Integrating Agricultural Waste Recycling with Sustainable Feed Production: Microbial and Enzymatic Dynamics During Pleurotus Cultivation on Maize Straw
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection
- One part was dried in an oven at 120 °C for 30 min, then at 65 °C for 48 h, and ground for nutritional and physicochemical analysis.
- Another part was stored at 4 °C for physiological enzyme activity testing.
- The third part was frozen at −80 °C for DNA extraction of the ITS1 amplicon.
2.3. Nutritional and Physicochemical Analysis of Cultivation Substrate
2.3.1. Nutritional Analysis
2.3.2. Measurement of Enzyme Activity
2.4. Sensory Quality and Yield Analysis of Pleurotus
2.5. DNA Extraction and Illumina MiSeq Sequencing of 16S rRNA and ITS1 Amplicons
2.6. Statistical Analysis
3. Results
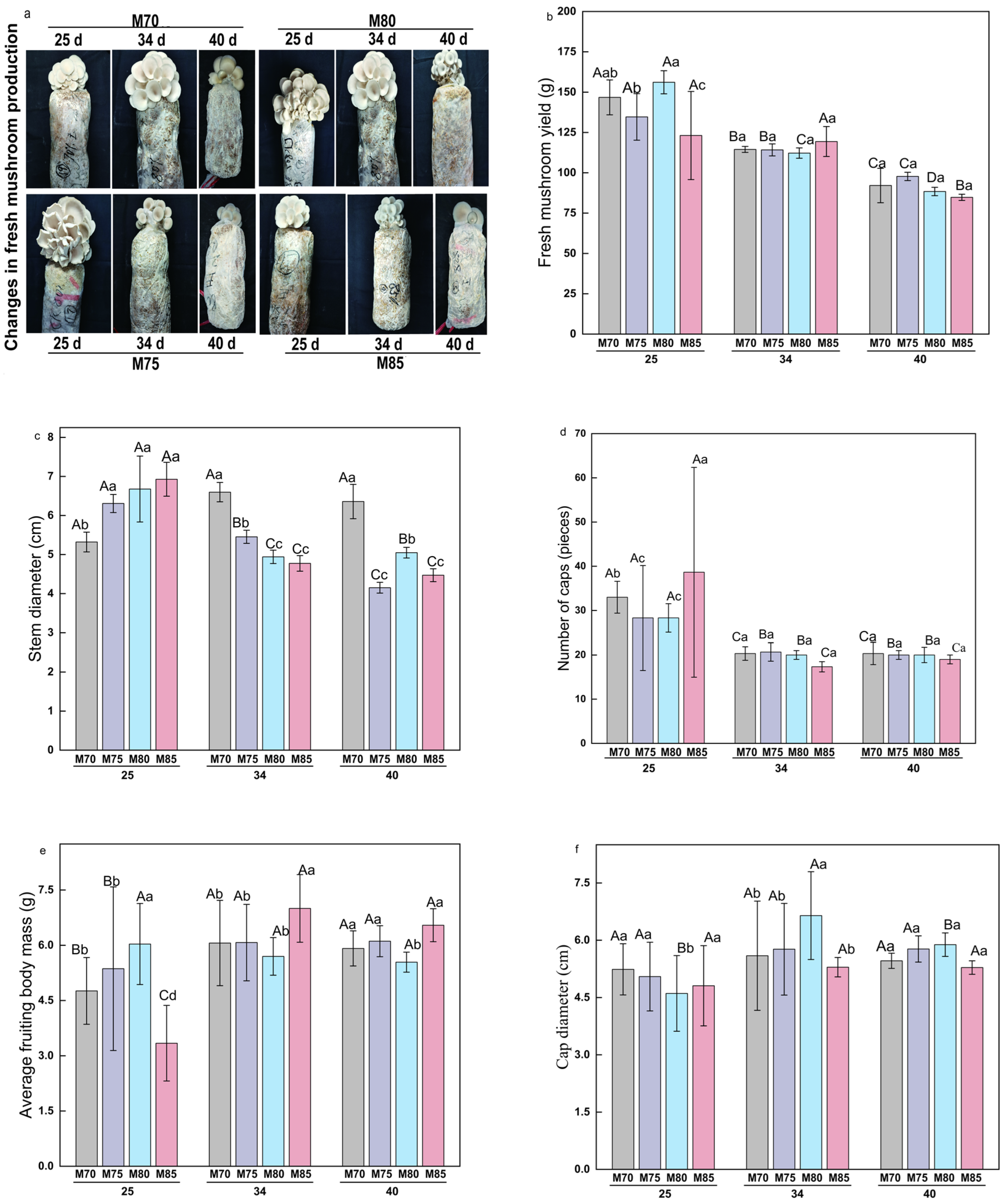
3.1. Yield and Sensory Quality Changes in Oyster Mushrooms After Fruiting
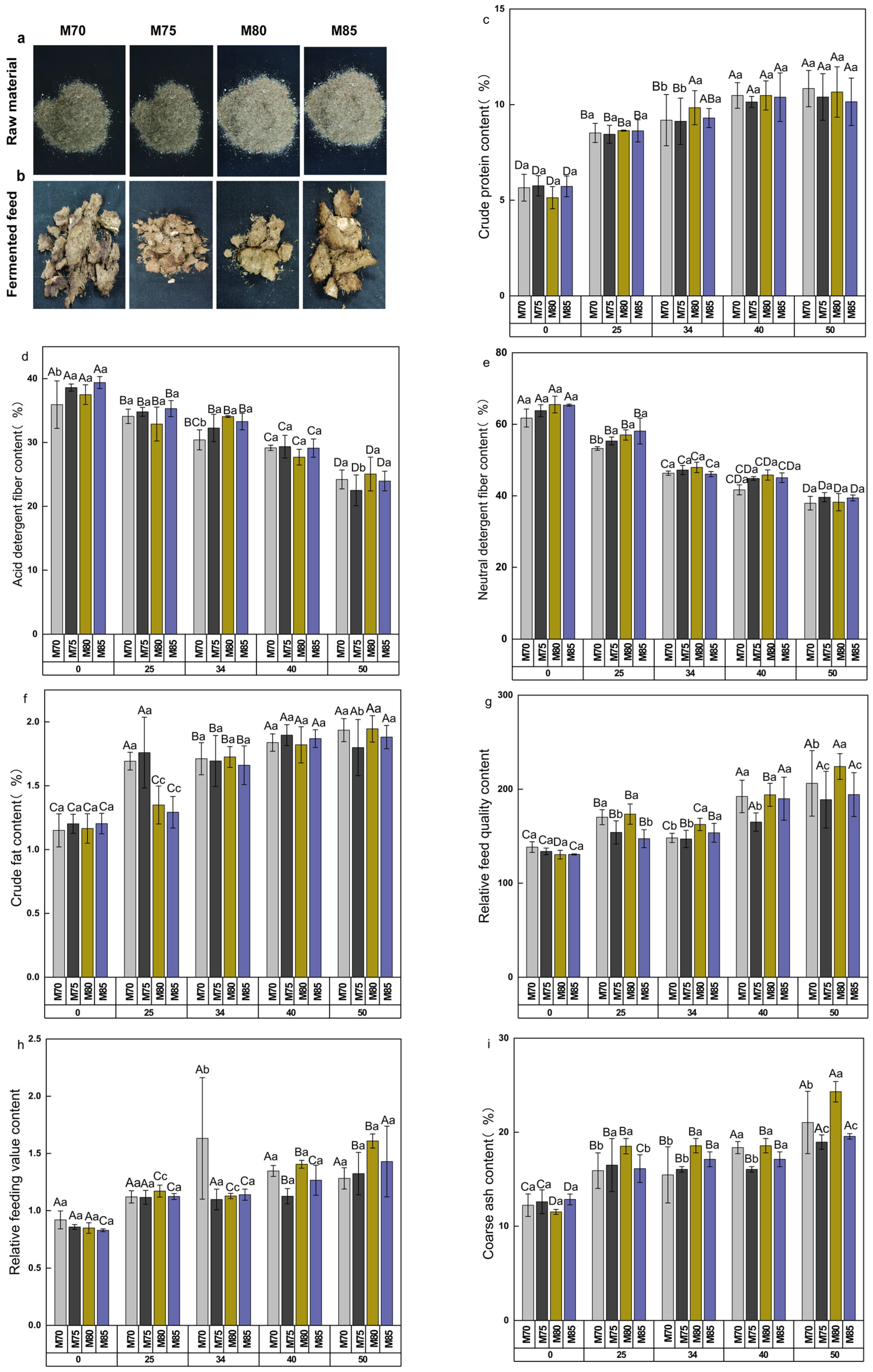
3.2. Sensory Evaluation, Nutritional Quality, and Feeding Value Assessment of Fermented Feed
3.2.1. Sensory Evaluation of Feed
3.2.2. Evaluation of Nutritional Quality of Feed
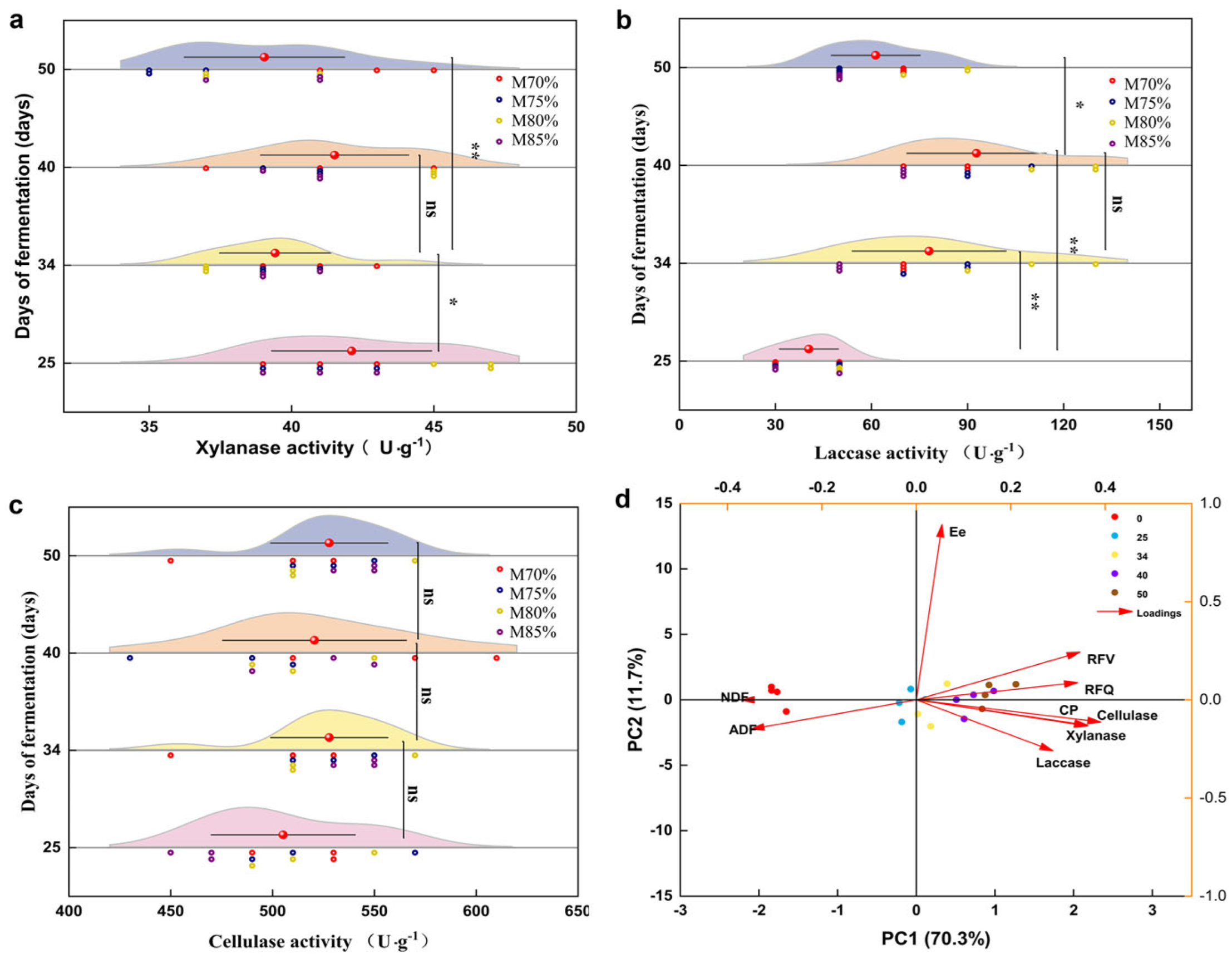
3.3. Changes in Enzyme Activity and Correlation of Nutritional Indicators in Cultivation Material over Fermentation Days
3.3.1. Changes in Enzyme Activity and Principal Component Analysis
3.3.2. Correlation Between Nutritional Parameters and Enzyme Activity
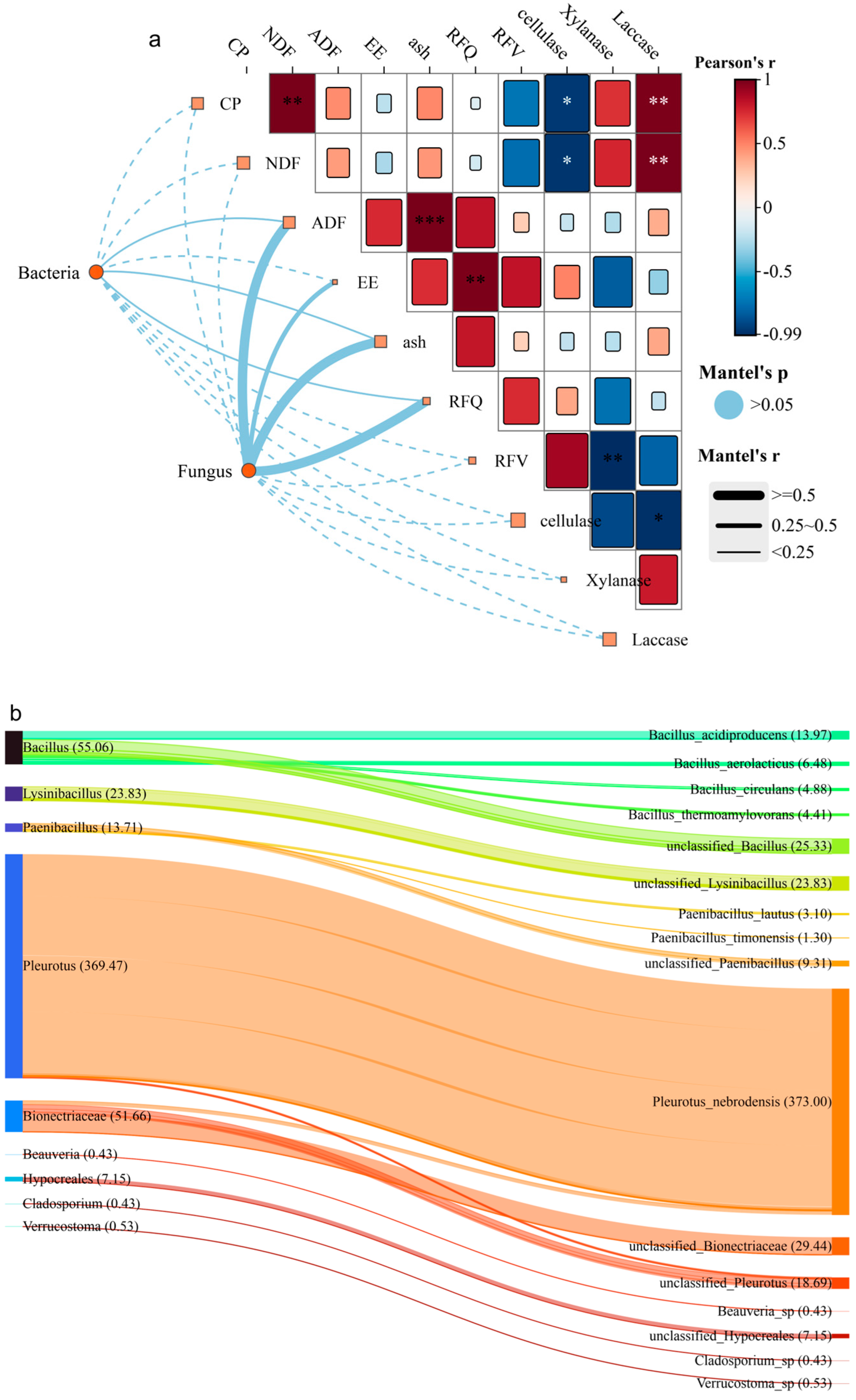
3.4. Changes in Microbial Community Structure and Functional Metabolic Pathway Predictions
3.4.1. Relationships Among Fungi, Bacteria, Feed Nutrition, Enzyme Activity, and Changes in Microbial Community Structure
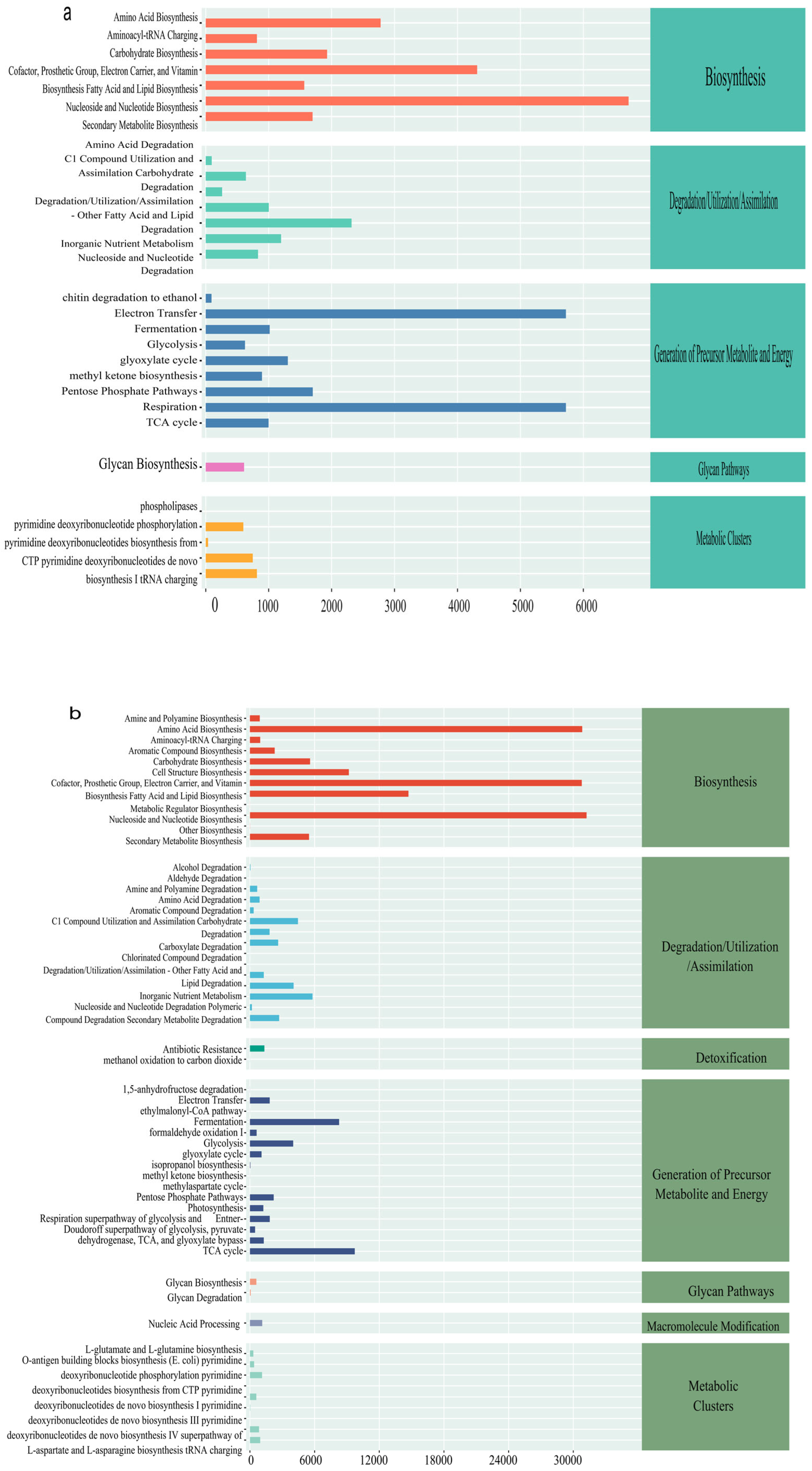
3.4.2. Prediction of Functional Metabolic Pathways in Bacteria and Fungi
