Entomopathogenic Fungus Treatment Affects Trophic Interactions by Altering Volatile Emissions in Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Insect Culture
2.2. Fungal Culture and Colonization on Tomato Plants
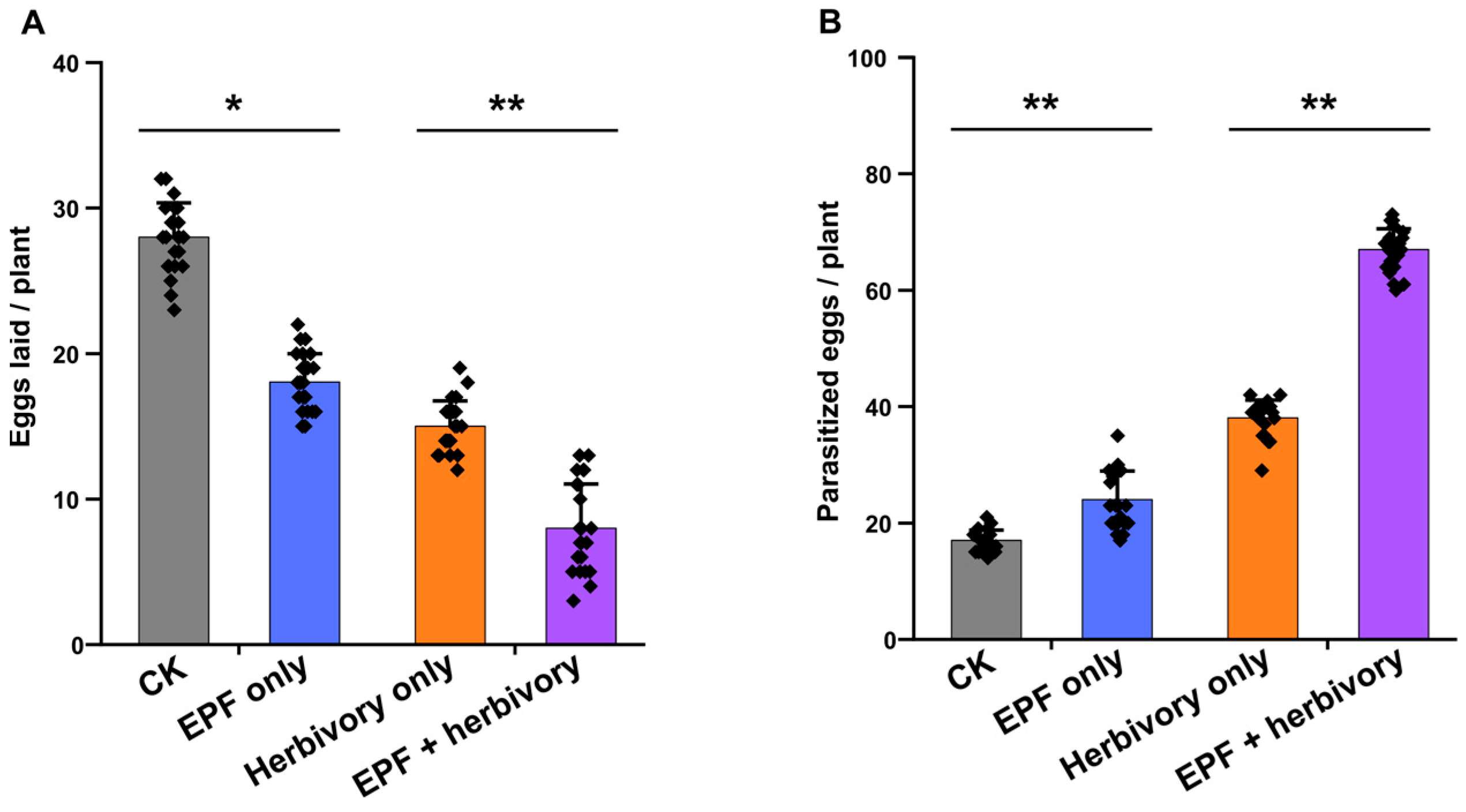
2.3. P. absoluta Oviposition and T. chilonis Parasitism Assays
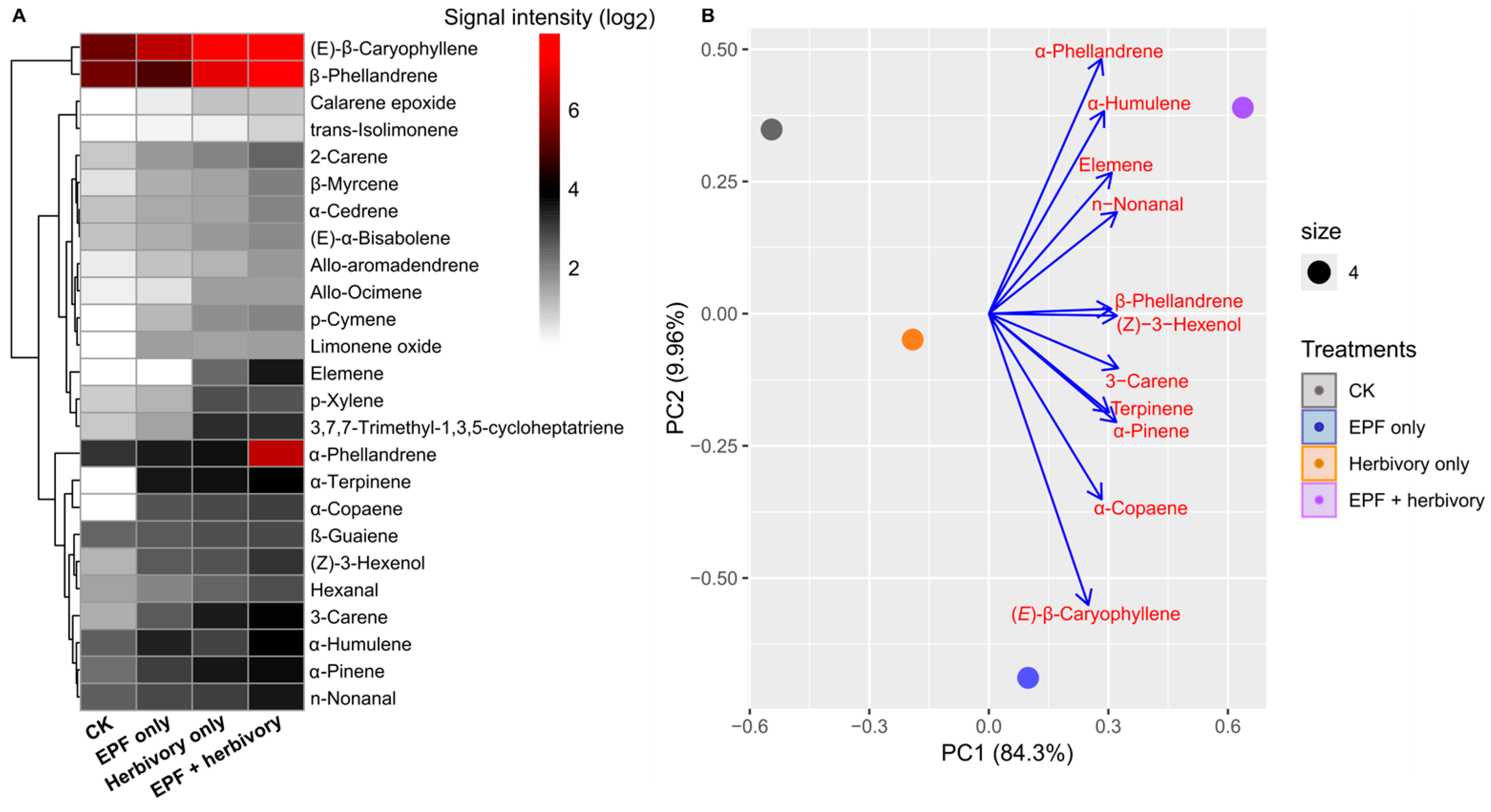
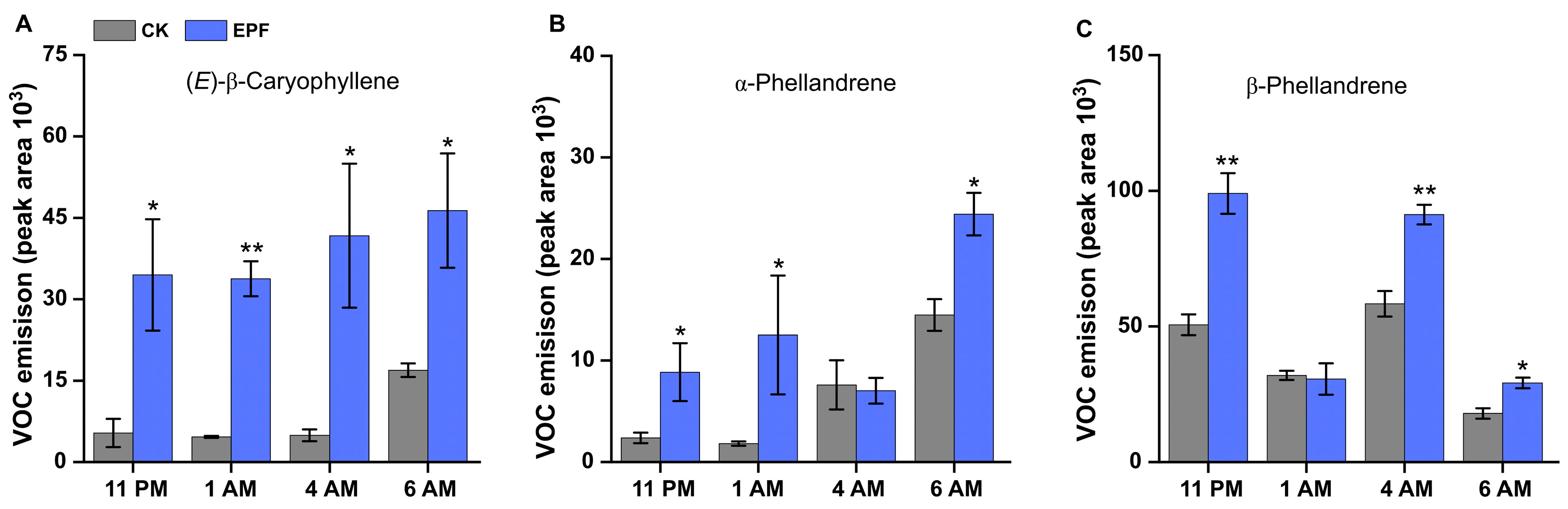
2.4. Sampling of Foliar VOC Blends
2.5. Phytohormone Extraction and Quantification
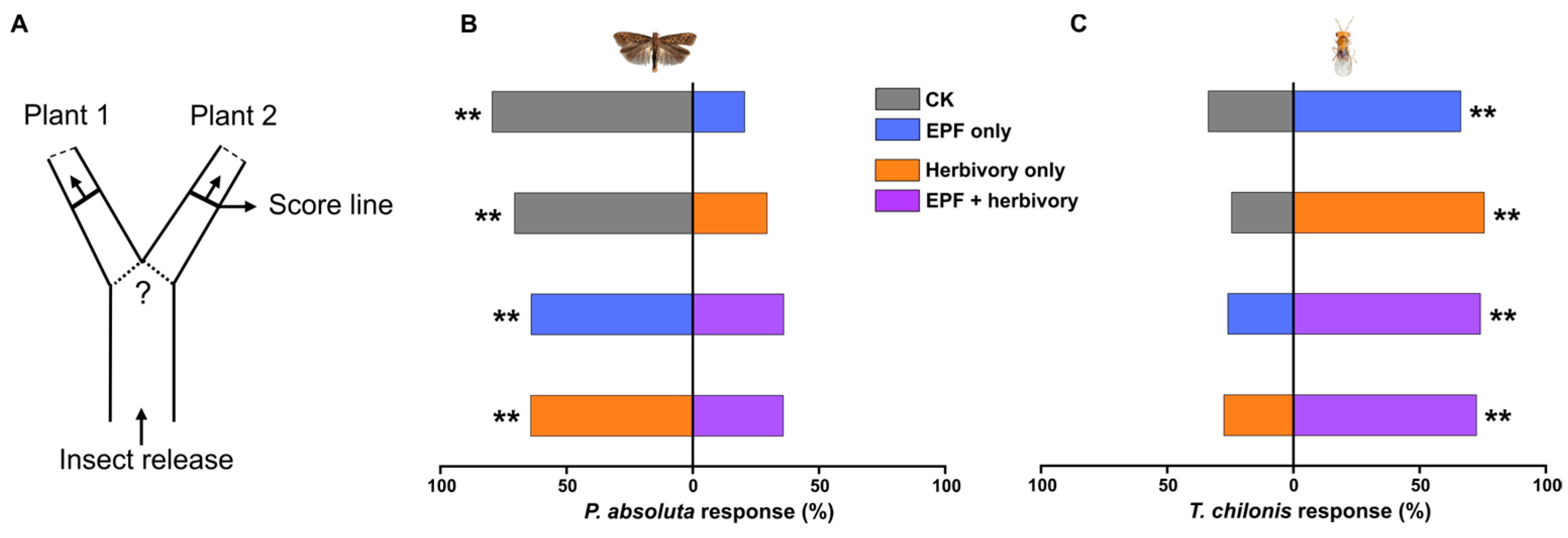
2.6. Insect Choice Assay with Y-Tube Olfactometer
2.7. Electroantennogram (EAG) and Odor Delivery
2.8. Synthetic VOC Applications and P. absoluta Behavior Assays
2.9. Statistical Analyses
3. Results
3.1. Effects of EPF Treatment on Herbivore and Parasitoid Behavioral Preferences
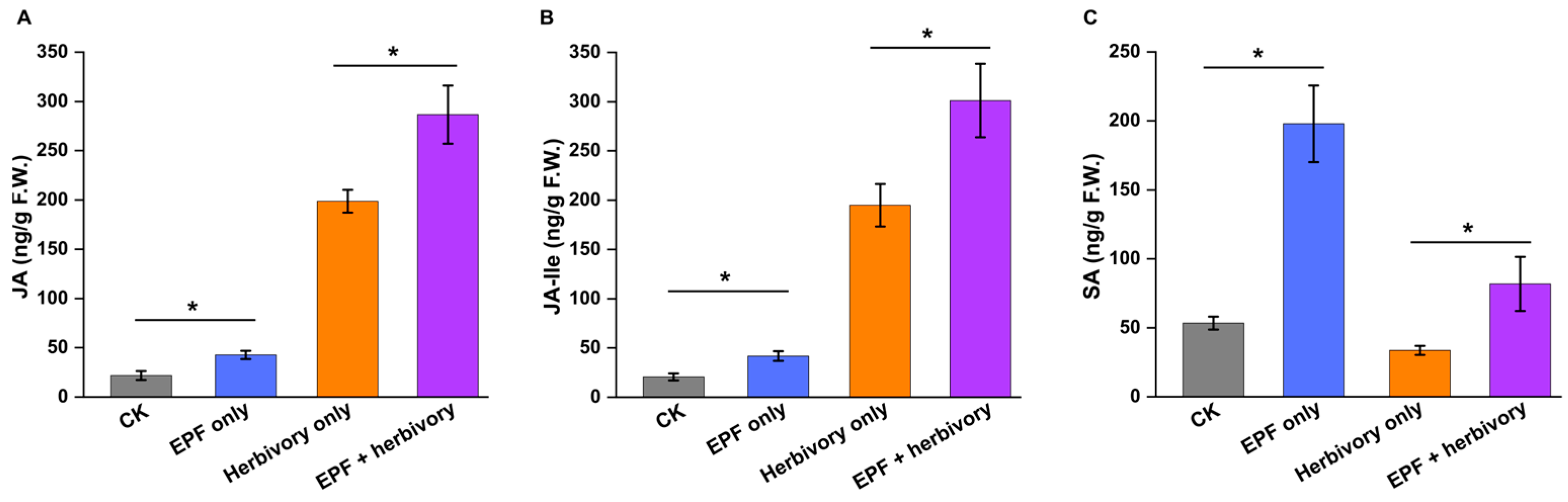
3.2. EPF Treatment Modulates VOC Emissions and Induces Phytohormone Accumulation
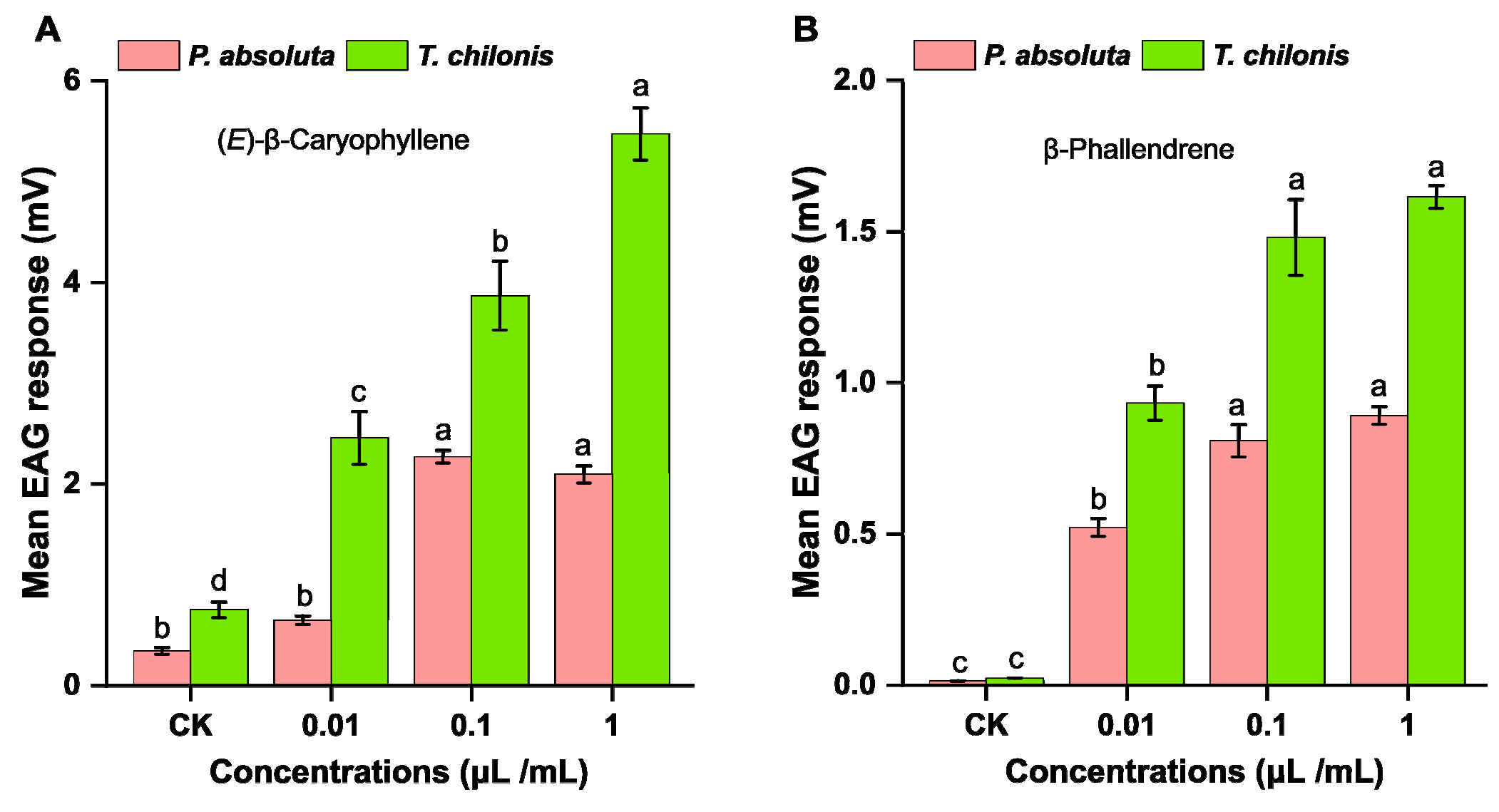
3.3. Behavioral and Electrophysiological Responses of Insects to Synthetic VOC Odors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Shaurav, S.; Yadav, P.K. Entomopathogenic fungi and their relevance in sustainable agriculture: A review. Cogent Food Agric. 2023, 9, 2180857. [Google Scholar] [CrossRef]
- Shang, S.-Q.; Wei-Zhen, L.I.; Chen, Y.-N.; Tong, Z.H.U. The influence of Ahy1 strain of Acremonium hansfodii on functional response of Neoseiulus barkeri to Tetranychus urticae. Syst. Appl. Acarol. 2023, 28, 903–911. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, V.; Kaur, A.; Kaur, S. Insecticidal potential of an endophytic fungus, Cladosporium uredinicola, against Spodoptera litura. Phytoparasitica 2013, 41, 373–382. [Google Scholar] [CrossRef]
- García-Estrada, C.; Cat, E.; Santamarta, I. Beauveria bassiana as Biocontrol Agent: Formulation and Commercialization for Pest Management. In Agriculturally Important Microorganisms: Commercialization and Regulatory Requirements in Asia; Singh, H.B., Sarma, B.K., Keswani, C., Eds.; Springer: Singapore, 2016; pp. 81–96. [Google Scholar]
