Abstract
The novel hydro-electro hybrid priming (HEHP) technique, which synergistically combines controlled hydration and electrostatic field application, represents an innovative chemical-free approach to improve seed germination synchrony. However, the regulatory mechanism of HEHP on cell wall remodeling during post-imbibition remains unclear. Here, we demonstrate that HEHP accelerates carrot (Daucus carota L.) seed germination by synchronizing cell wall hydrolysis and synthesis pathways. Comparative transcriptomics revealed 4591 differentially expressed genes (DEGs) between HEHP-treated and untreated seeds, with significant enrichment in cell wall organization (GO terms) and phenylpropanoid biosynthesis (KEGG pathway). HEHP significantly induced the expression of expansin (EXP), hydrolases (xyloglucan endotransglucosylase (XET), pectinesterase (PE), and phenylalanine ammonia lyase (PAE)), and synthases (cellulose synthase (CesA)), reducing endosperm rupture force considerably at S20 compared to hydropriming (HYD). Enzymatic assays confirmed earlier activity peaks for XET and PE in HEHP, correlating with the sustained expression of key genes. Notably, HEHP pre-activated germination-related metabolism, evidenced by fewer post-imbibition DEGs, and synchronized lignin deposition via transient phenylalanine ammonia lyase (PAL) and 4-coumarate/CoA ligase (4CL) activation. These synergies enabled faster radicle emergence than HYD. Our findings reveal that HEHP optimizes cell wall loosening–reinforcement dynamics through transcriptional priming, offering a tailored solution for mechanized sowing in Apiaceae crops.
1. Introduction
Seed germination marks the critical transition from quiescence to active plant growth, a process tightly regulated by the interplay of metabolic reactivation and cellular expansion [1,2]. As a crucial root vegetable, the cultivation area and yield of carrot (Daucus carota L.) in China rank first globally. However, due to differences in the quality of carrot seeds caused by the arrangement of umbels, and the fact that the seeds are leathery with prickly hairs and contain volatile oils, they have poor permeability and slow and irregular germination [3,4]. In addition, the traditional sowing method of carrots requires a large amount of seeds, which ultimately leads to the uneven emergence of carrots after sowing and time-consuming and laborious intercropping and seedling establishment. The mechanized precision sowing mode has been applied to the large-scale planting of carrots in recent years, but this sowing mode requires the higher speed and uniformity of seed germination. Seed priming technologies, such as hydropriming (HYD), have been widely adopted to enhance the germination characteristics by triggering pregermination metabolism without radicle protrusion [5,6,7]. However, conventional HYD often shows suboptimal efficacy in improving germination uniformity, particularly under mechanized sowing conditions [8,9].
Recently, physical priming methods combining hydration with electromagnetic stimuli (e.g., magnetic fields and cold plasma) have emerged as innovative strategies to enhance priming effects without any chemical intervention [10,11,12]. Electrostatic field treatment, as a low-cost technology, has been proven to rapidly improve germination performance in various crops in a short period of time [13,14,15]. Our previous study demonstrated that hydro-electro hybrid priming (HEHP), a novel technique integrating hydration with electrostatic field exposure, significantly accelerated reserve mobilization in dry carrot seeds by activating the glyoxylate cycle and respiratory pathways [16]. A transcriptomic analysis of pre-imbibed seeds revealed the upregulation of isocitrate lyase (ICL) and malate dehydrogenase (MDH), facilitating lipid-to-carbohydrate conversion [16,17]. While these findings explained the preparatory metabolic shifts during priming, the regulatory mechanisms governing post-imbibition events, particularly cell wall loosening and radicle emergence, remain unexplored.
The process of cell wall remodeling in the early stage of seed swelling has received widespread attention [18,19,20]. Due to the thickening of the cell wall, the endosperm of carrot seeds has high mechanical strength. The smooth completion of germination needs to be coordinated with the decrease in the mechanical strength of the endosperm after swelling, that is, the process of endosperm weakening [21]. The post-imbibition phase requires coordinated cell wall modification and energy homeostasis to enable embryo expansion. Another research study of our group has demonstrated that the rapid germination of carrot seeds is closely related to the enzymatic loosening mechanism of endosperm weakening and that β-mannanase plays a key role in this process [22]. In addition to this, other hydrolases (e.g., pectinesterase and xyloglucan endotransglucosylases) and the important nonenzymatic factors, expansins, are also known to mediate cell wall loosening during germination [23,24,25]. At present, there are few studies reporting the role of these mechanisms in regulating the seed germination of carrots. Moreover, there is also vigorous cell wall synthesis activity (synthesis of cellulose and lignin) during the germination stage [26,27]. The secondary metabolites, like phenylpropanoids, may participate in reshaping cell wall architecture during the germination process [28,29]. The synergy of cell wall decomposition and synthesis activities is of great significance for the smooth completion of seed germination. Nevertheless, how HEHP synergy modulates these processes during germination remains unknown.
Building upon our prior discovery of HEHP-induced metabolic priming before imbibition, it is hypothesized that HEHP synchronizes cell wall remodeling through the coordinated regulation of both hydrolytic and synthetic pathways. To further confirm the relationship between the rapid germination performance and cell wall remodeling of carrot seeds treated with HEHP, the present study investigates the post-imbibition effects through the comparative transcriptomics of germinating carrot seeds. Furthermore, by combining the determination of endosperm mechanical properties, the activity of key enzymes, and the analysis of the expression patterns of key genes involved in cell wall remodeling pathways, it was confirmed that the process of cell wall remodeling has a prominent response to the priming treatments, especially the HEHP treatment. This work not only deepens our understanding of the mechanism by which HEHP promotes rapid germination of carrot seeds but also provides molecular targets for optimizing HEHP technology in horticultural crops.
2. Materials and Methods
2.1. Seed Material and Priming Treatments
Carrot (Daucus carota L.) seeds (cultivar Naaisi) were obtained from Shanghai Wells Seeds Co., Ltd. (Shanghai, China). The seeds were harvested in 2022 and stored at 5 °C and 35% relative humidity prior to use. Three treatments were applied: control (CK), hydro-priming (HYD), and hydro-electro hybrid priming (HEHP).
The parameters for HYD and HEHP were optimized based on our previous studies [16,22]. For the HYD treatment, seeds were soaked in distilled water at 20 °C for 6 h, followed by incubation in a climate chamber (QHX-300BSH-III, Jiangnan Instrument Co., Ltd., Ningbo, China) at 22 °C and 98% relative humidity under dark conditions for 48 h. After imbibition, seeds were desiccated at 25 °C in a drum wind dryer until their initial weight was restored. For the HEHP treatment, following the 6 h soaking step (as in HYD), seeds were surface-dried and evenly spread on the cathode plate (10 cm × 10 cm) of a BM-201 electrostatic field generator (Boya Electric Co., Ltd., Wuxi, China). A high-voltage electrostatic field (2.0 kV/cm) was applied for 90 s. Subsequent imbibition and drying steps were identical to the HYD protocol. Untreated seeds served as the control (CK). All primed seeds were subjected to germination assays and physiological analyses.
