Fulvic Acid Enhances Oat Growth and Grain Yield Under Drought Deficit by Regulating Ascorbate–Glutathione Cycle, Chlorophyll Synthesis, and Carbon–Assimilation Ability
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Treatments
2.2. Growth Indicators and Yield
2.3. Ascorbic Acid-Glutathione Cycle
2.4. Chlorophyll Content
2.5. Carbon Assimilative Enzyme Activity
2.6. Carbon Metabolism-Related Enzyme Activities
2.7. Statistical Analysis
3. Results
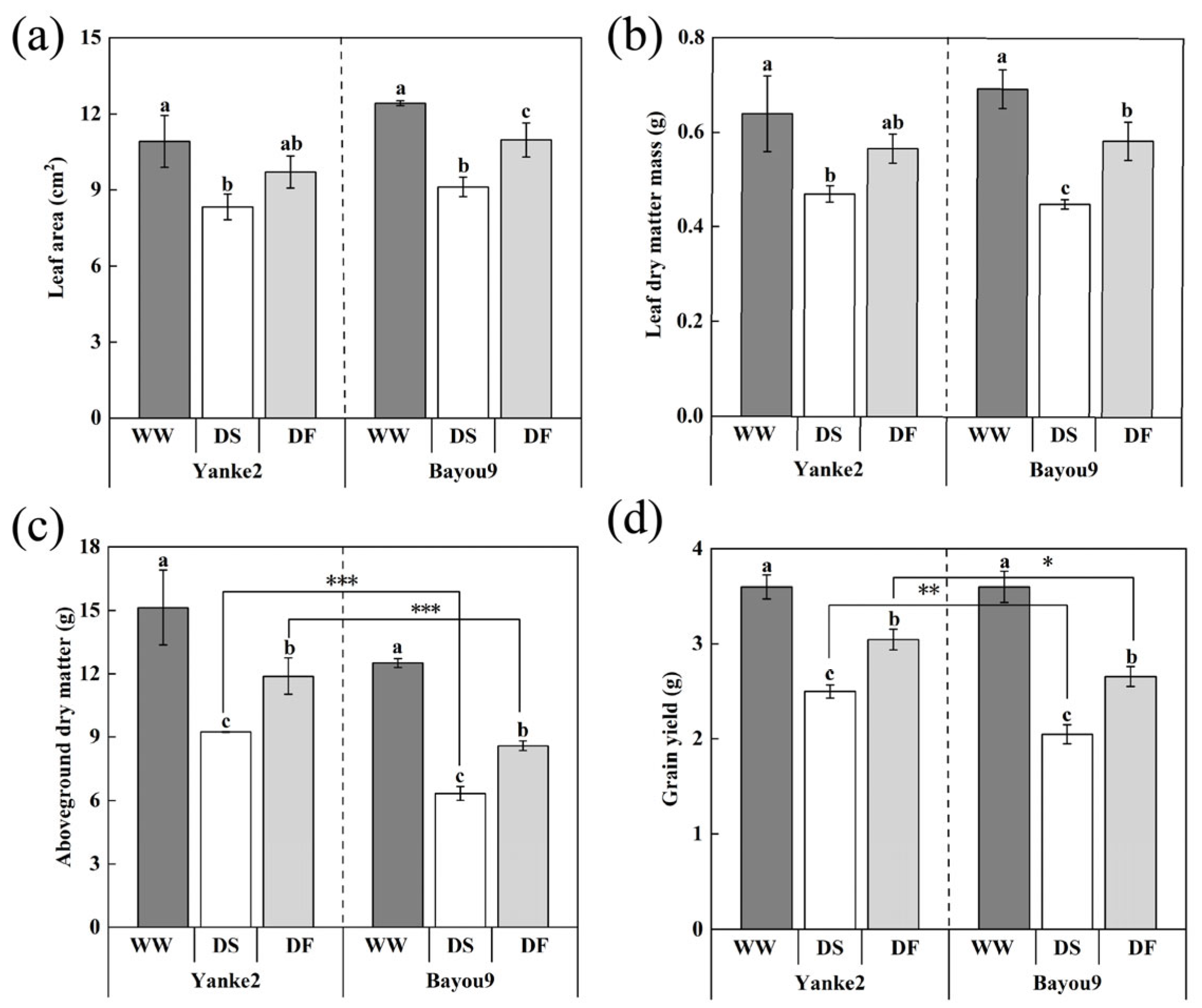
3.1. Effect of FA on Growth and Yield of Oats Under Drought Stress
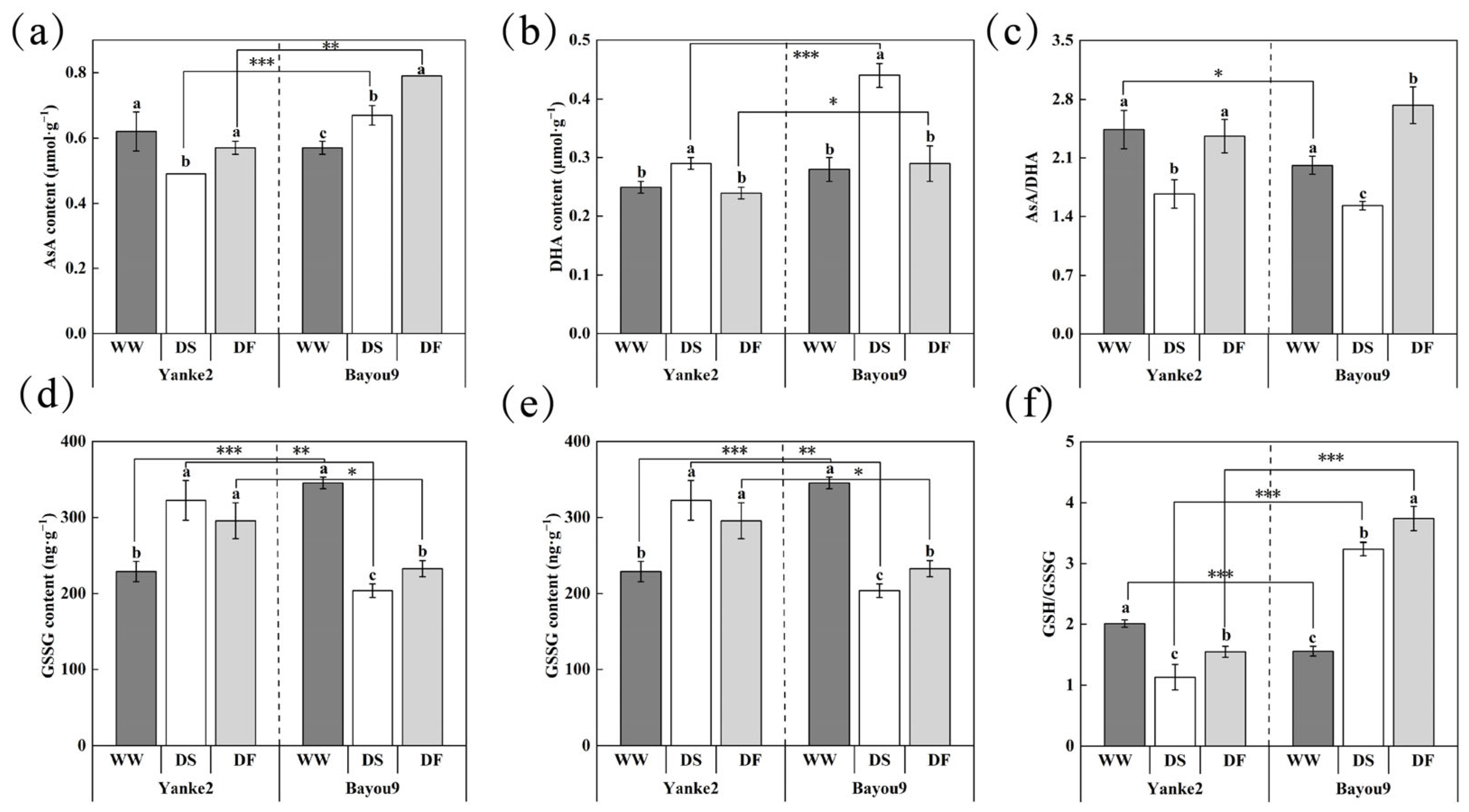
3.2. Effect of FA on Antioxidant Enzyme Activity in Oat Leaves Under Drought Stress
3.3. Effect of FA on Non-Enzymatic Antioxidants in Oat Leaves Under Drought Stress
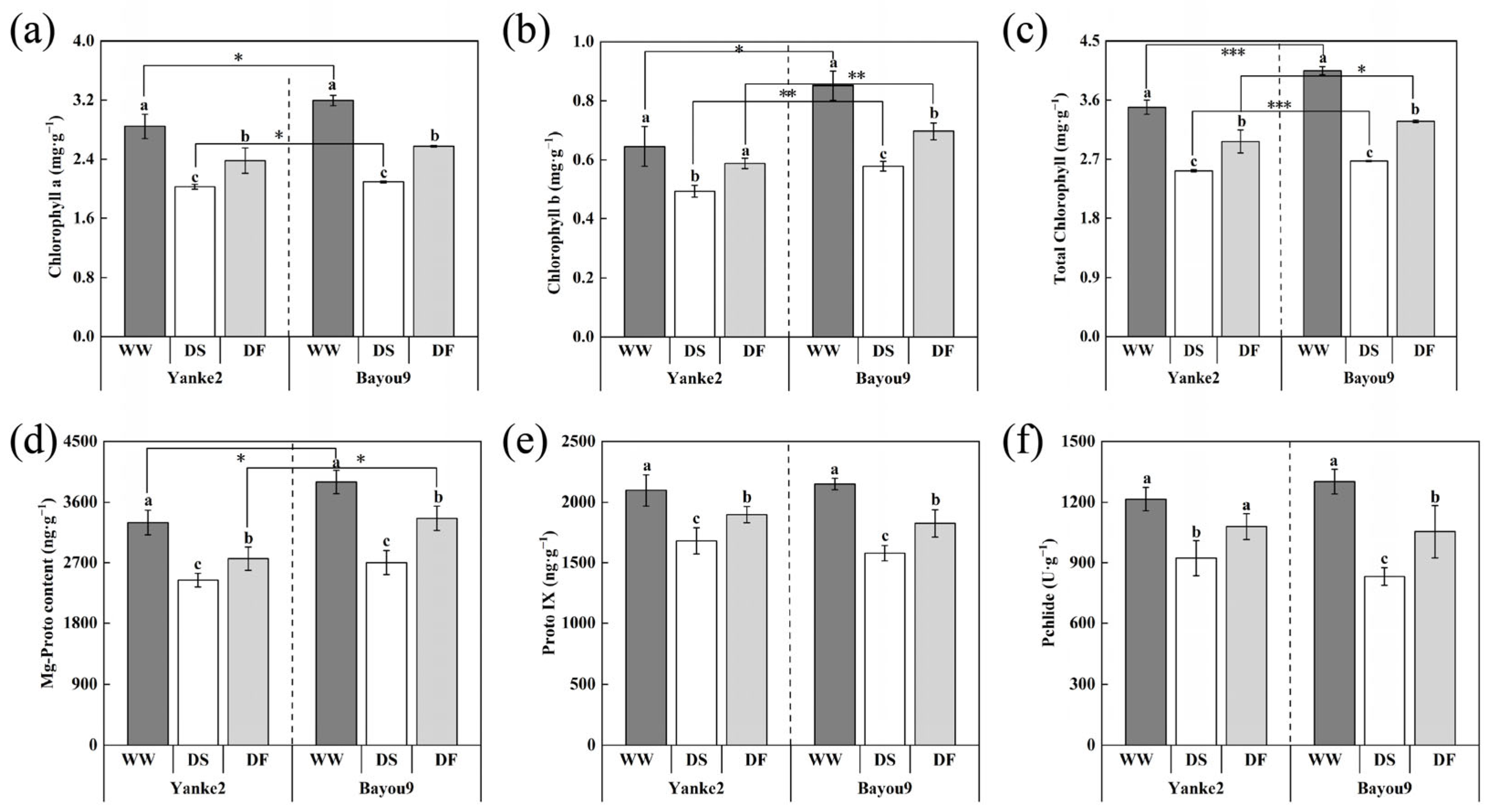
3.4. Effect of FA on Chlorophyll Content and Its Intermediates in Oat Leaves Under Drought Stress
3.5. Effects of FA on Carbon-Assimilating Enzymes in Oat Leaves Under Drought Stress
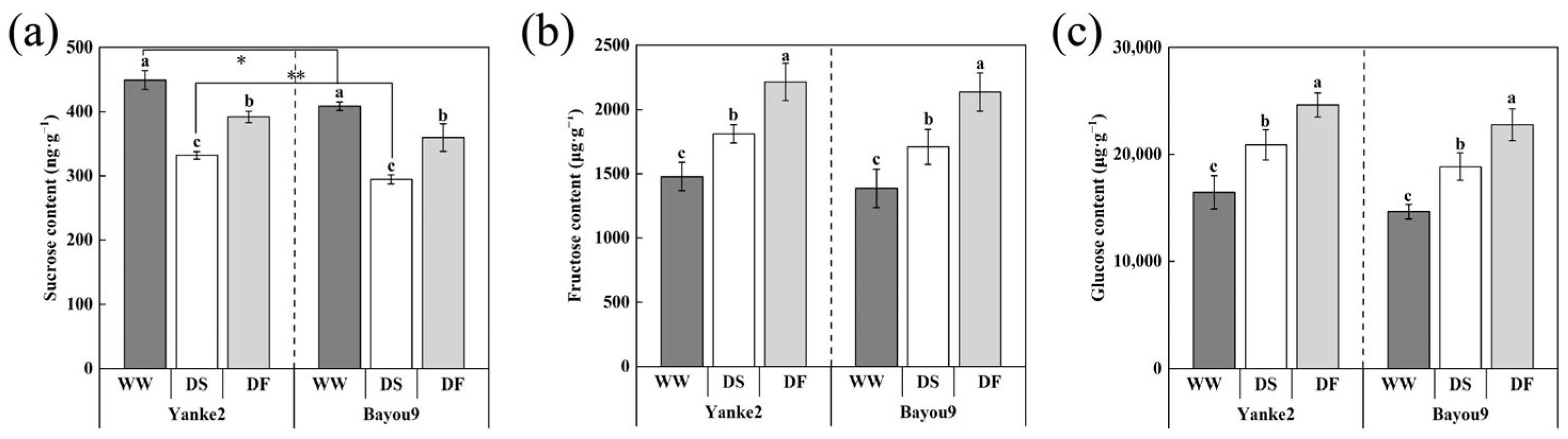
3.6. Effect of FA on Carbon Metabolism in Oat Leaves Under Drought Stress
3.7. Correlation Analysis of Growth and Physiological Indexes Under Different Treatments
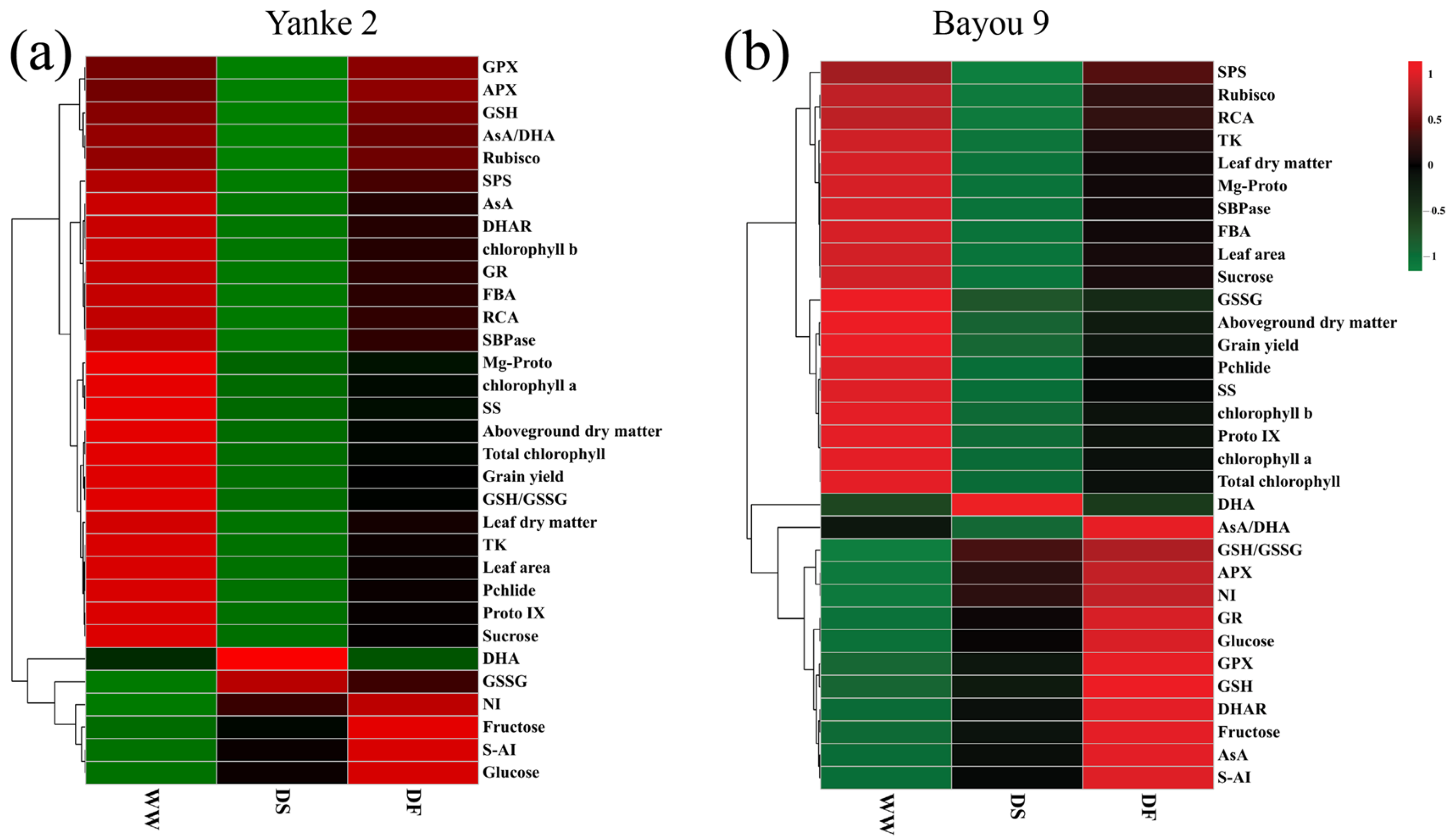
3.8. Hierarchical Cluster Analysis of Growth and Physiological Indicators Under Different Treatments
4. Discussion
4.1. Effect of FA Treatment on Growth and Yield Under Drought Stress
4.2. Effect of FA on AsA-GSH Cycling in Oat Leaves Under Drought Stress
4.3. Effect of FA on Chlorophyll Synthesis in Oat Leaves Under Drought Stress
4.4. Effect of FA on Carbon-Assimilating Enzymes in Oat Leaves Under Drought Stress
4.5. Effect of FA on Carbon Metabolism-Related Enzymes in Oat Leaves Under Drought Stress
4.6. Effect of FA on Carbohydrate Content in Oat Leaves Under Drought Stress
4.7. The Agronomic Implications of FA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Takano, T.; Liu, S. Screening and evaluation of saline–alkaline tolerant germplasm of rice (Oryza sativa L.) in soda saline–alkali soil. Agronomy 2018, 8, 205. [Google Scholar] [CrossRef]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A review of health-beneficial properties of oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Sehrawat, R.; Kong, Y. Oat proteins: A perspective on functional properties. LWT Food Sci. Technol. 2021, 152, 112307. [Google Scholar] [CrossRef]
