Detection of Leaf Miner Infestation in Chickpea Plants Using Hyperspectral Imaging in Morocco
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Data Collection
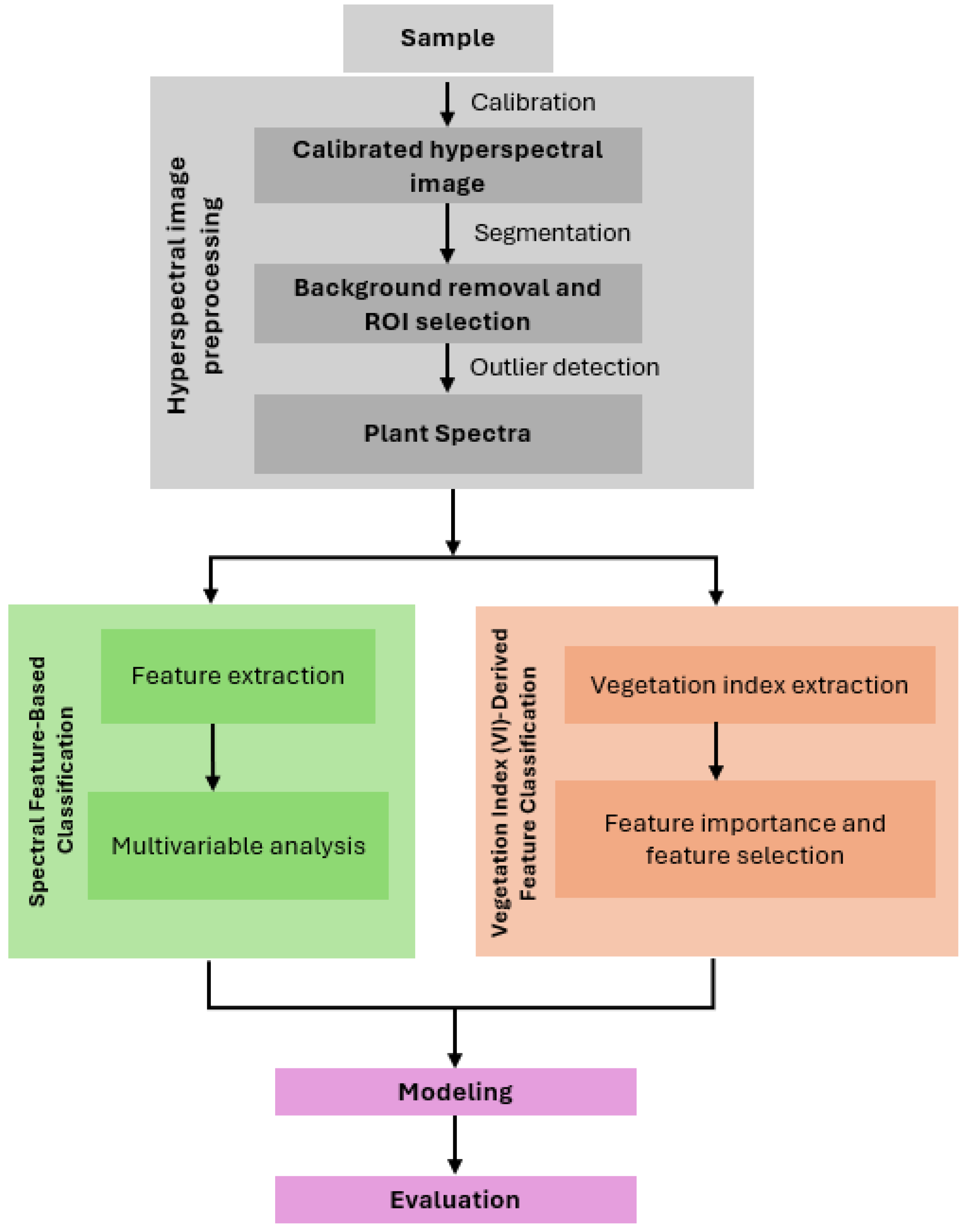
2.2. Methods
2.2.1. Image Preprocessing
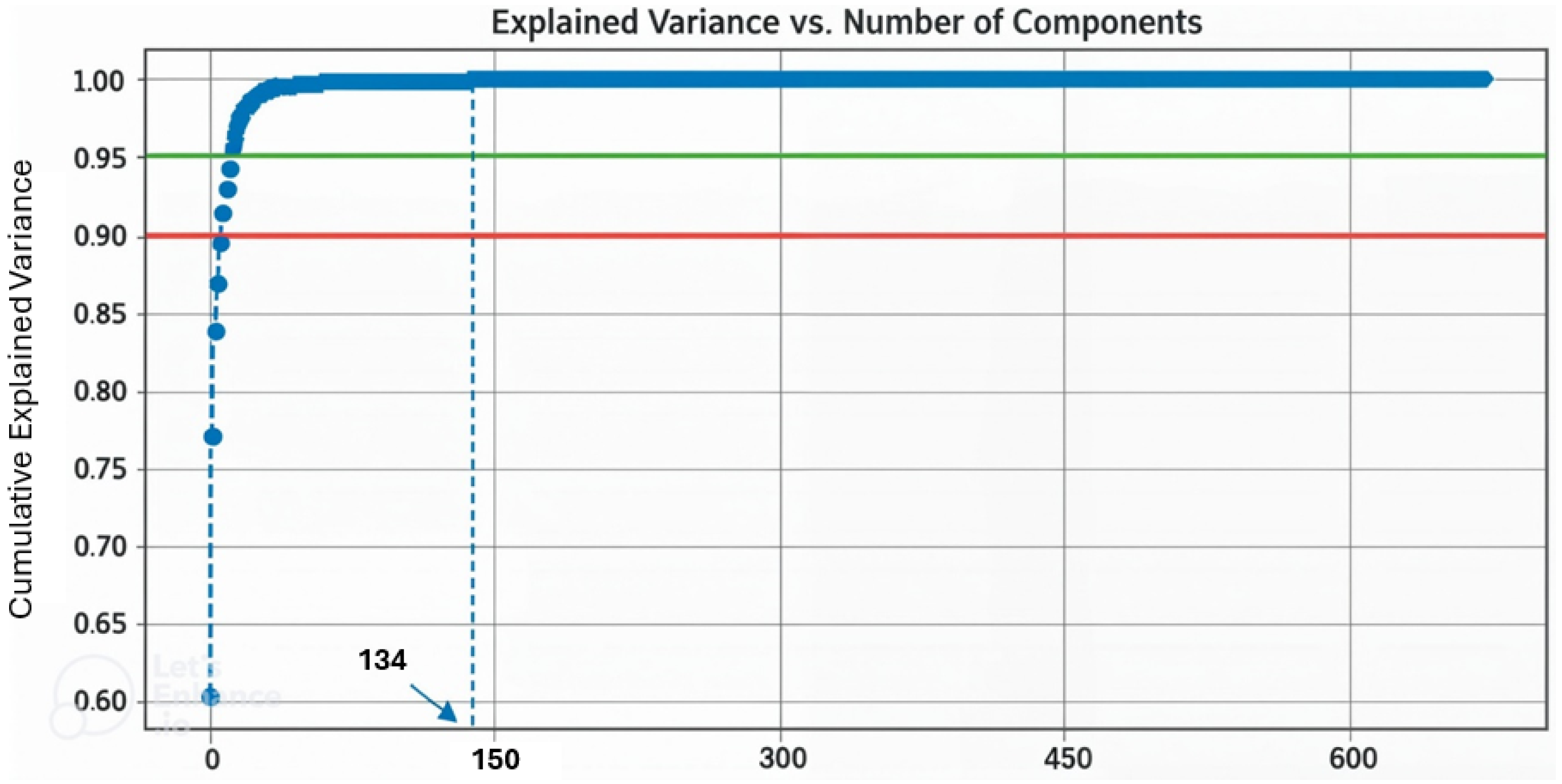
