Natural Product-Based Fungicides: Design, Synthesis, and Antifungal Activity of Rhein Derivatives Against Phytopathogenic Fungi
Abstract
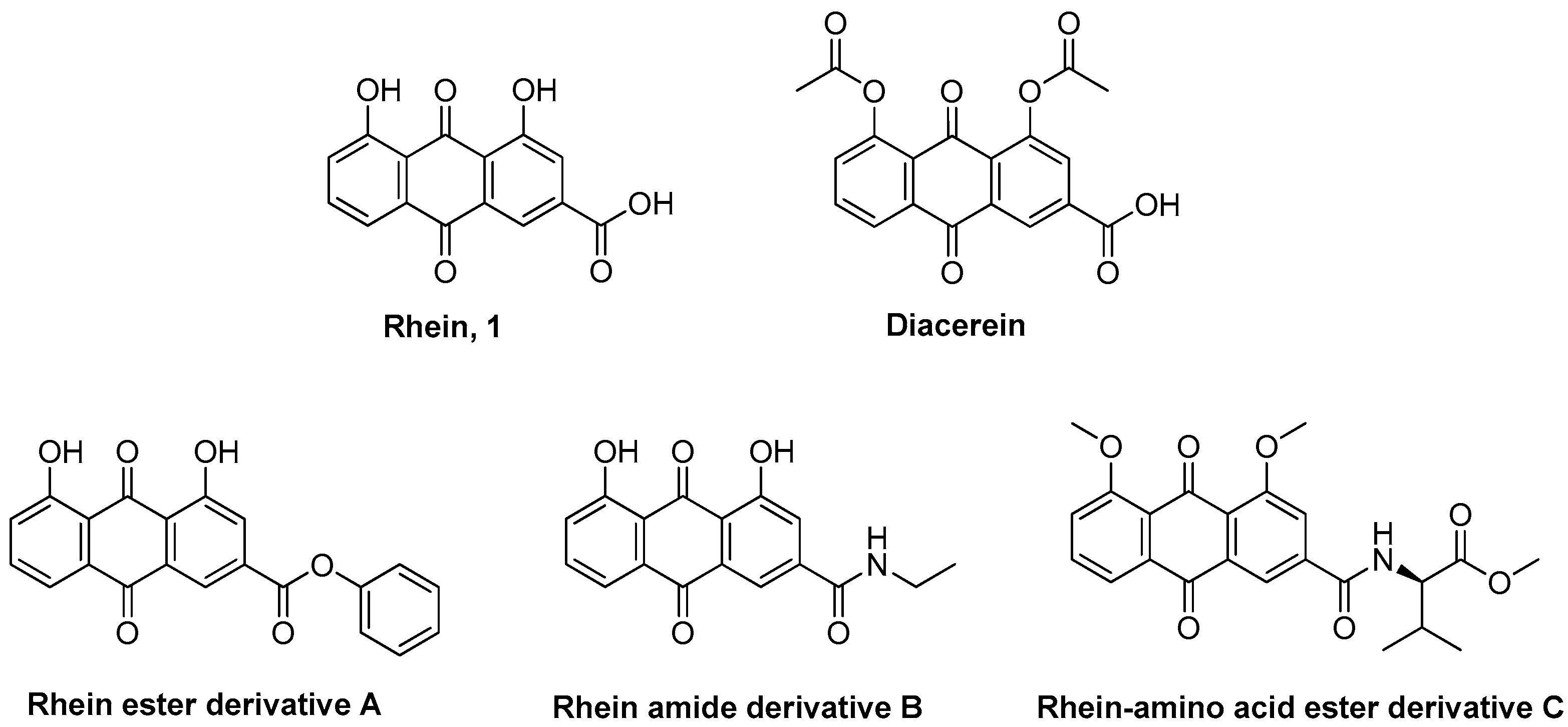
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Chemical Synthesis
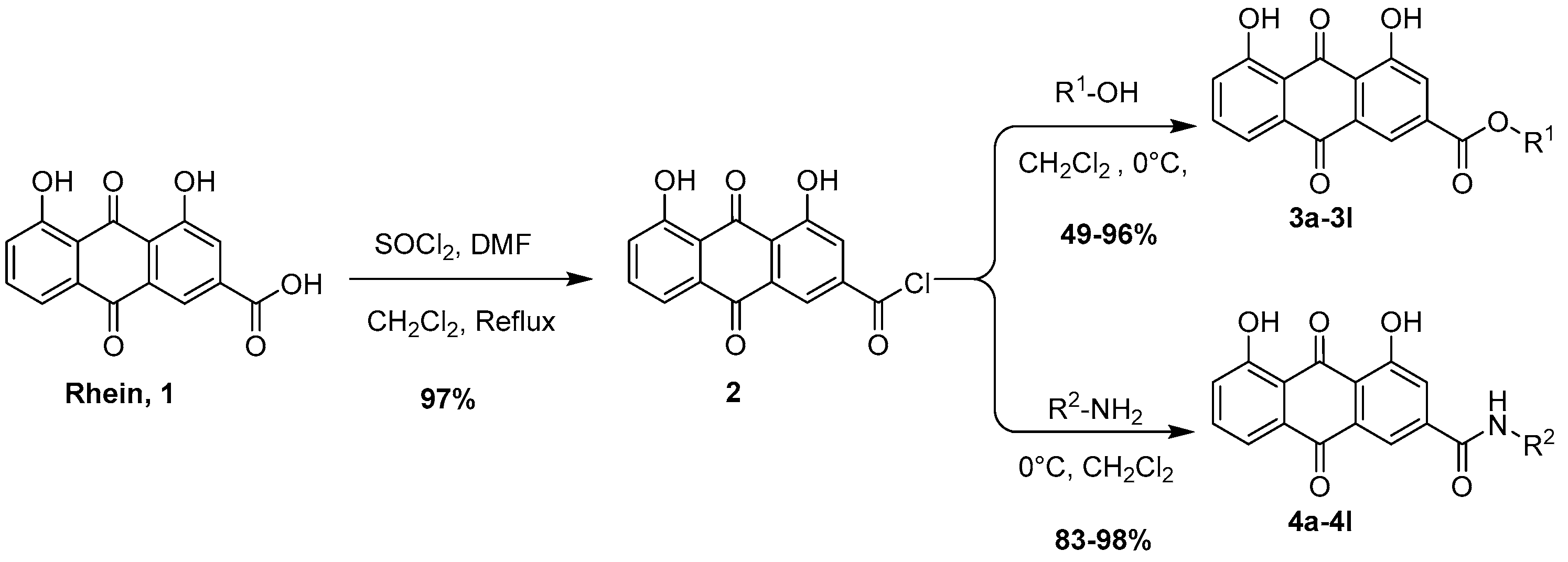
2.2.1. Synthesis of 4,5-Dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carbonyl Chloride (2)
2.2.2. General Procedures for the Synthesis of Compounds 3a–3l
Propyl 4,5-Dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylate (3a)
Isopropyl 4,5-Dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylate (3b)
2.2.3. General Procedures for the Synthesis of Compounds 4a–4l
N-Ethyl-4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (4a)
4,5-Dihydroxy-N-isopropyl-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (4b)
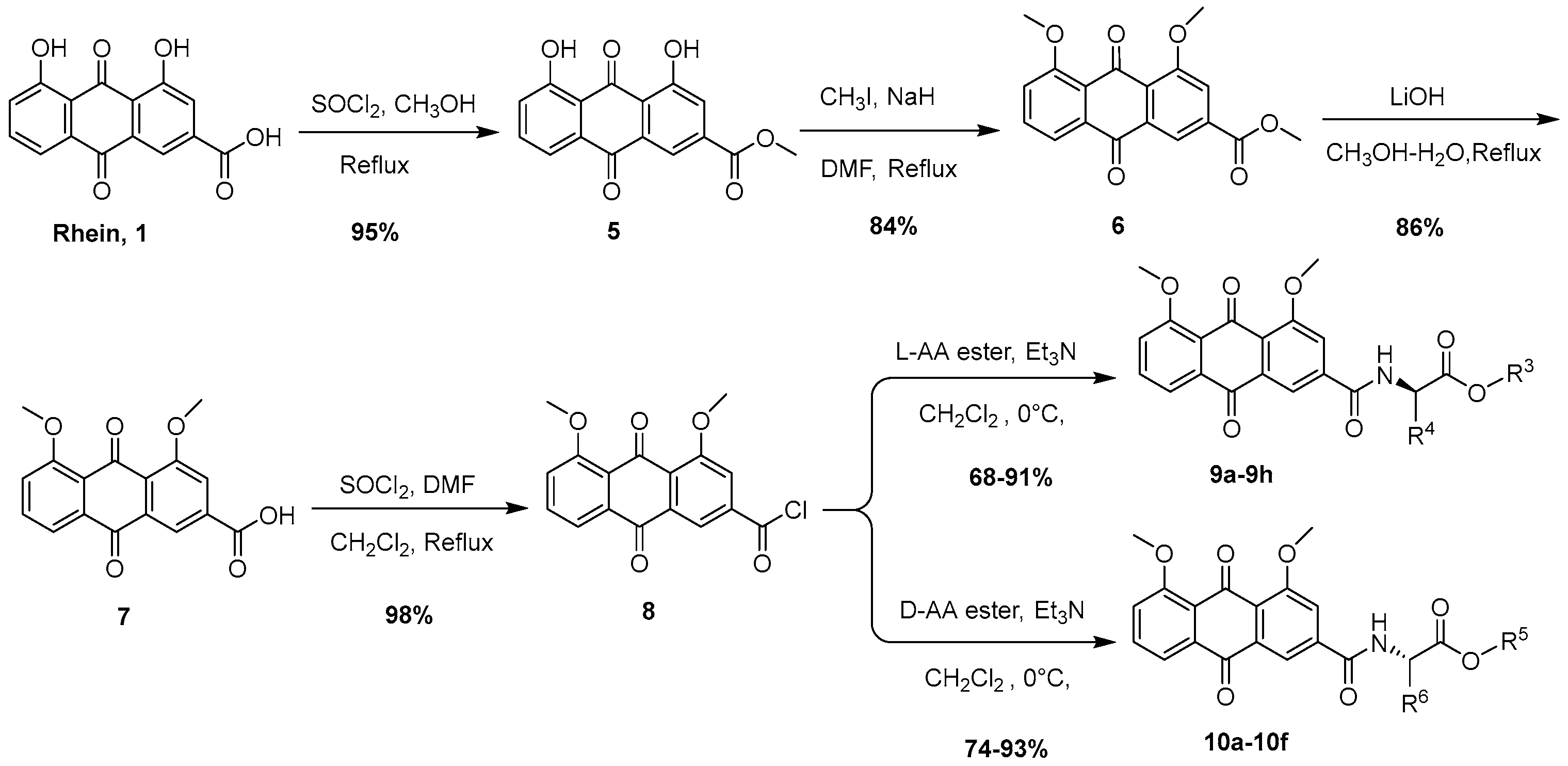
2.2.4. Synthesis of Methyl 4,5-Dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylate (5)
2.2.5. Synthesis of Methyl 4,5-Dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylate (6)
2.2.6. Synthesis of 4,5-Dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic Acid (7)
2.2.7. Synthesis of 4,5-Dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carbonyl Chloride (8)
2.2.8. General Procedures for the Synthesis of Compounds 9a–9h
(R)-Methyl 2-(4,5-Dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxamido)propanoate (9a)
(R)-Methyl 2-(1,8-Dimethoxy-9,10-dioxo-9,10-dihydroanthracene-6-carboxamido)-3-methylbutanoate (9b)
2.2.9. General Procedures for the Synthesis of Compounds 10a–10f
(S)-Ethyl 2-(4,5-Dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxamido)propanoate (10a)
(S)-Methyl 2-(4,5-Dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxamido)-3-methylbutanoate (10b)
2.3. In Vitro Antifungal Assay
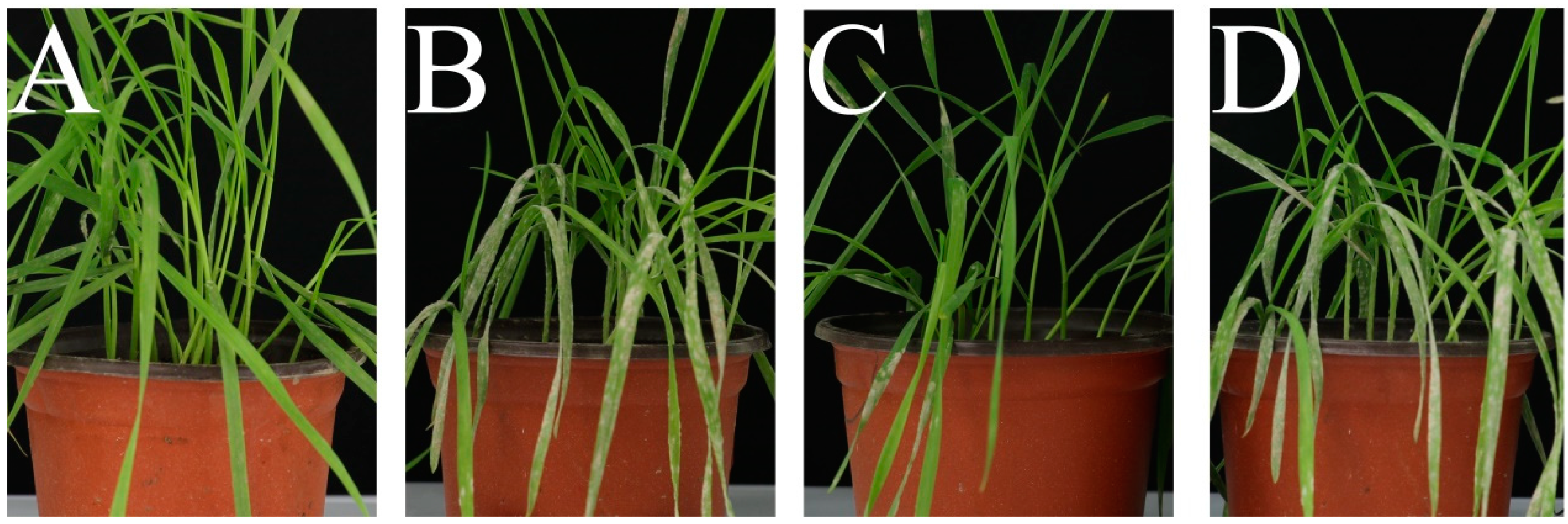
2.4. In Vivo Bioassay Against Wheat Powdery Mildew
2.5. Phytotoxic Bioassay