- Shah, R.S.; Senanayake, M.; Zhang, H.-H.; Pu, Y.; Biswal, A.K.; Pingali, S.V.; Davison, B.; O’Neill, H. Evidence for lignin–carbohydrate complexes from studies of transgenic switchgrass and a model lignin–pectin composite. ACS Sustain. Chem. Eng. 2023, 11, 15941–15950. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Bao, Y.; Liu, F.; Wang, H.; Wang, Y. Oat: Current state and challenges in plant-based food applications. Trends Food Sci. Tech. 2023, 134, 56–71. [Google Scholar] [CrossRef]
- FAO. 2024. Available online: https://www.fao.org/ (accessed on 18 November 2024).
- Salih, W.; Epule, T.E.; EL Khalki, E.M.; Ouatiki, H.; Erraki, S.; Achli, S.; Chehbouni, A. A comprehensive assessment of satellite precipitation products over a semi-arid region: Focus on extreme events. Nat. Hazards 2023, 120, 3037–3065. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, Y.; Zhao, B.; Zhao, Y.; Chen, Y.; Zhou, Q.; Wang, H. Photosynthetic performance and carbon metabolism in the ear organs of oats under drought stress. Front. Plant Sci. 2025, 15, 1463284. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought stress in wheat during flowering and grain-filling periods. Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, Y.; Ma, F. Integrating high-throughput phenotyping and genome-wide association studies for enhanced drought resistance and yield prediction in wheat. New Phytol. 2024, 243, 1758–1775. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, W.; Ma, J.; Cheng, Z.; Zhang, X.; Liu, X.; Wu, X.; Zhang, J. An integrated framework for drought stress in plants. Int. J. Mol. Sci. 2024, 25, 9347. [Google Scholar] [CrossRef]
- Hu, C.; Elias, E.; Nawrocki, W.J.; Croce, R. Drought affects both photosystems in Arabidopsis thaliana. New Phytol. 2023, 240, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.; Ratnitsyna, D.; Gromova, E.; Sukhov, V. Development of two-dimensional model of photosynthesis in plant leaves and analysis of induction of spatial heterogeneity of CO2 assimilation rate under action of excess light and drought. Plants 2022, 11, 3285. