Enhancing Growth and Nutrient Uptake in Chinese Cabbage Through the Application of Corn Steep Liquor and Myo-Inositol in Salt-Stressed Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Corn Steep Liquor and Myo-Inositol
2.2. Soil and Seedling
2.3. Pot Experiment Design
2.4. Physical and Biochemical Analyses
2.5. Statistical Analysis
3. Results
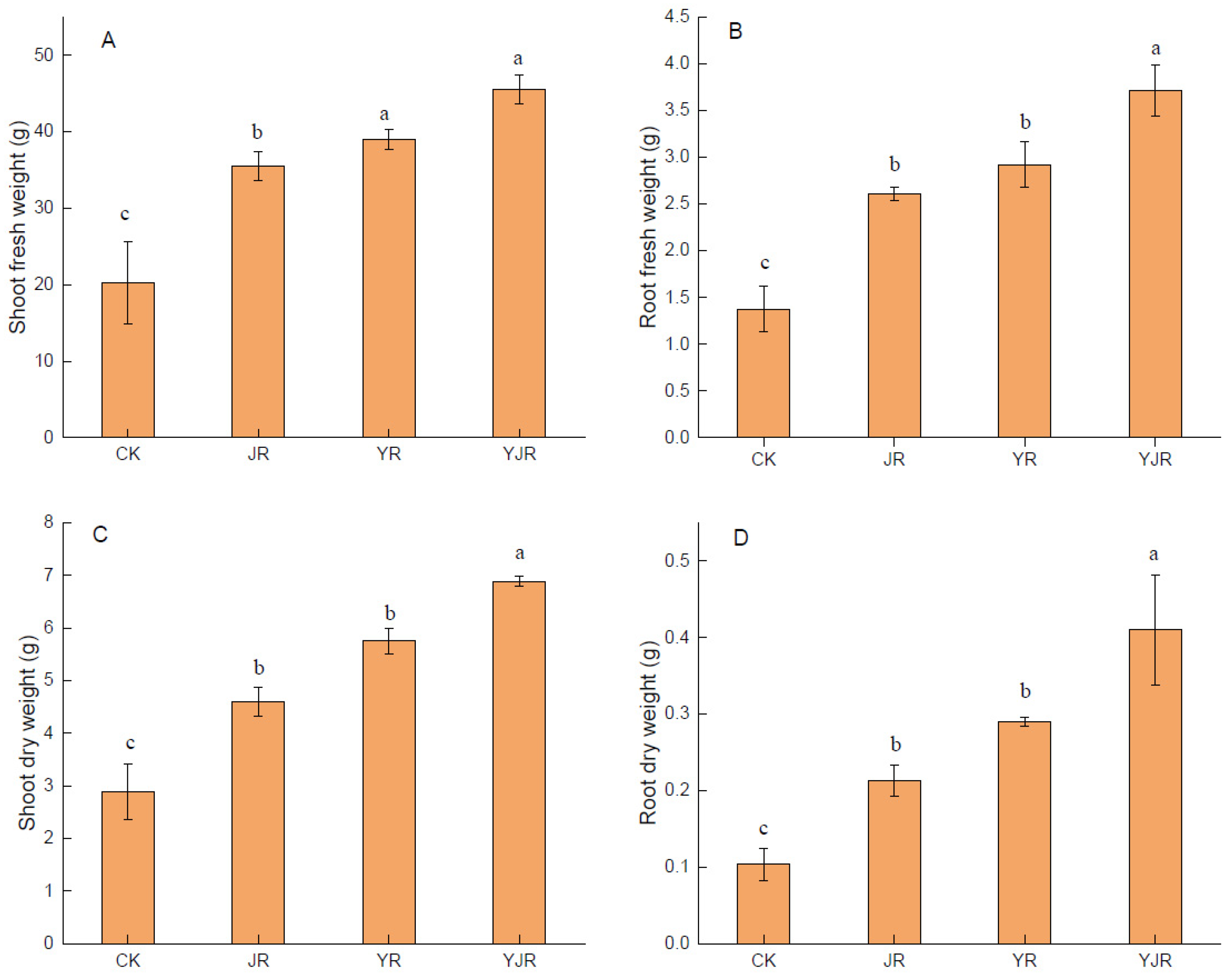
3.1. Effect of CSL and MI Application on Plant Growth
3.2. Effect of CSL and MI Application on Root Architecture
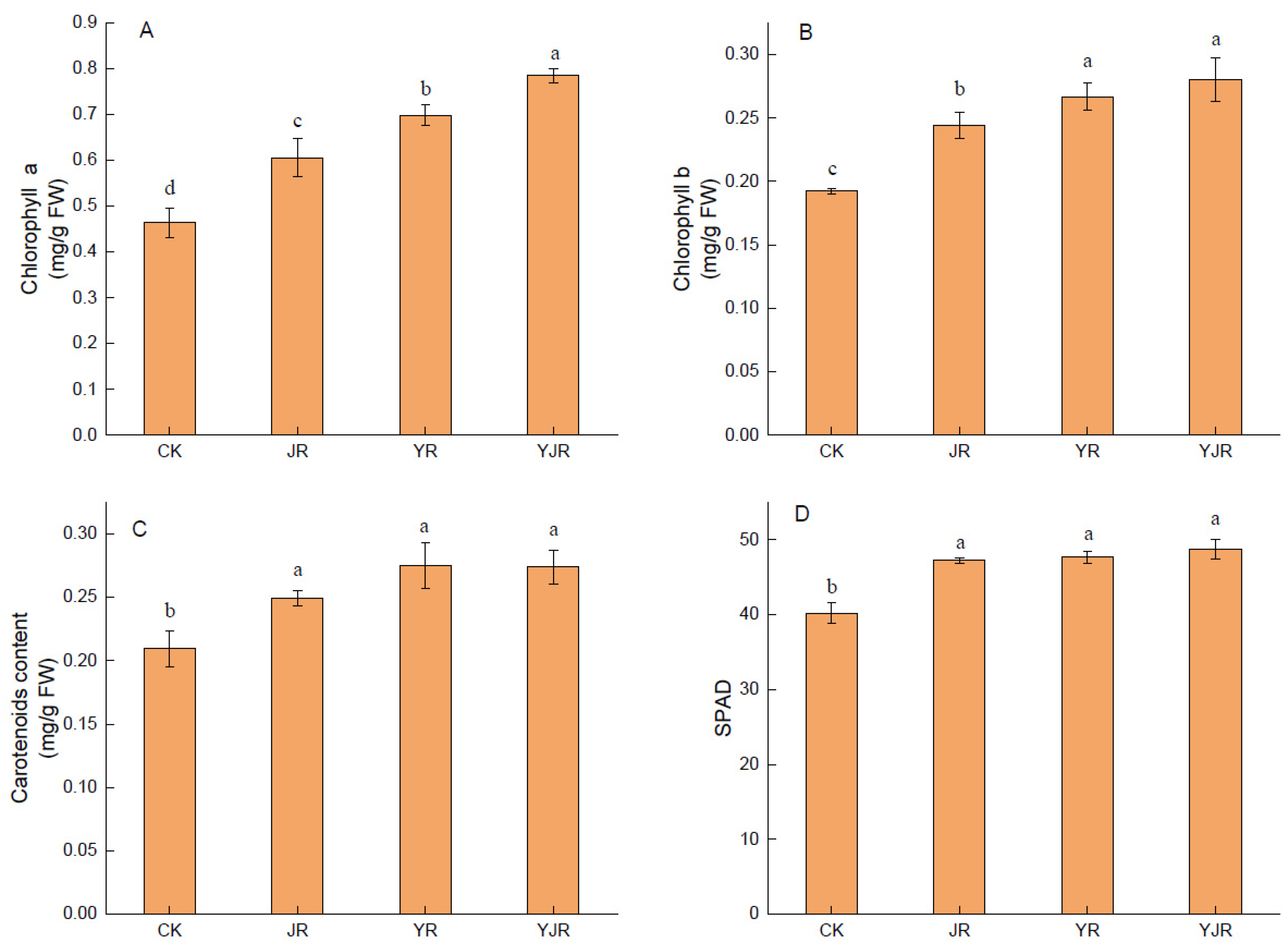
3.3. Effect of CSL and MI Application on Photosynthetic Pigment Contents and Photosynthesis
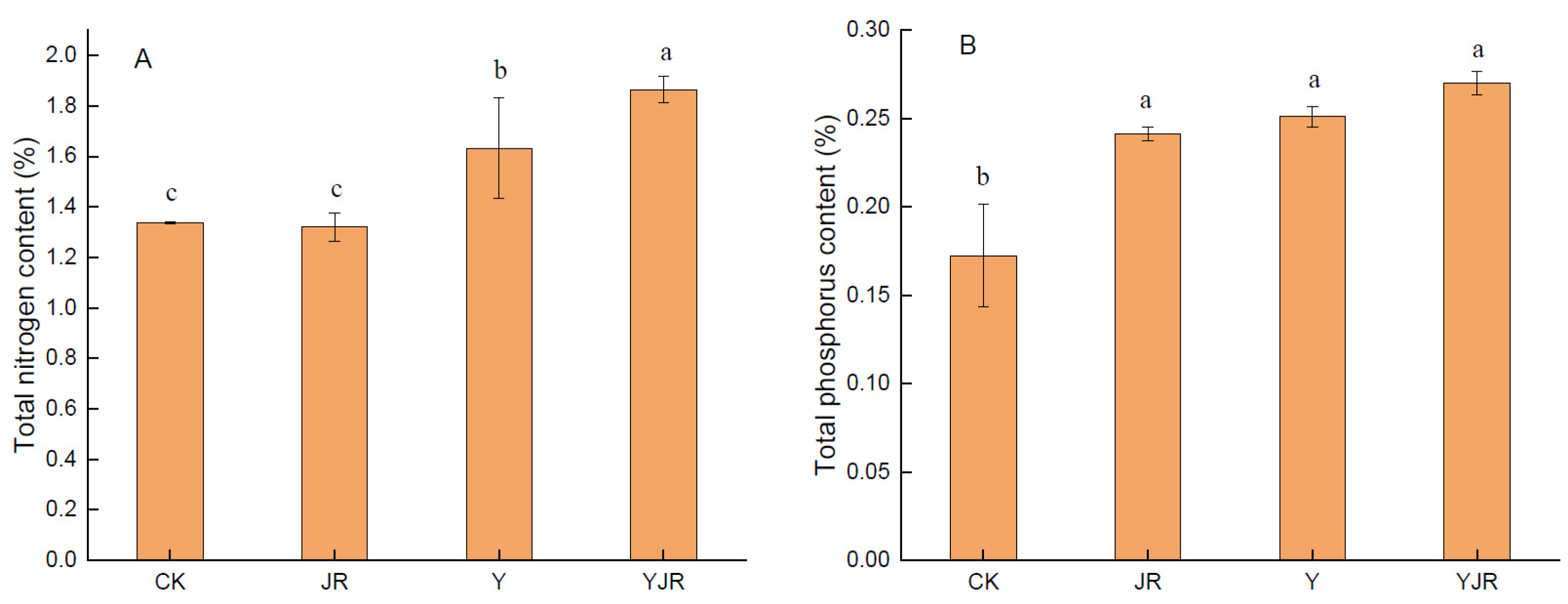
3.4. Effect of CSL and MI Application on Plant Nutrient Uptake
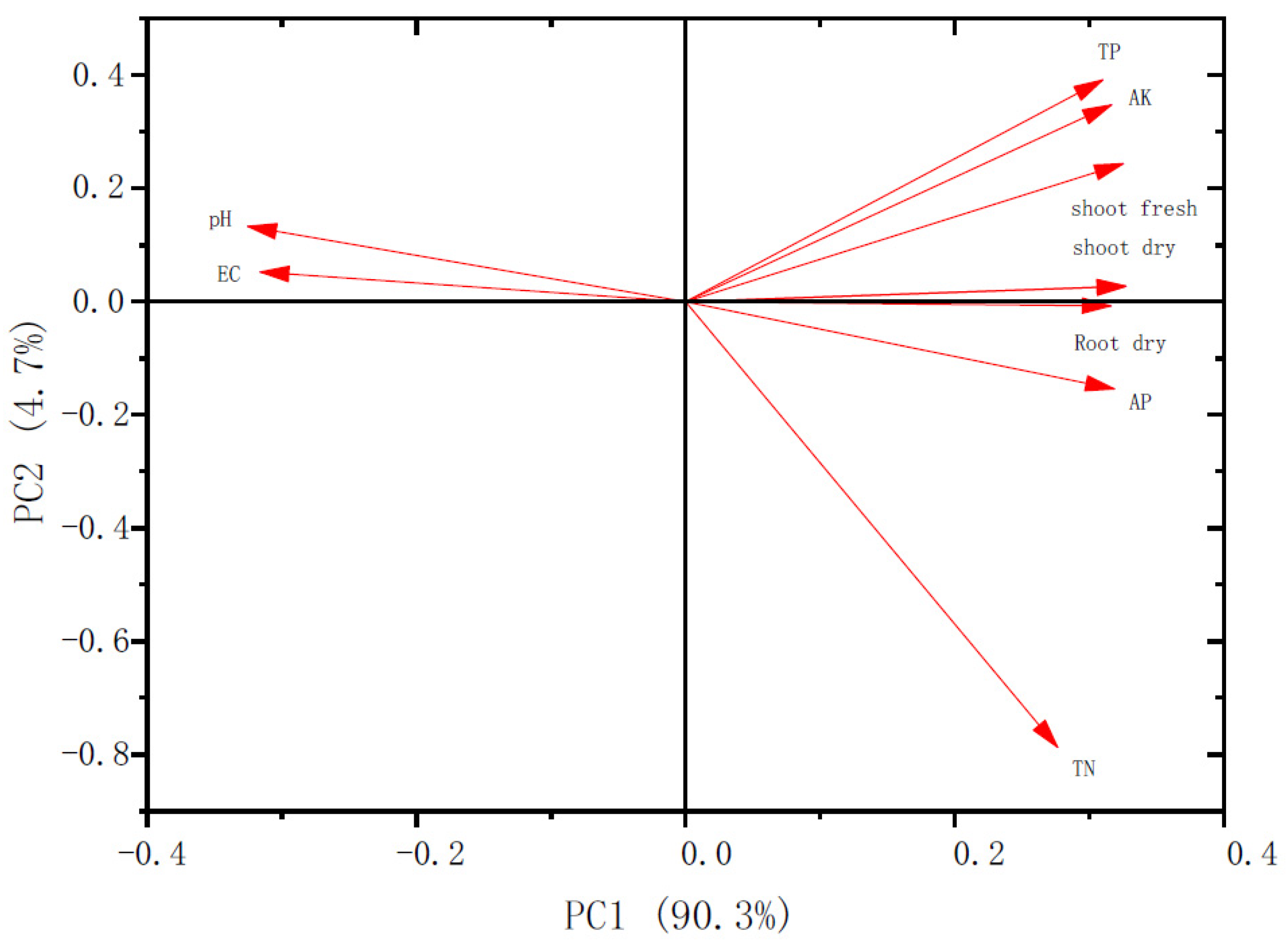
3.5. Principal Component and Correlation Analysis of Chinese Cabbage Growth and Nutrient Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussy, H.; Jang, G.; Verniquet, A.; Broeze, J.; et al. A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit. Rev. Environ. Sci. Technol. 2018, 48, 614–654. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, Q.; Du, M.; Wang, F.; Sun, W.; Chen, L.; Tang, H. Promoting the resource utilization of agricultural wastes in China with public-private-partnership mode: An evolutionary game perspective. J. Clean. Prod. 2024, 434, 140206. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, H.; Han, H.; Chang, Y. Development and utilization of corn processing by-products: A review. Foods. 2022, 11, 3709. [Google Scholar] [CrossRef]
- Tauqir, N.A.; Faraz, A.; Passantino, A.; Shahzad, M.A.; Bilal, R.M.; Tahir, A.; Ishaq, H.M.; Waheed, A. Impact of Corn Steep Liquor and Enzose Mixture on Growth Performance of Chicks. Pak. J. Zool. 2022, 54, 491–494. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, J.; Ma, Y.; Cai, L.; Zheng, L.; Gong, W.; Liu, Q. Corn Steep Liquor: Green Biological Resources for Bioindustry. Appl. Biochem. Biotech. 2022, 194, 3280–3295. [Google Scholar] [CrossRef]
