Abstract
Echinochloa crus-galli (L.) P. Beauv. (EC), E. crus-galli var. mitis (Pursh) Petermann (ECM), and E. glabrescens Munro ex Hook.f. (EG) are all troublesome weeds and frequently managed as one species on rice (Oryza sativa) fields. To examine inter- and intra-specific differences in seed germination responses to drought stresses, we conducted seed germination experiments with 57 EC, 112 ECM, and 92 EG populations. In all drought stress treatments, EC exhibited higher and faster germination than ECM and EG. Under 0 MPa, seed germinations of all populations initiated on 3 DAT (day after treatment). Accumulative seed germination percentages of EC, ECM, and EG under −0.1 MPa did not show significant differences with the same species treated with 0 MPa, while significantly decreased with the osmotic potential treated decreasing to −0.4 MPa or lower. OR50 values (the osmotic potential at which 50% germination occurs) for EC, ECM, and EG were −0.55 MPa, −0.49 MPa, and −0.45 MPa, respectively. Intra-specific variation within all three species increased as osmotic potential decreased from −0.1 MPa to −0.8 MPa. Moreover, seed germination was significantly correlated with 1000-seed weight and latitudes of population-collected locations. In four treatments, seeds produced by Echinochloa weeds growing in transplanted rice fields exhibited significantly higher germination percentages than those from direct-seeded rice fields.
1. Introduction
Weed management is a major challenge in rice cultivation worldwide. With the increasing seriousness of herbicide resistance, integrative weed management combining various chemical and non-chemical control strategies is increasingly important and practical for rice growers [1]. Considering that a majority of weed seedlings grow from seeds, knowledge on seed germination biology of troublesome weed species is the basis for effective integrative weed management strategies [2,3,4]. As one of the most traditional researching agendas in weed science, many references have reported seed germination biology of a great number of weed species. However, significant intra-species variation among populations has been observed in different weed species [5,6,7], and knowledge on seed germination biology of a certain weed species has to be derived from systematical studies with many populations collected from different areas [8]; therefore, integrated weed management strategies targeting specific troublesome weed species have to be raised from the seed germination biology of local representative multiple populations.
Weed infestations cause over three million tons of crop losses annually in China, with Echinochloa as a major contributor [9]. Rice yield loss from different Echinochloa species ranged from 10.6% to 46.5% [9]. Echinochloa crus-galli (L.) P. Beauv. (EC), E. crus-galli var. mitis (Pursh) Petermann (ECM), and E. glabrescens Munro ex Hook.f. (EG) are all serious rice weeds in China, which are usually treated as a single species in weed management practices. Our previous studies found differences in distribution [10] and seed germination responding to temperature regimes among EC, ECM, and EG [8]. By comparing 327 populations of EC, ECM, and EG collected from Jiangsu Province, China, seeds of EC were found to be significantly heavier than ECM and EG, and were germinated with significantly higher percentages under all temperature regimes tested. Moreover, intra-specific variations in seed germination responding to different temperature regimes were also testified in EC, ECM, and EG. Therefore, it is important to reveal inter- and intra-specific differences in seed germination responding to key environmental factors of the three Echinochloa species.
Osmotic potential is a vital component of the water potential of a solution, which determines the direction and magnitude of water movement [3]. The more concentrated the solution, the lower its osmotic potential and the greater its ability to draw water into the solution. Thus, osmotic potential treatments were frequently used to test the seed germination biology of weeds responding to drought stress [11]. In eastern China, arable lands are usually used in annual double crop systems; and farmers usually plant rice in June, which is the hottest period in the year [12]. Drought stress is one of the biggest challenges to rice growers here, due to drought and hot weather and the inconvenience of irrigation. Some farmers sow rice seeds and wait for rainy weather or unified irrigation, for several days [12]. Under these field conditions, soil water potential could be very low, under which some weeds might still adapt to the drought stress and germinate, and quickly grow to be tolerant of herbicides after rain or irrigation. Moreover, for the transplanting of rice fields, some farmers may use minimal tillage and then plant rice; however, many weed seedlings which emerged before rice planting may survive and quickly grow. Therefore, knowledge on seed germination responses to drought stress could be very important for predicting the seedling emergence patterns of troublesome weed species, and deciding on time windows to apply pre-emergence rice herbicides [4]. Inter- and intra-species variations were frequently observed in seed germination responses to different osmotic potentials in weeds. For example, the average OR50 (the osmotic potential at which the seed germination percentage reaches 50%) values of 242 Leptochloa chinensis populations ranged from −0.47 MPa to −0.07 MPa, with an average of −0.28 MPa [2]. We hypothesize on inter- and intra-specific variations in seed germination biological responses to drought stresses. To test the hypothesis, we tested seed germination under different osmotic potentials with 57 EC, 112 ECM, and 92 EG populations collected from rice fields in Jiangsu province, China, in 2022.
