Microbially Enhanced Biofertilizers: Technologies, Mechanisms of Action, and Agricultural Applications
Abstract
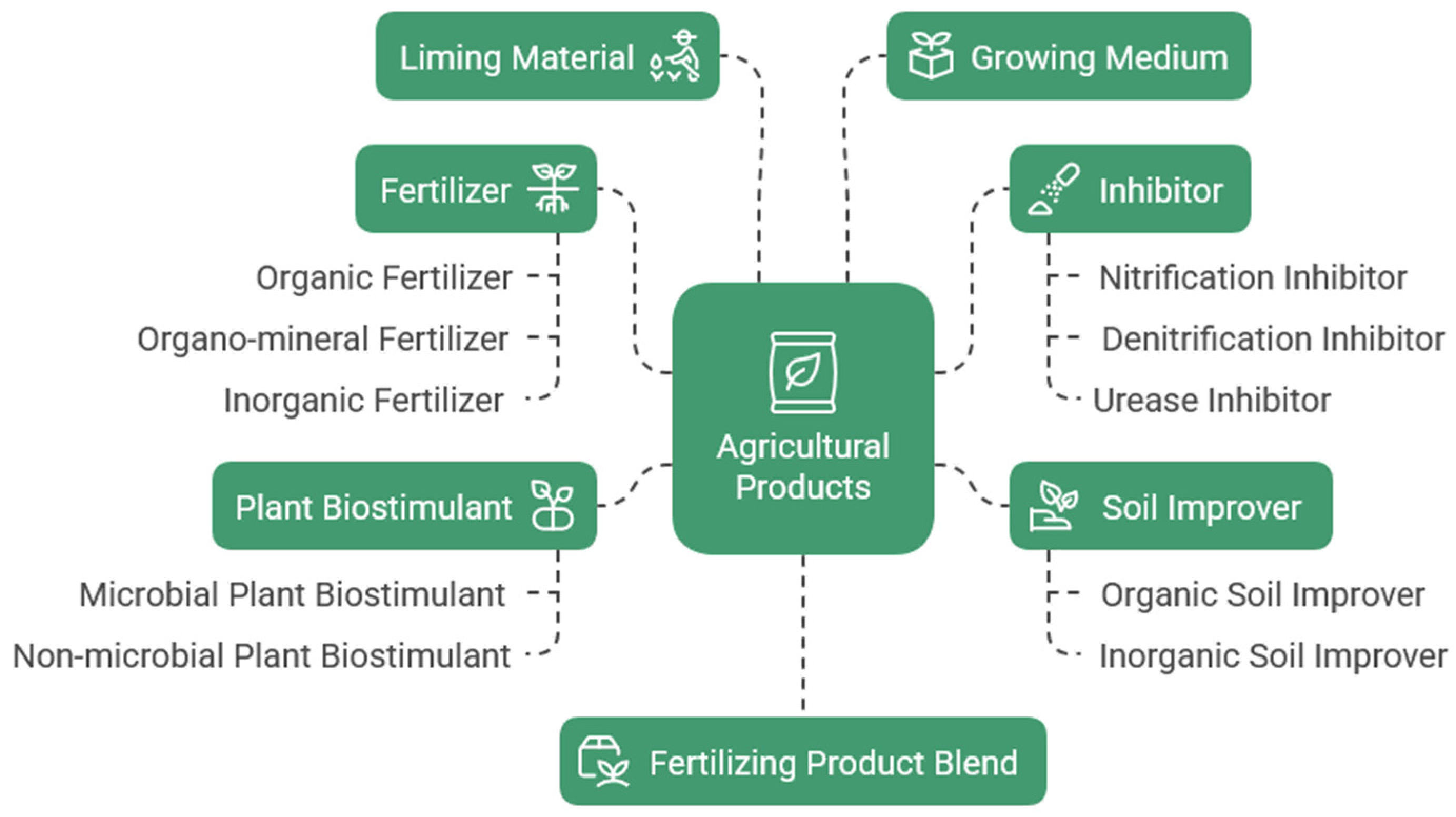
:1. Biofertilizers—Fertilizers Enhanced with Biologically Active Additives
- Nitrogen-fixing biofertilizers: Containing microorganisms that can fix atmospheric nitrogen, such as Rhizobium, Azotobacter, Azospirillum, and cyanobacteria.
- Phosphate-solubilizing biofertilizers: Containing microorganisms that solubilize insoluble phosphates, such as Bacillus, Pseudomonas, and Aspergillus.
- Phosphate-mobilizing biofertilizers: Containing mycorrhizal fungi that help plants access phosphorus from soil.
- Potassium-solubilizing biofertilizers: Containing microorganisms that release potassium from insoluble minerals, such as Bacillus mucilaginosus and Bacillus edaphicus.
- Sulfur-oxidizing biofertilizers: Containing microorganisms that oxidize sulfur to make it available to plants, such as Thiobacillus.
- Plant growth-promoting rhizobacteria (PGPR): Providing multiple benefits through various mechanisms, including hormone production, siderophore formation, and pathogen suppression [5].
- The selection of the appropriate carrier;
- The application of a particular formulation type for inoculated products;
- Soil materials—peat, clay, coal, and lignite;
- Organic material of plant origin—charcoal, manure, cellulose, soybean pellets, soybean oil and nut oil, wheat bran, corncobs, and sawdust;
- Inert materials: bentonite, kaolin, silicates, vermiculite, perlite, calcium sulfate, and polyacrylamide gels;
- Dry inoculants (powders): Dry inoculants are produced with the use of soil organic substances or inert carriers. Most frequently, the formulation of powder inoculants is carried out using peat. Peat ensures an environment that is rich in nutrients for the growth of a large variety of microorganisms. Peat should be non-toxic, highly adsorptive, and easily sterilized, and it should have a high content of organic matter and the capacity to absorb water, as well as being easily available at a reasonable price. The main drawback of peat is its variable composition. Peat inoculated with bacteria is usually introduced on seeds just before sowing [31,32].
- Liquid inoculants: Liquid inoculants are based on aqueous broth cultures in a polymer-based oil or water suspension. Liquid formulation gained great popularity because of the easy application of seeds into the soil [5,23]. Contrary to powder inoculants, the liquid formula allows the producer to include relevant amounts of nutrients and cell protection measures to improve the efficiency of bioproduct application [33]. Moreover, it was found that they do not contain impurities, and they are more field-efficient as compared to peat-based products [28]. Liquid inoculants require specific storage conditions (low temperatures) and have a limited durability time [34,35].
- Granules: Granules are made of peat lumps or marble, calcite and silicate grains coated or impregnated with microorganisms [29]. Granules have different sizes, but there is a correlation between the density of the matrix culture population and the quality of the final product [35]. Fertilizer granules are placed in furrows close to seeds to allow interaction between lateral roots, thanks to which they do not have direct contact with pesticides, which are toxic for microorganisms [23,26,30,34].
- Freeze-dried powders: Freeze-drying allows for obtaining a high bacteria survival rate without the necessity of using a carrier. To protect the cytoplasm and cell membranes of bacteria, cryoprotectants should be added, e.g., mannitol or microcrystal cellulose, which lead to slower degradation kinetics in soil and a higher stability of inoculums at room temperatures for a longer period [36].
2. Legislation
3. Assimilation of Nutrients by Cultivated Plants
- Soil properties (pH, organic matter content, and texture);
- Environmental conditions (temperature and moisture);
- Microbial community composition and activity;
- Plant species and growth stage;
- Application method and timing.
- Building elements: carbon, hydrogen, and oxygen, specified as biogenic elements;
- Macroelements: nitrogen, phosphorous, sulfur, potassium, calcium, and magnesium, present in soil and plants in the amount of 0.01–5.0%;
- Siderophore production: Many bacteria and fungi produce low-molecular-weight compounds called siderophores that chelate Fe3+ with high affinity, making them available for microbial and plant uptake. Key siderophore-producing microorganisms include Pseudomonas, Bacillus, and Trichoderma species.