- Eilenberg, J.; Keller, S.; Humber, R.A.; Jensen, A.H.; Jensen, A.B.; Görg, L.M.; Muskat, L.C.; Kais, B.; Gross, J.; Patel, A.V. Pandora cacopsyllae Eilenberg, Keller & Humber (Entomophthorales: Entomophthoraceae), a new species infecting pear psyllid Cacopsylla pyri L. (Hemiptera: Psyllidae). J. Invertebr. Pathol. 2023, 200, 107954. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Afolabi, O.G.; Hussain, M.; Qasim, M.; Wang, L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018, 217, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Santra, H.K.; Banerjee, D. Multifunctional Efficacy and Eco-friendly Applications of Fungal Endophytes. In Fungal Endophytes Volume II: Applications in Agroecosystems and Plant Protection; Abd-Elsalam, K.A., Hashem, A.H., Eds.; Springer Nature: Singapore, 2025; pp. 33–60. [Google Scholar]
- Hong, L.; Wang, Q.; Zhang, J.; Chen, X.; Liu, Y.; Asiegbu, F.O.; Wu, P.; Ma, X.; Wang, K. Advances in the beneficial endophytic fungi for the growth and health of woody plants. For. Res. 2024, 4, e028. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef]
- Ji, X.; Xia, Y.; Zhang, H.; Cui, J.L. The microscopic mechanism between endophytic fungi and host plants: From recognition to building stable mutually beneficial relationships. Microbiol. Res. 2022, 261, 127056. [Google Scholar] [CrossRef]
- Taheri, P.; Puopolo, G.; Santoyo, G. Plant growth-promoting microorganisms: New insights and the way forward. Microbiol. Res. 2025, 297, 128168. [Google Scholar] [CrossRef]
- Anjali; Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Saini, H.P.; Meena, M.; Sahoo, A.; Mehta, T. A review on fungal endophytes of the family Fabaceae, their metabolic diversity and biological applications. Heliyon 2025, 11, e42153. [Google Scholar] [CrossRef] [PubMed]
