Abstract
Optimizing fertilization practices can reduce the application of chemical nitrogen fertilizers, thereby enhancing crop yield while mitigating environmental impacts. In 2021–2022, we conducted field experiments in the Inner Mongolia region, evaluating the effects of different nitrogen application methods on sugar beet growth and productivity. A two-factor randomized complete block design was applied for the experiments with the nitrogen application rates (0, 75, 150, 225, and 300 kg ha−1, denoted as N0, N75, N150, N225, N300, respectively) as the primary factor and the nitrogen application methods (single basal application, S1; and split application, S2) as the second factor. The results indicate that increasing nitrogen application rates significantly enhances leaf growth, chlorophyll content, dry matter accumulation, nitrogen metabolism, and yield. In comparison with a nitrogen-free treatment, the N225S1 treatment significantly improved sugar beet development and nitrogen metabolism, resulting in an average yield increase of 29.36% over two years. Notably, by optimizing the root–shoot balance and carbon–nitrogen metabolism, N150S2 achieved root and sugar yields comparable to N225S1. Therefore, our study demonstrates that split nitrogen application can maintain sugar beet yield while reducing nitrogen fertilizer inputs, thereby providing valuable insights for sustainable sugar beet cultivation in Inner Mongolia, the main sugar beet growing area in China.
1. Introduction
Sugar beet originates from Mediterranean coastal regions and is an important sugar-producing crop with widespread global cultivation and significant economic value [1]. Sugar from sugar beets is one of the major sources in the world sugar market, accounting for approximately 25% of production, second only to sugarcane (hybrids of Saccharum spp.) [2]. In addition to being directly used for extracting sucrose, it can also be used to produce biofuels, animal feed, processed gelatin, and bioplastics, and is considered an important energy crop [3,4,5]. Nonetheless, despite the increasing demand for sugar beet, its production remains subject to significant challenges due to factors such as geographic limitations, constrained arable land, and economic considerations [6,7,8]. Therefore, implementing measures such as rational water and fertilizer management, optimized cropping systems, and mechanization can significantly enhance sugar beet yield [8,9].
Crop yield is closely related to fertilizer management, as optimized fertilization practices can enhance crop productivity while minimizing environmental impacts, thus contributing to the sustainability of agricultural systems. Sugar beet has high biomass production and requires substantial water and nutrient inputs for optimal growth [10]. In China, sugar beet is primarily cultivated in northern regions, including Inner Mongolia and Xinjiang, with Inner Mongolia alone accounting for over 100,000 hm2 of planting area, representing more than half of the country’s total planting area [11,12,13]. In recent years, improper water and fertilizer management in Inner Mongolia has led to soil degradation [12]. Additionally, long-term monocropping has resulted in continuous cropping obstacles (CCOs), severely restricting the sustainability of the sugar beet industry [13]. Therefore, it is important to explore appropriate cropping systems and reduce chemical fertilizer use for the environmentally friendly development of the sugar beet industry. As a sustainable amendment, biochar has shown great potential in improving sugar beet yield and reducing environmental hazards [14].
Carbon and nitrogen metabolism are important metabolic activities in plant cells [15,16]. Nitrogen is not only critical for protein synthesis but also a constituent element of amino acids, chlorophyll, and other biomolecules, playing a crucial role in plant carbon metabolism [15,17]. Insufficient nitrogen supply limits chlorophyll synthesis, thereby reducing photosynthetic efficiency, which ultimately exerts a negative influence on crop growth, yield, and quality [18,19]. The processes of carbon metabolism, including photosynthesis and respiration, provide the carbon skeletons and energy required for cellular activities, thereby influencing nitrogen metabolism [20,21,22,23]. Numerous studies on crops have demonstrated that nitrogen application significantly alters carbon and nitrogen metabolism [24,25,26,27,28]. For instance, a high nitrogen application rate increases crude protein content in rice but also reduces thousand-grain weight and amylose content [29]. However, optimal nitrogen application can enhance rice photosynthetic efficiency by increasing the abundance of the Rubisco enzyme [30]. With increasing nitrogen application rates, the activity of nitrogen metabolism-associated enzymes, such as glutamic oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT), increases, whereas the activity of carbon metabolism-associated enzymes, including granule-bound starch synthase (GBSS) and soluble starch synthase (SSS) decreases [24]. In addition to rice, nitrogen application has also been observed to regulate carbon and nitrogen metabolism in Coreopsis tinctoria, cotton, and tomato [24,25,26,31]. Therefore, an optimized nitrogen fertilizer application strategy is critical for increasing crop productivity [32,33]. However, few studies have been performed on how nitrogen application affects carbon and nitrogen metabolism in sugar beet and regulates sugar beet quality.
Moreover, rational nitrogen fertilization can optimize the physiological metabolic processes of sugar beet, ultimately influencing the final yield and quality [34]. Nitrogen deficiency significantly limits the development of sugar beet leaves and reduces chlorophyll biosynthesis, thereby decreasing photosynthetic efficiency and yield [35,36]. With increasing nitrogen application rates, enhancements in leaf area, root quality, and yield are observed [36,37]. However, excessive nitrogen application not only poses significant environmental risks but also promotes excessive leaf elongation, which depletes photosynthates and ultimately reduces sugar beet root yield [35,38,39,40,41]. In addition to being associated with the cultivation practices employed, the nitrogen demand of sugar beet is affected by local environmental conditions, including soil type and rainfall [39]. As an example, in Ethiopia, the optimal sugar beet yield was observed at 92 kg ha−1 of nitrogen, whereas in Croatia and Xinjiang (China), the ideal nitrogen application rate ranged between 130 and 160 kg ha−1. In contrast, in New Zealand, maximum yields were obtained at 320 kg ha−1 [36,37,39,42]. To our knowledge, however, such analyses have not yet been performed for Inner Mongolia, the biggest sugar beet production area in China.
In addition to the total nitrogen input, the timing of nitrogen application is essential for optimizing sugar beet yield. Nitrogen fertilization during the early growth phase of sugar beet can promote leaf development, increase chlorophyll content, the leaf area index (LAI), and support the accumulation of dry matter [43]. However, applying nitrogen fertilizer during the later growth stages can lead to an accumulation of impurities in the roots, which adversely affects sucrose extraction and reduces beet quality [39,44]. Although the critical role of nitrogen in enhancing sugar beet yield is well acknowledged, there is a noticeable lack of studies investigating how its application rate and timing influence carbon and nitrogen metabolism, especially in temperate semi-arid regions. In addition, most current research on the effects of nitrogen fertilization in sugar beet primarily focuses on one-time total nitrogen input, whereas the influence of split nitrogen application strategies remains largely understudied. In Inner Mongolia, a key agricultural region, fertilizer application intensity has surpassed the global average, posing potential environmental risks [45]. Thus, optimizing fertilization practices and appropriately reducing input levels is of critical importance for mitigating environmental impacts. In this study, we investigated optimal nitrogen management strategies by applying five nitrogen levels (0, 75, 150, 225, and 300 kg ha−1) in combination with two application methods (S1: single application; S2: split application). The results demonstrated that the N150S2 treatment significantly enhanced sugar beet yield by promoting leaf development, dry matter accumulation, and nitrogen metabolism. By splitting a portion of nitrogen fertilizer during the rapid leaf expansion stage of sugar beet, our findings provide practical insights into nitrogen management strategies that ensure high yields while reducing chemical fertilizer input and alleviating environmental pressure.
2. Materials and Methods
2.1. Experimental Site
Between April and October of 2021–2022, the field experiment was conducted in sugar beet growing region in Linxi County, Chifeng, Inner Mongolia, China (43°36′49″ N, 118°2′41″ E; refer to Figure 1A). The experimental field is located in a mid-temperate continental monsoon climate zone with sandy loam soil. The basic physicochemical properties of the soil are as follows: alkaline hydrolyzable nitrogen, 90.01 mg kg−1; available phosphorus, 174.94 mg kg−1; available potassium, 151.2 mg kg−1; organic matter, 17.72 g kg−1; and pH = 8.06. The monthly average temperature and total monthly precipitation in the area are presented in Figure 1B,C.
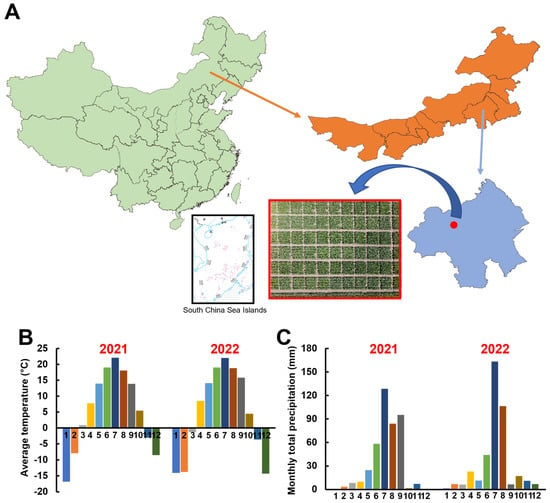
Figure 1.
Experimental site location (A), monthly average temperature (B), and total monthly precipitation (C) in 2021–2022.