2. Materials and Methods
2.1. Seed Materials
Seeds of 261 Echinochloa populations collected from 210 rice fields in Jiangsu province, China, were used in this study (Figure 1), including 57 EC, 112 ECM, and 92 EG populations. Sampling covered all rice-growing counties in Jiangsu Province, with a minimum interval of >5 km between adjacent populations. Panicles containing mature seeds were randomly collected from over 100 individuals of each population with a pollen bag (100 mesh, 30 cm × 45 cm), and mature seeds were collected by hand. The rice planting method (direct-seeding or transplanting) and location of each population-collection field were also recorded. The collecting locations of these populations ranged from 117.85° to 121.62° in longitude, in a range of 3.77°, and from 31.28° to 34.4° in latitude, in a range of 3.18°. The collected seeds were stored in kraft paper envelopes at room temperature. EC, ECM, and EG are very similar in morphology, which could be identified by spikelets and inflorescences. Spikelets of EC are 3 to 4 mm in length, and the lower lemma extends into an awn to 3 cm. Spikelets of ECM are about 3 mm in length and awnless, or with awns shorter than 5 mm, while inflorescences of ECM are usually branched. The lower lemma of EG is convex, coriaceous, and shining, while the lower lemmas of EC and ECM are flat and herbaceous [8,10,13]. The 1000-seed weight of each population was determined by weighing 5 replicates of 100 mature seeds.
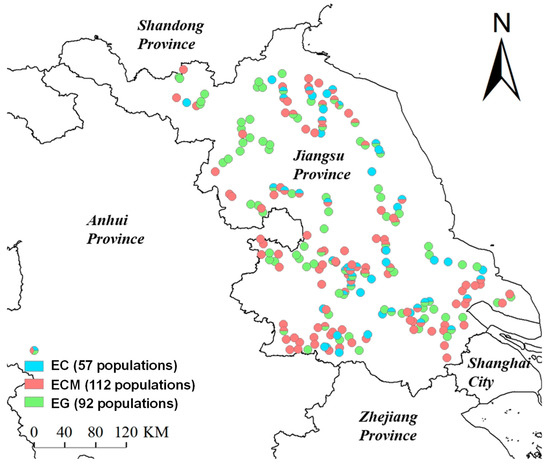
Figure 1.
Collection sites of Echinochloa crus galli (L.) P. Beauv. (EC), E. crus-galli var. mitis (Pursh) Petermann (ECM), and E. glabrescens Munro ex Hook.f. (EG) populations in rice fields from Jiangsu province, China.
2.2. Germination Experiments
All of 261 Echinochloa populations were tested in 2023. In 2024, a repeated experiment was conducted with 76 populations, including 24 EC, 26 ECM, and 26 EG populations. Polyethylene glycol 6000 (PEG-6000) was used to simulate drought stress due to its inability to penetrate plant cell membranes and walls [14]. PEG-6000 solutions with osmotic potential of −0.1, −0.4, and −0.6 MPa were used to simulate various levels of drought stress, with sterile water serving as the control (CK), and −0.8 MPa treatment was conducted with 81 populations in 2023, including 24 EC, 33 ECM and 24 EG populations to verify the germination limit. For each Petri dish, 50 undamaged seeds were evenly placed in 9 cm diameter Petri dishes with two layers of filter paper moistened with 6 mL Polyethylene glycol solution. The dishes were sealed with parafilm to prevent evaporation and placed in a growth chamber with a constant temperature of 30/20 °C (day/night, light 12 h/dark 12 h, 12,000 lux). Each treatment replicated three Petri dishes. Percentage germination of each Petri dish was determined daily for 9 d, and the final germination percentage was recorded at 21 days. Seeds were considered germinated once the coleoptile or radicle had appeared.
2.3. Data Analysis
To analyze variations among populations across different indices, the coefficient of variation (CV) was calculated [15]. Data were analyzed using one-way analysis of variance (ANOVA) in SPSS (version 16.0, IBM, Armonk, NY, USA). After log transformation, data were checked for constant variance and normality. Non-transformed means are presented alongside statistical interpretation based on transformed data. Treatment means were separated using the LSD test at p < 0.05 to distinguish the difference in germination percentage/germination index among species and treatment. The germination index (GI), representing the germination ability of each population, was calculated as follows:
where GT is the number of seeds germinated per day, and DT is the corresponding germination day. Independent sample t-tests in SPSS 16.0 were used to compare indices determined between populations collected direct-seeded and transplanted rice fields.
GI = ∑ (GT/DT)
A three-parameter logistic function was fitted to model the response of germination percentage to osmotic potential treatments using the ‘drc’ add-on package in R 3.1.3:
where Y represents the cumulative germination percentage; x is the applied osmotic potential; a is the upper limit; d is the lower limit; e is the osmotic potential at which the germination percentage reaches 50% (OR50). To analyze the influences of the treated osmotic potential, Echinochloa species, and the rice planting method used in seed collecting sites on the germination percentage and GI, a generalized linear model (GLM) univariate analysis in SPSS 16.0 was applied. To further analyze the correlation between the latitude of population collection sites, 1000-seed weight, seed germination percentage, and the germination index (GI) of three Echinochloa species under different osmotic potential treatment, Pearson analysis was performed with SPSS 16.0. Data on 1000-seed weight for each population were cited from our previous study [8].
Y = (a − d)/[1 + (x/e)b]
3. Results
3.1. Seed Germination Rhythm
Germination was inhibited as osmotic potential decreased. Under 0 MPa, all 261 Echinochloa populations began germinating on the third day (Figure 2A,E), with most populations germinating between 3 and 6 days after treatment (DAT) (Figure 2A). The average cumulative germination percentages of EC, ECM, and EG were 61.8%, 38.6%, and 34.5% at 3 DAT; 82.9%, 65.2%, and 62.1% at 4 DAT; and 89.1%, 78.0%, and 78.6% at 9 DAT, respectively. A total of 90% of the 261 populations initiated germination on the third day, with most populations germinating between 3 and 7 DAT (Figure 2B,F). The cumulative germination percentages of EC, ECM, and EG were 14.7%, 9.4%, and 8.3%, respectively. At −0.4 MPa, 42% of the populations began germinating on the fourth day, with mean germination percentages of 50.2% (EC), 35.5% (ECM), and 33.9% (EG) at 9 DAT in 2023 (Figure 2C). The mean germination percentage of EC was significantly higher than that of ECM and EG at all-time points (Figure 2C,G). At −0.6 MPa, four populations germinated on the fourth day, while 51% of the populations began germinating on the fifth day across all three Echinochloa species (Figure 2D,H). Germination was significantly inhibited, with cumulative germination percentages of 29.7%, 25.8%, and 18.3% at 9 DAT for EC, ECM, and EG in 2023, respectively.