- Toppo, P.; Kagatay, L.L.; Gurung, A.; Singla, P.; Chakraborty, R.; Roy, S.; Mathur, P. Endophytic fungi mediates production of bioactive secondary metabolites via modulation of genes involved in key metabolic pathways and their contribution in different biotechnological sector. 3 Biotech 2023, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Xu, Y.; Abou El-Ela, A.S.; Zhang, Y.; Zhong, J.; Mao, Z.; Chen, X.; Guo, H.; Zhang, C.; Sun, Y.; et al. Tissue-specific regulation of volatile emissions moves predators from flowers to attacked leaves. Curr. Biol. 2023, 33, 2321–2329.e2325. [Google Scholar] [CrossRef]
- Munawar, A.; Zhu, Z.; Machado, R.A.R.; Zhou, W. Beyond ‘push–pull’: Unraveling the ecological pleiotropy of plant volatile organic compounds for sustainable crop pest management. Crop Health 2023, 1, 18. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Munawar, A.; Zhu, J.; Zhong, J.; Zhang, Y.; Guo, H.; Zhu, Z.; Baldwin, I.T.; Zhou, W. Manipulating stomatal aperture by silencing StSLAC1 affects potato plant–herbivore–parasitoid tritrophic interactions under drought stress. New Phytol. 2025, 245, 2133–2149. [Google Scholar] [CrossRef]
- Nawrocka, J.; Szymczak, K.; Skwarek-Fadecka, M.; Małolepsza, U. Toward the Analysis of Volatile Organic Compounds from Tomato Plants (Solanum lycopersicum L.) Treated with Trichoderma virens or/and Botrytis cinerea. Cells 2023, 12, 1271. [Google Scholar] [CrossRef]
- Rubio, M.B.; Monti, M.M.; Gualtieri, L.; Ruocco, M.; Hermosa, R.; Monte, E. Trichoderma harzianum Volatile Organic Compounds Regulated by the THCTF1 Transcription Factor Are Involved in Antifungal Activity and Beneficial Plant Responses. J. Fungi 2023, 9, 654. [Google Scholar] [CrossRef]
- Stříbrská, B.; Hradecký, J.; Čepl, J.; Modlinger, R.; Tomášková, I.; Jirošová, A. Physiological and biochemical indicators in Norway spruces freshly infested by Ips typographus: Potential for early detection methods. Front. For. Glob. Change 2023, 6, 1197229. [Google Scholar] [CrossRef]
- van Zijll de Jong, E.; Kandula, J.; Rostás, M.; Kandula, D.; Hampton, J.; Mendoza-Mendoza, A. Fungistatic Activity Mediated by Volatile Organic Compounds Is Isolate-Dependent in Trichoderma sp. “atroviride B”. J. Fungi 2023, 9, 238. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Sadhukhan, S. Unearthing the alteration in plant volatiles induced by mycorrhizal fungi: A shield against plant pathogens. Physiol. Plant 2023, 175, e13845. [Google Scholar] [CrossRef]
