Abstract
Despite substantial progress in elucidating the stress-responsive mechanisms of 4-coumarate-CoA ligases (4CL) in various plant species, the maize (Zea mays L.) 4CL gene family remains underexplored, leaving a significant gap in our comprehension of its potential roles in abiotic stress tolerance and adaptive strategies. Through comprehensive genome-wide analysis, we identified and characterized 32 putative 4CL genes in maize, which were phylogenetically classified into seven distinct clades. Members within the same clade exhibited conserved gene structures and motif compositions. Expression profiling across various maize tissues and under multiple abiotic stress conditions revealed specific 4CL genes associated with stress tolerance. Notably, promoter analysis identified numerous stress-responsive cis-regulatory elements in Zm4CL genes. Among the identified genes, six exhibited significant induction under salt stress, while five showed upregulation during drought conditions. Particularly, Zm4CL8, a member of the 4CL clade, demonstrated dual responsiveness to both drought and salt stresses. Functional characterization through virus-induced gene silencing (VIGS) revealed that Zm4CL8-silenced plants displayed enhanced sensitivity to both drought and salt stresses, as evidenced by significantly reduced chlorophyll content and survival rate, which collectively suggests its positive regulatory role in stress adaptation mechanisms. These findings establish Zm4CL8 as a promising molecular target for enhancing drought and salt tolerance in maize, while significantly advancing our understanding of the functional characterization of 4CL genes in this crucial crop species.
1. Introduction
The phenylpropanoids are biosynthesized from aromatic amino acids, such as phenylalanine (Phe) and tyrosine (Tyr), through the phenylpropanoid pathway [1,2]. This pathway is crucial for the survival of plants and provides them with a plethora of precursors for secondary metabolites, thereby contributing to their growth, development, and resistance to environmental stress [3]. The enzyme 4-coumarate:CoA ligase (4CL; EC 6.2.1.12) plays a crucial role in phenylpropanoid metabolism by producing CoA esters of hydroxycinnamic acids [4]. Existing research indicates that 4CL exhibits specificity towards 4-coumaric acid, cinnamic acids, and other derivatives of cinnamic acid [5]. These cinnamyl CoA esters serve as crucial intermediates in the biosynthesis of a wide range of phenolic secondary natural products, including monolignols and flavonoids [6].
Previous research has indicated the presence of several conserved polypeptide sequences within 4CL protein sequences, notably including BOX I (SSGTTGLPKGV) and BOX II (GEICIRG) [7]. These findings underscore the remarkable conservation of specific amino acid motifs in this protein family, shedding light on their functional significance and evolutionary history. BOX I represents the adenosine monophosphate (AMP) binding domain, which is highly conserved across all members of the adenylate-forming enzyme family [8]. BOX II does not directly participate in catalytic reactions; however, the insufficiency of this conserved sequence leads to a near-complete depletion of 4CL enzyme activity [9,10]. The 4CL gene family found in various plant species encompasses both the AMP-binding enzyme domain (AMP-binding; PF00501) and the AMP-binding enzyme C-terminal domain (AMP-binding_C; PF13193) [11].
Subsequent to the generation of genomic sequence data from Arabidopsis, a multitude of genes encoding adenylate-forming enzymes were identified as being closely associated with authentic 4CLs, yet the majority of their biochemical functions still remain unclear [12]. A total of nine Arabidopsis genes encoding enzymes, which bear the closest resemblance to genes encoding 4CL, have been identified and classified as either 4CL or 4CL-like by various research groups [13,14,15]. Genes similar to 4CL have also been detected within the genomes of Poplar, rice, and Physcomitrella species [12]. It is plausible that these genes may participate in various pathways within plant metabolism and the biosynthesis of natural products.
Several reports indicate that the expression of 4CL is altered in response to both biotic and abiotic stresses [16,17,18]. The mitigation of stress was primarily accomplished through the regulation of lignin, flavonoids, and other secondary metabolites [19,20,21]. This intricate process effectively safeguarded against the detrimental effects of stress. Functional characterization demonstrates that Gh4CL7 acts as a positive regulator of drought tolerance, with Gh4CL7-silenced cotton plants showing increased drought sensitivity and Gh4CL7-overexpressing Arabidopsis lines exhibiting enhanced drought resistance [22]. Similarly, salt stress induces substantial upregulation of multiple Euc4CLs in Eucommia ulmoides, particularly Euc4CL9, Euc4CL17, and Euc4CL27, suggesting their involvement in salinity adaptation [23]. In potatoes, distinct 4CL members exhibit differential regulation under various stresses: St4CL6 and St4CL8 are upregulated while St4CL5 is downregulated in response to white light, UV irradiation, and ABA and PEG treatments. This coordinated expression pattern may promote lignin and flavonoid biosynthesis, thereby enhancing reactive oxygen species (ROS) scavenging capacity and ultimately strengthening abiotic stress resistance [24]. The conserved stress-protective role of 4CLs is further exemplified by Zm4CL-like9 in maize, which confers drought tolerance through ROS mitigation [25], highlighting the family’s universal importance in plant stress adaptation. Although the stress-responsive roles of 4CLs have been well characterized in many plants, the functions of the 4CL gene family in maize remain poorly understood, limiting insights into their contribution to abiotic stress adaptation.
