Potential of Salvia discolor Extract Against Common Agricultural Pathogens †
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Chemicals
2.4. Extraction and Isolation
2.5. Microorganisms
2.6. Poisoned Food Technique
2.7. Efficacy of S. discolor Extract Against Post-Harvest Diseases in Tomato Fruit
2.8. Antibacterial Assay
2.9. Statistical Analysis
3. Results
3.1. Chemical Analysis
3.2. Antifungal Assays
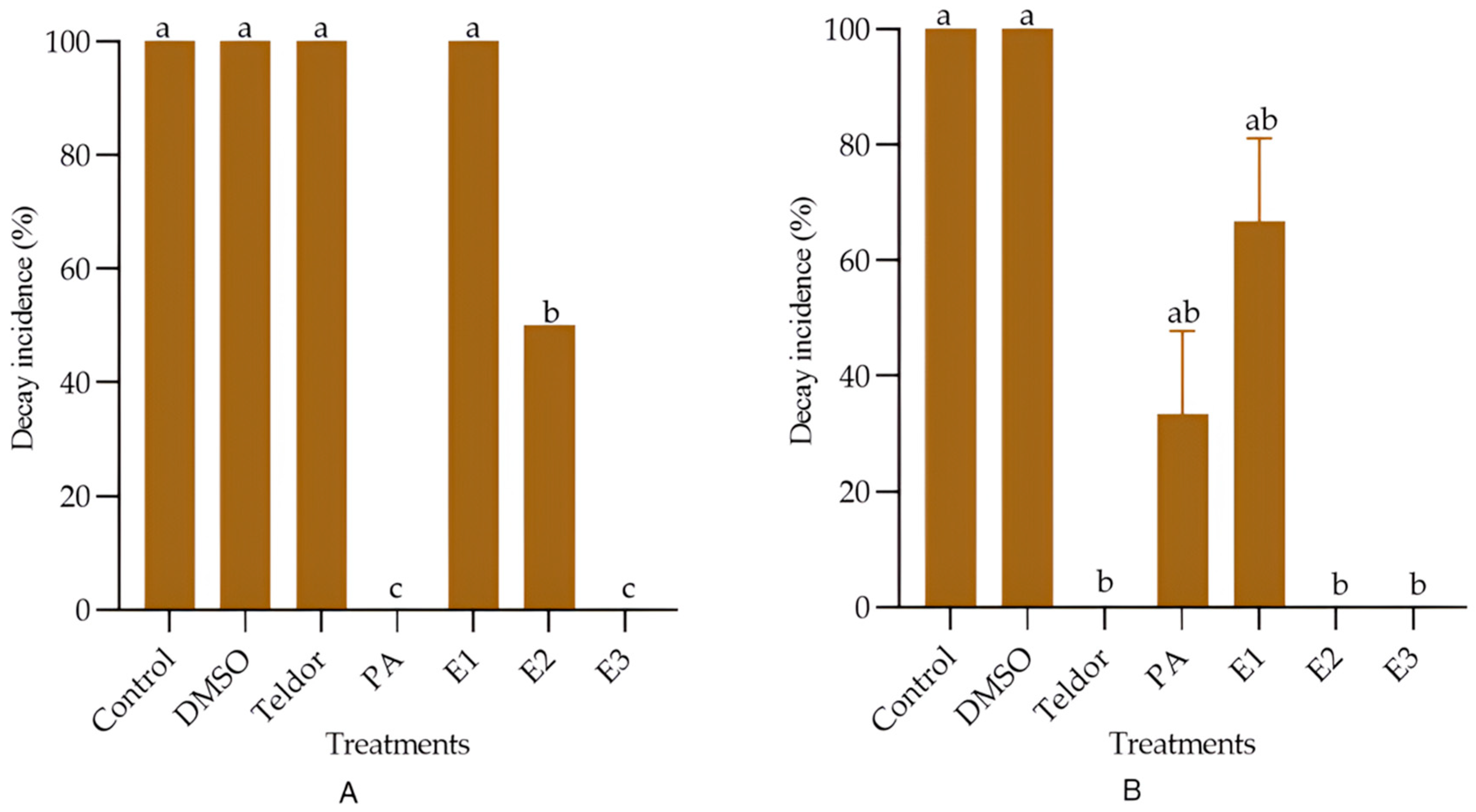
3.3. Effect of the Dichloromethane Extract Against Post-Harvest Diseases in Tomato Fruit
3.4. Antibacterial Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. In The Fungal Kingdom; Heitman, J., Howlett, B.J., Crous, P.W., Stukenbrock, E.H., James, T.Y., Gow, N.A.R., Eds.; American Society for Microbiology: Washington, DC, USA, 2017; pp. 701–726. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, The Pathogen and Its Management in Agricultural Systems; Springer: Heidelberg, Germany, 2016. [Google Scholar]
- Yang, L.-N.; He, M.-H.; Ouyang, H.-B.; Zhu, W.; Pan, Z.-C.; Sui, Q.-J.; Shang, L.-P.; Zhan, J. Cross-resistance of the pathogenic fungus Alternaria alternata to fungicides with different modes of action. BMC Microbiol. 2019, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Zuriegat, Q.; Zheng, Y.; Liu, H.; Wang, Z.; Yun, Y. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Wagacha, J.M.; Dossaji, S.F.; Wanzala, W. Chemical composition and antibacterial activity of essential oils of Tagetes minuta (Asteraceae) against selected plant pathogenic bacteria. Int. J. Microbiol. 2016, 2016, 7352509. [Google Scholar] [CrossRef]
- López-Cabeza, R.; Rodríguez-Sabina, S.; Reyes, C.P.; Expósito, D.G.; Giménez, C.; Jiménez, I.A.; Cabrera, R.; Bazzocchi, I.L. Bio-guided isolation of aromatic abietane diterpenoids from Salvia canariensis as biopesticides in the control of phytopathogenic fungi. Pest Manag. Sci. 2024, 80, 2199–2207. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Amara, A. Recent approaches towards control of fungal diseases in plants: An updated review. J. Fungi 2021, 7, 900. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Al-Goul, S.T. The impact of eco-friendly nanoparticles on the management of phytopathogenic fungi: A comprehensive review. J. Plant Pathol. 2025, 107, 265–290. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Kalliora, C.; Mamoulakis, C.; Vasilopoulos, E.; Stamatiades, G.A.; Kalafati, L.; Barouni, R.; Karakousi, T.; Abdollahi, M.; Tsatsakis, A. Association of pesticide exposure with human congenital abnormalities. Toxicol. Appl. Pharmacol. 2018, 346, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wang, J.-W.; Li, J.; Han, B. Designing future crops: Challenges and strategies for sustainable agriculture. Plant J. 2021, 105, 1165–1178. [Google Scholar] [CrossRef]
- PAN. PAN 2023–2024 Annual Report; PAN: Berkeley, CA, USA, 2024. [Google Scholar]
- Košćak, L.; Lamovšek, J.; Đermić, E.; Prgomet, I.; Godena, S. Microbial and plant-based compounds as alternatives for the control of phytopathogenic bacteria. Horticulturae 2023, 9, 1124. [Google Scholar] [CrossRef]
- Jiménez-Reyes, M.F.; Carrasco, H.; Olea, A.; Silva-Moreno, E. Natural compounds: A sustainable alternative for controlling phytopathogens. J. Chil. Chem. Soc. 2019, 64, 4459–4465. [Google Scholar] [CrossRef]
