Abstract
Tomato (Solanum lycopersicum) is highly susceptible to both high and low temperatures, which threaten its growth, yield, and quality. Ascorbate peroxidase (APX) plays a pivotal role in plant responses to abiotic stresses. In this study, we unveil the positive involvement of heat- and cold-induced SlAPX2 in bolstering tomato resilience to temperature extremes. Knockout of SlAPX2 using the CRISPR/Cas9 technique exacerbated oxidative stress under heat and cold conditions, as evidenced by reduced Fv/Fm and increased electrolyte leakage (REL), malondialdehyde (MDA) content, and hydrogen peroxide (H2O2) levels. Furthermore, SlAPX2 expression was modulated by abscisic acid (ABA), and the transcription factor ABF4 in the ABA signaling pathway positively regulated SlAPX2 transcription. Using yeast one-hybrid (Y1H) and dual luciferase (LUC) assays, we found that ABF4 directly bound to the SlAPX2 promoter, thereby activating its transcription. Additionally, silencing of SlABF4 compromised tomato’s tolerance to heat or cold. Collectively, these findings reveal a regulatory module, SlABF4–SlAPX2, that enhances tomato tolerance to temperature extremes by detoxifying excessive reactive oxygen species (ROS). This study advances our understanding of ABA-mediated stress responses and highlights the SlABF4–SlAPX2 module as a promising target for breeding temperature-resilient tomato cultivars.
1. Introduction
Throughout their lifespan, plants confront a myriad of environmental challenges, including extreme temperatures, drought, waterlogging, salinity, metal toxicity, and UV radiation [1,2,3,4]. Among these, high and low temperatures have emerged as critical stressors due to the increasing frequency of extreme weather events [5,6,7]. Aberrant temperatures adversely affect growth, development, and yield, posing a substantial threat to economically important crops [8,9,10,11]. Consequently, the study of heat and cold tolerance is paramount and warrants consideration in the breeding of new crop varieties.
Extreme temperature conditions often trigger the overproduction of reactive oxygen species (ROS), including superoxide radicals, hydrogen peroxide, hydroxyl radicals, alkoxyl radicals, and singlet oxygen. Excessive ROS accumulation leads to oxidative stress, causing indiscriminate damage to various cellular components such as DNA/RNA, proteins, lipids, and carbohydrates [12,13,14]. To counteract temperature-induced oxidative stress, plants have developed a sophisticated antioxidant defense system comprising a combination of enzymatic and non-enzymatic components. Key enzymatic antioxidants encompass ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), and peroxidase (POX), while non-enzymatic antioxidant compounds include ascorbate, glutathione, prolines, glycine betaine, flavonoids, phenols, tocopherols, polyamines, carotenoids, and others [15,16]. Through the coordinated action of these enzymes and compounds, plants effectively reduce the accumulation of ROS and alleviate oxidative stress induced by temperature fluctuations.
Being a key antioxidant enzyme, ascorbate peroxidase (APX) functions in scavenging intracellular H2O2 through the ascorbate–glutathione cycle [17]. In this cycle, APX primarily detoxifies H2O2 by converting it into H2O, utilizing ascorbate as a substrate [18]. The significance of APX is underscored by the presence of various isoforms distributed across different cellular compartments. For example, sAPX and tAPX are localized in chloroplasts, cAPX in the cytosol, and mitAPX in mitochondria [17,19,20,21]. Numerous studies have highlighted the crucial role of APX in plant responses to various abiotic stresses. Overexpression of cAPX in tobacco plants enhances tolerance to oxidative stress induced by paraquat [22]. Mutation in cAPX leads to increased sensitivity to salt stress, while its overexpression improves tolerance to salt stress in plums [23]. Similarly, ectopic expression of a pea cAPX enhances APX activity and increases salt stress tolerance in transgenic tomatoes [24]. Knockout of a cytosolic APX gene enhances rice susceptibility to drought stress [25]. Additionally, APX plays a crucial role in adaptation or tolerance to temperature stresses. Overexpression of tAPX in tobacco plants enhances tolerance to chilling stress [17,26], while increasing cAPX expression in rice substantially increases cold tolerance [27]. Similarly, heterologous expression of sAPX from Cyanidioschyzon merolae confers increased tolerance to heat stress in Arabidopsis [28], and overexpression of APX and Cu/ZnSOD in potato plants enhances tolerance to high temperature [29]. Some studies have also highlighted the role of APX in tomato’s response to temperature stress. Suppression of the tomato’s thylakoidal APX gene leads to increased H2O2 accumulation and oxidative damage during chilling stress [30]. Knockout of a cytosolic APX gene via CRISPR/Cas9-mediated gene editing compromises tomato thermotolerance [31]. Recent studies have further demonstrated increased APX activities under heat or cold conditions in tomatoes [32,33], further substantiating the critical function of APX in tomato’s response to temperature stress. However, APX genes capable of conferring both heat and cold tolerance in tomato have yet to be explored fully.
Phytohormones play a pivotal role in the control of developmental processes, and stress responses in plants. Abscisic acid (ABA) is a key hormone, intricately involved in modulating various physiological processes and facilitating plant responses to a wide array of environmental stresses [34,35]. The ABA signaling pathway in plants is well elucidated, commencing with the recognition of ABA by a group of receptors, commonly referred to as PYR1/PYR1-LIKE receptors. The formation of the ABA-receptor complex leads to the inhibition of protein phosphatase 2Cs (PP2Cs), culminating in the activation of SNF1-related protein kinases 2s (SnRK2s). Subsequently, SnRK2s phosphorylate downstream transcription factors, such as ABRE-binding factors (ABFs), ultimately triggering the transcriptional activation of ABA-responsive genes [36]. While the indispensable role of ABA in drought response is well-established, emerging evidence suggests its involvement in plant responses to temperature stresses. Numerous studies have reported a significant increase in endogenous ABA levels in response to heat or cold stress, and the application of exogenous ABA has been shown to enhance plant resistance to temperature extremes [32,37,38]. Mutants deficient in ABA biosynthesis or signaling exhibit heightened sensitivity to high temperature stress, whereas transgenic plants with enhanced ABA signaling demonstrate elevated thermotolerance [35,39,40,41]. Moreover, the inhibition of ABA biosynthesis using a biosynthesis inhibitor results in diminished cold stress response in tomato seedlings [38]. Despite the existence of several models depicting the role of ABA in temperature stress tolerance, our understanding of the underlying mechanisms of ABA-mediated tolerance to heat or cold stress in plants remains largely unexplored. Particularly intriguing is the potential interplay between ABA signaling and the expression of APX genes, given the pivotal role of APX-mediated ROS detoxification during environmental stress. Investigating whether and how ABA signaling regulates APX gene expression and APX activity under abnormal temperature conditions holds promise for unraveling novel mechanisms underlying plant adaptation to temperature stress.
