In Vitro Propagation of Endangered Vanda coerulea Griff. ex Lindl.: Asymbiotic Seed Germination, Genetic Homogeneity Assessment, and Micro-Morpho-Anatomical Analysis for Effective Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Micropropagation
2.1.1. In Vitro Culture Establishment and Propagation
2.1.2. Acclimatization and Transplantation
2.1.3. Culture Data Recording and Statistical Analysis
2.2. Anatomical Study
2.3. Genetic Stability Assessment
2.3.1. DNA Extraction
2.3.2. PCR Amplification
2.3.3. Data Analysis
2.3.4. Flow Cytometry (FCM) Analysis
3. Results
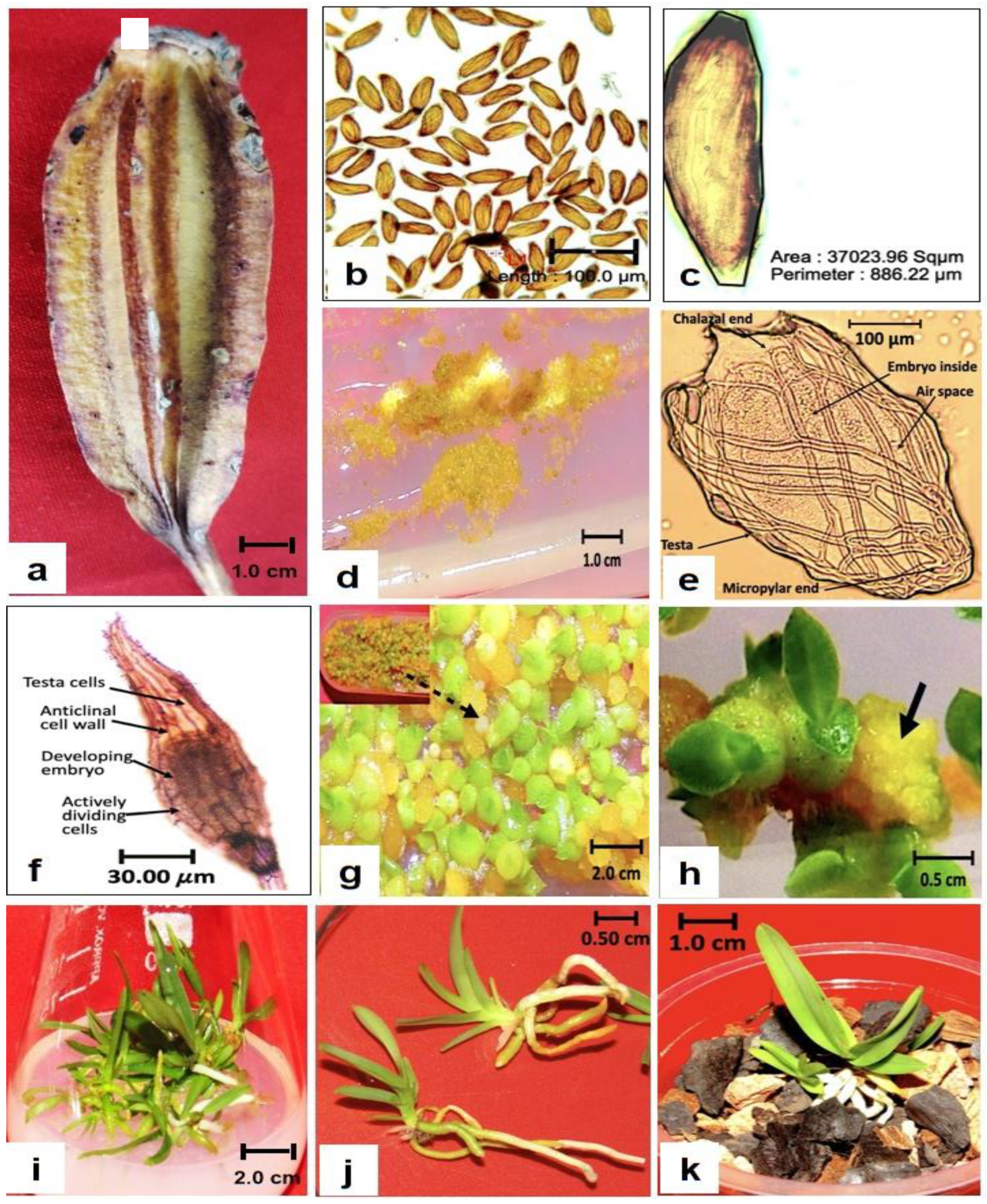
3.1. Development of Protocorm and Shoot Apical Meristem
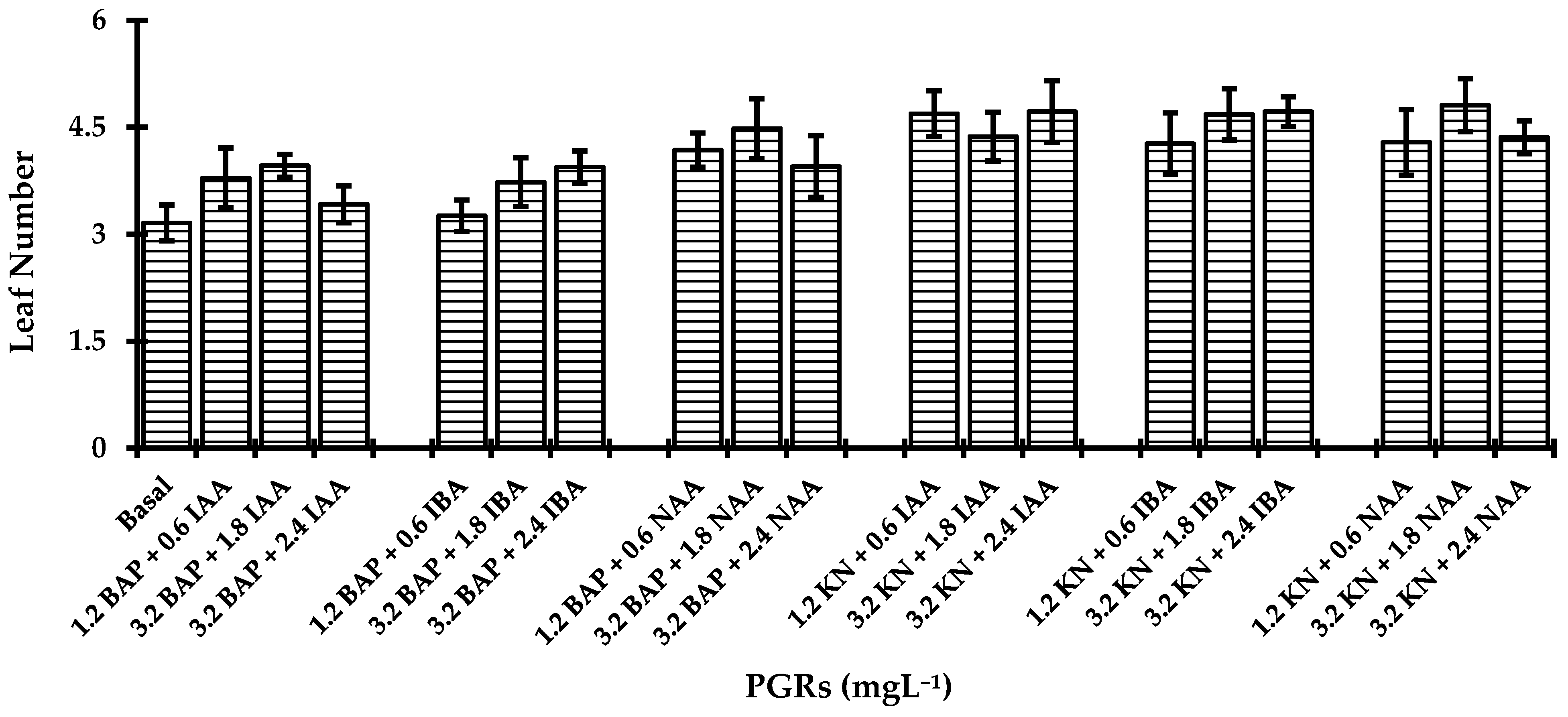
3.2. Shoot Induction and Leaf Formation
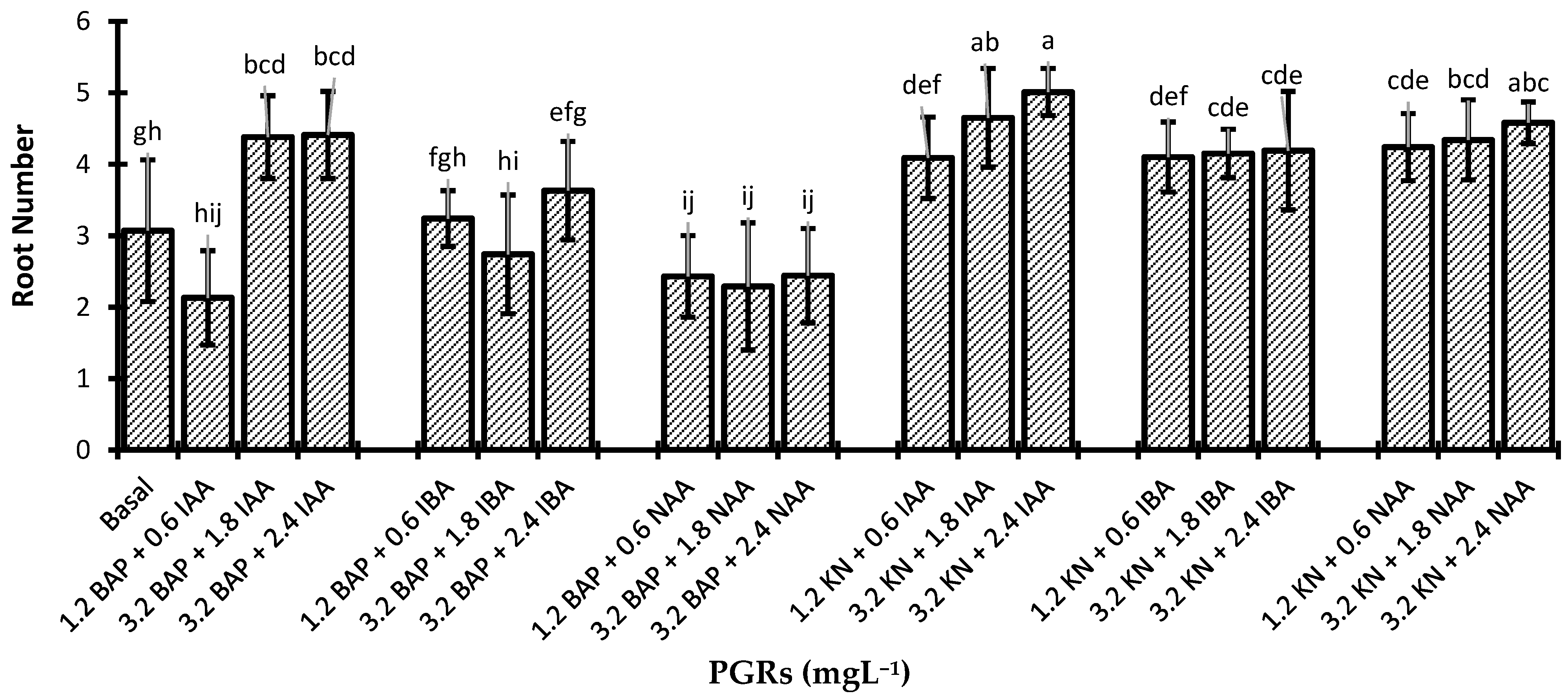
3.3. Rooting and Hardening of Regenerated Plantlets
3.4. Morpho-Anatomical Characterization of Micropropagated Plants
3.4.1. Seed Germination, Shoot, and Root Morphology
3.4.2. Anatomy of the Acclimatized In Vitro-Propagated Plants
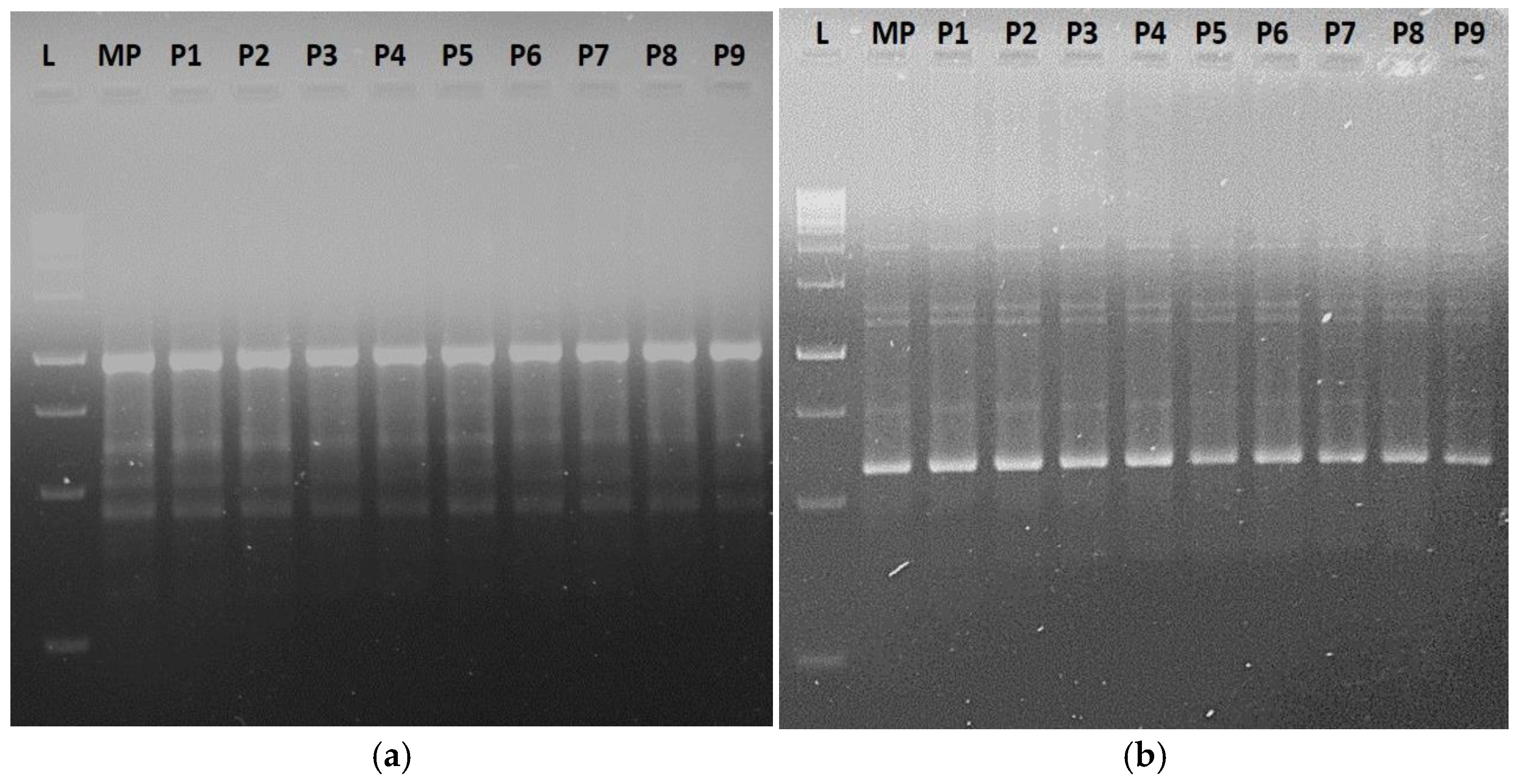
3.5. Genetic Stability Assessment of In Vitro-Propagated Plants
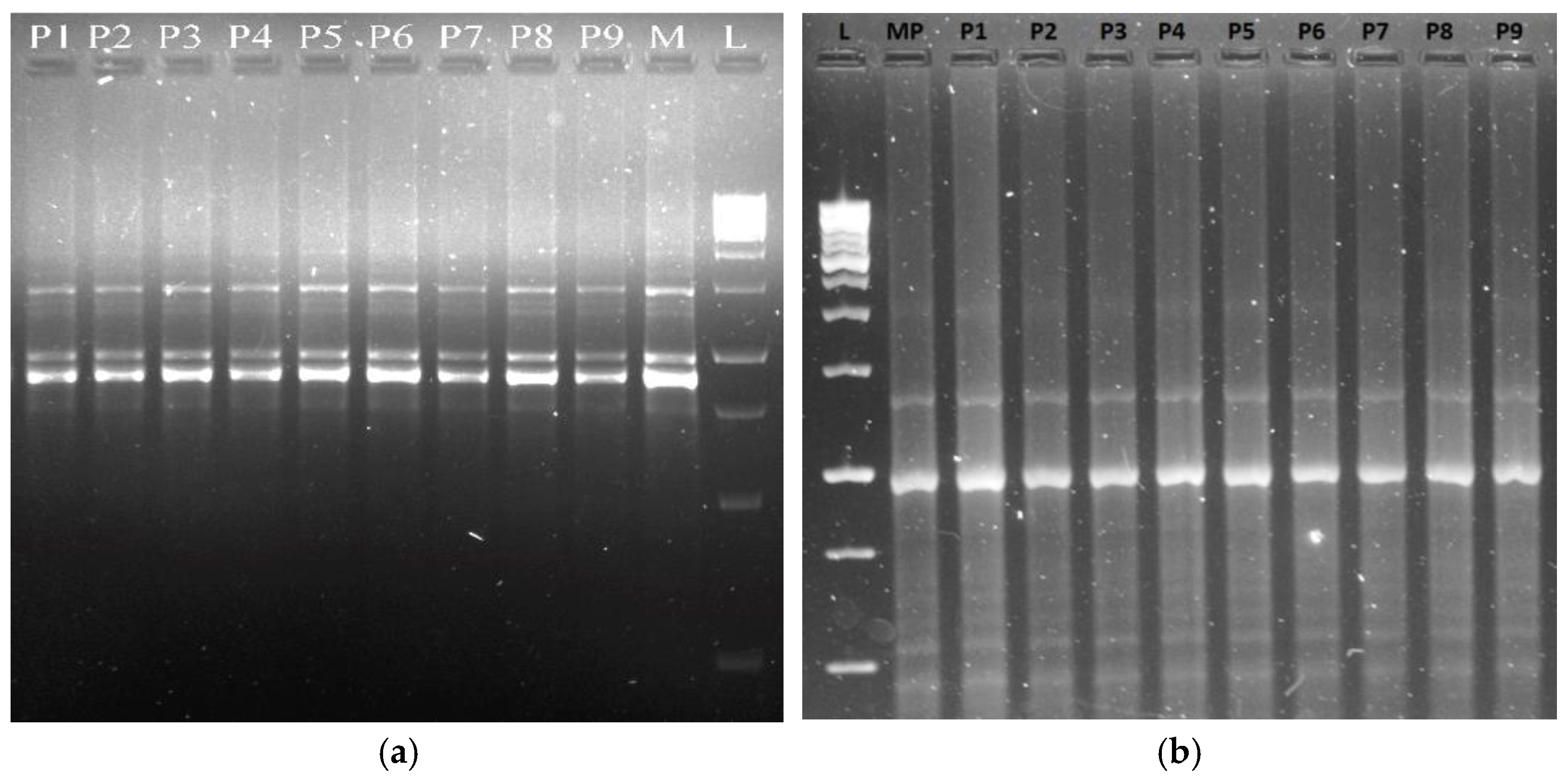
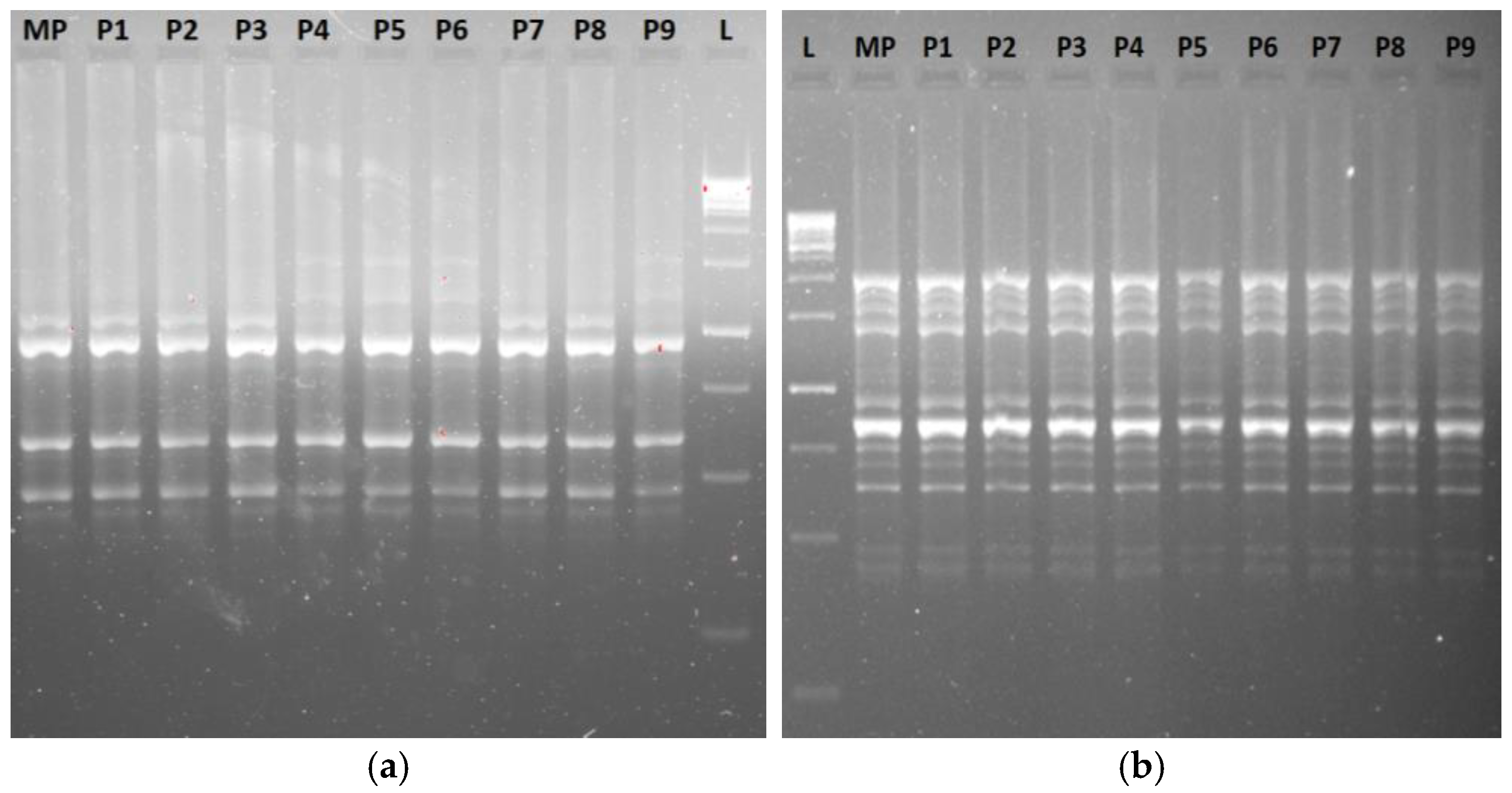
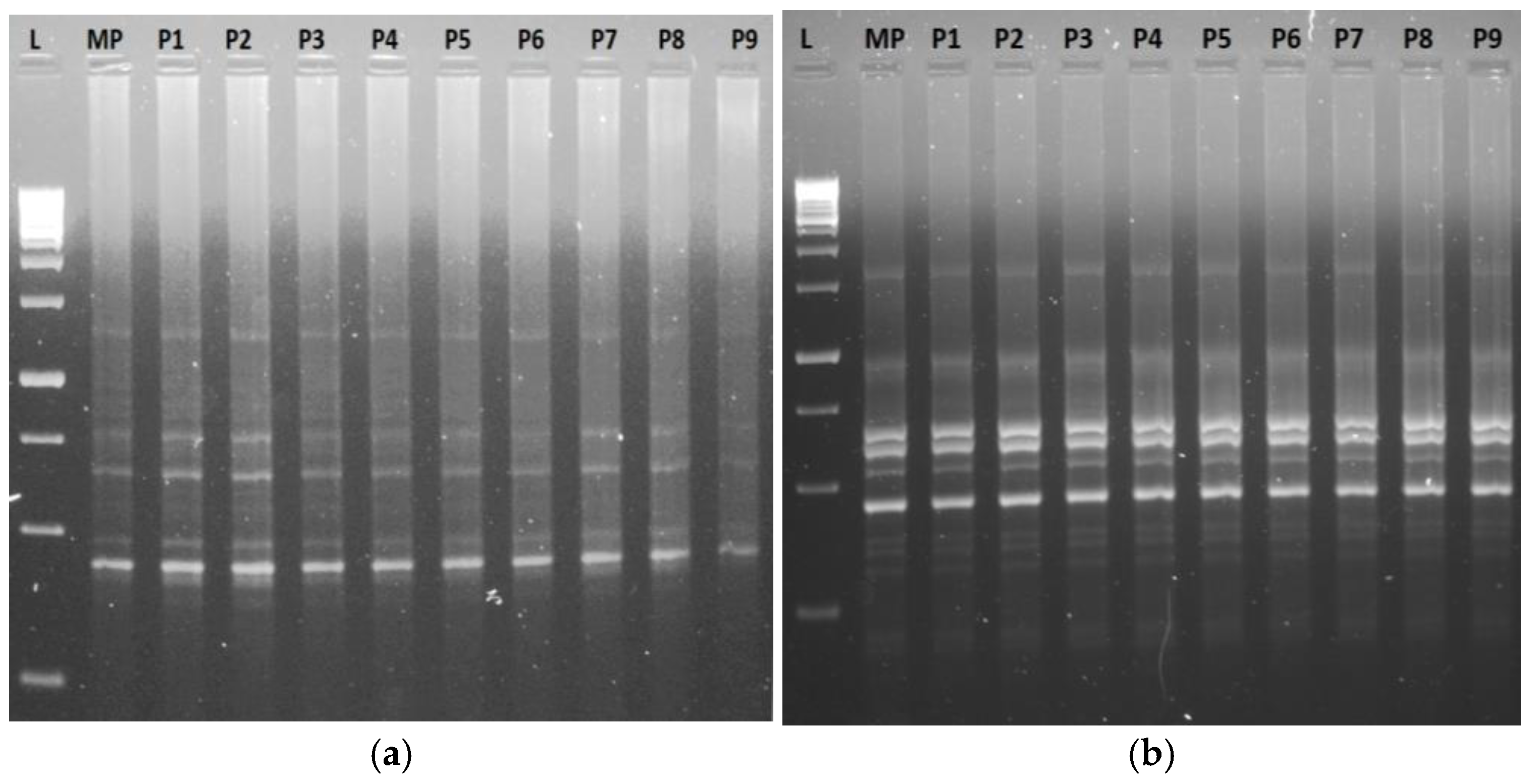
3.5.1. RAPD, ISSR, iPBS, and SCoT Markers’ Profiles and Molecular Polymorphism
3.5.2. Genetic Distance and Cluster Analysis
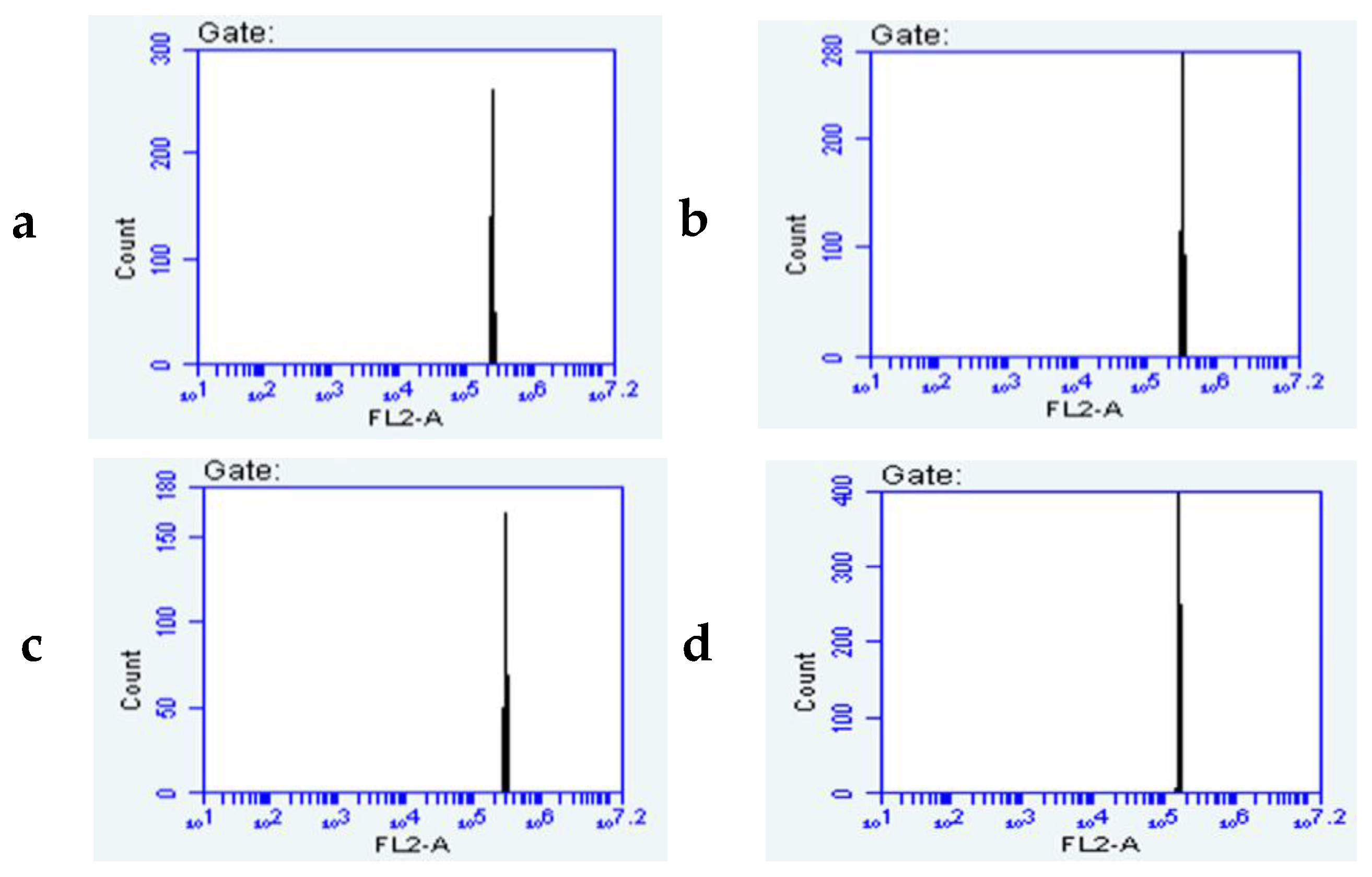
3.5.3. Flow Cytometry Analysis of the Micropropagated Plants and the Mother Plant
4. Discussion
4.1. In Vitro Propagation of V. coerulea
4.2. Morpho-Anatomical Characterization of Micropropagated Plants
4.3. Genetic Stability Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sripotar, D. (Ed.) Non-detrimental finding of Vanda coerulea. In NDF Workshop Case Studies WG 4- Geophytes and Epiphytes Case Study 4, Vanda coerulea; CITES: Bangkok, Thailand, 2008; pp. 1–8. [Google Scholar]
- Manners, V.; Kumaria, S.; Tandon, P. Propagation of Vanda coerulea via in vitro asymbiotic seed germination. Seed Tech. 2011, 33, 79–87. [Google Scholar]
- Roy, A.R.; Patel, R.S.; Patel, V.V.; Sajeev, S.; Deka, B.C. Asymbiotic seed germination, mass propagation and seedling development of Vanda coerulea Griff ex. Lindl. (Blue Vanda): An in vitro protocol for an endangered orchid. Sci. Hortic. 2011, 128, 325–331. [Google Scholar] [CrossRef]
- Simmler, C.; Antheaume, C.; Lobstein, A. Antioxidant biomarkers from Vanda coerulea stems reduce irradiated HaCaT PGE-2 production as a result of COX-2 inhibition. PLoS ONE 2010, 5, e13713. [Google Scholar] [CrossRef]
- Medhi, R.P.; Chakrabarti, S. Traditional knowledge of NE people on conservation of wild orchids. Indian J. Trad. Knowl. 2009, 8, 11–16. [Google Scholar]
- Hadi, H.; Razali, S.N.S.; Awadh, A.I. A comprehensive review of the cosmeceutical benefits of Vanda species (Orchidaceae). Nat. Prod. Commun. 2015, 10, 1934578X1501000842. [Google Scholar] [CrossRef]
- India Code. Available online: https://www.indiacode.nic.in/bitstream/123456789/1726/1/a1972-53.pdf (accessed on 17 March 2025).
- Khan, H.; Marya; Belwal, T.; Tariq, M.; Atanasov, A.G.; Devkota, H.P. Genus Vanda: A review on traditional uses, bioactive chemical constituents and pharmacological activities. J. Ethnopharmacol. 2019, 229, 46–53. [Google Scholar] [CrossRef]
- De, L.C. Morphological diversity in orchids. Int. J. Bot. Stud. 2020, 5, 229–238. [Google Scholar]
- Yang, Q.; Xu, L.; Xia, W.; Liand, L.; Bai, X.; Li, L.; Xu, L.; Liu, L. Mycorrhizal compatibility and germination-promoting activity of Tulasnella species in two species of orchid (Cymbidium mannii and Epidendrum radicans). Horticulturae 2021, 7, 472. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Terrestrialo Orchids, from Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar] [CrossRef]
- Merckx, V.; Bidartondo, M.I.; Hynson, N.A. Myco-heterotrophy: When fungi host plants. Ann. Bot. 2009, 104, 1255–1261. [Google Scholar] [CrossRef]
- Těšitelová, T.; Těšitel, J.; Jersáková, J.; Říhová, G.; Selosse, M.A. Symbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am. J. Bot. 2012, 99, 1020–1032. [Google Scholar] [CrossRef]
- Davis, B.J.; Phillips, R.D.; Wright, M.; Linde, C.C.; Dixon, K.W. Continent-wide distribution in mycorrhizal fungi: Implications for the biogeography of specialized orchids. Ann. Bot. 2015, 116, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Huang, W.; Wu, K.; Zhang, J.; da Silva, J.A.T.; Duan, J. In vitro propagation of Paphiopedilum orchids. Crit. Rev. Biotechnol. 2015, 36, 521–534. [Google Scholar] [CrossRef]
- Tikendra, L.; Amom, T.; Nongdam, P. Molecular genetic homogeneity assessment of micropropagated Dendrobium moschatum Sw.-a rare medicinal orchid, using RAPD and ISSR markers. Plant Gene 2019, 19, 100196. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Rasmussen, F.N. Seedling mycorrhiza: A discussion of origin and evolution in orchidaceae. Bot. J. Linn. Soc. 2014, 175, 313–327. [Google Scholar] [CrossRef]