- Kursa, W.; Jamiołkowska, A.; Wyrostek, J.; Kowalski, R. Antifungal effect of plant extracts on the growth of the cereal pathogen Fusarium spp.—An in vitro study. Agronomy 2022, 12, 3204. [Google Scholar] [CrossRef]
- Steglińska, A.; Bekhter, A.; Wawrzyniak, P.; Kunicka-Styczyńska, A.; Jastrząbek, K.; Fidler, M.; Śmigielski, K.; Gutarowska, B. Antimicrobial activities of plant extracts against Solanum tuberosum L. phytopathogens. Molecules 2022, 27, 1579. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Pedrelli, F.; D’Ambola, M.; Labanca, F.; Schito, A.M.; Govaerts, R.; De Tommasi, N.; Milella, L. Quinone diterpenes from Salvia species: Chemistry, botany, and biological activity. Phytochem. Rev. 2019, 18, 665–842. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Firuzi, O.; Xiao, J. Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem. Rev. 2016, 15, 829–867. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Bisio, A.; Damonte, G.; Fraternale, D.; Giacomelli, E.; Salis, A.; Romussi, G.; Cafaggi, S.; Ricci, D.; De Tommasi, N. Phytotoxic clerodane diterpenes from Salvia miniata Fernald (Lamiaceae). Phytochemistry 2011, 72, 265–275. [Google Scholar] [CrossRef]
- Bisio, A.; Fraternale, D.; Damonte, G.; Millo, E.; Lanteri, A.P.; Russo, E.; Romussi, G.; Parodi, B.; Ricci, D.; De Tommasi, N. Phytotoxic Activity of Salvia x jamensis. Nat. Prod. Commun. 2009, 4, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Fraternale, D.; Giacomini, M.; Giacomelli, E.; Pivetti, S.; Russo, E.; Caviglioli, G.; Romussi, G.; Ricci, D.; De Tommasi, N. Phytotoxicity of Salvia spp. exudates. Crop Prot. 2010, 29, 1434–1446. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Pane, C.; Caputo, M.; Durazzo, A.; Lucarini, M.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A.; De Feo, V. Sage species case study on a spontaneous Mediterranean plant to control phytopathogenic fungi and bacteria. Forests 2020, 11, 704. [Google Scholar] [CrossRef]
- LoPresti, E.F. Chemicals on plant surfaces as a heretofore unrecognized, but ecologically informative, class for investigations into plant defence. Biol. Rev. 2015, 91, 1102–1117. [Google Scholar] [CrossRef]
- Muller, C.; Riederer, M. Plant surface properties in chemical ecology. J. Chem. Ecol. 2005, 31, 2621–2651. [Google Scholar] [CrossRef]
- Marchev, A.; Haas, C.; Schulz, S.; Georgiev, V.; Steingroewer, J.; Bley, T.; Pavlov, A. Sage in vitro cultures: A promising tool for the production of bioactive terpenes and phenolic substances. Biotechnol. Lett. 2014, 36, 211–221. [Google Scholar] [CrossRef]
- Jenks, A.A.; Walker, J.B.; Kim, S.-C. Phylogeny of New World Salvia subgenus Calosphace (Lamiaceae) based on cpDNA (psbA-trnH) and nrDNA (ITS) sequence data. J. Plant Res. 2013, 126, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gordillo, M.; Martínez-Ambriz, E.; García-Peña, M.R.; Cantú-Morón, E.A.; Fragoso-Martínez, I. Lamiaceae Martinov; Universidad Nacional Autónoma de México: Ciudad de México, México, 2019; Volume 2019, p. 119. [Google Scholar]
- POWO. Plants of the World Online. Available online: https://powo.science.kew.org (accessed on 24 January 2025).