Tomato (Solanum lycopersicum) stands as a globally cultivated horticultural crop, offering a significant source of essential nutrients for human well-being. However, the susceptibility of tomatoes to both high and low temperatures throughout its vegetative and reproductive stages poses a significant challenge in tomato production [42,43]. High temperatures exceeding 35 °C or low temperatures dipping below 12 °C profoundly impact tomato quality and yield, emphasizing the critical need to understand its response to temperature stress. In the present study, we have identified an ascorbate peroxidase gene, SlAPX2, which was induced by heat, cold, and ABA. Through physiological and biochemical analyses, we have elucidated the positive role of SlAPX2 in enhancing heat and cold tolerance in tomato plants. Additionally, our study unveils the involvement of SlABF4, a pivotal component of the ABA signaling pathway, in directly regulating SlAPX2 expression and contributing positively to tomato’s tolerance to temperature extremes. Thus, our findings unveil a regulatory module, SlABF4–SlAPX2, which acts to detoxify excessive ROS and confer tolerance to heat and cold stress in tomato. This study not only expands our understanding of ABA-mediated responses to extreme temperatures but also sheds light on the intricate relationship between ABA and APX in tomato under temperature stress conditions. Leveraging the SlABF4–SlAPX2 module holds promise for the development of tomato cultivars with enhanced resilience to heat and cold stress.
2. Materials and Methods
2.1. Plant Materials
In this study, we utilized tomato plants (Solanum lycopersicum ‘MicroTom’) as plant materials. Both wild-type and mutant seeds were germinated and cultivated in phytotrons under a long-day photoperiod condition (16 h light/8 h dark; air temperature, 25 °C/22 °C, day/night; PPFD 400 µmol m−2 s−1). The relative air humidity was maintained at approximately 65%.
2.2. Generation of slapx2 Knockout Mutants
Loss-of-function of SlAPX2 mutants were generated using a CRIPSR/Cas9 gene editing system. The target sequence (GTTTCAGATGGCATTCAGCA) was selected using CRISPR-P [44]. Subsequently, the target fragment was synthesized and ligated into the vector AtU6-sgRNA-AtUBQ-Cas9. The reconstructed vector was then inserted into the binary vector pCAMBIA1301. The resulting recombinant vector was introduced into Agrobacterium tumefaciens (GV3101), which was utilized to generate slapx2 mutants. Successfully transformed plants were first screened using hygromycin. The mutants were further selected through sequencing.
2.3. VIGS-Mediated Suppression of SlABF4
Silencing of SlABF4 was performed using the VIGS technique as previously outlined [45]. In brief, to specifically silence SlABF4, a 300 bp fragment of its coding sequence (CDS) was amplified and introduced into the pTRV2 vector (tobacco rattle virus). The reconstructed vector pTRV2-SlABF4 was then introduced into Agrobacterium tumefaciens (GV3101). The empty vector pTRV2 served as a control. Agrobacterium harboring different vectors was used to inoculate tomato cotyledons. Four weeks post-inoculation, the relative expression levels of SlABF4 transcripts were evaluated by qRT-PCR.
2.4. Treatments
For heat treatment, tomato plants with four fully expanded leaves (4-leaf stage) were exposed to heat stress (42 °C) for a duration of 24 h. Similarly, for cold treatment, tomato plants at the same growth stage were subjected to low temperatures (4 °C). As controls, tomato plants were maintained at a constant temperature of 25 °C. All other growth conditions remained consistent with those described in Plant Materials. Following the treatments, tomato leaves were harvested and stored for subsequent experiments. To determine the transcript levels of SlAPX2 and SlABF4 in response to ABA, tomato leaves were detached and floated in 50 µM of ABA solution for a period of 24 h. To investigate ABA-induced tolerance to heat and cold stress, tomato plants with four fully expanded leaves were sprayed with a 100 µM ABA solution 24 and 12 h prior to the onset of heat or cold treatments.
2.5. Heat or Cold Tolerance Assay
Tolerance to heat or cold stress in tomato plants was evaluated by assessing the parameters of Fv/Fm, REL, malondialdehyde (MDA) content, and hydrogen peroxide (H2O2) levels. The assessment of Fv/Fm was conducted as previously reported [2]. Briefly, tomato plants were adapted in darkness for 30 min and Fo was measured with a portable fluorometer. Fm was obtained after a saturating pulse was imposed. Fo and Fm were used to calculate the maximum quantum yield (Fv/Fm). REL was determined in accordance with the protocol described by Ding et al. [46]. The measurement of MDA content was conducted following the procedure outlined by Wang et al. [2]. The quantification of H2O2 levels was performed as per the methodology described in the study by Ding et al. [9].
2.6. Measurement of Transcript Levels by qRT-PCR
The transcript levels were examined using qRT-PCR as described previously, following the methodology outlined in a previous study [47]. In brief, total RNA was extracted using an RNA extraction kit (RNAprep Pure Plant Kit, TIANGEN, Beijing, China). The total RNA was subsequently used to synthesize cDNA. The isolated total RNA was then reverse transcribed into cDNA. Subsequently, qRT-PCR reactions were conducted using a real-time PCR system and a Premix Ex Taq kit (TaKaRa, Dalian, China). SlACTIN2 served as the reference gene for normalization.
2.7. APX Activity Assay
APX activity assay was carried out following a method described previously [32]. Leaves (0.3 g) were ground with 50 mM PBS (pH 7.8) containing 0.2 mM EDTA, 2 mM AsA, and 2% (w/v) PVP. The homogenate was then centrifuged at 12,000× g for 20 min at 4 °C, and the resulting supernatants were collected for the APX activity assay. The reduction in absorbance at 290 nm was monitored using a spectrophotometer (Shimadzu, Kyoto, Japan) to determine APX activities.
2.8. Quantification of ABA
ABA quantification was conducted following the protocol outlined by Ding et al. [38]. Leaf samples (0.1 g) were homogenized with 1 mL of ethyl acetate (D6-ABA as internal standard) to extract ABA. The homogenate was then centrifuged at 18,000× g for 10 min at 4 °C. The supernatants were collected and dried using N2. The resulting pellets were resuspended in methanol (70%, v/v), followed by centrifugation. The supernatants were collected and subjected to analysis using HPLC–mass spectrometry.
2.9. Yeast One-Hybrid (Y1H) Assay
Yeast one-hybrid (Y1H) analysis was performed using the Clontech One-Hybrid System to validate the interaction between SlABF4 and the SlAPX2 promoter. Fragments of the SlAPX2 promoter, both with and without ABRE cis-elements, were cloned into the pAbAi vector, while the coding sequence (CDS) of SlABF4 was inserted into the pGADT7 vector. These reconstructed vectors were then co-transformed into Y1H Gold yeast strain. Yeast cells were cultured at 30 °C for 3 days in SD/-Ura/-Leu medium with or without 100 ng mL−1 AbA. The association of SlABF4 with the SlAPX2 promoter was determined by the growth ability of the cotransformants on the selection medium.
2.10. Dual Luciferase Assay
Two vectors were used for dual luciferase assay. The pGreenII 62-SK vector was reconstructed by ligating the CDS of SlABF4 to it. The resulting vector served as the effector. The promoter fragments of SlAPX2 with or without ABRE cis-elements were inserted into pGreenII0800-LUC to serve as the reporter. Subsequently, A. tumefaciens (GV3101) was transformed with the effector and the reporter constructs. A. tumefaciens was then used for the inoculation of young leaves of N. benthamiana. Three days after inoculation, LUC activities were evaluated with a commercial assay kit (Promega, Madison, WI, USA).
2.11. Statistical Analysis
At least three biological replicates were performed in this study. For Fv/Fm measurements, 20 leaves from different plants within each treatment group were sampled. Statistical differences between treatments were analyzed using Student’s t-test (* p < 0.05 and ** p < 0.01).