- Mitina, G.V.; Stepanycheva, E.A.; Choglokova, A.A.; Cherepanova, M.A. Features of Behavioral Reactions of the Peach Aphid Myzus persicae (Sulzer, 1776) (Hemiptera, Aphididae) to Volatile Organic Compounds of Entomopathogenic Fungi of the Genus Lecanicillium. Entomol. Rev. 2021, 101, 1015–1023. [Google Scholar] [CrossRef]
- Ballot, A.; Dore, J.; Rey, M.; Meiffren, G.; Langin, T.; Joly, P.; Dreux-Zigha, A.; Taibi, A.; Prigent-Combaret, C. Dimethylpolysulfides production as the major mechanism behind wheat fungal pathogen biocontrol, by Arthrobacter and Microbacterium actinomycetes. Microbiol. Spectr. 2023, 11, e0529222. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Shi, X.; Han, J.; Ren, Q.; Gao, Z.; Zhang, A.; Wang, H.; Du, Y. VOCs from fungi-infected apples attract and increase the oviposition of yellow peach moth Conogethes punctiferalis. Pest Manag. Sci. 2023, 79, 5208–5219. [Google Scholar] [CrossRef] [PubMed]
- Eckert, S.; Eilers, E.J.; Jakobs, R.; Anaia, R.A.; Aragam, K.S.; Bloss, T.; Popp, M.; Sasidharan, R.; Schnitzler, J.P.; Stein, F.; et al. Inter-laboratory comparison of plant volatile analyses in the light of intra-specific chemodiversity. Metabolomics 2023, 19, 62. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, Worldwide Spread, and Management of the Invasive South American Tomato Pinworm, Tuta absoluta: Past, Present, and Future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Sánchez-Hernández, C.; López, M.G.; Délano-Frier, J.P. Reduced levels of volatile emissions in jasmonate-deficient spr2 tomato mutants favour oviposition by insect herbivores. Plant Cell Environ. 2006, 29, 546–557. [Google Scholar] [CrossRef]
- Contreras, E.; Rodriguez-Herva, J.J.; Isabel, D.; Emilia, L.-S.; Martinez, M. Previous interaction with phytopathogenic bacteria alters the response of Arabidopsis against Tetranychus urticae herbivory. J. Plant Interact. 2023, 18, 2144651. [Google Scholar] [CrossRef]