- Iron reduction: Some microorganisms can reduce Fe3+ to the more soluble Fe2+ form, facilitating plant uptake. Iron-reducing bacteria include Geobacter and Shewanella species.
- Organic acid production: Similar to phosphate solubilization, the microbial production of organic acids can solubilize iron compounds by lowering pH and through chelation effects.
3.1. Microbial Conversions of Nitrogen
- Host specificity: Many nitrogen-fixing bacteria have narrow host ranges, limiting their applicability across different crops.
- Environmental sensitivity: Factors like soil acidity, temperature, and moisture significantly affect nitrogen fixation efficiency.
- Energy requirements: The process requires substantial energy from the plant, potentially reducing yield under certain conditions.
- Delayed nutrient availability: Unlike chemical fertilizers, biological nitrogen fixation provides nitrogen gradually over time.
- Competition with native soil microbiota: Introduced nitrogen-fixing bacteria must compete with established microbial communities.
- Variability in performance: Results can vary considerably across different field conditions and seasons.
- Symbiotic bacteria, including Rhizobiaceae (Rhizobium, Bradyrhizobium, Sinorhizobium, Azorhizobium, Mesorhizobium, and Allorhizobium) [67]. Approx. 20% of legume plants are capable of entering into symbiosis with microorganisms, as a result of which molecular nitrogen is reduced and it is incorporated into the plant’s metabolism. Actinobacteria Frankia living symbiotically with approx. 170 tree plants, mainly Betulaceae, fix approx. 10–200 kg N/ha [68].
- Non-symbiotic bacteria (free-living, associative, and endophytic), e.g., Acetobacter, Herbaspirillum, Azoarcus spp., Alcaligenes, Azospirillum, Bacillus, Enterobacter, Klebsiella, Pseudomonas, Azotobacter, Burkholderia, Beiferinckia, Clostridium, Serratia, and Erwinia [69]. Non-symbiotic bacteria provide insignificant amounts of nitrogen to the related plants [70].
- Cyanobacteria (blue-green algae)—Aulosira, Trichodesmium, Anabaena, Cylindrospermum, Nostoc plectonema, and Tolypothrix. The cyanobacterial nitrogen fixation by Azolla-Anabena bacteria was of key importance in rice cultivation until the end of the 1970’s [71].
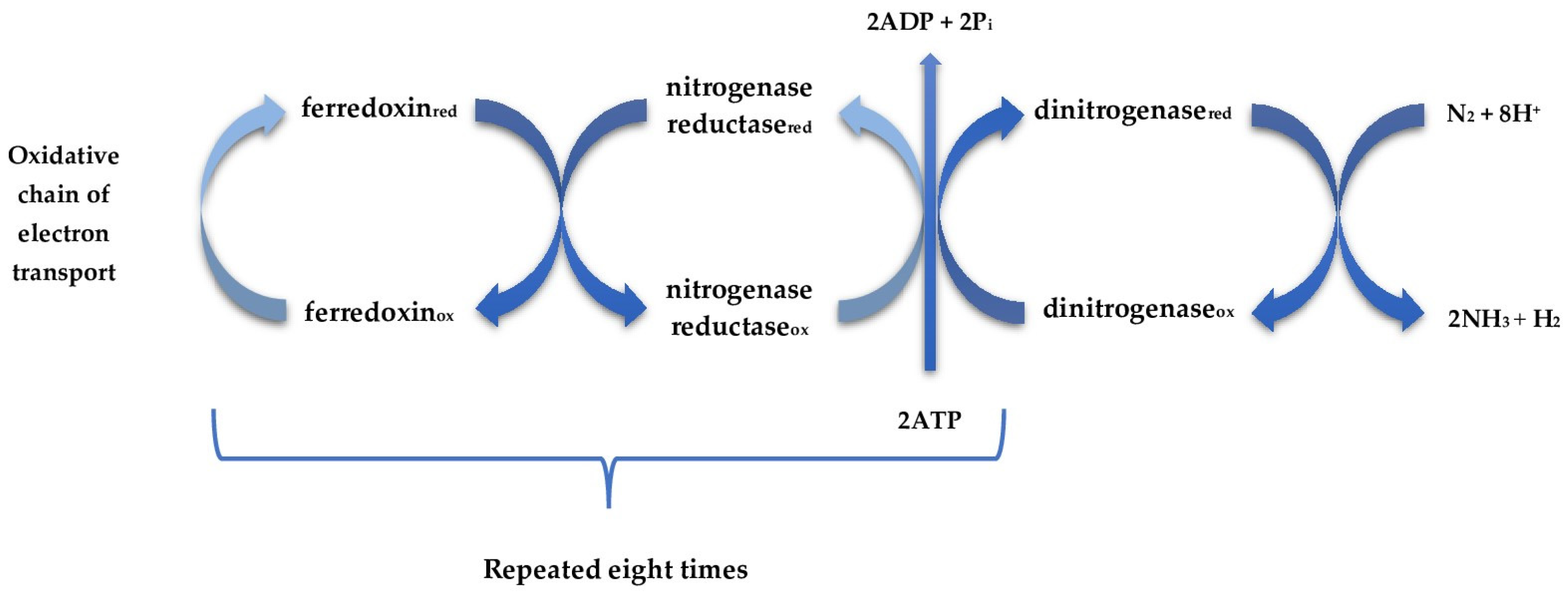
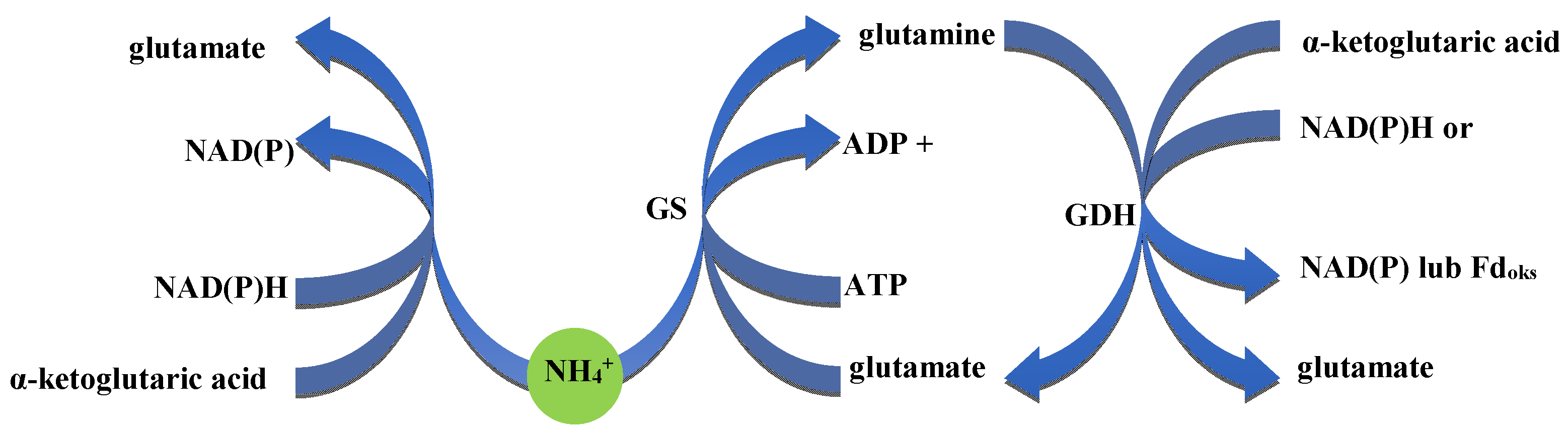
- Host specificity: Many nitrogen-fixing bacteria, particularly rhizobia, have high host specificity, limiting their application to specific plant species or varieties.