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Bureau of Statistics. Announcement of Grain Production Data for 2023 [EB/OL]. 11 December 2023. Available online: https://www.gov.cn/lianbo/bumen/202312/content_6919545 (accessed on 5 October 2024).
- Qian, B.; Shao, C.; Yang, F. Spatial Suitability Evaluation of the Conversion and Utilization of Crop Straw Resources in China. Environ. Impact Assess. Rev. 2024, 105, 107438. [Google Scholar] [CrossRef]
- Mu, G.; Xu, L.; Zhang, J. Study of the Utilization of Main Crop Straw Resources in Southern China and Its Potential as a Replacement for Chemical Fertilizers. Front. Plant Sci. 2024, 14, 1172689. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, Y.; Gao, C. The Assessment and Utilization of Straw Resources in China. Agric. Sci. China 2010, 9, 1807–1815. [Google Scholar] [CrossRef]
- Lin, B.-J.; Cheng, J.; Duan, H.-X.; Liu, W.-X.; Dang, Y.P.; Zhao, X.; Zhang, H.-L. Optimizing Straw and Nitrogen Fertilizer Resources for Low-Carbon Sustainable Agriculture. Resour. Conserv. Recycl. 2024, 209, 107743. [Google Scholar] [CrossRef]
- Sheikh, G.G.; Ganai, A.M.; Reshi, P.A.; Bilal, S.; Mir, S.; Masood, D. Improved Paddy Straw as Ruminant Feed: A Review. Agric. Rev. 2018, 39, 137–143. [Google Scholar] [CrossRef]
- Børsting, C.; Olijhoek, D.; Hellwing, A.; Moyes, K.; Østergaard, S.; Weisbjerg, M.; Lund, P.; Larsen, M.; Mogensen, L.; Raun, B.; et al. Replacing Silage with Large Amounts of Concentrate and Straw Affects Milk Production, Economics, and Climate Differently in Holstein and Jersey Cows. Livest. Sci. 2023, 275, 105293. [Google Scholar] [CrossRef]
