Mechanical Chiseling Versus Root Bio-Tillage on Soil Physical Quality and Soybean Yield in a Long-Term No-Till System
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Treatments and Experimental Design
2.3. Experiment Conduction
2.4. Soil and Plant Assessments
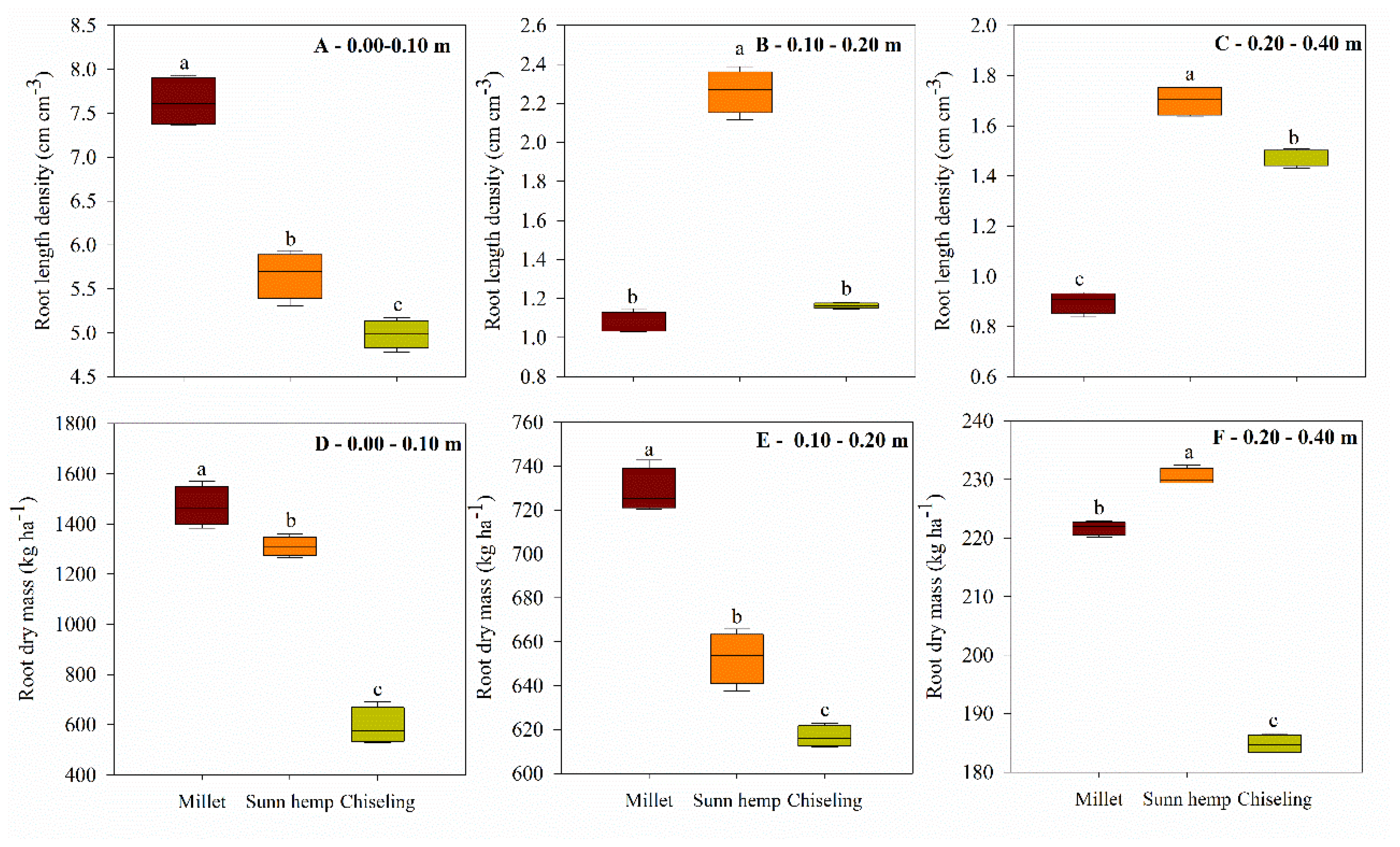
2.4.1. Root Development
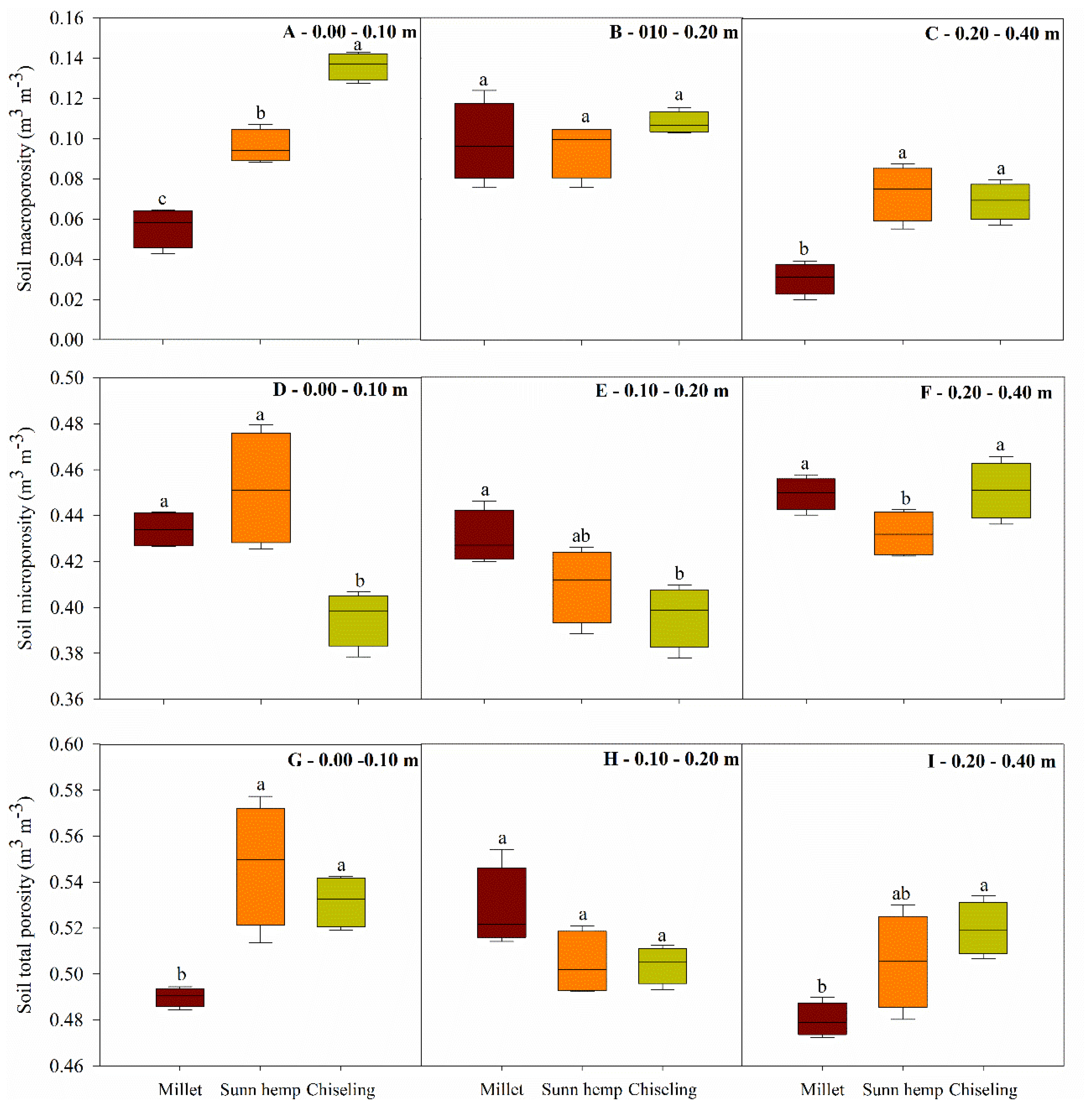
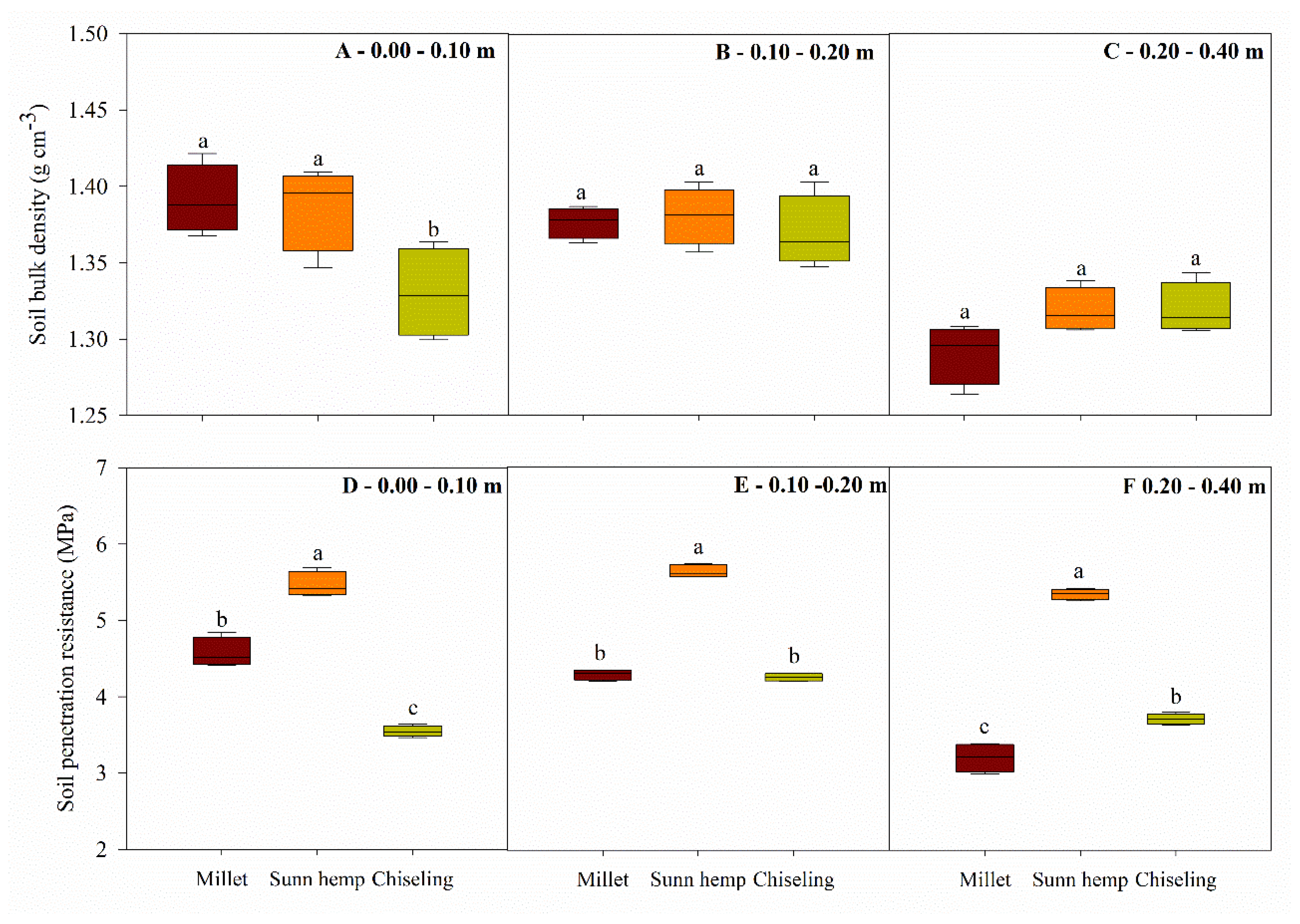
2.4.2. Soil Physical Properties
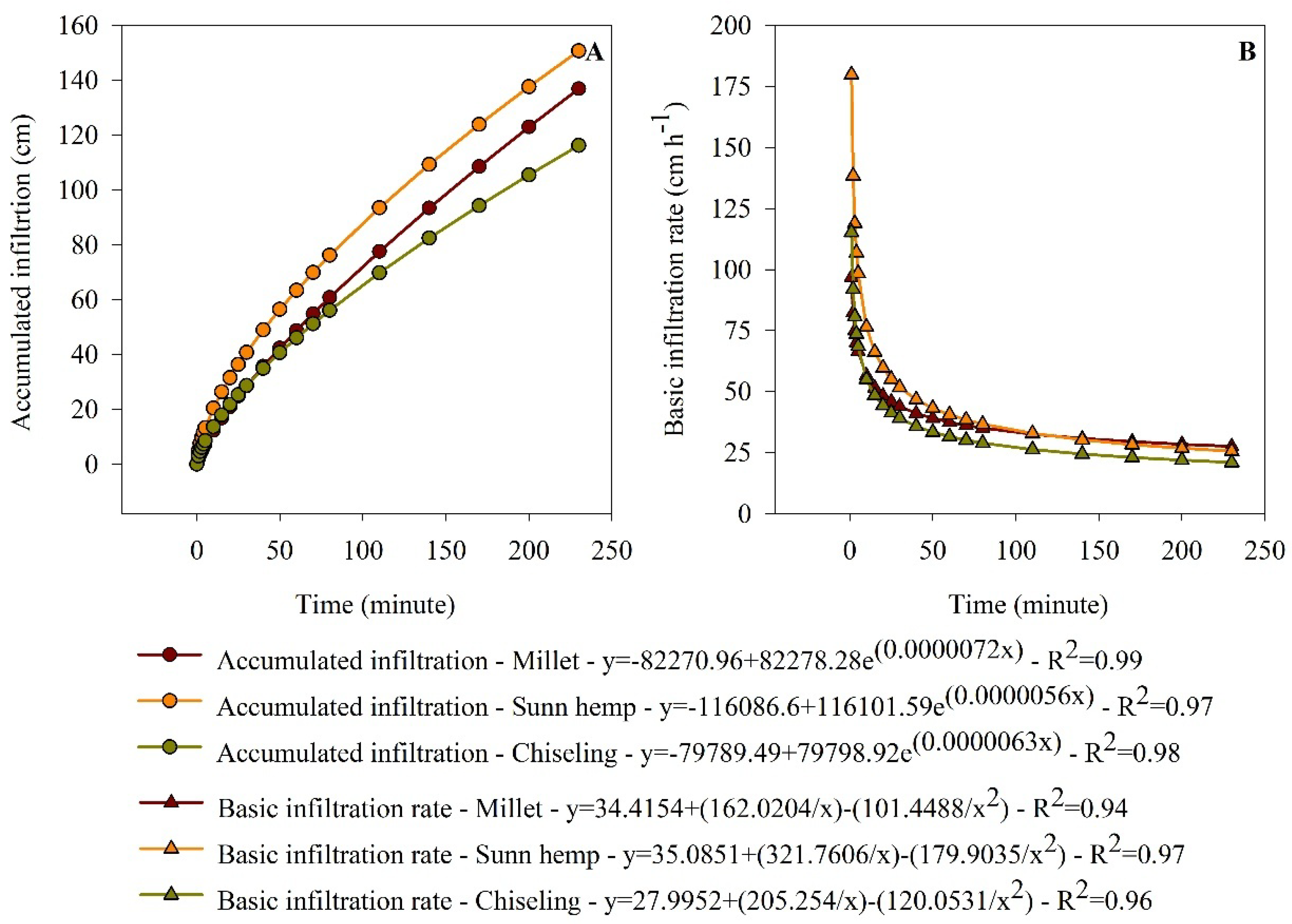
