Comparative Nutrient Study of Raphanus sativus L. Sprouts Microgreens, and Roots
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Standards, and Equipment
2.2. Plant Material
2.3. Moisture and Ash Content
2.4. Total Protein Content
2.5. Crude Fat Content
2.6. Dietary Fiber Content
2.7. Available Carbohydrate Content and Energy Value
2.8. Extraction and Analysis of Sugars
2.9. Extraction and Analysis of Organic Acids
2.10. Fatty Acid Composition
2.11. Statistical Analysis
3. Results and Discussion
3.1. Nutrient Composition of Radish Sprouts, Microgreens, and Roots
3.2. Composition of Dietary Fiber
3.3. Composition of Sugars
3.4. Composition of Organic Acids
3.5. Composition of Fatty Acids
3.6. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S. A comparative metabolomics study of flavonoids in radish with different skin and flesh colors (Raphanus sativus L.). J. Agric. Food Chem. 2020, 68, 14463–14470. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [CrossRef]
- Alloggia, F.P.; Bafumo, R.F.; Ramirez, D.A.; Maza, M.A.; Camargo, A.B. Brassicaceae microgreens: A novel and promissory source of sustainable bioactive compounds. Curr. Res. Food Sci. 2023, 6, 100480. [Google Scholar] [CrossRef]
- Aziz, A.; Noreen, S.; Khalid, W.; Mubarik, F.; Niazi, M.K.; Koraqi, H.; Ali, A.; Lima, C.M.G.; Alansari, W.S.; Eskandrani, A.A.; et al. Extraction of bioactive compounds from different vegetable sprouts and their potential role in the formulation of functional foods against various disorders: A literature-based review. Molecules 2022, 27, 7320. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and microgreens—Novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Tallei, T.E.; Kapantow, N.H.; Niode, N.J.; Sailah, I.; Savitri, M.; Lahay, M.Y.; Barasarathi, J. The therapeutic potential of red radish microgreens in modulating inflammation and cancer pathways. CyTA-J. Food 2025, 23, 2467410. [Google Scholar] [CrossRef]
- Vučetić, A.; Šovljanski, O.; Pezo, L.; Gligorijević, N.; Kostić, S.; Vulić, J.; Čanadanović-Brunet, J. A comprehensive antioxidant and nutritional profiling of Brassicaceae microgreens. Antioxidants 2025, 14, 191. [Google Scholar] [CrossRef]
- Khan, R.S.; Khan, S.S.; Siddique, R. Radish (Raphanus sativus): Potential antioxidant role of bioactive compounds extracted from radish leaves—A review. Pak. J. Med. Health Sci. 2022, 16, 2. [Google Scholar] [CrossRef]
- Younus, I.; Siddiq, A.; Baig, S.G.; Sadaf, R.; Fayyaz, T.B.; Ain, N.U.; Ahmer, A. An analytical report of phytochemical constituents, pharmacological potential, and toxic effects of radish. Pak. J. Med. Health Sci. 2022, 16, 3. [Google Scholar] [CrossRef]
- Tomas, M.; Zhang, L.; Zengin, G.; Rocchetti, G.; Capanoglu, E.; Lucini, L. Metabolomic insight into the profile, in vitro bioaccessibility and bioactive properties of polyphenols and glucosinolates from four Brassicaceae microgreens. Food Res. Int. 2021, 140, 110039. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, N.A.; Ahmed, A.S.; Al-Sandooq, D.L. Detection of active compounds in radish Raphanus sativus L. and their various biological effects. Plant Arch. 2020, 20, 1647–1650. [Google Scholar]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. microgreens as novel functional foods: Variation of nutritional and phytochemical profiles and their in vitro bioactive properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef]