2.2.2. Spectral Feature-Based Classification
2.2.3. VI-Based Classification
2.2.4. Model Selection, Training, and Evaluation
3. Results
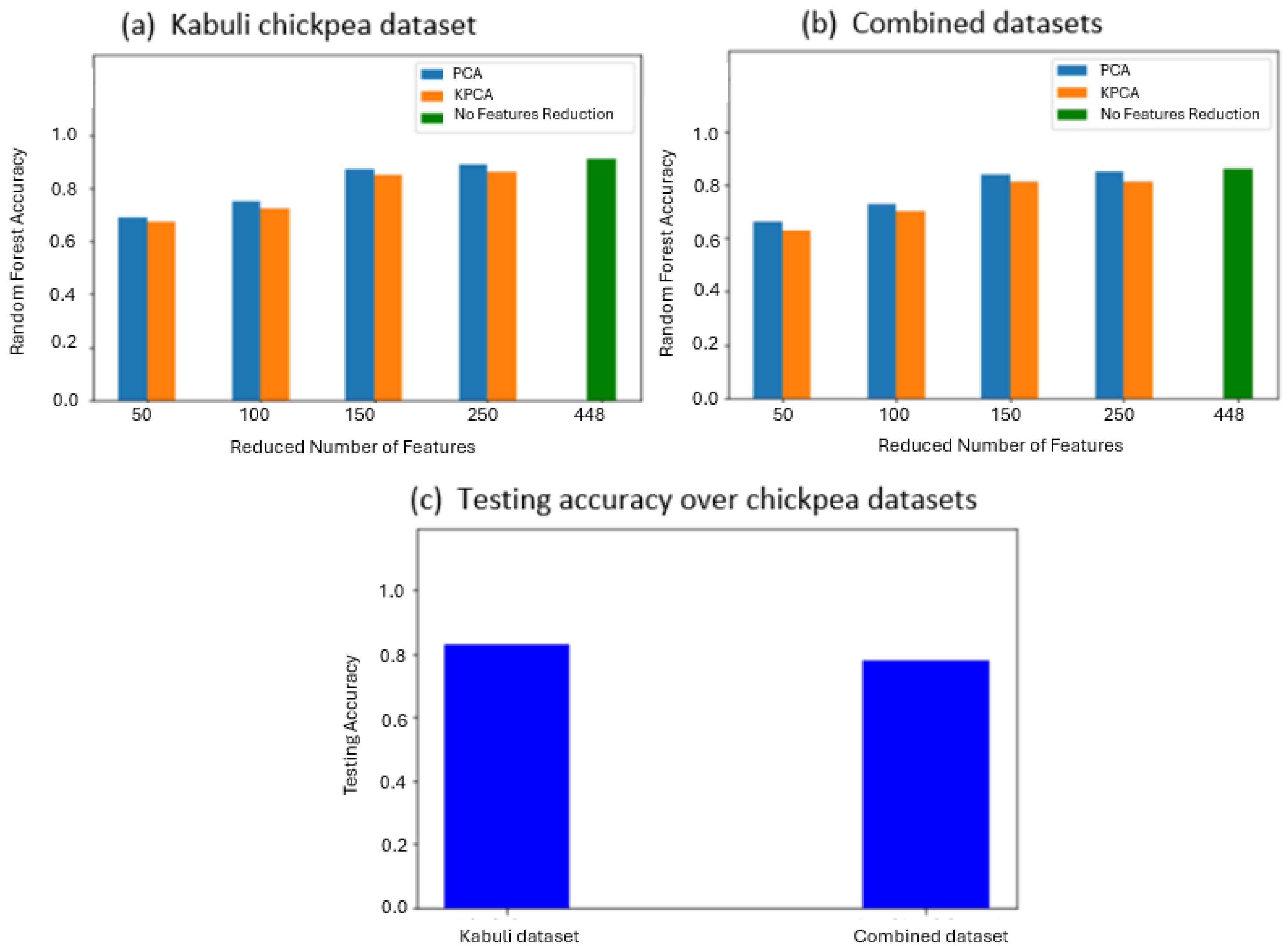
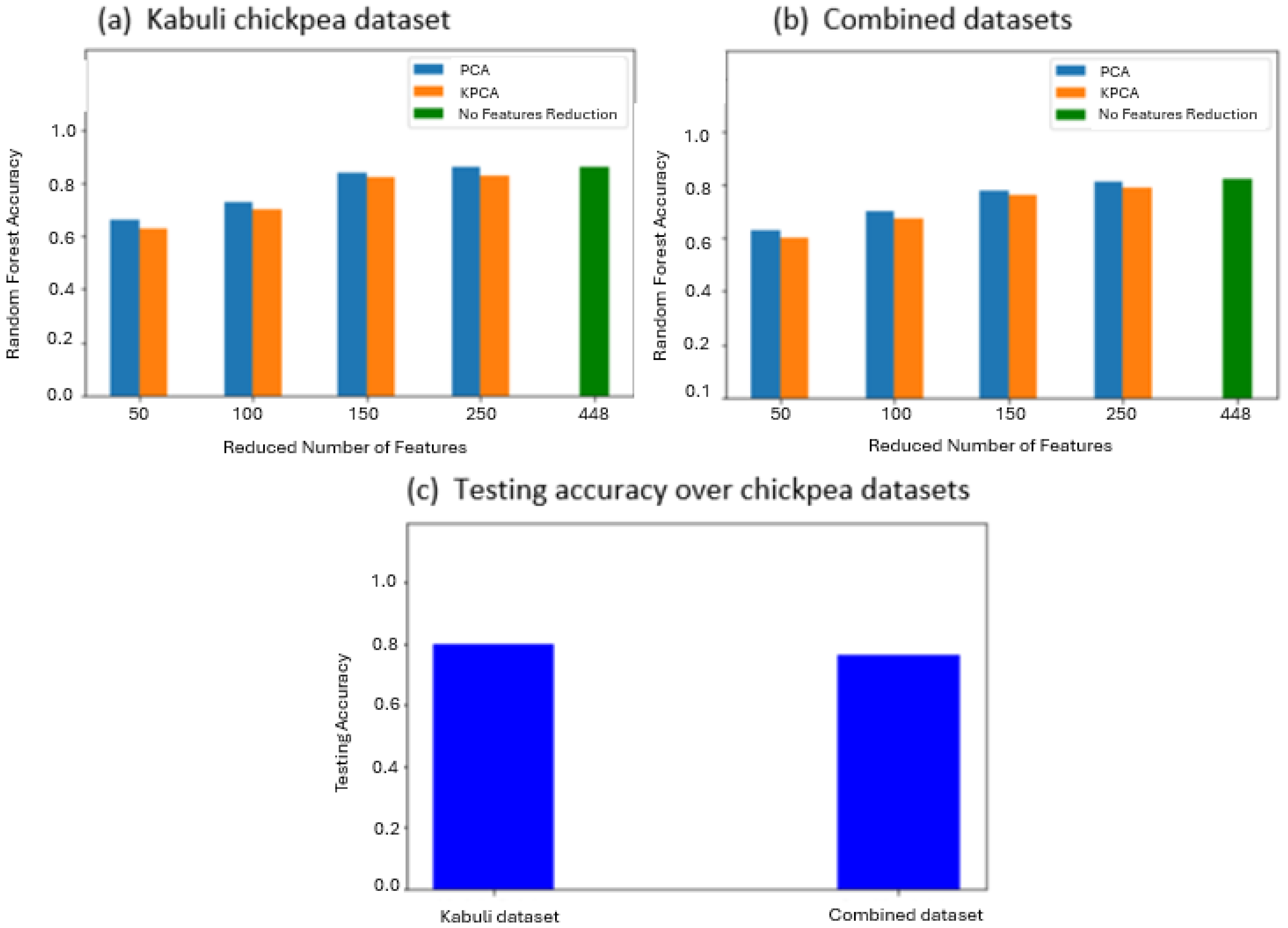
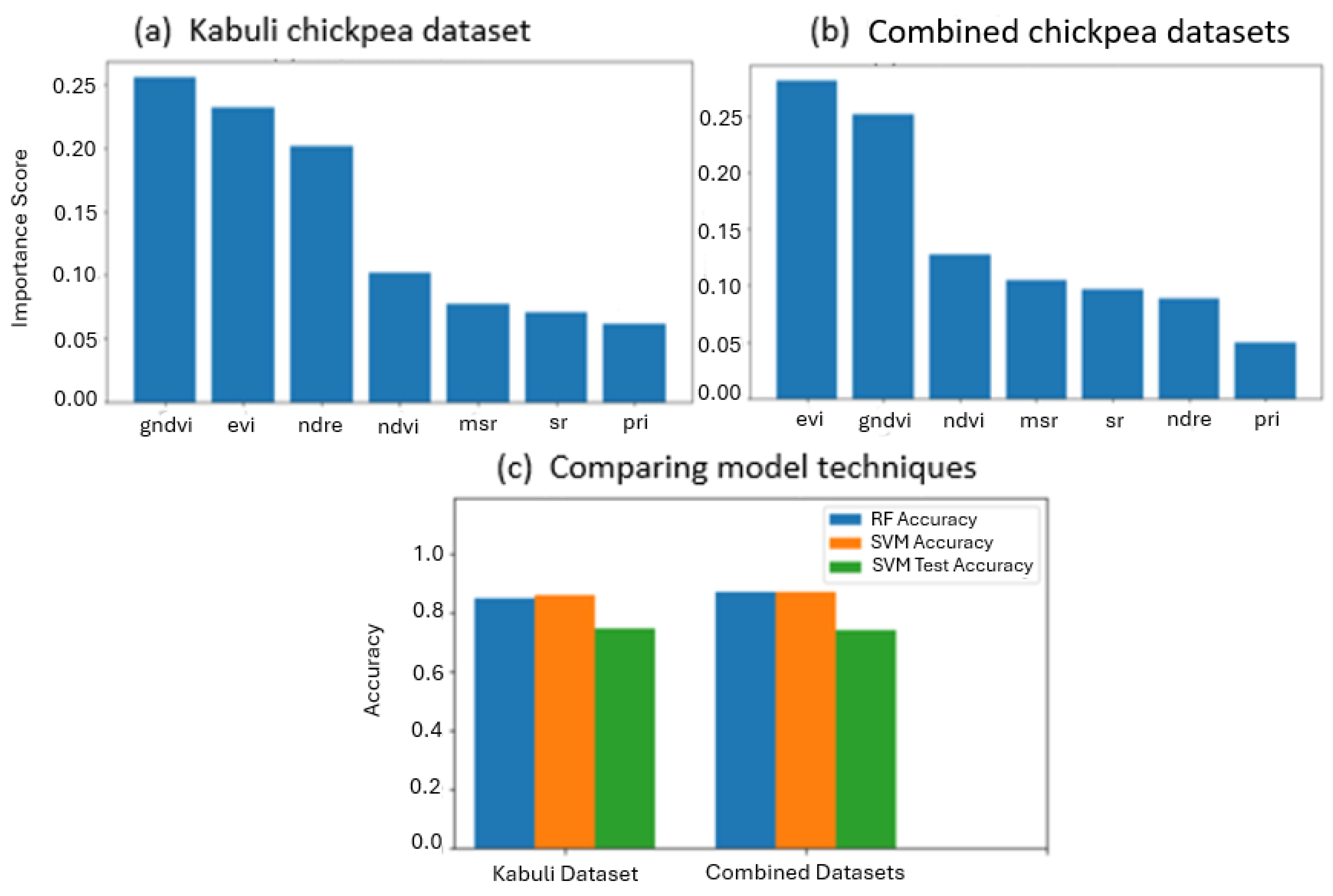