- Nag, S.; Kumaria, S. In vitro propagation of medicinally threatened orchid Vanda coerulea: An improved method for the production of phytochemicals, antioxidants and phenylalanine ammonia lyase activity. J. Pharmacogn. Phytochem. 2018, 7, 2973–2982. [Google Scholar]
- Mehta, Y.R.; Angra, D.C. Somaclonal variation for disease resistance in wheat and production of dihaploids through wheat x maize hybrids. Genet. Mol. Biol. 2000, 23, 617–622. [Google Scholar] [CrossRef]
- Predieri, S. Induced mutation and tissue culture in fruits. Plant Cell Tissue Organ Cult. 2001, 64, 185–210. [Google Scholar] [CrossRef]
- Unai, E.; Iselen, T.; de Garcia, E. Comparison of characteristics of bananas (Musa sp.) from the somaclone CIEN BTA-03 and its parental clone Williams. Fruits 2004, 59, 257–263. [Google Scholar] [CrossRef]
- Krishna, H.; Singh, D. Micropropagation of lasora (Cordia myxa Roxb.). Indian J. Hortic. 2013, 70, 323–327. [Google Scholar]
- Antony, J.J.J.; Shamshir, R.A.; Poobathy, R.; Chew, B.L.; Subramaniam, S. Somaclonal variations were not induced by the cryopreservation: Levels of somaclonal variations of in vitro and thawed protocorms of Dendrobium Bobby Messina analysed by SCoT and TRAP DNA markers. S. Afr. J. Bot. 2015, 100, 148–157. [Google Scholar] [CrossRef]
- Rathore, M.S.; Mastan, S.G.; Yadav, P.; Bhatt, V.D.; Shekhawat, N.S.; Chikara, J. Shoot regeneration from leaf explants of Withania coagulans (stocks) Dunal and genetic stability evaluation of regenerates with RAPD and ISSR markers. S. Afr. J. Bot. 2016, 102, 12–17. [Google Scholar] [CrossRef]
- Al-Qurainy, F.; Nadeem, M.; Khan, S.; Alansi, S.; Tarroum, M.; Al-Ameri, A.A.; Gaafar, A.R.Z.; Alshameri, A. Rapid plant regeneration, validation of genetic integrity by ISSR markers and conservation of Reseda pentagyna an endemic plant growing in Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 111–116. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Amom, T.; Nongdam, P. The use of molecular marker methods in plants: A review. Int. J. Cur. Res. Rev. 2017, 9, 1–7. [Google Scholar]
- Amom, T.; Tikendra, L.; Nandeibam, A.; Goutam, M.; Paonam, S.; Koijam, A.S.; Potshangbam, A.M.; Hamidu, R.; Nongdam, P. Efficiency of RAPD, ISSR, iPBS, SCoT and phytochemical markers in the genetic relationship study of five native and economical important bamboos of North-East India. Phytochem. 2020, 174, 112330. [Google Scholar] [CrossRef]
- Tikendra, L.; Rahaman, H.; Dey, A.; Sahoo, M.R.; Nongdam, P. Applicability of molecular markers in ascertaining genetic diversity and relationship between five edible bamboos of North-East India. In Molecular Marker Techniques; Kumar, N., Ed.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Tikendra, L.; Dey, A.; Jamir, I.; Sahoo, M.R.; Nongdam, P. Cytokinin influence on in vitro shoot induction and genetic stability assessment of Dendrocalamus latiflorus Munro: A commercially important bamboo in Manipur, North East India. Vegetos 2022, 35, 1085–1095. [Google Scholar] [CrossRef]
- Goto, S.; Thakur, R.C.; Ishii, K. Determination of genetic stability in long-term micropropagated shoots of Pinus thunbergii Parl. using RAPD markers. Plant Cell Rep. 1998, 18, 193–197. [Google Scholar] [CrossRef]
- Palombi, M.; Damiano, C. Comparison between RAPD and SSR molecular markers in detecting genetic variation in kiwifruit (Actinidia deliciosa A. Chev). Plant Cell Rep. 2002, 20, 1061–1066. [Google Scholar] [CrossRef]
- Joshi, P.R.; Pandey, S.; Maharjan, L.; Pant, B. Micropropagation and assessment of genetic stability of Dendrobium transparens Wall. Ex Lindl. using RAPD and ISSR markers. Front. Conserv. Sci. 2023, 3, 1083933. [Google Scholar] [CrossRef]
- Novikova, T.I.; Asbaganov, S.V.; Ambros, E.V.; Zaytseva, Y.G. TDZ-induced axillary shoot proliferation of Rhododendron mucronulatum Turcz and assessment of clonal fidelity using DNA-based markers and flow cytometry. Vitr. Cell. Dev. Biol. Plant 2020, 56, 307–317. [Google Scholar] [CrossRef]
- Mamgain, J.; Mujib, A.; Ejaz, B.; Gulzar, B.; Malik, M.Q.; Syeed, R. Flow cytometry and start codon targeted (SCoT) genetic fidelity assessment of regenerated plantlets in Tylophora indica (Burm. f.) Merrill. Plant Cell Tissue Organ Cult. 2022, 150, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.C.; Marques, D.D.A.; Siqueira, W.J.; Latado, R.R. Micropropagation of Jatropha curcas superior genotypes and evaluation of clonal fidelity by target region amplification polymorphism (TRAP) molecular marker and flow cytometry. Afr. J. Biotechnol. 2014, 13, 3872–3880. [Google Scholar] [CrossRef]