- Navarro-Morillo, I.; Blasco, B.; Cámara-Zapata, J.M.; Muñoz-Acero, J.; Simón-Grao, S.; Alfosea-Simón, M.; Plasencia, F.; García-Sanchez, F. Corn Steep Liquor application on pepper plants (Capsicum annum L.) stimulates growth under nitrogen-deficient growing conditions. Sci. Hortic. 2024, 328, 112955. [Google Scholar] [CrossRef]
- Cornejo-Villegas, M.D.L.Á.; Rincón-Londoño, N.; Del Real-López, A.; Rodríguez-García, M.E. The effect of Ca2+ ions on the pasting, morphological, structural, vibrational, and mechanical properties of corn starch–water system. J. Cereal Sci. 2018, 79, 174–182. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, E.; Bao, Y.; Duan, S.; She, J.; Liu, H.; Wu, T.; Cao, X.; Zhang, J.; Li, B. Low concentration of corn steep liquor promotes seed germination, plant growth, biomass production and flowering in soybean. Plant Growth Regul. 2019, 87, 29–37. [Google Scholar] [CrossRef]
- Case, K.C.; Salsaa, M.; Yu, W.; Greenberg, M.L. Regulation of Inositol Biosynthesis: Balancing Health and Pathophysiology. Handb. Exp. Pharmacol. 2020, 259, 221–260. [Google Scholar]
- Klages, K.; Boldingh, H.; Smith, G.S. Accumulation of myo-inositol in Actinidia seedlings subjected to salt stress. Ann. Bot. 1999, 84, 521–527. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.; Zhang, H.; Chen, Y. Effects of myo-inositol on NaCl stress in Tamarix ramosissima: Insights from transcriptomics and metabolomics. Forests 2023, 14, 1686. [Google Scholar] [CrossRef]
- Ye, W.; Ren, W.; Kong, L.; Zhang, W.; Wang, T. Transcriptomic profiling analysis of Arabidopsis thaliana treated with exogenous myo-inositol. PLoS ONE 2016, 11, e0161949. [Google Scholar] [CrossRef]