- Environmental sensitivity: Nitrogen fixation is inhibited by factors such as high soil nitrogen levels, low pH, drought, extreme temperatures, and oxygen exposure, making performance inconsistent across different agroecosystems.
- Competition with indigenous microflora: Introduced nitrogen-fixing bacteria must compete with native soil microorganisms, often resulting in poor establishment.
- Formulation challenges: Maintaining viability and activity during production, storage, and after application remains technically challenging.
- Delayed benefits: Unlike mineral nitrogen fertilizers that provide immediately available nutrients, biological nitrogen fixation may take time to establish and provide significant amounts of fixed nitrogen.
- Quantification difficulties: Measuring the actual contribution of biologically fixed nitrogen under field conditions is challenging, making it difficult to determine appropriate application rates.
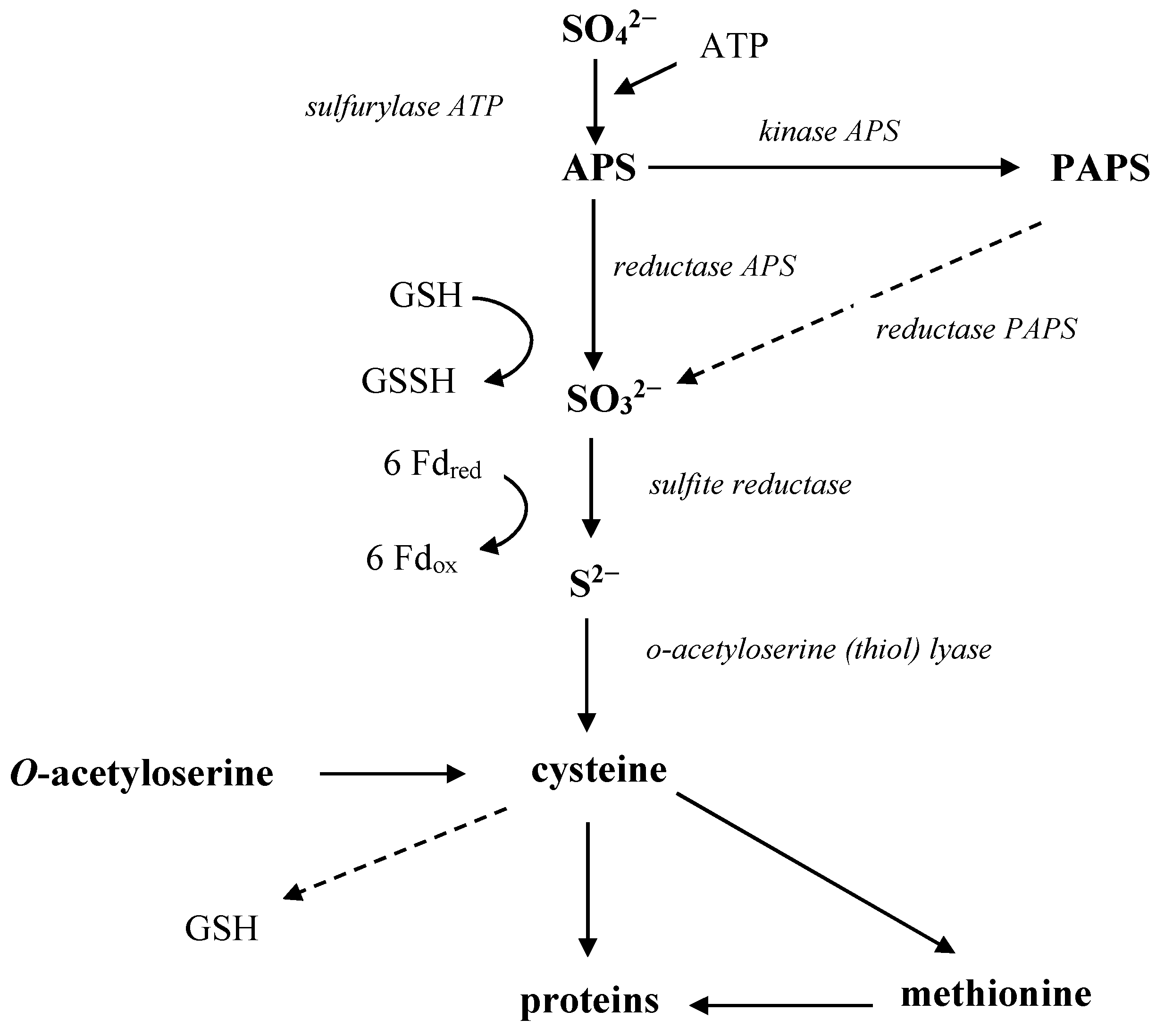
3.2. Microbial Conversions of Sulfur
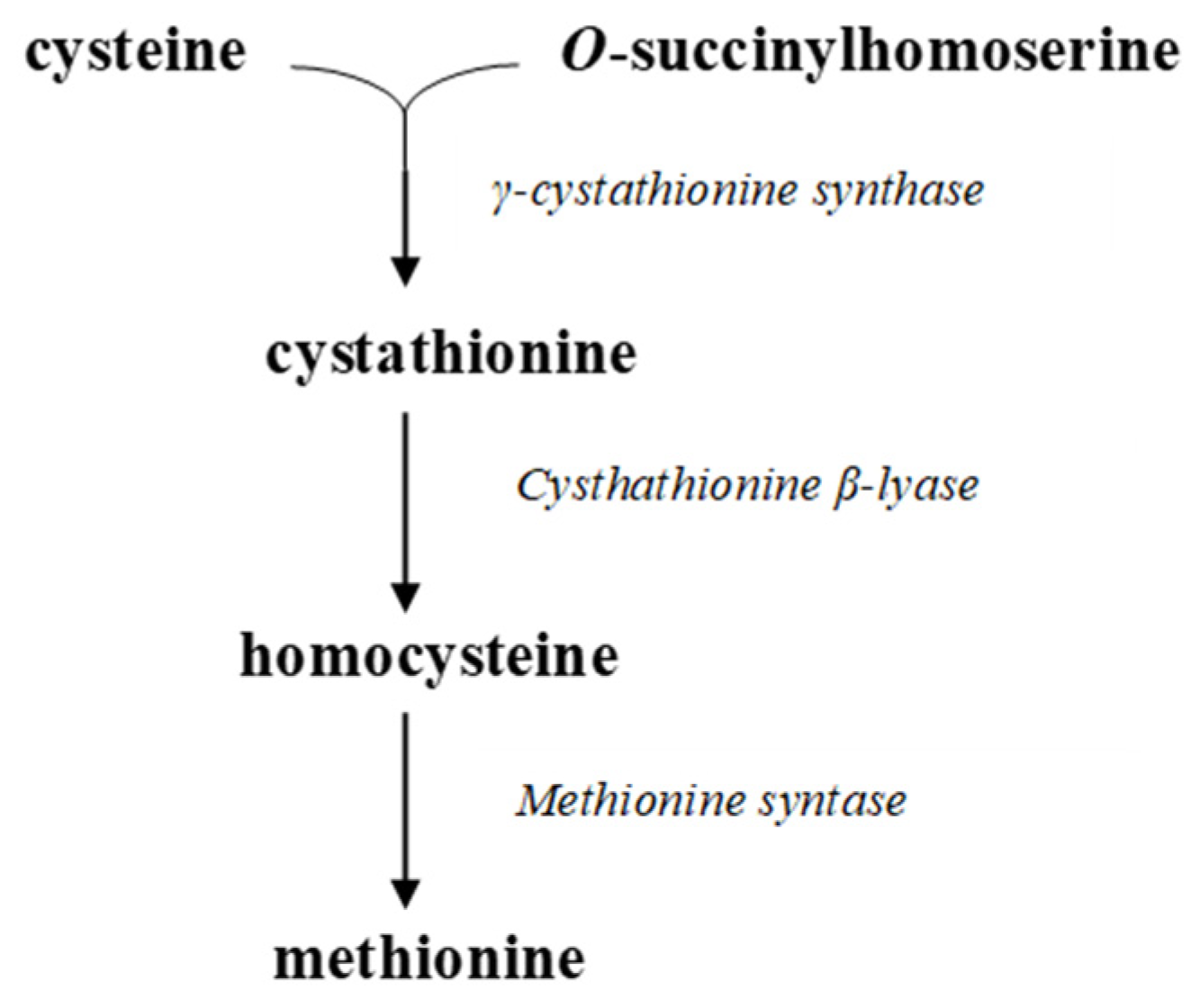
3.3. Phosphorus Availability and Its Role in Plant Nutrition: Mechanisms of Assimilation and Solubilization by Microorganisms
- Active phosphorous present in soils as PO43−, HPO42−, and H2PO4−, which are three levels of the dissociation of orthophosphoric acid;
- Assimilable phosphorous—tricalcium phosphate, iron phosphate, aluminum phosphate, dicalcium phosphate, dimagnesium phosphate, and vivianite—Fe3(PO4)2∙8H2O;
- Auxiliary phosphorous, which involves apatites—Ca10(PO4)6 (OH− or F−)2, variscite—AlPO4∙2H2O, and strengite—FePO4∙2H2O [88].