2.2. Germination Assay and Determination of Mechanical Properties of Endosperm
The carrot seeds of each treatment were spread evenly in 90 mm culture dishes with two layers of filter paper (50 seeds per dish); 3 mL of distilled water was added to each dish, and 0.5 mL of distilled water was replenished every 20 h. The dishes were placed in an artificial climate chamber (QHX-300BSH-III) at 20 °C and 80% relative humidity, and a 14 h/10 h optical period was set. The max rupture force of carrot seed endosperm tissue was determined using an electronic universal testing machine (Instron 5543) produced by Instron Corporation (Norwood, MA, USA). The experimental machine has a range of 500 N and a force sensor accuracy of 0.0001 N. The seeds were placed horizontally. When loading, the lower loading plate is fixed and stationary, while the upper loading plate moves downward at a constant speed set to 1.5 mm/min in this experiment. The deadline for loading is to detect a rapid decrease in force on the loading plate, which indicates that damage has occurred at a certain position inside the seed [30]. Each treated seed sample was measured once before sowing, and every 10 h after sowing, samples were taken and measured until 40 h of imbibition, for a total of 5 measurements (S0, S10, S20, S30, and S40), with 10 seeds measured per treatment.
2.3. Transcriptome Sequencing and Analysis
2.3.1. RNA Extraction and Library Construction
Carrot seeds of 20 h post-imbibition from different treatments (approximately 0.1 g of seeds per replication, three biological replicates per treatment) were flash-frozen in liquid nitrogen and ground into powder. RNA isolation was performed with a TRIzol reagent (Invitrogen, Waltham, MA, USA) following the manufacturer’s protocol. RNA integrity was verified using an Agilent 2100 Bioanalyzer (RIN ≥ 7.5), and concentration/purity (OD260/280 = 1.8–2.2) was measured using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Strand-specific cDNA libraries were constructed using the TruSeq Stranded mRNA Library Prep Kit (Illumina, San Diego, CA, USA).
2.3.2. Sequencing and Data Preprocessing
Libraries were quantified using a Qubit 3.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) and quality-checked with an Agilent 2100 system (Agilent Technologies, Santa Clara, CA, USA). Paired-end 150 bp (PE150) sequencing was performed on an Illumina NovaSeq 6000 platform. Raw data were processed using Fastp software (v0.23.1) to remove low-quality reads (Q < 20), adapter contaminants, and reads with >5% ambiguous bases (N content), yielding high-quality clean reads.
2.3.3. Sequence Alignment and Differential Expression Analysis
Clean reads were aligned to the carrot reference genome (https://www.ncbi.nlm.nih.gov/genome/?term=Daucuscarota (accessed on 1 November 2023)). Alignment rates were calculated using SAMtools. Gene expression levels were quantified as TPM (Transcripts Per Million) using StringTie. Differentially expressed genes (DEGs) were identified with DESeq2 (v1.34.0) using thresholds of |log2(fold change)| ≥ 1 and an adjusted p-value (FDR) ≤ 0.05. Differential expression analysis between three key comparisons was conducted (CK vs. HYD, CK vs. HEHP, and HYD vs. HEHP).
2.3.4. Enrichment Analysis of DEGs
The software Goatools (v0.9.9, https://github.com/tanghaibao/GOatools (accessed on 1 November 2023)) and KOBAS (v0.9.9, http://kobas.cbi.pku.edu.cn/home.do (accessed on 1 November 2023)) were used for the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of the DEGs.
2.4. Activity Determination of Key Enzymes in Cell Wall Remodeling Pathways
Carrot seeds subjected to priming treatments were sampled at three stages: before sowing (S0), 20 h post-imbibition (S20), and 40 h post-imbibition (S40). Carrot seeds (approximately 0.2 g per replication) were rapidly snap-frozen in liquid nitrogen and stored at −80 °C. All enzyme activity assays were performed with four biological replicates. The activities of pectinesterase (PE, EC 3.1.1.11), xyloglucan endotransglucosylase (XET, EC 2.4.1.207), phenylalanine ammonia lyase (PAL, EC 4.3.1.23, 4.3.1.24, and 4.3.1.25), and cellulose synthase (CesA, EC 2.4.1.12) were determined. The determination of PE activity was based on the spectrophotometric method (based on methanol release) [31]. In total, 0.1 mL of crude enzyme solution was mixed with 0.5 mL of 1% pectin (dissolved in 50 mM Tris HCl, pH 7.5) and incubated at 37 °C for 30 min, and 0.5 mL of 0.1 M NaOH was added to terminate the reaction. The control group consisted of an enzyme solution inactivated in a boiling water bath. After centrifugation, 0.2 mL of the supernatant was taken from the reaction solution, and 0.8 mL of hydroxylamine hydrochloric acid solution (0.5 M, pH 6.8) and 0.1 mL of 4 M HCl were added in sequence. After standing for 10 min, 0.5 mL of 5% FeCl3 was added, and the absorbance value at 550 nm was measured. The enzyme activity was expressed as the amount of methanol released per gram of fresh sample per unit time. The PAL activity was determined by detecting the amount of trans cinnamic acid produced [32]. The reaction system contained 0.1 mL of enzyme solution and 0.9 mL of 20 mM L-phenylalanine (dissolved in 50 mM boric acid buffer, pH 8.8). After reacting at 37 °C for 30 min, 0.1 mL of 6 M HCl was added to terminate the reaction. The control group replaced the substrate with a buffer solution. The absorbance change of the reaction solution was measured at 290 nm. The enzyme activity was expressed as the amount of cinnamic acid generated per minute per gram of sample. The activities of XET and CesA were determined by assay kits (Michy Biomedical Technology Co., Ltd., Suzhou, China) [33,34].
2.5. qRT-PCR Analysis of Key DEGs in Cell Wall Remodeling Pathways
The DEGs encoding XET, expansins (EXP), PE, pectin acetylesterase (PAE, EC 3.1.1.6), PAL, 4-coumarate/CoA ligase (4 CL, EC 6.2.1.12), and CesA were selected for the expression pattern research. Primers were designed using Primer 5.0 (Table S1), with actin-7 (LOC108202619) of carrot as the reference gene.
The total RNA of the seed samples at S0, S20, and S40 was extracted using TRIzol reagent (Invitrogen, USA). cDNA synthesis was performed with the Goldenstar RT6 cDNA Synthesis Kit (Tsingke Biotechnology, Shanghai, China). qPCR reactions containing 2× T5 Fast qPCR Mix (SYBR Green I) were run on a QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific, USA). Amplification conditions: 95 °C for 1 min (initial denaturation); 40 cycles of 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 30 s; followed by melt curve analysis (95 °C for 5 s, 60 °C for 1 min, and continuous fluorescence acquisition from 60 °C to 95 °C). Relative gene expression was calculated using the 2−ΔΔCt method with three technical replicates.
2.6. Statistical Analysis
SPSS 23.0 was used to process the enzyme activity index and qRT-PCR analysis data. Statistical analysis was performed via ANOVA, and Duncan’s multiple comparison test was used for multiple comparisons of mean values between treatments.
3. Results
3.1. Differences in Endosperm Mechanical Characteristics of Carrot Seeds
In order to understand the mechanical properties of the endosperm in the early stage of carrot seed germination, the maximum rupture force that the seeds can withstand before rupture was determined at different time points for each treatment. As shown in Figure 1A, the max rupture force of carrot seeds decreases the fastest during the initial 10 h of imbibition, indicating that the max rupture force is closely related to the moisture content of the seeds. Primed seeds (HYD and HEHP) showed consistently lower rupture force than CK from pre-sowing through imbibition. In addition, at the two time points of S10 and S20 in the early stage of imbibition, the max rupture force of HEHP treatment was considerably lower than that of HYD treatment. Based on the germination data from previous studies, HEHP has a significantly better promoting effect on carrot seed germination than HYD [16,22]. The germination phenomenon of seeds treated with HEHP could be observed at S20, earlier than the HYD treatment and CK (Figure 1B).