3. Results
3.1. Identification of Heat- and Cold-Induced APX Genes
APX is crucial for the maintenance of ROS homeostasis under stress conditions. We first aimed to identify APX genes responsive to heat and cold stresses. Transcriptome data analysis showed that tomato leaves exposed to heat stress (42 °C) revealed three heat-upregulated APX genes, including Solyc09g007270, Solyc06g005160, and Solyc06g005150, with Solyc09g007270 exhibiting the highest level of upregulation. Similarly, transcriptome data (SRA accession no. SRR18694252) of tomato leaves exposed to cold stress (4 °C) demonstrated significant up- or downregulation of three APX genes (Solyc09g007270, Solyc06g060260, and Solyc02g083630), with Solyc09g007270 being prominently upregulated (Figure 1A). Given its upregulation under both heat and cold stresses, Solyc09g007270 was selected for further functional characterization. The phylogenetic tree revealed that Solyc09g007270 closely clustered with Arabidopsis APX2 (Figure 1B), and hereafter Solyc09g007270 was referred to as SlAPX2. Sequence analysis showed that the full-length transcript of SlAPX2 spans 1245 bp, with nine coding exons (Figure 1C), and an open reading frame of 753 bp encoding a protein of 250 amino acids. SlAPX2 was predicted to have a molecular weight (MW) of 27.64 kDa and an isoelectric point (pI) of 9.84.
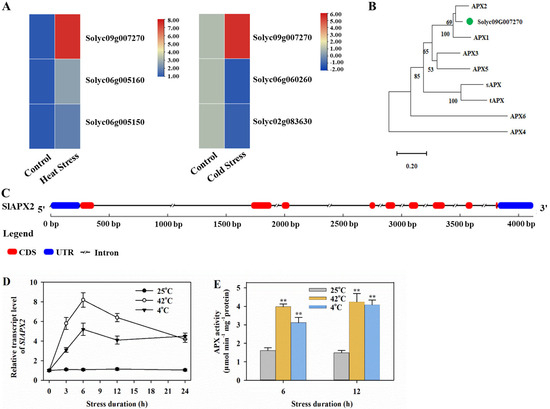
Figure 1.
Identification and expression analysis of SlAPX2. (A) Heatmap showing heat- and cold-induced APX genes based on transcriptome data. Data shown are log2 fold change after heat or cold treatment. (B) The phylogenetic tree of SlAPX2 and 8 Arabidopsis APX genes. The tree was constructed using MEGA. (C) SlAPX2 gene structure. (D) Time course of the transcript levels of SlAPX2 at 25, 42 or 4 °C. Leaf samples of tomato plants were collected at 0, 3, 6, 12, and 24 h after heat or cold treatment to determine SlAPX2 transcript levels. (E) APX activities at 25, 42, or 4 °C. Leaf samples were collected at 6 and 12 h after heat or cold treatment for enzyme activity assay. Results are presented as mean ± SD of three biological replicates. Student’s t-test was performed to compare the difference between tomato plants under temperature stresses and those under normal growth temperature. Significant differences are indicated by asterisks (** p < 0.01).
To investigate the role of SlAPX2 under heat or cold stress, we conducted a time course analysis of SlAPX2 transcript levels in tomato leaves exposed to 42 °C or 4 °C. Our results demonstrated a marked increase in SlAPX2 transcription in response to both heat and cold stress, with transcript levels peaking at 6 h after the initiation of heat or cold stress (Figure 1D). In addition, we examined changes in APX activities in tomato leaves under temperature stress. Our data revealed a significant enhancement in APX activities following exposure to both heat and cold treatments (Figure 1E). These findings indicate that SlAPX2 is induced by high or low temperatures, thereby contributing to increased APX activities during the early stages of temperature stress.
3.2. Knockout of SlAPX2 Impairs Tolerance to Heat and Cold in Tomato
Our observation that SlAPX2 is induced by temperature stresses led us to examine whether SlAPX2 functions in tolerance to heat and cold in tomato. To address this question, we employed CRISPR/Cas9-mediated gene editing to knock out SlAPX2. We obtained two loss-of-function transgenic lines, slapx2#3 with 1-bp deletion of G and slapx2#7 with a 2-bp deletion of C and A (Figure 2A). Enzyme activity assays showed that slapx2 mutant plants exhibited significantly lower APX activities compared to wild-type plants, particularly under heat or cold stress conditions (Figure 2B).
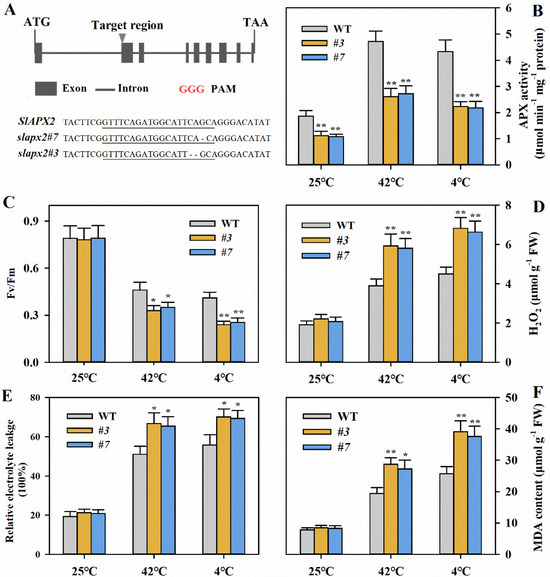
Figure 2.
Knockout mutation of SlAPX4 using the CRISPR/Cas9 technique reduces heat and cold tolerance in tomato. (A) Schematic illustration of the target site of SlAPX2 and two mutant alleles of T2 mutant lines. GGG in red are PAM. (B) APX activities in slapx2 mutant plants and their wild-type counterparts under temperature stresses. Leaf samples from slapx2 and WT plants were collected at 12 h after temperature treatments for enzyme activity assay. (C) The maximum quantum yield of PSII (Fv/Fm) of slapx2 and WT leaves after 12 h of heat at 42 °C or cold at 4 °C. (D) H2O2 levels in the leaves of slapx2 and WT plants after 24 h of heat at 42 °C or cold at 4 °C. (E) Relative electrolyte leakage (REL) in the leaves of slapx2 and WT plants after 24 h of heat at 42 °C or cold at 4 °C. (F) Malondialdehyde (MDA) content in the leaves of slapx2 and WT plants after 24 h of heat at 42 °C or cold at 4 °C. Results are presented as mean ± SD of three biological replicates. The results of Fv/Fm were from 15 replicates. Student’s t-test was performed to compare the difference between slapx2 mutants and WT plants under different temperature stresses. Significant differences are indicated by asterisks (* p < 0.05 and ** p < 0.01).
The maximum quantum yield of PSII (Fv/Fm), ROS levels, malondialdehyde (MDA) content, and relative electrolyte leakage (REL) are important indicators of temperature tolerance. Subsequently, we conducted analyses of these parameters. As anticipated, slapx2 mutant lines exhibited significantly lower Fv/Fm, accumulated markedly higher levels of H2O2, displayed substantially higher REL, and showed a significantly elevated level of MDA compared to their wild-type counterparts under temperature stress, despite no significant differences being observed in these parameters under normal temperature conditions (Figure 2C–F). These results demonstrate that SlAPX2 plays a positive role in heat and cold tolerance. Mutation in SlAPX2 leads to reduced APX activities, resulting in impaired ROS detoxification and compromised tolerance to temperature stress in tomato.