- Soil pH-buffering capacity: Highly buffered soils may neutralize the organic acids produced by PSMs, reducing their effectiveness in phosphate solubilization.
- Variable performance: The efficiency of PSMs varies considerably depending on soil type, environmental conditions, and crop species.
- Persistence issues: Many introduced PSMs show poor survival and colonization in field soils, particularly under stressful conditions.
- Compatibility challenges: Some PSMs may not function optimally when combined with certain pesticides or other agricultural inputs.
- Formulation stability: Maintaining viable populations of PSMs with consistent phosphate-solubilizing activity throughout the production, storage, and application process remains challenging.
- Limited understanding of consortia effects: The interactions between different PSM strains and with other soil microorganisms are not fully understood, making it difficult to predict performance in complex soil environments.
- Ectomycorrhizae, where the fungal hyphae form a mantle around the root surface and penetrate between cortical cells but do not enter them. Common ectomycorrhizal fungi include species from the genera Amanita, Boletus, and Tuber.
- Endomycorrhizae, where the fungal hyphae penetrate the cell walls of the root cells. The most common type is arbuscular mycorrhizal fungi (AMF), which form arbuscules (tree-like structures) within root cortical cells. AMF belong to the phylum Glomeromycota and include genera such as Glomus, Gigaspora, and Acaulospora.
- -
- Mycorrhizal fungi enhance phosphorus uptake through several mechanisms:
- -
- The extension of the root system through external hyphae, increasing the soil volume explored by up to 1000 times;
- -
- The production of phosphatase enzymes that hydrolyze organic phosphorus compounds;
- -
- The secretion of organic acids that solubilize mineral phosphates;
- -
- More efficient phosphorus uptake due to higher affinity transport systems.
3.4. Microbial Conversions of Potassium
3.4.1. Forms of Potassium in the Soil and Their Availability to Plants
- Potassium in soil solution—occurring as K+ ions, directly available to plants, accounting for only 0.1–0.2% of total soil potassium content;
- Exchangeable potassium—adsorbed on the soil sorption complex, readily available to plants, accounting for 1–2% of total potassium content;
- Non-exchangeable potassium—trapped in the inter-packet spaces of clay minerals, hardly available to plants, accounting for 1–10% of the total potassium content [113];
- Structural potassium—embedded in the structure of primary (feldspars and mica) and secondary (clay minerals) minerals, practically unavailable to plants, accounting for 90–98% of the total potassium content of the soil [114].
3.4.2. Potassium-Solubilizing Microorganisms and Their Mechanisms of Action
- -
- Organic acid production—Microorganisms secrete a variety of organic acids such as citric, oxalic, tartaric, succinic, lactic, gluconic, and α-ketogluconic acids [121]. These acids lower the pH of the environment, which promotes the dissolution of potassium minerals. In addition, anions of organic acids can form complexes with cations (Ca2+, Mg2+, Fe3+, and Al3+) present in the crystal lattice of minerals, leading to their destabilization and the release of potassium [122].
- -
- The production of chelating substances—Microorganisms secrete siderophores and other chelating compounds that bind metal cations in potassium minerals, leading to their breakdown and the release of potassium [123].
- -
- The acidification of the environment—During respiration, microorganisms secrete CO2, which forms carbonic acid (H2CO3) in soil solution, lowering the pH and increasing the solubility of potassium minerals [124].
- -
- The production of extracellular polysaccharides (EPSs)—The polysaccharides secreted by microorganisms form a biofilm on the surface of potassium minerals, which promotes potassium solubilization by creating a microenvironment with reduced pH and an increased concentration of organic acids [125].
- -
- The production of specific enzymes—Some microorganisms produce enzymes capable of catalyzing reactions that lead to the breakdown of potassium minerals [126].
- -
- Redox reactions—Microorganisms can oxidize or reduce iron and manganese ions present in potassium minerals, leading to changes in the crystal structure and the release of potassium [127].
3.4.3. Biofertilizers Containing Potassium-Solubilizing Microorganisms
- The selection of suitable microbial strains: Effective strains should be characterized by high potassium solubilization capacity, resistance to unfavorable environmental conditions, the ability to colonize the rhizosphere, and a lack of antagonism towards other beneficial soil microorganisms [129].
- Biofertilizer formulation: A suitable formulation should ensure the survival of the microorganisms during storage and application, facilitate their incorporation into the soil and stimulate their activity after application [2]. The most commonly used formulations are peat-based powders, granules, liquid formulations, and freeze-dried formulations [1].
- Soil and environmental conditions: The effectiveness of biofertilizers depends on the physicochemical properties of the soil (pH, organic matter content, and cation exchange capacity), climatic conditions (temperature and humidity), and interaction with the autochthonous soil microflora [130].
- The method and timing of application: The appropriate method of application (seed, soil, and foliar) and timing (before sowing or during the growing season) can significantly affect the effectiveness of a biofertilizer [28].
3.4.4. Interactions of Potassium-Solubilizing Microorganisms with Other Soil Microorganisms
- The mutual supply of growth factors: Different groups of microorganisms can supply growth factors such as vitamins, amino acids, and nucleotides to each other [139];
- The removal of inhibitory metabolites: Some microorganisms can remove metabolites that inhibit the growth of other microorganisms [140];
- Changes in environmental properties: The activities of some microorganisms can lead to changes in environmental properties (pH and redox potential) that favor the growth of other microorganisms [125];
- The creation of functional systems: Different groups of microorganisms can form functional systems in which the metabolic products of some microorganisms are substrates for others [141].
3.4.5. Assimilation of Potassium by Plants Assisted by Microorganisms
- Shaker-type K+ channels—potential difference-activated potassium channels, involved in the uptake of potassium from the soil at low external concentrations [144];
- KUP/HAK/KT transporters—proton–potassium transporters, particularly important at low environmental potassium concentrations [145];
- HKT transporters—potassium–sodium transporters, involved in potassium transport and the maintenance of ionic homeostasis under salt stress [146];
- Antiporter-type K+/H+ transporters maintain the cell’s potassium homeostasis [147].