- Devi, P. Chemical and Biological Investigation of Some Species of Salvia. Ph.D. Thesis, University of Genoa, Genova, Italy, 2025. [Google Scholar]
- Almasoudi, N.M.; Al-Qurashi, A.D.; Elsayed, M.I.; Abo-Elyousr, K.A.M. Native bacterial bioagents for management of potato soft rot disease caused by Pectobacterium carotovorum subsp. carotovorum. Egypt. J. Biol. Pest Control 2024, 34, 31. [Google Scholar] [CrossRef]
- Bodah, E. Root rot diseases in plants: A review of common causal agents and management strategies. Agric. Res. Technol. 2017, 5, 56–63. [Google Scholar] [CrossRef]
- Kong, W.; Huo, H.; Gu, Y.; Cao, Y.; Wang, J.; Liang, J.; Niu, S. Antifungal activity of camphor against four phytopathogens of Fusarium. S. Afr. J. Bot. 2022, 148, 437–445. [Google Scholar] [CrossRef]
- Sultana, F.; Hossain, M.M. Diseases of vegetables caused by Phoma spp. In Phoma: Diversity, Taxonomy, Bioactivities, and Nanotechnology; Rai, M., Zimowska, B., Kövics, G.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 91–119. [Google Scholar]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins produced by fungi associated with grapevine trunk diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef]
- Khaledi, N.; Hassani, F. Antifungal activity of Bunium persicum essential oil and its constituents on growth and pathogenesis of Colletotrichum lindemuthianum. J. Plant Prot. Res. 2018, 58, 431–441. [Google Scholar] [CrossRef]
- Rosado-Álvarez, C.; Molinero-Ruiz, L.; Rodríguez-Arcos, R.; Basallote-Ureba, M.J. Antifungal activity of asparagus extracts against phytopathogenic Fusarium oxysporum. Sci. Hortic. 2014, 171, 51–57. [Google Scholar] [CrossRef]
- Minerdi, D.; Sadeghi, S.J.; Pautasso, L.; Morra, S.; Aigotti, R.; Medana, C.; Gilardi, G.; Gullino, M.L.; Gilardi, G. Expression and role of CYP505A1 in pathogenicity of Fusarium oxysporum f. sp. lactucae. Biochim. Biophys. Acta 2020, 1868, 140268. [Google Scholar] [CrossRef]
- Martin, F.N.; Loper, J.E. Soilborne plant diseases caused by Pythium spp.: Ecology, epidemiology, and prospects for biological control. Crit. Rev. Plant Sci. 1999, 18, 111–181. [Google Scholar] [CrossRef]
- Kasteel, M.; Ketelaar, T.; Govers, F. Fatal attraction: How Phytophthora zoospores find their host. Semin. Cell Dev. Biol. 2023, 148, 13–21. [Google Scholar] [CrossRef]
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus species: Excellent biocontrol agents against tomato diseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef]
- Zorzi Tomazoni, E.; Schiavo Griggio, G.; Pessin Broilo, E.; da Silva Ribeiro, R.T.; Gonçalves Soares, G.L.; Schwambach, J. Screening for inhibitory activity of essential oils on fungal tomato pathogen Stemphylium solani Weber. Biocatal. Agric. Biotechnol. 2018, 16, 364–372. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Kumar, A.; Fadiji, A.E.; Babalola, O.O.; Puopolo, G.; Santoyo, G. Agroecological management of the grey mould fungus Botrytis cinerea by plant growth-promoting bacteria. Plants 2023, 12, 637. [Google Scholar] [CrossRef]
- Nikolova, M.T.; Yordanov, P.; Slavov, S.; Berkov, S. Antifungal activity of plant extracts against phytopathogenic fungi: Antifungal activity of plant extracts. J. Biosci. Biotechnol. 2017, 6, 155–161. [Google Scholar]