- Chatterjee, J.; Majumder, A.L. Salt-induced abnormalities on root tip mitotic cells of Allium cepa: Prevention by inositol pretreatment. Protoplasma 2010, 245, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.L.; Alford, S.R.; Torabinejad, J.; Kerwin, R.E.; Nourbakhsh, A.; Ray, W.K.; Hernick, M.; Huang, X.; Lyons, B.M.; Hein, P.P. The Arabidopsis thaliana myo-inositol 1-phosphate synthase1 gene is required for myo-inositol synthesis and suppression of cell death. Plant Cell. 2010, 22, 888–903. [Google Scholar] [CrossRef]
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhang, W.; Chen, Q. Combined Application of Myo-Inositol and Corn Steep Liquor from Agricultural Waste Alleviate Salt Stress in Brassica rapa. Plants 2023, 12, 4110. [Google Scholar] [CrossRef]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-source software for root image analysis and measurement standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef]
- Mardonova, M.; Han, Y. Environmental, hydrological, and social impacts of coal and nonmetal minerals mining operations. J. Environ. Manag. 2023, 332, 117387. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. 34Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Loupassaki, M.H.; Chartzoulakis, K.S.; Digalaki, N.B.; Androulakis, I.I. Effects of salt stress on concentration of nitrogen, phosphorus, potassium, calcium, magnesium, and sodium in leaves, shoots, and roots of six olive cultivars. J. Plant Nutr. 2002, 25, 2457–2482. [Google Scholar] [CrossRef]
- Li, Y.; Feng, H.; Dong, Q.; Xia, L.; Li, J.; Li, C.; Zang, H.; Andersen, M.N.; Olesen, J.E.; Jørgensen, U. Ammoniated straw incorporation increases wheat yield, yield stability, soil organic carbon and soil total nitrogen content. Field Crop Res. 2022, 284, 108558. [Google Scholar] [CrossRef]