2.6. Resistance Development Assay
3. Results and Discussion
3.1. Chemistry
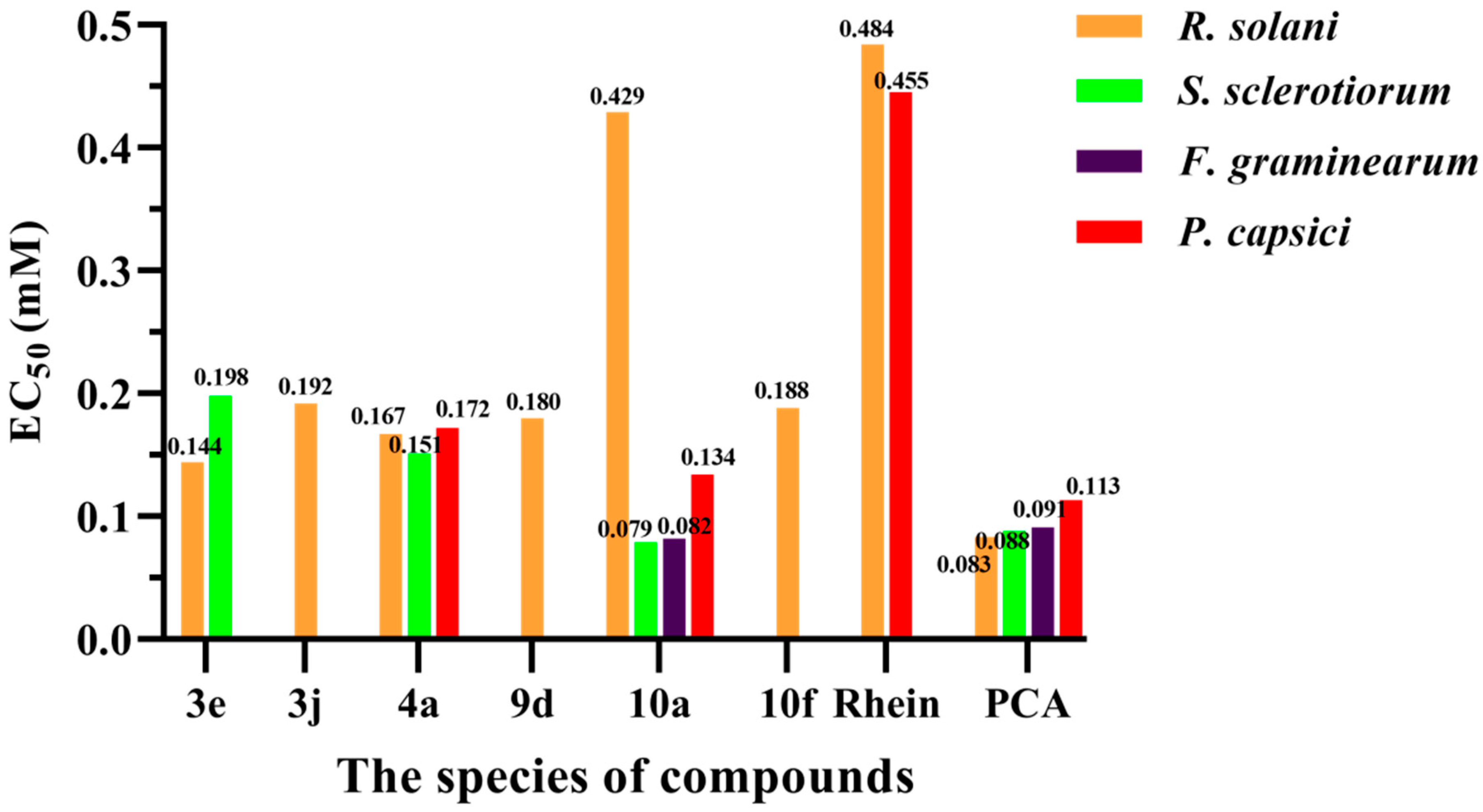
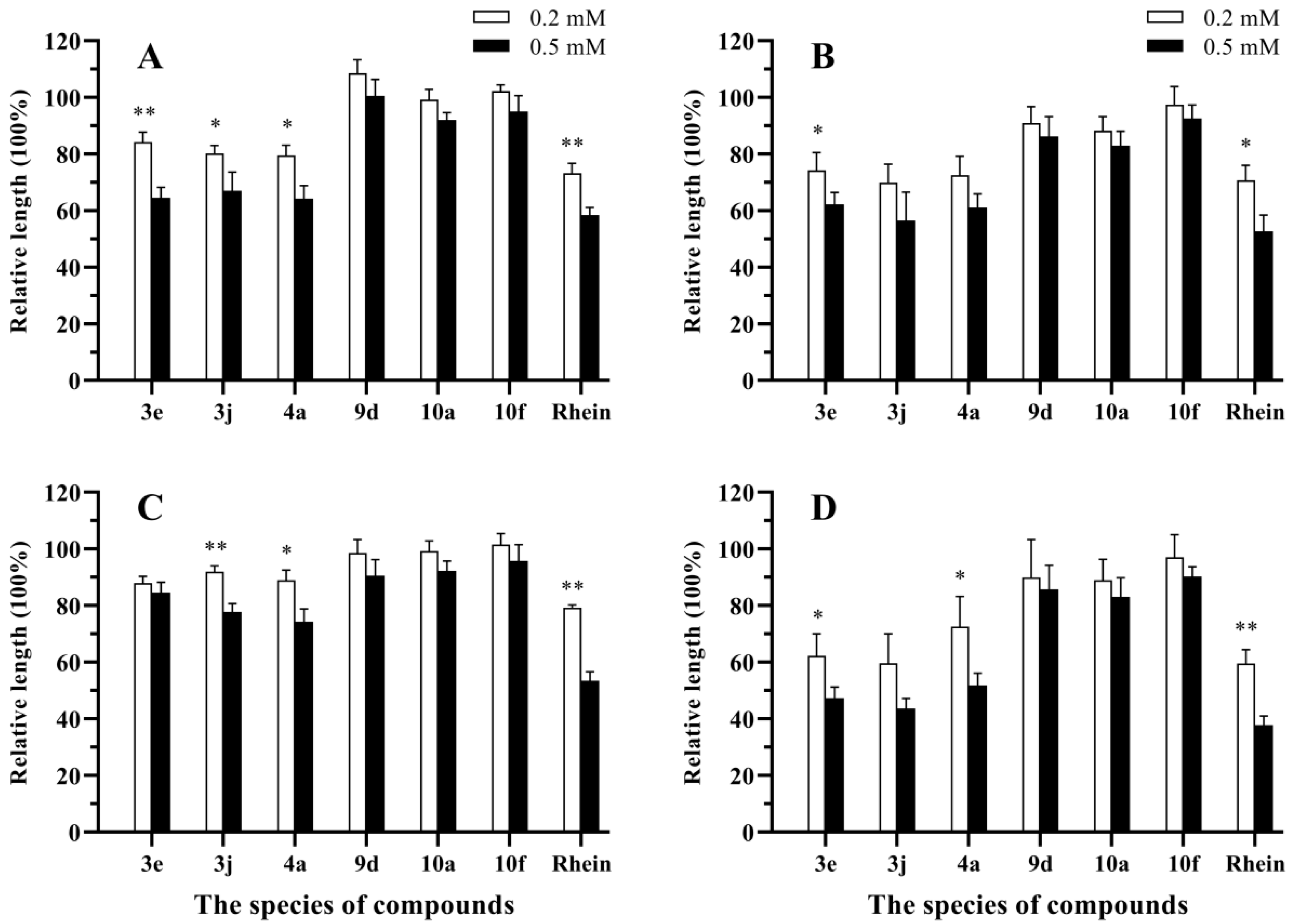
3.2. Antifungal Activities In Vitro
3.3. Antifungal Activities In Vivo
3.4. Phytotoxicity
3.5. Preliminary Resistance Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Knogge, W. Fungal Infection of Plants. Plant Cell 1996, 8, 1711–1722. [Google Scholar] [CrossRef]
- Bebber, D.P.; Gurr, S.J. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet. Biol. 2015, 74, 62–64. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Y.; Duan, X.; He, W.; Si, H.; Wang, P.; Chen, S.; Luo, H.; Rao, X.; Wang, Z.; et al. Novel Citral-thiazolyl Hydrazine Derivatives as Promising Antifungal Agents against Phytopathogenic Fungi. J. Agric. Food Chem. 2021, 69, 14512–14519. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 260, 739–742. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Faria-Ramos, I.; Cruz, L.; Pina-Vaz, C.; Rodrigues, A.G. Genesis of azole antifungal resistance from agriculture to clinical settings. J. Agric. Food Chem. 2015, 63, 7463–7468. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Lorsbach, B.A.; Sparks, T.C.; Cicchillo, R.M.; Garizi, N.V.; Hahn, D.R.; Meyer, K.G. Natural products: A strategic lead generation approach in crop protection discovery. Pest Manag. Sci. 2019, 75, 2301–2309. [Google Scholar] [CrossRef]
- Ayo, R.G. Phytochemical constituents and bioactivities of the extracts of Cassia nigricans Vahl: A review. J. Med. Plants Res. 2010, 4, 1339–1348. [Google Scholar]
- Agarwal, S.K.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef]
- Liang, Y.; Yue, Z.; Li, J.; Tan, C.; Miao, Z.; Tan, W. Natural product-based design, synthesis and biological evaluation of anthra [2,1-d]thiazole-6,11-dione derivatives from rhein as novel antitumour agents. Eur. J. Med. Chem. 2014, 84, 505–515. [Google Scholar] [CrossRef]