- Nandi, M.; Macdonald, J.; Liu, P.; Weselowski, B.; Yuan, Z.-C. Clavibacter michiganensis ssp. michiganensis: Bacterial canker of tomato, molecular interactions and disease management. Mol. Plant Pathol. 2018, 19, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Khair, H.; Abdel-Gaied, T.G.; Mikhail, M.S.; Abdel-Alim, A.I.; El-Nasr, H.I.S. Biological control of Pectobacterium carotovorum subsp. carotovorum, the causal agent of bacterial soft rot in vegetables, in vitro and in vivo tests. Bull. Natl. Res. Cent. 2021, 45, 37. [Google Scholar] [CrossRef]
- Iobbi, V.; Donadio, G.; Lanteri, A.P.; Maggi, N.; Kirchmair, J.; Parisi, V.; Minuto, G.; Copetta, A.; Giacomini, M.; Bisio, A.; et al. Targeted metabolite profiling of Salvia rosmarinus Italian local ecotypes and cultivars and inhibitory activity against Pectobacterium carotovorum subsp. carotovorum. Front. Plant Sci. 2024, 15, 1164859. [Google Scholar] [CrossRef]
- Iobbi, V.; Lo Vetere, M.; Lanteri, A.P.; Reinhardt, J.K.; Danton, O.; Keller, M.; Hamburger, M.; Salis, A.; Damonte, G.; Potterat, O.; et al. Antifungal potential of carnosic acid from Salvia somalensis against phytopathogenic fungi. Agronomy 2024, 14, 1444. [Google Scholar] [CrossRef]
- Bisio, A.; De Tommasi, N.; Romussi, G. Diterpenoids from Salvia wagneriana. Planta Med. 2004, 70, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Fraternale, D.; Schito, A.M.; Parricchi, A.; Dal Piaz, F.; Ricci, D.; Giacomini, M.; Ruffoni, B.; De Tommasi, N. Establishment and analysis of in vitro biomass from Salvia corrugata Vahl. and evaluation of antimicrobial activity. Phytochemistry 2016, 122, 276–285. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh. against selected Fusarium spp. Int. J. Microbiol. 2017, 2017, 8761610. [Google Scholar] [CrossRef]
- Bayar, Y.; Yilar, M. The antifungal and phytotoxic effect of different plant extracts of Salvia virgata Jacq. Fresenius Environ. Bull. 2019, 28, 3492–3497. [Google Scholar]
- Kalboush, Z.; Hassan, A. Antifungal potential and characterization of plant extracts against Fusarium fujikuroi on rice. J. Plant Prot. Pathol. 2019, 10, 369–376. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Grabke, A.; Bryson, P.K.; Amiri, A.; Peres, N.A.; Schnabel, G. Fungicide resistance profiles in Botrytis cinerea from strawberry fields of seven southern U.S. States. Plant Dis. 2014, 98, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Dogra, D.; Julka, J.M.; Kumar, A. Insight into antimicrobic evaluation techniques and their role in better assessment of antimicrobic agents: A review. Asian J. Pharm. Clin. Res. 2021, 15, 6–13. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Lüdecke, D. ggeffects: Tidy data frames of marginal effects from regression models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef]
- Tippannanavar, M.; Verma, A.; Kumar, R.; Gogoi, R.; Kundu, A.; Patanjali, N. Preparation of nanofungicides based on imidazole drugs and their antifungal evaluation. J. Agric. Food. Chem. 2020, 68, 4566–4578. [Google Scholar] [CrossRef]
- Grougnet, R.; Magiatis, P.; Mitaku, S.; Skaltsounis, A.-L.; Cabalion, P.; Tillequin, F.; Michel, S. Dammarane Triterpenes from Gardenia aubryi Vieill. Helv. Chim. Acta 2011, 94, 656–661. [Google Scholar] [CrossRef]
- Reza Jassbi, A.R.; Zamanizadehnajari, S.; Aberoomand Azar, P.; Tahar, S. Antibacterial Diterpenoids from Astragalus brachystachys. Z. Naturforsch. C 2002, 57, 1016–1021. [Google Scholar] [CrossRef]