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Siddique, K.H.; Ahmad, P. Understanding drought tolerance in plants. Physiol. Plant 2021, 172, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Li, X.; Chen, J.; Li, Y.; Tang, Y.; Lv, J. Photosynthetic and ascorbate-glutathione metabolism in the flag leaves as compared to spikes under drought stress of winter wheat (Triticum aestivum L.). PLoS ONE 2018, 13, e0194625. [Google Scholar] [CrossRef]
- Li, Y.; Hu, W.; Zou, J.; He, J.; Zhu, H.; Zhao, W.; Wang, Y.; Chen, B.; Meng, Y.; Wang, S. Effects of soil drought on cottonseed kernel carbohydrate metabolism and kernel biomass accumulation. Plant Physiol. Biochem. 2023, 195, 170–181. [Google Scholar] [CrossRef]
- Sasi, M.; Awana, M.; Samota, M.K.; Tyagi, A.; Kumar, S.; Sathee, L.; Krishnan, V.; Praveen, S.; Singh, A. Plant growth regulator induced mitigation of oxidative burst helps in the management of drought stress in rice (Oryza sativa L.). Environ. Exp. Bot. 2021, 185, 104413. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Hu, Y.; Hu, Q.; Wen, J.; Chen, Y.; Qian, R.; Zheng, J. Effects of exogenous spraying of melatonin on the growth of Platycrater arguta under drought stress. Front. Plant Sci. 2025, 15, 1516302. [Google Scholar] [CrossRef]
- Lv, X.; Li, Q.; Deng, X.; Ding, S.; Sun, R.; Chen, S.; Yun, W.; Dai, C.; Luo, B. Fulvic acid application increases rice seedlings performance under low phosphorus stress. BMC Plant Biol. 2024, 24, 703. [Google Scholar] [CrossRef]
- Gong, G.-Q.; Yuan, X.; Zhang, Y.-J.; Li, Y.-J.; Liu, W.-X.; Wang, M.; Zhao, Y.-F.; Xu, L.-W. Characterization of coal-based fulvic acid and the construction of a fulvic acid molecular model. RSC Adv. 2020, 10, 5468–5477. [Google Scholar] [CrossRef]
- De Castro, T.A.V.T.; de Oliveira Torchia, D.F.; de Lima, A.C.S.; de Abreu Lopes, S.; Cantarino, R.E.; Rodrigues, N.F.; García, A.C. Conversion of kappaphycus alvarezii macroalgae biomass enriched with fulvic acid into a foliar biostimulant for plant (Oryza sativa L.) growth and stress protection. Chem. Biol. Technol. Agric. 2024, 11, 172. [Google Scholar] [CrossRef]