- Manivannan, A.; Kim, J.H.; Kim, D.S.; Lee, E.S.; Lee, H.E. Deciphering the nutraceutical potential of Raphanus sativus—A comprehensive overview. Nutrients 2019, 11, 402. [Google Scholar] [CrossRef]
- Cai, D.; Dong, Y.; Wang, L.; Zhao, S. Integrated metabolomics and transcriptomics analysis provides insights into biosynthesis and accumulation of flavonoids and glucosinolates in different radish varieties. Curr. Res. Food Sci. 2025, 10, 100938. [Google Scholar] [CrossRef]
- Jambalsuren, B.; Dondog, P. Biochemical analysis of radish and beet species. Mong. J. Agric. Sci. 2023, 16, 31–36. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Maraei, R.W.; Shalaby, T.A.; Aly, A.A. Metabolites, Nutritional quality and antioxidant activity of red radish roots affected by gamma rays. Agronomy 2022, 12, 1916. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Michalak, I.; Biesiada, A.; Szumny, A.; Pachura, N.; Piszcz, U. Systematic investigation of the effects of seven plant extracts on the physiological parameters, yield, and nutritional quality of radish (Raphanus sativus var. sativus). Front. in Plant Sci. 2021, 12, 651152. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Ashraf, R.; Sultana, B.; Riaz, S.; Mushtaq, M.; Iqbal, M.; Nazir, A.; Zafar, Z. Fortification of phenolics, antioxidant activities and biochemical attributes of radish root by plant leaf extract seed priming. Biocatal. Agr. Biotech. 2018, 16, 115–120. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.; Armstrong, E.; Moses, A.; Fahlman, R.; Koosha, H.; Yager, J.Y. Broccoli, kale, and radish sprouts: Key phytochemical constituents and DPPH free radical scavenging activity. Molecules 2023, 28, 4266. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.; Debs, E.; Koubaa, M.; Alrayess, Z.; Maroun, R.G.; Louka, N. Sprouts use as functional foods. optimization of germination of wheat (Triticum aestivum L.), alfalfa (Medicago sativa L.), and radish (Raphanus sativus L.) seeds based on their nutritional content evolution. Foods 2022, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Singh, N.; Kaur, C.; Varghese, E. Measurement of phytochemical content and nutritional characteristics of microgreens grown in high altitude region of India. J. Food Meas. Charact. 2024, 18, 3113–3127. [Google Scholar] [CrossRef]
- Tilahun, S.; Baek, M.W.; An, K.-S.; Choi, H.R.; Lee, J.H.; Hong, J.S.; Jeong, C.S. Radish Microgreens Produced Without Substrate in a Vertical Multi-Layered Growing Unit Are Rich in Nutritional Metabolites. Front. Plant Sci. 2023, 14, 1236055. [Google Scholar] [CrossRef]
- Kowitcharoen, L.; Phornvillay, S.; Lekkham, P.; Pongprasert, N.; Srilaong, V. Bioactive composition and nutritional profile of microgreens cultivated in Thailand. Appl. Sci. 2021, 11, 7981. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Mobeen; Wang, X.; Saleem, M.H.; Parveen, A.; Mumtaz, S.; Hassan, A.; Adnan, M.; Fiaz, S.; Ali, S.; Iqbal Khan, Z.; et al. Proximate composition and nutritive value of some leafy vegetables from Faisalabad, Pakistan. Sustainability 2021, 13, 8444. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Dmochowska-Ślęzak, K.; Grembecka, M. Root vegetables—Composition, health effects, and contaminants. Int. J. Environ. Res. Public Health 2022, 19, 15531. [Google Scholar] [CrossRef]