- Pedreros, S.; Rodríguez, B.; De La Torre, M.C.; Bruno, M.; Savona, G.; Perales, A.; Torres, M.R. Dammarane triterpenes of Salvia hierosolymitana. Phytochemistry 1990, 29, 919–922. [Google Scholar] [CrossRef]
- Nagao, T.; Abe, F.; Kinjo, J.; Okabe, H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. Biol. Pharm. Bull. 2002, 25, 875–879. [Google Scholar] [CrossRef]
- Pukalskas, A.; van Beek, T.A.; de Waard, P. Development of a triple hyphenated HPLC-radical scavenging detection-DAD-SPE-NMR system for the rapid identification of antioxidants in complex plant extracts. J. Chromatogr. A 2005, 1074, 81–88. [Google Scholar] [CrossRef]
- Fan, X.; Zi, J.; Zhu, C.; Xu, W.; Cheng, W.; Yang, S.; Guo, Y.; Shi, J. Chemical Constituents of Heteroplexis micocephala. J. Nat. Prod. 2009, 72, 1184–1190. [Google Scholar] [CrossRef]
- Seto, M.; Miyase, T.; Akira, U. Ent-clerodane diterpenoids from Rhynchospermum verticillatum. Phytochemistry 1987, 26, 3289–3292. [Google Scholar] [CrossRef]
- Calvert, D.J.; Cambie, R.C.; Davis, B.R. 13C NMR spectra of polymethoxy- and methylenedioxyflavonols. Org. Magn. Reson. 1979, 12, 583–586. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Shi, L.; Luan, F.Q.; Wang, Q.A. Synthesis of kaempferide Mannich base derivatives and their antiproliferative activity on three human cancer cell lines. Acta Biochim. Pol. 2015, 62, 547–552. [Google Scholar] [CrossRef]
- Bigham, A.; Munro, T.; Rizzacasa, M.; Robins-Browne, R. Divinatorins A−C, new neoclerodane diterpenoids from the controlled sage Salvia divinorum. J. Nat. Prod. 2003, 66, 1242–1244. [Google Scholar] [CrossRef]
- Rivera, A.P.; Faini, F.; Castillo, M. 15α-Hydroxy-β-amyrin and patagonic acid from Baccharis magellanica and Baccharis patagonica. J. Nat. Prod. 1988, 51, 155–157. [Google Scholar] [CrossRef]
- Dowling, P.M. Peptide Antibiotics. In Antimicrobial Therapy in Veterinary Medicine; Dowling, P.M., Prescott, J.F., Baptiste, K.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024; pp. 187–201. [Google Scholar]
- Gustafson, R.H. Antibiotics use in agriculture: An overview. In Antibiotics use in Agriculture; American Chemical Society: Washington, DC, USA, 1986; pp. 1–6. [Google Scholar]
- Németh, J. Practice of applying streptomycin to control fireblight in Hungary. EPPO Bull. 2004, 34, 381–382. [Google Scholar] [CrossRef]
- Cameron, A.; Sarojini, V. Pseudomonas syringae pv. actinidiae: Chemical control, resistance mechanisms and possible alternatives. Plant Pathol. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Verhaegen, M.; Bergot, T.; Liebana, E.; Stancanelli, G.; Streissl, F.; Mingeot-Leclercq, M.-P.; Mahillon, J.; Bragard, C. On the use of antibiotics to control plant pathogenic bacteria: A genetic and genomic perspective. Front. Microbiol. 2023, 14, 1221478. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial use and resistance in plant agriculture: A one health perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Alewu, B.; Nosiri, C. Pesticides and human health. In Pesticides in the Modern World—Effects of Pesticides Exposure; Margarita, S., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 231–250. [Google Scholar]
- Sparks, T.C.; Bryant, R.J. Crop protection compounds—Trends and perspective. Pest Manag. Sci. 2021, 77, 3608–3616. [Google Scholar] [CrossRef]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current challenges and trends in the discovery of agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef]