- Desneux, N.; Han, P.; Mansour, R.; Arnó, J.; Brévault, T.; Campos, M.R.; Chailleux, A.; Guedes, R.N.C.; Karimi, J.; Konan, K.A.J.; et al. Integrated pest management of Tuta absoluta: Practical implementations across different world regions. J. Pest Sci. 2022, 95, 17–39. [Google Scholar] [CrossRef]
- Depenbusch, L.; Teresa, S.; Pepijn, S.; Mahin, S.; Krishnadas, M.; Nasir, U.; Hanson, P. Tomato pests and diseases in Bangladesh and India: Farmers’ management and potential economic gains from insect resistant varieties and integrated pest management. Int. J. Pest Manag. 2023, 69, 1–15. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Roditakis, E.; Campos, M.R.; Haddi, K.; Bielza, P.; Siqueira, H.A.A.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. J. Pest Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Koller, J.; Sutter, L.; Gonthier, J.; Collatz, J.; Norgrove, L. Entomopathogens and Parasitoids Allied in Biocontrol: A Systematic Review. Pathogens 2023, 12, 957. [Google Scholar] [CrossRef]
- Fortes, A.D.R.; Coelho, A.; Amorim, D.J.; Demetrio, C.G.B.; Parra, J.R.P. Biology and quality assessment of Telenomus remus (Hymenoptera: Scelionidae) and Trichogramma spp. (Hymenoptera: Trichogrammatidae) in eggs of Spodoptera spp. for augmentative biological control programs. J. Insect Sci. 2023, 23, 1–10. [Google Scholar] [CrossRef]
- Shi, R.; Yu, J.; Chang, X.; Qiao, L.; Liu, X.; Lu, L. Recent Advances in Research into Jasmonate Biosynthesis and Signaling Pathways in Agricultural Crops and Products. Processes 2023, 11, 736. [Google Scholar] [CrossRef]
- Munawar, A.; Zhang, Y.; Zhong, J.; Ge, Y.; Abou El-Ela, A.S.; Mao, Z.; Ntiri, E.S.; Mao, L.-J.; Zhu, Z.; Zhou, W. Heat stress affects potato’s volatile emissions that mediate agronomically important trophic interactions. Plant Cell Environ. 2022, 45, 3036–3051. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Liao, H.; Wang, K.; Ren, Y. The research hotspots and trends of volatile organic compound emissions from anthropogenic and natural sources: A systematic quantitative review. Environ. Res. 2023, 216, 114386. [Google Scholar] [CrossRef]