- Nabi, N.; Saffeullah, P.; Singh, S. Micropropagation using direct and indirect organogenesis in Artemisia maritima L.: Scanning electron microscopy of somatic embryos and genome size analysis by flow cytometry. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 1012–1024. [Google Scholar] [CrossRef]
- Tikendra, L.; Dey, A.; Sahoo, M.R.; Nongdam, P. Genetic stability in micropropagated orchids: Assessment by molecular markers and flow cytometry. In Genome Size and Genetic Homogeneity of Regenerated Plants: Methods and Applications; Mujib, A., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023; pp. 180–231. [Google Scholar]
- Mohapatra, P.; Ray, A.; Sandeep, I.S.; Parida, R.; Mohanty, S. Genetic and biochemical stability of in vitro raised and conventionally propagated Centella asiatica-a valuable medicinal herb. S. Afr. J. Bot. 2021, 140, 444–453. [Google Scholar] [CrossRef]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Sandhya, D.; Jogam, P.; Manokari, M.; Shekhawat, M.S.; Jadaun, J.S.; Allini, V.R.; Abbagani, S. High-frequency in vitro propagation and assessment of genetic uniformity and micro-morphological characterization of Origanum majorana L.—A highly traded aromatic herb. Biocatal. Agric. Biotechnol. 2021, 34, 102024. [Google Scholar] [CrossRef]
- Mood, K.; Jogam, P.; Sirikonda, A.; Sekhawat, M.S.; Rohela, G.K.; Manokari, M.; Allini, V.R. Micropropagation, morpho-anatomical characterization, and genetic stability studies in Lippia javanica (Burm.f.) Spreng: A multipurpose medicinal plant. Plant Cell Tissue Organ Cult. 2022, 150, 427–437. [Google Scholar] [CrossRef]
- Manokari, M.; Cokulraj, M.; Faisal, M.; Alatar, A.A.; Alok, A.; Dey, A.; Shekhawat, M.S. Polyethylene-glycol modulated foliar anatomical and histochemical traits in Coccoloba uvifera (L.) L.: A salt and drought tolerant tree species. S. Afr. J. Bot. 2023, 153, 28–36. [Google Scholar] [CrossRef]
- Rachna, S.; Natasha, S.; Nirmala, C. Propagation methods for conserving Vanda coerulea griff. ex Lindl., an endangered and commercially important orchid. Plant Cell Biotechnol. Mol. Biol. 2013, 14, 147–157. [Google Scholar]
- Mitra, G.C.; Prasad, R.N.; Roychowdhury, A. Inorganic salts and differentiation of protocorms in seed callus of orchid and correlative changes in its free aminoacid content. Indian J. Exp. Biol. 1976, 14, 350–351. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Tikendra, L.; Potshangbam, A.M.; Dey, A.; Devi, T.R.; Sahoo, M.R.; Nongdam, P. RAPD, ISSR, and SCoT markers based genetic stability assessment of micropropagated Dendrobium fimbriatum Lindl. var. oculatum Hk. f.-an important endangered orchid. Physiol. Mol. Biol. Plants 2021, 27, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P. GenAlEx 6.5: Genetic analysis in excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Fernández, A.; Gómez, S. Solving non-uniqueness in agglomerative hierarchical clustering using multidendrograms. J. Classif. 2009, 25, 43–46. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef]
- George, E.F.; Klerk, G.J. The components of plant tissue culture media I: Macro- and micro-nutrients. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., Klerk, G.J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 65–113. [Google Scholar]
- Na, L.; Li, Z.; Xiangxiang, M.; Ara, N. Effect of nitrate/ammonium ratios on growth, root morphology and nutrient elements uptake of watermelon (Citrullus lanatus) Seedlings. J. Plant Nutr. 2014, 37, 1859–1872. [Google Scholar] [CrossRef]
- Dutra, D.; Johnson, T.R.; Kauth, P.J.; Stewart, S.L.; Kane, M.E.; Richardson, L. Asymbiotic seed germination, in vitro seedling development, and greenhouse acclimatization of the threatened terrestrial orchid Bletia purpurea. Plant Cell Tissue Organ Cult. 2008, 94, 11–21. [Google Scholar] [CrossRef]
- Balilashaki, K.; Martinez-Montero, M.E.; Vahedi, M.; Cardoso, J.C.; Silva Agurto, C.L.; Leiva-Mora, M.; Feizi, F.; Musharof Hossain, M. Medicinal use, flower trade, preservation and mass propagation techniques of Cymbidium orchids—An overview. Horticulturae 2023, 9, 690. [Google Scholar] [CrossRef]
- Noggle, G.R.; Wynd, F.L. Effects of vitamins on germination and growth of orchids. Bot. Gaz. 1943, 104, 455–459. [Google Scholar] [CrossRef]
- Kumaria, S. In vitro propagation of Dendrobium fimbriatum var. oculatum Hk. F.: Some functional and biochemical aspects of its growth. In Biotechnological Approaches in Ex Situ Conservation of Plant Genetic Resources of Northeast India; Kumaria, S., Choudhury, H., Das, M.C., Eds.; Excel India Publishers: New Delhi, India, 2015; pp. 1–16. [Google Scholar]