- Lu, Z.L.; Liu, L.W.; Li, X.Y.; Gong, Y.Q.; Hou, X.L.; Zhu, X.W.; Yang, J.L.; Wang, L.Z. Analysis and evaluation of nutritional quality in Chinese radish (Raphanus sativus L.). Agr. Sci. China 2008, 7, 823–830. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. The nutritional quality potential of microgreens, baby leaves, and adult lettuce: An underexploited nutraceutical source. Foods 2022, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, M.C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Lo Scalzo, R.; Branca, F. Morphometric characteristics, polyphenols and ascorbic acid variation in Brassica oleracea L. Novel foods: Sprouts, microgreens and baby leaves. Agronomy 2020, 10, 782. [Google Scholar] [CrossRef]
- Yadav, L.P.; Koley, T.K.; Tripathi, A.; Singh, S. Antioxidant potentiality and mineral content of summer season leafy greens: Comparison at mature and microgreen stages using chemometric. Agric. Res. 2019, 8, 165–175. [Google Scholar] [CrossRef]
- Waterland, N.L.; Moon, Y.; Tou, J.C.; Kim, M.J.; Pena-Yewtukhiw, E.M.; Park, S. Mineral content differs among microgreen, baby leaf, and adult stages in three cultivars of kale. HortScience 2017, 52, 566–571. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT-Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- de Menezes, E.W.; Grande, F.; Giuntini, E.B.; Lopes, T.D.V.C.; Dan, M.C.T.; do Prado, S.B.R.; Lajolo, F.M. Impact of dietary fiber energy on the calculation of food total energy value in the Brazilian Food Composition Database. Food Chem. 2016, 193, 128–133. [Google Scholar] [CrossRef]
- van Tran, K.; Ross, E.; Hancock, P. Evaluation of XBridge™ BEH Amide Columns for the analysis of five sugars in food and beverages using an Arc HPLC, Coupled to a 2414 RI Detector. Waters Corp. 2022, 720007835. Available online: https://www.waters.com (accessed on 14 May 2025).
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of storage temperature, storage duration, and sub L.;sequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of western red nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef]
- Zieliński, H.; Frias, J.; Piskuła, M.K.; Kozłowska, H.; Vidal-Valverde, C. Vitamin B1 and B2, dietary fiber and minerals content of Cruciferae sprouts. Eur. Food Res. Technol. 2005, 221, 78–83. [Google Scholar] [CrossRef]
- Pant, Y.; Lingwan, M.; Masakapalli, S.K. Metabolic, biochemical, mineral and fatty acid profiles of edible Brassicaceae microgreens establish them as promising functional food. Food Chem. Adv. 2023, 3, 100461. [Google Scholar] [CrossRef]
- Drozdowska, M.; Leszczyńska, T.; Koronowicz, A.; Piasna-Słupecka, E.; Domagała, D.; Kusznierewicz, B. Young shoots of red cabbage are a better source of selected nutrients and glucosinolates in comparison to the vegetable at full maturity. Eur. Food Res. Technol. 2020, 246, 2505–2515. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 63, 8752–8767. [Google Scholar] [CrossRef] [PubMed]
- Kalala, G.; Kambashi, B.; Everaert, N.; Beckers, Y.; Richel, A.; Pachikian, B.; Bindelle, J. Characterization of fructans and dietary fibre profiles in raw and steamed vegetables. Int. J. Food Sci. Nutr. 2018, 69, 682–689. [Google Scholar] [CrossRef]