- Xin, X.; Nepal, J.; Wright, A.L.; Yang, X.; He, Z. Carbon nanoparticles improve corn (Zea mays L.) growth and soil quality: Comparison of foliar spray and soil drench application. J. Clean. Prod. 2022, 363, 132630. [Google Scholar] [CrossRef]
- Min, X.; Xu, D.; Hu, X.; Li, X. Changes in total organic carbon and organic carbon fractions of reclaimed minesoils in response to the filling of different substrates. J. Environ. Manag. 2022, 312, 114928. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Kirnak, H.; Higgs, D.; Saltali, K. Supplementary calcium enhances plant growth and fruit yield in strawberry cultivars grown at high (NaCl) salinity. Sci. Hortic. 2002, 93, 65–74. [Google Scholar] [CrossRef]
- Navarro-Morillo, I.; Pardo-Pina, S.; Garcia-Sánchez, F.; Ruiz, J.; Laserna-Arcas, S.; Plasencia, F.; Cámara-Zapata, J. Corn Steep Liquor Application Improves Pepper (Capsicum annum L.) Tolerance to Salinity. Horticulturae 2023, 9, 785. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Li, Y.; Chen, X.; Liu, B.; Li, C.; Gong, X.; Ma, F. Exogenous myo-inositol alleviates salinity-induced stress in Malus hupehensis Rehd. Plant Physiol. Bioch. 2018, 133, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yuan, Y.; Gong, P.; Imazumi, Y.; Na, R.; Shimizu, N. Comparative effects of mineral fertilizer and digestate on growth, antioxidant system, and physiology of lettuce under salt stress. Hortic. Environ. Biotechnol. 2023, 64, 379–391. [Google Scholar] [CrossRef]
- Joardar, S.; Duarah, P.; Purkait, M.K. Recent advances in myo-inositol recovery and purification from agricultural sources as potential dietary supplements: A review. Sustain. Chem. Pharm. 2023, 36, 101331. [Google Scholar] [CrossRef]