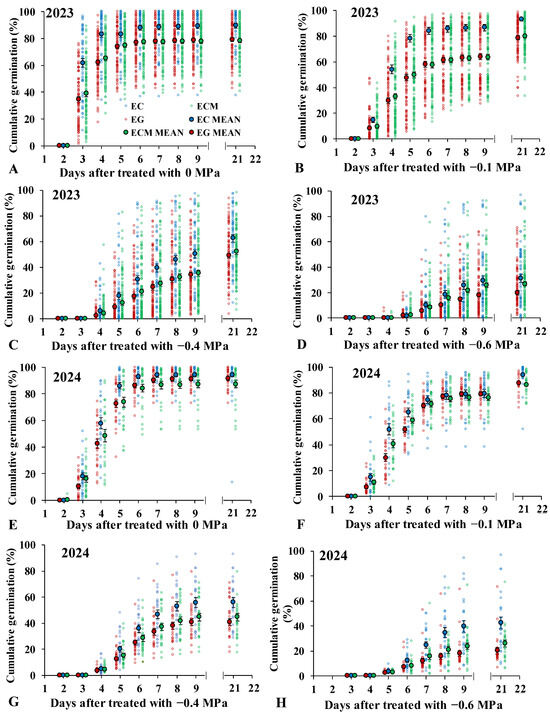
Figure 2.
Cumulative germination percentage of Echinochloa crus-galli (L.) P.Beauv. (EC), E. crus-galli var. mitis (Pursh) Petermann (ECM), and E. glabrescens Munro ex Hook.f. (EG) after treatment under different osmotic potentials at different days after treatment.
3.2. Seed Germination Percentage
At 21 DAT, the average germination percentages between 0 MPa and −0.1 MPa all did not show significant differences among the three Echinochloa species (Figure 3A,B), which all decreased significantly (p < 0.05) in treatments with −0.4 and −0.6 MPa (Figure 3C,D). The CVs among the three Echinochloa species first decreased (from 0 MPa to −0.1 MPa) and then increased (from −0.1 MPa to −0.6 MPa; Table S1). In experiments conducted in 2023 on treatments with osmotic potential from 0 to −0.4 MPa, the average germination percentage of 57 EC populations was significantly higher than that of 112 ECM populations and 92 EG populations, with average values of 89.6%, 79.2% and 78.4% at 0 MPa; 93.2%, 78.8% and 79.9% at −0.1 MPa; and 62.4%, 49.0% and 51.9% at −0.4 MPa, respectively (Figure 3A–C). At −0.6 MPa, the average germination percentage of EG (24.9%) was significantly lower than EC (36.5%) and ECM (31.2%), and the CVs value of 0.69, 0.61 and 0.66, respectively (Figure 3D; Table S1). At −0.8 MPa, the average germination percentage of EC, ECM, and EG were 11.9%, 10.3%, and 5.6%, respectively, with 50%, 55%, and 83% of populations showing germination rates below 10% (Figure 3E). In 2024 treatment, no significant differences were observed in the average germination percentage among the three Echinochloa species at 0 MPa (Figure 3F). From −0.1 MPa to −0.6 MPa, germination trends were consistent with those in 2023, with EC exhibiting a significantly higher germination percentage than ECM and EG (Figure 3G–I). Pooling data from 2023 and 2024 with total populations indicated that OR50 values (the osmotic potential at which the germination percentage reaches 50%) for EC, ECM, and EG were −0.52 MPa, −0.49 MPa, and −0.45 MPa, respectively (Figure 4; Table S2).
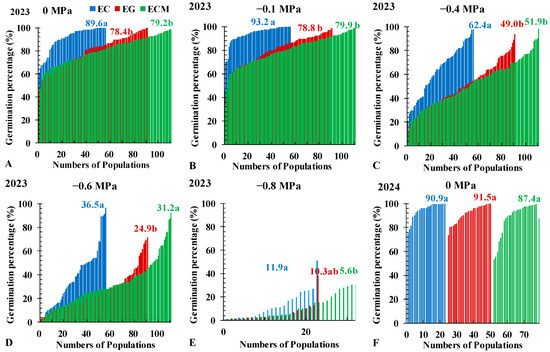
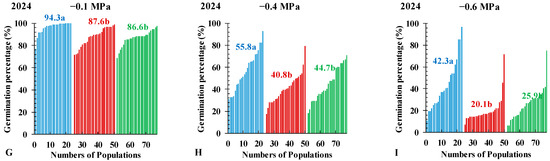
Figure 3.
Seed germination percentages of Echinochloa crus-galli (L.) P.Beauv. (EC), E. glabrescens Munro ex Hook.f. (EG) and E. crus-galli var. mitis (Pursh) Petermann (ECM) under 0 (A,F), −0.1 (B,G), −0.4 (C,H), or −0.6 MPa (D,I) or −0.8 MPa (E) osmotic potentials at 21 days after treatments. The illumination chamber was maintained at a constant 30/20 °C (day/night) and 12/12 light/dark cycle throughout the experiment. Numbers in different colors represent the average germination indices of the three Echinochloa species. Different letters indicate significant differences among species within the same figure.