- Schäfer, J.; Brett, A.; Trierweiler, B.; Bunzel, M. Characterization of cell wall composition of radish (Raphanus sativus L. var. sativus) and maturation related changes. J. Agric. Food Chem. 2016, 64, 8625–8632. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, X.; Shi, C.; Zhu, Y. Study on the effect of radish sprouts on short-chain fatty acids and gut microbial diversity in healthy individuals. Foods 2025, 14, 170. [Google Scholar] [CrossRef]
- Tang, L.; Li, M.; Zhao, G.; Ye, F. Characterization of a low-methoxyl pectin extracted from red radish (Raphanus sativus L.) pomace and its gelation induced by NaCl. Int. J. Biol. Macromol. 2024, 254, 127869. [Google Scholar] [CrossRef]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, Y. Comparative analysis of phytochemicals and antioxidant activities in seeds and sprouts of different varieties of radish (Raphanus sativus L.): TOPSIS-entropy weight method. Front. Plant Sci. 2025, 16, 1531570. [Google Scholar] [CrossRef]
- Schreiner, M.; Huyskens-Keil, S.; Peters, P.; Schonhof, I.; Krumbein, A.; Widell, S. Seasonal climate effects on root colour and compounds of red radish. J. Sci. Food Agric. 2002, 82, 1325–1333. [Google Scholar] [CrossRef]
- Priecina, L.; Karklina, D. Composition of major organic acids in vegetables and spices. In CBU International Conference Proceedings; ISE Research Institute: Tokyo, Japan, 2015; Volume 3, pp. 447–454. [Google Scholar] [CrossRef]
- Mei, S.; He, Z.; Zhang, J. Identification and analysis of major flavor compounds in radish taproots by widely targeted metabolomics. Front. Nutr. 2022, 9, 889407. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kim, H.K.; Kim, M.H.; Lee, J.S. Analysis of oxalic acid of various vegetables consumed in Korea. Food Sci. Biotechnol. 2007, 16, 650–654. [Google Scholar]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Zlatković, V.S.; Grahovac, N.L.; Banković Ilić, I.B.; Mitrović, P.M.; Troter, D.Z.; Todorović, Z.B.; Marjanović Jeromela, A.M.; Veljković, V.B. Effects of screw pressing conditions on fodder radish seed oil yield, throughput, and fatty acid profile. J. Food Process Eng. 2024, 47, e14588. [Google Scholar] [CrossRef]
- Mitrović, J.S.; Nikolić, N.Č.; Karabegović, I.T.; Todorović, Z.B.; Lazić, M.M.; Stojanović, G.S. The comparative study on the composition of acylglycerols and fatty acids in celery, parsnip and black radish roots. Adv. Technol. 2018, 7, 28–34. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]

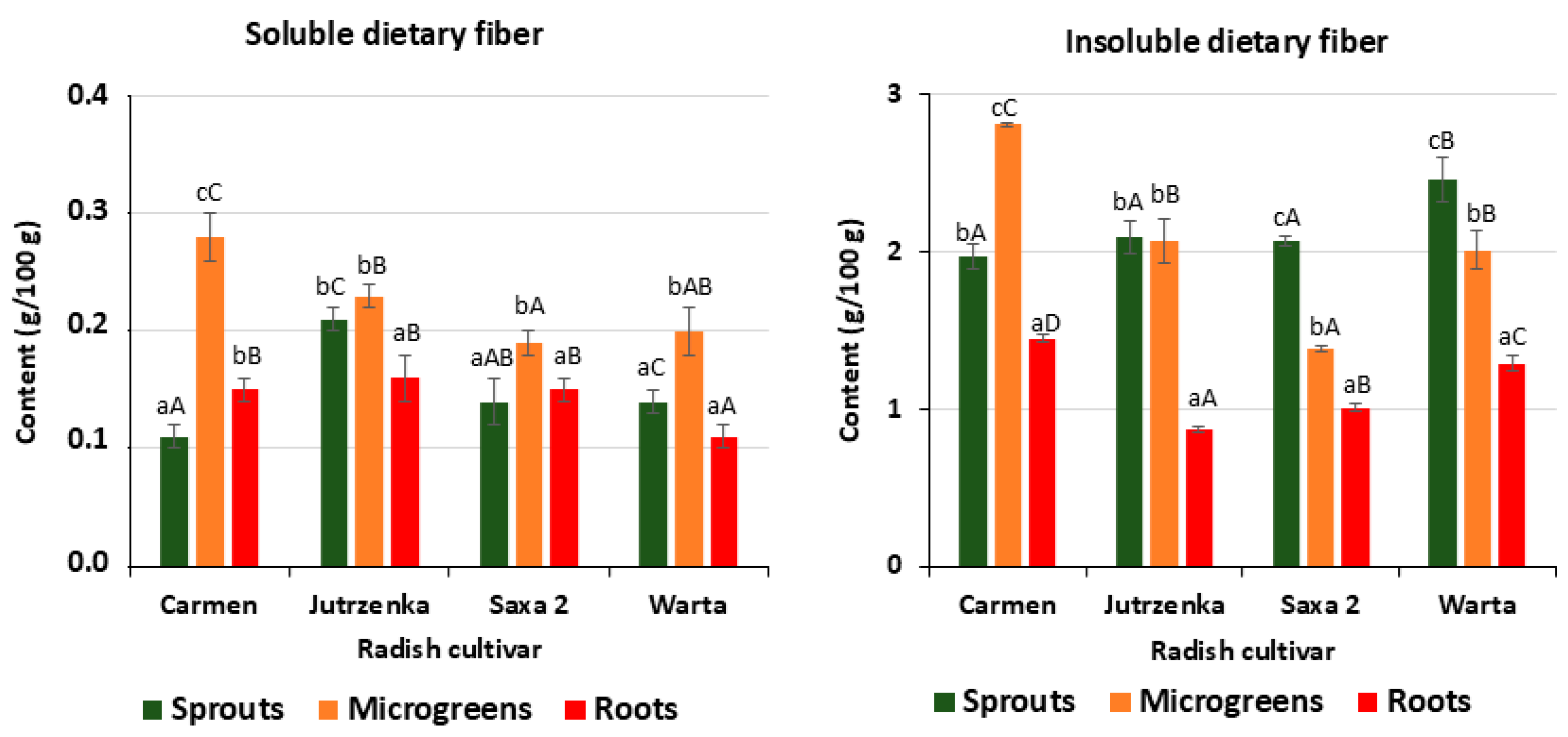
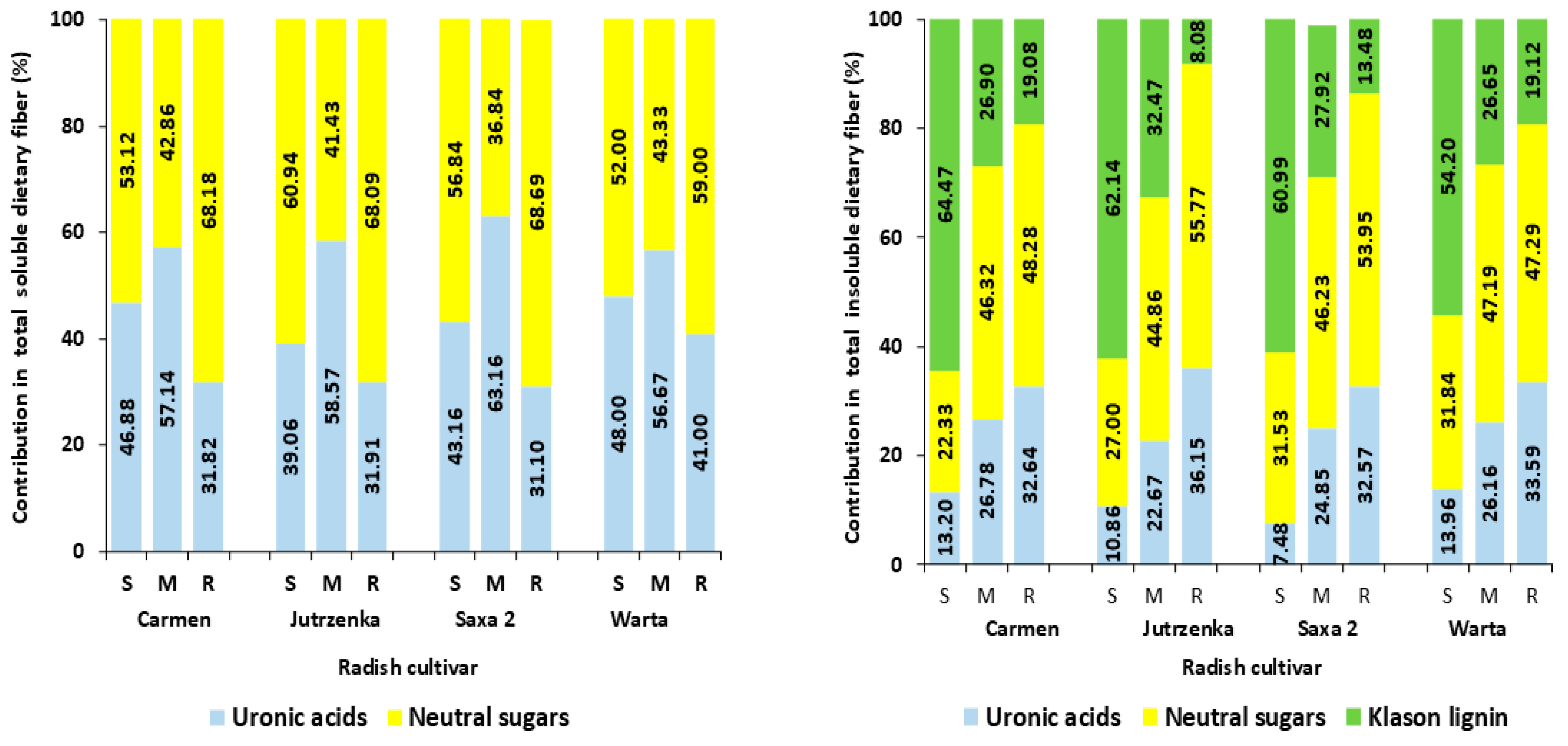



| Radish Cultivar | Stage of Development | Moisture | Total Protein | Crude Fat | Ash | Total Dietary Fiber | Available Carbohydrates | Energy Value |
|---|---|---|---|---|---|---|---|---|
| Carmen | Sprouts | 92.65 ± 0.36 bB | 2.90 ± 0.02 cA | 1.23 ± 0.04 cA | 0.56 ± 0.03 aA | 2.08 ± 0.08 bA | 0.58 ± 0.09 aB | 29.14 ± 0.67 cA |
| Microgreens | 91.54 ± 0.18 aB | 2.35 ± 0.03 bC | 0.55 ± 0.04 bC | 1.07 ± 0.04 bA | 3.09 ± 0.02 cC | 1.40 ± 0.18 bA | 26.12 ± 0.49 bB | |
| Roots | 94.81 ± 0.12 cB | 0.55 ± 0.01 aB | 0.11 ± 0.01 aA | 0.56 ± 0.01 aA | 1.60 ± 0.02 aD | 2.37 ± 0.12 cA | 15.87 ± 0.41 aA | |
| Jutrzenka | Sprouts | 91.35 ± 0.13 aA | 2.99 ± 0.02 cA | 2.05 ± 0.08 cB | 0.67 ± 0.03 aB | 2.30 ± 0.10 bA | 0.65 ± 0.10 aB | 37.58 ± 0.26 cC |