- Martinez-Arcos, A.; Moldes, A.B.; Vecino, X. Adding value to secondary streams of corn wet milling industry. Cyta-J. Food. 2021, 19, 675–681. [Google Scholar] [CrossRef]
- Carvalho, P.; Foulkes, M.J. Roots and Uptake of Water and Nutrients. In Sustainable Food Production; Christou, P., Savin, R., Costa-Pierce, B.A., Misztal, I., Whitelaw, C.B.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1390–1404. [Google Scholar]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci. Rep. 2021, 11, 354. [Google Scholar] [CrossRef]
- Al-Mushhin, A.A.; Qari, S.H.; Fakhr, M.A.; Alnusairi, G.S.; Alnusaire, T.S.; ALrashidi, A.A.; Latef, A.A.H.A.; Ali, O.M.; Khan, A.A.; Soliman, M.H. Exogenous myo-inositol alleviates salt stress by enhancing antioxidants and membrane stability via the upregulation of stress responsive genes in Chenopodium quinoa L. Plants 2021, 10, 2416. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Safe 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Chen, X.; Wang, Z.; Xia, Y.; Zheng, D.; Xue, S.; Zhao, H.; Huang, Z.; Niu, Y.; Zhang, G. Melatonin Alleviates Abscisic Acid Deficiency Inhibition on Photosynthesis and Antioxidant Systems in Rice under Salt Stress. Phyton 2024, 93, 0031–9457. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, H.; Zhou, J.; Smith, S.M.; Li, J. Malate circulation: Linking chloroplast metabolism to mitochondrial ROS. Trends Plant Sci. 2020, 25, 446–454. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Liang, D.; Wu, S.; Ma, F.; Lei, Y. Isolation and developmental expression analysis of L-myo-inositol-1-phosphate synthase in four Actinidia species. Plant Physiol. Biochem. 2013, 73, 351–358. [Google Scholar] [CrossRef]