3.1. Spectral Feature-Based Classification
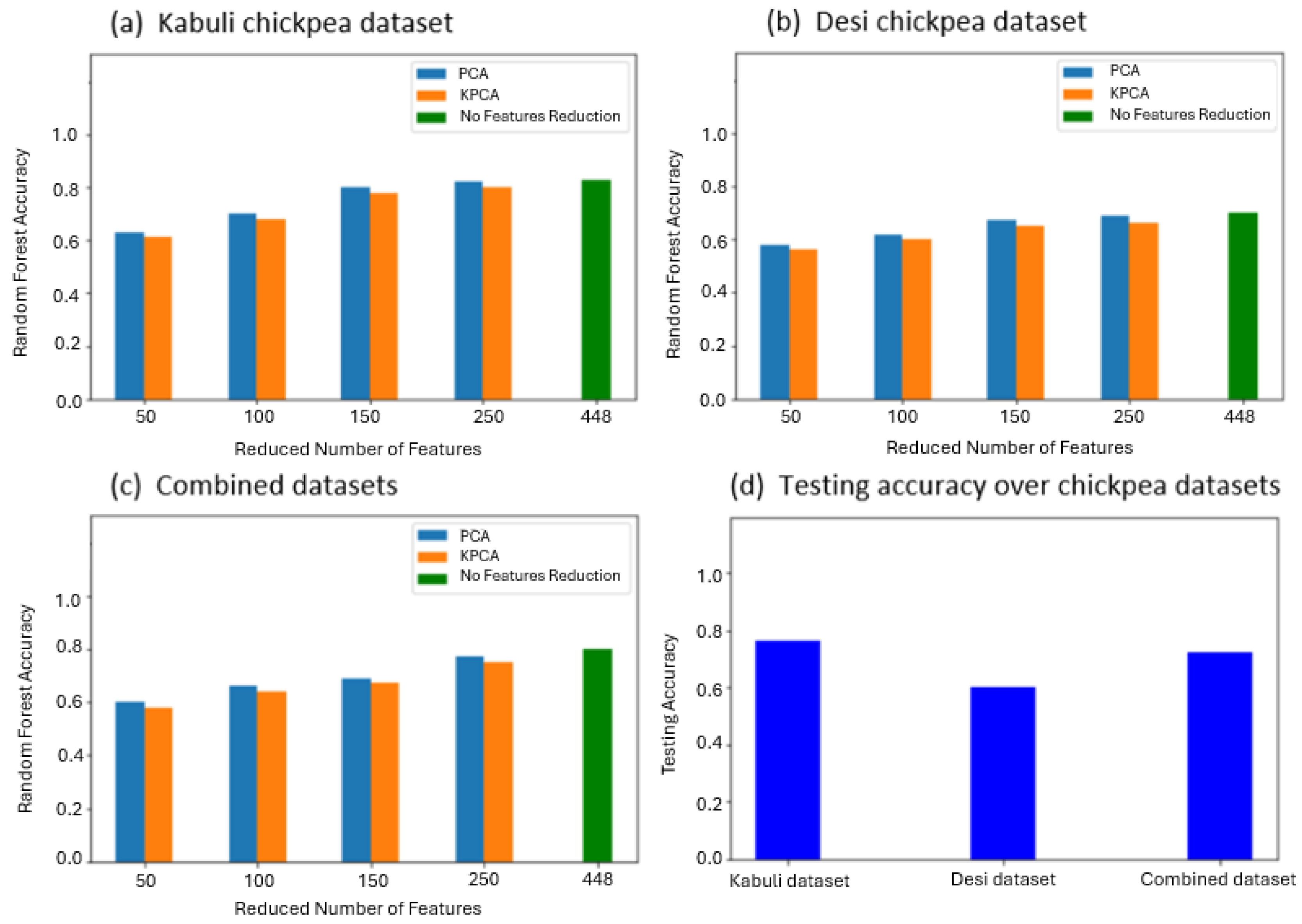
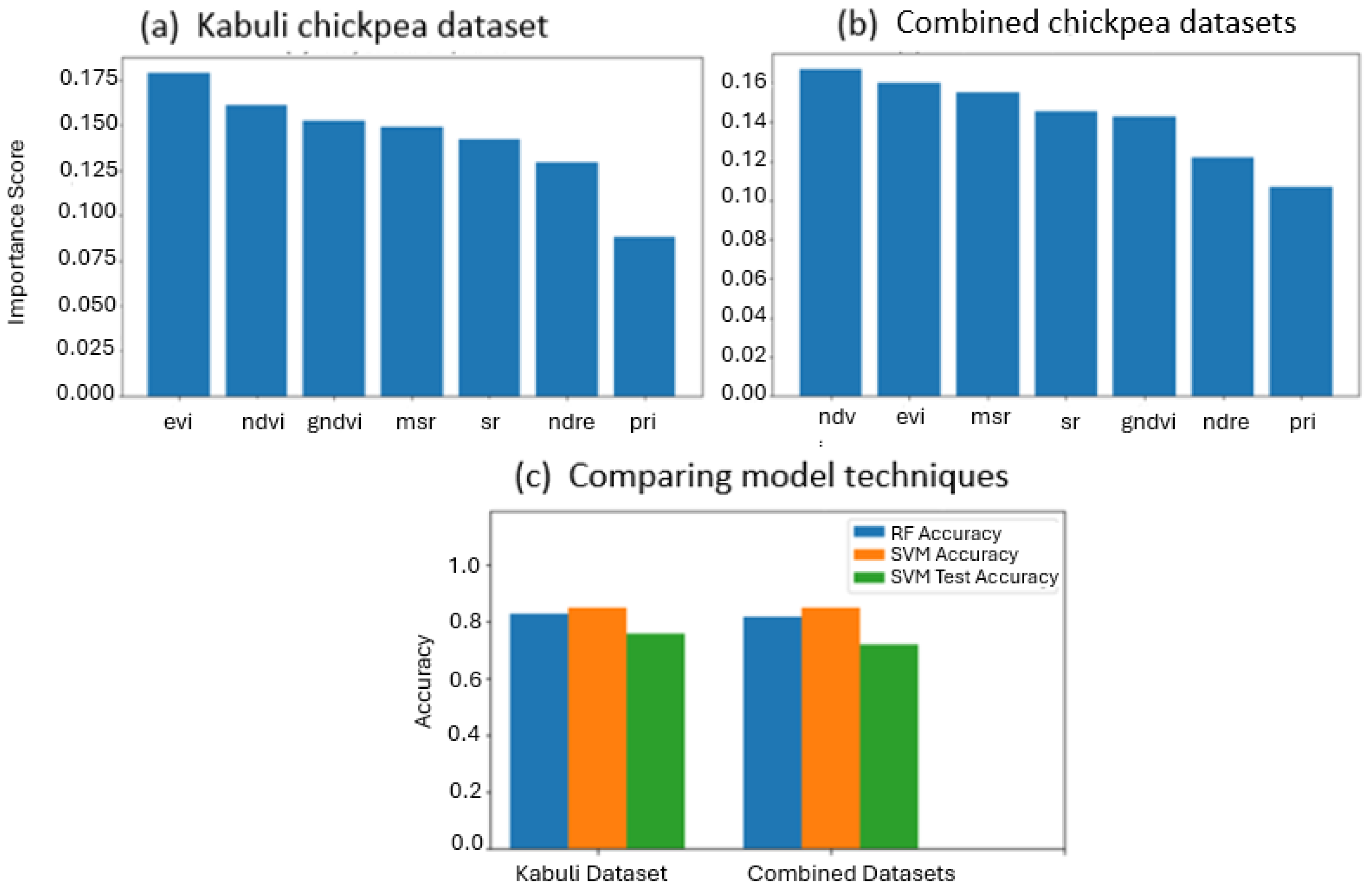
3.2. VI-Based Classification
4. Discussion
5. Conclusions
- High Accuracy in Classification: Machine learning applied to spectral and vegetation index-based features from hyperspectral data enabled the accurate distinction between healthy and infested chickpea plants. Notably, both approaches delivered high classification accuracies, confirming that the spectral information is a strong proxy for plant health and infestation status.