- Ahmad, H.; Matsubara, Y.-I. Antifungal effect of Lamiaceae herb water extracts against Fusarium root rot in Asparagus. J. Plant Dis. Prot. 2020, 127, 229–236. [Google Scholar] [CrossRef]
- Del Frari, G.; Gobbi, A.; Aggerbeck, M.R.; Oliveira, H.; Hansen, L.H.; Ferreira, R.B. Fungicides and the grapevine wood mycobiome: A case study on tracheomycotic ascomycete Phaeomoniella chlamydospora reveals potential for two novel control strategies. Front. Plant Sci. 2019, 10, 1405. [Google Scholar] [CrossRef]
- Cobos, R.; Mateos, R.M.; Álvarez-Pérez, J.M.; Olego, M.A.; Sevillano, S.; González-García, S.; Garzón-Jimeno, E.; Coque, J.J. Effectiveness of natural antifungal compounds in controlling infection by grapevine trunk disease pathogens through pruning wounds. Appl. Environ. Microbiol. 2015, 81, 6474–6483. [Google Scholar] [CrossRef]
- Goussous, S.J.; Mas’ad, I.S.; Abu El-Samen, F.M.; Tahhan, R.A. In vitro inhibitory effects of rosemary and sage extracts on mycelial growth and sclerotial formation and germination of Sclerotinia sclerotiorum. Arch. Phytopathol. Plant Protect. 2013, 46, 890–902. [Google Scholar] [CrossRef]
- Munir, S.; Azeem, A.; Sikandar Zaman, M.; Zia Ul Haq, M. From field to table: Ensuring food safety by reducing pesticide residues in food. Sci. Total Environ. 2024, 922, 171382. [Google Scholar] [CrossRef] [PubMed]
- Gartemann, K.H.; Kirchner, O.; Engemann, J.; Gräfen, I.; Eichenlaub, R.; Burger, A. Clavibacter michiganensis subsp. michiganensis: First steps in the understanding of virulence of a Gram-positive phytopathogenic bacterium. J. Biotechnol. 2003, 106, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Badosa, E.; Planas, M.; Feliu, L.; Montesinos, L.; Bonaterra, A.; Montesinos, E. Synthetic peptides against plant pathogenic bacteria. Microorganisms 2022, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; González-García, V.; Palacio-Bielsa, A.; Lorenzo-Vidal, B.; Buzón-Durán, L.; Martín-Gil, J.; Martín-Ramos, P. Antibacterial activity of Ginkgo biloba extracts against Clavibacter michiganensis subsp. michiganensis, Pseudomonas spp., and Xanthomonas vesicatoria. Horticulturae 2023, 9, 461. [Google Scholar] [CrossRef]
- Siddique, M.; Din, N.; Ahmad, M.; Ali, A.; Naz, I.; Alam, S.S.; Ullah, N. Bioefficacy of some aqueous phytoextracts against Clavibacter michiganensis subsp. michiganensis (Smith), the cause of bacterial canker of tomato. Gesunde Pflanz. 2020, 72, 207–217. [Google Scholar] [CrossRef]
- Talibi, I.; Amkraz, N.; Askarne, L.; Msanda, F.; Saadi, B.; Boudyach, H.; Boubaker, H. Antibacterial activity of moroccan plants extracts against Clavibacter michiganensis subsp. michiganensis, the causal agent of tomatoes’ bacterial canker. J. Med. Plant. Res. 2011, 5, 4332–4338. [Google Scholar]
- Temel, I.; Dönmez, M.; Temtek, E. Control of bacterial cancer and wilt agent Clavibacter michiganensis subsp. michiganensis using different plant essential oils. J. Essent. Oil Bear. Plants 2023, 26, 814–829. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.; Gao, Y.; Wang, Q. Antibacterial activity and mechanism of Polygonum orientale L. Essential oil against Pectobacterium carotovorum subsp. carotovorum. Foods 2022, 11, 1585. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, N.; Behiry, S.; Ali, H.; Salem, M. Evaluation of Tecoma stans and Callistemon viminalis extracts against potato soft rot bacteria in vitro. J. Pure Appl. Microbiol. 2014, 8, 667–673. [Google Scholar]