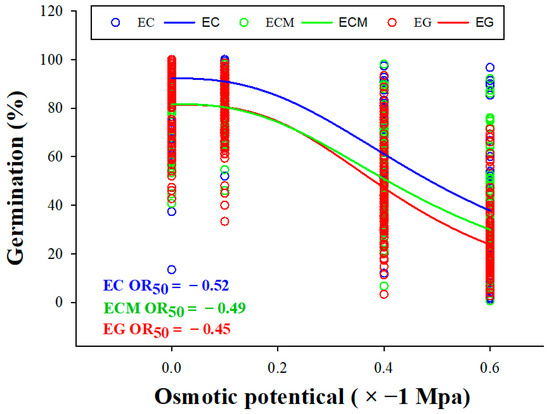
Figure 4.
OR50 (the osmotic potential at which germination percentage reaches 50%) curves of Echinochloa crus-galli (L.) P.Beauv. (EC), E. glabrescens Munro ex Hook.f. (EG) and E. crus-galli var. mitis (Pursh) Petermann (ECM).
3.3. Seed Germination Index
The average GIs of three Echinochloa species decreased significantly with decreasing osmotic potential (Figure 5A–I). The CVs of the three Echinochloa species decreased (from 0 MPa to −0.1 MPa) and then increased (from −0.1 MPa to −0.6 MPa; Table S3). In the 2024 experiment, no significant difference in average GI was observed among the three species at 0 MPa (Figure 5F). However, from −0.1 MPa to −0.6 MPa, EC exhibited significantly higher GIs than ECM and EG (Figure 5G–I). At −0.8 MPa, the average GI of EG was significantly lower than that of EC and ECM, with values of 1.5, 1.1, and 0.6, respectively (Figure 5E).
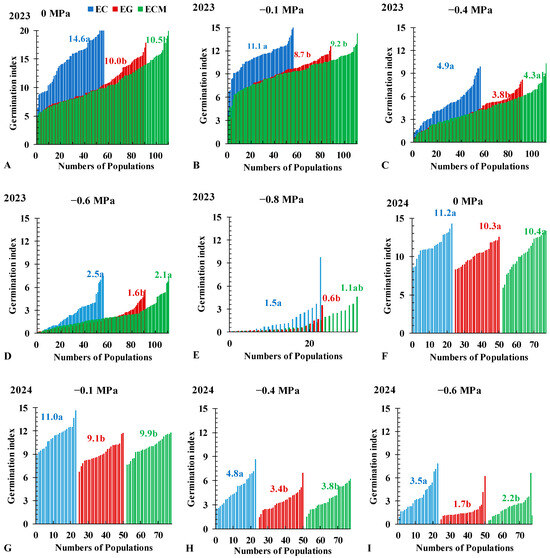
Figure 5.
Germination index of Echinochloa crus-galli (L.) P.Beauv. (EC), E. crus-galli var. mitis (Pursh) Petermann (ECM), and E. glabrescens Munro ex Hook.f. (EG) under different osmotic potentials. Different letters indicate significant differences among species within the same figure.
3.4. Influences of Latitude and Rice Planting Method
The results of GLMs suggested that the treated osmotic potentials, species (EC, ECM or EG), and rice planting method (direct-seeded or transplanted rice) showed significant influences on germination percentages and GI (Tables S4 and S5). Germination percentages and GI of ECM and EG were significantly (p < 0.05) influenced by rice planting methods (Figure 6). Under 0 MPa and −0.1 MPa of ECM, and −0.4 MPa and −0.6 MPa of EG, seeds from the transplanting field exhibited significantly higher germination percentages. Likewise, under −0.1 MPa of ECM, and −0.4 MPa and −0.6 MPa of EG, seeds from the transplanting field had significantly higher germination percentages and GI values (Figure 6).
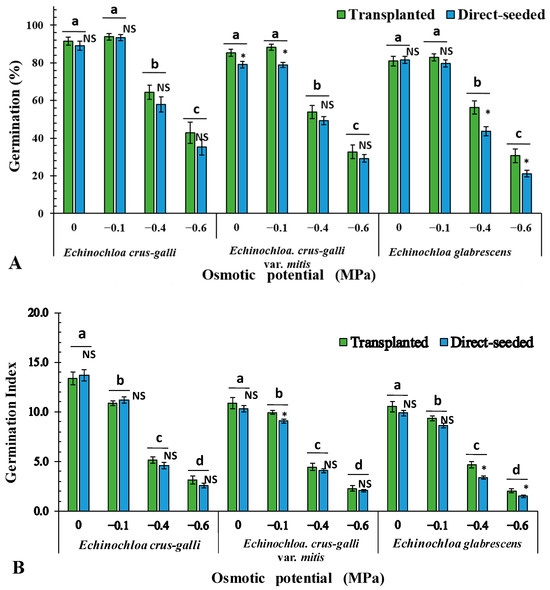
Figure 6.
Average germination percentage (A) and germination index (B) of different species populations collected from fields with varying rice planting methods under different osmotic potentials. Different letters indicate significant (p < 0.05) differences among treatments within the same species. *: significant (p < 0.05) difference between the two sets of data. NS: No significant (p < 0.05) difference between the two sets of data.