3.4.6. Challenges and Prospects for the Use of Potassium-Solubilizing Biofertilizers
- The variability of effects under different conditions—the effectiveness of biofertilizers can vary considerably depending on soil type, climatic conditions, plant species, and interaction with indigenous soil microflora [151];
- Microbial survival issues—maintaining microbial viability and activity during the production, storage, and application of biosolids is a significant technological challenge [29];
- Competition with indigenous microflora—introduced microorganisms must compete with natural soil microflora for ecological niche and substrates [152].
- A lack of quality standards—the lack of uniform quality standards and methods to assess the effectiveness of biofertilizers hinders their certification and marketing [28];
- Farmers’ insufficient knowledge—a lack of knowledge and awareness among farmers about the benefits and proper use of biofertilizers limits their adoption in agricultural practice [35].
4. Impact of Carrier Materials on Microbial Nutrient Transformation Efficiency
5. Challenges and Limitations of Microbially Enhanced Biofertilizers
5.1. Regulatory and Quality Control Hurdles
5.2. Technical Limitations in Strain Efficacy
5.3. Environmental and Application Risks
5.4. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.H. Future of bio-fertilizers in Indian agriculture: An overview. Int. J. Agric. Food Res. 2015, 3, 10–23. [Google Scholar] [CrossRef]
- Rusek, Ł.; Rusek, P.; Zdeb, Z.; Schab, S.; Borowik, K.; Brodowska, M. Technology for producing microbiologically enriched granular fertilizers using the coating method, along with the determination of process parameters. Przemysł Chem. 2025, 104, 91–104. [Google Scholar] [CrossRef]
- Malusá, E.; Vassilev, N. A contribution to set a legal framework for biofertilisers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607. [Google Scholar] [CrossRef]
- Nobbe, F.; Hiltner, L. Inoculation of the Soil for Cultivating Leguminous Plant. U.S. Patent 570 813, 3 November 1896. [Google Scholar]
- Hartmann, A.; Rothballer, M.; Schmid, M. Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 2008, 312, 7–14. [Google Scholar] [CrossRef]
- Ibáñez, A.; Garrido-Chamorro, S.; Vasco-Cárdenas, M.F.; Barreiro, C. From Lab to Field: Biofertilizers in the 21st Century. Horticulturae 2023, 9, 1306. [Google Scholar] [CrossRef]
- Zhu, H.J.; Liu, J.H.; Sun, L.F.; Hu, Z.F.; Qiao, J.J. Combined alkali and acid pretreatment of spent mushroom substrate for reducing sugar and biofertilizer production. Bioresour. Technol. 2013, 136, 257–266. [Google Scholar] [CrossRef]
- Mahdi, S.S.; Hassan, G.I.; Samoon, S.A.; Rather, H.A.; Dar, S.A.; Zehra, B. Bio-fertilizers in organic agriculture. J. Phytol. Res. 2010, 2, 42–54. [Google Scholar]
- Zandi, P.; Basu, S.K. Organic farming for sustainable agriculture. In Sustainable Development and Biodiversity; Nandwani, D., Ed.; Springer: Cham, Switzerland, 2016; Volume 9, pp. 71–87. [Google Scholar]
- Badura, L. Mikroorganizmy glebowe i ich znaczenie w ekosystemach degradowanych przez człowieka. In Inżynieria Ekologiczna; Polskie Towarzystwo Inżynierii Ekologicznej: Warszawa, Poland, 2005; Volume 12, pp. 14–15. [Google Scholar]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Grzebisz, W. Podstawy Nawożenia, Nawożenie Roślin Uprawnych; Państwowe Wydawnictwo Rolnicze i Leśne: Poznań, Poland, 2008. [Google Scholar]
- Bashan, Y.; De-Bashan, L.E. Bacteria/plant growth promotions. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; Volume 1, pp. 103–115. [Google Scholar]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 49, 109–114. [Google Scholar] [CrossRef]
- Barin, M.; Asadzadeh, F.; Hosseini, M.; Hammer, E.C.; Vetukuri, R.R.; Vahedi, R. Optimization of biofertilizer formulation for phosphorus solubilizing by Pseudomonas fluorescens Ur21 via response surface methodology. Processes 2022, 10, 650. [Google Scholar] [CrossRef]
- Król, M.J. Przemiany Mikrobiologiczne Fosforu w Glebie; Monografie i Rozprawy Naukowe IUNG-PIB: Puławy, Poland, 2012. [Google Scholar]
- Kalitkiewicz, A.; Kępczyńska, E. The use of rhizobacteria in plant growth promoting proces. Biotechnologia 2008, 2, 102–114. [Google Scholar]
- Bevivino, A.; Sarrocco, S.; Dalmastri, C.; Tabacchioni, S.; Cantale, C.; Chiarini, L. Characterization of a free-living maize-rhizosphere population of Burkholderia cepacia: Effect of seed treatment on disease suppression and growth promotion of maize. FEMS Microbiol. Ecol. 1998, 27, 225–237. [Google Scholar] [CrossRef]
- Smith, R.S. Legume inoculant formulation and application. J. Microbiol. 1992, 38, 85–492. [Google Scholar] [CrossRef]
- Muresu, R.; Sulas, L.; Caredda, S. Legume—Rhizobium symbiosis: Characteristics and prospects of inoculation. Rivol. Agron. 2003, 37, 33–45. [Google Scholar]
- Xavier, I.J.; Holloway, G.; Leggett, M.; Bios, P. Development of Rhizobial Inoculant Formulations. Crop Manag. 2004, 3, 1. [Google Scholar] [CrossRef]
- Keyser, H.H.; Somasegaran, P.; Bohlool, B.B. Soil Microbial Ecology: Applications in Agricultural and Environmental Management; Metting, E.B., Ed.; Marcel Dekker: New York, NY, USA, 1993; pp. 205–226. [Google Scholar]
- Bashan, Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 1998, 16, 729–770. [Google Scholar] [CrossRef]
- Herridge, D.F. Inoculation Technology For Legumes. In Nitrogen-Fixing Leguminous Symbioses. Nitrogen Fixation: Origins, Applications, and Research Progress; Dilworth, M.J., James, E.K., Sprent, J.I., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 7, pp. 77–115. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 2013, 97, 8859–8873. [Google Scholar] [CrossRef]
- Bashan, Y.; De Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- McQuilken, M.P.; Halmer, P.; Rhodes, D.J. Formulation of Microbial Biopesticides; Burges, H.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 255–285. [Google Scholar]
- Okon, Y.; Hadar, H. Microbial inoculants as crop-yield enhancers. Crit. Rev. Biotechnol. 1987, 6, 61–85. [Google Scholar] [CrossRef]
- Schulz, T.J.; Thelen, K.D. Soybean seed inoculant and fungicidal seed treatment effects on soybean. Crop Sci. 2008, 48, 1975–1983. [Google Scholar] [CrossRef]
- Sahu, P.K.; Brahmaprakash, G.P. Formulations of biofertilizers—Approaches and advances. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 179–198. [Google Scholar] [CrossRef]
- Date, R.A. Inoculated legumes in cropping systems of the tropics. Field Crops Res. 2000, 65, 123–136. [Google Scholar] [CrossRef]
- Stephens, J.H.G.; Rask, H.M. Inoculant production and formulation. Field Crops Res. 2000, 65, 249–258. [Google Scholar] [CrossRef]
- Hernandez, A.; Weekers, F.; Mena, J.; Borroto, C.; Thonart, P. Freeze-drying of the biocontrol agent Tsukamurlla paurometabola C-924: Predicted stability of formulated powders. Ind. Biotechnol. 2006, 2, 209–212. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003; Belgium, 2019. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009 (accessed on 14 March 2025).
- Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 Relating to Fertilisers. Available online: https://eur-lex.europa.eu/eli/reg/2003/2003/oj/eng (accessed on 14 January 2025).