- Correa, T.A.; Santos, F.S.; Camargo, M.G.; Quinelato, S.; Bittencourt, V.; Golo, P.S. Comparison of Methods for Isolating Entomopathogenic Fungi from Soil Samples. J. Vis. Exp. 2022, 179, e63353. [Google Scholar] [CrossRef]
- Wu, J.; Hettenhausen, C.; Meldau, S.; Baldwin, I.T. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 2007, 19, 1096–1122. [Google Scholar] [CrossRef]
- Song, J.; Bian, J.; Xue, N.; Xu, Y.; Wu, J. Inter-species mRNA transfer among green peach aphids, dodder parasites, and cucumber host plants. Plant Divers. 2022, 44, 1–10. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Schmelz, E.A.; Zhang, Z.P. Effects of octadecanoid metabolites and inhibitors on induced nicotine accumulation in Nicotiana sylvestris. J. Chem. Ecol. 1996, 22, 61–74. [Google Scholar] [CrossRef]
- Michael, J. Crawley, Generalized Linear Models. In The R Book; Crawley, M.J., Ed.; Weily: Hoboken, NJ, USA, 2012; pp. 557–578. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef]
- Sani, I.; Jamian, S.; Saad, N.; Abdullah, S.; Mohd Hata, E.; Jalinas, J.; Ismail, S.I. Inoculation and colonization of the entomopathogenic fungi, Isaria javanica and Purpureocillium lilacinum, in tomato plants, and their effect on seedling growth, mortality and adult emergence of Bemisia tabaci (Gennadius). PLoS ONE 2023, 18, e0285666. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Afolabi, O.G.; Siddiqui, J.A.; Xu, Y. Endophytic insect pathogenic fungi-host plant-herbivore mutualism: Elucidating the mechanisms involved in the tripartite interactions. World J. Microbiol. Biotechnol. 2023, 39, 326. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.E.; Cabral, C.; Enkegaard, A.; Steenberg, T. Influence of the plant interacting entomopathogenic fungus Beauveria bassiana on parasitoid host choice-behavior, development, and plant defense pathways. PLoS ONE 2020, 15, e0238943. [Google Scholar] [CrossRef] [PubMed]