3.3. ABA Promotes Tolerance to Heat and Cold Stress
It has been well established that ABA is involved in many aspects of growth and development, as well as stress responses. Previous results have revealed that ABA participates in the response to temperature stress in plants. To investigate the relevance of ABA to heat or cold tolerance in tomato, we initially examined the ABA content in plants subjected to heat or cold treatments. Our observations revealed a substantial increase in ABA content under both heat and cold stress conditions (Figure 3A).
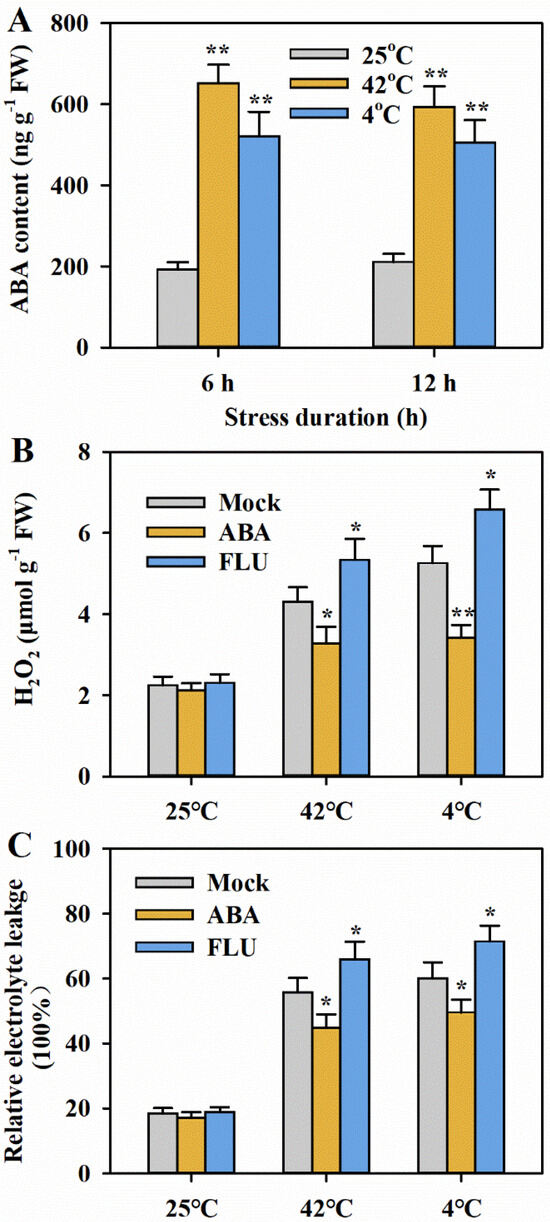
Figure 3.
ABA positively regulates heat and cold tolerance in tomato. (A) ABA contents in the leaves of tomato plants at 6 and 12 h after heat or cold treatment. (B) H2O2 levels in the leaves of tomato plants treated with ABA or an ABA biosynthesis inhibitor FLU after 24 h of heat at 42 °C or cold at 4 °C. (C) REL in the leaves of tomato plants treated with ABA or ABA biosynthesis inhibitor FLU after 24 h of heat at 42 °C or cold at 4 °C. Results are presented as mean ± SD of three biological replicates. Student’s t-test was conducted to compare the difference between ABA- or FLU-treated plants and mock-treated plants under different temperature stresses. Significant differences are indicated by asterisks (* p < 0.05 and ** p < 0.01).
Subsequently, we evaluated whether ABA affects heat or cold tolerance through the application of exogenous ABA and an inhibitor of ABA biosynthesis, fluridone (FLU). Under normal growth conditions, exogenous ABA and FLU induced slight changes in H2O2 content and REL levels. However, under heat or cold stress, exogenous ABA significantly reduced H2O2 content and REL levels, while FLU markedly elevated H2O2 content and REL levels compared to mock treatment (Figure 3B,C). These results imply that ABA is vital for tolerance to heat and cold in tomato.
3.4. ABA-Induced Tolerance to Temperature Stress Partly Depends on SlAPX2
Our findings indicate that both SlAPX2 and ABA contribute positively to heat and cold tolerance in tomato. Given this, it is reasonable to investigate whether ABA is associated with SlAPX2 in response to temperature stress. To test this hypothesis, we first examined whether SlAPX2 expression is responsive to ABA. Our results demonstrated that SlAPX2 expression was rapidly induced upon ABA treatment, reaching its peak transcript level at 3 h following ABA treatment (Figure 4A). Furthermore, under heat or cold conditions, exogenous ABA significantly enhanced SlAPX2 transcript levels compared to mock treatment (Figure 4B). In line with these results, ABA-treated plants displayed significantly higher APX activities than mock-treated plants, especially under temperature stresses (Figure 4C).
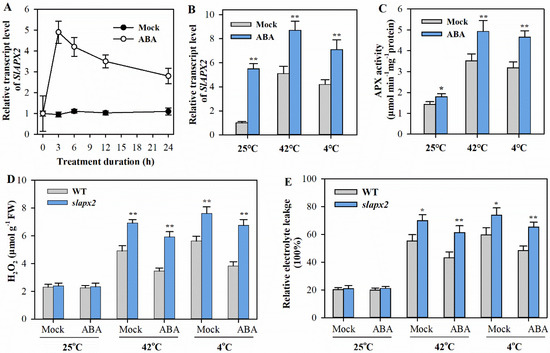
Figure 4.
ABA-induced heat and cold tolerance is partially dependent on SlAPX2. (A) Time course of the transcript levels of SlAPX2 in response to ABA. Leaf samples of tomato plants were collected at 0, 3, 6, 12, 24 h after ABA treatment to determine SlAPX2 transcript levels. (B) SlAPX2 transcript levels in the leaves of ABA-treated tomato plants after 6 h of heat at 42 °C or cold at 4 °C. (C) APX activities in the leaves of tomato plants pretreated with ABA after 6 h of heat at 42 °C or cold at 4 °C. (D) H2O2 levels in the leaves of ABA-treated tomato plants after 24 h of heat at 42 °C or cold at 4 °C. (E) REL in the leaves of ABA-treated tomato plants after 24 h of heat at 42 °C or cold at 4 °C. Results are presented as mean ± SD of three biological replicates. Student’s t-test was performed to compare the difference between ABA-treated plants and mock-treated plants under different temperature stresses. Significant differences are indicated by asterisks (* p < 0.05 and ** p < 0.01).
Next, we took advantage of slapx2 mutants to further elucidate the association of ABA with SlAPX2 in heat or cold tolerance. Our results revealed that under temperature stresses, ABA-treated plants accumulated significantly less H2O2 than mock-treated plants. However, the attenuating effect of ABA on H2O2 accumulation was diminished in slapx2 mutants (Figure 4D). Similarly, the reductions in REL levels mediated by ABA were also mitigated in slapx2 mutants (Figure 4E). Altogether, these observations demonstrate that ABA induces the expression of SlAPX2 under heat or cold stress, and ABA-mediated tolerance to heat and cold stress is partially dependent on SlAPX2.
3.5. ABF4 Activates the Expression of SlAPX2
Having established that ABA promotes SlAPX2 expression, we sought to elucidate the underlying mechanism of this upregulation. Transcription factors such as ABFs play crucial roles in the ABA signaling pathway, initiating the ABA response by activating various target genes. Previous studies have demonstrated that tomato ABF4 is induced by heat and cold stress [32,33], prompting us to investigate whether ABF4 activates the expression of SlAPX2. ABFs preferentially bind to cis-acting elements known as ABREs. Promoter analysis showed that the SlAPX2 promoter (2000 bp) contains 5 ABRE core sequences (Figure 5A).