- Ryszko, U.; Rusek, P.; Watros, A.; Ostrowski, J. Nawozy mineralne w świetle nowej unijnej regulacji nawozowej 2019/1009. Przem. Chem. 2020, 99, 1072–1078. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving Crop Yield and Nutrient Use Efficiency via Biofertilization—A Global Meta-analysis. Front. Plant Sci. 2017, 8, 2204. [Google Scholar] [CrossRef]
- Qiu, Z.; Paungfoo-Lonhienne, C.; Ye, J.; Garcia, A.G.; Petersen, I.; Di Bella, L.; Hobbs, R.; Ibanez, M.; Heenan, M.; Wang, W.; et al. Biofertilizers can enhance nitrogen use efficiency of sugarcane. Environ Microbiol 2022, 24, 3655–3671. [Google Scholar] [CrossRef]
- Czuba, R. (Ed.) Nawożenie Mineralne Roślin Uprawnych; Police: Zakłady Chemiczne, Poland, 1996. [Google Scholar]
- Vijay, K.; Shibasini, M.; Sivasakthivelan, P.; Kavitha, T. Microbial siderophores as molecular shuttles for metal cations: Sources, sinks and application perspectives. Arch Microbiol. 2023, 205, 322. [Google Scholar] [CrossRef] [PubMed]
- Nealson, K.H.; Myers, C.R. Microbial reduction of manganese and iron: New approaches to carbon cycling. Appl. Environ. Microbiol. 1992, 58, 439–443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Odoh, C.K.; Sam, K.; Zabbey, N.; Eze, C.N.; Nwankwegu, A.S.; Laku, C.; Dumpe, B.B. Microbial consortium as biofertilizers for crops growing under the extreme habitats. In Plant Microbiomes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 381–424. [Google Scholar]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Martínez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Sienko, M.J.; Plane, R.A. Chemia; Podstawy i zastosowania WNT: Warszawa, Poland, 2002. [Google Scholar]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Rees, D.C. Nitrogenase and biological nitrogen fixation. Biochemistry 1994, 33, 389–397. [Google Scholar] [CrossRef]
- Pudełko, K.; Narożna, D.; Króliczak, J.; Kidaj, D.; Wielbo, J.; Skorupska, A.; Mądrzak, C. Czynniki Nod Jako Potencjalne Stymulatory Procesu Brodawkowania Łubinu. Zesz. Nauk. UP Wroc. Rol. CXXIII 2017, 626, 115–132. [Google Scholar]
- Kopcewicz, J.; Lewak, S. (Eds.) Fizjologia Roślin; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2012. [Google Scholar]
- Maillet, F.; Poinsot, V.; André, O.; Puech-Pagès, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formel, D.; Niebel, A.; et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 2011, 469, 58–63. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Smith, B.E.; Richards, R.L. Catalysts for nitrogen fixation. In Nitrogenases, Relevant Chemical Models and Commercial Processes; Newton, W.E., Ed.; Springer: Dordrecht, The Netherlands, 2004; Volume 1. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef]
- Szwejkowska, A. Fizjologia Roślin; Wydawnictwo Naukowe UAM: Poznań, Poland, 1998. [Google Scholar]
- Kopcewicz, J.; Lewak, S.; Gabryś, H.; Kacperska, A.; Starck, Z.; Strzałka, K.; Tretyn, A. (Eds.) Fizjologia Roślin; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2005. [Google Scholar]
- Hames, B.D.; Hooper, N.M.; Wykłady, K. Biochemia; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006. [Google Scholar]
- Prell, J.; Poole, P. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 2006, 14, 161–168. [Google Scholar] [CrossRef]
- White, J.; Prell, J.; James, E.K.; Poole, P. Nutrient sharing between symbionts. Plant Physiol. 2007, 144, 604–614. [Google Scholar] [CrossRef]
- Prell, J.; White, J.P.; Bourdes, A.; Bunnewell, S.; Bongaerts, R.J.; Poole, P.S. Legumes regulate Rhizobium bacteroid development and persistence by the supply of branched-chain amino acids. Proc. Natl. Acad. Sci. USA 2009, 106, 12477–12482. [Google Scholar] [CrossRef] [PubMed]
- Czerwiński, W. Fizjologia Roślin; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1976. [Google Scholar]
- Karunakaran, R.; Ramachandran, V.K.; Seaman, J.C.; East, A.K.; Mouhsine, B.; Mauchline, T.H.; Poole, P.S. Transcriptomic analysis of Rhizobium leguminosarum Biovar viciae in symbiosis with host plants Pisum sativum and Vicia cracca. J. Bacteriol. 2009, 191, 4002–4014. [Google Scholar] [CrossRef]
- Mancinelli, R.L. The nature of nitrogen: An overview. Life Support Biosph. Sci. 1996, 3, 17–24. [Google Scholar]
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Lavakush, S.V. Impact of plant growth promoting Rhizobacteria on crop production. Int. J. Agric. Res. 2010, 5, 954–983. [Google Scholar] [CrossRef]
- Gentili, F.; Jumpponen, A. Potential and possible uses of bacterial and fungal biofertilizers. In Handbook of Microbial Biofertilizers; Rai, M.K., Ed.; The Haworth Press, Inc.: New York, NY, USA, 2006; pp. 1–28. [Google Scholar]
- Grzebisz, W. Nawożenie Roślin Uprawnych. Nawozy i Systemy Nawożenia; Państwowe Wydawnictwo Rolnicze i Leśne: Poznań, Poland, 2009. [Google Scholar]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting Rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Giordano, M.; Raven, J.A. Nitrogen and sulfur assimilation in plants and algae. Aquat. Bot. 2014, 118, 45–61. [Google Scholar] [CrossRef]
- Witte, C.P. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr Opin Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Pinton, R.; Tomasi, N.; Zanin, L. Molecular and physiological interactions of urea and nitrate uptake in plants. Plant Signal Behav. 2016, 11, e1076603. [Google Scholar] [CrossRef]
- Goyal, R.K.; Mattoo, A.K.; Schmidt, M.A. Rhizobial-Host Interactions and Symbiotic Nitrogen Fixation in Legume Crops Toward Agriculture Sustainability. Front Microbiol. 2021, 12, 669404. [Google Scholar] [CrossRef]
- Sadowsky, M.J.; Graham, P.H. Agricultural and Environmental Applications of Nitrogen Fixing Organisms. In Highlights of Nitrogen Fixation Research; Martĺnez, E., Hernández, G., Eds.; Springer: Boston, MA, USA, 1999. [Google Scholar] [CrossRef]
- Eichhorn, E.E. Sulfonate-Sulfur Assimilation in Escherichia Coli. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2000. [Google Scholar]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Kopriva, S.; Koprivova, A. Plant adenosine 5′-phosphosulphate reductase: The past, the present, and the future. J. Exp. Bot. 2004, 55, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko, G.; Sirko, A. Sulfur metabolism and its regulation in plants. Post. Bioch. 2008, 54, 402–411. [Google Scholar]
- Or-Rashid, M.; Onodera, R.; Wadud, S. Biosynthesis of methionine from homocysteine, cystathionine and homoserine plus cysteine by mixed rumen microorganisms in vitro. Appl. Microbiol. Biotechnol. 2001, 55, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Kredich, N.M. Escherichia coli and Salmonella, 2nd ed.; Neidhardt, F.C., Curtiss, R., Ingraham, J.L., Lin, E.C.C., Low, K.B., Magasanik, B., Reznikoff, W.S., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; ASM Press: Washington, DC, USA, 1996; pp. 514–527. [Google Scholar]
- Kessler, D. Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol. Rev. 2006, 30, 825–840. [Google Scholar] [CrossRef]
- Grundy, F.J.; Henkin, T.M. The S box regulon: A new global transcription termination control system for methionine and cysteine biosynthesis genes in gram-positive bacteria. Mol. Microbiol. 1998, 30, 737–749. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Erratum to: Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2011, 10, 105–106. [Google Scholar] [CrossRef]
- Jaśkiewicz, B. Potas i fosfor kształtuje plon. Nasza Rola 2011, 3, 34–35. [Google Scholar]