2.2. Experimental Design
The experiment was carried out using two-factor randomized complete block design. The primary factor was nitrogen fertilizer application rates with five levels: 0, 75, 150, 225, and 300 kg ha−1 (denoted as N0, N75, N150, N225, and N300, respectively). The second factor was the nitrogen application methods, including single application (S1), where 100% of the nitrogen was applied as basal fertilizer prior to transplanting, and a split application (S2), where 60% of the nitrogen was applied as basal fertilizer prior to transplanting and the remaining 40% was applied before the early rapid leaf growth stage. The sugar beet cultivar MA097, extensively cultivated in Inner Mongolia and bred by MARIBO (Denmark) through hybridization of the parental lines M-020 and P2-33, is characterized by its high yield, strong disease resistance, and excellent adaptability to cold environmental conditions. The applied fertilizers were urea (46.4% N), triple superphosphate (46% P), and potassium sulfate (52% K2O). Before transplanting the sugar beet seedlings, based on the experimental design, either all or 60% of the nitrogen fertilizer was applied as basal fertilizer, together with all of the phosphorus (300 kg ha−1) and potassium (225 kg ha−1) fertilizers. The experiment consisted of nine treatments with three replications each, totaling 27 experiment plots. Each plot had an area of 20 m2 (4 × 5 m), with a plant spacing of 0.2 m and a row spacing of 0.55 m. The sugar beet seedlings were cultivated using paper tube seeding technology on 2 April 2021, and 5 April 2022, and healthy seedlings were subsequently transplanted on 7 May 2021, and 8 May 2022. A drip irrigation system supplied water throughout the experimental period.
2.3. Sampling Period
Sugar beet samples were collected at essential growth stages (Figure 2), including the rapid leaf growth stage (LG, ~95 days after emergence), root growth stage (RG, ~130 days after emergence), sugar accumulation stage (SA, ~160 days after emergence), and harvest stage (HA, ~180 days after emergence). For each treatment, eight healthy sugar beet plants were randomly harvested and transported to the laboratory for thorough washing. Fresh tissues intended for soluble protein determination were immediately frozen in liquid nitrogen and stored at −80 °C. The remaining sugar beet samples were weighed to determine fresh biomass and then oven-dried at 75 °C to a constant weight for further physiological and biochemical analyses.
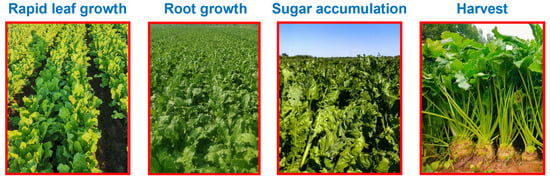
Figure 2.
Sampling period of sugar beet. Rapid leaf growth (LG) stage, root growth (RG) stage, sugar accumulation (SA) stage, and harvest (HA) stage.
2.4. Leaf Area Index and SPAD Value
During the four important growth stages (LG, RG, SA, and HA) of the sugar beet, the chlorophyll content was measured with a chlorophyll meter (SPAD-502, Tokyo, Japan). In each experimental plot, five healthy sugar beet plants were randomly selected, and ten measurements were taken per plant.
Subsequently, LAI was determined for these selected plants via the hole-punch drying method. For each plant, 25 leaf samples were collected using a 2 cm diameter punch. These samples, along with all the leaves from the respective plant, were placed in paper bags, oven-dried, and weighed. LAI was computed using the following formula [46,47]:
where N is the number of sugar beet plants per experimental plot; W1 is the dry weight of the 25 leaf samples per plant; W2 represents the dry weight of all of the leaves per plant; r is the radius of the punch used for leaf sampling; and S is the total area of the experimental plot.
2.5. Dry Matter Accumulation
As described in Section 2.3, the aboveground parts and roots of five healthy sugar beet plants from each plot were first dried in electrically heated blast drying ovens (101-2DB, Tianjin, China) at 105 °C for 30 min, and then dried at 75 °C until reaching a constant weight. Finally, the dry weights of the aboveground parts and roots were recorded, and the root–crown ratio was determined by dividing the root dry weight by the dry weight of the aboveground part.
2.6. Carbon and Nitrogen Metabolism Analysis
Fresh sugar beet roots (1 g) were finely ground in a mortar with 10 mL of phosphate buffer (pH 7.8), and the resulting homogenate was transferred into a 10 mL Eppendorf tube (Cotaus, Taicang, Jiangsu, China), where it was incubated at room temperature for one hour. Subsequently, the homogenate was centrifuged at 5000 rpm for 30 min, and the supernatant was used for soluble protein measurement, following the method described by Sun et al. [48]. The total nitrogen content in the sugar beet roots was determined using the Kjeldhal analysis method [49]. For this purpose, 0.12 g of dried sugar beet root from Section 2.5 was treated with H2SO4-H2O2 to prepare the test solution, and the total nitrogen content was quantified using an automatic Kjeldahl nitrogen analyzer (K06, Shanghai, China).
To quantify total soluble sugars, sucrose, and fructose in sugar beet roots at four different growth stages, sugars were extracted from dried roots using 80% ethanol, and the supernatant was used for analysis. The total soluble sugar content was determined according to the method described by Lei et al. [50], the sucrose content was measured using the resorcinol method [51], and the fructose content was determined following the method of Cai et al. [52]. The carbon–nitrogen (C/N) ratio was calculated as the ratio of total soluble sugar content to total nitrogen content, following the protocol described by Feng et al. [27]. Three independent biological replicates were included for each treatment.
2.7. Determination of Agronomic Nitrogen Use Efficiency, Sugar Content, Root Yield, and Sugar Yield
At the harvest stage, on 13 October 2021 and 15 October 2022, ten sugar beets were randomly selected from each experimental plot, washed, and weighed to calculate the root yield. The formula is as follows [44]:
Root Yield (t ha−1) = Single Plant Fresh Root Weight (kg) × Plant Density (plants ha−1) × 10−3
The sugar content was determined using a portable handheld refractometer (PAL-1, Tokyo, Japan), with the measured value multiplied by 0.83 to obtain the sugar content. The sugar yield was determined according to the following formula [44]:
Sugar yield (t ha −1) = Root yield (t ha −1) × Sugar content
The agronomic nitrogen use efficiency (ANUE) of the sugar beet was calculated following the method of Wan et al. [53]:
2.8. Statistical Analysis
Data processing and visualization were performed using Microsoft Excel 2021. Analysis of variance (ANOVA) and multiple comparisons were performed using SPSS Statistics 20.0, with a significance level of p ≤ 0.05. Correlation relationships were analyzed and plotted using the Correlation Plot program in Origin 2024. Schematic diagrams were created using Adobe Photoshop 25.0.
3. Results
3.1. Leaf-Related Parameters During the Growth Stages of Sugar Beet
LAI is an important dynamic parameter for assessing leaf development and acts as an essential indicator of crop development and potential yield [54]. Over the two-year experimental period, the LAI of sugar beet showed a bell-shaped trend, with an initial rise to a maximum level, followed by a gradual decrease, with relatively stable values observed during the SA and HA growth stages (Figure 3A,B). Specifically, LAI initially ranged from 1.67 to 3.45 during the early growth stage, peaked at over 6.00, and then gradually decreased to between 2.18 and 3.96 at harvest (Figure 3A,B). Across both years, increasing nitrogen application rates significantly enhanced LAI under both the single (S1) and split (S2) application methods, with the highest values observed under the 300 kg ha−1 treatment. During the early LG stage, the single nitrogen application resulted in significantly higher LAI values compared to the split application, indicating a stronger promotion of early canopy development. However, as the growth progressed, the total nitrogen input rather than the application method became the primary factor determining LAI (Figure 3A,B), indicating that nitrogen availability plays a more critical role than its timing during the later stages.
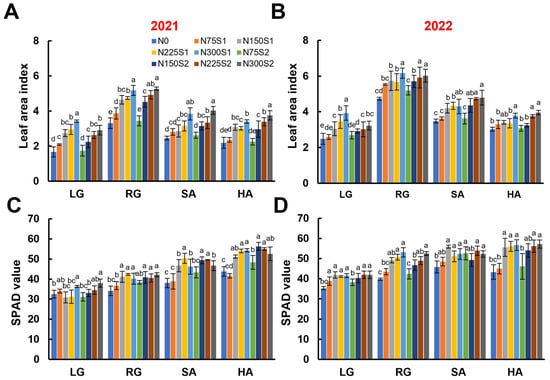
Figure 3.
The effects of nitrogen application levels (N0, N75, N150, N225, and N300) and methods (S1 and S2) on the leaf area index (LAI) and chlorophyll content of sugar beet at different growth stages (LG, RG, SA, and HA). (A,B) The LAI of sugar beet in 2021 and 2022. (C,D) The SPAD values of sugar beet leaves in 2021 and 2022. Error bars represent standard deviations. Different lowercase letters indicate statistically significant differences between treatments (p ≤ 0.05). Rapid leaf growth (LG) stage; root growth (RG) stage; sugar accumulation (SA) stage; harvest (HA) stage.