- Yang, X.; Sun, G.; Yang, C.; Wang, B. Novel Rhein Analogues as Potential Anticancer Agents. ChemMedChem 2011, 6, 2294–2301. [Google Scholar] [CrossRef]
- Wang, Q.; Yun, S.; Sheng, J.; Gu, L.; Ying, Z.; Chen, X.; Chen, C.; Li, W.; Li, K.; Dai, J. Anti-influenza A virus activity of rhein through regulating oxidative stress, TLR4, Akt, MAPK, and NF-κB signal pathways. PLoS ONE 2018, 13, e0191793. [Google Scholar] [CrossRef]
- Sun, H.; Luo, G.; Chen, D.; Xiang, Z. A Comprehensive and System Review for the Pharmacological Mechanism of Action of Rhein, an Active Anthraquinone Ingredient. Front. Pharmacol. 2016, 7, 247. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, W.; Yue, W.; Peng, C.; Rahman, K.; Zhang, H. Rhein: A Review of Pharmacological Activities. Evid. Based Complement. Altern. Med. 2015, 1, 578107. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Ignacimuthu, S.; Paulraj, M.G. Antifeedant and larvicidal activities of Rhein isolated from the flowers of Cassia fistula L. Saudi J. Biol. Sci. 2011, 18, 129–133. [Google Scholar] [CrossRef]
- Yang, Y.; Lim, M.; Lee, H. Emodin Isolated from Cassia obtusifolia (Leguminosae) Seed Shows Larvicidal Activity against Three Mosquito Species. J. Agric. Food Chem. 2003, 51, 7629–7631. [Google Scholar] [CrossRef]
- Nicolas, P.; Tod, D.M.; Padoin, C.; Petitjean, O. Clinical pharmacokinetics of diacerein. Clin. Pharmacokinet. 1998, 35, 347–359. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, S.; Zhen, Y.; Zhang, Y.; Hsiang, T.; Huang, R.; Qi, J.; Gan, T.; Chang, Y.; Li, J. Antifungal and insecticidal activities of rhein derivatives: Synthesis, characterization and preliminary structure-activity relationship studies. Nat. Prod. Res. 2022, 36, 4140–4146. [Google Scholar] [CrossRef]