- Integration with Precision: The demonstrated success of both pixel-level spectral features and vegetation indices (e.g., NDVI, EVI, NDRE) in this study supports the integration of HSI with precision agriculture technologies. Such integration allows for non-invasive, real-time monitoring of crop health, reducing dependence on traditional pest control methods and promoting more sustainable practices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koul, B.; Sharma, K.; Sehgal, V.; Yadav, D.; Mishra, M.; Bharadwaj, C. Chickpea (Cicer arietinum L.) biology and biotechnology: From domestication to biofortification and biopharming. Plants 2022, 11, 2926. [Google Scholar] [CrossRef] [PubMed]
- Merga, B.; Haji, J. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Li, A.; Liu, R.H.; Gao, X.; Li, D.; Kou, X.; Xue, Z. Nutritional constituent and health benefits of chickpea (Cicer arietinum L.): A review. Food Res. Int. 2021, 150, 110790. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.E.; Shakappa, D.; Rengel, Z. A review of the nutritional and antinutritional constituents of chickpea (Cicer arietinum) and its health benefits. Crop Pasture Sci. 2022, 73, 401–414. [Google Scholar] [CrossRef]
- Houasli, C.; Idrissi, O.; Nsarellah, N. Chickpea genetic improvement in Morocco: State of the art, progress and prospects. Moroc. J. Agric. Sci. 2020, 1. [Google Scholar]
- Houasli, C.; Sahri, A.; Nsarellah, N.; Idrissi, O. Chickpea (Cicer arietinum L.) breeding in Morocco: Genetic gain and stability of grain yield and seed size under winter planting conditions. Euphytica 2021, 217, 159. [Google Scholar] [CrossRef]
- El Fakhouri, K.; Boulamtat, R.; Sabraoui, A.; El Bouhssini, M. The chickpea Pod borer, Helicoverpa armigera (Hübner): Yield loss estimation and biorational insecticide assessment in Morocco. Agronomy 2022, 12, 3017. [Google Scholar] [CrossRef]
- Manjunatha, L.; Puyam, A.; Prema, G.U.; Sanjay Bandi, M.; Kumar, R.; Keerthi, M.C.; Dixit, G.P.; Kavitha, T.R. Chickpea Biotic Stresses. In Genomic Designing for Biotic Stress Resistant Pulse Crops; Springer International Publishing: Cham, Switzerland, 2022; pp. 117–159. [Google Scholar]
- Tankodara, K.D.; Gohil, G.R.; Khunt, K.R. Problems faced by the chickpea growers in adoption of recommended chickpea production technology. J. Pharmacogn. Phytochem. 2020, 9, 388–389. [Google Scholar]
- Soltani, A.; Ben Abda, M.; Amri, M.; Carapelli, A.; Ben Jemâa, J.M. Seasonal incidence of the leaf miner Liriomyza cicerina Rond (Diptera: Agromyzidae) in chickpea fields and effects of climatic parameters, chickpea variety, and planting date on the leaf miner infestation rate. Euro-Mediterr. J. Environ. Integr. 2020, 5, 58. [Google Scholar] [CrossRef]
- Sabraoui, A.; Lhaloui, S.; Bouchelta, A.; El Fakhouri, K.; El Bouhssini, M. Grain yield losses due to leaf miner (Liriomyza cicerina R.) in winter- and spring-planted chickpea in Morocco. Crop Prot. 2019, 117, 115–120. [Google Scholar] [CrossRef]
- Mahesha, H.S.; Keerthi, M.C.; Shivakumar, K.V.; Bhargavi, H.A.; Saini, R.P.; Manjunatha, L.; Hickok, D.; Blair, M.W. Development of biotic stress resistant cowpea. In Genomic Designing for Biotic Stress Resistant Pulse Crops; Springer: Cham, Switzerland, 2022; pp. 213–251. [Google Scholar]
- Chrigui, N.; Sari, D.; Sari, H.; Eker, T.; Cengiz, M.F.; Ikten, C.; Toker, C. Introgression of resistance to leafminer (Liriomyza cicerina Rondani) from Cicer reticulatum Ladiz. to C. arietinum L. and relationships between potential biochemical selection criteria. Agronomy 2020, 11, 57. [Google Scholar] [CrossRef]
- Saxena, H.; Bandi, S.; Devindrappa, M. Pests of pulses. In Pests and Their Management; Springer: Singapore, 2018; pp. 99–136. [Google Scholar]
- Ngugi, L.C.; Abelwahab, M.; Abo-Zahhad, M. Recent advances in image processing techniques for automated leaf pest and disease recognition—A review. Inf. Process. Agric. 2021, 8, 27–51. [Google Scholar] [CrossRef]
- Thakur, K.; Sharma, A.; Sharma, K. Management of agricultural insect pests with physical control methods. Pharma Innov. J. 2021, 10, 306–314. [Google Scholar]