The germination percentage and GI value of three Echinochloa species were significantly influenced by the latitude of the seed collection site and the 1000-seed weight of seeds (Tables S6 and S7). For latitude, the germination percentage and GI of EC and ECM under −0.4 and −0.6 MPa, and the GI of EC under 0 MPa and the ECM under −0.6 MPa showed significantly positively with latitude. While the germination percentage of EG under 0 MPa and the germination percentage and GI of EG under −0.1 MPa were negatively correlated with latitude. For 1000-seed weight, the germination percentage of EC and ECM under 0 MPa, and ECM and EG under −0.1 MPa, were significantly positively correlated with 1000-seed weight. The GI of ECM under −0.1 MPa showed significantly positive correlation with 1000-seed weight.
4. Discussion
4.1. Commonality in Seed Germination of the Three Echinochloa Species
The three Echinochloa species exhibited similar germination rhythms, but slower rates than other rice weeds. This may be related to base temperature and seed dormancy levels [16]. The slower germination rhythm likely allows Echinochloa species seeds to escape pre-emergence chemical control practices in paddy fields [12]. Specifically, under suitable conditions, Echinochloa species seeds began to germinate on the third day, peaking between the sixth and seventh days. Under relatively dry conditions, germination started on the fourth or fifth day and continued thereafter. Our previous study found that most Leptochloa chinensis seeds germinated within two days under suitable conditions [8]. Similarly, Digitaria sanguinalis reached 80% germination within two days under suitable conditions [17]. Weedy rice began germinating within two days after treatment, with peak germination occurring between two and six days [18].
For paddy fields with EC, ECM, or EG occurrence, pre-emergence herbicides should be applied according to soil moisture conditions accordingly. Considering that seeds of EC, ECM, and EG all germinated non-homogeneously within species, multiple pre-emergence chemical controls should be conducted at different times [12]. Specifically, for rice fields well prepared for transplanting rice seedlings, or those well prepared for direct-seeding with good moisture conditions, pre-emergence rice herbicides should be applied at 4–6 d after irrigated or rain weather as the first time of pre-emergence chemical control; and a second pre-emergence chemical control might be necessary at about 10 d after the first time. For direct-seeding rice fields finishing seeding and waiting for irrigation or rain weather, pre-emergence rice herbicides should be applied at 5–7 d after seeding as the first time of pre-emergence chemical control; and a second pre-emergence chemical control should be applied according to seedling emergence situations of fields.
In general, the three Echinochloa species showed high drought tolerance during germination. For all of EC, ECM, and EG, cumulative germination percentages at −0.1 MPa were all similar to those at 0 MPa, while the GIs were significantly lower than those at 0 MPa treatments. A similar pattern was observed in Glycyrrhiza uralensis, where low concentrations of PEG solution had no effect on germination percentage but significantly reduced the GI [19], as well as results in Suaeda vermiculata [20] and Camelina sativa [21]. Mild PEG treatment might enhance osmotic adjustment and antioxidant capacity by regulating peroxidase, superoxide dismutase, catalase, and other enzymes, and thereby promoting seed germination, which could be diminished by severe drought stress [22]. Moreover, mild PEG treatment slows down water absorption, preventing timely enzyme activation and inhibiting GI [23]. In our study, osmotic potential of −0.4 MPa, −0.6 MPa, and −0.8 MPa significantly inhibited both germination percentage and speed in all Echinochloa populations tested. Under these conditions, seeds struggle to absorb water and hormone imbalance in seeds, leading to inhibited respiration and energy production, which ultimately prevents germination [24,25]. In addition, higher adaptation of EC, ECM, and EG seed germination to lower osmotic potential may possibly facilitate their infestation in rice fields under drought stress [5]. Specifically, in eastern China, some farmers sow rice seeds and wait on rainy weather or irrigation for several days, during which EC, ECM, and EG seeds germinated may quickly grow after rain or irrigating, and form competitive advantages to rice seedlings emerged later; as well become big enough to be tolerant to pre-emergence herbicides [12]. For example, pretichlor is one of the most frequently used pre-emergence rice herbicides for control grassy weeds including EC, ECM, and EG, whereas, its efficacy decreases greatly against Echinochloa seedlings after the two-leaf stage [12].
4.2. Inter-Specific Difference in Drought Stress
Despite morphological similarities, the three Echinochloa species differed significantly in drought response during germination [10,26]. Regardless of osmotic potential, EC germinated with a higher percentage and faster speed than ECM and EG on average, while EG showed the lowest average germination percentage and speed. Similar patterns were observed in our previous study on germination biology responding to different temperatures [8]. Similarly, in a study on drought tolerance with three Glycyrrhiza species, G. glabra demonstrated the highest drought resistance, while G. uralensis was the least tolerant [12]. Seed germination relies on stored nutrients, and thus smaller seeds may struggle to support germination under unfavorable conditions [27,28]. In this study, EC had a significantly higher 1000-seed weight than ECM and EG, while ECM was slightly heavier than EG [8]. The OR50 values for EC, ECM, and EG were −0.52 MPa, −0.49 MPa, and −0.45 MPa, respectively.