- Loewus, F.A.; Murthy, P.P.N. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, L. myo-Inositol-1-phosphate Synthase Is Required for Polar Auxin Transport and Organ Development*. J. Biol. Chem. 2010, 285, 24238–24247. [Google Scholar] [CrossRef]
- Kano, K.; Kitazawa, H.; Suzuki, K.; Widiastuti, A.; Odani, H.; Zhou, S.; Chinta, Y.D.; Eguchi, Y.; Shinohara, M.; Sato, T. Effects of Organic Fertilizer on Bok Choy Growth and Quality in Hydroponic Cultures. Agronomy 2021, 11, 491. [Google Scholar] [CrossRef]
- Chinta, Y.D.; Kano, K.; Widiastuti, A.; Fukahori, M.; Kawasaki, S.; Eguchi, Y.; Misu, H.; Odani, H.; Zhou, S.; Narisawa, K.; et al. Effect of corn steep liquor on lettuce root rot (Fusarium oxysporum f.sp. lactucae) in hydroponic cultures. J. Sci. Food Agric. 2014, 94, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yu, D. The significance of lateral roots in phosphorus (P) acquisition of water hyacinth (Eichhornia crassipes). Aquat. Bot. 2003, 75, 311–321. [Google Scholar] [CrossRef]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Teo, W.F.A.; Teoh, E.Y.; Tan, B.C. Microbiome engineering and plant biostimulants for sustainable crop improvement and mitigation of biotic and abiotic stresses. Discover Food. 2022, 2, 9. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Garcia-Perez, P.; Cardarelli, M.; Senizza, B.; Miras-Moreno, B.; Colla, G.; Lucini, L. Plant biostimulants from seaweeds or vegetal proteins enhance the salinity tolerance in greenhouse lettuce by modulating plant metabolism in a distinctive manner. Sci. Hortic. 2022, 305, 111368. [Google Scholar] [CrossRef]