- Chen, S.; Wang, M.; Yu, L.; Shi, J.; Zhang, Y.; Tian, Y.; Li, L.; Zhu, X.; Li, J. Rhein–Amino Acid Ester Conjugates as Potential Antifungal Agents: Synthesis and Biological Evaluation. Molecules 2023, 28, 2074. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, M.; Yu, L.; Xu, Z.; Yang, D.; Du, X.; Wu, Q.; Li, J. Synthesis and bioactivities of diamide derivatives containing a phenazine-1-carboxamide scaffold. Nat. Prod. Res. 2019, 33, 2453–2460. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, L.; Hsiang, T.; Huang, D.; Xu, Z.; Wu, Q.; Du, X.; Li, J. The influence of steric configuration of phenazine-1-carboxylic acid-amino acid conjugates on fungicidal activity and systemicity. Pest Manag. Sci. 2019, 75, 3323–3330. [Google Scholar] [CrossRef]
- He, L.; Cui, K.; Ma, D.; Shen, R.; Huang, X.; Jiang, J.; Mu, W.; Liu, F. Activity, Translocation, and Persistence of Isopyrazam for Controlling Cucumber Powdery Mildew. Plant Dis. 2017, 101, 1139–1144. [Google Scholar] [CrossRef]
- Wu, H.; Lu, X.; Xu, J.; Zhang, X.; Li, Z.; Yang, X.; Ling, Y. Design, Synthesis and Fungicidal Activity of N-(thiophen-2-yl) Nicotinamide Derivatives. Molecules 2022, 27, 8700. [Google Scholar] [CrossRef]
- Guan, A.; Wang, M.; Yang, J.; Wang, L.; Xie, Y.; Lan, J.; Liu, C. Discovery of a new fungicide candidate through lead optimization of Pyrimidinamine derivatives and its activity against cucumber downy mildew. J. Agric. Food Chem. 2017, 65, 10829–10835. [Google Scholar] [CrossRef]
- Sui, G.; Song, X.; Zhang, B.; Wang, Y.; Liu, R.; Guo, H.; Wang, J.; Chen, Q.; Yang, X.; Hao, H.; et al. Design, synthesis and biological evaluation of novel neuchromenin analogues as potential antifungal agents. Eur. J. Med. Chem. 2019, 173, 228–239. [Google Scholar] [CrossRef]
| Compd. | Average Inhibition Rate ±SD (%) (n = 3) | |||||||
|---|---|---|---|---|---|---|---|---|
| R. solani | S. sclerotiorum | F. graminearum | P. capsici | |||||
| 0.2 (mM) | 0.5 (mM) | 0.2 (mM) | 0.5 (mM) | 0.2 (mM) | 0.5 (mM) | 0.2 (mM) | 0.5 (mM) | |
| 3a | 41.3 ± 3.9 | 61.4 ± 2.6 | 14.9 ± 0.7 | 38.2 ± 0.6 | 15.5 ± 2.1 | 31.2 ± 1.2 | <10 | 20.7 ± 0.5 |