| Microgreens | 91.96 ± 0.08 bB | 1.49 ± 0.18 bA | 0.54 ± 0.01 bC | 1.02 ± 0.08 bA | 2.31 ± 0.13 bB | 2.69 ± 0.22 bC | 26.18 ± 0.42 bB | |
| Roots | 94.33 ± 0.22 cA | 0.49 ± 0.01 aA | 0.12 ± 0.01 aA | 0.56 ± 0.01 aA | 1.02 ± 0.02 aA | 3.47 ± 0.20 cB | 18.97 ± 0.88 aB | |
| Saxa 2 | Sprouts | 91.18 ± 0.31 aA | 3.24 ± 0.19 cB | 2.06 ± 0.04 cB | 0.82 ± 0.01 aC | 2.21 ± 0.03 cA | 0.49 ± 0.04 aAB | 37.86 ± 0.26 cC |
| Microgreens | 90.90 ± 0.11 aA | 1.50 ± 0.01 bB | 0.32 ± 0.02 bA | 1.51 ± 0.20 bB | 1.57 ± 0.03 bA | 4.20 ± 0.16 cD | 28.84 ± 0.47 bC | |
| Roots | 94.97 ± 0.09 bB | 0.51 ± 0.01 aA | 0.11 ± 0.02 aA | 0.65 ± 0.04 aB | 1.17 ± 0.02 aB | 2.59 ± 0.04 bA | 15.72 ± 0.31 aA | |
| Warta | Sprouts | 91.54 ± 0.16 aA | 3.52 ± 0.03 cC | 1.32 ± 0.03 cA | 0.68 ± 0.07 aB | 2.60 ± 0.14 cB | 0.35 ± 0.04 aA | 32.53 ± 0.10 cB |
| Microgreens | 92.49 ± 0.11 bC | 1.64 ± 0.03 bAB | 0.43 ± 0.03 bB | 1.18 ± 0.01 bA | 2.21 ± 0.12 bB | 2.05 ± 0.17 bB | 23.03 ± 0.35 bA | |
| Roots | 94.63 ± 0.12 cAB | 0.51 ± 0.00 aA | 0.13 ± 0.02 aA | 0.75 ± 0.05 aC | 1.41 ± 0.05 aC | 2.58 ± 0.11 cA | 16.33 ± 0.45 aA |
| Radish Cultivar | Radish Form | Fructose | Glucose | Sucrose | Total |
|---|---|---|---|---|---|
| Carmen | Sprouts | 0.16 ± 0.00 bB | 0.21 ± 0.00 aB | 0.05 ± 0.00 aA | 0.42 ± 0.01 aB |
| Microgreens | 0.09 ± 0.00 aC | 0.47 ± 0.02 bC | 0.05 ± 0.00 aC | 0.61 ± 0.02 bC | |
| Roots | 0.40 ± 0.02 cB | 1.34 ± 0.02 cB | 0.08 ± 0.01 bBC | 1.82 ± 0.04 cB | |
| Jutrzenka | Sprouts | 0.19 ± 0.02 bB | 0.37 ± 0.03 aC | 0.11 ± 0.01 cB | 0.67 ± 0.05 bC |
| Microgreens | 0.06 ± 0.00 aB | 0.34 ± 0.00 aB | 0.04 ± 0.01 aB | 0.44 ± 0.01 aB | |
| Roots | 0.73 ± 0.02 cD | 1.75 ± 0.06 bD | 0.08 ± 0.01 bAB | 2.56 ± 0.07 cD | |
| Saxa 2 | Sprouts | 0.12 ± 0.00 bA | 0.16 ± 0.01 aA | 0.10 ± 0.00 cB | 0.38 ± 0.02 bB |
| Microgreens | 0.04 ± 0.01 aA | 0.15 ± 0.02 aA | 0.03 ± 0.01 aA | 0.22 ± 0.02 aA | |
| Roots | 0.55 ± 0.01 cC | 1.07 ± 0.01 bA | 0.07 ± 0.01 bA | 1.69 ± 0.02 cA | |
| Warta | Sprouts | 0.12 ± 0.01 bA | 0.14 ± 0.01 aA | 0.05 ± 0.01 bA | 0.31 ± 0.02 aA |
| Microgreens | 0.09 ± 0.01 aC | 0.34 ± 0.02 bB | 0.02 ± 0.00 aA | 0.45 ± 0.03 bB | |
| Roots | 0.34 ± 0.02 cA | 1.51 ± 0.03 cC | 0.08 ± 0.02 cC | 1.93 ± 0.04 cC |
| Radish Cultivar | Radish Form | Oxalic Acid | Citric Acid | Tartaric Acid | Malic Acid | Succinic Acid | Fumaric Acid | Total Content |
|---|---|---|---|---|---|---|---|---|
| Carmen | Sprouts | 32.35 ± 0.37 bB | 4.35 ± 0.14 bA | 1.10 ± 0.02 bA | 1.18 ± 0.03 aA | 0.00 aA | 12.73 ± 0.51 bC | 51.71 ± 0.44 bB |
| Microgreens | 59.92 ± 4.29 cB | 7.73 ± 0.16 cC | 1.85 ± 0.13 cC | 21.57 ± 0.42 cB | 8.91 ± 0.47 cC | 38.88 ± 1.51 cD | 138.86 ± 3.45 cC | |
| Roots | 15.60 ± 0.65 aB | 1.63 ± 0.17 aC | 0.00 aA | 7.54 ± 0.23 bB | 2.21 ± 0.04 bAB | 3.61 ± 0.11 aB | 30.59 ± 0.76 aA | |
| Jutrzenka | Sprouts | 29.59 ± 0.60 bA | 5.73 ± 0.21 cB | 1.61 ± 0.12 cB | 4.52 ± 0.20 aC | 0.00 aA | 15.24 ± 0.39 cD | 56.69 ± 1.19 bC |
| Microgreens | 63.75 ± 1.59 cB | 3.91 ± 0.09 bA | 0.93 ± 0.08 bA | 20.43 ± 0.36 cB | 7.05 ± 0.26 bB | 11.63 ± 0.18 bA | 107.70 ± 1.58 cB | |
| Roots | 20.14 ± 0.36 aC | 1.05 ± 0.06 aAB | 0.00 aA | 6.26 ± 0.16 bA | 3.05 ± 0.12 bC | 5.38 ± 0.16 aC | 35.88 ± 0.52 aB | |
| Saxa 2 | Sprouts | 31.62 ± 0.88 bB | 9.97 ± 0.79 cC | 2.26 ± 0.09 cC | 7.61 ± 0.30 aD | 0.00 aA | 11.65 ± 0.42 bB | 63.11 ± 2.11 bD |