Numerous investigations have demonstrated that chlorophyll serves as an important indicator for evaluating crop growth, because its synthesis is markedly influenced by nitrogen application [55,56,57]. The SPAD meter provides a rapid and non-destructive method for assessing SPAD values in plant leaves, which serves as an effective proxy for estimating chlorophyll content [58]. Overall, the SPAD values for sugar beet remain relatively stable throughout the growth periods, ranging from 30.86 to 57.18, with higher values observed during the later growth stages (Figure 3C,D). In the case of the single nitrogen application, the SPAD values generally increased as nitrogen application rates increased (Figure 3C,D). Conversely, under the split nitrogen application, the SPAD values showed no significant differences across various nitrogen application rates, except for the treatment of 75 kg ha−1 with a significantly lower SPAD value (Figure 3C,D).
3.2. Dry Matter Accumulation for the Aboveground Parts and Roots During the Growth Stages of Sugar Beet
Similarly to LAI, the dry matter accumulation for the aboveground parts displayed a dynamic pattern characterized by an increase in the early stages and a decrease in the later stages. Compared to the control, the nitrogen applications significantly enhanced the dry matter accumulation for the aboveground parts of sugar beet, with this enhancement becoming more pronounced as the nitrogen application rates increased (Figure 4A,B). At harvest, the dry matter accumulation of the aboveground parts under the nitrogen application of 300 kg ha−1 was 1.55 times (in 2021) and 2.05 times (in 2022) that of the nitrogen-free treatment (Figure 4A,B).
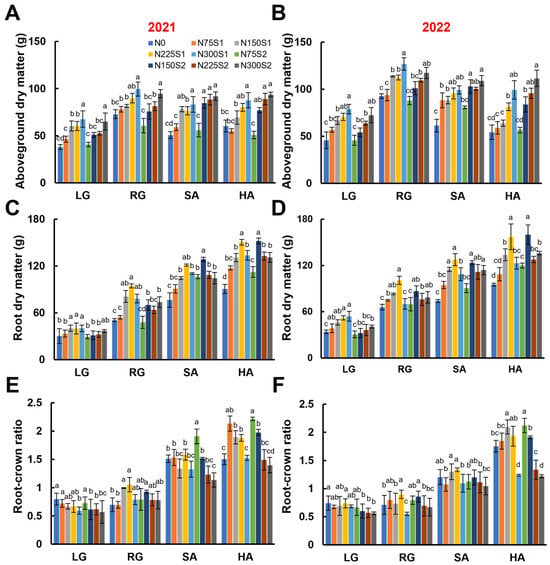
Figure 4.
Effects of nitrogen application levels (N0, N75, N150, N225, and N300) and methods (S1 and S2) on dry matter accumulation and root-crown ratio in sugar beet at different growth stages (LG, RG, SA, and HA). (A,B) Aboveground dry matter accumulation per plant in 2021 and 2022. (C,D) Root dry matter accumulation per plant in 2021 and 2022. (E,F) Sugar beet root–crown ratio in 2021 and 2022. Error bars represent standard deviations. Different lowercase letters indicate statistically significant differences between treatments (p ≤ 0.05). Rapid leaf growth (LG) stage; root growth (RG) stage; sugar accumulation (SA) stage; harvest (HA) stage.
The dry matter accumulation in sugar beet roots gradually increased throughout the growing season, reaching a peak at the harvest stage (Figure 4C,D). In general, nitrogen applications significantly improved root dry matter accumulation, with the optimal nitrogen application rate with the treatment of N150S2, which achieved a 1.6-fold increase relative to the nitrogen-free treatment (Figure 4C,D). Notably, the maximum root dry matter accumulation was achieved by the treatment of 225 kg ha−1 for the single nitrogen application (N225S1), whereas for the split nitrogen application it was achieved by the treatment of 150 kg ha−1 (N150S2).
The root–crown ratio increased progressively throughout the growing season and reached its maximum at the harvest stage, which can exceed a ratio of 2.00 (Figure 4E,F). During the early growth stages (LG and RG), the root–crown ratio was generally less than 1.00 due to the rapid expansion of the leaves. In the later stages, as the roots underwent rapid enlargement and accumulated considerable photosynthates, the root–crown ratio increased substantially. In addition, the root–crown ratio of the sugar beet gradually decreased with increasing nitrogen application rates, regardless of the nitrogen application methods.
3.3. Nitrogen Metabolism During the Growth Stages of Sugar Beet
The soluble protein content in sugar beet roots reached its maximum at the LG stage, then sharply declined by approximately 50% and remained relatively stable throughout the RG, SA, and HA stages. In general, under single nitrogen application, the soluble protein content in sugar beet roots increased with rising nitrogen application rates, reaching a maximum at 300 kg ha−1. However, under split nitrogen application, the soluble protein content in sugar beet roots initially increased as the nitrogen application rates increased to 225 kg ha−1, then decreased when the nitrogen application rates further increased. At the harvest stage, compared to the nitrogen-free (N0) control, the soluble protein content with the treatments N300S1 and N225S2 was improved by 51.65% and 53.32% in 2021, respectively, and it was improved by 47.82% and 38.50% in 2022, respectively (Figure 5A,B). Similarly, the total nitrogen content in sugar beet roots showed the same trend as the soluble protein content, increasing with the nitrogen application rates (Figure 5C,D). These findings suggest that split nitrogen applications can effectively modulate nitrogen metabolism in sugar beet roots.
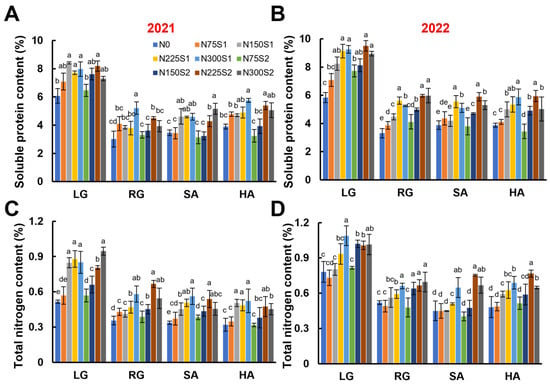
Figure 5.
Effects of nitrogen application levels (N0, N75, N150, N225, and N300) and methods (S1 and S2) on root nitrogen metabolism in sugar beet at different growth stages (LG, RG, SA, and HA). (A,B) Root soluble protein content in 2021 and 2022. (C,D) Root total nitrogen content in 2021 and 2022. Error bars represent standard deviations. Different lowercase letters indicate statistically significant differences between treatments (p ≤ 0.05). Rapid leaf growth (LG) stage; root growth (RG) stage; sugar accumulation (SA) stage; harvest (HA) stage.
3.4. Carbon Metabolism During the Growth Stages of Sugar Beet
The total soluble sugar content in the roots was lowest during the LG stage, increasing during the growth stages, and eventually reached a plateau. In general, as the nitrogen application rates increased, the total soluble sugar content decreased, which was particularly pronounced in 2022 (Figure 6B). Notably, at 75 kg ha−1, the split nitrogen application (N75S2) led to a substantial reduction in total soluble sugar content compared to the single nitrogen application (N75S1). Sucrose and fructose, the primary constituents of total soluble sugars, showed a similar trend (Figure 6C–F). The C/N ratio in plants is a measure of carbon and nitrogen metabolism, providing valuable insights into the overall physiological status of plants [59]. During the LG stage, the C/N ratio was relatively low but increased gradually with further development, ultimately stabilizing during the SA and HA stages. In a manner similar to total soluble sugar content, the C/N ratio decreased with increasing nitrogen application rates, with the ratio at 300 kg ha−1 being approximately 70% of that observed in the nitrogen-free treatment (Figure 6G,H).
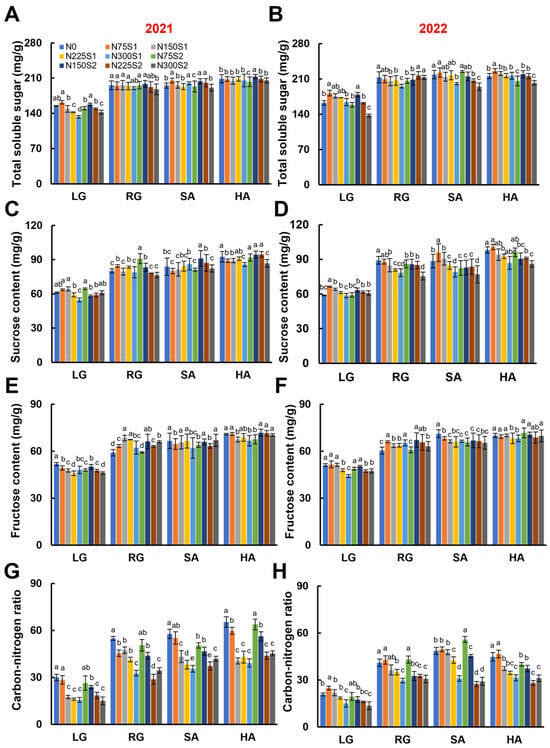
Figure 6.
Effects of nitrogen application levels (N0, N75, N150, N225, and N300) and methods (S1 and S2) on root carbon metabolism and carbon–nitrogen ratio in sugar beet at different growth stages (LG, RG, SA, and HA). (A,B) Root total soluble sugar content in 2021 and 2022. (C,D) Root sucrose content in 2021 and 2022. (E,F) Root fructose content in 2021 and 2022. (G,H) Root carbon–nitrogen ratio in 2021 and 2022. Error bars represent standard deviations. Different lowercase letters indicate statistically significant differences between treatments (p ≤ 0.05). Rapid leaf growth (LG) stage; root growth (RG) stage; sugar accumulation (SA) stage; harvest (HA) stage.