All three Echinochloa species germinations were more drought-tolerant than those of other common rice weeds like Leptochloa chinensis(OR50 = −0.28 MPa) [2] and Digitaria sanguinalis(OR50 = −0.41 MPa) [17]. Sedges in rice fields in eastern China mainly include Cyperus iria, Cyperus difformis, and Fimbristylis miliacea, which showed OR50 values of −0.46 MPa, −0.12 MPa, and −0.69 MPa, respectively [29]. Moreover, the OR50 for broad-leaved rice weed species Ludwigia prostrata was −0.4 MPa [30]. This ability to be resistant to drought stress seen in EC, ECM, and EG may contribute to their persistence and dominance in rice fields. Specifically, famers frequently finish seeding, and then leave the rice fields waiting for irrigation or rainy weather for several days to even longer than one week [12]. Seed banks of EC, ECM, and EG may germinate in relatively dry soil, and quickly grow with dense seedlings, in fields after irrigation or rainfall, which could hold more than two leaves in 2–3 d in hot season and become tolerant to many pre-emergence rice herbicides [31]; afterwards, they can become too old to be controlled by post-emergence herbicides normally applied later. Therefore, in rice fields with EC, ECM, and/or EG—in particular for EC—pre-emergence chemical control should be highlighted according to its germination biology traits [8].
4.3. Intra-Specific Variations
Our results suggest that EC, ECM, and EG all exhibit intra-specific variation in germination biology under different osmotic potentials. Intra-specific variation in all of the three Echinochloa species increased with osmotic potential decreasing from −0.1 MPa to −0.5 MPa, and the OR50 values of the three Echinochloa species follow the same order as previous results, but are lower [5]. Seed germination indices under osmotic stress were significantly influenced by 1000-seed weight, the latitude of the collection site, and the rice planting method used in the source fields. Geographic factors such as average maximum temperatures during rice growing season (from about 28 °C to 31 °C) and annual precipitation (from about 600 mm to 1200 mm) vary across latitudes in the studying area [4]. Maternal environmental effects may shape seed traits [32]. The annual precipitation in Jiangsu province is negatively correlated with latitude [4], which in turn affects seed size and germination ability [33,34,35]. Similarly, Leptochloa chinensis seeds collected from higher latitudes exhibited higher germination capacity at 20 °C [4]. Matzrafi et al. (2021) [36] reported that Amaranthus palmeri seeds collected from water-stressed plants were larger and exhibited greater drought resistance than those from well-watered plants. Similarly, in sunflowers, drought stress during seed development reduced pericarp thickness, increased soluble electrolyte leakage, and altered the accumulation of ABA, oligosaccharides, and polyphenols, contributing to enhanced seed tolerance to abiotic stress [37]. Interestingly, EC and ECM seed germination percentages and speeds at 0 and −0.1 MPa were positively correlated with latitude, whereas EG exhibited the opposite trend. Species-specific maternal effects may explain these differences, though the underlying mechanisms require further investigations.
Additionally, in ECM under favorable conditions (0 MPa and −0.1 MPa) and in EG under drought conditions (−0.4 MPa and −0.6 MPa), seeds from transplanted fields showed significantly higher germination percentages than those from direct-seeded fields. This may be related to herbicide application strategies, as herbicide use in direct-seeded fields is typically more frequent and intense than in transplanted fields [8]. Consequently, Echinochloa plants that escape herbicide control in direct-seeded fields may produce seeds of uneven quality and maturation time, further influencing germination performance.
5. Conclusions
As the three most troublesome rice weeds in eastern China, EC, ECM, and EG showed quite different germination biology compared with the other most common rice weed species, such as Leptochloa chinensis [4,8] and Digitaria sanguinalis [16]. Specifically, seeds of EC, ECM, and EG germinated non-homogeneously even under suitable conditions, while most seeds of Leptochloa chinensis and Digitaria sanguinalis could germinate in 2–4 d under suitable conditions. This difference challenges the commonly applied chemical control strategies which involve one-time pre-emergence chemical control in rice fields in eastern China. Under suitable conditions, one pre-emergence chemical control is sufficient for most populations of L. chinensis and D. sanguinalis, while at least two applications of pre-emergence chemical control should be undergone for rice fields with EC, ECM and/or EG. Moreover, the seed germination of the three Echinochloa species was highly resistant to drought stress compared to various common rice weed species. The three Echinochloa species exhibited significant inter-specific differences in germination biology responding to drought stresses, although the three species are highly similar in morphography. Specifically, EC exhibited higher germination rates and faster germination than ECM and EG on average, while EG showed the lowest average germination percentage and speed. Intra-specific variation in all of the three Echinochloa species increased with osmotic potential decreasing from −0.1 MPa to −0.6 MPa. Moreover, 1000-seed weight, latitude, and rice planting method showed significant influences on seed germination biology of the three Echinochloa weed species.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15051169/s1: Table S1: Coefficient of variations (CVs) in germination percentages of Echinochloa crus-galli (L.) P.Beauv. (EC), Echinochloa glabrescens (L.) P.Beauv. (EG), and Echinochloa crus-galli var. mitis (Pursh) Petermann (ECM) populations treated with different osmotic potentials; Table S2: Estimated parameters of osmotic potential treatment response models for three Echinochloa species; Table S3: Coefficient of variations (CVs) in germination indices of Echinochloa crus-galli (L.) P.Beauv. (EC), Echinochloa crus-galli var. mitis (Pursh) Petermann (ECM), and Echinochloa glabrescens (L.) P.Beauv. (EG) populations treated with different osmotic potentials; Table S4: Results (F-values) of GLMs among germination percentages of Echinochloa crus-galli (L.) P.Beauv. (EC), Echinochloa crus-galli var. mitis (Pursh) Petermann (ECM), and Echinochloa glabrescens (L.) P.Beauv. (EG) seeds at different osmotic potentials, Echinochloa species, and rice planting methods in seed collecting fields; Table S5: Results (F-values) of GLMs among germination index of Echinochloa crus-galli (L.) P.Beauv. (EC), Echinochloa crus-galli var. mitis (Pursh) Petermann (ECM), and Echinochloa glabrescens (L.) P.Beauv. (EG) seeds at different osmotic potentials, Echinochloa species, and rice planting methods of seed collecting fields; Table S6: The correlation between the latitude of the populations collection sites and seed germination percentage and germination index (GI) of three Echinochloa species under different osmotic potential treatment (MPa); Table S7: The correlation between the 1000-seed weight and seed germination percentage and germination index (GI) of three Echinochloa species under different osmotic potential treatment (MPa).