In this study, we classified both 4CL and 4CL-like genes into the 4CL gene family and designated them collectively under the 4CL nomenclature. Through genome-wide analysis, we identified 32 Zm4CL genes, among which Zm4CL8 showed significant transcriptional upregulation under salt and drought stress. Functional characterization using virus-induced gene silencing (VIGS) revealed that Zm4CL8-knockdown plants exhibited reduced stress tolerance, demonstrating its positive regulatory role in drought and salt stress responses. These findings establish Zm4CL8 as a promising candidate for the development of stress-resistant maize varieties and provide fundamental insights into the functional characterization of 4CL genes in maize.
2. Materials and Methods
2.1. Genome-Wide Identification of 4CL Genes in Maize
Two hidden Markov models (HMMs) representing the AMP-binding (PF00501) and AMP-binding_C (PF13193) domains were acquired from the Pfam database (http://pfam-legacy.xfam.org/, accessed on 18 April 2024) [26]. These domain-specific HMM profiles were subsequently utilized as search queries to comprehensively screen and identify potential Zm4CLs proteins within the target proteome. In order to ascertain the involvement of candidate members in the 4CL gene family, their protein domains were examined using the NCBI CDD and Pfam databases (accessed on 18 April 2024). Those candidate members containing both the 4CL domain and AFD_class_I superfamily domain in the CDD database, as well as the AMP-binding domain and AMP-binding_C domain in the Pfam database, were ultimately identified as belonging to the 4CL gene family.
2.2. Phylogenetic Analysis and Prediction of the Physicochemical Properties of Zm4CL Proteins
MEGA7 (v7.0.26) (https://www.megasoftware.net/, accessed on 6 June 2024) was utilized for conducting multiple sequence alignment [27] and phylogenetic tree construction of the identified 32 protein sequences with Arabidopsis’s 4CL proteins. The phylogenetic tree was constructed using the Neighbor-Joining (NJ) method, with a bootstrap value set to 1000. Furthermore, itol (https://itol.embl.de/, accessed on 6 June 2024) online tool was utilized to enhance the esthetic appeal of the phylogenetic tree. The prediction of physicochemical properties of Zm4CL protein sequences was conducted using the online tool ProtParam on ExPASy (https://web.expasy.org/protparam/, accessed on 12 June 2024) [28].
2.3. Chromosomal Localization and Collinearity Analysis
The chromosomal location information of the maize 4CL gene family is based on gene annotation information (Zm-B73-REFERENCE-NAM-5.0), and the physical distribution of candidate genes on maize chromosomes was annotated using TBtools (v2.210) (https://github.com/CJ-Chen/TBtools-II/releases, accessed on 30 April 2024) [29]. To facilitate further studies, the 4CL genes were renamed based on their chromosomal position order. Utilizing the genomic and structural annotation datasets of maize, Arabidopsis, and rice, collinearity maps of maize 4CL genes were constructed within and across species. These intricate maps were then visually represented using TBtools (v2.210) (accessed on 9 July 2024).
2.4. Analysis of the Gene Structure and Protein Domain, and Motif Detection of the Zm4CL Gene Family
The analysis of the gene structure is facilitated by the utilization of maize genome annotation information. In order to validate the existence of conserved domains, the batch CD-search tool [30] provided by NCBI (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi, accessed on 6 June 2024) was employed. Subsequently, the resulting hitdata.txt file was acquired for further in-depth analysis. The conserved motifs of the candidate proteins were meticulously analyzed utilizing the advanced MEME suite version 5.5.5 (https://meme-suite.org/meme/tools/meme, accessed on 6 June 2024) [31]. The analysis was conducted with a set of specific parameters, including a maximum allowance of 10 motifs, a minimum motif width of 6, and a maximum motif width of 50. The resultant meme.xml file was acquired for further in-depth analysis. The TBtools (v2.210) software was utilized to visualize the aforementioned findings (accessed on 6 June 2024).
2.5. Analysis of the cis-Acting Elements in the Promoters of the Zm4CL Gene Family
In order to explore the cis-acting elements present in the promoters of the Zm4CL genes, we retrieved the 2000 bp upstream promoter regions adjacent to the start codon of the coding sequence (CDS) for each Zm4CL gene from maize genomic data (https://maizegdb.org, accessed on 20 May 2024). The promoter sequences of Zm4CL genes were analyzed using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 21 May 2024) [32]. The positions of anticipated cis-elements were visualized using TBtools (v2.210) (accessed on 21 May 2024) and subsequently refined through Adobe Illustrator (v24.2.1) to prepare the final figure.
2.6. Expression Analysis of Zm4CL Genes in Different Tissues
The expression data for the Zm4CL genes were acquired from a diverse array of tissues, encompassing roots, leaves, seeds, shoot tip, internodes, tassel, cob, coleoptile, pericarp, silks, endosperm, embryo, and anthers. These valuable data were procured from the qTeller (https://qteller.maizegdb.org/, accessed on 3 June 2024). The TPM (Transcripts Per Million) values of the Zm4CL genes were utilized to generate a heat map using TBtools (v2.210) (accessed on 5 June 2024). Then, the correlation coefficients of gene expression among Zm4CL genes were computed utilizing the expression profiles obtained from qTeller (https://qteller.maizegdb.org/, accessed on 3 June 2024).
2.7. Prediction of the Upstream Transcription Factors for Zm4CL Genes in Clade 4CL
The PlantTFdb (v5.0) (https://planttfdb.gao-lab.org/, accessed on 30 May 2024) [33] was utilized to retrieve the upstream regulators of Zm4CL genes within clade 4CL. Subsequently, all predicted upstream transcription factors were visually represented using Cytoscape software (version: 3.10.2) (accessed on 31 May 2024) [34]. Following this, the expression correlation coefficients between potential upstream transcription factors and Zm4CL genes were computed utilizing the expression profiles obtained from qTeller (accessed on 4 June 2024).