3.5. Agronomic Nitrogen Use Efficiency
For the single nitrogen applications, the ANUE decreased as the nitrogen application rates increased. However, for the split nitrogen applications, the ANUE initially increased but decreased with further increases in nitrogen application rates. For the treatment with 300 kg ha−1, the ANUE for both nitrogen application methods was extremely low. Compared to the single application, the split nitrogen application significantly enhanced the ANUE at an application rate of 150 kg ha−1 (Figure 7), where the ANUE increased significantly by 66.01% (2021) and 23.83% (2022) in contrast to the single nitrogen application (Figure 7).
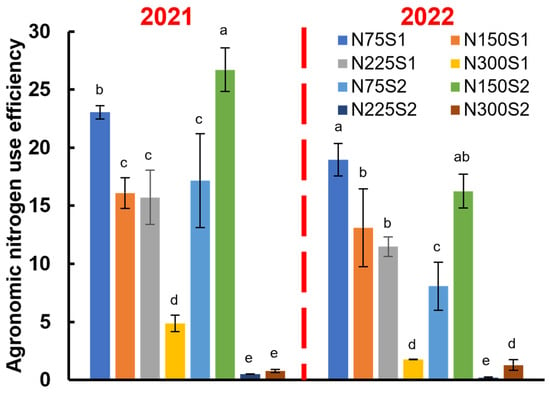
Figure 7.
Effects of nitrogen application levels (N0, N75, N150, N225, and N300) and methods (S1 and S2) on agronomic nitrogen use efficiency. Different lowercase letters indicate statistically significant differences between treatments (p ≤ 0.05).
3.6. Root Yield and Quality
Regardless of the nitrogen application methods, nitrogen application rates significantly enhanced the final yield of the sugar beet, with a yield increase of up to 37.93% compared to the nitrogen-free treatment (Figure 8A). However, the rate of yield improvement decreased when nitrogen application surpassed 225 kg ha−1 (Figure 8A). Our findings indicate that maximum yield was achieved with a nitrogen application rate of 225 kg ha−1 for the single nitrogen application (N225S1) or 150 kg ha−1 for the split nitrogen application (N150S2). The split nitrogen application (N150S2) increased sugar beet yield by 12.42% in 2021 and 7.43% in 2022 relative to the corresponding single application (N150S1, Figure 8A).
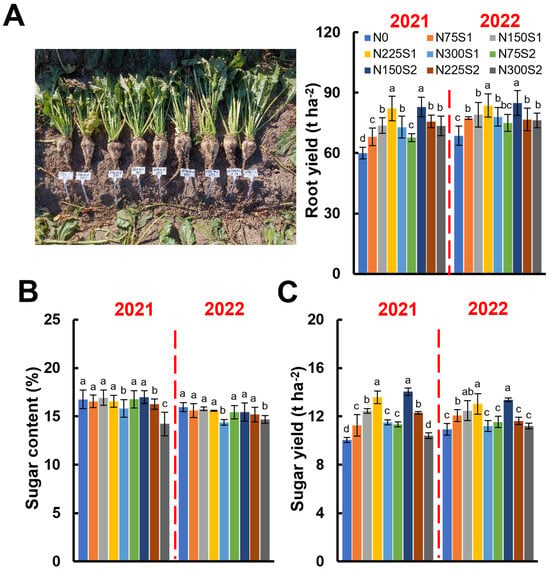
Figure 8.
The effects of nitrogen application levels (N0, N75, N150, N225, and N300) and methods (S1 and S2) on sugar beet root yield, sugar content, and sugar yield. (A) The root yield in 2021 and 2022. Representative images of sugar beet plants from each treatment are presented. In the figures, the sugar beet plants are displayed sequentially from left to right, representing the N0, N75S2, N75S1, N150S1, N150S2, N225S1, N225S2, N300S1, and N300S2 treatments. (B) The sugar content in 2021 and 2022. (C) The sugar yield in 2021 and 2022. The error bars represent the standard deviations. Different lowercase letters indicate statistically significant differences between treatments (p ≤ 0.05).
In general, sugar content in sugar beet tends to decrease with increasing nitrogen application levels. At 300 kg ha−1, the sugar content in the N300S2 treatment was significantly reduced relative to the nitrogen-free treatment, with reductions of up to 15.12% in 2021 and 7.85% in 2022 (Figure 8B). Furthermore, regardless of the nitrogen application method, for nitrogen application rates lower than225 kg ha−1, the sugar content showed non-significant variation, consistently ranging from 16.24% to 16.98% in 2021 and from 15.18% to 15.94% in 2022 (Figure 8B).
Compared to the nitrogen-free treatment, the application of nitrogen significantly enhanced sugar yield. However, this positive effect diminished once the nitrogen application rate exceeded 225 kg ha−1, with non-significant differences observed between the treatment with 300 kg ha−1 and the nitrogen-free treatment, except for the N300S1 treatment in 2021 (Figure 8C). Compared to the nitrogen-free treatment, the nitrogen application rate of 225 kg ha−1 led to a substantial increase in sugar yield, ranging from 6.24% (N225S2, 2022) to 35.21% (N225S1, 2021). In addition, at the nitrogen application rate of 150 kg ha−1, the split nitrogen application (N150S2) markedly improved sugar yield compared to the single nitrogen application (N150S1), with improvements ranging from 7.21% to 12.79% achieving a yield comparable to the N225S1 treatment (Figure 8C).
3.7. Correlation Analysis Between Physiological Parameters, Yield, and Quality of Sugar Beet
Correlation analysis based on two-year datasets showed a strong positive correlation between sugar beet root yield and key physiological parameters, including chlorophyll content, dry matter accumulation in both aboveground parts and roots, total root nitrogen content, and sugar yield. Similarly, sugar yield was positively associated with chlorophyll content, root dry matter accumulation, total soluble sugar content, root sugar content, and root yield (Figure 9A,B). Furthermore, the root total nitrogen content was significantly and positively associated with leaf area index, chlorophyll content, aboveground and root dry matter accumulation, soluble protein content, and root yield. Conversely, the C/N ratio displayed a negative correlation with these physiological parameters. We also observed interannual differences in the correlations among physiological and yield-related traits. For example, the negative correlations of LAI, chlorophyll content, and aboveground dry matter accumulation with sucrose content were only detected in 2022, while the positive associations of chlorophyll content and root–crown ratio with total soluble sugar content were observed exclusively in 2021 (Figure 9A,B).
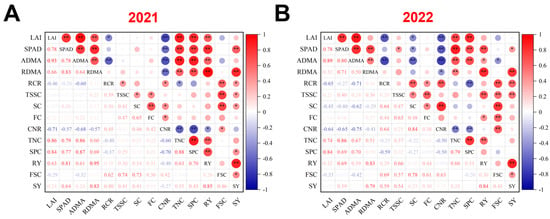
Figure 9.
Correlation analysis between physiological parameters, yield, and the quality of sugar beet under different nitrogen application levels (N0, N75, N150, N225, and N300) and methods (S1 and S2). (A) Correlation analysis conducted in 2021. (B) Correlation analysis conducted in 2022. LAI: leaf area index; ADMA: aboveground dry matter accumulation; RDMA: root dry matter accumulation; RCR: root–crown ratio; TSSC: total soluble sugar content; SC: sucrose content; FC: fructose content; CNR: carbon–nitrogen ratio; TNC: total nitrogen content; SPC: soluble protein content; RY: root yield; FSC: final sugar content; SY: sugar yield. * indicates p ≤ 0.05, and ** indicates p ≤ 0.01.
4. Discussion
Sugar beet, as a high-biomass crop, requires substantial water and nutrient inputs throughout its growth stages. Nitrogen, a critical fundamental element in plants, significantly influences crop yield and quality when applied in optimal quantities [37,60,61]. Our findings in this study demonstrate that optimal nitrogen application rates significantly increase sugar beet yield by enhancing the biomass accumulation of aboveground parts and regulating carbon and nitrogen metabolism, thus increasing LAI and chlorophyll content (Figure 10). In accordance with previous studies, our results further verified the essential role of nitrogen application in enhancing sugar beet productivity [62,63,64]. This study investigated the effects of split nitrogen application on sugar beet growth, ANUE, and yield from the perspective of carbon and nitrogen metabolism, aiming to identify optimal fertilization strategies for sugar beet production in Inner Mongolia, China.
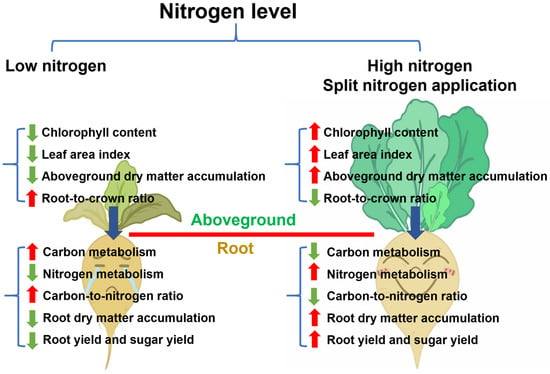
Figure 10.