| 3b | 38.1 ± 1.1 | 56.5 ± 1.5 | 18.1 ± 0.8 | 35.6 ± 1.1 | <10 | 11.7 ± 0.5 | <10 | 21.6 ± 0.3 |
| 3c | 34.8 ± 1.5 | 55.1 ± 2.1 | <10 | 21.9 ± 0.7 | <10 | <10 | <10 | <10 |
| 3d | 24.1 ± 1.6 | 38.7 ± 1.5 | 36.4 ± 1.9 | 59.7 ± 2.1 | 14.8 ± 3.8 | 31.7 ± 0.8 | 12.4 ± 1.9 | 34.4 ± 2.5 |
| 3e | 66.9 ± 1.7 | 82.7 ± 2.3 | 50.3 ± 2.1 | 76.4 ± 3.1 | 35.2 ± 1.3 | 56.8 ± 1.7 | 41.6 ± 2.1 | 71.6 ± 3.8 |
| 3f | 43.0 ± 2.2 | 63.4 ± 2.5 | 31.1 ± 1.0 | 55.3 ± 1.1 | 13.9 ± 1.6 | 36.6 ± 0.8 | 29.1 ± 2.3 | 54.1 ± 2.6 |
| 3g | 13.6 ± 1.9 | 31.3 ± 1.2 | 35.7 ± 2.2 | 55.9 ± 1.1 | <10 | 21.4 ± 1.1 | 29.8 ± 2.1 | 54.6 ± 3.8 |
| 3h | 47.5 ± 1.7 | 69.7 ± 2.4 | 23.9 ± 0.7 | 47.1 ± 0.9 | 28.8 ± 1.3 | 46.9 ± 1.5 | 11.8 ± 1.5 | 37.8 ± 2.6 |
| 3i | 37.4 ± 1.9 | 55.4 ± 1.9 | 19.5 ± 2.1 | 45.1 ± 1.7 | 10.1 ± 0.9 | 29.8 ± 1.3 | 19.2 ± 1.8 | 41.5 ± 3.5 |
| 3j | 51.5 ± 4.9 | 73.9 ± 3.5 | 18.6 ± 1.6 | 34.9 ± 1.2 | <10 | 19.5 ± 0.4 | 13.1 ± 0.8 | 21.5 ± 1.5 |
| 3k | 42.1 ± 0.8 | 61.5 ± 1.3 | <10 | 22.9 ± 1.1 | <10 | 22.3 ± 0.7 | <10 | <10 |
| 3l | 39.7 ± 2.5 | 58.2 ± 3.1 | 14.3 ± 2.0 | 29.1 ± 2.1 | <10 | 19.9 ± 0.3 | 11.6 ± 1.8 | 25.1 ± 2.5 |
| 4a | 58.4 ± 1.1 | 75.7 ± 1.3 | 62.4 ± 0.6 | 87.9 ± 1.2 | 12.8 ± 1.1 | 33.9 ± 1.7 | 56.4 ± 1.5 | 83.4 ± 3.7 |
| 4b | 44.7 ± 1.6 | 61.8 ± 1.7 | <10 | 18.3 ± 0.6 | <10 | 27.0 ± 0.8 | <10 | <10 |
| 4c | 32.7 ± 1.7 | 46.9 ± 1.8 | 21.4 ± 0.6 | 37.1 ± 1.1 | 16.9 ± 2.1 | 34.27% | 11.3 ± 2.7 | 29.5 ± 2.1 |
| 4d | 35.5 ± 0.8 | 51.5 ± 1.1 | 12.1 ± 1.0 | 32.4 ± 0.8 | <10 | 20.8 ± 0.6 | <10 | 10.9 ± 1.8 |
| 4e | 40.7 ± 1.7 | 57.9 ± 2.2 | <10 | 24.7 ± 0.4 | <10 | 20.3 ± 0.8 | <10 | 14.8 ± 2.1 |
| 4f | 36.7 ± 2.0 | 53.7 ± 3.2 | <10 | 28.4 ± 0.7 | 12.9 ± 1.2 | 28.9 ± 1.5 | <10 | 17.7 ± 2.1 |
| 4g | 37.7 ± 3.3 | 53.8 ± 2.1 | <10 | 15.7 ± 0.8 | <10 | 15.8 ± 0.7 | <10 | <10 |
| 4h | 43.5 ± 2.7 | 60.5 ± 1.6 | <10 | 25.1 ± 0.8 | <10 | <10 | <10 | <10 |