- Sheng, P.; Bai, B.; Liu, M.; Ma, W.; Liu, J.; Song, C.; Du, S.; Ge, G.; Jia, Y.; Wang, Z. Effects of Different Additives and Ratios on Broom Sorghum Straw Silage Characteristics and Bacterial Communities. Microorganisms 2024, 12, 2062. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Gao, L. New Strategy for the Biosynthesis of Alternative Feed Protein: Single-Cell Protein Production from Straw-Based Biomass. GCB Bioenergy 2024, 16, e13120. [Google Scholar] [CrossRef]
- Murillo, W.; Suárez, H. Evaluation of Nutritional Values of Wild Mushrooms and Spent Substrate of Lentinus crinitus (L.) Fr. Heliyon 2020, 6, e03502. [Google Scholar] [CrossRef]
- Kalač, P. A Review of Chemical Composition and Nutritional Value of Wild-Growing and Cultivated Mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef]
- Zhao, Y.; Shakeel, U.; Rehman, M.S.U.; Li, H.; Xu, X.; Xu, J. Lignin-Carbohydrate Complexes (LCCs) and Its Role in Biorefinery. J. Clean. Prod. 2020, 253, 120076. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic Biomass: Acid and Alkaline Pretreatments and Their Effects on Biomass Recalcitrance–Conventional Processing and Recent Advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Rahikainen, J.; Leskinen, T.; Pihlajaniemi, V.; Mattinen, M.-L.; Lange, H.; Crestini, C.; Österberg, M. Structural Changes of Lignin in Biorefinery Pretreatments and Consequences to Enzyme-Lignin Interactions. Nord. Pulp Pap. Res. J. 2017, 32, 550–571. [Google Scholar] [CrossRef]
- Nlandu, H.; Belkacemi, K.; Chorfa, N.; Elkoun, S.; Robert, M.; Hamoudi, S. Laccase-Mediated Grafting of Phenolic Compounds onto Lignocellulosic Flax Nanofibers. J. Nat. Fibers 2022, 19, 222–231. [Google Scholar] [CrossRef]
- Steinmetz, V.; Villain-Gambier, M.; Klem, A.; Ziegler, I.; Dumarcay, S.; Trebouet, D. In-Situ Extraction of Depolymerization Products by Membrane Filtration Against Lignin Condensation. Bioresour. Technol. 2020, 311, 123530. [Google Scholar] [CrossRef]