- Shaheen, H.A.; Issa, M.Y. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic. 2020, 264, 108940. [Google Scholar] [CrossRef]
- Suaza-Gaviria, V.; Mesa-Vanegas, A.M.; Ocampo-Jiménez, O.; Monsalve-Fonnegra, Z.I. Antioxidant activity and phytopathogenic control of extracts and fraction from Struthanthus calophyllus A.C.Sm. (Loranthaceae). Chem. Biodivers. 2023, 20, e202200830. [Google Scholar] [CrossRef] [PubMed]
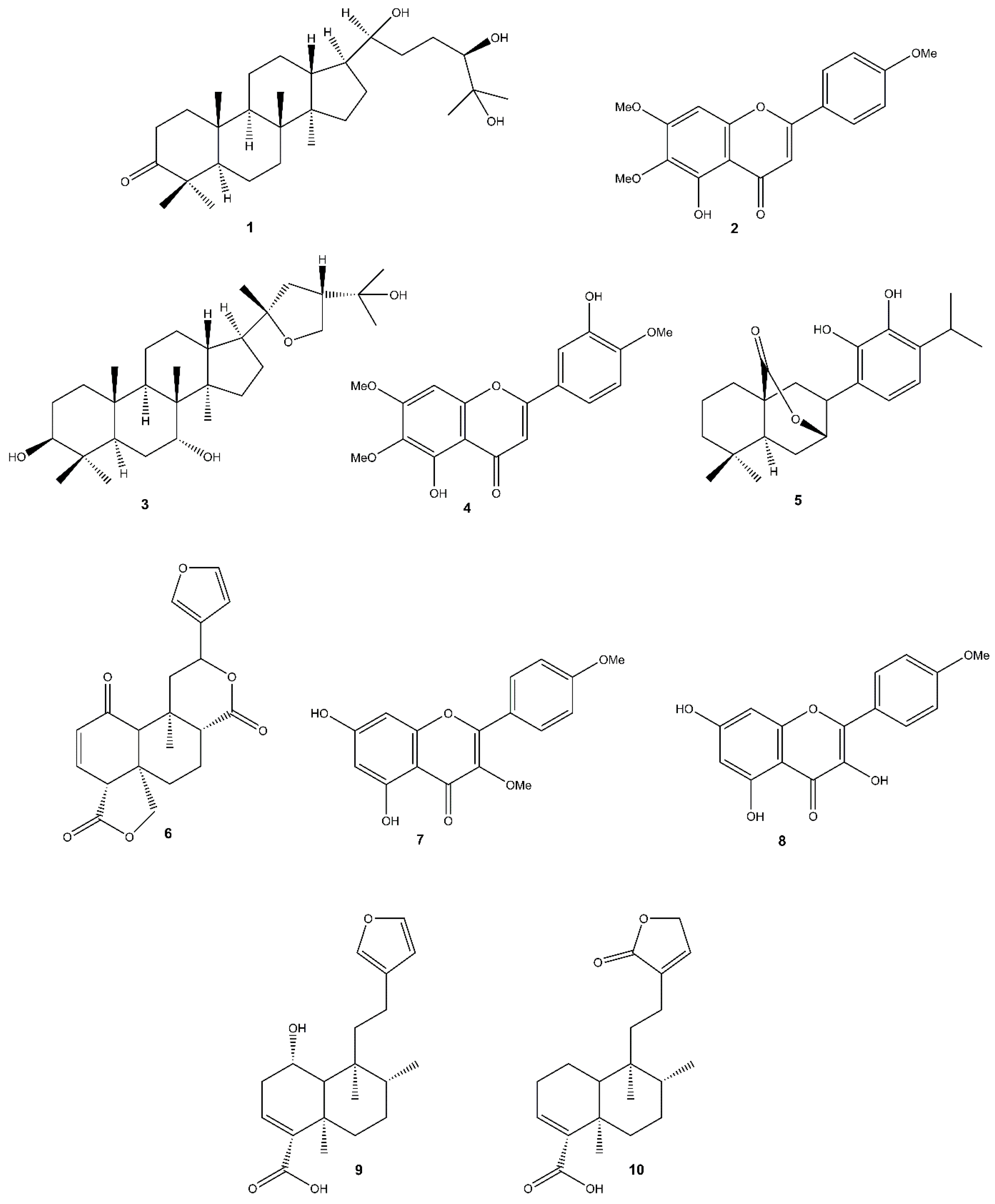
| Treatment | Concentration (μg mL−1) | Fusarium solani | Phoma betae | Phaeomoniella chlamydospora | Colletotrichum lindemuthianum | Fusarium oxysporum f. sp. lactucae Race 1 | Pythium dissotocum | Alternaria solani | Stemphylium sp. | Botrytis cinerea (Strain 4) |
|---|---|---|---|---|---|---|---|---|---|---|
| Crude extract | 5 | 6.67 ± 1.55 b | 11.42 ± 0.04 b | 31.42 ± 0.04 b | 5.71 ± 0.03 b | 0.95 ± 1.64 a | 16.19 ± 1.64 b | 2.85 ± 0.04 b | 4.74 ± 1.56 b | 4.76 ± 1.63 b |
| Crude extract | 100 | 43.81 ± 1.60 c | 28.57 ± 0.03 c | 59.04 ± 1.60 c | 15.23 ± 1.56 c | 8.57 ± 0.04 b | 27.61 ± 1.60 c | 19.40 ± 1.59 c | 28.57 ± 0.04 c | 14.28 ± 0.01 c |
| Crude extract | 250 | 54.29 ± 0.04 d | 48.57 ± 0.04 d | 77.14 ± 0.04 d | 18.09 ± 1.63 d | 8.57 ± 0.03 b | 27.61 ± 1.55 c | 29.52 ± 1.55 d | 28.57 ± 0.04 c | 18.09 ± 1.61 d |
| Crude extract | 500 | 71.43 ± 0.04 e | 57.14 ± 0.03 e | 80.86 ± 1.61 e | 22.85 ± 0.04 e | 11.42 ± 0.04 c | 51.42 ± 0.02 d | 37.14 ± 0.03 e | 40.0 ± 0.03 d | 34.28 ± 0.04 e |
| Crude extract | 750 | 80.00 ± 0.03 f | 57.14 ± 0.03 e | 88.57 ± 0.04 f | 28.54 ± 0.03 f | 23.80 ± 0.03 d | 64.74 ± 1.59 e | 40.00 ± 0.04 f | 51.42 ± 0.02 e | 37.14 ± 0.02 f |
| Crude extract | 1000 | 91.43 ± 0.04 g | 70.51 ± 0.02 f | 100 ± 0.00 g | 31.42 ± 0.04 g | 28.57 ± 0.04 e | 91.42 ± 0.0 fg | 55.23 ± 1.63 g | 57.14 ± 0.01 f | 42.54 ± 0.02 g |
| Cyprodinil + Fludioxonil | 375 + 250 | 100 ± 0.00 i | 100 ± 0.00 i | 100 ± 0.00 g | 100 ± 0.00 j | 91.42 ± 0.04 h | 100 ± 0.00 g | 100 ± 0.00 h | 100 ± 0.00 g | 100 ± 0.00 j |
| Metalaxyl-m + copper | 20 + 141.9 | 97.14 ± 0.03 h | 94.28 ± 0.00 h | 100 ± 0.00 g | 100 ± 0.00 j | 88.57 ± 0.04 g | 100 ± 0.00 g | 100 ± 0.00 h | 100 ± 0.00 g | 97.14 ± 0.00 ij |
| Difenoconazole | 250 | 97.14 ± 0.03 h | 100 ± 0.00 i | 100 ± 0.00 g | 82.85 ± 0.0 i | 88.57 ± 0.04 g | 84.66 ± 1.63 f | 100 ± 0.00 h | 98.08 ± 0.02 g | 93.34 ± 0.02 i |
| Azoxystrobin | 250 | 80.01 ± 0.04 f | 88.57 ± 0.03 g | 80.00 ± 0.04 e | 42.85 ± 0.04 h | 55.23 ± 0.03 f | 100 ± 0.00 g | 28.57 ± 0.04 d | 100 ± 0.00 g | 65.71 ± 0.03 h |
| DMSO | 10 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Control | - | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.0 ± 0.000 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Fungal Species | Equation | ED50 (μg mL−1) a | R2 |
|---|---|---|---|
| Fusarium solani | y = 2.291 × 10−7x3 − 0.0004312x2 + 0.2835x + 10.78 | 185.55 (127.11–263.02) | 0.97262 |
| Phoma betae | y = 2.229 × 10−7x3 − 0.0004022x2 + 0.24x + 9.606 | 277.41 (258.11–299.41) | 0.99896 |
| Phaeomoniella chlamydospora | y = 2.693 × 10−7x3 − 0.0004788x2 + 0.278x + 32.43 | 71.73 (45.63–100.84) | 0.98336 |
| Colletotrichum lindemuthianum | y = 2.693 × 10−7x3 − 0.0004788x2 + 0.278x + 32.43 | NA b | 0.97038 |