| 4i | 37.3 ± 2.4 | 52.5 ± 1.7 | <10 | 13.0 ± 0.2 | 13.3 ± 1.0 | 29.8 ± 1.6 | <10 | 10.7 ± 0.9 |
| 4j | 48.6 ± 0.6 | 64.3 ± 1.3 | <10 | 25.2 ± 0.5 | <10 | 14.3 ± 0.5 | <10 | <10 |
| 4k | 32.0 ± 3.8 | 46.9 ± 4.1 | 11.7 ± 0.7 | 35.1 ± 1.2 | <10 | 25.3 ± 0.6 | <10 | 12.6 ± 1.5 |
| 4l | 35.3 ± 2.1 | 53.6 ± 1.7 | 10.4 ± 1.3 | 31.3 ± 1.3 | <10 | 17.4 ± 0.5 | <10 | 10.2 ± 1.8 |
| 9a | <10 | 29.4 ± 1.8 | <10 | 19.2 ± 0.8 | <10 | <10 | <10 | 15.7 ± 2.5 |
| 9b | <10 | 31.5 ± 1.5 | <10 | 19.4 ± 0.5 | <10 | <10 | 12.1 ± 0.7 | 37.7 ± 2.7 |
| 9c | 20.2 ± 3.0 | 51.6 ± 3.5 | <10 | 24.5 ± 0.8 | <10 | 16.0 ± 1.2 | 11.8 ± 1.2 | 28.8 ± 1.7 |
| 9d | 55.4 ± 2.2 | 83.2 ± 3.5 | 13.2 ± 0.5 | 41.5 ± 1.2 | 12.5 ± 0.6 | 37.6 ± 1.3 | 15.6 ± 1.1 | 35.3 ± 1.7 |
| 9e | 19.8 ± 0.8 | 46.3 ± 2.5 | 34.6 ± 1.4 | 60.5 ± 1.5 | 11.1 ± 0.3 | 32.2 ± 1.1 | 22.7 ± 1.3 | 46.4 ± 1.9 |
| 9f | 28.2 ± 2.1 | 57.5 ± 2.5 | 24.4 ± 0.5 | 47.4 ± 1.0 | <10 | 22.1 ± 1.0 | 11.3 ± 3.0 | 31.3 ± 2.1 |
| 9g | 24.8 ± 1.8 | 50.4 ± 2.3 | 16.7 ± 1.2 | 39.49 ± 1.5 | <10 | 22.6 ± 0.8 | <10 | 29.0 ± 1.7 |
| 9h | 23.9 ± 0.9 | 52.0 ± 2.8 | <10 | 28.6 ± 1.3 | <10 | 13.23 ± 0.3 | 11.2 ± 0.6 | 30.3 ± 1.5 |
| 10a | 38.8 ± 1.2 | 62.1 ± 2.3 | 86.5 ± 0.8 | 100 ± 0.0 | 84.2 ± 1.2 | 100 ± 0.0 | 62.7 ± 1.7 | 85.6 ± 2.3 |
| 10b | 24.9 ± 0.5 | 51.8 ± 1.9 | 19.1 ± 1.0 | 45.1 ± 2.3 | 12.1 ± 0.5 | 31.26 ± 0.8 | 17.6 ± 1.1 | 36.1 ± 1.6 |
| 10c | 13.5 ± 1.5 | 34.4 ± 1.1 | <10 | 29.2 ± 1.1 | <10 | <10 | <10 | 21.7 ± 1.8 |
| 10d | <10 | 28.7 ± 1.8 | <10 | 16.1 ± 0.4 | <10 | 15.2 ± 0.3 | <10 | 15.6 ± 0.7 |
| 10e | 27.1 ± 1.3 | 47.3 ± 1.8 | <10 | 29.9 ± 1.1 | 12.1 ± 0.4 | 36.68 ± 0.6 | 14.7 ± 0.8 | 35.7 ± 2.2 |
| 10f | 54.6 ± 2.1 | 80.0 ± 3.2 | 25.6 ± 0.5 | 55.2 ± 1.2 | 24.5 ± 0.9 | 57.5 ± 1.8 | 12.8 ± 0.4 | 31.7 ± 1.6 |
| Rhein | 28.6 ± 1.3 | 47.8 ± 1.8 | 27.0 ± 1.2 | 45.4 ± 3.1 | 16.1 ± 0.5 | 35.8 ± 1.5 | 35.4 ± 2.4 | 52.6 ± 3.4 |
| PCA | 86.3 ± 0.9 | 100 ± 0.0 | 84.6 ± 1.2 | 100 ± 0.0 | 83.2 ± 0.8 | 100 ± 0.0 | 79.8 ± 1.6 | 97.5 ± 1.3 |