- Rustia, D.J.A.; Lin, C.E.; Chung, J.-Y.; Zhuang, Y.-J.; Hsu, J.-C.; Lin, T.-T. Application of an image and environmental sensor network for automated greenhouse insect pest monitoring. J. Asia-Pac. Èntomol. 2020, 23, 17–28. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Shariff, A.R.M.; Moslim, R. Monitoring insect pest infestation via different spectroscopic techniques. Appl. Spectrosc. Rev. 2018, 53, 836–853. [Google Scholar] [CrossRef]
- Lima, M.C.F.; de Almeida Leandro, M.E.D.; Valero, C.; Coronel, L.C.P.; Bazzo, C.O.G. Automatic detection and monitoring of insect pests—A review. Agriculture 2020, 10, 161. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, C.; Xiao, D.; Huang, Q. Automatic monitoring of flying vegetable insect pests using an RGB camera and YOLO-SIP detector. Precis. Agric. 2023, 24, 436–457. [Google Scholar] [CrossRef]
- Duarte, A.; Borralho, N.; Cabral, P.; Caetano, M. Recent advances in forest insect pests and diseases monitoring using UAV-based data: A systematic review. Forests 2022, 13, 911. [Google Scholar] [CrossRef]
- Adedeji, A.A.; Ekramirad, N.; Rady, A.; Hamidisepehr, A.; Donohue, K.D.; Villanueva, R.T.; Parrish, C.A.; Li, M. Non-destructive technologies for detecting insect infestation in fruits and vegetables under postharvest conditions: A critical review. Foods 2020, 9, 927. [Google Scholar] [CrossRef]
- Júnior, T.D.C.; Rieder, R.; Di Domênico, J.R.; Lau, D. InsectCV: A system for insect detection in the lab from trap images. Ecol. Inform. 2022, 67, 101516. [Google Scholar] [CrossRef]
- Júnior, T.D.C.; Rieder, R. Automatic identification of insects from digital images: A survey. Comput. Electron. Agric. 2020, 178, 105784. [Google Scholar] [CrossRef]
- Kashyap, B.; Kumar, R. Sensing methodologies in agriculture for monitoring biotic stress in plants due to pathogens and pests. Inventions 2021, 6, 29. [Google Scholar] [CrossRef]
- Simović, I.; Šikoparija, B.; Panić, M.; Radulović, M.; Lugonja, P. Remote sensing of poplar phenophase and leaf miner attack in urban forests. Remote Sens. 2022, 14, 6331. [Google Scholar] [CrossRef]
- Lu, B.; Dao, P.D.; Liu, J.; He, Y.; Shang, J. Recent advances of hyperspectral imaging technology and applications in agriculture. Remote Sens. 2020, 12, 2659. [Google Scholar] [CrossRef]
- Wang, B.; Sun, J.; Xia, L.; Liu, J.; Wang, Z.; Li, P.; Guo, Y.; Sun, X. The applications of hyperspectral imaging technology for agricultural products quality analysis: A review. Food Rev. Int. 2023, 39, 1043–1062. [Google Scholar] [CrossRef]
- Soltani, A.; Zouali, Y.; Haoual-Hamdi, S.; Saadouni, D.; Amri, M.; Carapelli, A.; Ben Jemâa, J.M. Relationship between secondary metabolites and infestations caused by chickpea leafminer Liriomyza cicerina (Diptera:Agromyzidae). Int. J. Trop. Insect Sci. 2020, 41, 251–259. [Google Scholar] [CrossRef]
- Soltani, A.; Beyareslan, A.; Gençer, L.; Hamdi, S.H.; Bousselmi, A.; Amri, M.; Ben Jemâa, J.M. Parasitoids of chickpea leafminer Liriomyza cicerina (Diptera: Agromyzidae) and their parasitism rate on chickpea fields in North Tunisia. J. Asia-Pac. Èntomol. 2018, 21, 1215–1221. [Google Scholar] [CrossRef]
- Nawaz, R.; Abbasi, N.A.; Hafiz, I.A.; Khan, M.F.; Khalid, A. Environmental variables influence the developmental stages of the citrus leafminer, infestation level and mined leaves physiological response of Kinnow mandarin. Sci. Rep. 2021, 11, 7720. [Google Scholar] [CrossRef]
- Li, Y.-H.; Tan, X.; Zhang, W.; Jiao, Q.-B.; Xu, Y.-X.; Li, H.; Zou, Y.-B.; Yang, L.; Fang, Y.-P. Research and application of several key techniques in hyperspectral image preprocessing. Front. Plant Sci. 2021, 12, 627865. [Google Scholar] [CrossRef]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral imaging: A review on UAV-based sensors, data processing and applications for agriculture and forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Kordestani, H.; Zhang, C. Direct use of the savitzky–golay filter to develop an output-only trend line-based damage detection method. Sensors 2020, 20, 1983. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, L.W.; Meyer, H.; Obermeier, W.A.; Silva, B.; Regeling, B.; Bendix, J. Hyperspectral data analysis in R: The hsdar package. arXiv 2018, arXiv:1805.05090. [Google Scholar] [CrossRef]