- Sharma, S.; Kumawat, K.C.; Kaur, S. Potential of Indigenous Ligno-Cellulolytic Microbial Consortium to Accelerate Degradation of Heterogeneous Crop Residues. Environ. Sci. Pollut. Res. 2022, 29, 88331–88346. [Google Scholar] [CrossRef]
- Kazige, O.K.; Chuma, G.B.; Lusambya, A.S.; Mondo, J.M.; Balezi, A.Z.; Mapatano, S.; Mushagalusa, G.N. Valorizing Staple Crop Residues through Mushroom Production to Improve Food Security in Eastern Democratic Republic of Congo. J. Agric. Food Res. 2022, 8, 100285. [Google Scholar] [CrossRef]
- Leadbeater, D.R.; Oates, N.C.; Bennett, J.P.; Li, Y.; Dowle, A.A.; Taylor, J.D.; Alponti, J.S.; Setchfield, A.T.; Alessi, A.M.; Helgason, T.; et al. Mechanistic Strategies of Microbial Communities Regulating Lignocellulose Deconstruction in a UK Salt Marsh. Microbiome 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Thomas, A.T. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Franco, M.; Stefański, T.; Jalava, T.; Lehto, M.; Kahala, M.; Järvenpää, E.; Mäntysaari, P.; Rinne, M. Effect of potato by-product on production responses of dairy cows and total mixed ration stability. Dairy 2021, 2, 218–230. [Google Scholar] [CrossRef]
- Jia, Z.; Zhou, J.; Yang, M.; Wang, M.; Li, L.; Fan, X. Preparation and evaluation of certified reference materials for crude protein, crude fat, and crude ash in feed. Microchem. J. 2023, 191, 108854. [Google Scholar] [CrossRef]
- Prinz, A.; Hönig, J.; Schüttmann, I.; Zorn, H.; Zeiner, T. Separation and Purification of Laccases from Two Different Fungi Using Aqueous Two-Phase Extraction. Process Biochem. 2014, 49, 335–346. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bajaj, P.; Mahajan, R. Cellulase and Xylanase Synergism in Industrial Biotechnology. Appl. Microbiol. Biotechnol. 2019, 103, 8711–8724. [Google Scholar] [CrossRef]