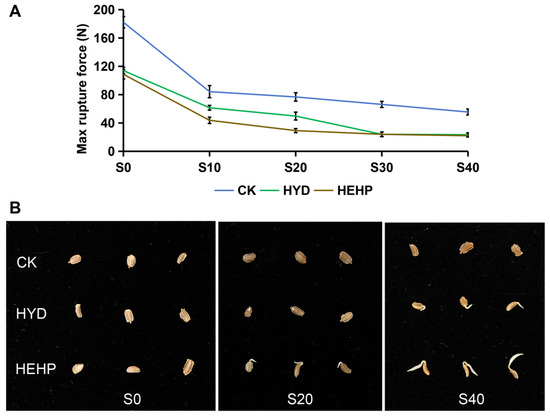
Figure 1.
Endosperm mechanical characteristics and germination phenotypes of carrot (Daucus carota L.) seeds under CK, HYD, and HEHP treatments. (A) The variation in maximum rupture of carrot seeds treated with different treatment force over time. (B) The germination phenotype of carrot seeds at different sampling time points.
3.2. Statistics of DEGs and Comparison with the Number of DEGs Sampled Before Imbibition
According to the comparison results, the alignment efficiency between the reads of each sample and the reference genome ranges from 87.28% to 88.05% (Table S2). The quantitative analysis of the gene expression level by the TPM quantitative index showed that 20,500, 21,088, and 21,117 genes were identified in the CK, HYD, and HEHP treatments, respectively. Among them, 519, 159, and 350 genes were specifically expressed in CK, HYD, and HEHP, respectively, while 19,667 genes were co-expressed in all treatments (Figure 2). Principal component analysis (PCA) demonstrated clear segregation among treatment groups, with HEHP samples clustering distantly from both HYD and CK (Figure S1), indicating substantial transcriptional reprogramming induced by the hybrid priming protocol (Figure S1). The detailed information on all the identified DEGs is shown in Table S3. As shown in Table 1 and Figure S2, for DEGs between CK and HEHP, with the screening threshold of |log2FC| > 1 and p-adjust values ≤ 0.05, the maximum number of DEGs appeared between CK and HEHP (4591 DEGs including 3168 upregulated and 1423 downregulated), while the minimum number of DEGs appeared between HYD and HEHP (90 DEGs, including 58 upregulated and 32 downregulated). This result indicated that the difference in gene expression patterns between seeds treated with HEHP and HYD rapidly decreased after imbibition for 24 h, which showed a gap with the transcriptome data from samples before imbibition [9]. So, for each treatment, the comparison with the number of DEGs sampled before imbibition was also conducted. As shown in Table 1, it can be seen that after 24 h of imbibition, the largest number of DEGs appeared in CK compared with that before imbibition, followed by HYD treatment and HEHP treatment, which confirms that HEHP has a stronger effect on the early initiation of germination-related metabolism. Furthermore, in each comparison group, DEGs were mainly upregulated.
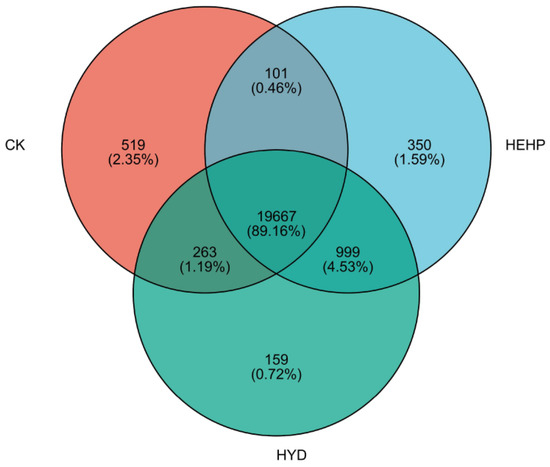
Figure 2.
Venn diagram showing specific and overlapping identified genes in carrot (Daucus carota L.) seeds under CK, HYD, and HEHP treatments.

Table 1.
The number and comparison of DEGs with different treatments applied to carrot (Daucus carota L.) seeds.
3.3. GO and KEGG Enrichment Analysis of DEGs
A GO enrichment analysis was performed on the DEGs among the CK, HYD, and HEHP treatments to reveal significant biological functions associated with the DEGs. The top 20 GO terms enriched in CK vs. HEHP, CK vs. HYD, HYD vs. HEHP, and all the DEGs are shown in Figure 3. The complete enriched GO terms and related DEGs are listed in Table S4. It is noteworthy that for all the DEGs identified in the three group, the GO terms “cell wall macromolecule metabolic process”, “hemicellulose metabolic process”, “xyloglucan metabolic process”, “cell wall polysaccharide metabolic process”, and “cell wall organization” have the highest enrichment levels (Figure 3D), indicating that the priming treatment is likely to regulate the process of cell remodeling during the imbibition. In addition, the GO term named “extracellular region” was enriched in both the CK vs. HEHP and CK vs. HYD groups. Except for the above GO terms, “anchored component of plasma membrane”, “anchored component of membrane”, “terpene synthase activity”, and “oxidoreductase activity” were top enriched in CK vs. HEHP, while “microtubule-based movement”, “movement of cell or subcellular component”, “microtubule-based process”, and “monolayer-surrounded lipid storage body” were top enriched in CK vs. HYD (Figure 3A,B). In HYD vs. HEHP, “terpene synthase activity”, “carbon-oxygen lyase activity”, “lyase activity”, “magnesium ion binding”, and “plant-type cell wall organization” were top-enriched GO terms (Figure 3C). The above results indicate that the activation of certain extracellular enzyme expression and cell wall movement was triggered by the priming treatments, especially the HEHP treatment.
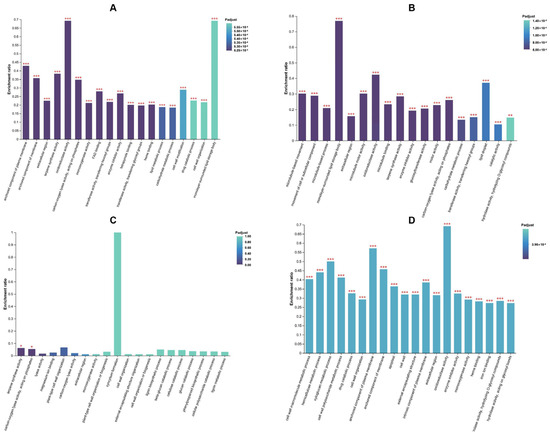
Figure 3.
GO enrichment analysis of DEGs in carrot (Daucus carota L.) seeds under CK, HYD, and HEHP treatments. (A) Top 20 enriched GO terms of the DEGs identified in CK vs. HEHP. (B) Top 20 enriched GO terms of the DEGs identified in CK vs. HYD. (C) Top 20 enriched GO terms of the DEGs identified in HYD vs. HEHP. (D) Top 20 enriched GO terms of all the DEGs identified in CK vs. HEHP, CK vs. HYD, and HYD vs. HEHP. * means significant at the level of p < 0.05. ** means significant at the level of p < 0.01. *** means significant at the level of p < 0.001.