- Miao, Y.; Luo, X.; Gao, X.; Wang, W.; Li, B.; Hou, L. Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci. Hortic. 2020, 272, 109577. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots Withstanding their Environment: Exploiting Root System Architecture Responses to Abiotic Stress to Improve Crop Tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramirez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Zhang, Y.; Sun, H.; Zhang, K.; Bai, Z.; Dong, H.; Li, C. Fine root and root hair morphology of cotton under drought stress revealed with RhizoPot. J. Agron. Crop Sci. 2020, 206, 679–693. [Google Scholar] [CrossRef]
- Zhu, J.; Lynch, J.P. The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays) seedlings. Funct. Plant Biol. FPB 2004, 31, 949–958. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, P.; Li, Y.; Ma, L.; Alva, A.; Dou, Z.; Chen, Q.; Zhang, F. Phosphorus in China’s Intensive Vegetable Production Systems: Overfertilization, Soil Enrichment, and Environmental Implications. J. Environ. Qual. 2013, 42, 982–989. [Google Scholar] [CrossRef] [PubMed]



| pH | Organic Matter (g·L−1) | Organic Acid (g·L−1) | Amino Acid (g·L−1) | Sugar (g·L−1) | TN (g·L−1) | TK (g·L−1) | TP (g·L−1) |
|---|---|---|---|---|---|---|---|
| 4.50 | 200 | 297.81 | 80 | 100 | 30.72 | 52.37 | 17.35 |
| pH | EC (μS·cm−1) | TN (g·kg−1) | AK (mg·kg−1) | AP (mg·kg−1) | SOM (g·kg−1) | Na Content (g·kg−1) |
|---|---|---|---|---|---|---|
| 8.93 | 1373 | 2.4 | 33.14 | 411.41 | 10.52 | 0.47 |
| Treatment | NaCl (g·kg−1) | Corn Steep Liquor (g·kg−1) | Myo-Inositol (mg·kg−1) |
|---|---|---|---|
| CK | 2.25 | 0 | 0 |
| JR | 2.25 | 0 | 45 |
| YR | 2.25 | 0.7 | 0 |
| YJR | 2.25 | 0.7 | 45 |
| Total Root Length (cm) | Average Diameter (mm) | Network Area (cm2) | Number of Root Tips | Number of Branch Points | |
|---|---|---|---|---|---|
| CK | 634.26 ± 255.57 c | 0.27 ± 0.03 ab | 54.45 ± 23.58 c | 3763 ± 1076.39 c | 3385 ± 2381 b |
| JR | 1420.73 ± 36.73 b | 0.26 ± 0.02 b | 116.07 ± 23.97 bc | 9085 ± 3234.01 b | 8417 ± 3378 b |