- Huang, L.; Sun, N.; Ban, L.; Wang, Y.; Yang, H. Ability of Different Edible Fungi to Degrade Crop Straw. AMB Express 2019, 9, 4. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical Composition and Nutritional Value of the Most Widely Appreciated Cultivated Mushrooms: An Inter-Species Comparative Study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef]
- Buswell, J.A.; Cai, Y.J.; Chang, S.T.; Peberdy, J.F.; Fu, S.Y.; Yu, H.-S. Lignocellulolytic Enzyme Profiles of Edible Mushroom Fungi. World J. Microbiol. Biotechnol. 1996, 12, 537–542. [Google Scholar] [CrossRef]
- Mears, L.; Stocks, S.M.; Sin, G.; Gernaey, K.V. A Review of Control Strategies for Manipulating the Feed Rate in Fed-Batch Fermentation Processes. J. Biotechnol. 2017, 245, 34–46. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, P.; Leng, D.; Shang, L.; Zhang, C.; Wu, Z.; Wang, Z. Functional Roles of LaeA-Like Genes in Fungal Growth, Cellulase Activity, and Secondary Metabolism in Pleurotus ostreatus. J. Fungi 2022, 8, 902. [Google Scholar] [CrossRef]
- Hazelgrove, L.; Moody, S.C. Successful Cultivation of Edible Fungi on Textile Waste Offers a New Avenue for Bioremediation and Potential Food Production. Sci. Rep. 2024, 14, 11510. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, G.; Ezemaduka, A.N.; Luo, N.; Yu, H.; Wang, M. The Yields and Quality of Golden Oyster Mushroom Cultivated on Common Reed Substrates. J. Food Compos. Anal. 2023, 121, 105331. [Google Scholar] [CrossRef]
- Lin, H.; Li, P.; Ma, L.; Lai, S.; Sun, S.; Hu, K.; Zhang, L. Analysis and Modification of Central Carbon Metabolism in Hypsizygus marmoreus for Improving Mycelial Growth Performance and Fruiting Body Yield. Front. Microbiol. 2023, 14, 1233512. [Google Scholar] [CrossRef]
- Chen, S.; Ma, D.; Ge, W.; Buswell, J.A. Induction of Laccase Activity in the Edible Straw Mushroom, Volvariella volvacea. FEMS Microbiol. Lett. 2003, 218, 143–148. [Google Scholar] [CrossRef]