2.8. Analysis of Zm4CL Genes Expression in Maize Under Abiotic Stresses
An investigation into the expression levels of Zm4CL genes in response to drought, cold, heat, salt, and UV stress was performed using RNA-seq data acquired from qTeller (https://qteller.maizegdb.org/) on MaizeGDB (accessed on 5 June 2024). The expression levels of Zm4CL genes, represented as TPM values, were used to create a heatmap through TBtools (v2.210) software (accessed on 5 June 2024).
2.9. Stress Response Analysis of Selected Zm4CL Genes
The B73 inbred line was obtained from the State Key Laboratory of Plant Environmental Resilience, College of Biological Sciences, China Agricultural University. Prior to stress treatment, plants were cultivated for two weeks in a growth chamber (16/8 h light/dark photoperiod, 25 °C) using a 1:1 (v/v) mixture of nutrient soil and vermiculite as growth substrate. For stress treatment, maize seedlings were carefully removed from their growth medium, and the roots were thoroughly washed with distilled water. The plants were then transferred to a hydroponic system containing Hoagland solution, where they were subjected to combined stress using 5% (w/v) polyethylene glycol 6000 (PEG-6000) and 200 mM NaCl. Leaf tissues were harvested at 0, 1, 3, 6, and 12 h post-treatment and promptly frozen in liquid nitrogen for subsequent analysis.
2.10. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR Analysis
Total RNA was extracted using the RNAiso Easy Kit (TaKaRa, Kyoto, Japan), followed by the synthesis of the first strand of cDNA using the PrimeScript™ FAST RT Reagent Kit with gDNA Eraser (TaKaRa, Kyoto, Japan). Subsequently, RT-qPCR was performed using 2× SYBR Green qPCR Master Mix (Selleck, Houston, TX, USA) on the LineGene9600 fluorescence quantitative PCR instrument (Bioer Technology, Hangzhou, China). The relative transcript levels of the target genes were calculated using the 2−ΔΔCt method [35]. The maize Actin7 gene served as the internal reference, and the specific primers employed for RT-qPCR are detailed in Supplementary Table S3.
2.11. Construction of VIGS Vectors, Vacuum-Assisted Agroinfiltration, and Co-Cultivation Procedures
The VIGS assay was performed using Tobacco rattle virus (TRV)-mediated gene silencing. The pTRV1 and pTRV2 VIGS vectors were purchased from Fenghui Biotechnology Company (Changsha, China). The prediction of VIGS gene fragments was conducted using the pssRNAit online platform (https://www.zhaolab.org/pssRNAit/, accessed on 20 September 2024), a web-based tool specifically designed for plant small RNA analysis and VIGS fragment prediction. A 186 bp fragment derived from the coding sequence of Zm4CL8 and a 196 bp fragment from the coding sequence of ZmPDS were individually cloned into the pTRV2 vector. This was achieved through double digestion using EcoRI and BamHI restriction enzymes, resulting in the construction of the recombinant vectors pTRV2-Zm4CL8 and pTRV2-ZmPDS, respectively.
Seeds of maize cultivar B73 were subjected to surface sterilization through sequential treatments: initially immersed in 75% (v/v) ethanol for 1 min, followed by exposure to a 2.5% sodium hypochlorite solution supplemented with 0.1% Tween 20 for 6 min. Subsequently, the seeds were thoroughly washed with sterile deionized water through five consecutive rinses to ensure the complete removal of sterilizing agents.
The Agrobacterium-mediated transformation was performed using A. tumefaciens strain GV3101 harboring pTRV1 and GV3101 strains carrying different pTRV2-derived vectors (pTRV2, pTRV2-Zm4CL8, pTRV2-ZmPDS). The bacterial cultures were grown in a Luria–Bertani (LB) medium supplemented with 100 mg·L−1 rifampicin and 50 mg·L−1 kanamycin until reaching an OD600 of 0.3. For agroinfiltration, equal volumes (1:1 ratio) of pTRV1-containing Agrobacterium and pTRV2-derived vector-containing Agrobacterium were mixed in an infiltration solution containing 19.62 mg·L−1 acetosyringone (AS), 400 mg·L−1 cysteine (Cys), and 5 mL·L−1 Tween 20. Subsequently, 5 mL of the bacterial mixture was transferred into 10 mL medical glass bottles equipped with rubber plugs. Surface-sterilized maize seeds with the pericarp above the embryo gently scarified were placed in the bottles and subjected to vacuum infiltration for 60 s using a 20 mL syringe. Following vacuum treatment, the seeds were co-cultivated with the Agrobacterium suspension in a shaking incubator at 28 °C and 180 rpm for 15 h. After co-cultivation, the agroinfiltrated seeds were thoroughly washed with sterilized water to remove surface-adhered Agrobacteria and subsequently transferred to soil for growth. The detailed infection procedure was performed according to previously established protocols [36].
2.12. Drought and Salt Stress Tolerance Assays
For the natural drought treatment, two-week-old CK and VIGS maize seedlings were subjected to a 12-day water deprivation period. During this period, phenotypic observations and photographic documentation were conducted. Following the drought stress, the plants were rewatered, and subsequent assessments were performed, including survival rate determination and chlorophyll content measurement. In the salt stress treatment, two-week-old CK and VIGS maize seedlings were irrigated with a 200 mM NaCl solution. Phenotypic observations and photographic documentation were carried out at 12 and 14 days post-treatment. Additionally, survival rates and chlorophyll content were measured to evaluate the plants’ response to salt stress.