| YR | 1588.29 ± 186.03 b | 0.28 ± 0.01 ab | 140.78 ± 17.04 ab | 10,622 ± 1307.68 ab | 10,601 ± 1634 ab |
| YJR | 2292.19 ± 58.89 a | 0.38 ± 0.11 a | 217.07 ± 78.85 a | 14461 ± 2197.29 a | 17,666 ± 8208 a |
| OM (g/kg−1) | TN (g/kg−1) | AP (mg/kg−1) | AK (mg/kg−1) | |
|---|---|---|---|---|
| CK | 8.38 ± 0.24 b | 1.22 ± 0.08 a | 19.54 ± 0.55 d | 380.59 ± 3.27 c |
| JR | 8.52 ± 0.10 b | 1.23 ± 0.06 a | 21.52 ± 0.58 c | 400.50 ± 2.56 b |
| YR | 8.97 ± 0.26 a | 1.32 ± 0.06 a | 23.77 ± 0.44 b | 407.23 ± 0.27 a |
| YJR | 9.11 ± 0.20 a | 1.34 ± 0.08 a | 26.24 ± 1.42 a | 410.08 ± 3.23 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yan, X.; Zhang, X.; Li, L.; Qi, Q.; Cui, W.; Chen, Q.; Mu, K. Enhancing Growth and Nutrient Uptake in Chinese Cabbage Through the Application of Corn Steep Liquor and Myo-Inositol in Salt-Stressed Soils. Agronomy 2025, 15, 1196. https://doi.org/10.3390/agronomy15051196
Wang X, Yan X, Zhang X, Li L, Qi Q, Cui W, Chen Q, Mu K. Enhancing Growth and Nutrient Uptake in Chinese Cabbage Through the Application of Corn Steep Liquor and Myo-Inositol in Salt-Stressed Soils. Agronomy. 2025; 15(5):1196. https://doi.org/10.3390/agronomy15051196
Chicago/Turabian StyleWang, Xian, Xu Yan, Xinjun Zhang, Longcheng Li, Qingzhen Qi, Wenjing Cui, Qing Chen, and Kangguo Mu. 2025. "Enhancing Growth and Nutrient Uptake in Chinese Cabbage Through the Application of Corn Steep Liquor and Myo-Inositol in Salt-Stressed Soils" Agronomy 15, no. 5: 1196. https://doi.org/10.3390/agronomy15051196
APA StyleWang, X., Yan, X., Zhang, X., Li, L., Qi, Q., Cui, W., Chen, Q., & Mu, K. (2025). Enhancing Growth and Nutrient Uptake in Chinese Cabbage Through the Application of Corn Steep Liquor and Myo-Inositol in Salt-Stressed Soils. Agronomy, 15(5), 1196. https://doi.org/10.3390/agronomy15051196