| Microgreens | 88.87 ± 9.31 cC | 4.09 ± 0.07 bA | 1.59 ± 0.06 bB | 18.81 ± 0.80 cA | 4.27 ± 0.14 cA | 27.36 ± 1.10 cC | 144.99 ± 9.04 cC | |
| Roots | 13.36 ± 0.32 aA | 1.13 ± 0.07 aB | 0.24 ± 0.01 aC | 9.30 ± 0.45 bC | 2.46 ± 0.20 bB | 5.44 ± 0.25 aC | 31.93 ± 0.87 aA | |
| Warta | Sprouts | 28.45 ± 0.55 aA | 4.66 ± 0.26 bAB | 1.00 ± 0.09 bA | 2.32 ± 0.04 aB | 0.00 aA | 3.51 ± 0.26 bA | 39.94 ± 1.20 aA |
| Microgreens | 35.02 ± 1.63 bA | 6.39 ± 0.14 cB | 1.52 ± 0.09 cB | 20.70 ± 0.12 cB | 4.43 ± 0.19 cA | 15.97 ± 0.21 cB | 84.03 ± 1.80 bA | |
| Roots | 25.94 ± 0.77 aD | 0.83 ± 0.05 aA | 0.21 ± 0.01 aB | 9.61 ± 0.36 bC | 2.12 ± 0.08 bA | 2.58 ± 0.09 aA | 41.29 ± 1.31 aC |
| Fatty Acid | Carmen | Jutrzenka | Saxa 2 | Warta | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sprouts | Microgreens | Roots | Sprouts | Microgreens | Roots | Sprouts | Microgreens | Roots | Sprouts | Microgreens | Roots | |
| Lauric (C12:0) | 0.62 ± 0.02 | 1.93 ± 0.09 | 0.03 ± 0.00 | 0.00 ± 0.00 | 0.93 ± 0.03 | 0.00 ± 0.00 | 0.50 ± 0.03 | 1.38 ± 0.05 | 0.36 ± 0.02 | 0.03 ± 0.00 | 0.69 ± 0.04 | 0.09 ± 0.00 |
| Myristic (C14:0) | 1.23 ± 0.05 | 2.15 ± 0.05 | 0.63 ± 0.03 | 0.93 ± 0.03 | 1.74 ± 0.04 | 0.38 ± 0.01 | 1.49 ± 0.06 | 2.18 ± 0.09 | 0.23 ± 0.00 | 1.72 ± 0.06 | 4.98 ± 0.19 | 0.71 ± 0.02 |
| Pentadecanoic (C15:0) | 0.18 ± 0.01 | 0.62 ± 0.02 | 0.12 ± 0.00 | 0.11 ± 0.01 | 0.00 ± 0.00 | 0.58 ± 0.03 | 0.14 ± 0.00 | 0.67 ± 0.03 | 0.20 ± 0.01 | 0.80 ± 0.03 | 0.50 ± 0.02 | 0.24 ± 0.01 |
| Palmitic (C16:0) | 78.63 ± 3.93 cA | 55.57 ± 1.95 bB | 10.72 ± 0.47 aA | 143.02 ± 6.72 cC | 75.60 ± 2.57 bC | 16.35 ± 0.75 aBC | 114.83 ± 5.44 cB | 47.12 ± 1.98 bA | 14.94 ± 0.66 aB | 81.73 ± 2.45 cA | 46.52 ± 2.00 bA | 16.99 ± 0.85 aC |
| Heptadecanoic (C17:0) | 0.00 ± 0.00 | 1.24 ± 0.03 | 0.34 ± 0.01 | 0.78 ± 0.03 | 0.97 ± 0.02 | 0.01 ± 0.00 | 0.42 ± 0.02 | 0.46 ± 0.01 | 0.00 ± 0.00 | 1.11 ± 0.03 | 0.74 ± 0.03 | 0.52 ± 0.01 |
| Stearic (C18:0) | 10.34 ± 0.39 cA | 4.94 ± 0.25 bA | 2.31 ± 0.11 aB | 14.15 ± 0.68 cB | 10.37 ± 0.31 bC | 5.09 ± 0.24 aC | 20.52 ± 0.62 cC | 4.38 ± 0.21 bA | 1.89 ± 0.10 aA | 10.04 ± 0.45 cA | 7.13 ± 0.22 bB | 2.34 ± 0.10 aB |
| Arachidic Acid (C20:0) | 6.66 ± 0.20 | 3.06 ± 0.13 | 1,44 ± 0.04 | 17.64 ± 0.65 | 1.85 ± 0.04 | 3.26 ± 0.10 | 20.11 ± 0.62 | 2.70 ± 0.13 | 1.57 ± 0.04 | 8.32 ± 0.35 | 2.75 ± 0.09 | 1.39 ± 0.04 |
| Heneicosanoic (C21:0) | 0.00 ± 0.00 | 2.61 ± 0.07 | 2.38 ± 0.08 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.06 ± 0.05 | 0.00 ± 0.00 | 12.83 ± 0.36 | 4.07 ± 0.13 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.02 ± 0.08 |
| Behenic (C22:0) | 3.00 ± 0.08 | 3.41 ± 0.15 | 0.00 ± 0.00 | 3.42 ± 0.12 | 0.84 ± 0.02 | 1.02 ± 0.05 | 5.21 ± 0.25 | 3.74 ± 0.12 | 0.46 ± 0.01 | 3.92 ± 0.15 | 0.68 ± 0.02 | 0.52 ± 0.02 |
| Lignoceric (C24:0) | 1.35 ± 0.04 | 7.69 ± 0.36 | 0.00 ± 0.00 | 4.64 ± 0.18 | 5.16 ± 0.14 | 0.00 ± 0.00 | 6.35 ± 0.30 | 3.99 ± 0.14 | 0.00 ± 0.00 | 4.43 ± 0.16 | 4.20 ± 0.19 | 0.41 ± 0.01 |
| Cerotic (C26:0) | 0.00 ± 0.00 | 0.29 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.37 ± 0.14 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.73 ± 0.14 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Fatty Acid | Carmen | Jutrzenka | Saxa 2 | Warta | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sprouts | Microgreens | Roots | Sprouts | Microgreens | Roots | Sprouts | Microgreens | Roots | Sprouts | Microgreens | Roots | |