Author Contributions
G.C. and Q.D. designed the study; A.M., Y.C. and K.A. performed material seed collection and the germination treatment work; A.M., Y.C., K.A. and Q.D. performed data processing work; K.A. and G.C. wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Key Research and Development Program of Jiangsu Province (D21YFD17008), Jiangsu Key R&D Plan (BE2022338), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials.
Acknowledgments
We thank Hongcheng Zhang from Agricultural College, Yangzhou University, China for his guidance on this study.
Conflicts of Interest
The authors declare that they have no competing financial interests or personal relationships that could have influenced the work reported in this study.
References
- Délye, C.; Jasieniuk, M.; Le Corre, V. Deciphering the evolution of herbicide resistance in weeds. Trends Genet. 2013, 29, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; An, K.; Liu, Y.; Chen, L.; Lin, Y.; Wei, H. Seed germination and seedling emergence responding to different osmotic potentials and burial depths of 242 Leptochloa chinensis populations. Weed Res. 2025, 65, e12674. [Google Scholar] [CrossRef]
- Singh, M.; Thapa, R.; Kukal, M.S.; Irmak, S.; Mirsky, S.; Jhala, A.J. Effect of water stress on weed germination, growth characteristics, and seed production: A global meta-analysis. Weed Sci. 2022, 70, 621–640. [Google Scholar] [CrossRef]
- An, K.; Chen, L.; Liu, Y.Y.; Wei, H.Y.; Chen, G.Q. Seed Dormancy and Germination Responses to Different Temperatures of Leptochloa chinensis (L.) Nees: A Case Study with 242 Populations Collected from Rice Fields in East China. Agronomy 2024, 14, 2177. [Google Scholar] [CrossRef]
- Yuan, G.H.; Gao, Y.; Fang, J.P.; Shen, G.H.; Tian, Z.H. Environmental Influences on Seed Germination and Seedling Emergence in Four Echinochloa Taxa. Agronomy 2025, 15, 401. [Google Scholar] [CrossRef]
- Wu, L.M.; Fang, Y.; Yang, H.N.; Bai, L.Y. Effects of drought-stress on seed germination and growth physiology of quinclorac-resistant Echinochloa crusgalli. PLoS ONE 2019, 14, e0214480. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Wang, H.C.; Cao, J.J.; Li, G.; Chauhan, B.S. Seed biology of alkali barnyardgrass (Echinochloa crus-galli var. zelayensis) and junglerice (Echinochloa colona) for improved management in direct-seeded rice. Weed Sci. 2023, 71, 112–123. [Google Scholar] [CrossRef]
- Chen, Y.; Masoom, A.; Huang, Z.Y.; Xue, J.H.; Chen, G.Q. Interspecific and Intraspecific Differences in Seed Germination Response to Different Temperatures of Three Echinochloa Rice Weeds: A Case Study with 327 Populations. Weed Sci. 2025, 73, e23. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Cao, J.J.; Gu, T.; Yang, X.; Peng, Q.; Bai, L.Y.; Li, Y.F. Co-planted barnyardgrass reduces rice yield by inhibiting plant above- and belowground-growth during post-heading stages. Crop J. 2021, 9, 1198–1207. [Google Scholar] [CrossRef]
- Chen, G.; Tang, W.; Li, J.; Lu, Y.; Dong, L. Distribution Characteristics of Echinocloa Species in Rice Fields in China: A Case Survey on 73 Sites from Nine Provincial Administrative Regions. Chin. J. Rice Sci. 2019, 33, 368–376. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Chen, G.Q.; Yuan, S.Z.; Guo, B.W.; Dai, Q.G.; Huo, Z.Y.; Gao, H.; Wei, H.Y. Safe and Efficient Use Technology of Herbicide in Paddy Field, 1st ed.; China Agriculture Press: Beijing, China, 2021; pp. 6–9. [Google Scholar]
- Flora of China. Available online: https://www.iplant.cn/info/Echinochloa%20crus-galli%20var.%20mitis?t=foc (accessed on 1 May 2024).