- Novak, S.D.; Whitehouse, G.A. Auxin regulates first leaf development and promotes the formation of protocorm trichomes and rhizome-like structures in developing seedlings of Spathoglottis plicata (Orchidaceae). AoB Plants 2013, 5, pls053. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sharma, M.; Pathak, P. In vitro propagation of Dendrobium aphyllum (Orchidaceae)—Seed germination to flowering. J. Plant Biochem. Biotechnol. 2013, 22, 157–167. [Google Scholar] [CrossRef]
- Hrahsel, L.; Thangjam, R. Asymbiotic in vitro seed germination and regeneration of Vanda coerulea Giff. Ex. Lindl., an endangered orchid from Northeast India. J. Plant Sci. Res. 2015, 2, 1–5. [Google Scholar]
- Asghar, S.; Ahmad, T.; Hafiz, I.A.; Yaseen, M. In vitro propagation of orchid (Dendrobium nobile) var. Emma white. Afr. J. Biotechnol. 2011, 10, 3097–3103. [Google Scholar]
- Rahman, M.S.; Hasan, M.F.; Das, R.; Hossain, M.S.; Rahman, M. In vitro micropropagation of orchid (Vanda tessellata L.) from shoot tip explant. J. Bio-Sci. 2009, 17, 139–144. [Google Scholar] [CrossRef]
- Setiaji, A.; Annisa, R.R.R.; Santoso, A.D.; Kinasih, A.; Riyadi, A.D.R. In vitro propagation of Vanda orchid: A review. Com. Sci. 2021, 12, e3427. [Google Scholar]
- Maharjan, S.; Pradhan, S.; Thapa, B.B.; Pant, B. In vitro propagation of endangered orchid, Vanda pumila hook.f. through protocorms culture. Am. J. Plant Sci. 2019, 10, 1220–1232. [Google Scholar] [CrossRef]
- Kiaheirati, H.; Hashemabadi, D.; Kaviani, B. In vitro propagation of the orchid Phalaenopsis circus via organogenesis and somatic embryogenesis using protocorm and thin cell layer explants. Ital. Bot. 2024, 18, 29–50. [Google Scholar] [CrossRef]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- El-Showk, S.; Ruonala, R.; Helariutta, Y. Crossing paths: Cytokinin signalling and crosstalk. Development 2013, 140, 1373–1383. [Google Scholar] [CrossRef]
- Davies, P.J. (Ed.) Regulatory factors in hormone action: Level, location and signal transduction. In Plant Hormones: Biosynthesis, Signal Transduction, Action, 3rd ed.; Cornell University: Ithaca, NY, USA, 2010; pp. 16–35. [Google Scholar] [CrossRef]
- Tikendra, L.; Amom, T.; Nongdam, P. Effect of phytohormones on rapid in vitro propagation of Dendrobium thyrsiflorum Rchb.f.: An endangered medicinal orchid. Phcog. Mag. 2018, 14, 495–500. [Google Scholar] [CrossRef]
- Martins, C.M.; Nhacaa, F.J.; Bilaa, C.; Cossab, M.; Fernandesb, S.; Macieb, R.; Diasb, A.; Guilundoa, S.V.; Victorinoa, I.; Pinhoa, M.; et al. The effect of different concentrations of NAA and BAP in promoting in vitro regeneration and the acclimatization of the leopard orchid (Ansellia africana lindl). Front. Agric. Food Technol. 2022, 10, 01–08. [Google Scholar]
- Laplaze, L.; Benkova, E.; Casimiro, I.; Maes, L.; Vanneste, S.; Swarup, R.; Weijers, D.; Calvo, V.; Parizot, B.; Herrera-Rodriguez, M.B.; et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell. 2007, 19, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Makita, N.; Kojima, M.; Tokunaga, H.; Sakakibara, H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-O-glucosyltransferase in rice. Plant Physiol. 2012, 160, 319–331. [Google Scholar] [CrossRef]
- Šimášková, M.; O’Brien, J.A.; Khan, M.; Van Noorden, G.; Ötvös, K.; Vieten, A.; De Clercq, I.; Van Haperen, J.M.A.; Cuesta, C.; Hoyerová, K.; et al. Cytokinin response factors regulate PIN-FORMED auxin transporters. Nat. Commun. 2015, 6, 8717. [Google Scholar] [CrossRef]
- Márquez, G. Cytokinin inhibits lateral root development at the earliest stages of lateral root primordium initiation in maize primary root. J. Plant Growth Regu. 2019, 38, 83–92. [Google Scholar] [CrossRef]
- Waidmann, S.; Michel, R.R.; Schöller, M.; Sarkel, E.; Lindner, H.; LaRue, T.; Petřík, I.; Dünser, K.; Martopawiro, S.; Sasidharan, R.; et al. Cytokinin functions as an asymmetric and anti-gravitropic signal in lateral roots. Nat. Commun. 2019, 10, 3540. [Google Scholar] [CrossRef]
- van denBerg, C.; Willemsen, V.; Hendriks, G.; Weisbeek, P.; Scheres, B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 1997, 390, 287–289. [Google Scholar] [CrossRef]
- Zhang, W.; Swarup, R.; Bennett, M.; Schaller, G.E.; Kieber, J.J. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr. Biol. 2013, 23, 1979–1989. [Google Scholar] [CrossRef]
- Drisch, R.C.; Stahl, Y. Function and regulation of transcription factors involved in root apical meristem and stem cell maintenance. Front. Plant Sci. 2015, 6, 505. [Google Scholar] [CrossRef]
- Azlan, G.J.; David, D.; Marbawi, H.; Jawan, R. Asymbiotic seed germination and seedling development of Vanda dearei. Malays. Appl. Biol. 2014, 43, 25–33. [Google Scholar]
- Arditti, J.; Ernst, R. Physiology of germinating orchid seeds. In Orchidbiology, Reviews and Perspectives III; Arditti, J., Ed.; Cornell University Press: Ithaca, NY, USA, 1984; pp. 177–222. [Google Scholar]