A summary of the effects of nitrogen application levels and methods on sugar beet growth, carbon and nitrogen metabolism, and yield. The diagram summarizes effects under low versus high/split nitrogen treatments. Blue arrows indicate the transport of photosynthetic products; red and green arrows represent increased and decreased indicators measured in this study, respectively.
LAI and chlorophyll content are important dynamic parameters for assessing leaf development and serve as essential indicators of crop development and potential yield [54,65]. We observed a clear positive correlation between the nitrogen application rate and LAI, indicating that nitrogen significantly promotes canopy development (Figure 3A,B). Similar findings have been reported in previous studies, where LAI increased substantially with increasing nitrogen application rates [44,60,66]. At the LG stage, the LAI under a single nitrogen application was markedly higher than that under split applications. Nevertheless, this disparity diminished in the later growth stages following the application of the remaining nitrogen, suggesting that delayed nitrogen input rapidly stimulated leaf expansion and compensated for earlier differences. Our findings diverge from those of Su et al., as we observed no notable improvement in LAI with a split nitrogen application [44]. One possible explanation is the difference in fertilization timing, since our approach prioritized early-stage nitrogen application, while their strategy emphasized mid- to late-growth stage fertilization. The application of nitrogen fertilizer led to a marked increase in chlorophyll concentration in sugar beet leaves, aligning with prior studies and indicating enhanced photosynthetic capacity [10,67].
Our results indicate that when the nitrogen application rate was lower than 225 kg ha−1, sugar beet yields significantly increased as the nitrogen application rates increased. However, when the nitrogen application rate reached 300 kg ha−1, the yield did not increase anymore and was lower than that at 225 kg ha−1. This is in accordance with previous studies on many crops [48,68]. On the one hand, excessive nitrogen application promotes the overgrowth of stems and leaves, which consumes a large amount of photosynthetic products, thus reducing the accumulation of assimilate in storage organs [69]. On the other hand, when excessive amounts of nitrogen are applied all at once, plants are unable to absorb it completely, leading to its accumulation in the soil. Therefore, excess nitrogen in the soil can induce soil acidification, which deteriorates the soil environment and adversely affects root development [61,70,71]. Such impairment in root growth compromises nutrient absorption, thereby limiting plant development and ultimately causing a decrease in crop yield [72]. In our study, a similar phenomenon was observed, wherein increasing nitrogen application rates showed a stronger promotive effect on the aboveground dry matter accumulation compared to the roots (Figure 4E,F). This disproportionate allocation ultimately resulted in a decreased yield of the roots, the primary harvestable organ (Figure 8A,C). This observation is consistent with previous studies, where excessive nitrogen application was found to divert assimilates towards leaf growth, thereby inhibiting root development in sugar beet [42,44]. Therefore, for sugar beet, identifying the appropriate nitrogen application is crucial for optimizing the root–shoot ratio and maximizing root yield.
Given the varying types and quantities of nutrients required at the different developmental stages of crops, split fertilizer application based on the actual growth conditions is generally more effective than single fertilizer application [73,74]. Studies on crops such as rice, maize, sweet potato, and winter wheat have demonstrated that split nitrogen application, compared to single nitrogen application, can significantly enhance nitrogen use efficiency and increase crop yield [75,76,77,78]. However, the proportion and timing of split nitrogen application should be optimized according to the crop’s growth stages, as inappropriate splitting strategies may adversely affect yield improvement [79]. For instance, compared to a two-split nitrogen application, a three-split application has been shown to be more effective in enhancing maize yield [64]. However, for winter wheat, a two-split nitrogen application is most beneficial for improving leaf photosynthetic capacity and yield [77]. In our study, compared to the single nitrogen application, a split application called for 40% of the total nitrogen to be applied before the LG stage, ensuring a timely nitrogen supply and thus contributing to yield improvement. Nonetheless, as nitrogen application rates increased, the yield-enhancing effect gradually diminished, possibly due to soil degradation caused by excessive nitrogen input, which had a negative impact on sugar beet growth. Similarly, Su et al. found that although a two-time nitrogen application was more effective than a single application, further splitting into three applications reduced the yield advantage [44]. Their research also demonstrated that surpassing optimal nitrogen levels failed to further enhance sugar beet yield and, in some cases, even reduced it.
Plants continuously exchange the metabolites involved in carbon and nitrogen metabolism via the phloem and xylem, thereby coordinating the growth of both the aboveground parts and the roots [20,80]. Our results revealed that, during the early growth stage (LG), sugar beet roots exhibited a high total nitrogen content and a low C/N ratio, indicating a strong nitrogen metabolic capacity for synthesizing the proteins and enzymes essential for root development. In contrast, during the later stages, the roots shifted to enhance carbon metabolism as they began to accumulate and store sugars. Similar trends have been observed in rice, maize, and other crops, where the grain starch content increases with prolonged post-anthesis periods [27,63,81,82]. In addition, our results indicated that, as the nitrogen application levels increased, the total nitrogen content in roots increased significantly, whereas the levels of total soluble sugars decreased to varying degrees, resulting in a reduced C/N ratio (Figure 6G,H). This finding suggests that high nitrogen inputs enhance nitrogen metabolism while suppressing carbon metabolism in sugar beet roots. This is consistent with numerous previous studies that show that a high nitrogen application significantly enhances grain nitrogen content, while simultaneously inhibiting carbohydrate biosynthesis by reducing the activity of the key enzymes involved in carbon metabolism [48,62,83].
For sugar beet production, sugar yield directly influences final economic returns. Therefore, maximizing sugar yield is a fundamental objective for sugar beet production to enhance productivity and profitability. Sugar content represents a crucial determinant of sugar yield in sugar beet, and is influenced by water availability, fertilization, and climatic conditions [10,43]. In general, there is a negative correlation between root yield and sugar content in sugar beet—as nitrogen application increases, root yield tends to increase while sugar content decreases [84]. Our findings demonstrated that sugar content was significantly reduced by high nitrogen applications, whereas lower application rates had no significant influence (Figure 8B). However, our study did not observe any significant effect of the split nitrogen applications on sugar content, in contrast to the findings of Su et al., who reported a significant increase in sugar content under appropriately split nitrogen treatments [44]. This difference could be attributed to variations in cultivation regions, including climate and soil conditions, as well as differences in experimental design across the studies.
In this study, our results showed that the split nitrogen application with a rate of 150 kg ha−1 can reach comparable root and sugar yields with the single nitrogen application at a rate of 225 kg ha−1, which was the highest yield observed in this study, indicating that a split nitrogen application can optimize sugar beet root growth and sugar metabolism. This means that a split nitrogen application can maintain both a taproot yield and final sugar yield compared to the single nitrogen application, while greatly reducing nitrogen fertilizer input by 33.33%. Moreover, the split nitrogen application can substantially enhance ANUE and sugar yield, ensuring high sugar production without additional nitrogen fertilizer inputs. Although this study provides valuable insights into the role of split nitrogen application in enhancing sugar beet yield, several limitations should be acknowledged. First, the field experiments were conducted in Inner Mongolia, which may limit the generalizability of the results to other agroecological zones. Second, the analysis focused primarily on conventional physiological indicators such as LAI, SPAD, and dry matter accumulation, without incorporating more detailed physiological and biochemical assessments, such as key enzyme activities. Mechanistic insights at the molecular or metabolic level were thus lacking. Third, this study did not evaluate key quality-related parameters of sugar beet roots, such as the content of sodium (Na+), potassium (K+), and α-amino nitrogen, which are crucial for sugar extraction and processing quality. Fourth, although water and fertilizer are two primary factors influencing sugar beet growth, water availability was not considered in the present study, which may have interacted with nitrogen effects. Finally, the potential environmental impacts associated with different nitrogen management strategies, such as nitrogen leaching and gaseous emissions were not assessed. These limitations highlight the need for further investigations that incorporate more comprehensive physiological, agronomic, and environmental parameters across diverse geographical conditions.
5. Conclusions
In this study, field experiments conducted in Inner Mongolia over two growing seasons demonstrated that increasing nitrogen application significantly improved chlorophyll content and LAI in sugar beet, thereby enhancing photosynthetic capacity and resulting in the highest sugar yield at 225 kg ha−1. However, an elevated nitrogen input also led to a marked decline in ANUE and disrupted the carbon and nitrogen balance in roots. Notably, compared to single nitrogen applications, a split application strategy, where 60% of the nitrogen was applied as basal fertilizer and the remaining 40% before the rapid leaf growth stage, significantly improved ANUE and optimized carbon and nitrogen metabolism. This approach maintained high sugar beet yield while reducing nitrogen input from 225 to 150 kg ha−1. These findings highlight the effectiveness of split nitrogen management in balancing productivity and input efficiency. By reducing chemical fertilizer use without compromising yield or sugar content, this strategy contributes to more sustainable agricultural practices, with the potential to mitigate environmental impacts associated with excessive nitrogen use and promote crop production in semi-arid regions.