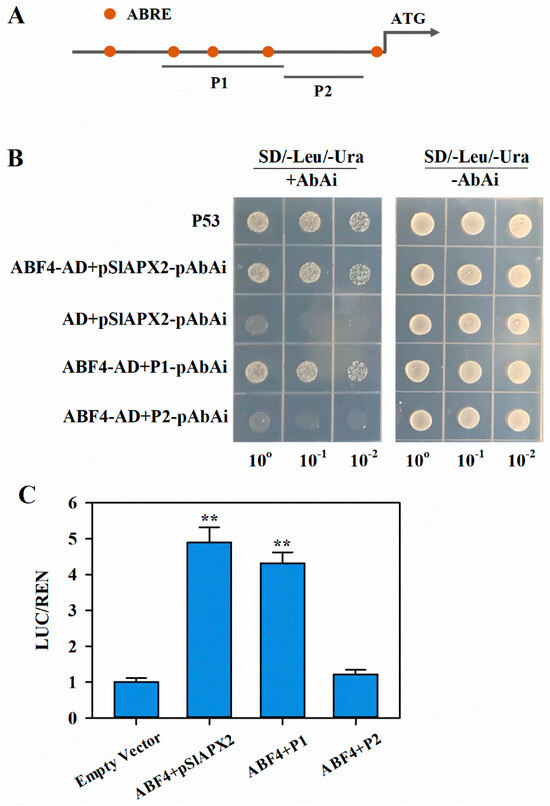
Figure 5.
SlABF4 activates the transcription of SlAPX2. (A) Schematic illustration of ABRE cis-acting elements within the promoter of SlAPX2. P1, promoter fragment of SlAPX2 containing three cis-acting elements of ABRE. P2, promoter fragment of SlAPX2 without ABRE cis-acting element. (B) Growth of yeast cells co-transformed with SlABF4-pADT7 and pSlAPX2-pAbAi/P1-pAbAi/P2-pAbAi, along with the positive control (p53-AbAi + pGAD-p53) on SD/–Ura/–Leu selection medium with or without 100 ng mL−1 AbAi. (C) Dual LUC assay in tobacco leaves co-transformed with the pGreenII0800-LUC reporter driven by pSlAPX2/P1/P2 and the effector SK-SlABF4, alone or together. The relative LUC activity (LUC/REN) of the combination of empty SK vectors with SlAPX2 promoter was set at 1. ** p < 0.01.
To confirm the association of ABF4 with the SlAPX2 promoter, we conducted a yeast one-hybrid (Y1H) assay. Yeast cells harboring both ABF4 and the full-length promoter sequence (2000 bp) were able to grow on the selection medium supplemented with AbA (Figure 5B). Similarly, yeast cells containing ABF4 and a promoter fragment (P1) containing three ABRE core sequences also exhibited growth on the selection medium. However, substitution of P1 with a fragment lacking ABRE core sequences (P2) completely inhibited yeast growth. These results suggest that ABF4 is capable of binding to the SlAPX2 promoter through ABRE cis-acting elements.
To further confirm that ABF4 activates SlAPX2 expression in planta, we conducted a dual luciferase (LUC) assay. Introduction of effector constructs containing ABF4 and reporter constructs containing the full-length SlAPX2 promoter sequence into Nicotiana benthamiana leaves resulted in markedly enhanced LUC/REN ratios. Conversely, when the reporter was inserted with a promoter fragment lacking ABRE elements, the LUC/REN ratios were reduced to control levels (Figure 5C). Taken together, these results indicate that ABF4 activates SlAPX2 transcription by directly binding to the ABRE elements.
3.6. Silencing of SlABF4 Decreases Tolerance to Heat or Cold Stress
In parallel with SlAPX2, SlABF4 exhibited apparent induction in response to heat, cold, and ABA treatment (Figure 6A,B). This observation prompted us to hypothesize that SlABF4 may play a positive role in regulating SlAPX2 expression and enhancing tolerance to temperature stress. To validate this hypothesis, we employed the virus-induced gene silencing (VIGS) approach to knock down SlABF4 expression. TRV-SlABF4 plants with reductions in the abundance of SlABF4 transcripts by approximately 70% were selected to assess SlAPX2 expression and temperature stress tolerance.
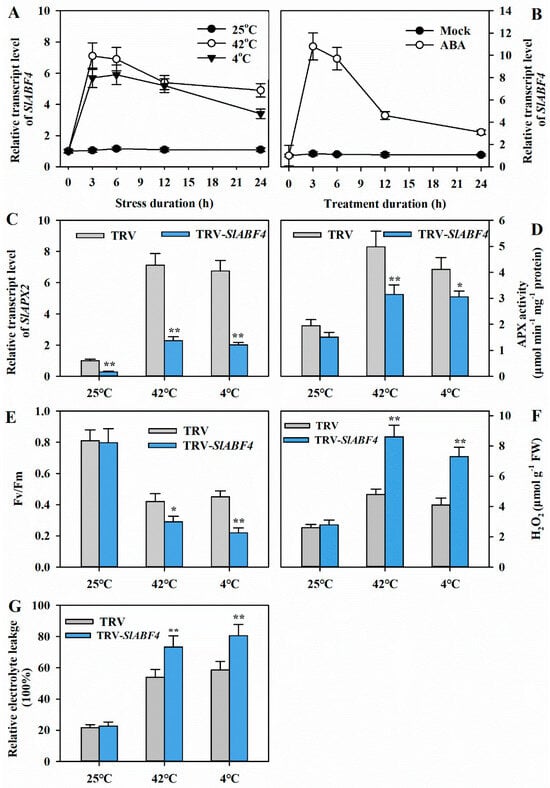
Figure 6.
Silencing of SlABF4 reduces tolerance to heat and cold in tomato. (A) Time course of the transcript levels of SlABF4 at 25, 42 or 4 °C. Leaf samples of tomato plants were collected at 0, 3, 6, 12, and 24 h after heat or cold treatment for the determination of SlABF4 transcript levels. (B) Time course of the transcript levels of SlABF4 in response to ABA. Leaf samples of tomato plants were collected at 0, 3, 6, 12, and 24 h after ABA treatment to determine SlABF4 transcript levels. (C) SlAPX2 transcript levels in the leaves of TRV-SlABF4 and TRV plants after 12 h of heat at 42 °C or cold at 4 °C. (D) APX activities in the leaves of TRV-SlABF4 and TRV plants after 12 h of heat at 42 °C or cold at 4 °C. (E) Fv/Fm in the leaves of TRV-SlABF4 and TRV plants after 12 h of heat at 42 °C or cold at 4 °C. (F) H2O2 levels in the leaves of TRV-SlABF4 and TRV plants after 12 h of heat at 42 °C or cold at 4 °C. (G) REL in the leaves of TRV-SlABF4 and TRV plants after 12 h of heat at 42 °C or cold at 4 °C. Results are presented as mean ± SD of three biological replicates. The results of Fv/Fm were from 20 replicates. Student’s t-test was conducted to compare the difference between TRV-SlABF4 and TRV plants under different temperature stresses. Significant differences are indicated by asterisks (* p < 0.05 and ** p < 0.01).