- Guo, M.F.; Zong, J.; Zhang, J.X.; Wei, L.; Wei, W.G.; Fan, R.Y.; Zhang, T.T.; Tang, Z.H.; Zhang, G. Effects of temperature and drought stress on the seed germination of a peatland lily (Lilium concolor var. megalanthum). Front. Plant Sci. 2024, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Oliveira, J.P.; Ranal, M.A. Sample size in studies on the germination process. Botany 2016, 94, 103–115. [Google Scholar] [CrossRef]
- Gardarin, A.; Daürr, C.; Colbach, N. Prediction of germination rates of weed species: Relationships between germination speed parameters and species traits. Ecol. Model. 2011, 222, 626–636. [Google Scholar] [CrossRef]
- Roseberg, R.J. Herbicide tolerance of Euphorbia lagascae Spreng., (Euphorbiaceae). Ind. Crop. Prod. 2016, 90, 56–64. [Google Scholar] [CrossRef]
- Zhang, H.; Irving, L.J.; Tian, Y.; Zhou, D. Influence of salinity and temperature on seed germination rate and the hydrotime model parameters for the halophyte, Chloris virgata, and the glycophyte, Digitaria sanguinalis. S. Afr. J. Bot. 2012, 78, 203–210. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Gao, H.T.; Xu, J.Y.; Liu, Q.H.; Dong, L.Y. Weedy rice (Oryza sativa L.) seed dormancy features and potential usage for management. Crop Prot. 2023, 169, 106240. [Google Scholar] [CrossRef]
- Hou, M.C.; Ma, M. Effect of PEG-simulated Drought Stress on Seed Germination of Three Medicinal Liquorice (Glycyrrhiza) Species. Legume Res. 2022, 45, 1388–1393. [Google Scholar] [CrossRef]
- Al-Shamsi, N.; El-Keblawy, A.; Mosa, K.A.; Navarro, T. Drought tolerance and germination response to light and temperature for seeds of saline and non-saline habitats of the habitat-indifferent desert halophyte Suaeda vermiculata. Acta Physiol. Plant. 2018, 40, 13. [Google Scholar] [CrossRef]
- Canak, P.; Zanetti, F.; Jovicic, D.; Vujosevic, B.; Miladinov, Z.; Stanisavljevic, D.; Mirosavljevic, M.; Alberghini, B.; Facciolla, E.; Jeroela, A.M. Camelina germination under osmotic stress- Trend lines, time-courses and critical points. Ind. Crop. Prod. 2022, 181, 114761. [Google Scholar] [CrossRef]
- Ma, L.Y.; Wei, J.G.; Han, G.J.; Sun, X.M.; Yang, X.B. Seed osmopriming with polyethylene glycol (PEG) enhances seed germination and seedling physiological traits of Coronilla varia L. under water stress. PLoS ONE 2024, 19, e0303145. [Google Scholar] [CrossRef]
- Yan, M. Seed priming stimulate germination and early seedling growth of Chinese cabbage under drought stress. S. Afr. J Bot. 2015, 99, 88–92. [Google Scholar] [CrossRef]
- Ren, X.M.; Yang, C.K.; Zhu, X.X.; Yi, P.F.; Jiang, X.Z.; Yang, J.S.; Xiang, S.P.; Li, Y.X.; Yu, B.; Yan, W.J.; et al. Insights into drought stress response mechanism of tobacco during seed germination by integrated analysis of transcriptome and metabolome. Plant Physiol. Biochem. 2024, 209, 108526. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.L.; Zhang, J.; Peng, T.; Sun, H.W.; Zhao, Q.Z. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Institute of Botany, the Chinese Academy of Sciences. Available online: https://www.iplant.cn/foc (accessed on 28 March 2025).
- Burmeier, S.; Donath, T.W.; Otte, A.; Eckstein, R.L. Rapid burial has differential effects on germination and emergence of small- and large-seeded herbaceous plant species. Seed Sci. Res. 2010, 20, 189–200. [Google Scholar] [CrossRef]
- Albert, D.; Vijayaraghavareddy, P.; Sreeman, S. Seed size, an imperative trait for seed vigor and drought tolerance in rice. Cereal Res. Commun. 2024, 52, 559–568. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Ecological studies on Cyperus difformis, Cyperus iria and Fimbristylis miliacea: Three troublesome annual sedge weeds of rice. Ann. Appl. Biol. 2009, 155, 103–112. [Google Scholar] [CrossRef]
- Li, T.; Qian, H.W.; Yuan, G.H.; Fan, J.Q.; Guo, S.L. Germination ecology and response to herbicides of Ludwigia prostrata and their implication for weed control in paddy fields. Weed Technol. 2023, 37, 197–204. [Google Scholar] [CrossRef]
- Mir, M.S.; Singh, P.; Kanth, R.H.; Bhat, T.A.; Shah, Z.A.; Dar, E.A.; Farooq, S.; Ali, M.A.; Elshikh, M.S. Impact of different sowing dates and weed management strategies on phenological development, productivity, and thermal efficiencies of direct seeded rice. Adv. Weed Sci. 2024, 42, 13. [Google Scholar] [CrossRef]
- Galloway, L.F. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol. 2005, 166, 93–99. [Google Scholar] [CrossRef]
- Gorden, N.L.S.; Winkler, K.J.; Jahnke, M.R.; Marshall, E.; Horky, J.; Huddelson, C.; Etterson, J.R. Geographic patterns of seed mass are associated with climate factors, but relationships vary between species. Am. J. Bot. 2016, 103, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.F.; Yu, H.W.; Yang, K.W.; Chen, L.; Yin, W.D.; Ding, J.Q. Latitudinal and Longitudinal Trends of Seed Traits Indicate Adaptive Strategies of an Invasive Plant. Front. Plant Sci. 2021, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Matzrafi, M.; Osipitan, O.A.; Ohadi, S.; Mesgaran, M.B. Under pressure: Maternal effects promote drought tolerance in progeny seed of Palmer amaranth (Amaranthus palmeri). Weed Sci. 2021, 69, 31–38. [Google Scholar] [CrossRef]
- Vancostenoble, B.; Blanchet, N.; Langlade, N.B.; Bailly, C. Maternal drought stress induces abiotic stress tolerance to the progeny at the germination stage in sunflower. Environ. Exp. Bot. 2022, 201, 104939. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).