Author Contributions
S.S. and X.X. designed the project. X.X. and S.D. measured the indicators related to carbon and nitrogen metabolism. X.X., S.D. and M.G. collaboratively determined the soil physicochemical properties, SPAD values, LAI, dry matter accumulation, and yield. Data processing and manuscript drafting were performed by X.X. and L.W., with S.S. revising and reviewing the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the National Natural Science Foundation of China (32360537), the National Modern Agricultural Industrial Technology System Project (CARS-17), the Inner Mongolia Autonomous Region Major Science and Technology Project (2022JBGS0029), and the Central Guidance for Local Science and Technology Development Fund Project (2024ZY0023).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Wenxue Sui, Weidong Cui, and Zeyu Yang of Inner Mongolia Biohymn New Agricultural Technology Co., Ltd. We also sincerely thank Linxi County Meteorological Bureau for supplying local temperature and precipitation data for the experimental and data processing stages. We are particularly grateful to Jinquan Li and Mohsin Ali from Strube GmbH for their insightful feedback and constructive suggestions during the manuscript revision process. During the preparation of this manuscript, the authors used ChatGPT (OpenAI, GPT-4, 2025 version) for grammar checking, vocabulary enhancement, and improving academic expression.
Conflicts of Interest
Author Xuming Xing was employed by the company Inner Mongolia Biohymn New Agricultural Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| LAI | Leaf area index |
| CCOs | Continuous cropping obstacles |
| GOT | Glutamic oxaloacetate transaminase |
| GPT | Glutamate pyruvate transaminase |
| GBSS | Granule-bound starch synthase |
| SSS | Soluble starch synthase |
| LG | Leaf growth stage |
| RG | Root growth stage |
| SA | Sugar accumulation stage |
| HA | Harvest stage |
| C/N | Carbon–nitrogen |
| ANUE | Agronomic nitrogen use efficiency |
| ADMA | Aboveground dry matter accumulation |
| RDMA | Root dry matter accumulation |
| RCR | Root–crown ratio |
| TSSC | Total soluble sugar content |
| SC | Sucrose content |
| FC | Fructose content |
| CNR | Carbon–nitrogen ratio |
| TNC | Total nitrogen content |
| SPC | Soluble protein content |
| RY | Root yield |
| FSC | Final sugar content |
| SY | Sugar yield |
References
- Ebrahimi, P.; Khamirikar, F.; Lante, A. Unlocking the Biorefinery Approaches to Valorize Sugar Beet Leaves (B. vulgaris L.) for Food Industry Applications: A Critical Review. Food Res. Int. 2024, 197, 115145. [Google Scholar] [CrossRef]
- Gruska, R.M.; Baryga, A.; Kunicka-Styczyńska, A.; Brzeziński, S.; Rosicka-Kaczmarek, J.; Miśkiewicz, K.; Sumińska, T. Fresh and Stored Sugar Beet Roots as a Source of Various Types of Mono- and Oligosaccharides. Molecules 2022, 27, 5125. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Bieliński, D.; Binczarski, M.; Berlowska, J.; Dziugan, P.; Piotrowski, J.; Stanishevsky, A.; Witońska, I.A. Products of Sugar Beet Processing as Raw Materials for Chemicals and Biodegradable Polymers. RSC Adv. 2018, 8, 3161–3177. [Google Scholar] [CrossRef]
- Xiao, S.; Chai, H.; Shao, K.; Shen, M.; Wang, Q.; Wang, R.; Sui, Y.; Ma, Y. Image-Based Dynamic Quantification of Aboveground Structure of Sugar Beet in Field. Remote Sens. 2020, 12, 269. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of Sugar Beet Pulp to Value-Added Products: A Review. Bioresour. Technol. 2022, 346, 126580. [Google Scholar] [CrossRef]
- Yousefabadi, V.A.; Mehdikhani, P.; Nadali, F.; Sharifi, M.; Azizi, H.; Ahmadi, M.; Fasahat, P. Evaluation of Yield and Stability of Sugar Beet (Beta vulgaris L.) Genotypes Using GGE Biplot and AMMI Analysis. Sci. Rep. 2024, 14, 27384. [Google Scholar] [CrossRef]
- McGinnis, E.E.; Meyer, M.H.; Smith, A.G. Sweet and Sour: A Scientific and Legal Look at Herbicide-Tolerant Sugar Beet. Plant Cell 2010, 22, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Khusnitdinova, M.; Abdrakhmanova, A.; Pozharskiy, A.; Kapytina, A.; Kerimbek, N.; Nizamdinova, G.; Taskuzhina, A.; Adilbayeva, K.; Kolchenko, M.; Gritsenko, D. Problems and Prospects of Sugar Beet Cultivation in Kazakhstan. Agron. Res. 2023, 21, 1174–1185. [Google Scholar] [CrossRef]
- Tayyab, M.; Wakeel, A.; Mubarak, M.U.; Artyszak, A.; Ali, S.; Hakki, E.E.; Mahmood, K.; Song, B.; Ishfaq, M. Sugar Beet Cultivation in the Tropics and Subtropics: Challenges and Opportunities. Agronomy 2023, 13, 1213. [Google Scholar] [CrossRef]
- Wang, N.; Fu, F.; Wang, H.; Wang, P.; He, S.; Shao, H.; Ni, Z.; Zhang, X. Effects of Irrigation and Nitrogen on Chlorophyll Content, Dry Matter and Nitrogen Accumulation in Sugar Beet (Beta vulgaris L.). Sci. Rep. 2021, 11, 16651. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, K.; Cai, Z.; Che, Y.; Chen, H.; Xiao, S.; Wang, R.; Liu, Y.; Li, B.; Ma, Y. Prediction of Sugar Beet Yield and Quality Parameters Using Stacked-LSTM Model with Pre-Harvest UAV Time Series Data and Meteorological Factors. Artif. Intell. Agric. 2025, 15, 252–265. [Google Scholar] [CrossRef]
- Chang, Y.; Li, G.; Jian, C.; Zhang, B.; Sun, Y.; Li, N.; Zhang, S. Influence of Water and Fertilizer Reduction on Respiratory Metabolism in Sugar Beet Taproot (Beta vulgaris L.). Plants 2024, 13, 2282. [Google Scholar] [CrossRef]
- Guo, X.-X.; Tian, L.; Li, Y.-H.; Huang, C.-Y.; Li, Z.; Zhang, P.; Jian, C.-Y.; Han, K.; Zhou, J.-C. Effects of Continuous Cropping and Application of Bio-Organic Fertilizer on Photosynthetic Performance, Dry Matter Accumulation and Distribution of Sugar Beet. Sci. Rep. 2025, 15, 1512. [Google Scholar] [CrossRef] [PubMed]
- Amirahmadi, E.; Ghorbani, M.; Krexner, T.; Hörtenhuber, S.J.; Bernas, J.; Neugschwandtner, R.W.; Konvalina, P.; Moudrý, J. Life Cycle Assessment of Biochar and Cattle Manure Application in Sugar Beet Cultivation—Insights into Root Yields, White Sugar Quality, Environmental Aspects in Field and Factory Phases. J. Clean. Prod. 2024, 476, 143772. [Google Scholar] [CrossRef]
- Huang, W.; Han, S.; Wang, L.; Li, W. Carbon and Nitrogen Metabolic Regulation in Freshwater Plant Ottelia Alismoides in Response to Carbon Limitation: A Metabolite Perspective. Front. Plant Sci. 2022, 13, 962622. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and Signaling Aspects Underpinning the Regulation of Plant Carbon Nitrogen Interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Wang, Z.; Wang, L.; Li, X.; Fan, Z.; Zhang, Y.; Li, J.; Gao, X.; Shi, J.; et al. Effects of Nitrogen Fertilizer on Photosynthetic Characteristics, Biomass, and Yield of Wheat under Different Shading Conditions. Agronomy 2021, 11, 1989. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Q.; Zeng, X.P.; Liu, Y.; Li, Y.R. Fate of Nitrogen in Agriculture and Environment: Agronomic, Eco-Physiological and Molecular Approaches to Improve Nitrogen Use Efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef]
- Hu, W.; Coomer, T.D.; Loka, D.A.; Oosterhuis, D.M.; Zhou, Z. Potassium Deficiency Affects the Carbon-Nitrogen Balance in Cotton Leaves. Plant Physiol. Biochem. 2017, 115, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Liu, Y.; Sun, G.; Kong, D.; Guo, W.; Sun, H. Regulatory Effect of Graphene on Growth and Carbon/Nitrogen Metabolism of Maize (Zea mays L.). J. Sci. Food Agric. 2024, 104, 1572–1582. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant Carbon Metabolism and Climate Change: Elevated CO2 and Temperature Impacts on Photosynthesis, Photorespiration and Respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Yang, S.; Ma, A.; Lunzhu, C.; Wang, M.; Wang, G.; Guo, S. Grain Chalkiness Is Reduced by Coordinating the Biosynthesis of Protein and Starch in Fragrant Rice (Oryza sativa L.) Grain under Nitrogen Fertilization. Field Crop. Res. 2023, 302, 109098. [Google Scholar] [CrossRef]