2.13. Determination of Relative Water Content
To determine the relative water content (RWC), leaf samples were collected from plants and their fresh weight (FW) was immediately measured. The leaves were then immersed in distilled water and maintained at 25 °C in complete darkness for 8 h to achieve full turgidity, after which the turgid weight (TW) was recorded. Subsequently, the samples were oven-dried at 65 °C until a constant weight was obtained to determine the dry weight (DW). The RWC was calculated using the following formula: RWC (%) = [(FW − DW)/(TW − DW)] × 100 [37].
3. Results
3.1. Identification and Phylogenetic Relationship of 4CL Genes in Maize
Employing the genome-wide data of maize inbred line B73 from Ensemblplants, we utilized two hidden Markov models corresponding to the AMP-binding (PF00501) and AMP-binding_C (PF13193) domains as queries for screening protein sequences containing these domains. Through analyzing the initially screened protein sequences with NCBI CDD and Pfam databases, a total of 32 Zm4CL genes were ultimately identified (Table 1, Figure 1). In order to facilitate subsequent studies, we systematically renamed all the genes based on their chromosomal position. Table 1 presents a comprehensive overview of all the members within the 4CL gene family, providing essential baseline information for further research and analysis. The physicochemical properties of these proteins were assessed utilizing the ExPASy-ProtParam tool. The Zm4CLs encode a variety of proteins, ranging in length from 118 to 1168 amino acids. These proteins exhibit molecular weights spanning from 13.00 to 128.45 kDa, and theoretical isoelectric points ranging from 4.58 to 9.07. Notably, among these proteins, the pI values of 21 were found to be less than 7, indicating their predominantly acidic nature (pI < 7). The aliphatic index varies between 75.65 and 103.60, reflecting the varying degrees of thermal stability exhibited by different Zm4CL proteins. Meanwhile, the instability index ranges from 23.87 to 56.41, with 22 proteins having values below 40 and 10 proteins having values above 40, indicating a lower level of stability in the latter group of proteins. The grand average hydropathy (GRAVY) index encompasses a spectrum ranging from −0.247 to 0.257, denoting the level of hydrophobicity or hydrophilicity. This signifies the molecule’s propensity for interaction with water molecules and reflects the Zm4CL protein’s inclination towards either repulsion by or attraction to water.

Table 1.
Identification and physicochemical property analysis of 4CL gene family members in maize.
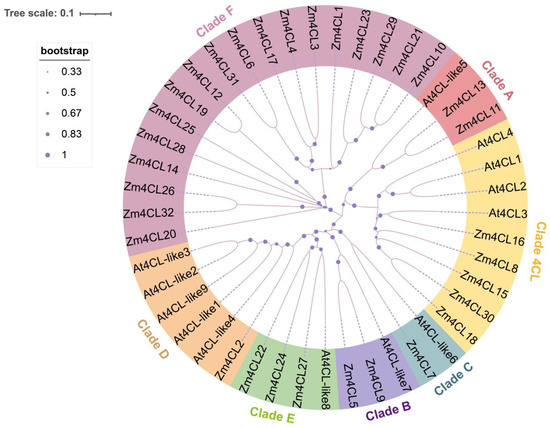
Figure 1.
Phylogenetic analysis of the 4CL genes from Zea mays (Zm) and Arabidopsis thaliana (At). Phylogenetic tree represents the evolutionary relationship between the 4CL gene family members of maize and Arabidopsis. All 4CL genes were divided into seven clades and shown in different colors.
In order to further elucidate the phylogeny and functional characteristics of the maize 4CL family, a cluster analysis was conducted on 4CL gene homologs from both maize and Arabidopsis (Figure 1). This analysis utilized the Neighbor-Joining (NJ) method within the MEGA (v7.0.26) software, aiming to provide a comprehensive understanding of the evolutionary relationships and biological functions inherent in this gene family. Following this, we proceeded to categorize their evolutionary relationships based on the classification methodology of the 4CL gene family as outlined by De Azevedo Souza [12]. All 4CL gene members were categorized into seven clades (Clade 4CL and A-F). The five maize 4CL genes, namely Zm4CL8, Zm4CL15, Zm4CL16, Zm4CL18, and Zm4CL30, exhibit close homology to the At4CL1-4 genes in Arabidopsis. They are clustered within the 4CL clade and are likely involved in the biosynthesis of phenylpropanoid. The Clade A–E all contain the Arabidopsis At4CL-like genes, with Zm4CL11 and Zm4CL13 clustering into Clade A, and Zm4CL5 and Zm4CL9 clustering into Clade B. Meanwhile, Zm4CL7 and Zm4CL2 cluster into Clade C and D, respectively. Additionally, Zm4CL22, Zm4CL24, and Zm4CL27 are grouped within Clade E. It is worth noting that Clade F exhibits a notable absence of At4CL-like genes, with only the presence of Zm4CL genes giving rise to a distinct branch. According to De Azevedo Souza’s description [12], it is possible that Clade F contains AAE (acyl activating enzymes) or AAEL (acyl activating enzyme-like) proteins. It is conceivable that these genes, akin to 4CL, may play a role in diverse pathways within plant metabolism and the synthesis of natural compounds.