- Braziene, Z.; Paltanavicius, V.; Avizienytė, D. The influence of fulvic acid on spring cereals and sugar beets seed germination and plant productivity. Environ. Res. 2021, 195, 110824. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, J.; Wang, Z.; Hu, D.; Jiang, Y.; Han, Y.; Wang, Y. Fulvic acid alleviates the stress of low nitrogen on maize by promoting root development and nitrogen metabolism. Physiol. Plant. 2024, 176, e14249. [Google Scholar] [CrossRef] [PubMed]
- Capstaff, N.M.; Morrison, F.; Cheema, J.; Brett, P.; Hill, L.; Muñoz-García, J.C.; Khimyak, Y.Z.; Domoney, C.; Miller, A.J. Fulvic acid increases forage legume growth inducing preferential up-regulation of nodulation and signalling-related genes. J. Exp. Bot. 2020, 71, 5689–5704. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Chen, H.; Xu, X. Physiology and proteomics reveal fulvic acid mitigates cadmium adverse effects on growth and photosynthetic properties of lettuce. Plant. Sci. 2022, 323, 111418. [Google Scholar] [CrossRef]
- Zhu, S.; Mi, J.; Zhao, B.; Wang, Z.; Yang, Z.; Wang, M.; Liu, J. Integrative transcriptome and metabolome analysis reveals the mechanism of fulvic acid alleviating drought stress in oat. Front. Plant Sci. 2024, 15, 1439747. [Google Scholar] [CrossRef]
- Yu, B.; Xue, X.; Nie, P.; Lu, N.; Wang, L. Fulvic acid alleviates cadmium-induced root growth inhibition by regulating antioxidant enzyme activity and carbon–nitrogen metabolism in apple seedlings. Front. Plant Sci. 2024, 15, 1370637. [Google Scholar] [CrossRef]
- Hareem, M.; Danish, S.; Obaid, S.A.; Ansari, M.J.; Datta, R. Mitigation of drought stress in chili plants (Capsicum annuum L.) using mango fruit waste biochar, fulvic acid and cobalt. Sci. Rep. 2024, 14, 14270. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef]
- Iftikhar, F.; Zulfiqar, A.; Kamran, A.; Saleem, A.; Arshed, M.Z.; Zulfiqar, U.; Djalovic, I.; Vara Prasad, P.; Soufan, W. Antioxidant responses in chromium-stressed maize as influenced by foliar and root applications of fulvic acid. Sci. Rep. 2025, 15, 1289. [Google Scholar] [CrossRef]
- Jarošová, M.; Klejdus, B.; Kováčik, J.; Babula, P.; Hedbavny, J. Humic acid protects barley against salinity. Acta Physiol. Plant. 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Liu, C.; Lv, C.; Ai, X.; Bi, H. Effects of fulvic acid on photosynthetic characteristics, yield and quality of cucumber under drought stress. Chin. J. Appl. Ecol. 2022, 33, 1300–1310. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, X.; Zhang, X.; Zhao, D.; Tao, J. Effects of fulvic acid on the photosynthetic and physiological characteristics of Paeonia ostii under drought stress. Plant Signal. Behav. 2020, 15, 1774714. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Mi, J.; Zhao, B.; Wu, J.; Wang, Y.; Liu, J. Effect of fulvic acid on photosynthesis and antioxidant enzyme activities of Avena sativa under drought stress. Acta Bot. Boreal.-Occident. Sin. 2022, 42, 1902–1909. [Google Scholar] [CrossRef]
- Yanming, L. A preliminary study on the determination of leaf area of oat. J. Hebei Agric. Univ. 1993, 16, 25–28. [Google Scholar] [CrossRef]
- Zou, Q. Experimental Guidance on Plant Physiology; China Agriculture Press: Beijing, China, 2000; pp. 33–36. [Google Scholar]
- Park, E.; Cho, M.; Ki, C.-S. Correct use of repeated measures analysis of variance. Korean J. Lab. Med. 2009, 29, 1–9. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Alsudays, I.M.; Alshammary, F.H.; Alabdallah, N.M.; Alatawi, A.; Alotaibi, M.M.; Alwutayd, K.M.; Alharbi, M.M.; Alghanem, S.M.; Alzuaibr, F.M.; Gharib, H.S. Applications of humic and fulvic acid under saline soil conditions to improve growth and yield in barley. BMC Plant Biol. 2024, 24, 191. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, K.; Guo, Y.; Li, T.; Kuang, N.; Liu, Z.; Yang, H. Investigating the mechanism of auxin-mediated fulvic acid-regulated root growth in Oryza sativa through physiological and transcriptomic analyses. Planta 2025, 261, 1–15. [Google Scholar] [CrossRef]
- Bocanegra, M.P.; Lobartini, J.C.; Orioli, G.A. Plant uptake of iron chelated by humic acids of different molecular weights. Commun. Soil Sci. Plant Anal. 2006, 37, 239–248. [Google Scholar] [CrossRef]
- Lalas, S.; Athanasiadis, V.; Dourtoglou, V.G. Humic and fulvic acids as potentially toxic metal reducing agents in water. Clean Soil Air Water 2018, 46, 1700608. [Google Scholar] [CrossRef]