The results of the KEGG pathway enrichment analysis for the DEGs are shown in Figure 4. The complete enriched KEGG pathways and related DEGs are listed in Table S5. “Phenylpropanoid biosynthesis” emerged as the most significantly enriched pathway across comparisons, particularly in CK vs. HEHP (Figure 4A,B,D), prompting the biosynthesis of phenylpropanoid to be activated by the priming treatments, especially the HEHP treatment, which is directly related to the synthesis of lignin, the main component of the cell wall. Furthermore, “biosynthesis of unsaturated fatty acids”, “monoterpenoid biosynthesis”, and “flavonoid biosynthesis” were also the top five enriched pathways in both the CK vs. HEHP and CK vs. HYD groups. In HYD vs. HEHP, “monoterpenoid biosynthesis”, “fatty acid biosynthesis”, “isoflavonoid biosynthesis”, and “terpenoid backbone biosynthesis” were also top-enriched KEGG pathways (Figure 4C).
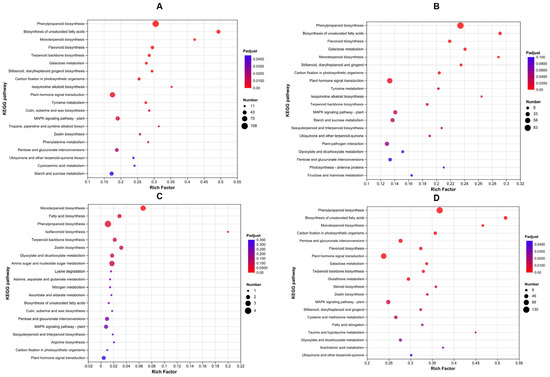
Figure 4.
KEGG pathway enrichment analysis of DEGs in carrot (Daucus carota L.) seeds under CK, HYD, and HEHP treatments. (A) Top 20 enriched KEGG pathways of the DEGs identified in CK vs. HEHP. (B) Top 20 enriched KEGG pathways of the DEGs identified in CK vs. HYD. (C) Top 20 enriched KEGG pathways of the DEGs identified in HYD vs. HEHP. (D) Top 20 enriched KEGG pathways of all the DEGs identified in CK vs. HEHP, CK vs. HYD, and HYD vs. HEHP.
3.4. DEGs Involved in Cell Wall Remodeling Pathways
The key genes encoding processes related to cell wall remodeling in all DEGs were identified, such as those associated with endosperm weakening, including key enzymes and proteins involved in cell wall polysaccharide degradation (XET and EXP), as well as key enzymes involved in pectin degradation (PE and PAE). In addition, there are also genes related to lignin synthesis key enzymes (PAL and 4CL) and cellulose synthesis key enzymes (CesA). The differential expression levels of these DEGs between treatments are shown in Table 2. It can be found that the DEGs were mainly upregulated in the priming treatments, especially the HEHP treatment. For the cell wall polysaccharide degradation-related DEGs, among the nine EXPs and the fourteen XETs, only two EXPs and two XETs showed downregulation, respectively. For the pectin degradation-related DEGs, out of the eighteen PEs, only one PE showed downregulation, and all four PAEs were upregulated by the priming treatment. For the lignin synthesis and cellulose synthesis related DEGs, one PAL and three CesAs showed downregulation, and all four CLs were upregulated by the priming treatment. Furthermore, among the 61 DEGs under consideration, 42 DEGs showed greater expression differences in the HEHP treatment compared to the HYD treatment.

Table 2.
Differential expressions of cell wall remodeling-related DEGs in carrot (Daucus carota L.) seeds under CK, HYD, and HEHP treatments.
3.5. Analysis of Key Enzyme Activities in Cell Wall Remodeling Pathways
The activities of the key enzymes involved in cell wall polysaccharide degradation (XET), pectin degradation (PE), lignin synthesis (PAL), and cellulose synthesis (CesA) of carrot seeds under different treatments were determined at three selected time points, as shown in Figure 5. At S0, the activities of XET and CesA treated with HEHP were significantly higher than those treated with HYD and CK, while the activities of PE and PAL showed no significant difference between treatments. At S20, when HEHP-treated seeds initiated radicle protrusion (Figure 1B), XET and PE activities in HEHP reached their peak levels (Figure 5A,B), coinciding with the critical phase of endosperm weakening. This surge in hydrolase activity aligned with the observed reduction in endosperm rupture force (Figure 1A) and the onset of germination in HEHP. At S40, except for PAL, the activities of the other three enzymes treated with the priming treatments were no longer significantly higher than those of CK, and the XET and PAL activities of CK were significantly higher than those of the priming treatments. The CK seeds exhibited delayed enzymatic activation, with XET and PE activities remaining low at S20 and only peaking at S40, corresponding to their later germination timing. Notably, CesA activity in HEHP was significantly elevated even at S0 (pre-imbibition) compared to CK (Figure 5D), suggesting that HEHP pre-activated cellulose synthesis machinery during priming to prepare for post-germination cell wall reinforcement. The transient rise in PAL activity at S20 (Figure 5C) mirrored the timing of radicle emergence.

Figure 5.
Activity dynamics of cell wall remodeling enzymes in carrot (Daucus carota L.) seeds during imbibition (S0–S40) under CK, HYD, and HEHP treatments. The different lowercase letters above the bars indicate significant differences in 95% probability level (p < 0.05, Duncan test was performed after ANOVA analysis).
3.6. Analysis of Expression Patterns of Key Genes in Cell Wall Remodeling Pathways
Gene expression dynamics further supported the temporal coordination between cell wall remodeling and germination progression. At S20, HEHP-treated seeds exhibited the maximal upregulation of genes encoding XET (LOC108197383), PE (LOC108222122), and CesA (LOC108222895) (Figure 6A), directly correlating with their enzymatic activity peaks (Figure 5). This transcriptional burst at S20 synchronized with the mechanical weakening of the endosperm (Figure 1A) and the initiation of radicle protrusion (Figure 1B). In contrast, CK seeds showed a delayed expression of these genes, with significant upregulation only at S40, consistent with their slower germination. It is noteworthy that genes encoding EXP (LOC108206602) and 4CL (LOC108193188) were already upregulated in HEHP at S0 (Figure 6A), indicating that HEHP induced transcriptional “priming” during pretreatment to expedite post-imbibition responses. Moreover, the expression pattern of these DEGs at S20 obtained from qRT-PCR was positively correlated with the transcriptome results, reflecting that the transcriptome data were reliable (Figure 6B).
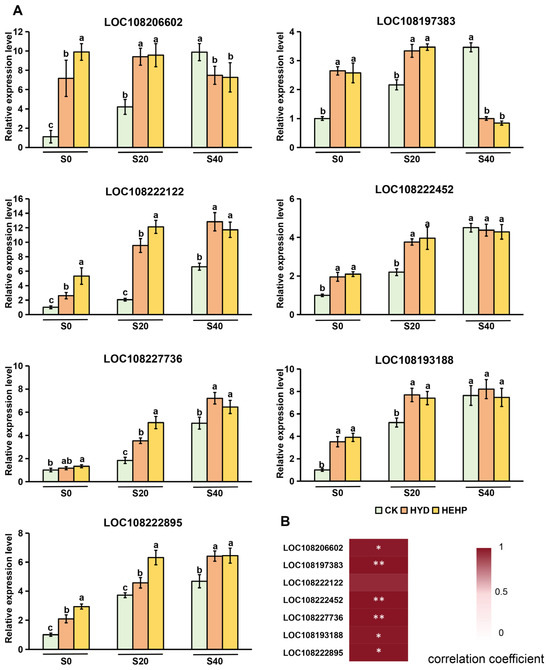
Figure 6.