- Bao, X.; Feng, H.; Guo, G.; Huo, W.; Li, Q.; Xu, Q.; Liu, Q.; Wang, C.; Chen, L. Effects of Laccase and Lactic Acid Bacteria on the Fermentation Quality, Nutrient Composition, Enzymatic Hydrolysis, and Bacterial Community of Alfalfa Silage. Front. Microbiol. 2022, 13, 1035942. [Google Scholar] [CrossRef] [PubMed]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Cheng, J.; Tan, Q.; Pan, Y. Effect of Nutritional Parameters on Laccase Production by the Culinary and Medicinal Mushroom, Grifola frondosa. World J. Microbiol. Biotechnol. 2006, 22, 799–806. [Google Scholar] [CrossRef]
- Qin, P.; Li, T.; Liu, C.; Liang, Y.; Sun, H.; Chai, Y.; Yang, T.; Gong, X.; Wu, Z. Extraction and Utilization of Active Substances from Edible Fungi Substrate and Residue: A Review. Food Chem. 2023, 398, 133872. [Google Scholar] [CrossRef]
- Strong, P.J.; Self, R.; Allikian, K.; Szewczyk, E.; Speight, R.; O’hara, I.; Harrison, M.D. Filamentous Fungi for Future Functional Food and Feed. Curr. Opin. Biotechnol. 2022, 76, 102729. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; da Silva, B.P.; Castoldi, R.; Kato, C.G.; de Sá-Nakanishi, A.B.; Peralta, R.A.; de Souza, C.G.M.; Bracht, A. Spent Mushroom Substrate of Pleurotus pulmonarius: A Source of Easily Hydrolyzable Lignocellulose. Folia Microbiol. 2016, 61, 439–448. [Google Scholar] [CrossRef]
- Gonani, Z.; Riahi, H.; Sharifi, K. Impact of Using Leached Spent Mushroom Compost as a Partial Growing Media for Horticultural Plants. J. Plant Nutr. 2011, 34, 337–344. [Google Scholar] [CrossRef]
- Wood, P.L. Metabolic and Lipid Biomarkers for Pathogenic Algae, Fungi, Cyanobacteria, Mycobacteria, Gram-Positive Bacteria, and Gram-Negative Bacteria. Metabolites 2024, 14, 378. [Google Scholar] [CrossRef]