- Gao, X.; Wang, Y.; Deng, D.; Luo, Y.; Shao, S.; Luo, Y. Morphogenesis Changes in protocorm development during symbiotic seed germination of Dendrobium chrysotoxum (Orchidaceae) with its mycobiont, Tulasnella sp. Horticulturae 2023, 9, 531. [Google Scholar] [CrossRef]
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 58, 33. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.C.; Li, Y.-Y.; Lee, Y.-I. Understanding seed and protocorm development in orchids. In Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols; Lee, Y.I., Yeung, E.C., Eds.; Springer: New York, NY, USA, 2018; pp. 3–26. [Google Scholar]
- Hazarika, B.N.; Teixeira da Silva, J.A.; Talukdar, A. Effective acclimatization of in vitro cultured plants: Methods, physiology and genetics. Floricult. Ornament. Plant Biotechnol. 2006, 2, 427–438. [Google Scholar]
- Yokota, S.; Karim, M.Z.; Azad, M.A.K.; Rahman, M.M.; Eizawa, J.; Saito, Y.; Yshiguri, F.; Iizuka, K.; Yahara, S.; Yoshizawa, N. Histological observation of changes in leaf structure during successive micropropagation stages in Aralia elata and Phellodendron Amurense. Plant Biotechnol. 2007, 24, 221–226. [Google Scholar] [CrossRef][Green Version]
- Hazarika, B.N. Acclimatization of tissue-cultured plants. Curr. Sci. 2003, 85, 1704–1712. [Google Scholar]
- Hronková, M.; Zahradníčková, H.; Šimková, M.; Šimek, P.; Heydová, A. The role of abscisic acid in acclimation of plants cultivated in vitro to ex vitro conditions. Biol. Plant. 2003, 46, 535–541. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, X. Leaf epidermal characters and taxonomic revision of Schizophragma and Pileostegia (Hydrangeaceae). Bot. J. Linn. Soc. 2011, 165, 285–314. [Google Scholar] [CrossRef]
- Yang, S.J.; Sun, M.; Yang, Q.Y.; Ma, R.Y.; Zhang, J.L.; Zhang, S.B. Two strategies by epiphytic orchids for maintaining water balance: Thick cuticles in leaves and water storage in pseudobulbs. AoB Plants 2016, 8, plw046. [Google Scholar] [CrossRef]
- Kowsalya, A.; Rojamala, K.; Muthukumar, T. Comparative vegetative anatomy of South Indian Vandas (Orchidaceae). Flora 2017, 235, 59–75. [Google Scholar] [CrossRef]
- Thangavelu, M.; Muthu, S. Vegetative anatomical adaptations of Epidendrum radicans (Epidendroideae, Orchidaceae) to epiphytic conditions of growth. Mod. Phytomorphol. 2017, 11, 117–130. [Google Scholar]
- Mani, M.; Rasangam, L.; Selvam, P.; Shekhawat, M.S. Micro-morpho-anatomical mechanisms involve in epiphytic adaptation of micropropagated plants of Vanda tessellata (Roxb.) Hook. ex G. Don. Microsc. Res. Tech. 2020, 84, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Olivera, V.D.C.; Sajo, M.D.G. Anatomia foliar de espécies epífitas de Orchidaceae. Brazilian J. Bot. 1999, 22, 365–374. [Google Scholar] [CrossRef]
- Dycus, A.M.; Knudson, L. The role of the velamen of the aerial roots of orchids. Bot. Gaz. 1957, 119, 78–87. [Google Scholar] [CrossRef]
- Pridgeon, A.M.; Arditti, J. Orchid Biology: Reviews and Perspectives; Cornell University Press: Ithaca, NY, USA, 1987; pp. 139–192. [Google Scholar]
- Porembski, S.; Barthlott, W. Velamen radicum micromorphology and classification of orchidaceae. Nord. J. Bot. 1988, 8, 117–137. [Google Scholar] [CrossRef]
- Moreira, A.S.F.P.; dos Santos Isaias, R.M. Comparative anatomy of the absorption roots of terrestrial and epiphytic orchids. Braz. Arch. Biol. Technol. 2008, 51, 83–93. [Google Scholar] [CrossRef]
- Balachandar, M.; Ravi, R.K.; Nagaraj, K.; Muthukumar, T. Vegetative anatomy and mycorrhizal morphology of Schoenorchis nivea (Lindl.) Schltr., (Orchidaceae) and their adaptive significance. Acta Biol. Szeged. 2019, 63, 1–13. [Google Scholar] [CrossRef]
- Tikendra, L.; Koijam, A.S.; Nongdam, P. Molecular markers based genetic fidelity assessment of micropropagated Dendrobium chrysotoxum Lindl. Meta Gene 2019, 20, 100562. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumar, V.; Staden, J.V. Assessment of genetic stability amongst micropropagated Ansellia africana, a vulnerable medicinal orchid species of Africa using SCoT markers. S. Afr. J. Bot. 2017, 108, 294–302. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumaria, S.; Diengdoh, R.; Tandon, P. Genetic stability and phytochemical analysis of the in vitro regenerated plants of Dendrobium nobile Lindl., an endangered medicinal orchid. Meta Gene 2014, 2, 489–504. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Yang, Z.; Luo, C.; Zhang, W.; Xu, F.; Ye, J.; Liao, Y. Genetic diversity analysis and DNA fingerprint construction of Zanthoxylum species based on SSR and iPBS markers. BMC Plant Biol. 2024, 24, 843. [Google Scholar] [CrossRef]
- Bidyananda, N.; Jamir, I.; Nowakowska, K.; Varte, V.; Vendrame, W.A.; Devi, R.S.; Nongdam, P. Plant genetic diversity studies: Insights from DNA marker analyses. Int. J. Plant Biol. 2024, 15, 607–640. [Google Scholar] [CrossRef]