2.4.3. Soil Water Infiltration
2.4.4. Storage and Availability of Water in the Soil
2.4.5. Soybean Grain Yield
2.5. Statistical Analysis
3. Results
3.1. Root Development
3.2. Soil Physical Properties
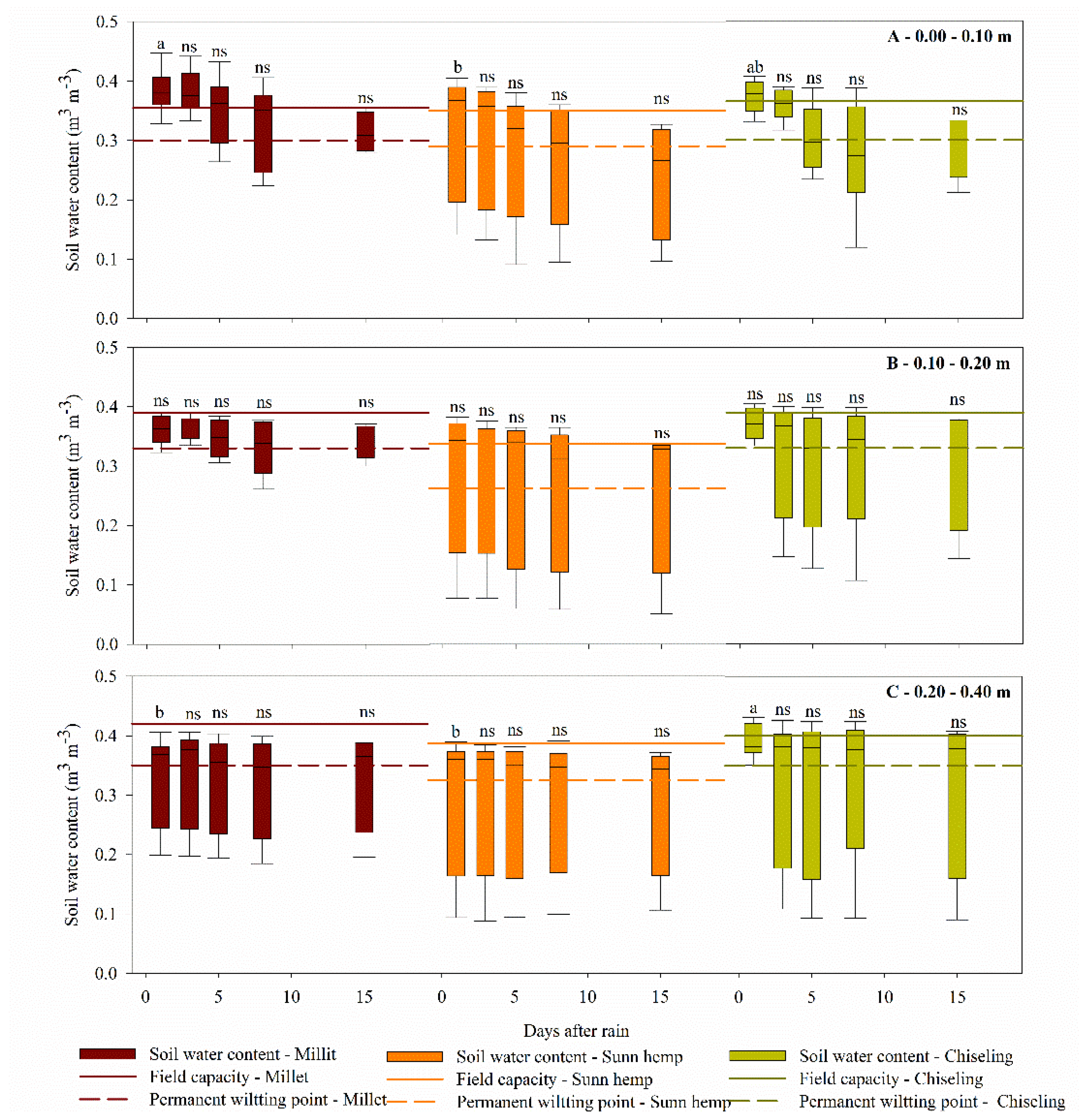
3.3. Infiltration, Storage, and Availability of Water in the Soil
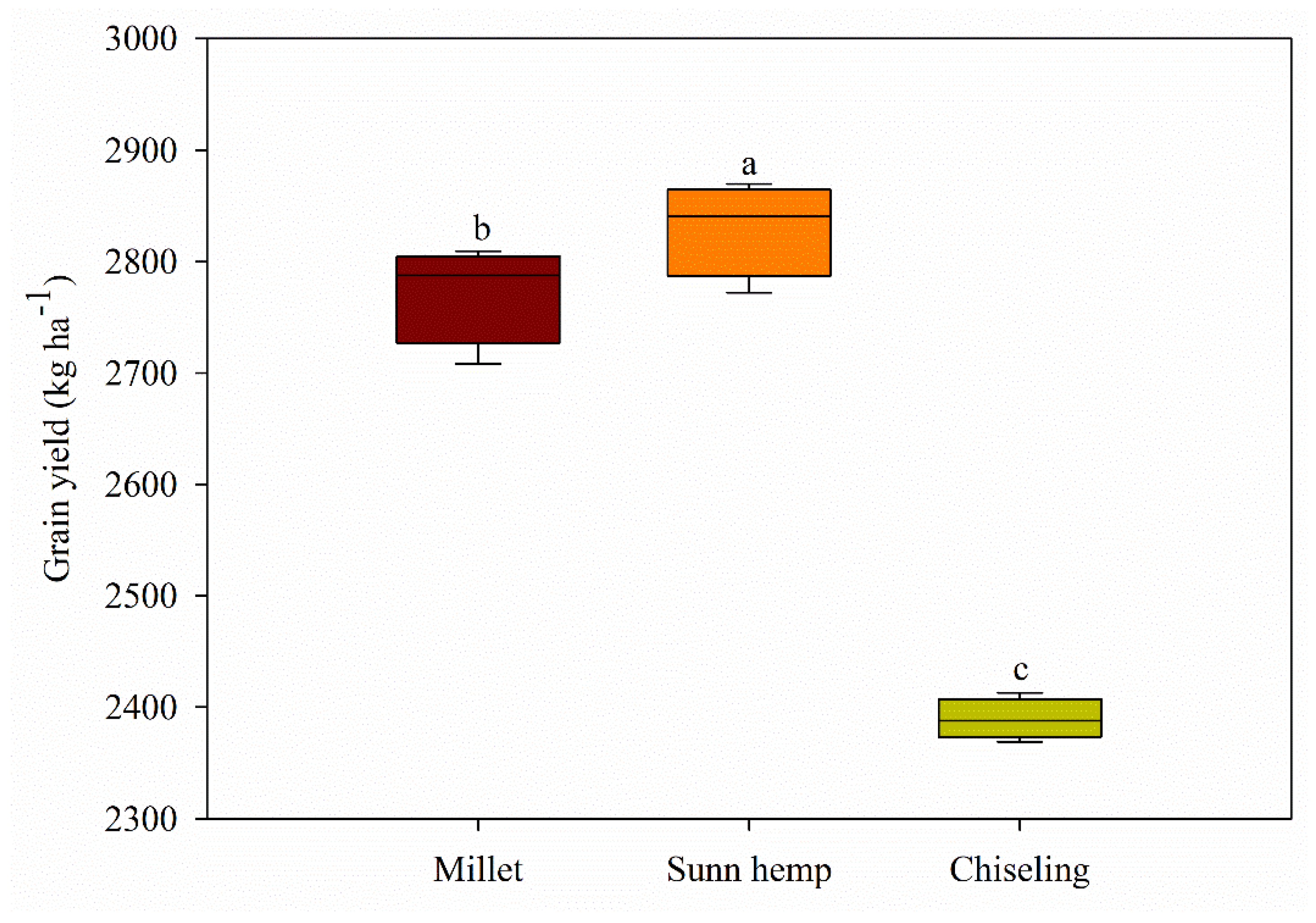
3.4. Soybean Grain Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIR | basic infiltration rate |
| DAR | days after rainfall |
| FC | field capacity |
| PWP | permanent wilting point |
| WI | soil water infiltration |
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a Cultivated Planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Pawlak, K.; Kołodziejczak, M. The Role of Agriculture in Ensuring Food Security in Developing Countries: Considerations in the Context of the Problem of Sustainable Food Production. Sustainability 2020, 12, 5488. [Google Scholar] [CrossRef]
- Cárceles Rodríguez, B.; Durán-Zuazo, V.H.; Soriano Rodríguez, M.; García-Tejero, I.F.; Gálvez Ruiz, B.; Cuadros Tavira, S. Conservation Agriculture as a Sustainable System for Soil Health: A Review. Soil Syst. 2022, 6, 87. [Google Scholar] [CrossRef]
- ELD Initiative. The Value of Land: Prosperous Lands and Positive Rewards Through Sustainable Land Management, 1st ed.; Stewart, N., Ed.; German Federal Ministry for Economic Cooperation and Development: Bonn Germany, 2015.