3.2. Chromosomal Localization and Syntenic Analysis of Zm4CL Genes
In maize, a total of 32 4CLs were distributed in a random and irregular manner across 10 different chromosomes (Figure S1). Chromosome 1 exhibited the highest Zm4CL density at 28.13%, followed by chromosomes 7 and 9, which had a Zm4CL density of 12.5%. Next up were chromosomes 2 and 5, showing a Zm4CL density of 9.38%. Chromosomes 3, 4, 6, and 8 all contain two Zm4CLs with a gene density of 6.25%. Chromosome 10 contains only one Zm4CL, with a gene density of 3.13%. Intraspecific collinearity analysis unveiled the presence of three pairs of genomic synteny among six genes belonging to the maize 4CL gene family (Figure 2A). More specifically, the genes Zm4CL12 on Chr2 and Zm4CL19 on Chr5, as well as Zm4CL18 on Chr5 and Zm4CL30 on Chr9, along with Zm4CL21 on Chr6 and Zm4CL29 on Chr9, were found to be collinear. This suggests that segmental duplication played a pivotal role in propelling the evolution of the maize 4CL gene family. In order to elucidate the selection pressure among Zm4CL homologous gene pairs, we conducted an analysis of the Ka and Ks parameters (Table S1). The Ka/Ks ratios of all Zm4CL homologous gene pairs were determined to be less than 1, signifying that all gene pairs underwent purifying selection.
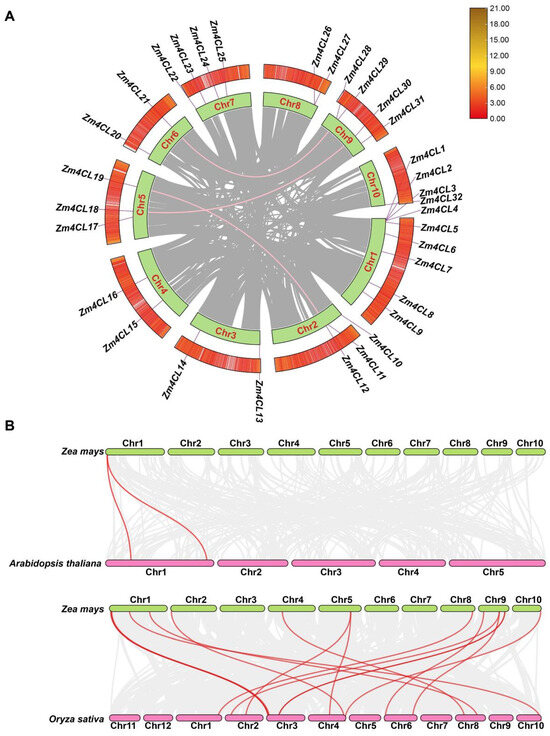
Figure 2.
Synteny analysis of 4CL genes. (A) Intraspecific synteny analysis of Zm4CL genes. The green areas represent chromosomes, while the gray areas indicate collinear regions. The pink lines denote syntenic relationships between Zm4CL genes of maize. (B) Interspecific synteny of 4CL genes across various species. The collinearity maps above and below represent the collinear relationships between maize and Arabidopsis genes, as well as between maize and rice genes. The gray lines indicate the regions of collinearity, while the red lines connect the collinear 4CL gene pairs.
In order to further elucidate the phylogenetic mechanisms underlying the maize 4CL gene family, we constructed a comprehensive genome-wide collinearity map of maize and Arabidopsis, as well as maize and rice (Figure 2B). This map highlights the members of the 4CL gene family that exhibit collinear relationships across these diverse species. The results revealed that one maize 4CL gene and two Arabidopsis 4CL genes formed two syntenic gene pairs, while fourteen maize 4CL genes and sixteen rice 4CL genes formed a total of sixteen syntenic gene pairs. This suggests that the 4CL family between maize and rice exhibits an exceptionally high level of homology. These 4CL syntenic gene pairs may harbor similar potential functions or even trace their origins back to a common ancestor.
3.3. Conserved Motif, Domain, and Gene Structure Analysis of 4CL Gene Family Members in Maize
In order to gain deeper insights into the Zm4CL gene members, we conducted a comprehensive survey of the conserved motif, domain, and gene structure associated with each individual Zm4CL gene. The conserved protein motifs within the Zm4CL gene family were anticipated through the utilization of the MEME tool. A total of ten distinct motifs, designated as motif 1 through motif 10, were discerned within the Zm4CL gene family (Table S2). The number of conserved motifs within each Zm4CL protein exhibited a range from 3 to 9, with all members possessing motif 1 and the majority also containing motifs 2–8 (Figure 3B). This observation suggests a relatively high level of conservation among the Zm4CL proteins. The conservative domain analysis reveals that all members of the Zm4CL exhibit the 4CL domain from the NCBI CDD database, as well as the AFD_class_I superfamily domain. Additionally, they also possess the AMP-binding domain and AMP-binding_C domain from the Pfam database (Figure 3C). The analysis of gene structure revealed significant differences in the identified Zm4CL genes. The exon count of Zm4CLs ranged from 1 to 18, and the intron-exon structures of Zm4CL genes indicated a normal distribution of introns and exons (Figure 3D).
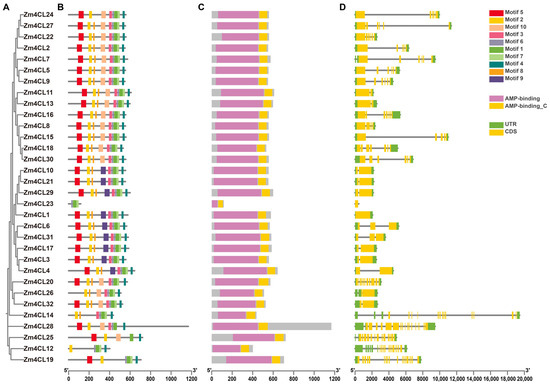
Figure 3.