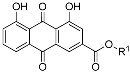 | 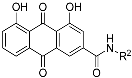 | ||
|---|---|---|---|
| Compd. | R1 | Compd. | R2 |
| 3a |  | 4a |  |
| 3b | 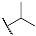 | 4b | 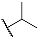 |
| 3c |  | 4c |  |
| 3d | 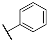 | 4d |  |
| 3e | 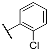 | 4e | 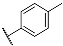 |
| 3f | 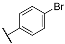 | 4f |  |
| 3g | 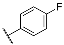 | 4g | 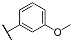 |
| 3h | 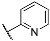 | 4h | 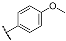 |
| 3i | 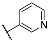 | 4i | 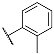 |
| 3j | 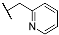 | 4j | 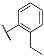 |
| 3k |  | 4k | 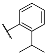 |
| 3l |  | 4l |  |
 | 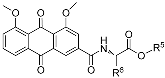 | ||||
|---|---|---|---|---|---|
| Compd. | R3 | R4 | Compd. | R5 | R6 |
| 9a | CH3 | 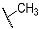 | 10a | CH2CH3 | 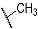 |
| 9b | CH3 | 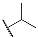 | 10b | CH3 | 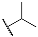 |
| 9c | CH2CH3 |  | 10c | CH3 |  |
| 9d | CH2CH3 | 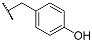 | 10d | CH2CH3 | 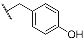 |
| 9e | CH3 | 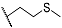 | 10e | CH3 | 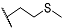 |
| 9f | CH3 |  | 10f | CH3 | 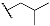 |
| 9g | CH2CH3 | 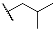 | |||
| 9h | CH3 | 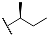 | |||
| Compd. | Curative Activity | Protective Activity | ||
|---|---|---|---|---|
| Disease Index (%) | Control Efficiency (%) | Disease Index (%) | Control Efficiency (%) | |