Expression patterns of cell wall remodeling genes in carrot (Daucus carota L.) seeds during imbibition (S0–S40) under CK, HYD, and HEHP treatments. (A) qRT-PCR analysis of selected DEGs in cell wall remodeling pathways at different time points. The different lowercase letters above the bars indicate significant differences in 95% probability level (p < 0.05, Duncan test was performed after ANOVA analysis) (B) Correlation analysis between the results of qRT-PCR and transcriptome (Pearson correlation coefficient was used. * means significant at the level of p < 0.05. ** means significant at the level of p < 0.01).
4. Discussion
4.1. HEHP Synergistically Coordinates Cell Wall Decomposition and Synthesis for Enhanced Endosperm Weakening
Our findings demonstrate that HEHP significantly accelerates carrot seed germination by modulating the mechanical properties of the endosperm through dynamic cell wall remodeling. The rapid decline in endosperm rupture force during early imbibition (Figure 1) aligns with the transcriptional activation of genes encoding cell wall loosening enzymes and nonenzymatic factors, including EXP, XET, and pectin-modifying enzymes (PE and PAE) (Table 2). As a group of nonenzymatic factors, EXPs disrupt hydrogen bonds between cellulose and hemicellulose to loosen the cell wall, facilitating radicle extension [35,36]. In addition, XET cleaves and reconnects xyloglucan chains, remodeling the cell wall network to coordinate expansion and reinforcement [19,37,38]. PE removes methyl esters from pectin, reducing esterification to enhance calcium-mediated crosslinking and regulate wall rigidity and porosity, while PAE hydrolyzes acetyl groups, eliminating steric hindrances for pectinases like polygalacturonase to degrade homogalacturonan [23,39]. They synergistically remodel cell wall architecture to drive endosperm weakening. Notably, HEHP-treated seeds exhibited earlier germination (Figure 1) and the sustained upregulation of these genes compared to HYD and CK (Table 2), suggesting that electrostatic field exposure during priming amplifies the hydration-induced activation of cell wall remodeling machinery. Our findings corroborate previous mechanistic insights into endosperm modification, particularly the established role of β-mannanase activity in facilitating carrot seed germination [22], but the present study extends our understanding by showing that HEHP synchronizes both hydrolytic (XET and PE) and synthetic (CesA, PAL/4CL) pathways. The co-upregulation of CesA and PAL/4CL (Table 2) implies that HEHP balances cell wall loosening with localized reinforcement, a strategy likely to maintain structural integrity while permitting embryo expansion, as hypothesized in rice [26].
The pronounced activity of XET and PE at S20 (Figure 5) correlates with the data showing sustained expression of LOC108197383 (XET) and LOC108222122 (PE) in HEHP (Figure 6). XETs are known to remodel hemicellulose networks during the extension of the cell wall [40], while PEs demethoxylate pectin to enhance wall flexibility [41]. The early peak in the activity (S20) suggests that HEHP activates enzymatic reservoirs during imbibition, enabling rapid wall modification post-imbibition. This aligns with reports that physical priming methods, such as cold plasma, enhance enzyme stability [42], but HEHP uniquely integrates hydration and electrostatic stimuli to amplify this effect. The mechanistic insights from this study have direct applications in seed priming technology. The ability of HEHP to synchronize germination (Figure 1B) aligns with the requirements of mechanized sowing, where uniform emergence reduces thinning labor and improves yield predictability. By targeting specific cell wall components (e.g., xyloglucan via XETs), HEHP protocols could be customized for other Apiaceae species with similar endosperm constraints, such as celery.
4.2. Phenylpropanoid Biosynthesis as a Pivotal Link Between Priming and Cell Wall Reinforcement
The KEGG enrichment analysis highlighted phenylpropanoid biosynthesis as a central pathway activated by HEHP (Figure 4). PAL and 4CL are key enzymes in the phenylpropanoid synthesis pathway, and PAL is also the rate-limiting enzyme [43,44,45]. The upregulation of PAL and 4CL (Table 2) correlates with increased PAL activity at S20 (Figure 5), indicating enhanced lignin precursor synthesis. Lignin deposition is critical for mechanical support during cell wall expansion [46,47,48], yet excessive lignification can impede germination [49]. HEHP appears to resolve this paradox by temporally regulating PAL/4CL expression, in which the peak activity appearing at S20 (Figure 5) coincides with embryo emergence, while the downregulation was observed at S40 (Figure 6A) to prevent over-lignification. This temporal precision mirrors findings in foxtail millet, where phenylpropanoid pathways are transiently activated under drought stress to balance wall rigidity and growth [29]. Intriguingly, the “extracellular region” GO term was enriched in HEHP-treated seeds (Figure 3), suggesting apoplastic phenylpropanoid transport. Monolignol polymerization at specific wall domains could reinforce radicle tip walls during protrusion. Furthermore, the correlation between PAL activity and CesA upregulation (Figure 5) implies crosstalk between lignin and cellulose synthesis, potentially mediated by ROS signaling triggered by electrostatic exposure [16,50]. This synergy may explain the superior germination uniformity of HEHP-treated seeds compared to HYD.
4.3. Temporal Dynamics of Cell Wall Remodeling Underpin Germination Synchronization
The transcriptome comparison between pre- and post-imbibition samples revealed a striking reduction in DEG numbers for HEHP (HEHP0 vs. HEHP, 4069 DEGs) relative to CK (CK0 vs. CK, 11,095 DEGs) (Table 1), which indicates that HEHP pre-activates germination-related pathways during priming, reducing transcriptional “lag” of post-imbibition. For instance, CesA and EXP genes were already upregulated at S0 in HEHP (Figure 6), inducing the seed for immediate cell wall restructuring upon hydration. Such “transcriptional memory” has been documented in hydroprimed cereals [51], but HEHP extends this by coupling metabolic priming with cell wall remodeling. The biphasic enzyme activity profiles (Figure 5), such as a rapid increase in S20 and a decrease in S40, reflect a strict time regulation program. For example, XET and PE activities peaked earlier in HEHP than in HYD, aligning with its faster germination. Conversely, the late rise in PAL activity in CK (Figure 5) may represent a stress response to incomplete endosperm weakening, delaying radicle emergence. These dynamics underscore the importance of timing in cell wall remodeling. The premature enzyme activation tends to waste resources, while delayed activity prolongs mechanical constraints. HEHP optimizes this balance, likely via electrostatic field-enhanced membrane permeability, which accelerates signal transduction for coordinated gene expression. The temporal alignment between enzymatic/transcriptional peaks (S20) and radicle protrusion demonstrates that HEHP precisely coordinates cell wall remodeling phases to match the biomechanical demands of germination.