- UNCCD. United Nations Convention to Combat Desertification—Global Land Outlook, 1st ed.; UNCCD Secretariat: Bonn, Germany, 2017. [Google Scholar]
- Coppus, R. The Global Distribution of Human-Induced Land Degradation and Areas at Risk—SOLAW21 Technical Background Report; FAO: Rome, Italy, 2023; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/0e82eb25-237f-4f9c-8e7e-9007cabd4bde/content (accessed on 20 May 2025).
- Ferdinand, M.S.; Baret, P.V. A Method to Account for Diversity of Practices in Conservation Agriculture. Agron. Sustain. Dev. 2024, 44, 31. [Google Scholar] [CrossRef]
- Bernoux, M.; Cerri, C.C.; Cerri, C.E.P.; Neto, M.S.; Metay, A.; Perrin, A.-S.; Scopel, E.; Tantely, R.; Blavet, D.; de Piccolo, M.C.; et al. Cropping Systems, Carbon Sequestration and Erosion in Brazil: A Review. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 75–85. [Google Scholar]
- Peixoto, D.S.; da Silva, L.D.C.M.; de Melo, L.B.B.; Azevedo, R.P.; Araújo, B.C.L.; de Carvalho, T.S.; Moreira, S.G.; Curi, N.; Silva, B.M. Occasional Tillage in No-Tillage Systems: A Global Meta-Analysis. Sci. Total Environ. 2020, 745, 140887. [Google Scholar] [CrossRef] [PubMed]
- Vizioli, B.; Cavalieri-Polizeli, K.M.V.; Tormena, C.A.; Barth, G. Effects of Long-Term Tillage Systems on Soil Physical Quality and Crop Yield in a Brazilian Ferralsol. Soil Tillage Res. 2021, 209, 104935. [Google Scholar] [CrossRef]
- Keller, T.; Sandin, M.; Colombi, T.; Horn, R.; Or, D. Historical Increase in Agricultural Machinery Weights Enhanced Soil Stress Levels and Adversely Affected Soil Functioning. Soil Tillage Res. 2019, 194, 104293. [Google Scholar] [CrossRef]
- Sattolo, T.M.S.; Pereira, L.M.; Otto, R.; Francisco, E.; Duarte, A.P.; Kappes, C.; Prochnow, L.I.; Cherubin, M.R. Effects of Land Use, Tillage Management, and Crop Diversification on Soil Physical Quality in Cerrado Agricultural Systems. Soil Sci. Soc. Am. J. 2021, 85, 1799–1813. [Google Scholar] [CrossRef]
- Tian, M.; Whalley, W.R.; Zhou, H.; Ren, T.; Gao, W. Does No-Tillage Mitigate the Negative Effects of Harvest Compaction on Soil Pore Characteristics in Northeast China? Soil Tillage Res. 2023, 233, 105787. [Google Scholar] [CrossRef]
- Zhang, B.; Jia, Y.; Fan, H.; Guo, C.; Fu, J.; Li, S.; Li, M.; Liu, B.; Ma, R. Soil Compaction Due to Agricultural Machinery Impact: A Systematic Review. Land Degrad. Dev. 2024, 35, 3256–3273. [Google Scholar] [CrossRef]
- Munkholm, L.J.; Schjønning, P.; Rüegg, K. Mitigation of Subsoil Recompaction by Light Traffic and On-Land Ploughing. Soil Tillage Res. 2005, 80, 149–158. [Google Scholar] [CrossRef]
- Schneider, F.; Don, A.; Hennings, I.; Schmittmann, O.; Seidel, S.J. The Effect of Deep Tillage on Crop Yield—What Do We Really Know? Soil Tillage Res. 2017, 174, 193–204. [Google Scholar] [CrossRef]
- Keller, T.; Colombi, T.; Ruiz, S.; Schymanski, S.J.; Weisskopf, P.; Koestel, J.; Sommer, M.; Stadelmann, V.; Breitenstein, D.; Kirchgessner, N.; et al. Soil Structure Recovery Following Compaction: Short-term Evolution of Soil Physical Properties in a Loamy Soil. Soil Sci. Soc. Am. J. 2021, 85, 1002–1020. [Google Scholar] [CrossRef]
- Håkansson, I.; Reeder, R.C. Subsoil Compaction by Vehicles with High Axle Load—Extent, Persistence and Crop Response. Soil Tillage Res. 1994, 29, 277–304. [Google Scholar] [CrossRef]
- Arvidsson, J.; Keller, T. Soil Stress as Affected by Wheel Load and Tyre Inflation Pressure. Soil Tillage Res. 2007, 96, 284–291. [Google Scholar] [CrossRef]
- Fernández-Ugalde, O.; Virto, I.; Bescansa, P.; Imaz, M.J.; Enrique, A.; Karlen, D.L. No-Tillage Improvement of Soil Physical Quality in Calcareous, Degradation-Prone, Semiarid Soils. Soil Tillage Res. 2009, 106, 29–35. [Google Scholar] [CrossRef]
- Dang, Y.P.; Balzer, A.; Crawford, M.; Rincon-Florez, V.; Liu, H.; Melland, A.R.; Antille, D.; Kodur, S.; Bell, M.J.; Whish, J.P.M.; et al. Strategic Tillage in Conservation Agricultural Systems of North-Eastern Australia: Why, Where, When and How? Environ. Sci. Pollut. Res. 2018, 25, 1000–1015. [Google Scholar] [CrossRef] [PubMed]
- Li, G.D.; Conyers, M.K.; Helyar, K.R.; Lisle, C.J.; Poile, G.J.; Cullis, B.R. Long-Term Surface Application of Lime Ameliorates Subsurface Soil Acidity in the Mixed Farming Zone of South-Eastern Australia. Geoderma 2019, 338, 236–246. [Google Scholar] [CrossRef]
- Rusu, T. Energy Efficiency and Soil Conservation in Conventional, Minimum Tillage and No-Tillage. Int. Soil Water Conserv. Res. 2014, 2, 42–49. [Google Scholar] [CrossRef]