In line with the decrease in SlABF4 transcript abundance, the relative expression of SlAPX2 markedly decreased in TRV-SlABF4 lines. The differences in SlAPX2 expression levels between TRV-SlABF4 plants and the control were particularly pronounced under heat or cold stress (Figure 6C). Correspondingly, APX activities were significantly diminished, especially under temperature stresses, in TRV-SlABF4 lines compared to control plants (Figure 6D). Furthermore, TRV-SlABF4 plants displayed significantly lower Fv/Fm and remarkably higher levels of H2O2 and REL than TRV control plants under temperature stresses (Figure 6E–G). All these results indicate that SlABF4 acts positively in tolerance to heat or cold stress by modulating the expression of SlAPX2 and the activities of APX in tomato.
4. Discussion
Growing in dynamically changing environments, plants are often challenged by a plethora of adverse factors, such as extreme temperatures, flooding, drought, and salinity. These environmental stresses typically cause various forms of damage, with oxidative damage being particularly detrimental. Oxidative damage arises from the excessive accumulation of ROS under stressful conditions [48]. To cope with oxidative stress, plants have evolved an intricate antioxidant system composed of different antioxidant enzymes and numerous soluble non-enzymatic antioxidant compounds. Ascorbate peroxidase (APX) is a key enzyme in this antioxidant system, responsible for detoxifying intracellular H2O2 [17,49]. While numerous studies have highlighted the essential role of APX in response to various abiotic stresses, its specific role in tomato, an important horticultural crop, under temperature stress remains to be elucidated. Moreover, the transcriptional regulation of APX genes in response to temperature stresses remains largely unknown. In this study, we identified a specific APX gene, SlAPX2, which confers tolerance to heat and cold in tomato plants. Notably, we found that the phytohormone ABA regulates the expression of SlAPX2 and mediates tolerance to heat and cold. Furthermore, we demonstrated that ABF4, a transcription factor downstream of the ABA signaling pathway, activates SlAPX2 transcription by binding to its promoter. Our study thus establishes a regulatory module involving ABF4 and SlAPX2 that detoxifies H2O2 to mitigate oxidative stress induced by heat and cold.
APX plays a vital role in maintaining ROS homeostasis and preventing ROS-triggered oxidative stress. Previous studies have established the functions of APX in the elimination of excessive ROS in plants under stress conditions. APX isoenzymes confer tolerance to different forms of environmental stresses mainly by diminishing oxidative stress [17,21,50]. In this work, we concluded that a tomato APX isoenzyme, SlAPX2, functions positively in the alleviation of extreme temperature-induced oxidative stress. Multiple lines of evidence were provided to support this conclusion. First, SlAPX2 transcripts accumulated substantially upon heat or cold stress in tomato leaves, accompanied by increased APX activities, suggesting the critical role of SlAPX2 in the temperature stress response in tomato. Furthermore, knockout mutation of SlAPX2 reduced APX activities and compromised tolerance to heat and cold stress, which is evidenced by a decrease in Fv/Fm and increases in the levels of H2O2, MDA, and REL in tomato plants. However, it should be noted that while the functions of APX in H2O2 homeostasis under stress conditions have been implicated in multiple studies, its role could be more nuanced. For instance, Arabidopsis plants deficient in APX display tolerance to heat stress at the reproductive stage, but exhibited increased sensitivity at the seedling stage [51]. Furthermore, the knockout of an APX gene (Solyc06g005150) reduces APX activities under normal growth conditions; however, mutant plants failed to increase APX activities under heat conditions [31]. In contrast to these findings, in the current work, knockout mutants, slapx2, were able to respond to high temperature and increase APX activity, albeit to a lesser degree compared with their wild-type counterparts. Interestingly, rice APX2 is essential for carbohydrate metabolism, plant architecture and fertility [52]; however, we did not observe any phenotypic changes in slapx2. These results further highlight the complexity of APX function, suggesting that the functional role of APX may be closely related to developmental stages, and some isoenzymes may play a dominant role depending on developmental stages and growth conditions. These observations should be considered in future functional studies on APX in plants under stress conditions.
ABA is a vital phytohormone involved in stress response. Prior studies have shown that, in addition to dehydration, ABA promotes tolerance to temperature stresses in plants. As early as 1981, Daie and Campbell observed increased levels of ABA in tomato treated with high or low temperatures, suggesting the potential function of ABA in the response to temperature stresses [53]. Recent studies have further revealed that ABA regulates both heat and cold tolerance in tomato [32,33,38]. Consistently, in the present study, we also observed increases in ABA levels in tomato leaves exposed to heat or cold. Physiological analyses demonstrate that ABA is required for the tolerance to temperature stresses in tomato. Both SlAPX2 and ABA contribute to tolerance to temperature stresses in tomato; however, their potential association during stress remains elusive and intriguing to explore. Our results demonstrate that ABA rapidly induces the expression of SlAPX2 and exogenous ABA substantially enhances SlAPX2 transcript levels compared with the mock treatment under heat or cold stress. Interestingly, the knockout of SlAPX2 significantly attenuated ABA-induced heat or cold tolerance. These results confirm that ABA upregulates SlAPX2 expression, and ABA-induced tolerance to temperature stresses is partially dependent on SlAPX2.
ABFs are basic leucine zipper (bZIP) transcription factors that act downstream in the ABA signaling pathway and play important roles in the modulation of abiotic stress response. PtrABF4, for example, is responsive to drought stress and promotes drought tolerance by activating PtrBAM3 expression in Poncirus trifoliata [54]. MeABFs stimulate dehydration resistance by elevating the biosynthesis of glycine betaine in cassava [55]. Tomato ABFs have been demonstrated to be induced by heat and cold stresses. Consistent with previous reports, in the current study, SlABF4 was identified as a positive regulator of SlAPX2-mediated tolerance to temperature stresses in tomato. It is established that ABFs bind to the cis-acting elements of ABRE in the promoter region. We identified four ABRE elements in the SlAPX2 promoter. The interactions between ABF4 and ABRE elements were well supported by our Y1H assays. Furthermore, dual luciferase assays verify that ABF4 acts as a transcriptional activator of SlAPX2. In addition, SlABF4 was prominently induced by heat, cold, and ABA treatment. Lastly, the silencing of SlABF4 substantially abated tolerance to heat or cold in tomato plants. These data thus support the notion that the SlABF4-SlAPX2 module is of great importance for the response to temperature stresses in tomato.
5. Conclusions
In summary, we have unveiled a regulatory module comprising the ABA signaling component SlABF4 and the antioxidant enzyme SlAPX2, which collectively detoxify H2O2 and confer tolerance to heat and cold stresses (Figure 7). In essence, exposure to temperature stresses triggers the accumulation of ABA, which, via the ABA signaling pathway, stimulates the expression of SlABF4. Subsequently, SlABF4 transcriptionally activates SlAPX2, leading to the reduction in ROS levels and the enhancement of tolerance to heat and cold stresses.
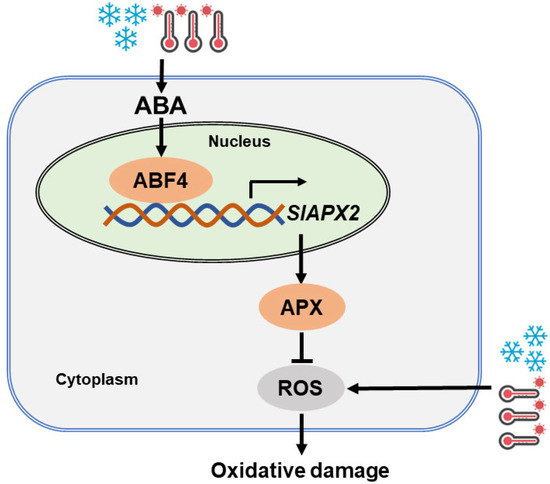
Figure 7.