Phylogenetic tree, conserved motifs, protein domains, and gene structure of the 4CL gene family in maize. (A) The phylogenetic tree was constructed using the Neighbor-Joining (NJ) method in MEGA7. (B) The motif composition of Zm4CL proteins was visualized using TBtools, with the utilization of distinctively colored boxes to represent motifs containing specific amino acid sequences. (C) AMP-binding and AMP-binding_C domains of Zm4CL proteins were visualized using TBtools, with the former displayed in pink and the latter in yellow. (D) The gene structure of Zm4CLs was analyzed using maize genome annotation information and visualized with TBtools. In the visualization, UTR regions are shown as green boxes, CDS regions as yellow boxes, and introns as gray lines.
3.4. Analysis of Promoter cis-Elements in Zm4CL Genes
To investigate the potential transcriptional regulatory mechanism of the maize 4CL gene family, we conducted cis-acting element prediction on the upstream 2000 bp promoter sequences of each member (Figure 4). The promoter regions of the maize 4CL gene family were found to be rich in a multitude of cis-acting elements, including those that are responsive to abiotic stresses (low temperature, drought, etc.), plant hormones (Abscisic Acid, methyl jasmonate, auxin, gibberellin, and salicylic acid), as well as growth and development (flavonoid biosynthesis, circadian rhythm, etc.). Additionally, there also exists a presence of light-responsive elements within these regions. All identified 4CL genes are found to contain cis-acting elements associated with light responsiveness, suggesting that the maize 4CL genes may play a crucial role in the establishment of photomorphogenesis. This also reflects the significance of light exposure in the growth and development of maize. A notable observation has been made regarding a substantial portion of identified 4CL members, which exhibit cis-acting elements linked to Abscisic Acid (ABA) hormone and drought response. This signifies the pivotal role of Zm4CLs in conferring resistance to abiotic stress.
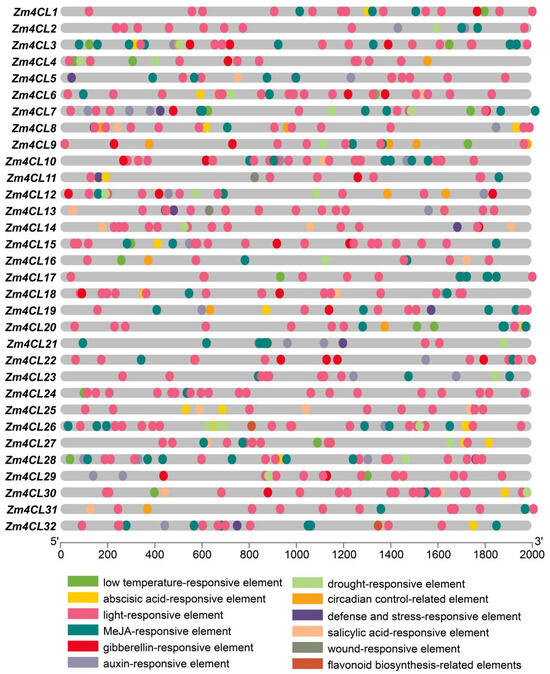
Figure 4.
Prediction of cis-regulatory elements within the promoter regions of Zm4CL genes. The distribution diagram of cis-elements for Zm4CL gene promoters displays diverse colored ellipses representing distinct cis-elements. The promoter regions of different Zm4CL genes are represented by thick gray lines.
3.5. Expression Patterns and Correlation Analysis of 4CL Genes in Maize
The exploration of tissue-specific gene expression patterns provides valuable insights into the potential biological functions of the Zm4CL genes. Examination of the expression patterns within the Zm4CL gene family unveiled distinct and diverse profiles among its various members, thus underscoring their multifaceted functionalities. Specifically, within clade 4CL, the Zm4CL30 and Zm4CL18 genes display elevated expression levels in various tissues, including the pericarp, root, internode, silks, seed, and anthers. Notably, the Zm4CL19 gene, located in clade F, exhibits a significantly higher expression across a broad spectrum of tissues. Additionally, the Zm4CL12 gene, also within clade F, shows pronounced expression in the leaf, anthers, and embryo. Furthermore, other genes in clade F, such as Zm4CL14, Zm4CL25, and Zm4CL31, demonstrate increased expression levels in the pericarp, internode, seed, and coleoptile. Certain Zm4CL genes exhibit tissue-specific high expression; for instance, Zm4CL5, Zm4CL10, and Zm4CL16 are exclusively highly expressed in leaves, while Zm4CL27 is specifically highly expressed in anthers. (Figure 5A). It is possible that they play crucial roles in these tissues. The diverse and distinct tissue-specific expression patterns displayed by the Zm4CL genes emphasize their multifaceted biological significance and potential contributions to a wide range of developmental processes and physiological functions in maize.
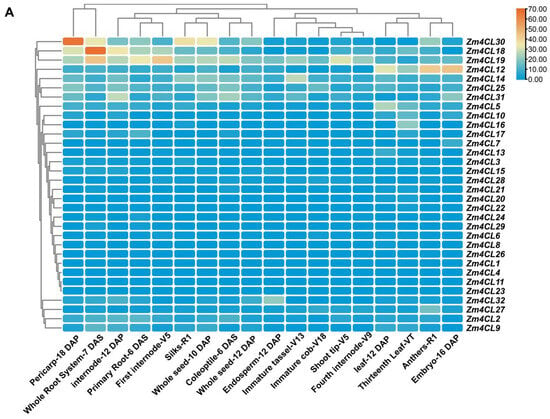
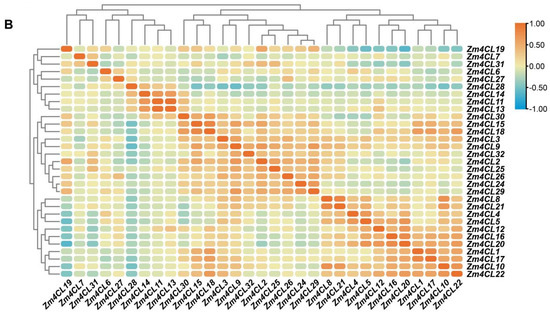
Figure 5.