| 10a | 7.9 | 92.1 a | 7.3 | 91.1 a |
| Rhein | 87.9 | 12.1 c | 70.0 | 15.4 c |
| Physcion | 61.6 | 38.4 b | 27.1 | 67.2 b |
| CK | 100 | - | 82.7 | - |
| Compd. | Average Inhibition Rate ± SD (%) (n = 3) | |||||
|---|---|---|---|---|---|---|
| R. solani | S. sclerotiorum | |||||
| Generation I | Generation II | Generation III | Generation I | Generation II | Generation III | |
| 3e | 84.3 ± 1.3 | 82.3 ± 2.2 | 83.8 ± 1.7 | 77.4 ± 2.3 | 76.5 ± 1.8 | 76.1 ± 2.5 |
| 4a | 74.8 ± 0.9 | 75.6 ± 1.2 | 74.4 ± 1.1 | 89.2 ± 1.7 | 88.5 ± 2.1 | 88.1 ± 1.5 |
| 9d | 82.7 ± 2.4 | 81.3 ± 1.8 | 81.7 ± 2.1 | - | - | - |
| 10a | - | - | - | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| 10f | 81.0 ± 3.1 | 79.6 ± 2.3 | 80.2 ± 2.4 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Li, L.; Shi, J.; Tian, Y.; Mao, G.; Zhang, X.; Yu, L.; Li, J. Natural Product-Based Fungicides: Design, Synthesis, and Antifungal Activity of Rhein Derivatives Against Phytopathogenic Fungi. Agronomy 2025, 15, 1148. https://doi.org/10.3390/agronomy15051148
Zhu X, Li L, Shi J, Tian Y, Mao G, Zhang X, Yu L, Li J. Natural Product-Based Fungicides: Design, Synthesis, and Antifungal Activity of Rhein Derivatives Against Phytopathogenic Fungi. Agronomy. 2025; 15(5):1148. https://doi.org/10.3390/agronomy15051148
Chicago/Turabian StyleZhu, Xiang, Li Li, Jinchao Shi, Yao Tian, Guoqing Mao, Xiaojun Zhang, Linhua Yu, and Junkai Li. 2025. "Natural Product-Based Fungicides: Design, Synthesis, and Antifungal Activity of Rhein Derivatives Against Phytopathogenic Fungi" Agronomy 15, no. 5: 1148. https://doi.org/10.3390/agronomy15051148
APA StyleZhu, X., Li, L., Shi, J., Tian, Y., Mao, G., Zhang, X., Yu, L., & Li, J. (2025). Natural Product-Based Fungicides: Design, Synthesis, and Antifungal Activity of Rhein Derivatives Against Phytopathogenic Fungi. Agronomy, 15(5), 1148. https://doi.org/10.3390/agronomy15051148