- Wilberts, L.; Vuts, J.; Caulfield, J.C.; Thomas, G.; Birkett, M.A.; Herrera-Malaver, B.; Verstrepen, K.J.; Sobhy, I.S.; Jacquemyn, H.; Lievens, B. Impact of endophytic colonization by entomopathogenic fungi on the behavior and life history of the tobacco peach aphid Myzus persicae var. nicotianae. PLoS ONE 2022, 17, e0273791. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Cárdenas, P.D.; Pattison, D.I.; Jensen, B.; Meyling, N.V. Isolate-Specific Effect of Entomopathogenic Endophytic Fungi on Population Growth of Two-Spotted Spider Mite (Tetranychus urticae Koch) and Levels of Steroidal Glycoalkaloids in Tomato. J. Chem. Ecol. 2021, 47, 476–488. [Google Scholar] [CrossRef]
- Huang, X.Z.; Xiao, Y.T.; Köllner, T.G.; Jing, W.X.; Kou, J.F.; Chen, J.Y.; Liu, D.F.; Gu, S.H.; Wu, J.X.; Zhang, Y.J.; et al. The terpene synthase gene family in Gossypium hirsutum harbors a linalool synthase GhTPS12 implicated in direct defence responses against herbivores. Plant Cell Environ. 2018, 41, 261–274. [Google Scholar] [CrossRef]
- McCallum, E.J.; Cunningham, J.P.; Lücker, J.; Zalucki, M.P.; De Voss, J.J.; Botella, J.R. Increased plant volatile production affects oviposition, but not larval development, in the moth Helicoverpa armigera. J. Exp. Biol. 2011, 214, 3672–3677. [Google Scholar] [CrossRef]
- Proffit, M.; Birgersson, G.; Bengtsson, M.; Reis, R., Jr.; Witzgall, P.; Lima, E. Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J. Chem. Ecol. 2011, 37, 565–574. [Google Scholar] [CrossRef]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 2021, 44, 1030–1043. [Google Scholar] [CrossRef]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef]
- Xin, K.; Wu, Y.; Ikram, A.U.; Jing, Y.; Liu, S.; Zhang, Y.; Chen, J. Salicylic acid cooperates with different small molecules to control biotic and abiotic stress responses. J. Plant Physiol. 2025, 304, 154406. [Google Scholar] [CrossRef]
- Benjamin, G.; Pandharikar, G.; Frendo, P. Salicylic Acid in Plant Symbioses: Beyond Plant Pathogen Interactions. Biology 2022, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.; Van Wees, S.C. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of Plant Defense System in Response to Microbial Interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M. Chapter 4—Upscaling plant defense system through the application of plant growth-promoting fungi (PGPF). In Microbial Technology for Agro-Ecosystems; Kumar, V., Iram, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 61–95. [Google Scholar]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Fan, R.; Naz, H.; Bamisile, B.S.; Hafeez, M.; Ghani, M.I.; Wei, Y.; Xu, Y.; Chen, X. Insights into insecticide-resistance mechanisms in invasive species: Challenges and control strategies. Front. Physiol. 2022, 13, 1112278. [Google Scholar] [CrossRef]
- Boldorini, G.X.; McCary, M.A.; Romero, G.Q.; Mills, K.L.; Sanders, N.J.; Reich, P.B.; Michalko, R.; Gonçalves-Souza, T. Predators control pests and increase yield across crop types and climates: A meta-analysis. Proc. Biol. Sci. 2024, 291, 20232522. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munawar, A.; Zhang, H.; Zhang, J.; Zhang, X.; Shi, X.-X.; Chen, X.; Li, Z.; He, X.; Zhong, J.; Zhu, Z.; et al. Entomopathogenic Fungus Treatment Affects Trophic Interactions by Altering Volatile Emissions in Tomato. Agronomy 2025, 15, 1161. https://doi.org/10.3390/agronomy15051161
Munawar A, Zhang H, Zhang J, Zhang X, Shi X-X, Chen X, Li Z, He X, Zhong J, Zhu Z, et al. Entomopathogenic Fungus Treatment Affects Trophic Interactions by Altering Volatile Emissions in Tomato. Agronomy. 2025; 15(5):1161. https://doi.org/10.3390/agronomy15051161
Chicago/Turabian StyleMunawar, Asim, Haonan Zhang, Jinyi Zhang, Xiangfen Zhang, Xiao-Xiao Shi, Xuan Chen, Zicheng Li, Xiaoli He, Jian Zhong, Zengrong Zhu, and et al. 2025. "Entomopathogenic Fungus Treatment Affects Trophic Interactions by Altering Volatile Emissions in Tomato" Agronomy 15, no. 5: 1161. https://doi.org/10.3390/agronomy15051161
APA StyleMunawar, A., Zhang, H., Zhang, J., Zhang, X., Shi, X.-X., Chen, X., Li, Z., He, X., Zhong, J., Zhu, Z., Zheng, Y., & Zhou, W. (2025). Entomopathogenic Fungus Treatment Affects Trophic Interactions by Altering Volatile Emissions in Tomato. Agronomy, 15(5), 1161. https://doi.org/10.3390/agronomy15051161