- Jitsopakul, N.; Thammasiri, K.; Ishikawa, K. Cryopreservation of Vanda coerulea protocorm-like bodies by droplet-vitrification. Acta Hortic. 2011, 908, 207–213. [Google Scholar] [CrossRef]
- Kishor, R.; Devi, H.S. Induction of multiple shoots in a monopodial orchid hybrid (Aerides vandarum Reichb.f × Vanda stangeana Reichb.f) using thidiazuron and analysis of their genetic stability. Plant Cell Tissue Organ Cult. 2009, 97, 121–129. [Google Scholar] [CrossRef]
- Pathak, P.; Anamika, K.; Chandler, B.D.; Zettler, L.W. In vitro propagation and phytochemical analysis of Vanda cristata Exll. ex Lianl.: An endangered medicinal orchid of biopharmaceutical importance. S. Afr. J. Bot. 2023, 153, 109–123. [Google Scholar] [CrossRef]
- Lal, A.; Pant, M.; Pant, G.; Palni, L.M.S.; Kumar, A.; Kumar, G. ISSR marker assisted genetic diversity assessment in natural populations of two endemic orchids Aerides multiflora and Rhynchostylis retusa from Uttarakhand, India. S. Afr. J. Bot. 2023, 157, 151–158. [Google Scholar] [CrossRef]
- Vukich, M.; Schulman, A.H.; Giordani, T.; Natali, L.; Kalendar, R.; Cavallini, A. Genetic variability in sunflower (Helianthus annuus L.) and in the Helianthus genus as assessed by retrotransposon-based molecular markers. Theor. Appl. Genet. 2009, 119, 1027–1038. [Google Scholar] [CrossRef]
- Çetin, B. Plant regeneration from hypocotyls of black carrot via direct somatic embryogenesis and determination of its genetic stability by RAPD and iPBS methods. Indian J. Genet. Plant Breed. 2018, 78, 373–377. [Google Scholar] [CrossRef]
- Siringam, T.; Vanijajiva, O. The effect of plant growth regulators on micropropagation of Melientha suavis Pierre. and assessment of genetic fidelity of regenerants based on iPBS and SRAP markers. Biodiversitas 2023, 24, 4628–4634. [Google Scholar] [CrossRef]
- Longchar, T.B.; Deb, C.R. Optimization of in vitro propagation protocol of Dendrobium heterocarpum Wall. ex. Lindl. and clonal genetic fidelity assessment of the regenerates: An orchid of horticultural and medicinal importance. S. Afr. J. Bot. 2022, 149, 67–78. [Google Scholar] [CrossRef]
- Sonia, P.; Apana, N.; Tikendra, L.; Dey, A.; Jamir, I.; Nongdam, P. Molecular clonal fidelity assessment of micropropagated orchids using DNA markers. In Biotechnology and Crop Improvement: Tissue Culture and Transgenic Approaches; Kumar, N., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 143–179. [Google Scholar] [CrossRef]
- Khoddamzadeh, A.A.; Sinniah, U.R.; Kadir, M.A.; Kadzimin, S.B.; Mahmood, M.; Sreeramanan, S. Detection of somaclonal variation by random amplified polymorphic DNA analysis during micropropagation of Phalaenopsis bellina (Rchb. f.) Christenson. Afr. J. Biotechnol. 2010, 9, 6632–6639. [Google Scholar]
- Ioannidis, K.; Tomprou, I.; Mitsis, V.; Koropouli, P. Genetic evaluation of in vitro micropropagated and regenerated plants of Cannabis sativa L. using SSR molecular markers. Plants 2022, 11, 2569. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Saraçli, S.; Doğan, N.; Doğan, İ. Comparison of hierarchical cluster analysis methods by cophenetic correlation. J. Inequal. Appl. 2013, 2013, 203. [Google Scholar] [CrossRef]
- Costa, G.F.d.; Cabral, P.D.S.; Silva, F.G.; Rubio Neto, A.; Mendonça, M.A.C. Clonal fidelity and genetic diversity of micropropagated Hancornia speciosa Gomes (Apocynaceae) as evaluated by molecular markers. Forests 2022, 13, 1645. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification; W.H. Freeman: New York, NY, USA, 1973. [Google Scholar]
- Legendre, P.; Legendre, L. (Eds.) Ordination in reduced space. In Numerical Ecology; Elsevier: Dordrecht, The Netherlands, 2012; pp. 425–520. [Google Scholar]
- Sarmast, M.K.; Salehi, H.; Ramezani, A.; Abolomoghadam, A.A.; Niazi, A.; Khui, M.K. RAPD Fingerprint to appraise the genetic fidelity of in vitro propagated Araucaria excelsa R. Br. var. glauca plantlets. Mol. Biotechnol. 2012, 50, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.K.; Chandel, P.; Gupta, S.; Gopal, J.; Singh, B.P.; Bhardwaj, V. Analysis of genetic stability of in vitro propagated potato microtubers using DNA markers. Physiol. Mol. Biol. Plants. 2013, 19, 587–595. [Google Scholar] [CrossRef]
- Nandeibam, A.; Amom, T.; Tikendra, L.; Potshangbam, A.M.; Dey, A.; Nongdam, P. Genetic diversity and population structure of Clerodendrum serratum (L.) Moon using CBDP, iPBS and SCoT markers. J. App. Res. Med. Arom. Plants 2021, 25, 100349. [Google Scholar] [CrossRef]
- Leitch, I.J.; Kahandawala, I.; Suda, J.; Hanson, L.; Ingrouille, M.J.; Chase, M.W.; Fay, M.F. Genome size diversity in orchids: Consequences and evolution. Ann. Bot. 2009, 104, 469–481. [Google Scholar] [CrossRef]