- Alskaf, K.; Mooney, S.J.; Sparkes, D.L.; Wilson, P.; Sjögersten, S. Short-Term Impacts of Different Tillage Practices and Plant Residue Retention on Soil Physical Properties and Greenhouse Gas Emissions. Soil Tillage Res. 2021, 206, 104803. [Google Scholar] [CrossRef]
- Peixoto, D.S.; Silva, B.M.; de Oliveira, G.C.; Moreira, S.G.; da Silva, F.; Curi, N. A Soil Compaction Diagnosis Method for Occasional Tillage Recommendation under Continuous No Tillage System in Brazil. Soil Tillage Res. 2019, 194, 104307. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, S.; Sun, Z.; Wang, H.; Qu, S.; Lei, N.; He, J.; Dong, Q. Tillage Effects on Soil Properties and Crop Yield after Land Reclamation. Sci. Rep. 2021, 11, 4611. [Google Scholar] [CrossRef]
- Guedes Filho, O.; da Silva, A.P.; Giarola, N.F.B.; Tormena, C.A. Structural Properties of the Soil Seedbed Submitted to Mechanical and Biological Chiseling under No-Tillage. Geoderma 2013, 204–205, 94–101. [Google Scholar] [CrossRef]
- Nunes, M.R.; Denardin, J.E.; Pauletto, E.A.; Faganello, A.; Pinto, L.F.S. Effect of Soil Chiseling on Soil Structure and Root Growth for a Clayey Soil under No-Tillage. Geoderma 2015, 259–260, 149–155. [Google Scholar] [CrossRef]
- Calonego, J.C.; Raphael, J.P.A.; Rigon, J.P.G.; de Oliveira Neto, L.; Rosolem, C.A. Soil Compaction Management and Soybean Yields with Cover Crops under No-till and Occasional Chiseling. Eur. J. Agron. 2017, 85, 31–37. [Google Scholar] [CrossRef]
- Silva, G.F.D.; Matusevicius, A.P.O.; Calonego, J.C.; Chamma, L.; Luperini, B.C.O.; Alves, M.D.S.; Leite, H.M.F.; Pinto, E.D.J.; Silva, M.D.A.; Putti, F.F. Soil–Plant Relationships in Soybean Cultivated under Crop Rotation after 17 Years of No-Tillage and Occasional Chiseling. Plants 2022, 11, 2657. [Google Scholar] [CrossRef] [PubMed]
- Wiesmeier, M.; Hübner, R.; Barthold, F.; Spörlein, P.; Geuß, U.; Hangen, E.; Reischl, A.; Schilling, B.; von Lützow, M.; Kögel-Knabner, I. Amount, Distribution and Driving Factors of Soil Organic Carbon and Nitrogen in Cropland and Grassland Soils of Southeast Germany (Bavaria). Agric. Ecosyst. Environ. 2013, 176, 39–52. [Google Scholar] [CrossRef]
- Colombi, T.; Torres, L.C.; Walter, A.; Keller, T. Feedbacks between Soil Penetration Resistance, Root Architecture and Water Uptake Limit Water Accessibility and Crop Growth—A Vicious Circle. Sci. Total Environ. 2018, 626, 1026–1035. [Google Scholar] [CrossRef]
- Inagaki, T.M.; Sá, J.C.D.M.; Tormena, C.A.; Dranski, A.; Muchalak, A.; Briedis, C.; de Oliveira Ferreira, A.; Giarola, N.F.B.; da Silva, Á.P. Mechanical and Biological Chiseling Impacts on Soil Organic C Stocks, Root Growth, and Crop Yield in a Long-Term No-till System. Soil Tillage Res. 2021, 211, 104993. [Google Scholar] [CrossRef]
- Çelik, İ.; Günal, H.; Acar, M.; Acir, N.; Bereket Barut, Z.; Budak, M. Strategic Tillage May Sustain the Benefits of Long-Term No-till in a Vertisol under Mediterranean Climate. Soil Tillage Res. 2019, 185, 17–28. [Google Scholar] [CrossRef]
- Secco, D.; Bassegio, D.; Villa, B.D.; Marins, A.C.D.; Zanão Junior, L.A.; Silva, T.R.B.D.; de Souza, S.N.M. Crambe Oil Yield and Soil Physical Properties Responses to No-Tillage, Cover Crops and Chiseling. Ind. Crops Prod. 2021, 161, 113174. [Google Scholar] [CrossRef]
- Cresswell, H.; Kirkegaard, J. Subsoil Amelioration by Plant-Roots—The Process and the Evidence. Soil Res. 1995, 33, 221. [Google Scholar] [CrossRef]
- Pulido-Moncada, M.; Katuwal, S.; Ren, L.; Cornelis, W.; Munkholm, L. Impact of Potential Bio-Subsoilers on Pore Network of a Severely Compacted Subsoil. Geoderma 2020, 363, 114154. [Google Scholar] [CrossRef]
- Galdos, M.V.; Pires, L.F.; Cooper, H.V.; Calonego, J.C.; Rosolem, C.A.; Mooney, S.J. Assessing the Long-Term Effects of Zero-Tillage on the Macroporosity of Brazilian Soils Using X-Ray Computed Tomography. Geoderma 2019, 337, 1126–1135. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, X. Bio-Tillage: A New Perspective for Sustainable Agriculture. Soil Tillage Res. 2021, 206, 104844. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, Z.; Peng, X. Root and Root-derived Biopore Interactions in Soils: A Review. J. Plant Nutr. Soil Sci. 2022, 185, 643–655. [Google Scholar] [CrossRef]
- Waring, E.R.; Pederson, C.; Lagzdins, A.; Clifford, C.; Helmers, M.J. Water and Soil Quality Respond to No-Tillage and Cover Crops Differently through 10 Years of Implementation. Agric. Ecosyst. Environ. 2024, 360, 108791. [Google Scholar] [CrossRef]