| Fusarium oxysporum f. sp. lactucae race 1 | y = 2.693 × 10−7x3 − 0.0004788x2 + 0.278x + 32.43 | NA b | 0.94038 |
| Pythium dissotocum | y = 3.037 × 10−8x3 − 2.067 × 10−5x2 + 0.06409x + 17.26 | 530.82 (430.21–623.86) | 0.98556 |
| Alternaria solani | y = 1.812 × 10−7x3 − 0.0003042x2 + 0.1756x + 2.757 | 945.87 (923.57–965.98) | 0.99751 |
| Stemphylium sp. | y = 8.645 × 10−8x3 − 0.0001666x2 + 0.1295x + 8.679 | 803.63 (412.44 c) | 0.93221 |
| Botrytis cinerea (strain 4) | y = 8.645 × 10−8x3 − 0.0001666x2 + 0.1295x + 8.679 | NA b | 0.98048 |
| Treatment | Concentration μg mL−1 | Botrytis cinerea (Strain 4) | Botrytis cinerea (Strain 1) | Botrytis cinerea (Strain 2) | Botrytis cinerea (Strain 11) |
|---|---|---|---|---|---|
| Crude extract | 5 | 4.76 ± 1.63 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Crude extract | 100 | 14.28 ± 0.01 c | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 5.71 ± 0.00 b |
| Crude extract | 250 | 18.09 ± 1.61 d | 2.85 ± 0.00 a | 2.85 ± 0.00 b | 5.71 ± 0.00 b |
| Crude extract | 500 | 34.28 ± 0.04 e | 8.57 ± 0.00 a | 48.57 ± 0.04 c | 37.14 ± 0.04 c |
| Crude extract | 750 | 37.14 ± 0.02 f | 8.57 ± 0.04 a | 65.73 ± 0.03 e | 71.42 ± 0.01 f |
| Crude extract | 1000 | 42.54 ± 0.02 g | 20.0 ± 0.03 b | 74.97 ± 0.02 f | 77.14 ± 0.04 g |
| Cyprodinil + Fludioxonil | 375 + 250 | 100 ± 0.00 j | 100 ± 0.00 e | 100 ± 0.00 g | 100 ± 0.00 h |
| Metalaxyl-m + copper | 20 + 141.9 | 97.14 ± 0.00 ij | 68.56 ± 0.02 d | 58.19 ± 0.03 d | 59.04 ± 1.59 e |
| Difenoconazole | 250 | 93.34 ± 0.02 i | 100 ± 0.00 e | 100 ± 0.00 g | 100 ± 0.00 h |
| Azoxystrobin | 250 | 65.71 ± 0.03 h | 45.71 ± 0.04 c | 48.57 ± 0.00 c | 51.4 ± 0.00 d |
| DMSO | 10 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Control | - | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Fungal Species | Equation | ED50 (μg mL−1) a | R2 |
|---|---|---|---|
| Botrytis cinerea (strain 4) | y = 1.436 × 10−8x3 − 5.365 × 10−5x2 + 0.07685x + 4.984 | NA b | 0.98048 |
| Botrytis cinerea (strain 1) | y = 3.963 × 10−8x3 − 4.762 × 10−5x2 + 0.0287x − 1.159 | NA b | 0.95867 |
| Botrytis cinerea (strain 2) | y = −2.678 × 10−7x3 + 0.0003832x2 − 0.0418x − 0.5586 | 566.10 (496.26–641.74) | 0.97996 |
| Botrytis cinerea (strain 11) | y = −2.617 × 10−7x3 + 0.0004002x2 − 0.06393x + 3.087 | 583.20 (540.15–627.90) | 0.99245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, P.; Lanteri, A.P.; Minuto, A.; Parisi, V.; Iobbi, V.; Tommasi, N.D.; Bisio, A. Potential of Salvia discolor Extract Against Common Agricultural Pathogens. Agronomy 2025, 15, 1268. https://doi.org/10.3390/agronomy15061268
Devi P, Lanteri AP, Minuto A, Parisi V, Iobbi V, Tommasi ND, Bisio A. Potential of Salvia discolor Extract Against Common Agricultural Pathogens. Agronomy. 2025; 15(6):1268. https://doi.org/10.3390/agronomy15061268
Chicago/Turabian StyleDevi, Poonam, Anna Paola Lanteri, Andrea Minuto, Valentina Parisi, Valeria Iobbi, Nunziatina De Tommasi, and Angela Bisio. 2025. "Potential of Salvia discolor Extract Against Common Agricultural Pathogens" Agronomy 15, no. 6: 1268. https://doi.org/10.3390/agronomy15061268
APA StyleDevi, P., Lanteri, A. P., Minuto, A., Parisi, V., Iobbi, V., Tommasi, N. D., & Bisio, A. (2025). Potential of Salvia discolor Extract Against Common Agricultural Pathogens. Agronomy, 15(6), 1268. https://doi.org/10.3390/agronomy15061268