| Myristoleic (C14:1) | 1.11 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.57 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.69 ± 0.03 | 0.00 ± 0.00 | 2.36 ± 0.07 | 0.56 ± 0.03 | 0.00 ± 0.00 |
| Palmitoleic (C16:1 n7) | 6.73 ± 0.28 | 7.56 ± 0.28 | 0.55 ± 0.01 | 7.13 ± 0.24 | 5.74 ± 0.18 | 0.47 ± 0.01 | 7.40 ± 0.28 | 5.23 ± 0.15 | 0.62 ± 0.03 | 11.14 ± 0.51 | 1.62 ± 0.07 | 1.34 ± 0.04 |
| cis-10-Heptadecenoic (C17:1 n7) | 1.23 ± 0.05 | 1.63 ± 0.07 | 0.00 ± 0.00 | 2.01 ± 0.08 | 2.48 ± 0.11 | 0.00 ± 0.00 | 2.07 ± 0.07 | 2.01 ± 0.10 | 0.00 ± 0.00 | 2.42 ± 0.12 | 1.36 ± 0.07 | 0.00 ± 0.00 |
| Petroselinic (C18:1 n6) | 0.00 ± 0.00 aA | 21.76 ± 0.59 bC | 0.00 ± 0.00 aA | 0.00 ± 0.00 aA | 12.81 ± 0.34 bB | 22.58 ± 0.70 cB | 0.00 ± 0.00 aA | 14.25 ± 0.73 bB | 0.00 ± 0.00 aA | 0.00 ± 0.00 aA | 10.25 ± 0.54 bA | 30.78 ± 0.92 cC |
| Elaidic (C18:1 n9t) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.65 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.52 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.06 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Oleic Acid (C18:1 n9c) | 253.43 ± 11.91 bA | 30.83 ± 1.29 aA | 30.42 ± 1.03 aD | 544.79 ± 22.56 cC | 46.75 ± 1.78 bB | 7.37 ± 0.03 aA | 553.60 ± 32.41 bC | 31.67 ± 0.98 aA | 26.42 ± 0.98 aC | 323.56 ± 10.35 cB | 33.53 ± 1.14 bA | 12.92 ± 0.39 aB |
| cis-11-Eicosenoic (C20:1 n9) | 52.08 ± 1.51 | 2.64 ± 0.13 | 0.94 ± 0.03 | 149.58 ± 5.09 | 2.28 ± 0.06 | 0.42 ± 0.01 | 119.16 ± 7.95 | 3.39 ± 0.16 | 0.52 ± 0.01 | 60.24 ± 2.71 | 2.43 ± 0.09 | 0.68 ± 0.02 |
| Erucic (C22:1 n9) | 164.63 ± 6.26 bA | 5.88 ± 0.17 aA | 0.80 ± 0.03 aB | 446.92 ± 25.16 bC | 4.84 ± 0.21 aA | 0.00 ± 0.00 aA | 490.77 ± 25.30 bC | 16.52 ± 0.63 aC | 0.00 ± 0.00 aA | 279.28 ± 9.78 bB | 10.08 ± 0.52 aB | 0.00 ± 0.00 aA |
| Nervonic (C24:1n9) | 3.47 ± 0.15 | 4.55 ± 0.17 | 0.60 ± 0.02 | 13.45 ± 0.60 | 1.86 ± 0.07 | 0.00 ± 0.00 | 16.17 ± 0.81 | 1.42 ± 0.05 | 0.00 ± 0.00 | 8.90 ± 0.27 | 2.38 ± 0.07 | 0.08 ± 0.00 |
| Fatty Acid | Carmen | Jutrzenka | Saxa 2 | Warta | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sprouts | Microgreens | Roots | Sprouts | Microgreens | Roots | Sprouts | Microgreens | Roots | Sprouts | Microgreens | Roots | |
| Linolelaidic (C18:2 n6t) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.33 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.08 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Linoleic (C18:2 n6c) | 183.01 ± 8.61 cA | 100.22 ± 2.80 bD | 15.56 ± 0.76 aB | 349.15 ± 20.65 cD | 77.38 ± 3.25 bC | 12.16 ± 0.58 aA | 309.55 ± 17.32 cC | 52.76 ± 2.64 bB | 14.61 ± 0.56 aB | 222.39 ± 7.12 cB | 42.63 ± 2.33 bA | 15.04 ± 0.46 aB |
| cis-11,14-Eicosadienoic (C20:2 n6) | 3.15 ± 0.13 | 1.72 ± 0.05 | 0.00 ± 0.00 | 4.26 ± 0.14 | 0.80 ± 0.02 | 0.00 ± 0.00 | 4.86 ± 0.17 | 0.90 ± 0.03 | 0.00 ± 0.00 | 2.76 ± 0.08 | 0.44 ± 0.02 | 0.00 ± 0.00 |
| cis-13,16-Docosadienoic (C22:2 n6) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.94 ± 0.07 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.11 ± 0.10 | 0.86 ± 0.02 | 0.00 ± 0.00 | 0.85 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| alpha-Linolenic (C18:3n3) | 158.48 ± 7.29 bA | 222.25 ± 6.23 cB | 29.72 ± 0.83 aB | 264.34 ± 9.52 bC | 260.06 ± 12.21 bC | 34.88 ± 1.36 aC | 274.31 ± 16.47 cC | 98.62 ± 5.75 bA | 24.98 ± 0.82 aA | 232.38 ± 7.44 cB | 213.67 ± 7.94 bB | 38.35 ± 1.95 aC |