- Patil, A.; Sharma, Y.; Khandelwal, V.; Rajamohan, N.; Arya, M. Biochemical, Molecular Characteristics, and Bioremediation Properties of Mn2+-Resistant Thermophilic Bacillus Strains. Waste Biomass Valorization 2024, 16, 175–190. [Google Scholar] [CrossRef]
- Lu, M.L.; Yuan, G.H.; Rehemujiang, H.; Li, C.-C.; Hu, L.-H.; Duan, P.-P.; Zhang, L.-D.; Diao, Q.-Y.; Deng, K.-D.; Xu, G.-S. Effects of Spent Substrate of Oyster Mushroom (Pleurotus ostreatus) on Ruminal Fermentation, Microbial Community and Growth Performance in Hu Sheep. Front. Microbiol. 2024, 15, 1425218. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.; Fru-Nji, F.; Steinert, R.; Kluenter, A.-M.; Verlhac, V. Gastrointestinal Functionality in Animal Nutrition and Health: New Opportunities for Sustainable Animal Production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Chu, Z.; Hu, Z.; Luo, Y.; Zhou, Y.; Yang, F.; Luo, F. Targeting Gut-Liver Axis by Dietary Lignans Ameliorate Obesity: Evidences and Mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 65, 243–264. [Google Scholar] [CrossRef]
- Jiménez, M.; González, A.; Martínez, M.; Martínez, A.; Dale, B. Screening of Yeasts Isolated from Decayed Wood for Lignocellulose-Degrading Enzyme Activities. Mycol. Res. 1991, 95, 1299–1302. [Google Scholar] [CrossRef]
- Makusheva, Y.; Goncharova, E.; Bets, V.; Korel, A.; Arzhanova, E.; Litvinova, E. Restoration of Lactobacillus johnsonii and Enterococcus faecalis Caused the Elimination of Tritrichomonas sp. in a Model of Antibiotic-Induced Dysbiosis. Int. J. Mol. Sci. 2024, 25, 5090. [Google Scholar] [CrossRef]
- Dubos, R.J. Studies on a Bactericidal Agent Extracted from a Soil Bacillus: I. Preparation of the Agent. Its Activity In Vitro. J. Exp. Med. 1939, 70, 1. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides Utilization for Dietary Polysaccharides and Their Beneficial Effects on Gut Health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Bailén, M.; Díaz-Castellanos, I.; Azami-Conesa, I.; Fernández, S.A.; Martínez-Díaz, R.A.; Navarro-Rocha, J.; Gómez-Muñoz, M.T.; González-Coloma, A. Anti-Trichomonas gallinae Activity of Essential Oils and Main Compounds from Lamiaceae and Asteraceae Plants. Front. Vet. Sci. 2022, 9, 981763. [Google Scholar] [CrossRef] [PubMed]

| Charge Mixture% | Maize Straw | Wheat Bran | Seedcake | Lime | Gyp |
|---|---|---|---|---|---|
| M70% | 70 | 22 | 5 | 2 | 1 |
| M75% | 75 | 17 | 5 | 2 | 1 |
| M80% | 80 | 12 | 5 | 2 | 1 |
| M85% | 85 | 7 | 5 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Lin, G.; Wang, S.; Wu, T.; Dan, Z.; Yang, J.; Lv, M.; Zhao, Y. Integrating Agricultural Waste Recycling with Sustainable Feed Production: Microbial and Enzymatic Dynamics During Pleurotus Cultivation on Maize Straw. Agronomy 2025, 15, 1171. https://doi.org/10.3390/agronomy15051171
Yang H, Lin G, Wang S, Wu T, Dan Z, Yang J, Lv M, Zhao Y. Integrating Agricultural Waste Recycling with Sustainable Feed Production: Microbial and Enzymatic Dynamics During Pleurotus Cultivation on Maize Straw. Agronomy. 2025; 15(5):1171. https://doi.org/10.3390/agronomy15051171
Chicago/Turabian StyleYang, Hang, Gang Lin, Shitao Wang, Tao Wu, Zhiwangjia Dan, Junjuan Yang, Min Lv, and Yajiao Zhao. 2025. "Integrating Agricultural Waste Recycling with Sustainable Feed Production: Microbial and Enzymatic Dynamics During Pleurotus Cultivation on Maize Straw" Agronomy 15, no. 5: 1171. https://doi.org/10.3390/agronomy15051171
APA StyleYang, H., Lin, G., Wang, S., Wu, T., Dan, Z., Yang, J., Lv, M., & Zhao, Y. (2025). Integrating Agricultural Waste Recycling with Sustainable Feed Production: Microbial and Enzymatic Dynamics During Pleurotus Cultivation on Maize Straw. Agronomy, 15(5), 1171. https://doi.org/10.3390/agronomy15051171