- Shreya, S.; Supriya, L.; Padmaja, G. Melatonin induces drought tolerance by modulating lipoxygenase expression, redox homeostasis and photosynthetic efficiency in Arachis hypogaea L. Front. Plant Sci. 2022, 13, 1069143. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Glutathione-induced drought stress tolerance in mung bean: Coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants 2015, 7, plv069. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Pan, C.; Du, Y.; Li, D.; Liu, W. Exogenous salicylic acid regulates reactive oxygen species metabolism and ascorbate–glutathione cycle in nitraria tangutorum bobr. under salinity stress. Physiol. Mol. Biol. Plants 2018, 24, 577–589. [Google Scholar] [CrossRef]
- Aguiar, N.O.; Medici, L.O.; Olivares, F.L.; Dobbss, L.B.; Torres-Netto, A.; Silva, S.F.; Novotny, E.; Canellas, L.P. Metabolic profile and antioxidant responses during drought stress recovery in sugarcane treated with humic acids and endophytic diazotrophic bacteria. Ann. Appl. Biol. 2016, 168, 203–213. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.G.; Guo, P.Y.; Yuan, X.Y. Effects of humic acid on ascorbate-glutathione cycle in the leaves of foxtail millet seedlings under drought stress. Crops 2021, 2, 173–177. [Google Scholar] [CrossRef]
- Pyngrope, S.; Bhoomika, K.; Dubey, R. Reactive oxygen species, ascorbate-glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma 2013, 250, 585–600. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Dong, B.; Chen, Y.; Kuang, C.; Da, F.; Ding, X. Phytic acid delays the senescence of rosa roxburghii fruit by regulating antioxidant capacity and the ascorbate–glutathione Cycle. Int. J. Mol. Sci. 2024, 26, 98. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Q.; Feng, Y.; Dong, Y.; Zhang, Z.; Wang, Y.; Liu, W. Responsive mechanism of hemerocallis citrina baroni to complex saline-alkali stress revealed by photosynthetic characteristics and antioxidant regulation. Plant Cell Rep. 2024, 43, 176. [Google Scholar] [CrossRef]
- Niu, K.; Ma, H. The positive effects of exogenous 5-aminolevulinic acid on the chlorophyll biosynthesis, photosystem and calvin cycle of Kentucky bluegrass seedlings in response to osmotic stress. Environ. Exp. Bot. 2018, 155, 260–271. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, R.; Yoshida, J.; Cougnon, M.; Reheul, D.; Van Labeke, M.-C. Morpho-physiological responses to dehydration stress of perennial ryegrass and tall fescue genotypes. Funct. Plant Biol. 2017, 44, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Wang, J.; Yang, Q. Exogenous salicylic acid alleviates water deficit stress by protecting photosynthetic system in maize seedlings. Agronomy 2023, 13, 2443. [Google Scholar] [CrossRef]