4.4. Redox Signals May Serve as a Bridge Between Electrostatic Fields and Metabolic Reprogramming
While our current data show the coordinated upregulation of ROS-sensitive PAL (Figure 5C) and peroxidases (Figure 3), previous studies have established that electrostatic fields induce ROS bursts through NADPH oxidase activation [47]. This aligns with our observed upregulation of respiratory genes (e.g., MDH in [16]) that maintain redox homeostasis during wall remodeling. The mechanism of HEHP in enhancing germination may also involve its modulation of redox homeostasis. Static electric fields can induce transient ROS bursts in seeds [52,53], which act as second messengers to activate Ca2⁺ signaling and cascade with MAP kinase [54]. ROS bursts in seeds can act as secondary messengers to activate Ca2⁺ signaling and MAP kinase cascades [10]. In the present work, the coordinated upregulation of ROS-sensitive genes like PAL (Figure 5) and peroxidases (enriched in GO terms; Figure 3) supports this hypothesis. ROS may directly oxidize cell wall polysaccharides, increasing their susceptibility to enzymatic cleavage [55,56], while simultaneously inducing antioxidant systems to prevent oxidative damage. This balance mechanism has also been observed in kidney beans under salt stress [27]. Moreover, electrostatic exposure could enhance NADPH oxidase activity, linking ROS production to the observed upregulation of respiratory genes (e.g., malate dehydrogenase) reported in our prior work [16]. This metabolic coupling ensures that the ATP demand for wall remodeling is met, addressing a critical bottleneck in conventional priming methods [5]. Future research should explore the role of ROS as secondary messengers in HEHP-induced signaling.
While HEHP shows laboratory efficacy, its agronomic value lies in reducing chemical priming dependency, particularly for organic carrot production [4]. Field validation should assess its performance across soil types, as seed–soil contact affects electromagnetic efficacy [12]. Environmental variability, such as fluctuating humidity and temperature during mechanized sowing, may also influence HEHP outcomes. Future research should focus on field validations to assess HEHP’s robustness under variable environmental conditions, and multi-omics integration could unravel cross-talk between redox signaling and wall remodeling, further refining HEHP protocols for horticultural applications.
5. Conclusions
The present study establishes that HEHP enhances carrot seed germination by orchestrating spatiotemporal cell wall remodeling through the synergistic activation of hydrolysis and synthesis pathways (Figure 7). The electrostatic field component of HEHP amplifies the effects of HYD, accelerating endosperm weakening via the coordinated upregulation of EXP and hydrolases (XET and PE) and targeted reinforcement by synthases (CesA, PAL/4CL). The rapid decline in endosperm rupture force and the earlier germination in HEHP-treated seeds correlate with the transcriptional priming of cell wall-related genes and biphasic enzyme activities, demonstrating that HEHP establishes a “metabolic memory” during pretreatment. By dynamically balancing lignin deposition and wall loosening, HEHP ensures structural flexibility for radicle emergence while avoiding excessive lignification, which may be a mechanism supported by transient phenylpropanoid pathway activation and ROS-mediated signaling. Notably, the superiority of HEHP over HYD lies in the ability to activate germination-related genes (e.g., EXP and CesA), reducing transcriptional lag post-imbibition. This aligns with the observed reduction in DEGs for HEHP compared to CK, indicating optimized resource allocation. These findings advance our understanding of physical priming technologies by revealing how electromagnetic stimuli synergize with hydration to reprogram seed metabolism. From an applied perspective, HEHP addresses critical challenges in carrot cultivation, particularly the demand for uniform germination under mechanized sowing. The capacity of HEHP to synchronize cell wall remodeling offers a template for improving seed vigor in other Apiaceae species. In conclusion, HEHP demonstrates potential as an eco-friendly priming strategy, though field-scale validation remains a prerequisite for mechanized sowing adoption. By decoding the response of the cell wall remodeling pathway to HEHP, this work provides actionable insights for developing precision agriculture technologies tailored to horticultural crops.
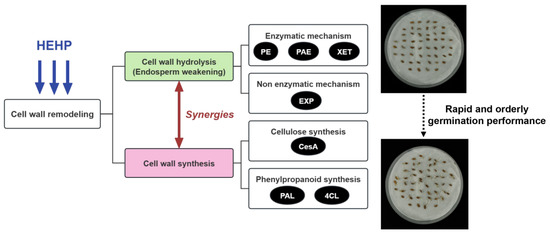
Figure 7.
HEHP activates the cell wall remodeling pathway to promote carrot seed germination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15051147/s1, Table S1: Selected DEGs and primers used for qRT-PCR; Table S2: Statistics of sequence alignment results; Table S3: (A) DEGs between CK and HEHP; (B) DEGs between CK and HYD; (C) DEGs between HYD and HEHP; Table S4: (A) Enriched GO terms in CK vs. HEHP; (B) Enriched GO terms in CK vs. HYD; (C) Enriched GO terms in HYD vs. HEHP; Table S5: (A) Enriched KEGG terms in CK vs. HEHP; (B) Enriched KEGG terms in CK vs. HYD; (C) Enriched KEGG terms in HYD vs. HEHP; Figure S1. Transcriptomic divergence revealed by PCA of carrot (Daucus carota L.) seeds under CK, HYD and HEHP treatments; Figure S2. Volcano plots of DEGs in carrot (Daucus carota L.) seeds under CK, HYD, and HEHP treatments.
