberi, R.; Yeap, S.K.; Satharasinghe, D.; Jaganath, I.B.; Jamaluddin, A.B.; Tan, S.W.; Ho, W.Y.; Alitheen, N.B.; et al. In vitro characterization and in vivo toxicity, antioxidant and immunomodulatory effect of fermented foods; Xeniji™. BMC Complement Altern. Med. 2017, 17, 344. [Google Scholar] [CrossRef]
- Kuwaki, S.; Nakajima, N.; Tanaka, H.; Ishihara, K. Plant-based paste fermented by lactic acid bacteria and yeast: Functional analysis and possibility of application to functional foods. Biochem. Insights 2012, 5, 21–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.H.; Zheng, Z.H.; Meng, X.Y.; Cai, Y.F.; Liu, J.B.; Hu, Y.G.; Yan, S.D.; Wang, X.F. A microbial ecosystem: Agricultural Jiaosu achieves effective and lasting antifungal activity against Botrytis cinerea. AMB Express 2020, 10, 216. [Google Scholar] [CrossRef]
- Gao, Y.H.; Zhang, Y.; Cheng, X.Q.; Zheng, Z.H.; Wu, X.H.; Dong, X.H.; Hu, Y.G.; Wang, X.F. Agricultural Jiaosu: An Eco-Friendly and Cost-Effective Control Strategy for Suppressing Fusarium Root Rot Disease in Astragalus membranaceus. Front. Microbiol. 2022, 13, 823704. [Google Scholar] [CrossRef]
- Fang, S.; Mao, J.W.; Shan, Z.C.; Mao, Y.C.; Li, H.H. Research progress and development suggestions on plant-derived enzyme nutrient solution. Agric. Eng. 2017, 7, 181–186. [Google Scholar]
- Yu, K.; Liu, Y.; Tichelaar, R.; Savant, N.; Lagendijk, E.; van Kuijk, S.J.L.; Stringlis, I.A.; van Dijken, A.J.H.; Pieterse, C.M.J.; Bakker, P.; et al. Rhizosphere-associated pseudomonas suppress local root immune responses by gluconic acid-mediated lowering of environmental pH. Curr. Biol. 2019, 29, 3913–3920.e4. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, Z.; Cheng, X.; Zhang, Y.; Liu, X.; Hu, Y.; Cai, Y.; Wang, X. An innovative way to treat cash crop wastes: The fermentation characteristics and functional microbial community using different substrates to produce Agricultural Jiaosu. Environ. Res. 2023, 227, 115727. [Google Scholar] [CrossRef] [PubMed]
- Van Wees, S.C.M.; Van der Ent, S.; Pieterse, C.M.J. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef]
- Singh, S.B.; Young, K.; Silver, L.L. What is an “ideal” antibiotic? Discovery challenges and path forward. Biochem. Pharmacol. 2017, 133, 63–73. [Google Scholar] [CrossRef]
- Akter, S.; Huq, M.A. Biologically rapid synthesis of silver nanoparticles by Sphingobium sp. MAH-11(T) and their antibacterial activity and mechanisms investigation against drug-resistant pathogenic microbes. Artif. Cells Nanomed. Biotechnol. 2020, 48, 672–682. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Yang, L.; Yang, F.Y.; Bai, W.J.; Zhang, X.Q.; Li, H.T.; Duan, G.G.; Xu, Y.T.; Li, Y.W. A bioinspired antibacterial and photothermal membrane for stable and durable clean water remediation. Mater. Horiz. 2023, 10, 268–276. [Google Scholar] [CrossRef]
- Hu, N.; Lei, M.; Zhao, X.; Zhang, Z.; Gu, Y.; Zhang, Y.; Wang, S. Analysis of the Microbial Diversity and Characteristics of Fermented Blueberry Beverages from Different Regions. Foods 2020, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Naseer, Q.A.; Xue, X.; Wang, X.; Dang, S.; Din, S.U.; Kalsoom; Jamil, J. Synthesis of silver nanoparticles using Lactobacillus bulgaricus and assessment of their antibacterial potential. Braz. J. Biol. 2021, 82, e232434. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Xin, W.G.; Yang, L.Y.; Ying, J.P.; Zhao, Z.S.; Lin, L.B.; Zhang, X.; Zhang, Q.L. A novel bacteriocin against Staphylococcus aureus from Lactobacillus paracasei isolated from Yunnan traditional fermented yogurt: Purification, antibacterial characterization, and antibiofilm activity. J. Dairy Sci. 2022, 105, 2094–2107. [Google Scholar] [CrossRef]
- Luan, C.; Jiang, N.; Zhou, X.L.; Zhang, C.; Zhao, Y.P.; Li, Z.Q.; Li, C. Antibacterial and anti-biofilm activities of probiotic Lactobacillus curvatus BSF206 and Pediococcus pentosaceus AC1-2 against Streptococcus mutans. Microb. Pathog. 2022, 164, 105446. [Google Scholar] [CrossRef] [PubMed]
- Gil, N.Y.; Gwon, H.M.; Yeo, S.H.; Kim, S.Y. Metabolite profile and immunomodulatory properties of bellflower root vinegar produced using Acetobacter pasteurianus A11-2. Foods 2020, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, M.; Sajnaga, E.; Kazimierczak, W.; Lis, M.; Wiater, A. Screening and Molecular Identification of Bacteria from the Midgut of Amphimallon solstitiale Larvae Exhibiting Antagonistic Activity against Bacterial Symbionts of Entomopathogenic Nematodes. Int. J. Mol. Sci. 2021, 22, 12005. [Google Scholar] [CrossRef] [PubMed]