- Bezak-Mazur, E.; Stoińska, R. The importance of phosphorus in the environment. Arch. Waste Manag. Environ. Prot. 2013, 15, 33–42. [Google Scholar]
- Górecki, R.J.; Grzesiuk, S. (Eds.) Fizjologia Plonowania Roślin; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Poland, 2002. [Google Scholar]
- Ros, M.B.H.; Koopmans, G.F.; van Groenigen, K.J.; Abalos, D.; Oenema, O.; Vos, H.M.J.; van Groenigen, J.W. Towards optimal use of phosphorus fertiliser. Sci. Rep. 2020, 10, 17804. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A Critical Review on Soil Chemical Processes that Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Bedassa, M. Fractionation and distribution of phosphorus in acid soils: Review. Int. J. Hortic. Food Sci. 2023, 5, 64–70. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Rodrıguez, H.; Fraga, F. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Salimpour, S.; Khavazi, K.; Nadian, H.; Besharati, H.; Miransari, M. Enhancing phosphorous availability to canola (Brassica napus L.) using P. solubilizing and sulfur oxidizing bacteria. Aust. J. Crop Sci. 2010, 4, 330. [Google Scholar]
- Jastrzębska, M.; Kostrzewska, M.K.; Makowski, P.; Treder, K.; Jastrzębski, W.P. Functional properties of granulated ash and bone-based phosphorus biofertilizers in the field assessment. Przem. Chem. 2016, 8, 1591–1594. [Google Scholar] [CrossRef]
- Mohammadi, K. Phosphorus Solubilizing Bacteria, Occurrence, Mechanisms and Their Role in Crop Production. Resour. Environ. 2012, 2, 80–85. [Google Scholar]
- Omar, S.A. The role of rock-phosphate-solubilizing fungi and vesicular–arbuscular-mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World J. Microbiol. Biotechnol. 1998, 14, 211–219. [Google Scholar] [CrossRef]
- Khan, M.S.; Ahmad, E.; Zaidi, A.; Oves, M. Bacteria in Agrobiology: Crop Productivity; Maheshwari, D., Saraf, M., Aeron, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Saeid, A.; Labuda, M.; Chojnacka, K.; Górecki, H. Zastosowanie Bacillus megaterium w solubilizacji fosforu. Przem. Chem. 2012, 91, 837–840. [Google Scholar]
- Gamalero, A.; Glick, B.R. Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–46. [Google Scholar]
- Pang, F.; Li, Q.; Solanki, M.K.; Wang, Z.; Xing, Y.X.; Dong, D.F. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 2024, 15, 1383813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vassilev, N.; Eichler-Löbermann, B.; Vassileva, M. Stress-tolerant P-solubilizing microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 851–859. [Google Scholar] [CrossRef]
- Moreno-Ramírez, L.; González-Mendoza, D.; Cecena-Duran, C.; Grimaldo-Juarez, O. Molecular identification of phosphate-solubilizing native bacteria isolated from the rhizosphere of Prosopis glandulosa in Mexicali valley. Genet. Mol. Res. 2015, 14, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Johir, M.A.; Pradhan, M.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Phosphate adsorption from wastewater using zirconium (IV) hydroxide: Kinetics, thermodynamics and membrane filtration adsorption hybrid system studies. J. Environ. Manag. 2016, 167, 167–174. [Google Scholar] [CrossRef]
- Herzel, H.; Kruger, O.; Hermann, L.; Adam, C. Sewage sludge ash—A promising secondary phosphorus source for fertilizer production. Sci. Total Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef]
- Kalmykova, Y.; Fedje, K.K. Phosphorus recovery from municipal solid waste incineration fly ash. Waste Manag. 2013, 33, 1403–1410. [Google Scholar] [CrossRef]
- Smol, M.; Kulczycka, J.; Kowalski, Z. Sewage sludge ash (SSA) from large and small incineration plants as a potential source of phosphorus—Polish case study. J. Environ. Manag. 2016, 184, 617–628. [Google Scholar] [CrossRef]
- Saeid, A.; Chojnacka, K. Innovative phosphorus bio-fertilizers. In Innovative Bio-Products for Agriculture; Chojnacka, K., Saeid, A., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2019. [Google Scholar]
- Abbas, K.; Javed, M.; Aslam, S.; Butt, F.M.; Al-Ansari, M.M.; Elshikh, M.S.; Ijaz, M.K.; Ali, H.; Aziz, M.; Mahmood, U.; et al. Co-application of potassium and thiourea for mitigating salinity stress in wheat seedlings. Sci. Rep. 2025, 15, 14689. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Sparks, D.L. Potassium dynamics in soils. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1987; Volume 6, pp. 1–63. [Google Scholar] [CrossRef]
- Grzebisz, W.; Diatta, J.; Barlog, P. Evaluation of soil and plant potassium tests for winter wheat on light soil. Potato Res. 1998, 41, 171–182. [Google Scholar] [CrossRef]
- Meena, V.S.; Bahadur, I.; Maurya, B.R.; Kumar, A.; Meena, R.K.; Meena, S.K.; Verma, J.P. Potassium-solubilizing microorganisms in agriculture: Their role in potassium nutrition of crops and its mobilization in soils. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 1–16. [Google Scholar] [CrossRef]
- Shakeel, M.; Ahmad, M.; Aslam, M.; Iqbal, J. Evaluation of plant growth promoting rhizobacteria for improving growth, yield and mineral uptake of wheat under salt-stressed conditions. J. Appl. Agric. Biotechnol. 2018, 3, 23–32. [Google Scholar]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects—A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Parmar, P.; Sindhu, S.S. Potassium solubilization by rhizosphere bacteria: Influence of nutritional and environmental conditions. J. Microbiol. Res. 2013, 3, 25–31. [Google Scholar] [CrossRef]
- Badr, M.A. Efficiency of K-feldspar combined with organic materials and silicate dissolving bacteria on tomato yield. J. Appl. Sci. Res. 2006, 2, 1191–1198. [Google Scholar]
- Sheng, X.F.; He, L.Y. Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can. J. Microbiol. 2006, 52, 66–72. [Google Scholar] [CrossRef]
- Liu, D.; Lian, B.; Dong, H. Isolation of Paenibacillus sp. and assessment of its potential for enhancing mineral weathering. Geomicrobiol. J. 2012, 29, 413–421. [Google Scholar] [CrossRef]
- Saiyad, S.A.; Jhala, Y.K.; Vyas, R.V. Comparative efficiency of five potash and silicate solubilizing bacteria and their key enzymes useful for enhancing potassium availability in cultivable soils. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 880–889. [Google Scholar]
- Liu, W.; Xu, X.; Wu, X.; Yang, Q.; Luo, Y.; Christie, P. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture. Environ. Geochem. Health 2006, 28, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kong, F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014, 82, 18–25. [Google Scholar] [CrossRef]
- Hu, X.; Chen, J.; Guo, J. Two phosphate- and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 2006, 22, 983–990. [Google Scholar] [CrossRef]
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, I.; Meena, V.S.; Kumar, S. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 161–176. [Google Scholar] [CrossRef]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Potassium-solubilizing microorganisms: Mechanism and their role in potassium solubilization and uptake. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 203–219. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M.; et al. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Zhao, F.; Sheng, X.; Huang, Z.; He, L. Isolation of mineral potassium-solubilizing bacterial strains from agricultural soils in Shandong Province. Biodivers. Sci. 2008, 16, 593–600. [Google Scholar] [CrossRef]