Author Contributions
Conceptualization, D.H. and S.Z.; methodology, Y.S., Y.Y. and S.Z.; validation, Y.Y. and G.W.; formal analysis, Y.S.; data curation, X.H.; writing—original draft preparation, Y.S.; writing—review and editing, Y.L., X.H. and S.Z.; project administration, G.W. and S.Z.; funding acquisition, G.W., Y.L. and S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (32402557), Shandong Provincial Natural Science Foundation (ZR2023QC213), Ningxia key research and development plan (2024BBF01013), and College Student Innovation Training Program Project of Shandong Province (S202410433065).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jhanji, S.; Goyal, E.; Chumber, M.; Kaur, G. Exploring fine tuning between phytohormones and ROS signaling cascade in regulation of seed dormancy, germination and seedling development. Plant Physiol. Biochem. 2024, 207, 108352. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Luby, C.H.; Maeda, H.A.; Goldman, I.L. Genetic and phenological variation of tocochromanol (vitamin E) content in wild (Daucus carota L. var. carota) and domesticated carrot (D. carota L. var. sativa). Hortic. Res. 2014, 1, 14015. [Google Scholar] [CrossRef]
- Que, F.; Hou, X.L.; Wang, G.L.; Xu, Z.S.; Xiong, A.S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I. Seed priming: What’s next? Seed Sci. Technol. 2023, 51, 379–405. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ju, Y.H.; Nakamichi, A.; Cho, S.W.; Woo, S.H.; Sakagami, J.I. Effect of seed hydropriming on the elongation of plumule and radicle during the germination process and changes in enzyme activity under water-deficient conditions. Plants 2024, 13, 3537. [Google Scholar] [CrossRef]
- Jiang, B.W.; Wang, L.Y.; Xu, C.T.; Yan, M. Hydropriming enhances the germination of aged ultra-dry wheat seeds. Seed Sci. Technol. 2020, 48, 57–63. [Google Scholar] [CrossRef]
- Thakur, M.; Tiwari, S.; Kataria, S.; Anand, A. Recent advances in seed priming strategies for enhancing planting value of vegetable seeds. Sci. Hortic. 2022, 305, 111355. [Google Scholar] [CrossRef]
- Aasim, M.; Akin, F.; Ali, S.A. Synergizing LED Technology and Hydropriming for Intelligent Modeling and Mathematical Expressions to Optimize Chickpea Germination and Growth Indices. J. Plant Growth Regul. 2024, 43, 2340–2359. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Hu, M.H.; Gao, Z.; Chen, X.X.; Huang, D.F. Biological mechanisms of a novel hydro-electro hybrid priming recovers potential vigor of onion seeds. Environ. Exp. Bot. 2018, 150, 260–271. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Samarah, N.H.; Hani, M.; Makhadmeh, I.M. Effect of magnetic treatment of water or seeds on germination and productivity of tomato plants under salinity stress. Horticulturae 2021, 7, 220. [Google Scholar] [CrossRef]
- Ries, A.; Benitez, J.V.; Samudio, A.; Armoa, R.; Nakayama, H.D. Germination of bean seeds (Vigna unguiculata L. Walp.) in strong electric fields. MethodsX 2023, 11, 102490. [Google Scholar] [CrossRef]
- Mamlic, Z.; Maksimovic, I.; Canak, P.; Mamlic, G.; Djukic, V.; Vasiljevic, S.; Dozet, G. The use of electrostatic field to improve soybean seed germination in organic production. Agronomy 2021, 11, 1473. [Google Scholar] [CrossRef]
- Wang, G.; Huang, J.; Gao, W.; Lu, J.; Li, J.; Liao, R.; Jaleel, C.A. The effect of high-voltage electrostatic field (HVEF) on aged rice (Oryza sativa L.) seeds vigor and lipid peroxidation of seedlings. J. Electrost. 2009, 67, 759–764. [Google Scholar] [CrossRef]
- Zhao, S.; Garcia, D.; Zhao, Y.; Huang, D. Hydro-electro hybrid priming promotes carrot (Daucus carota L.) seed germination by activating lipid utilization and respiratory metabolism. Int. J. Mol. Sci. 2021, 22, 11090. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, H.; Jia, Y.; Pan, X.; Huang, D. Carrot (Daucus carota L.) seed germination was promoted by hydro-electro hybrid priming through regulating the accumulation of proteins involved in carbohydrate and protein metabolism. Front. Plant Sci. 2022, 13, 824439. [Google Scholar] [CrossRef]
- Shigeyama, T.; Watanabe, A.; Tokuchi, K.; Toh, S.; Sakurai, N.; Shibuya, N.; Kawakami, N. α-Xylosidase plays essential roles in xyloglucan remodelling, maintenance of cell wall integrity, and seed germination in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 5615–5629. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, L.; Dong, D.H.; Xu, J.Y.; Li, H.X.; Deng, Q.L.; Zhang, Y.; Huang, W.; Zhang, H.J.; Guo, Y.D. The transcription factor SlLBD40 regulates seed germination by inhibiting cell wall remodeling enzymes during endosperm weakening. Plant Physiol. 2025, 197, kiaf022. [Google Scholar] [CrossRef]
- Jemmat, A.M.; Ranocha, P.; Le Ru, A.; Neel, M.; Jauneau, A.; Raggi, S.; Ferrari, S.; Burlat, V.; Dunand, C. Coordination of five class III peroxidase-encoding genes for early germination events of Arabidopsis thaliana. Plant Sci. 2020, 298, 110565. [Google Scholar] [CrossRef]
- Chandrasekaran, U.; Zhao, X.; Luo, X.; Wei, S.; Shu, K. Endosperm weakening: The gateway to a seed’s new life. Plant Physiol. Biochem. 2022, 178, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Han, X.; Yin, Y.L.; Wang, G.B.; Huang, D.F.; Lan, Y.B. Enzymatic loosening mechanism of endosperm weakening plays a key role in promoting carrot (Daucus carota L.) seed germination by hydro-electro hybrid priming. Sci. Hortic. 2024, 333, 113255. [Google Scholar] [CrossRef]
- Cao, X.L.; Li, M.L.; Li, J.; Song, Y.X.; Zhang, X.N.; Yang, D.L.; Li, M.F.; Wei, J.H. Co-expression of hydrolase genes improves seed germination of Sinopodophyllum hexandrum. Ind. Crops Prod. 2021, 164, 113414. [Google Scholar] [CrossRef]
- Muthusamy, M.; Kim, J.Y.; Yoon, E.K.; Kim, J.A.; Lee, S.I. BrEXLB1, a Brassica rapa Expansin-Like B1 Gene Is Associated with Root Development, Drought Stress Response, and Seed Germination. Genes 2020, 11, 404. [Google Scholar] [CrossRef]
- Chen, S.K.; Luo, Y.X.; Wang, G.J.; Feng, C.Z.; Li, H.F. Genome-wide identification of expansin genes in Brachypodium distachyon and functional characterization of BdEXPA27. Plant Sci. 2020, 296, 110490. [Google Scholar] [CrossRef]
- Lin, F.; Manisseri, C.; Fagerström, A.; Peck, M.L.; Vega-Sánchez, M.E.; Williams, B.; Chiniquy, D.M.; Saha, P.; Pattathil, S.; Conlin, B.; et al. Cell wall composition and candidate biosynthesis gene expression during rice development. Plant Cell Physiol. 2016, 57, 2058–2075. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, B.; Wang, G.D.; Zhang, W.J.; Li, M.; Yin, Z.G.; Yuan, X.K.; Sun, H.Y.; Du, J.D.; Du, Y.L.; et al. Exogenous melatonin enhances cell wall response to salt stress in common bean (Phaseolus vulgaris) and the development of the associated predictive molecular markers. Front. Plant Sci. 2022, 13, 1012186. [Google Scholar] [CrossRef]
- Tong, Y.; Yi, S.C.; Liu, S.Y.; Xu, L.; Qiu, Z.X.; Zeng, D.Q.; Tang, W.W. Bruceine D may affect the phenylpropanoid biosynthesis by acting on ADTs thus inhibiting Bidens pilosa L. seed germination. Ecotoxicol. Environ. Saf. 2022, 242, 113943. [Google Scholar] [CrossRef]