- Andreolli, M.; Lampis, S.; Tosi, L.; Marano, V.; Zapparoli, G. Fungicide sensitivity of grapevine bacteria with plant growth-promoting traits and antagonistic activity as non-target microorganisms. World J. Microbiol. Biotechnol. 2023, 39, 121. [Google Scholar] [CrossRef]
- Xie, T.; Shen, S.; Hu, R.; Li, W.; Wang, J. Screening, Identification, and Growth Promotion of Antagonistic Endophytes Associated with Chenopodium quinoa Against Quinoa Pathogens. Phytopathology 2023, 113, 1839–1852. [Google Scholar] [CrossRef]
- Yan, H.H.; Qiu, Y.; Yang, S.; Wang, Y.Q.; Wang, Y.K.; Jiang, L.L.; Wang, H.Y. Antagonistic activity of Bacillus velezensis SDTB038 against Phytophthora infestans in potato. Plant Dis. 2021, 105, 1738–1747. [Google Scholar] [CrossRef]
- Lopes, R.; Tsui, S.; Gonçalves, P.J.R.O.; de Queiroz, M.V. A look into a multifunctional toolbox: Endophytic Bacillus species provide broad and underexploited benefits for plants. World J. Microbiol. Biotechnol. 2018, 34, 94. [Google Scholar] [CrossRef]
- Zhang, J.M.; Huang, X.Q.; Yang, S.D.; Huang, A.R.; Ren, J.; Luo, X.G.; Feng, S.; Li, P.H.; Li, Z.G.; Dong, P. Endophytic Bacillus subtilis H17-16 effectively inhibits Phytophthora infestans, the pathogen of potato late blight, and its potential application. Pest Manag. Sci. 2023, 79, 5073–5086. [Google Scholar] [CrossRef]
- Cho, M.S.; Jin, Y.J.; Kang, B.K.; Park, Y.K.; Kim, C.K.; Park, D.S. Understanding the ontogeny and succession of Bacillus velezensis and B. subtilis subsp. subtilis by focusing on kimchi fermentation. Sci. Rep. 2018, 8, 7045. [Google Scholar] [CrossRef]
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef]
- Kovács, Á.T. Bacillus subtilis. Trends Microbiol. 2019, 27, 724–725. [Google Scholar] [CrossRef]
- Karpov, D.S.; Domashin, A.I.; Kotlov, M.I.; Osipova, P.G.; Kiseleva, S.V.; Seregina, T.A.; Goncharenko, A.V.; Mironov, A.S.; Karpov, V.L.; Poddubko, S.V. Biotechnological potential of the Bacillus subtilis 20 strain. Mol. Biol. 2020, 54, 137–145. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, C.Y.; Liang, J.; Wu, L.F.; Gao, W.B.; Jiang, J.Z. Iturin a extracted from Bacillus subtilis WL-2 affects Phytophthora infestans via cell structure disruption, oxidative stress, and energy supply dysfunction. Front. Microbiol. 2020, 11, 536083. [Google Scholar] [CrossRef]
- Alfiky, A.; L’Haridon, F.; Abou-Mansour, E.; Weisskopf, L. Disease inhibiting effect of strain Bacillus subtilis EG21 and Its metabolites against potato pathogens Phytophthora infestans and Rhizoctonia solani. Phytopathology 2022, 112, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Song, W.R.; Chen, H.; Qian, Z.H.; Zeng, J.; Dong, S.M. Status and prospects of chemical prevention and control of potato late blight. China Plant Prot. 2021, 41, 33–39. [Google Scholar]
- Rondeau, S.; Raine, N.E. Fungicides and bees: A review of exposure and risk. Environ. Int. 2022, 165, 107311. [Google Scholar] [CrossRef] [PubMed]
- Kelbessa, B.G.; Ghadamgahi, F.; Kumar, P.L.; Ortiz, R.; Whisson, S.C.; Bhattacharjee, R.; Vetukuri, R.R. Antagonistic and plant growth promotion of rhizobacteria against Phytophthora colocasiae in taro. Front. Plant Sci. 2022, 13, 1035549. [Google Scholar] [CrossRef]
- Kiewnick, S.; Jacobsen, B.J.; Braun-Kiewnick, A.; Eckhoff, J.L.A.; Bergman, J.W. Integrated Control of Rhizoctonia Crown and Root Rot of Sugar Beet with Fungicides and Antagonistic Bacteria. Plant Dis. 2001, 85, 718–722. [Google Scholar] [CrossRef]
- Su, X.; Wu, S.; Liu, L.; Lu, G.; Liu, H.; Jin, X.; Wang, Y.; Guo, H.; Wang, C.; Cheng, H. Potential Antagonistic Bacteria against Verticillium dahliae Isolated from Artificially Infested Nursery. Cells 2021, 10, 3588. [Google Scholar] [CrossRef]
- Saimi, A.; Zhang, Q.; Liu, Q.; Li, G.; Gao, H.; Chen, J. Screening of endophytic antagonistic bacteria in wheat and evaluation of biocontrol potential against wheat stripe rust. Plants 2024, 13, 1366. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.F. Ecological role of agricultural plant jiaosu in organic agricultural production. J. Poyang Lake 2019, 11, 70–74+125–126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).