- Ahmad, N.; Iqbal, J.; Shaheen, A.; Ghfar, A.; Al-Anazy, M.M.; Ouladsmane, M. Spatio-temporal analysis of chickpea crop in arid environment by comparing high-resolution UAV image and LANDSAT imagery. Int. J. Environ. Sci. Technol. 2022, 19, 6595–6610. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, H. Plant disease detection based on region-based segmentation and KNN classifier. In Proceedings of the International Conference on ISMAC in Computational Vision and Bio-Engineering 2018 (ISMAC-CVB), Palladam, India, 16–17 May 2018; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Tamilselvi, P.; Kumar, K.A. Unsupervised machine learning for clustering the infected leaves based on the leaf-colours. In Proceedings of the 2017 Third International Conference on Science Technology Engineering & Management (ICONSTEM), Chennai, India, 23–24 March 2017; IEEE: Piscataway, NJ, USA, 2017. [Google Scholar]
- Cheng, Z.; Zou, C.; Dong, J. Outlier detection using isolation forest and local outlier factor. In Proceedings of the Conference on Research in Adaptive and Convergent Systems, Chongqing, China, 24–27 September 2019. [Google Scholar]
- Forkman, J.; Josse, J.; Piepho, H.-P. Hypothesis tests for principal component analysis when variables are standardized. J. Agric. Biol. Environ. Stat. 2019, 24, 289–308. [Google Scholar] [CrossRef]
- Reddy, G.T.; Reddy, M.P.K.; Lakshmanna, K.; Kaluri, R.; Rajput, D.S.; Srivastava, G.; Baker, T. Analysis of dimensionality reduction techniques on big data. IEEE Access 2020, 8, 54776–54788. [Google Scholar] [CrossRef]
- Binol, H. Ensemble learning based multiple kernel principal component analysis for dimensionality reduction and classification of hyperspectral imagery. Math. Probl. Eng. 2018, 2018, 9632569. [Google Scholar] [CrossRef]
- Giovos, R.; Tassopoulos, D.; Kalivas, D.; Lougkos, N.; Priovolou, A. Remote sensing vegetation indices in viticulture: A critical review. Agriculture 2021, 11, 457. [Google Scholar] [CrossRef]
- Akkara, M.S.; Pimpale, A.R.; Wadatkar, S.B.; Rajankar, P.B.; Ramteke, I.K. Multispectral vegetation indices profiles of chickpea. Pharma Innov. J. 2023, 12, 729–734. [Google Scholar]
- Polivova, M.; Brook, A. Detailed investigation of spectral vegetation indices for fine field-scale phenotyping. In Vegetation Index and Dynamics; IntechOpen: London, UK, 2021; p. 103. [Google Scholar]
- De Santis, M.A.; Satriani, A.; De Santis, F.; Flagella, Z. Water use efficiency, spectral phenotyping and protein composition of two chickpea genotypes grown in Mediterranean environments under different water and nitrogen supply. Agriculture 2022, 12, 2026. [Google Scholar] [CrossRef]
- Indore, N.S.; Chaudhry, M.; Jayas, D.S.; Paliwal, J.; Karunakaran, C. Non-destructive assessment of microstructural changes in Kabuli chickpeas during storage. Foods 2024, 13, 433. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Sankaran, S. High-throughput field phenotyping of Ascochyta blight disease severity in chickpea. Crop Prot. 2019, 125, 104885. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Q.; Yin, X.; Wang, Y.; Tian, W. Multi-parameter health assessment of jujube trees based on unmanned aerial vehicle hyperspectral remote sensing. Agriculture 2023, 13, 1679. [Google Scholar] [CrossRef]
- Gujarkar, V.A.; Kulkarni, P.D.; Pal, A.; Chaudhari, K.; Mohite, V.A. Remote Sensing Based Crop Monitoring System. In Proceedings of the 2022 IEEE Region 10 Symposium (TENSYMP), Mumbai, India, 1–3 July 2022; IEEE: Piscataway, NJ, USA, 2022. [Google Scholar]
- Raczko, E.; Zagajewski, B. Comparison of support vector machine, random forest and neural network classifiers for tree species classification on airborne hyperspectral APEX images. Eur. J. Remote Sens. 2017, 50, 144–154. [Google Scholar] [CrossRef]
- Nguyen, C.; Sagan, V.; Maimaitiyiming, M.; Maimaitijiang, M.; Bhadra, S.; Kwasniewski, M.T. Early detection of plant viral disease using hyperspectral imaging and deep learning. Sensors 2021, 21, 742. [Google Scholar] [CrossRef]
- Sheykhmousa, M.; Mahdianpari, M.; Ghanbari, H.; Mohammadimanesh, F.; Ghamisi, P.; Homayouni, S. Support vector machine versus random forest for remote sensing image classification: A meta-analysis and systematic review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 6308–6325. [Google Scholar] [CrossRef]