- Sun, J.; Jin, L.; Li, R.; Meng, X.; Jin, N.; Wang, S.; Xu, Z.; Liu, Z.; Lyu, J.; Yu, J. Effects of Different Forms and Proportions of Nitrogen on the Growth, Photosynthetic Characteristics, and Carbon and Nitrogen Metabolism in Tomato. Plants 2023, 12, 4175. [Google Scholar] [CrossRef]
- Iqbal, A.; Jing, N.; Qiang, D.; Kayoumu, M.; Wang, X.; Gui, H.; Zhang, H.; Zhang, X.; Song, M. Genotypic Variation in Carbon and Nitrogen Metabolism in the Cotton Subtending Leaves and Seed Cotton Yield under Various Nitrogen Levels. J. Sci. Food Agric. 2023, 103, 2602–2617. [Google Scholar] [CrossRef]
- Feng, W.; Xue, W.; Zhao, Z.; Shi, Z.; Wang, W.; Bai, Y.; Wang, H.; Qiu, P.; Xue, J.; Chen, B. Nitrogen Fertilizer Application Rate Affects the Dynamic Metabolism of Nitrogen and Carbohydrates in Kernels of Waxy Maize. Front. Plant Sci. 2024, 15, 1416397. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Mu, B.; Wang, F.; Feng, N.; Zheng, D. Nitrogen Limitation Affects Carbon and Nitrogen Metabolism in Mung Bean (Vigna radiata L.). J. Plant Physiol. 2023, 290, 154105. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Wang, X.; Xu, G.; Luo, Z.; Peng, J.; Luo, Q.; Lu, W.; Han, Z. High Nitrogen Input Increases the Total Spikelets but Decreases the High-Density Grain Content in Hybrid Indica Rice. Field Crop. Res. 2022, 288, 108679. [Google Scholar] [CrossRef]
- Tanaka, M.; Keira, M.; Yoon, D.K.; Mae, T.; Ishida, H.; Makino, A.; Ishiyama, K. Photosynthetic Enhancement, Lifespan Extension, and Leaf Area Enlargement in Flag Leaves Increased the Yield of Transgenic Rice Plants Overproducing Rubisco Under Sufficient N Fertilization. Rice 2022, 15, 10. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Yan, H.; Jiang, X.; Ma, Y.; Qin, Y. Carbon and Nitrogen Metabolism under Nitrogen Variation Affects Flavonoid Accumulation in the Leaves of Coreopsis tinctoria. PeerJ 2021, 9, e12152. [Google Scholar] [CrossRef]
- Shi, Z.; Li, D.; Jing, Q.; Cai, J.; Jiang, D.; Cao, W.; Dai, T. Effects of Nitrogen Applications on Soil Nitrogen Balance and Nitrogen Utilization of Winter Wheat in a Rice-Wheat Rotation. Field Crop. Res. 2012, 127, 241–247. [Google Scholar] [CrossRef]
- Hou, W.; Xue, X.; Li, X.; Khan, M.R.; Yan, J.; Ren, T.; Cong, R.; Lu, J. Interactive Effects of Nitrogen and Potassium on: Grain Yield, Nitrogen Uptake and Nitrogen Use Efficiency of Rice in Low Potassium Fertility Soil in China. Field Crop. Res. 2019, 236, 14–23. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Yao, Q.; Xu, L.; Li, W.; Tan, W.; Wang, Q.; Xing, W.; Liu, D. Tolerance and Adaptation Characteristics of Sugar Beet (Beta vulgaris L.) to Low Nitrogen Supply. Plant Signal. Behav. 2023, 18, e2159155. [Google Scholar] [CrossRef]
- Malnou, C.S.; Jaggard, K.W.; Sparkes, D.L. Nitrogen Fertilizer and the Efficiency of the Sugar Beet Crop in Late Summer. Eur. J. Agron. 2008, 28, 47–56. [Google Scholar] [CrossRef]
- Sinta, Z.; Garo, G. Influence of Plant Density and Nitrogen Fertilizer Rates on Yield and Yield Components of Beetroot (Beta vulgaris L.). Int. J. Agron. 2021, 2021, 670243. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Xu, P.; Liu, D.; Zhang, L.; Hao, Y.; Wang, K.; Fan, H. Nitrogen Use Efficiency of Drip Irrigated Sugar Beet as Affected by Sub-Optimal Levels of Nitrogen and Irrigation. Agric. Water Manag. 2024, 298, 108849. [Google Scholar] [CrossRef]
- Mekdad, A.A.A.; Shaaban, A. Integrative Applications of Nitrogen, Zinc, and Boron to Nutrients-Deficient Soil Improves Sugar Beet Productivity and Technological Sugar Contents under Semi-Arid Conditions. J. Plant Nutr. 2020, 43, 1935–1950. [Google Scholar] [CrossRef]
- Varga, I.; Jović, J.; Rastija, M.; Markulj Kulundžić, A.; Zebec, V.; Lončarić, Z.; Iljkić, D.; Antunović, M. Efficiency and Management of Nitrogen Fertilization in Sugar Beet as Spring Crop: A Review. Nitrogen 2022, 3, 170–185. [Google Scholar] [CrossRef]
- Tarkalson, D.D.; Bjorneberg, D.L.; Lentz, R.D. Effects of Manure History and Nitrogen Fertilizer Rate on Sugar Beet Production in the Northwest US. Crop. Forage Turfgrass Manag. 2018, 4, 170083. [Google Scholar] [CrossRef]
- Last, P.J.; Draycott, A.P.; Messem, A.B.; Webb, D.J. Effects of Nitrogen Fertilizer and Irriǵation on Suǵar Beet at Broom’s Barn 1973-8. J. Agric. Sci. 1983, 101, 185–205. [Google Scholar] [CrossRef]
- Reid, J.B.; Hunt, A.G.; Johnstone, P.R.; Searle, B.P. Beetroot (Beta vulgaris L.) Growth and Response to N Supply—A Case Study. N. Z. J. Crop. Hortic. Sci. 2020, 48, 191–212. [Google Scholar] [CrossRef]
- Idris, B.E.M.; Marajan, W.A.; Adam, A.H.M. Effect of Nitrogen Fertilizer and Plant Spacing on Vegetative Growth of Sugar Beet (Beta vulgaris L.). J. Agron. Res. 2021, 4, 6–13. [Google Scholar] [CrossRef]
- Su, J.; Zhou, H.; Wang, K.; Fan, H.; Hou, Z. Effects of Nitrogen Fertilizer Management on Dry Matter Accumulation and Yield of Drip-Irrigated Sugar Beet in Arid Areas. Agronomy 2024, 14, 1010. [Google Scholar] [CrossRef]
- Da, B.; Wu, Y.; Bao, W. Analysis of Spatial Distribution and Spillover Effects of Fertilizer Application Intensity in Inner Mongolia, China. Sustainability 2024, 16, 4697. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, J.; Li, X.; Jiang, Z.; Dong, S. Effects of DA-6 and MC on the Growth, Physiology, and Yield Characteristics of Soybean. BMC Plant Biol. 2025, 25, 304. [Google Scholar] [CrossRef]
- Su, B.Y.; Song, Y.X.; Song, C.; Cui, L.; Yong, T.W.; Yang, W.Y. Growth and Photosynthetic Responses of Soybean Seedlings to Maize Shading in Relay Intercropping System in Southwest China. Photosynthetica 2014, 52, 332–340. [Google Scholar] [CrossRef]
- Sun, J.; Li, W.; Li, C.; Chang, W.; Zhang, S.; Zeng, Y.; Zeng, C.; Peng, M. Effect of Different Rates of Nitrogen Fertilization on Crop Yield, Soil Properties and Leaf Physiological Attributes in Banana Under Subtropical Regions of China. Front. Plant Sci. 2020, 11, 613760. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, X.; Hu, J.; Zhu, L.; Zhang, J.; Huang, J.; Jin, Q. Nitrogen Metabolism in Adaptation of Photosynthesis to Water Stress in Rice Grown under Different Nitrogen Levels. Front. Plant Sci. 2017, 8, 1079. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, B.; Yuan, Z.; Su, X. Changes in Carbohydrate Content and Membrane Stability of Two Ecotypes of Calamagrostis arundinacea Growing at Different Elevations in the Drawdown Zone of the Three Gorges Reservoir. PLoS ONE 2014, 9, e91394. [Google Scholar] [CrossRef]
- Shi, H.; Wang, B.; Yang, P.; Li, Y.; Miao, F. Differences in Sugar Accumulation and Mobilization between Sequential and Non-Sequential Senescence Wheat Cultivars under Natural and Drought Conditions. PLoS ONE 2016, 11, e0166155. [Google Scholar] [CrossRef]
- Cai, Y.; Shao, L.; Li, X.; Liu, G.; Chen, S. Gibberellin Stimulates Regrowth after Defoliation of Sheepgrass (Leymus chinensis) by Regulating Expression of Fructan-Related Genes. J. Plant Res. 2016, 129, 935–944. [Google Scholar] [CrossRef]
- Wan, X.; Wu, W.; Shah, F. Nitrogen Fertilizer Management for Mitigating Ammonia Emission and Increasing Nitrogen Use Efficiencies by 15N Stable Isotopes in Winter Wheat. Sci. Total Environ. 2021, 790, 147587. [Google Scholar] [CrossRef]
- Yang, S.; Ge, Y.; Wang, J.; Liu, R.; Tang, D.; Li, A.; Zhu, Z. A Dataset for Estimating Alfalfa Leaf Area and Predicting Leaf Area Index. Front. Plant Sci. 2024, 15, 1290920. [Google Scholar] [CrossRef]