- Abrahão, G.M.; Costa, M.H. Evolution of Rain and Photoperiod Limitations on the Soybean Growing Season in Brazil: The Rise (and Possible Fall) of Double-Cropping Systems. Agric. For. Meteorol. 2018, 256–257, 32–45. [Google Scholar] [CrossRef]
- Silva, G.F.D.; Calonego, J.C.; Luperini, B.C.O.; Chamma, L.; Alves, E.R.; Rodrigues, S.A.; Putti, F.F.; da Silva, V.M.; de Almeida Silva, M. Soil—Plant Relationships in Soybean Cultivated under Conventional Tillage and Long-Term No-Tillage. Agronomy 2022, 12, 697. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014; Volume 12, ISBN 0926487221. [Google Scholar]
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação Da Fertilidade de Solos Tropicais; IAC: Campinas, Brazil, 2001. [Google Scholar]
- EMBRAPA. Manual de Métodos de Análise de Solo, 3rd ed.; Teixeira, P.C., Donagemma, G.K., Fontana, A., Teixeira, W.G.T., Eds.; Embrapa: Brasílis, Brazil, 2017; ISBN 9788570357717. [Google Scholar]
- Franco, J.R.; Dal Pai, E.; Calça, M.V.C.; Raniero, M.R.; Dal Pai, A.; Sarnighausen, V.C.R.; Sanchéz-Román, R.M. Update of Climatological Normal and Köppen Climate Classification for the Municipality of Botucatu-SP. Irriga 2023, 28, 77–92. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Campos, L.J.M.; Menna, P.; Brandi, F.; Ramos, Y.G. Seed Pre-inoculation with Bradyrhizobium as Time-optimizing Option for Large-scale Soybean Cropping Systems. Agron. J. 2020, 112, 5222–5236. [Google Scholar] [CrossRef]
- Quaggio, J.A.; Cantarella, H.; Rosolem, C.A.; Crusciol, C.A.C. Soja. In Boletim 100: Recomendações de Adubação E Calagem Para O Estado de São Paulo; Cantarella, H., Quaggio, J.A., Mattos, D., Jr., Boaretto, R.M., Raij, B.V., Eds.; IAC: Campinas, Brazil, 2022; pp. 209–212. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. In Methods of Soil Analysis: Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 2018; pp. 635–662. [Google Scholar]
- Smith, K.A.; Mullins, C.E. Soil Analysis: Physical Methods; Marcel Dekker: New York, NY, USA, 1991; ISBN 0824783611. [Google Scholar]
- Stolf, R.; Murakami, J.H.; Brugnaro, C.; Silva, L.G.; Silva, L.C.F.D.; Margarido, L.A.C. Penetrômetro de Impacto Stolf—Programa Computacional de Dados Em EXCEL-VBA. Rev. Bras. Cienc. Solo 2014, 38, 774–782. [Google Scholar] [CrossRef]
- Bernardo, S.; Mantovani, E.C.; Da Silva, D.D.; Soares, A.A. Manual de Irrigação, 9th ed.; UFV: Viçosa, Brazil, 2019; ISBN 9788572696104. [Google Scholar]
- Kostiakov, A.N. On the Dynamics of the Coefficient of Water—Percolation in Soils and on the Necessity for Studying It from a Dynamic Point of View for Purposes of Ameliation. Soc. Soil Sci. 1932, 14, 17–31. [Google Scholar]
- Lewis, M.R. The Rate of Infiltration of Water in Irrigation-Practice. Trans. Am. Geophys. Union 1937, 18, 361. [Google Scholar] [CrossRef]
- Müller, M.; Schneider, J.R.; Klein, V.A.; da Silva Júnior, J.P.; Chavarria, G. Root Growth and Crop Performance of Soybean under Chemical, Physical, and Biological Changes after Subsoiling. Agron. J. 2020, 112, 932–947. [Google Scholar] [CrossRef]
- Silva, G.F.; Calonego, J.C.; Luperini, B.C.O.; Silveira, V.B.; Chamma, L.; Soratto, R.P.; Putti, F.F. No-Tillage System Can Improve Soybean Grain Production More Than Conventional Tillage System. Plants 2023, 12, 3762. [Google Scholar] [CrossRef] [PubMed]
- Calonego, J.C.; Rosolem, C.A. Soybean Root Growth and Yield in Rotation with Cover Crops under Chiseling and No-Till. Eur. J. Agron. 2010, 33, 242–249. [Google Scholar] [CrossRef]
- Wendel, A.S.; Bauke, S.L.; Amelung, W.; Knief, C. Root-Rhizosphere-Soil Interactions in Biopores. Plant Soil 2022, 475, 253–277. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-Smart Soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Poffenbarger, H.J.; Olk, D.C.; Cambardella, C.; Kersey, J.; Liebman, M.; Mallarino, A.; Six, J.; Castellano, M.J. Whole-Profile Soil Organic Matter Content, Composition, and Stability under Cropping Systems That Differ in Belowground Inputs. Agric. Ecosyst. Environ. 2020, 291, 106810. [Google Scholar] [CrossRef]
- Pandey, B.K.; Huang, G.; Bhosale, R.; Hartman, S.; Sturrock, C.J.; Jose, L.; Martin, O.C.; Karady, M.; Voesenek, L.A.C.J.; Ljung, K.; et al. Plant Roots Sense Soil Compaction through Restricted Ethylene Diffusion. Science 2021, 371, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Tisdall, J.M. Formation of Soil Aggregates and Accumulation of Soil Organic Matter. In Structure and Organic Matter Storage in Agricultural Soils; CRC Press: Boca Raton, FL, USA, 2020; pp. 57–96. [Google Scholar]