Expression patterns and correlation analysis of Zm4CL genes. (A) Analysis of Zm4CL gene expression in various tissues. The genes are marked on the right and the tissues are shown at the bottom of each column. Different colors on the map represent TPM (Transcripts Per Kilobase per Million mapped reads) values, as indicated in the bar to the right. (B) Analysis of the correlation among Zm4CL genes based on expression profiles collected from qTeller. The correlation coefficients were displayed in various colors, with the numerical values shown in the right-side bar.
Subsequently, an analysis was conducted to examine the correlation between the expression levels of the Zm4CL genes (Figure 5B). Among these, there exists a strong correlation in the gene expression levels between Zm4CL1 and Zm4CL17(r = 0.8023), as well as between Zm4CL8 and Zm4CL21(r = 0.8046). Additionally, a significant correlation is observed between Zm4CL11 and Zm4CL13 (r = 0.9761), along with that of Zm4CL15 and Zm4CL18 (r = 0.8098). These findings imply a potential intrinsic correlation among these genes in terms of their functional manifestation, suggesting an intricate interplay and interconnectedness within their biological pathways.
3.6. Prediction of Potential Upstream Regulators for Zm4CL Genes Within the 4CL Clade
The members of the Zm4CL gene family located in the 4CL clade are most likely to play crucial roles in lignin and flavonoid biosynthesis. Furthermore, we utilized the plantTFdb (v5.0) website to retrieve the upstream regulators of the Zm4CL genes located in the 4CL clade. Through analysis, we identified a total of 22 transcription factors located upstream of Zm4CL8, Zm4CL16, Zm4CL18, and Zm4CL30 (Figure 6A). Subsequently, an examination was conducted to assess the correlation between the expression levels of these predicted upstream regulators and Zm4CL genes. The correlation between Zm1 and Zm4CL18 (r = 0.8211, p < 0.0001) is notably strong (Figure 6B), with Zm1 also demonstrating a significant association with Zm4CL30 (r = 0.5011, p = 0.0003) (Figure 6C). Additionally, there is a strong correlation between AP2/EREBP105 and Zm4CL30 (r = 0.5148, p = 0.0002) (Figure 6D). Anthocyanin belongs to the class of flavonoids, and both Zm1 and 4CL are involved in the biosynthesis of anthocyanins. Given the results of the correlation analysis, it is highly likely that Zm1 plays a regulatory role upstream of Zm4CL18 or Zm4CL30. AP2/EREBP105, a member of the ERF class transcription factor, is likely to play a crucial role in non-biological stress such as salt or drought stress. Therefore, it is highly probable that it functions upstream of Zm4CL30 to participate in the regulation of non-biological stress in maize. These findings offer valuable insights into the intricate regulatory network involving Zm4CL genes and their upstream regulators in maize, contributing to a deeper understanding of their functional roles in maize.
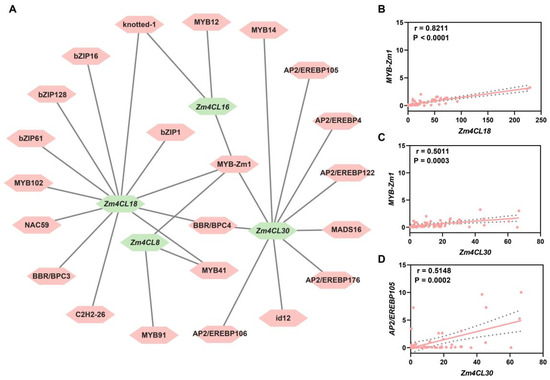
Figure 6.
Prediction of upstream regulators for the Zm4CL genes within clade 4CL. (A) The upstream regulators of Zm4CLs were predicted using the plantTFdb (v5.0) website. The green hexagon represents the presence of Zm4CLs in clade 4CL, while the pink hexagon signifies the potential upstream regulatory factors of Zm4CLs. (B–D) Correlation analysis was conducted to examine the relationship between the expression levels of (B) Zm1 and Zm4CL18, (C) Zm1 and Zm4CL30, as well as (D) AP2/EREBP105 and Zm4CL30. The p values were calculated using the test of Pearson correlation coefficient in the Graphpad Prism (v8.0.2) software.