A proposed model of SlABF4–SlAPX2 in tolerance to heat and cold in tomato. SlABF4 downstream of the ABA signaling pathway is induced by heat, cold and ABA. SlABF4 directly activates the transcription of SlAPX2 by binding to ABRE cis-acting elements within the promoter region, leading to enhanced APX activity. Thus, the SlABF4–SlAPX2 module regulates APX-mediated ROS detoxification and confers heat and cold tolerance in tomato.
The discovery of the SlABF4–SlAPX2 regulatory module in tomato provides a significant step forward in understanding how plants cope with temperature extremes. Future research should focus on translating these findings into practical applications, such as developing tomato varieties with enhanced SlAPX2 expression or fine-tuned ABA signaling to bolster resilience against heat and cold stress. Exploring whether this regulatory mechanism extends to other solanaceous crops could amplify its relevance for agriculture. Additionally, assessing SlAPX2’s involvement in other abiotic stresses, such as drought or salinity, might uncover its wider contributions to plant stress tolerance. By expanding our knowledge of this regulatory network, we can unlock innovative breeding and biotechnological strategies to safeguard tomato production amid growing climate unpredictability.
Author Contributions
Conceptualization, Z.S. and F.D.; methodology, X.F., K.L., Y.L., R.T. and M.W.; formal analysis, X.F., K.L., M.W., Z.S. and F.D.; investigation, X.F., K.L., Y.L. and R.T.; resources, Z.S. and F.D.; data curation, X.F., K.L., Y.L. and R.T.; writing—original draft preparation, M.W. and F.D.; writing—review and editing, Z.S., M.W. and F.D.; supervision, Z.S., M.W. and F.D.; project administration, M.W., Z.S. and F.D.; funding acquisition, M.W., Z.S. and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Liaocheng University, China (318052040, 318052039, 318042402), Shandong Provincial Natural Science Foundation (ZR202103070240), and National Natural Science Foundation of China (3247180862).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, S.; Ding, F. Melatonin Mitigates Chilling-Induced Oxidative Stress and Photosynthesis Inhibition in Tomato Plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ding, F.; Zhang, S. Mutation of SlSBPASE Aggravates Chilling-Induced Oxidative Stress by Impairing Glutathione Biosynthesis and Suppressing Ascorbate-Glutathione Recycling in Tomato Plants. Front. Plant Sci. 2020, 11, 565701. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A. Plant Abiotic Stress Challenges from the Changing Environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.M.; Lin, H.X.; Chong, K. Crop Improvement Through Temperature Resilience. Annu. Rev. Plant Biol. 2019, 70, 753–780. [Google Scholar] [CrossRef]
- Jung, J.H.; Seo, P.J.; Oh, E.; Kim, J. Temperature Perception by Plants. Trends Plant Sci. 2023, 28, 924–940. [Google Scholar] [CrossRef]
- Wang, M.; Fan, X.; Ding, F. Jasmonate: A Hormone of Primary Importance for Temperature Stress Response in Plants. Plants 2023, 12, 4080. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, S. Surviving and Thriving: How Plants Perceive and Respond to Temperature Stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Xu, N.; Wang, M.; Zhang, S. Jasmonic Acid-Regulated Putrescine Biosynthesis Attenuates Cold-Induced Oxidative Stress in Tomato Plants. Sci. Hortic. 2021, 288, 110373. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, Y.; He, Y. Molecular Epigenetic Mechanisms for the Memory of Temperature Stresses in Plants. J. Genet. Genom. 2022, 49, 991–1001. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, H.; Li, M.; Wang, D.; Xiang, H.; Zeng, R.; Chen, L.; Zhang, X.; Zuo, J.; Yang, S.; et al. Genetic and Lipidomic Analyses Reveal the Key Role of Lipid Metabolism for Cold Tolerance in Maize. J. Genet. Genom. 2023, 51, 326–337. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Van Breusegem, F. Improving Oxidative Stress Resilience in Plants. Plant J. 2021, 109, 359–372. [Google Scholar] [CrossRef]
- Maurya, A.K. Oxidative Stress in Crop Plants. Agron. Crops 2020, 3, 349–380. [Google Scholar]
- Xu, Y.; Zhang, S.; Zhang, M.; Jiao, S.; Guo, Y.; Jiang, T. The Role of Reactive Oxygen Species in Plant-Virus Interactions. Plant Cell Rep. 2024, 43, 197. [Google Scholar] [CrossRef]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic Acid—The Little-Known Antioxidant in Woody Plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, B.; Han, Y.; Li, S. The Pivotal Function of Dehydroascorbate Reductase in Glutathione Homeostasis in Plants. J. Exp. Bot. 2020, 71, 3405–3416. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- De Leonardis, S.; Dipierro, N.; Dipierro, S. Purification and Characterization of an Ascorbate Peroxidase from Potato Tuber Mitochondria. Plant Physiol. Biochem. 2000, 38, 773–779. [Google Scholar] [CrossRef]
- Jiménez, A.; Hernández, J.A.; Del Río, L.A.; Sevilla, F. Evidence for the Presence of the Ascorbate-Glutathione Cycle in Mitochondria and Peroxisomes of Pea Leaves. Plant Physiol. 1997, 114, 275–284. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant Responses to Stresses: Role of Ascorbate Peroxidase in the Antioxidant Protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Dabrowska, G.; Kata, A.; Goc, A.; Szechyńska-Hebda, M.; Skrzypek, E. Characteristics of the Plant Ascorbate Peroxidase Family. Acta Biol. Crac. Ser. Bot. 2007, 49, 7–17. [Google Scholar]
- Diaz-Vivancos, P.; Faize, M.; Barba-Espin, G.; Faize, L.; Petri, C.; Hernández, J.A.; Burgos, L. Ectopic Expression of Cytosolic Superoxide Dismutase and Ascorbate Peroxidase Leads to Salt Stress Tolerance in Transgenic Plums. Plant Biotechnol. J. 2013, 11, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wisniewski, M.; Meilan, R.; Cui, M.; Webb, R.; Fuchigami, L. Overexpression of Cytosolic Ascorbate Peroxidase in Tomato Confers Tolerance to Chilling and Salt Stress. J. Am. Soc. Hortic. Sci. 2005, 130, 167–173. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wu, J.; Zheng, X.; Zheng, S.; Sun, X.; Qiu, Q.; Lu, T. Gene Knockout Study Reveals That Cytosolic Ascorbate Peroxidase 2(OsAPX2) Plays a Critical Role in Growth and Reproduction in Rice under Drought, Salt and Cold Stresses. PLoS ONE 2013, 8, e57472. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, Y.; Motoki, T.; Yoshimura, K.; Takeda, T.; Ishikawa, T.; Shigeoka, S. Thylakoid Membrane-Bound Ascorbate Peroxidase Is a Limiting Factor of Antioxidative Systems under Photo-Oxidative Stress. Plant J. 2002, 32, 915–925. [Google Scholar] [CrossRef]