- Zhao, F.; Sheng, X.; Huang, Z.; He, L. Growth and mineral K solubilization of Bacillus mucilaginosus K02 in batch culture. Soil Sci. Plant Nutr. 2015, 15, 739–745. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Meena, S.K.; Meena, R.K.; Kumar, A.; Verma, J.P.; Singh, N.P. Can Bacillus species enhance nutrient availability in agricultural soils? In Bacilli and Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Jha, C.K., Aeron, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 367–395. [Google Scholar] [CrossRef]
- Ahmad, M.; Nadeem, S.M.; Naveed, M.; Zahir, Z.A. Potassium-solubilizing bacteria and their application in agriculture. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 293–313. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Aeron, A.; Kumar, A.; Kim, K.; Bajpai, V.K. Potassium solubilizing rhizobacteria (KSR): Isolation, identification, and K-release dynamics from waste mica. Ecol. Eng. 2015, 81, 340–347. [Google Scholar] [CrossRef]
- Wu, S.C.; Cao, Z.H.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, K.D. Phosphate and potassium solubilizing bacteria effect on mineral uptake, soil availability and growth of eggplant. Res. J. Agric. Biol. Sci. 2005, 1, 176–180. [Google Scholar]
- Zahedi, H. Growth-promoting effect of potassium-solubilizing microorganisms on some crop species. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 31–42. [Google Scholar] [CrossRef]
- Basak, B.B.; Biswas, D.R. Influence of potassium solubilizing microorganism (Bacillus mucilaginosus) and waste mica on potassium uptake dynamics by Sudan grass (Sorghum vulgare Pers.) grown under two Alfisols. Plant Soil 2009, 317, 235–255. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 1–331. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, K.D. Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ. 2006, 52, 130–136. [Google Scholar] [CrossRef]
- Zhang, A.M.; Zhao, G.Y.; Gao, T.G.; Wang, W.; Li, J.; Zhang, S.F.; Zhu, B.C. Solubilization of insoluble potassium and phosphate by Paenibacillus kribensis CX-7: A soil microorganism with biological control potential. Afr. J. Microbiol. Res. 2013, 7, 41–47. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and transport of sodium and potassium in plants. In Metal Ions in Life Sciences; Springer: Berlin/Heidelberg, Germany, 2016; Volume 16, pp. 291–324. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K⁺ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef]
- Ahn, S.J.; Shin, R.; Schachtman, D.P. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K⁺ uptake. Plant Physiol. 2004, 134, 1135–1145. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.; Wu, H.; Hua, S.; Liu, J.; Yuan, Y.; Xiao, H.; Deng, L.; et al. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557, 785–795. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Yuan, Y.; Liang, H.; Tian, Y. The influence of potassium-solubilizing bacteria on potassium uptake and yield in wheat. Plant Soil 2019, 447, 567–583. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Wang, B.; Wang, X.; Franks, A.E.; Teng, Y.; Li, Z.; Luo, Y. Changes in the abundance and structure of bacterial communities under long-term fertilization treatments in a peanut monocropping system. Plant Soil 2015, 395, 415–427. [Google Scholar] [CrossRef]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Enhancing grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil Environ. 2013, 59, 89–94. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Bahadur, I. Potassium solubilization by bacterial strain in waste mica. Bangladesh J. Bot. 2014, 43, 235–237. [Google Scholar] [CrossRef]
- Pathirana, B.K.W.; Padmini, Y. Evaluation of Different Carrier Substances for the Development of an Effective Pelleted Biofertilizer for Rice (Oryza sativa L.) Using Co-inoculated Bacteria and Arbuscular Mycorrhizal Fungi. Asian J. Biotechnol. Bioresour. Technol. 2020, 6, AJB2T.53857. [Google Scholar] [CrossRef]
- Nayak, S.; Kale, S. Chemical and Microbiological Analysis of Organic Manure of Nisargruna Biogas Plant and its Applications as Carrier Materials for Biofertilizers. Curr. World Environ. J. 2020, 15, 535–543. [Google Scholar] [CrossRef]
- Saliha, B.B.; Banupriya, B.; Balasubramaniam, P.; Indirani, R. Study of Soil Microbial Population and Enzyme Activities under Jasmine Cultivation as Influenced by Nutrient Sources. Curr. J. Appl. Sci. Technol. 2021, 40, 32–42. [Google Scholar] [CrossRef]
- Rahman, T.; Abdurrahim; Rintu, K.A.; Sarkar, R.; Kabir, A.; Islam, D. Hasanuzzaman Improvement of in vitro dissolution profile of poorly aqueous soluble anti-parasitic agent ivermectin using novel hydrophilic polymeric carriers. Bangladesh J. Sci. Ind. Res. 2023, 58, 209–220. [Google Scholar]
- Marschner, P. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; pp. 178–189. [Google Scholar]
- Wang, H. Directional bio-synthesis and bio-transformation technology using mixed microbial culture. Microb. Biotechnol. 2021, 15, 26–28. [Google Scholar] [CrossRef]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.-H.; Kirkham, M.; et al. Multifunctional applications of biochar beyond carbon storage. Int. Mater. Rev. 2021, 67, 150–200. [Google Scholar] [CrossRef]
- Ghanney, P.; Kugbe, J.X.; Anning, D.K. Role of Microbial Biomechanics in Composting with Special Reference to Lignocellulose Biomass Digestion. Asian J. Biotechnol. Bioresour. Technol. 2021, 7, 30–46. [Google Scholar] [CrossRef]
- Eze, V.C.; Okoronkwo, E.C.; Ngene, A.C.; Odo, E.S. Biofertilizer potentials of Rhizobium leguminosarum on two common tropical vegetable plants Talinium triangulare (waterleaf) and Telfairia occidentalis (pumpkin). Bangladesh J. Sci. Ind. Res. 2023, 58, 231–240. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Kannan, J.; Janaki, D. Liquid Biofertilizer—A Boon to Sustainable Agriculture. Biotica Res. Today 2020, 2, 915–918. [Google Scholar]
- Baumann, K.B.L.; Thoma, R.; Callbeck, C.M.; Niederdorfer, R.; Schubert, C.J.; Müller, B.; Lever, M.A.; Bürgmann, H. Trophic status and local conditions affect microbial potential for denitrification versus internal nitrogen cycling in lake sediments. arXiv 2021. [Google Scholar] [CrossRef]
- Prisa, D.; Fresco, R.; Spagnuolo, D. Microbial Biofertilisers in Plant Production and Resistance: A Review. Agriculture 2023, 13, 1666. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Cruz, C.; Babalola, O.O. Agricultural Sustainability: Microbial Biofertilizers in Rhizosphere Management. Agriculture 2021, 11, 163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figiel, S.; Rusek, P.; Ryszko, U.; Brodowska, M.S. Microbially Enhanced Biofertilizers: Technologies, Mechanisms of Action, and Agricultural Applications. Agronomy 2025, 15, 1191. https://doi.org/10.3390/agronomy15051191
Figiel S, Rusek P, Ryszko U, Brodowska MS. Microbially Enhanced Biofertilizers: Technologies, Mechanisms of Action, and Agricultural Applications. Agronomy. 2025; 15(5):1191. https://doi.org/10.3390/agronomy15051191
Chicago/Turabian StyleFigiel, Sylwia, Piotr Rusek, Urszula Ryszko, and Marzena Sylwia Brodowska. 2025. "Microbially Enhanced Biofertilizers: Technologies, Mechanisms of Action, and Agricultural Applications" Agronomy 15, no. 5: 1191. https://doi.org/10.3390/agronomy15051191
APA StyleFigiel, S., Rusek, P., Ryszko, U., & Brodowska, M. S. (2025). Microbially Enhanced Biofertilizers: Technologies, Mechanisms of Action, and Agricultural Applications. Agronomy, 15(5), 1191. https://doi.org/10.3390/agronomy15051191