- Kubar, M.S.; Wang, C.; Noor, R.S.; Feng, M.; Yang, W.; Kubar, K.A.; Soomro, K.; Yang, C.; Sun, H.; Mohamed, H.; et al. Nitrogen Fertilizer Application Rates and Ratios Promote the Biochemical and Physiological Attributes of Winter Wheat. Front. Plant Sci. 2022, 13, 1011515. [Google Scholar] [CrossRef]
- Lan, T.; Du, L.; Wang, X.; Zhan, X.; Liu, Q.; Wei, G.; Lyu, C.; Liu, F.; Gao, J.; Feng, D.; et al. Synergistic Effects of Planting Density and Nitrogen Fertilization on Chlorophyll Degradation and Leaf Senescence after Silking in Maize. Crop. J. 2024, 12, 605–613. [Google Scholar] [CrossRef]
- Linders, K.M.; Santra, D.; Schnable, J.C.; Sigmon, B. Variation in Leaf Chlorophyll Concentration in Response to Nitrogen Application across Maize Hybrids in Contrasting Environments. Micropubl. Biol. 2024, 2024, 001115. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.M. Effects of SPAD Value Variations According to Nitrogen Application Levels on Rice Yield and Its Components. Front. Plant Sci. 2024, 15, 1437371. [Google Scholar] [CrossRef]
- Xu, X.; Yang, G.; Yang, X.; Li, Z.; Feng, H.; Xu, B.; Zhao, X. Monitoring Ratio of Carbon to Nitrogen (C/N) in Wheat and Barley Leaves by Using Spectral Slope Features with Branch-and-Bound Algorithm. Sci. Rep. 2018, 8, 10034. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Lončarić, Z.; Kristek, S.; Kulundžić, A.M.; Rebekić, A.; Antunović, M. Sugar Beet Root Yield and Quality with Leaf Seasonal Dynamics in Relation to Planting Densities and Nitrogen Fertilization. Agriculture 2021, 11, 407. [Google Scholar] [CrossRef]
- Duan, J.; Shao, Y.; He, L.; Li, X.; Hou, G.; Li, S.; Feng, W.; Zhu, Y.; Wang, Y.; Xie, Y. Optimizing Nitrogen Management to Achieve High Yield, High Nitrogen Efficiency and Low Nitrogen Emission in Winter Wheat. Sci. Total Environ. 2019, 697, 134088. [Google Scholar] [CrossRef] [PubMed]
- Noor, H.; Ding, P.; Ren, A.; Sun, M.; Gao, Z. Effects of Nitrogen Fertilizer on Photosynthetic Characteristics and Yield. Agronomy 2023, 13, 1550. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, X.; Yan, F.; Xiang, K.; Wu, Y.; Zhang, Q.; Wang, Z.; He, L.; Fan, P.; Yang, Z.; et al. Nitrogen Application Rate Affects the Accumulation of Carbohydrates in Functional Leaves and Grains to Improve Grain Filling and Reduce the Occurrence of Chalkiness. Front. Plant Sci. 2022, 13, 921130. [Google Scholar] [CrossRef]
- Debele, M.; Taressa, B. Urea Split Application to Maize (Zea mays L.) Growth Stages of Medium Maturities, Influenced on Grain Yield and Parameter for Yield at Bako, East Wollega, Ethiopia. Int. J. Agron. 2023, 2023, 6673773. [Google Scholar] [CrossRef]
- Priyanka; Srivastava, P.K.; Rawat, R. Retrieval of Leaf Chlorophyll Content Using Drone Imagery and Fusion with Sentinel-2 Data. Smart Agric. Technol. 2023, 6, 100353. [Google Scholar] [CrossRef]
- Mekdad, A.A.A.; Rady, M.M. Response of Beta Vulgaris L. to Nitrogen and Micronutrients in Dry Environment. Plant Soil Environ. 2016, 62, 23–29. [Google Scholar] [CrossRef]
- Elsayed, S.; El-Hendawy, S.; Elsherbiny, O.; Okasha, A.M.; Elmetwalli, A.H.; Elwakeel, A.E.; Memon, M.S.; Ibrahim, M.E.M.; Ibrahim, H.H. Estimating Chlorophyll Content, Production, and Quality of Sugar Beet under Various Nitrogen Levels Using Machine Learning Models and Novel Spectral Indices. Agronomy 2023, 13, 2743. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Chen, Y.; Jiang, Y.; Dai, Q.; Huo, Z.; Shi, Y.; Zhao, L.; Liao, P.; Wang, W.; et al. Excessive Nitrogen Application Leads to Lower Rice Yield and Grain Quality by Inhibiting the Grain Filling of Inferior Grains. Agriculture 2022, 12, 962. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, Z.; Wen, X.; Liao, Y.; Liu, Y. Effect of Non-Structural Carbohydrate Accumulation in the Stem Pre-Anthesis on Grain Filling of Wheat Inferior Grain. Field Crop. Res. 2017, 211, 66–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil Acidification Caused by Excessive Application of Nitrogen Fertilizer Aggravates Soil-Borne Diseases: Evidence from Literature Review and Field Trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Zhu, F.; Gilliam, F.S.; Mulder, J.; Yoh, M.; Mo, J.; Lu, X. Effects of Excess Nitrogen (N) on Fine Root Growth in Tropical Forests of Contrasting N Status. Forests 2022, 13, 1328. [Google Scholar] [CrossRef]
- Huang, K.; Li, M.; Li, R.; Rasul, F.; Shahzad, S.; Wu, C.; Shao, J.; Huang, G.; Li, R.; Almari, S.; et al. Soil Acidification and Salinity: The Importance of Biochar Application to Agricultural Soils. Front. Plant Sci. 2023, 14, 1206820. [Google Scholar] [CrossRef]
- Kabir, T.; De Laporte, A.; Nasielski, J.; Weersink, A. Adjusting Nitrogen Rates with Split Applications: Modelled Effects on N Losses and Profits Across Weather Scenarios. Eur. J. Agron. 2021, 129, 126328. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, M.; Liu, J.; Wang, W.; Liu, S. Fertilizer and Soil Nitrogen Utilization of Pear Trees as Affected by the Timing of Split Fertilizer Application in Rain-Fed Orchard. Sci. Hortic. 2019, 252, 363–369. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Shi, Y.; Yu, Z. Optimized Split Nitrogen Fertilizer Increase Photosynthesis, Grain Yield, Nitrogen Use Efficiency and Water Use Efficiency under Water-Saving Irrigation. Sci. Rep. 2020, 10, 20310. [Google Scholar] [CrossRef]
- Du, X.; Xi, M.; Kong, L. Split Application of Reduced Nitrogen Rate Improves Nitrogen Uptake and Use Efficiency in Sweetpotato. Sci. Rep. 2019, 9, 14058. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Abubakar, S.A.; Si, Z.; Kama, R.; Gao, Y.; Duan, A. Suitable Split Nitrogen Application Increases Grain Yield and Photosynthetic Capacity in Drip-Irrigated Winter Wheat (Triticum aestivum L.) under Different Water Regimes in the North China Plain. Front. Plant Sci. 2023, 13, 1105006. [Google Scholar] [CrossRef]
- Abo, A.A.; Mamo, M.A.; Handiso, Y.E. Influence of Nitrogen Fertilizer Rate and Its Time of Application on Productivity of Maize (Zea mays L.) in Hadero Tunto District, Kambata Tambaro Zone Southern Ethiopia. Int. J. Agric. Nat. Sci. 2022, 15, 149–162. Available online: https://ijans.org/index.php/ijans/article/view/577 (accessed on 15 February 2025).
- Panison, F.; Sangoi, L.; Durli, M.M.; Leolato, L.S.; Coelho, A.E.; Kuneski, H.F.; de Liz, V.O. Timing and Splitting of Nitrogen Side-Dress Fertilization of Early Corn Hybrids for High Grain Yield. Rev. Bras. Cienc. Solo 2019, 43, e0170338. [Google Scholar] [CrossRef]
- Bao, A.; Zhao, Z.; Ding, G.; Shi, L.; Xu, F.; Cai, H. Accumulated Expression Level of Cytosolic Glutamine Synthetase 1gene (OsGS1;1 or OsGS1;2) Alter Plant Development and the Carbon-Nitrogen Metabolic Status in Rice. PLoS ONE 2014, 9, e95581. [Google Scholar] [CrossRef]
- Ma, Z.; Cao, J.; Chen, X.; Yu, J. Differences in Carbon and Nitrogen Metabolism of Soft Japonica Rice in Southern China during Grain Filling Stage under Different Light and Nitrogen Fertilizer Conditions and Their Relationship with Rice Eating Quality. Front. Plant Sci. 2025, 16, 1534625. [Google Scholar] [CrossRef]
- Li, W.; Shan, Y.; Xiao, X.; Zheng, J.; Luo, Q.; Ouyang, S.; Zhang, G. Effect of Nitrogen and Sulfur Fertilization on Accumulation Characteristics and Physicochemical Properties of A- and B-Wheat Starch. J. Agric. Food Chem. 2013, 61, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Olmedo Pico, L.B.; Vyn, T.J. Dry Matter Gains in Maize Kernels Are Dependent on Their Nitrogen Accumulation Rates and Duration during Grain Filling. Plants 2021, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, D.; Maharjan, B. Optimizing Nitrogen Management to Enhance Irrigated Sugar Beet Yield and Quality. Agron. J. 2024, 16, 2564–2572. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).