- Gozukirmizi, N.; Yilmaz, S.; Marakli, S.; Temel, A. Retrotransposon-based molecular markers; tools for variation analysis in plants. In Applications of Molecular Markers in Plant Genome Analysis and Breeding; Ajduković, K.T., Ed.; Research Signpost: Kerala, India, 2015; pp. 19–45. [Google Scholar]
- Doležel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
- Kandaiah, V.; Singaram, N.; Kandasamy, K.I. Optimized flow cytometric protocol and genome size estimation of Sabah snake grass (Clinacanthus nutans). J. Med. Plants Res. 2021, 15, 531–539. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, L.L.; Bai, C. Estimation of nuclear DNA content in tannin-rich medicinal plant Cornus officinalis by flow cytometry. Chin. Herb. Med. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- Obae, S.G.; West, T.P. Nuclear DNA content of Hydrastis canadensis L. and genome size stability of in vitro regenerated plantlets. Plant Cell Tissue Organ Cult. 2010, 102, 259–263. [Google Scholar] [CrossRef]
- Choudury, R.R.; Basak, S.; Ramesh, A.M.; Rangan, L. Nuclear DNA content of Pongamia pinnata L. and genome size stability of in vitro regenerated plantlets. Protoplasma 2013, 215, 703–709. [Google Scholar] [CrossRef] [PubMed]





| Mitra Medium (M) + PGRs (mgL−1) | Time Taken in Days for the Formation of | |||||
|---|---|---|---|---|---|---|
| BAP | KN | IAA | IBA | NAA | Protocorm | Shoot Apical Meristem |
| 0 | 0 | 0 | 0 | 0 | 27.53 ± 0.98 ab | 38.44 ± 1.12 ab |
| 1.2 | 0.6 | 26.69 ± 1.46 abc | 36.43 ± 0.79 bc | |||
| 3.2 | 1.8 | 24.40 ± 1.43 bcd | 36.66 ± 1.21 bc | |||
| 3.2 | 2.4 | 27.48 ± 1.01 ab | 36.32 ± 0.89 bc | |||
| 1.2 | 0.6 | 26.42 ± 0.21 abc | 40.45 ± 1.32 ab | |||
| 3.2 | 1.8 | 26.57 ± 0.76 abc | 39.30 ± 0.99 ab | |||
| 3.2 | 2.4 | 24.67 ± 1.19 bcd | 36.53 ± 0.93 bc | |||
| 1.2 | 0.6 | 16.39 ± 0.77 fg | 29.23 ± 0.62 b | |||
| 3.2 | 1.8 | 23.59 ± 0.91 cd | 36.43 ± 0.81 de | |||
| 3.2 | 2.4 | 28.39 ± 0.82 a | 41.35 ± 1.16 a | |||
| 1.2 | 0.6 | 16.49 ± 1.23 fg | 26.69 ± 0.99 e | |||
| 3.2 | 1.8 | 18.40 ± 0.85 ef | 32.63 ± 1.12 cd | |||
| 3.2 | 2.4 | 16.43 ± 0.86 fg | 30.54 ± 1.01 de | |||
| 1.2 | 0.6 | 21.67 ± 1.02 de | 35.29 ± 0.59 cd | |||
| 3.2 | 1.8 | 17.63 ± 1.32 fg | 32.31 ± 0.63 de | |||
| 3.2 | 2.4 | 16.38 ± 0.92 fg | 29.38 ± 0.76 de | |||
| 1.2 | 0.6 | 16.53 ± 1.18 fg | 28.42 ± 0.91 de | |||
| 3.2 | 1.8 | 14.42 ± 1.17 g | 26.35 ± 0.86 e | |||
| 3.2 | 2.4 | 19.39 ± 1.01 ef | 31.47 ± 1.17 d | |||
| PGRs (mgL−1) | Shoot Length (cm) | Leaf Length (cm) | Root Length (cm) | ||||
|---|---|---|---|---|---|---|---|
| BAP | KN | IAA | IBA | NAA | |||
| 0 | 0 | 0 | 0 | 0 | 0.38 ± 0.23 ik | 2.14 ± 0.26 ef | 1.24 ± 0.61 ef |
| 1.2 | 0.6 | 0.48 ± 0.07 k | 2.01 ± 0.32 ef | 1.38 ± 0.32 ef | |||
| 3.2 | 1.8 | 0.57 ± 0.12 ik | 2.22 ± 0.83 bcdef | 1.11 ± 0.09 f | |||
| 3.2 | 2.4 | 0.42 ± 0.14 k | 1.95 ± 0.04 f | 2.35 ± 0.32 bcd | |||
| 1.2 | 0.6 | 0.68 ± 0.07 dhi | 2.14 ± 0.68 bcdef | 1.41 ± 0.25 ef | |||
| 3.2 | 1.8 | 0.76 ± 0.08 d | 2.21 ± 0.59 bcdef | 1.82 ± 0.27 de | |||
| 3.2 | 2.4 | 0.71 ± 0.13 dhi | 2.30 ± 0.31 def | 2.11 ± 0.47 bcde | |||
| 1.2 | 0.6 | 0.55 ± 0.13 ghik | 2.78 ± 0.85 bcdef | 1.99 ± 0.35 cde | |||
| 3.2 | 1.8 | 0.53 ± 0.08 ik | 2.77 ± 0.16 bcde | 1.76 ± 0.18 e | |||
| 3.2 | 2.4 | 0.48 ± 0.10 k | 2.76 ± 0.87 bcdef | 1.31 ± 0.14 f | |||
| 1.2 | 0.6 | 1.27 ± 0.23 af | 2.97 ± 0.53 bcd | 3.21 ± 0.65 ab | |||
| 3.2 | 1.8 | 1.62 ± 0.30 ab | 4.37 ± 0.21 a | 3.96 ± 0.29 a | |||
| 3.2 | 2.4 | 1.18 ± 0.28 bcfg | 2.92 ± 0.31 bcde | 4.41 ± 0.56 a | |||
| 1.2 | 0.6 | 1.38 ± 0.21 ac | 2.42 ± 0.63 bcdef | 2.64 ± 0.35 bc | |||
| 3.2 | 1.8 | 1.34 ± 0.20 ae | 2.39 ± 0.15 def | 3.36 ± 0.61 ab | |||
| 3.2 | 2.4 | 1.83 ± 0.30 a | 2.81 ± 0.31 bcd | 3.87 ± 0.28 a | |||
| 1.2 | 0.6 | 0.73 ± 0.32 dfghijk | 2.68 ± 0.43 bcde | 3.71 ± 0.35 a | |||
| 3.2 | 1.8 | 0.90 ± 0.22 cdefgh | 3.17 ± 0.51 b | 4.08 ± 0.56 a | |||
| 3.2 | 2.4 | 1.36 ± 0.61 ad | 2.73 ± 0.61 bcde | 4.28 ± 0.52 a | |||
| RAPD | Primer Sequence (5′→3′) | Tm (°C) | No. of Scorable Bands | No. of Bands | Percentage of | Band Size (bp) | ||
|---|---|---|---|---|---|---|---|---|
| Monomorphic | Polymorphic | Monomorphism | Polymorphism | |||||
| OPA-01 | 5′-CAG2C3T2C-3′ | 34.0 | 04 | 04 | - | 100 | - | 1500–500 |
| OPA-04 | 5′-A2TCG3CTG-3′ | 32.0 | 05 | 05 | - | 100 | - | 2000–250 |
| OPA-05 | 5′-AG4TCT2G-3′ | 32.0 | 04 | 03 | 01 | 75 | 25 | 1500–250 |
| OPA-11 | 5′-CA2TCGC2GT-3′ | 32.0 | 05 | 05 | - | 100 | - | 2000–250 |
| OPA-13 | 5′-CAGCAC3AC-3′ | 34.0 | 05 | 05 | - | 100 | - | 2000–500 |
| OPB-02 | 5′-TGATC3TG2-3′ | 32.0 | 04 | 04 | - | 100 | - | 1500–250 |
| OPC-07 | 5′-GTC3GACGA-3′ | 34.0 | 04 | 04 | - | 100 | - | 1000–250 |
| OPD-01 | 5′-AC2GCGA2CG-3′ | 34.0 | 06 | 06 | - | 100 | - | 1500–250 |