| gamma-Linolenic (C18:3n6) | 0.30 ± 0.01 | 2.52 ± 0.13 | 0.12 ± 0.00 | 0.42 ± 0.02 | 5.17 ± 0.26 | 0.00 ± 0.00 | 0.56 ± 0.02 | 2.02 ± 0.08 | 0.15 ± 0.01 | 0.17 ± 0.01 | 0.65 ± 0.02 | 0.07 ± 0.00 |
| cis-11,14,17-Eicosatrienoic (C20:3 n3) | 0.00 ± 0.00 | 2.01 ± 0.07 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.44 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.37 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.44 ± 0.06 | 0.00 ± 0.00 |
| Principal Component | Eigenvalue | Variability (%) | Cumulative Variance % |
|---|---|---|---|
| PC1 | 17.41 | 56.17 | 56.17 |
| PC2 | 8.56 | 27.62 | 83.79 |
| No. | Parameter | PC1 | PC2 | No. | Parameter | PC1 | PC2 |
|---|---|---|---|---|---|---|---|
| 1 | Energy value | 0.963 | 0.005 | 17 | Succinic acid | −0.277 | 0.867 |
| 2 | Total protein | 0.947 | −0.037 | 18 | Fumaric acid | 0.314 | 0.791 |
| 3 | Crude fat | 0.941 | −0.323 | 19 | Total organic acids | 0.229 | 0.934 |
| 4 | Ash | 0.103 | 0.880 | 20 | Palmitic acid | 0.961 | −0.092 |
| 5 | Available carbohydrates | −0.768 | 0.363 | 21 | Stearic acid | 0.892 | −0.216 |
| 6 | Total dietary fiber | 0.744 | 0.418 | 22 | Petroselinic acid | −0.490 | 0.373 |
| 7 | Soluble dietary fiber | 0.206 | 0.751 | 23 | Oleic acid | 0.870 | −0.469 |
| 8 | Insoluble dietary fiber | 0.750 | 0.430 | 24 | Erucic acid | 0.858 | −0.469 |
| 9 | Fructose | −0.693 | −0.571 | 25 | Linoleic acid | 0.936 | −0.317 |
| 10 | Glucose | −0.823 | −0.384 | 26 | alpha-Linolenic acid | 0.906 | 0.260 |
| 11 | Sucrose | 0.155 | −0.744 | 27 | Total saturated fatty acids | 0.972 | −0.035 |
| 12 | Total sugars | −0.789 | −0.464 | 28 | Total monounsaturated fatty acids | 0.868 | −0.459 |
| 13 | Oxalic acid | 0.210 | 0.846 | 29 | Total diunsaturated fatty acids | 0.937 | −0.317 |
| 14 | Citric acid | 0.853 | 0.305 | 30 | Total triunsaturated fatty acids | 0.900 | 0.274 |
| 15 | Tartaric acid | 0.842 | 0.421 | 31 | Total fatty acids | 0.939 | −0.315 |
| 16 | Malic acid | −0.139 | 0.927 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajszczak, D.; Sosnowska, D.; Bonikowski, R.; Szymczak, K.; Frąszczak, B.; Pielech-Przybylska, K.; Podsędek, A. Comparative Nutrient Study of Raphanus sativus L. Sprouts Microgreens, and Roots. Agronomy 2025, 15, 1216. https://doi.org/10.3390/agronomy15051216
Kajszczak D, Sosnowska D, Bonikowski R, Szymczak K, Frąszczak B, Pielech-Przybylska K, Podsędek A. Comparative Nutrient Study of Raphanus sativus L. Sprouts Microgreens, and Roots. Agronomy. 2025; 15(5):1216. https://doi.org/10.3390/agronomy15051216
Chicago/Turabian StyleKajszczak, Dominika, Dorota Sosnowska, Radosław Bonikowski, Kamil Szymczak, Barbara Frąszczak, Katarzyna Pielech-Przybylska, and Anna Podsędek. 2025. "Comparative Nutrient Study of Raphanus sativus L. Sprouts Microgreens, and Roots" Agronomy 15, no. 5: 1216. https://doi.org/10.3390/agronomy15051216
APA StyleKajszczak, D., Sosnowska, D., Bonikowski, R., Szymczak, K., Frąszczak, B., Pielech-Przybylska, K., & Podsędek, A. (2025). Comparative Nutrient Study of Raphanus sativus L. Sprouts Microgreens, and Roots. Agronomy, 15(5), 1216. https://doi.org/10.3390/agronomy15051216