- Zahoor, R.; Dong, H.; Abid, M.; Zhao, W.; Wang, Y.; Zhou, Z. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017, 137, 73–83. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Ourry, A.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F. Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil. 2012, 359, 297–319. [Google Scholar] [CrossRef]
- Doron, L.; Xu, L.; Rachmilevitch, S.; Stern, D.B. Transgenic overexpression of rubisco subunits and the assembly factor RAF1 are beneficial to recovery from drought stress in maize. Environ. Exp. Bot. 2020, 177, 104126. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Mukherjee, S.; Kumar, R.; Alansi, S.; Shah, A.A.; Kalaji, H.M.; Javed, T.; Raza, A. Potassium and melatonin-mediated regulation of fructose-1, 6-bisphosphatase (FBPase) and sedoheptulose-1, 7-bisphosphatase (SBPase) activity improve photosynthetic efficiency, carbon assimilation and modulate glyoxalase system accompanying tolerance to cadmium stress in tomato seedlings. Plant Physiol. Biochem. 2022, 171, 49–65. [Google Scholar] [CrossRef]
- Raines, C.A.; Harrison, E.P.; Ölçer, H.; Lloyd, J.C. Investigating the role of the thiol-regulated enzyme sedoheptulose-1, 7-bisphosphatase in the control of photosynthesis. Physiol. Plant. 2000, 110, 303–308. [Google Scholar] [CrossRef]
- Li, T.H.; Li, S.H. Leaf responses of micropropagated apple plants to water stress: Nonstructural carbohydrate composition and regulatory role of metabolic enzymes. Tree Physiol. 2005, 25, 495–504. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of drought stress during soybean R2–R6 growth stages on sucrose metabolism in leaf and seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, C.; Sun, T.; Wu, Y.; He, M.; Chen, W.; Zhang, P.; Jiang, H. Beneficial effects of triadimefon in overcoming drought stress in soybean at fluorescence stage. J. Plant Physiol. 2023, 287, 154015. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.; Pollock, C.; Gallagher, J. Sucrose and the integration of metabolism in vascular plants. Plant. Sci. 2000, 154, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, W.; Yang, L.; Zou, Z.; Zhang, X.; Addo-Danso, S.D.; Zhou, L.; Li, S. Effects of drought stress on non-structural carbohydrates in different organs of Cunninghamia lanceolata. Plants 2023, 12, 2477. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, R.M.; Al-Saif, A.M.; Ahmed, M.E.; El-Bary, T.S.A.; Sharma, A.; El-Sheshtawy, A.-N.A.; El-Serafy, R.S.; El-Ghany, T.S.A. Evaluation of two different methods of fulvic acid application (seed priming and foliar spray) on growth, yield, and nutritional quality of Pea (Pisum sativum L.). Plants 2024, 13, 3380. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Xiemuxiding, A.; Zhang, X.; Duan, L.; Li, R. Fulvic acid, brassinolide, and uniconazole mediated regulation of morphological and physiological traits in maize seedlings under water stress. J. Plant Growth Regul. 2023, 42, 1762–1774. [Google Scholar] [CrossRef]




| PH | Total Nitrogen (g kg−1) | Available Phosphorus (mg kg−1) | Available Potassium (mg kg−1) |
|---|---|---|---|
| 7.32 | 1.32 | 18.5 | 145.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Mi, J.; Zhao, B.; Kang, Y.; Wang, M.; Liu, J. Fulvic Acid Enhances Oat Growth and Grain Yield Under Drought Deficit by Regulating Ascorbate–Glutathione Cycle, Chlorophyll Synthesis, and Carbon–Assimilation Ability. Agronomy 2025, 15, 1153. https://doi.org/10.3390/agronomy15051153
Zhu S, Mi J, Zhao B, Kang Y, Wang M, Liu J. Fulvic Acid Enhances Oat Growth and Grain Yield Under Drought Deficit by Regulating Ascorbate–Glutathione Cycle, Chlorophyll Synthesis, and Carbon–Assimilation Ability. Agronomy. 2025; 15(5):1153. https://doi.org/10.3390/agronomy15051153
Chicago/Turabian StyleZhu, Shanshan, Junzhen Mi, Baoping Zhao, Yongjian Kang, Mengxin Wang, and Jinghui Liu. 2025. "Fulvic Acid Enhances Oat Growth and Grain Yield Under Drought Deficit by Regulating Ascorbate–Glutathione Cycle, Chlorophyll Synthesis, and Carbon–Assimilation Ability" Agronomy 15, no. 5: 1153. https://doi.org/10.3390/agronomy15051153
APA StyleZhu, S., Mi, J., Zhao, B., Kang, Y., Wang, M., & Liu, J. (2025). Fulvic Acid Enhances Oat Growth and Grain Yield Under Drought Deficit by Regulating Ascorbate–Glutathione Cycle, Chlorophyll Synthesis, and Carbon–Assimilation Ability. Agronomy, 15(5), 1153. https://doi.org/10.3390/agronomy15051153