- Miller, R.M.; Jastrow, J.D. Hierarchy of Root and Mycorrhizal Fungal Interactions with Soil Aggregation. Soil Biol. Biochem. 1990, 22, 579–584. [Google Scholar] [CrossRef]
- Poirier, V.; Roumet, C.; Munson, A.D. The Root of the Matter: Linking Root Traits and Soil Organic Matter Stabilization Processes. Soil Biol. Biochem. 2018, 120, 246–259. [Google Scholar] [CrossRef]
- Bai, T.; Wang, P.; Ye, C.; Hu, S. Form of Nitrogen Input Dominates N Effects on Root Growth and Soil Aggregation: A Meta-Analysis. Soil Biol. Biochem. 2021, 157, 108251. [Google Scholar] [CrossRef]
- Sullivan, P.L.; Billings, S.A.; Hirmas, D.; Li, L.; Zhang, X.; Ziegler, S.; Murenbeeld, K.; Ajami, H.; Guthrie, A.; Singha, K.; et al. Embracing the Dynamic Nature of Soil Structure: A Paradigm Illuminating the Role of Life in Critical Zones of the Anthropocene. Earth Sci. Rev. 2022, 225, 103873. [Google Scholar] [CrossRef]
- King, A.E.; Ali, G.A.; Gillespie, A.W.; Wagner-Riddle, C. Soil Organic Matter as Catalyst of Crop Resource Capture. Front. Environ. Sci. 2020, 8, 50. [Google Scholar] [CrossRef]
- Gerke, J. The Central Role of Soil Organic Matter in Soil Fertility and Carbon Storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- Ning, T.; Liu, Z.; Hu, H.; Li, G.; Kuzyakov, Y. Physical, Chemical and Biological Subsoiling for Sustainable Agriculture. Soil Tillage Res. 2022, 223, 105490. [Google Scholar] [CrossRef]
- Bengough, A.G.; Bransby, M.F.; Hans, J.; McKenna, S.J.; Roberts, T.J.; Valentine, T.A. Root Responses to Soil Physical Conditions; Growth Dynamics from Field to Cell. J. Exp. Bot. 2006, 57, 437–447. [Google Scholar] [CrossRef]
- Luxmoore, R.J. Micro-, Meso-, and Macroporosity of Soil. Soil Sci. Soc. Am. J. 1981, 45, 671–672. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Werner, A.D.; Li, Y.; Jiang, S.; Tan, Z. Root-Induced Changes of Soil Hydraulic Properties—A Review. J. Hydrol. 2020, 589, 125203. [Google Scholar] [CrossRef]
- Raphael, J.P.A.; Calonego, J.C.; Milori, D.M.B.P.; Rosolem, C.A. Soil Organic Matter in Crop Rotations under No-Till. Soil Tillage Res. 2016, 155, 45–53. [Google Scholar] [CrossRef]
- Bortoluzzi, E.C.; Bortoluzzi, M.P.; Chiomento, J.L.T.; Cassel, J.L.; Werner, H.A.; Petry, C. Improving Water Infiltration in Croplands Mitigates the Effects of Extreme Rainfall Events. Environ. Earth Sci. 2025, 84, 124. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Q.; Lai, X.; Liao, K.; Guo, C. Response of Soil Hydrological Processes to Soil Rock Fragments: A Global Meta-Analysis. Sci. China Earth Sci. 2023, 66, 2066–2080. [Google Scholar] [CrossRef]
| Spring Management | pH | P | OM | H + Al | Ca | Mg | K | Sand | Silt | Clay |
|---|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | mg dm−3 | mmolc dm−3 | g kg−1 | |||||||
| 0.00–0.20 m | ||||||||||
| Millet | 4.8 | 47.7 | 27.3 | 55.0 | 7.7 | 2.5 | 5.7 | 110 | 245 | 645 |
| Sunn hemp | 5.2 | 43.2 | 31.1 | 46.5 | 8.4 | 3.5 | 5.1 | |||
| Chiseling | 5.3 | 42.5 | 26.7 | 41.5 | 8.3 | 3.5 | 6.9 | |||
| 0.20–0.40 m | ||||||||||
| Millet | 4.4 | 29.4 | 22.3 | 59.0 | 7.1 | 1.7 | 5.2 | 116 | 220 | 664 |
| Sunn hemp | 4.7 | 22.9 | 21.3 | 50.4 | 4.6 | 2.9 | 4.1 | |||
| Chiseling | 4.8 | 30.1 | 23.1 | 44.9 | 4.8 | 1.7 | 6.0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.F.d.; Luperini, B.C.O.; Barcelos, J.P.d.Q.; Putti, F.F.; Mooney, S.J.; Calonego, J.C. Mechanical Chiseling Versus Root Bio-Tillage on Soil Physical Quality and Soybean Yield in a Long-Term No-Till System. Agronomy 2025, 15, 1249. https://doi.org/10.3390/agronomy15051249
Silva GFd, Luperini BCO, Barcelos JPdQ, Putti FF, Mooney SJ, Calonego JC. Mechanical Chiseling Versus Root Bio-Tillage on Soil Physical Quality and Soybean Yield in a Long-Term No-Till System. Agronomy. 2025; 15(5):1249. https://doi.org/10.3390/agronomy15051249
Chicago/Turabian StyleSilva, Gustavo Ferreira da, Bruno Cesar Ottoboni Luperini, Jéssica Pigatto de Queiroz Barcelos, Fernando Ferrari Putti, Sacha J. Mooney, and Juliano Carlos Calonego. 2025. "Mechanical Chiseling Versus Root Bio-Tillage on Soil Physical Quality and Soybean Yield in a Long-Term No-Till System" Agronomy 15, no. 5: 1249. https://doi.org/10.3390/agronomy15051249
APA StyleSilva, G. F. d., Luperini, B. C. O., Barcelos, J. P. d. Q., Putti, F. F., Mooney, S. J., & Calonego, J. C. (2025). Mechanical Chiseling Versus Root Bio-Tillage on Soil Physical Quality and Soybean Yield in a Long-Term No-Till System. Agronomy, 15(5), 1249. https://doi.org/10.3390/agronomy15051249