- Adnan, M.; Alarood, A.A.S.; Uddin, M.I.; Rehman, I.U. Utilizing grid search cross-validation with adaptive boosting for augmenting performance of machine learning models. PeerJ Comput. Sci. 2022, 8, e803. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M.; Abd El-Aziz, S.E.; Marei, S.S. A review: Application of remote sensing as a promising strategy for insect pests and diseases management. Environ. Sci. Pollut. Res. 2020, 27, 33503–33515. [Google Scholar] [CrossRef]
- Xiao, Z.; Yin, K.; Geng, L.; Wu, J.; Zhang, F.; Liu, Y. Pest identification via hyperspectral image and deep learning. Signal Image Video Process. 2022, 16, 873–880. [Google Scholar] [CrossRef]
- Pham, H.T.; Awange, J.; Kuhn, M.; Van Nguyen, B.; Bui, L.K. Enhancing crop yield prediction utilizing machine learning on satellite-based vegetation health indices. Sensors 2022, 22, 719. [Google Scholar] [CrossRef]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and future perspectives of multi-/Hyperspectral thermal infrared remote sensing for crop water-stress detection: A review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Rumpf, T.; Welke, P.; Dehne, H.-W.; Plümer, L.; Steiner, U.; Oerke, E.-C. Development of spectral indices for detecting and identifying plant diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Steiner, U.; Hillnhütter, C.; Dehne, H.-W.; Oerke, E.-C. Hyperspectral imaging for small-scale analysis of symptoms caused by different sugar beet diseases. Plant Methods 2012, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Qin, J.; Burks, T.F.; Ritenour, M.A.; Bonn, W.G. Detection of citrus canker using hyperspectral reflectance imaging with spectral information divergence. J. Food Eng. 2009, 93, 183–191. [Google Scholar] [CrossRef]
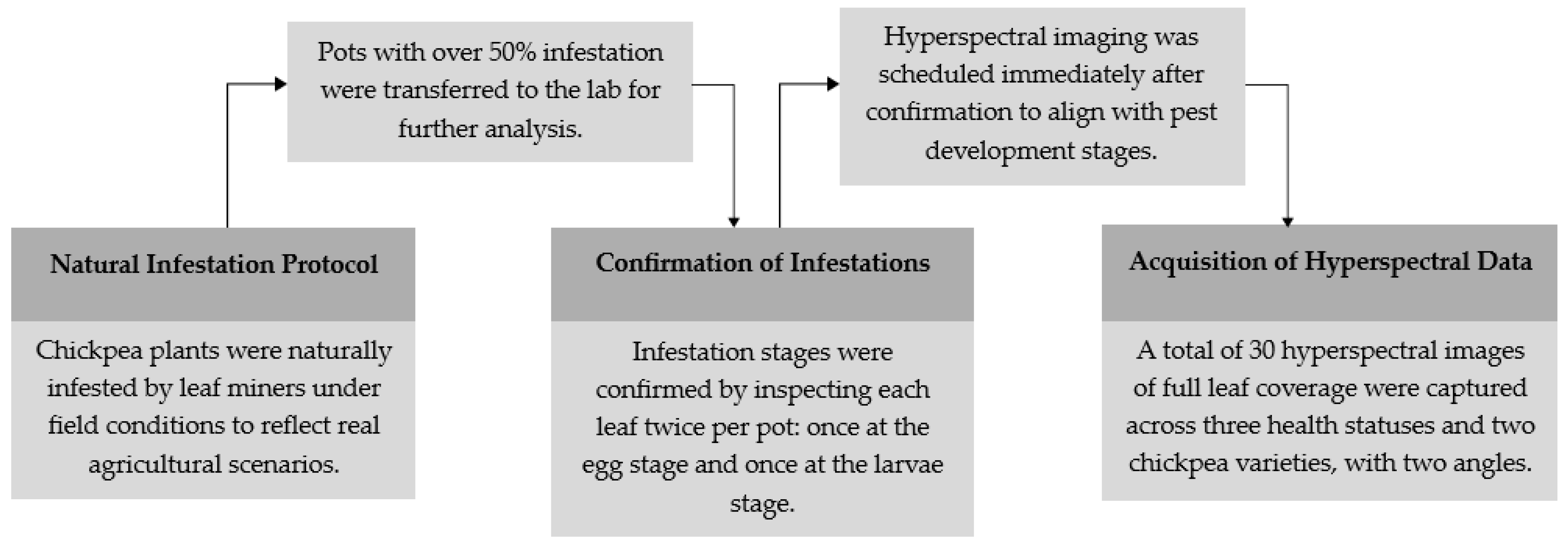

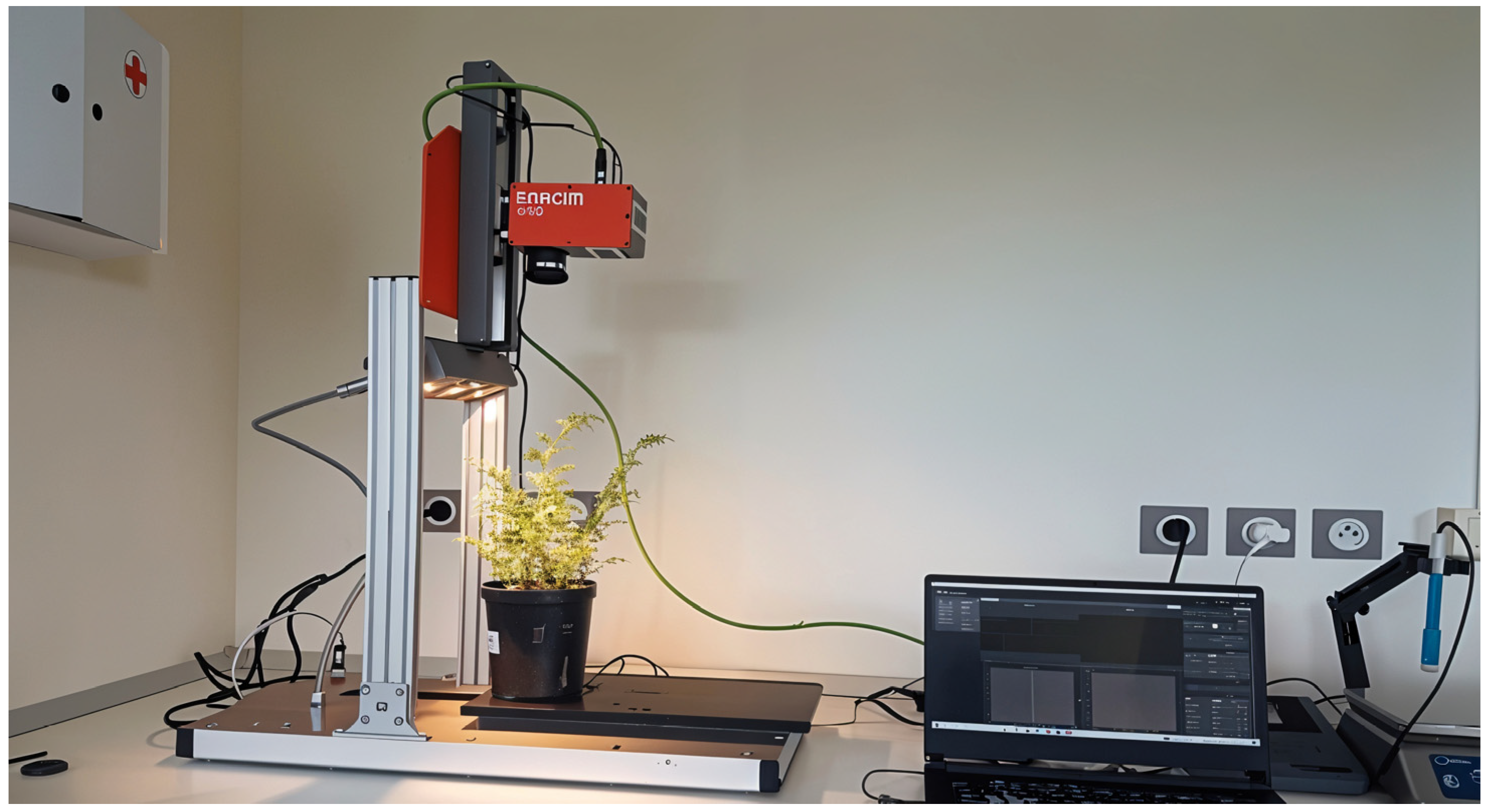



| Status/Period | Kabuli Variety Pots | Desi Variety Pots | Total |
|---|---|---|---|
| Healthy | 3 | 3 | 6 |
| Egg Period | 3 | 3 | 6 |
| Larvae Period | 3 | 3 | 6 |
| Total | 9 | 9 | 18 |
| Status/Period | Data Measurement Time (GMT Central Time) | Kabuli Variety Pots | Desi Variety Pots | Total |
|---|---|---|---|---|
| Egg Period | 6 May 2024 8:30–12:00 | 6 | 6 | 12 |
| Healthy | 6 May 2024 8:30–12:00 | 3 | 3 | 12 |
| 20 May 2024 8:30–12:00 | 3 | 3 | ||
| Larvae Period | 20 May 2024 8:30–12:00 | 6 | 0 | 6 |
| Total | 18 | 12 | 30 |
| Status/Period | Kabuli Variety | Desi Variety | Total | ||
|---|---|---|---|---|---|
| Train Set | Test Set | Train Set | Test Set | ||
| Healthy | 4 | 2 | 4 | 2 | 12 |
| Eggs Period | 4 | 2 | 4 | 2 | 12 |
| Larvae Period | 4 | 2 | 0 | 0 | 6 |
| Total | 12 | 6 | 8 | 4 | 30 |
| Vegetation Index (VI) | Acronym | Equation | References |
|---|---|---|---|
| Normalized Difference Vegetation Index | NDVI | NDVI = (NIR − Red)/(NIR + Red) | [36,44] |
| Enhanced Vegetation Index | EVI | EVI = G × (NIR − Red)/(NIR + C1 × Red − C2 × Blue + L) | [45,46] |
| Simple Ratio | SR | SR = NIR/Red | [47] |
| Green Normalized Difference Vegetation Index | GNDVI | GNDVI = (NIR − Green)/(NIR + Green) | [45,48] |
| Modified Simple Ratio | MSR | MSR = [(NIR/Red) − 1]/√[(NIR/Red) + 1] | [49] |
| Photochemical Reflectance Index | PRI | PRI = (Red531 − Red570)/(Red531 + Red570) | [46] |
| Normalized Difference Red Edge | NDRE | NDRE = (NIR − RedEdge)/(NIR + RedEdge) | [48,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arame, M.; Kadmiri, I.M.; Bourzeix, F.; Zennayi, Y.; Boulamtat, R.; Chehbouni, A. Detection of Leaf Miner Infestation in Chickpea Plants Using Hyperspectral Imaging in Morocco. Agronomy 2025, 15, 1106. https://doi.org/10.3390/agronomy15051106
Arame M, Kadmiri IM, Bourzeix F, Zennayi Y, Boulamtat R, Chehbouni A. Detection of Leaf Miner Infestation in Chickpea Plants Using Hyperspectral Imaging in Morocco. Agronomy. 2025; 15(5):1106. https://doi.org/10.3390/agronomy15051106
Chicago/Turabian StyleArame, Mohamed, Issam Meftah Kadmiri, Francois Bourzeix, Yahya Zennayi, Rachid Boulamtat, and Abdelghani Chehbouni. 2025. "Detection of Leaf Miner Infestation in Chickpea Plants Using Hyperspectral Imaging in Morocco" Agronomy 15, no. 5: 1106. https://doi.org/10.3390/agronomy15051106
APA StyleArame, M., Kadmiri, I. M., Bourzeix, F., Zennayi, Y., Boulamtat, R., & Chehbouni, A. (2025). Detection of Leaf Miner Infestation in Chickpea Plants Using Hyperspectral Imaging in Morocco. Agronomy, 15(5), 1106. https://doi.org/10.3390/agronomy15051106