- Sato, Y.; Masuta, Y.; Saito, K.; Murayama, S.; Ozawa, K. Enhanced Chilling Tolerance at the Booting Stage in Rice by Transgenic Overexpression of the Ascorbate Peroxidase Gene, OsAPXa. Plant Cell Rep. 2011, 30, 399–406. [Google Scholar] [CrossRef]
- Hirooka, S.; Misumi, O.; Yoshida, M.; Mori, T.; Nishida, K.; Yagisawa, F.; Yoshida, Y.; Fujiwara, T.; Kuroiwa, H.; Kuroiwa, T. Expression of the Cyanidioschyzon Merolae Stromal Ascorbate Peroxidase in Arabidopsis Thaliana Enhances Thermotolerance. Plant Cell Rep. 2009, 28, 1881–1893. [Google Scholar] [CrossRef]
- Kim, M.D.; Kim, Y.H.; Kwon, S.Y.; Yun, D.J.; Kwak, S.S.; Lee, H.S. Enhanced Tolerance to Methyl Viologen-Induced Oxidative Stress and High Temperature in Transgenic Potato Plants Overexpressing the CuZnSOD, APX and NDPK2 Genes. Physiol. Plant 2010, 140, 153–162. [Google Scholar] [CrossRef]
- Duan, M.; Ma, N.N.; Li, D.; Deng, Y.S.; Kong, F.Y.; Lv, W.; Meng, Q.W. Antisense-Mediated Suppression of Tomato Thylakoidal Ascorbate Peroxidase Influences Anti-Oxidant Network during Chilling Stress. Plant Physiol. Biochem. 2012, 58, 37–45. [Google Scholar] [CrossRef]
- Hu, Z.; Li, J.; Ding, S.; Cheng, F.; Li, X.; Jiang, Y.; Yu, J.; Foyer, C.H.; Shi, K. The Protein Kinase CPK28 Phosphorylates Ascorbate Peroxidase and Enhances Thermotolerance in Tomato. Plant Physiol. 2021, 186, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Xu, X.; Wang, M.; Zhang, H.; Fang, P.; Zhou, J.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Strigolactones Positively Regulate Abscisic Acid-Dependent Heat and Cold Tolerance in Tomato. Hortic. Res. 2021, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, J.; Lin, R.; Tang, M.; Shao, S.; Yu, J.; Zhou, Y. Tomato SlMYB15 Transcription Factor Targeted by SlymiR156e-3p Positively Regulates ABA-Mediated Cold Tolerance. J. Exp. Bot. 2022, 73, 7538–7551. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, X.; Yu, Q.; Ding, Y.; Li, X.; Yang, Y. Responses of PYR/PYL/RCAR ABA Receptors to Contrasting Stresses, Heat and Cold in Arabidopsis. Plant Signal. Behav. 2019, 14, 1670596. [Google Scholar] [CrossRef]
- Huang, Y.C.; Niu, C.Y.; Yang, C.R.; Jinn, T.L. The Heat Stress Factor HSFA6b Connects ABA Signaling and ABA-Mediated Heat Responses. Plant Physiol. 2016, 172, 1182–1199. [Google Scholar] [CrossRef]
- Soon, F.F.; Ng, L.M.; Zhou, X.E.; West, G.M.; Kovach, A.; Tan, M.H.E.; Suino-Powell, K.M.; He, Y.; Xu, Y.; Chalmers, M.J.; et al. Molecular Mimicry Regulates ABA Signaling by SnRK2 Kinases and PP2C Phosphatases. Science 2012, 335, 85–88. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Crosstalk between Abscisic Acid and Nitric Oxide under Heat Stress: Exploring New Vantage Points. Plant Cell Rep. 2021, 40, 1429–1450. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, X.; Li, Z.; Wang, M. Jasmonate Positively Regulates Cold Tolerance by Promoting ABA Biosynthesis in Tomato. Plants 2023, 12, 60. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against Heat Stress-Induced Oxidative Damage in Arabidopsis Involves Calcium, Abscisic Acid, Ethylene, and Salicylic Acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.Y.; Cho, D.I.; Park, J.H.; Soo, Y.K. ABF2, an ABRE-Binding BZIP Factor, Is an Essential Component of Glucose Signaling and Its Overexpression Affects Multiple Stress Tolerance. Plant J. 2004, 40, 75–87. [Google Scholar] [CrossRef]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA Is Required for Plant Acclimation to a Combination of Salt and Heat Stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Xu, N.; Wang, M. The Ethylene Response Factor SlERF.B8 Triggers Jasmonate Biosynthesis to Promote Cold Tolerance in Tomato. Environ. Exp. Bot. 2022, 203, 105073. [Google Scholar] [CrossRef]
- Fan, X.; Lin, H.; Ding, F.; Wang, M. Jasmonates Promote β-Amylase-Mediated Starch Degradation to Confer Cold Tolerance in Tomato Plants. Plants 2024, 13, 1055. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, L.; Liu, H.Y.; Li, S.; Xing, F.; Chen, L.L. CRISPR-P: A Web Tool for Synthetic Single-Guide RNA Design of CRISPR-System in Plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Zhang, S.; Wang, M. A Jasmonate-Responsive Glutathione S-Transferase Gene SlGSTU24 Mitigates Cold-Induced Oxidative Stress in Tomato Plants. Sci. Hortic. 2022, 303, 111231. [Google Scholar] [CrossRef]
- Ding, F.; Ren, L.; Xie, F.; Wang, M.; Zhang, S. Jasmonate and Melatonin Act Synergistically to Potentiate Cold Tolerance in Tomato Plants. Front. Plant Sci. 2022, 12, 763284. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, C.; Xu, N.; Zhang, S.; Wang, M. SlMYC2 Mediates Jasmonate-Induced Tomato Leaf Senescence by Promoting Chlorophyll Degradation and Repressing Carbon Fixation. Plant Physiol. Biochem. 2022, 180, 27–34. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogenperoxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Al Mahmud, J.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Sejima, H.; Harper, J.; Mittler, R. Enhanced Seed Production under Prolonged Heat Stress Conditions in Arabidopsis Thaliana Plants Deficient in Cytosolic Ascorbate Peroxidase 2. J. Exp. Bot. 2013, 64, 253–263. [Google Scholar] [CrossRef]
- Wu, B.; Li, L.; Qiu, T.; Zhang, X.; Cui, S. Cytosolic APX2 Is a Pleiotropic Protein Involved in H2O2 Homeostasis, Chloroplast Protection, Plant Architecture and Fertility Maintenance. Plant Cell Rep. 2018, 37, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Daie, J.; Campbell, W.F. Response of Tomato Plants to Stressful Temperatures. Plant Physiol. 1981, 67, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, J.; Khan, M.; Wang, Y.; Xiao, W.; Fang, T.; Qu, J.; Xiao, P.; Li, C.; Liu, J.H. Transcription Factors ABF4 and ABR1 Synergistically Regulate Amylase-Mediated Starch Catabolism in Drought Tolerance. Plant Physiol. 2023, 191, 591–609. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.J.; Ren, M.Y.; Lu, L.F.; Peng, M.; Guan, X.; Zhou, D.B.; Zhang, M.Y.; Qi, D.F.; Li, K.; Tang, W.; et al. Involvement of Abscisic Acid-Responsive Element-Binding Factors in Cassava (Manihot Esculenta) Dehydration Stress Response. Sci. Rep. 2019, 9, 126619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).