- Yu, A.L.; Zhao, J.F.; Wang, Z.H.; Cheng, K.; Zhang, P.; Tian, G.; Liu, X.; Guo, E.H.; Du, Y.W.; Wang, Y.W. Transcriptome and metabolite analysis reveal the drought tolerance of foxtail millet significantly correlated with phenylpropanoids-related pathways during germination process under PEG stress. BMC Plant Biol. 2020, 20, 274. [Google Scholar] [CrossRef]
- Alibas, I.; Koksal, N. The effect of moisture content on physical, mechanical and rheological properties of soybean (Glycine max cv. ATAEM-II) seed. Legume Res. 2015, 38, 324–333. [Google Scholar] [CrossRef]
- Grsic-Rausch, S.; Rausch, T. A coupled spectrophotometric enzyme assay for the determination of pectin methylesterase activity and its inhibition by proteinaceous inhibitors. Anal. Biochem. 2004, 333, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Huang, Y.H.; Lin, Z.Y.; Hsieh, L.S. Insights into the substrate selectivity of Bambusa oldhamii phenylalanine ammonia-lyase 1 and 2 through mutational analysis. Phytochem. Lett. 2020, 38, 140–143. [Google Scholar] [CrossRef]
- Wu, Z.C.; Cui, C.L.; Xing, A.Q.; Xu, X.H.; Sun, Y.; Tian, Z.Q.; Li, X.Y.; Zhu, J.Y.; Wang, G.M.; Wang, Y.H. Identification and response analysis of xyloglucan endotransglycosylase/hydrolases (XTH) family to fluoride and aluminum treatment in Camellia sinensis. BMC Genom. 2021, 22, 761. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Sun, S.-J.; Horikawa, Y.; Wada, M.; Sugiyama, J. Functional reconstitution of cellulose synthase in Escherichia coli. Biomacromolecules 2014, 15, 4206–4213. [Google Scholar] [CrossRef]
- Duan, Y.H.; Ma, Y.Y.; Zhao, X.D.; Huang, R.L.; Su, R.X.; Qi, W.; He, Z.M. Real-time adsorption and action of expansin on cellulose. Biotechnol. Biofuels 2018, 11, 317. [Google Scholar] [CrossRef]
- Montechiarini, N.H.; Delgado, L.; Morandi, E.N.; Carrillo, N.J.; Gosparini, C.O. The expansin EXP1 gene in the elongation zone is induced during soybean embryonic axis germination and differentially expressed in response to ABA and PEG treatments. Seed Sci. Res. 2021, 31, 60–68. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Tang, G.Y.; Xu, P.L.; Li, G.W.; Ma, C.L.; Li, P.X.; Jiang, C.Y.; Shan, L.; Wan, S.B. Genome-wide identification of xyloglucan endotransglucosylase/hydrolase gene family members in peanut and their expression profiles during seed germination. PeerJ 2022, 10, e13428. [Google Scholar] [CrossRef]
- Ducatti, K.R.; Batista, T.B.; Hirai, W.Y.; Luccas, D.A.; Moreno, L.D.; Guimaraes, C.C.; Bassel, G.W.; da Silva, E.A.A. Transcripts expressed during germination sensu stricto are associated with vigor in soybean seeds. Plants 2022, 11, 1310. [Google Scholar] [CrossRef]
- Liu, H.N.; Pei, M.S.; Ampomah-Dwamena, C.; Shang, Y.X.; Yu, Y.H.; Wei, T.L.; Shi, Q.F.; Guo, D.L. Alternative splicing of the PECTINESTERASE gene encoding a cell wall-degrading enzyme affects postharvest softening in grape. J. Integr. Agric. 2024, 23, 863–875. [Google Scholar] [CrossRef]
- Ishida, K.; Yokoyama, R. Reconsidering the function of the xyloglucan endotransglucosylase/hydrolase family. J. Plant Res. 2022, 135, 145–156. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.Q.; Li, X.Y.; Weng, X.A.; Feng, D.F.; Ying, J.F.; Wang, Z.F. An integrated RNA-Seq and physiological study reveals gene responses involving in the initial imbibition of seed germination in rice. Plant Growth Regul. 2020, 90, 249–263. [Google Scholar] [CrossRef]
- Pourbagher, M.; Pourbagher, R.; Abbaspour-Fard, M.H. Reduction of Adverse Effects of Drought Stress on Germination Indices and Antioxidant Enzymes of Licorice Seeds (Glycyrrhiza) Using Cold Plasma. J. Plant Growth Regul. 2025, 44, 1032–1042. [Google Scholar] [CrossRef]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants 2016, 2, 16050. [Google Scholar] [CrossRef]
- Fang, L.; Xu, X.; Li, J.; Zheng, F.; Li, M.Z.; Yan, J.W.; Li, Y.; Zhang, X.H.; Li, L.; Ma, G.H.; et al. Transcriptome analysis provides insights into the non-methylated lignin synthesis in Paphiopedilum armeniacum seed. BMC Genom. 2020, 21, 524. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, S.Y.; Yi, S.C.; Qiu, Z.X.; Wang, Y.H.; Zeng, D.Q.; Tang, W.W. Bruceine D, the main active ingredient of Brucea javanica (L.) residue inhibits the germination of Bidens pilosa L. seeds by suppressing phenylpropanoid biosynthesis. Ind. Crops Prod. 2021, 172, 114079. [Google Scholar] [CrossRef]
- Seyfferth, C.; Wessels, B.A.; Vahala, J.; Kangasjärvi, J.; Delhomme, N.; Hvidsten, T.R.; Tuominen, H.; Lundberg-Felten, J. Populus PtERF85 Balances Xylem Cell Expansion and Secondary Cell Wall Formation in Hybrid Aspen. Cells 2021, 10, 1971. [Google Scholar] [CrossRef]
- Peng, C.Y.; Wu, Y.; Hua, Q.L.; Shen, Y.B. Hydrological transport and endosperm weakening mechanisms during dormancy release in Tilia henryana seeds. J. Plant Physiol. 2025, 304, 154405. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Niu, Y.N.; Bai, X.D.; Mao, T.T. Transcriptomic and metabolic profiling reveals a lignin metabolism network involved in mesocotyl elongation during maize seed germination. Plants 2022, 11, 1034. [Google Scholar] [CrossRef]
- Zhang, W.H.; Wang, G.H.; Zhang, B.; Sui, W.J.; Si, C.L.; Zhou, L.P.; Jia, H.Y. Green potassium fertilizer from enzymatic hydrolysis lignin: Effects of lignin fractionation on wheat seed germination and seedling growth. Int. J. Biol. Macromol. 2024, 262, 130017. [Google Scholar] [CrossRef]
- Qi, M.Y.; Liu, Y.; Shi, S.S.; Xian, Y.H.; Liu, Q.Y.; Yan, H.Y.; Zhang, Y.; Yuan, Y. Inhibition mechanism of high voltage prick electrostatic field (HVPEF) on Staphylococcus aureus through ROS-mediated oxidative stress. Lwt-Food Sci. Technol. 2022, 155, 112990. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W.L.; Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: An overview. Planta 2022, 255, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Zheng, X.Y.; Tao, Y.; Xie, C.; Li, D.D.; Han, Y.B. Moderate electric field-stimulated brown rice germination: Insights into membrane permeability modulation and antioxidant system activation. Food Chem. 2025, 479, 143737. [Google Scholar] [CrossRef] [PubMed]
- Billah, M.; Karmakar, S.; Mina, F.B.; Haque, M.N.; Rashid, M.M.; Hasan, M.F.; Acharjee, U.K.; Talukder, M.R. Investigation of mechanisms involved in seed germination enhancement, enzymatic activity and seedling growth of rice (Oryza sativa L.) using LPDBD (Ar plus Air) plasma. Arch. Biochem. Biophys. 2021, 698, 108726. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Hu, P.G.; Li, F.J.; Wu, H.H.; Shen, Y.; White, J.C.; Tian, X.L.; Li, Z.H.; Giraldo, J.P. Emerging investigator series: Molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kato, M.; Ma, G.; Zhang, L.C.; Uthairatanakij, A.; Srilaong, V.; Laohakunjit, N.; Jitareerat, P. Electron beam radiation delayed the disassembly of cell wall polysaccharides in harvested mangoes. Postharvest Biol. Technol. 2021, 178, 111544. [Google Scholar] [CrossRef]
- Moles, T.M.; Guglielminetti, L.; Reyes, T.H. Differential effects of sodium chloride on germination and post-germination stages of two tomato genotypes. Sci. Hortic. 2019, 257, 108730. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).