| OPE-07 | 5′-AGATGCAGC2-3′ | 32.0 | 08 | 08 | - | 100 | - | 2000–500 |
| OPF-14 | 5′-TGC2AG2T-3′ | 32.0 | 06 | 06 | - | 100 | - | 2000–250 |
| Total | 51 | 50 | 01 | 97.50 | 2.5 | - | ||
| ISSR | Primer Sequence (5′→3′) | Tm (°C) | No. of Scorable Bands | No. of Bands | Percentage Degree of | Band Size (bp) | ||
|---|---|---|---|---|---|---|---|---|
| Monomorphic | Polymorphic | Monomorphism | Polymorphism | |||||
| UBC-807 | 5′-(AG)8T-3′ | 47.0 | 05 | 05 | - | 100 | - | 2000–250 |
| UBC-810 | 5′-(GA)8T-3′ | 45.4 | 03 | 03 | - | 100 | - | 1500–750 |
| UBC-815 | 5′-(CT)8G-3′ | 46.8 | 04 | 04 | - | 100 | - | 1500–250 |
| UBC-824 | 5′-(TC)8G-3′ | 48.5 | 11 | 08 | 03 | 72.73 | 27.27 | 1500–250 |
| UBC-827 | 5′-(AC)9G-3′ | 53.0 | 08 | 08 | - | 100 | - | 2000–250 |
| UBC-830 | 5′-(TG)8G-3′ | 52.7 | 06 | 06 | - | 100 | - | 1000–200 |
| UBC-835 | 5′-(AG)8YC-3′ | 50.2 | 05 | 05 | - | 100 | - | 1500–250 |
| UBC-848 | 5′-(CA)7CRG-3′ | 52.6 | 05 | 05 | - | 100 | - | 2000–500 |
| UBC-860 | 5′-(TG)8RA-3′ | 53.1 | 07 | 07 | - | 100 | - | 2000–250 |
| UBC-868 | 5′-(TG)8RA-3′ | 43.2 | 11 | 11 | - | 100 | - | 2000–250 |
| Total | 65 | 62 | 03 | 97.27% | 2.73% | |||
| iPBS | Primer Sequence (5′→3′) | Tm (°C) | No. of Scorable Bands | No. of Bands | Percentage of | Band Size (bp) | ||
|---|---|---|---|---|---|---|---|---|
| Monomorphic | Polymorphic | Monomorphism | Polymorphism | |||||
| 2077 | 5′-CTCACGATGCCA-3′ | 42.5 | 08 | 07 | 1 | 87.5 | 12.5 | 1500–250 |
| 2083 | 5′-CTTCTAGCGCCA-3′ | 42.0 | 05 | 05 | - | 100 | - | 2000–500 |
| 2220 | 5′-ACCTGGCTC(ATG)2CCA-3′ | 56.8 | 06 | 06 | - | 100 | - | 1000–250 |
| 2249 | 5′-AACCGACCTCTGATACCA-3′ | 52.4 | 05 | 05 | - | 100 | - | 2000–500 |
| 2271 | 5′-GGCTCGGATGCCA-3′ | 51.2 | 05 | 05 | - | 100 | - | 2000–250 |
| 2392 | 5′-TAGATGGTGCCA-3′ | 45.4 | 13 | 13 | - | 100 | - | 1500–500 |
| 2398 | 5′-(CAA)2TGGCTACCACCG-3′ | 45.4 | 04 | 04 | - | 100 | - | 2000–500 |
| 2399 | 5′-(A)3CTGGCAACGGCGCCA-3′ | 45.4 | 06 | 06 | - | 100 | - | 1500–250 |
| 2402 | 5′-TCTAAGCTCTTGATACCA-3′ | 45.4 | 07 | 07 | - | 100 | - | 1500–250 |
| 2408 | 5′-(CAA)2TGGCTACCACGT-3′ | 45.4 | 09 | 09 | - | 100 | - | 2000–250 |
| 2415 | 5′-CATCGTAGGTG3CGCCA-3′ | 60.5 | 05 | 05 | - | 100 | - | 1500–250 |
| Total | 73 | 72 | 01 | 98.86 | 1.14 | |||
| SCOT Primer | Primer Sequence (5′→3′) | Tm (°C) | No. of Scorable Bands | No. of Bands | Percentage of | Band Size (bp) | ||
|---|---|---|---|---|---|---|---|---|
| Monomorphic | Polymorphic | Monomorphism | Polymorphism | |||||
| S1 | 5′-(CAA)2TGGCTA(CCA)2-3′ | 52.6 | 03 | 03 | - | 100 | - | 2000–500 |
| S3 | 5′-(CAA)2TGGCTACCACCG-3′ | 53.9 | 04 | 04 | - | 100 | - | 1500–250 |
| S5 | 5′-(CAA)2TGGCTACCACGA-3′ | 52.6 | 08 | 06 | 02 | 75.0 | 25 | 1500–250 |
| S6 | 5′-(CCA)2TGGCTACCACGC-3′ | 54.4 | 05 | 05 | - | 100 | - | 1500–250 |
| S10 | 5′-(CCA)2TGGCTACCAGCC-3′ | 53.9 | 07 | 07 | - | 100 | - | 2000–250 |
| S12 | 5′-ACGACATGGCGACCAACG-3′ | 58.4 | 04 | 04 | - | 100 | - | 2000–250 |
| S17 | 5′-ACCATGGCT(ACC)2GAG-3′ | 57.1 | 04 | 04 | - | 100 | - | 2000–500 |
| S18 | 5′-ACCATGGCT(ACC)2GCC-3′ | 60.7 | 05 | 05 | - | 100 | - | 2000–500 |
| S28 | 5′-CCATGGCTACCACCGCCA-3′ | 60.7 | 05 | 05 | - | 100 | - | 2000–250 |
| S30 | 5′-CCATGGCTACCACCGGCG-3′ | 61.8 | 04 | 04 | - | 100 | - | 1500–250 |
| S35 | 5′-CATGGCTACCAC3GC3-3′ | 61.7 | 09 | 09 | - | 100 | - | 2000–250 |
| Total | 58 | 56 | 02 | 97.73 | 2.27 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikendra, L.; Singh, A.R.; Vendrame, W.A.; Nongdam, P. In Vitro Propagation of Endangered Vanda coerulea Griff. ex Lindl.: Asymbiotic Seed Germination, Genetic Homogeneity Assessment, and Micro-Morpho-Anatomical Analysis for Effective Conservation. Agronomy 2025, 15, 1195. https://doi.org/10.3390/agronomy15051195
Tikendra L, Singh AR, Vendrame WA, Nongdam P. In Vitro Propagation of Endangered Vanda coerulea Griff. ex Lindl.: Asymbiotic Seed Germination, Genetic Homogeneity Assessment, and Micro-Morpho-Anatomical Analysis for Effective Conservation. Agronomy. 2025; 15(5):1195. https://doi.org/10.3390/agronomy15051195
Chicago/Turabian StyleTikendra, Leimapokpam, Asem Robinson Singh, Wagner Aparecido Vendrame, and Potshangbam Nongdam. 2025. "In Vitro Propagation of Endangered Vanda coerulea Griff. ex Lindl.: Asymbiotic Seed Germination, Genetic Homogeneity Assessment, and Micro-Morpho-Anatomical Analysis for Effective Conservation" Agronomy 15, no. 5: 1195. https://doi.org/10.3390/agronomy15051195
APA StyleTikendra, L., Singh, A. R., Vendrame, W. A., & Nongdam, P. (2025). In Vitro Propagation of Endangered Vanda coerulea Griff. ex Lindl.: Asymbiotic Seed Germination, Genetic Homogeneity Assessment, and Micro-Morpho-Anatomical Analysis for Effective Conservation. Agronomy, 15(5), 1195. https://doi.org/10.3390/agronomy15051195