3.7. Expression Analysis of Zm4CLs Under Abiotic Stress
In order to explore the potential involvement of Zm4CL genes in modulating the abiotic stress responses of maize, we conducted an analysis on the transcriptional levels of Zm4CL genes under conditions of drought (Figure 7A), cold, heat, salt, and UV stress (Figure 7B) using RNA-seq data obtained from qTeller at MaizeGDB. The findings revealed that the transcription of the Zm4CL genes demonstrates differential responsiveness to various environmental stressors, indicating that different Zm4CLs play distinct roles in different types of stress. Specifically, the expression levels of genes Zm4CL8, Zm4CL13, Zm4CL21, Zm4CL26, and Zm4CL31 exhibited a more than twofold upregulation in response to drought treatment. Similarly, under salt treatment, the expression levels of Zm4CL8, Zm4CL10, Zm4CL25, Zm4CL26, Zm4CL31, and Zm4CL32 showed a significant increase. Furthermore, Zm4CL2, Zm4CL7, Zm4CL8, Zm4CL17, Zm4CL26, Zm4CL31, and Zm4CL32 displayed a more than two-fold upregulation following UV treatment. Notably, the expression levels of Zm4CL8 in clade 4CL, as well as Zm4CL26 and Zm4CL31 in clade F, were significantly upregulated under drought, salt, and UV treatments. Additionally, Zm4CL17, Zm4CL26, Zm4CL31, and Zm4CL32 were upregulated by at least two-fold in response to heat stress. Similarly, Zm4CL26 and Zm4CL31 showed significant upregulation under heat stress, suggesting that these two 4CL genes may play a pivotal role in regulating abiotic stress responses in maize. Moreover, Zm4CL1, Zm4CL13, Zm4CL17, and Zm4CL20 were all upregulated by at least two-fold following cold stress. The expression levels of Zm4CL17 and Zm4CL32, located within clade F, were also markedly elevated under multiple abiotic stress conditions, highlighting their potential importance in stress adaptation. The diverse expression patterns exhibited by the Zm4CL genes in response to various stressors, such as drought, salt, cold, heat, and UV radiation, indicate their potential as key regulators in the plant’s non-biological stress adaptive mechanisms.
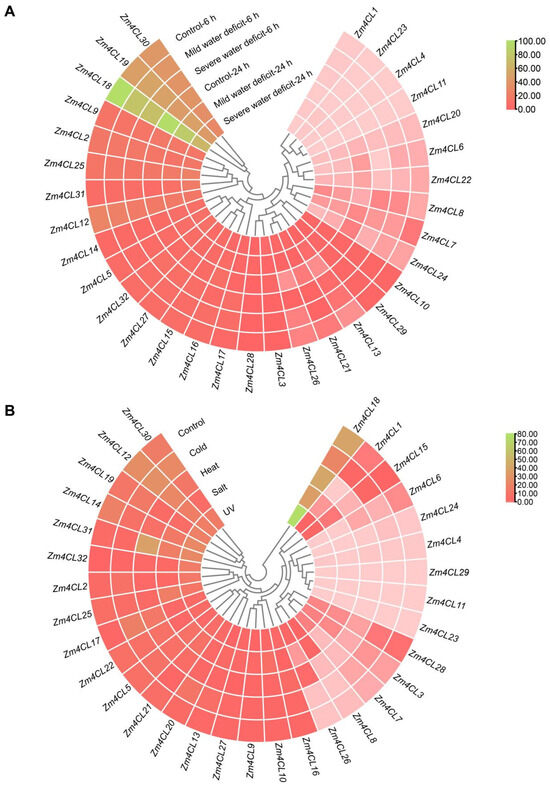
Figure 7.
Expression analysis of Zm4CLs in response to drought, cold, heat, salt, and UV stresses. (A) Expression analysis of Zm4CL genes under varying degrees of drought stress. (B) Expression analysis of Zm4CL genes under cold, heat, salt, and UV stresses. The TPM values of the Zm4CL genes were utilized to generate a heat map using TBtools. The varied colors depicted on the map correspond to different levels of TPM values, as delineated in the accompanying bar to the right.
3.8. Expression Profiling of Selected Zm4CL Genes Under Stress Conditions Using RT-qPCR
To further analyze the transcriptional changes in Zm4CLs under drought and salt stress, the drought and salt stress-responsive Zm4CL genes identified through transcriptome analysis were verified using RT-qPCR experiments. The results showed that the transcription of Zm4CL8, Zm4CL10, Zm4CL25, Zm4CL26, Zm4CL31, and Zm4CL32 was upregulated after salt treatment (Figure 8A–F), while the transcription of Zm4CL8, Zm4CL13, Zm4CL21, Zm4CL26, and Zm4CL31 was upregulated after drought treatment (Figure 8G–K). Notably, Zm4CL8, Zm4CL26, and Zm4CL31 were upregulated under both salt and drought treatments, suggesting that they may play a more significant role in abiotic stress responses in maize.
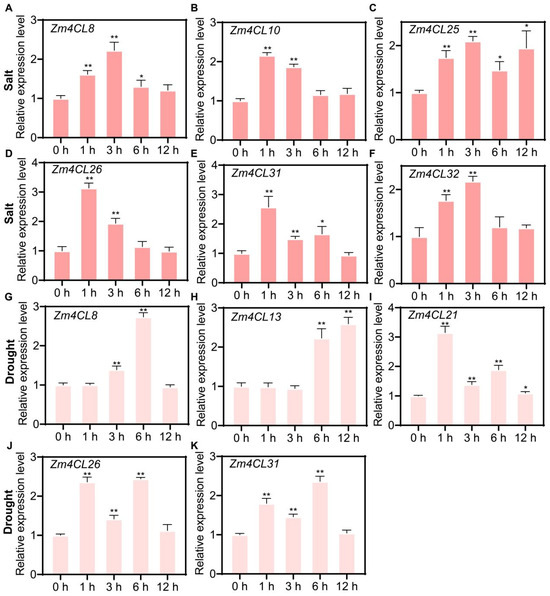
Figure 8.
Transcriptional alterations of selected Zm4CL genes in response to salinity and drought stress. (A–F) Transcriptional dynamics of Zm4CL8 (A), Zm4CL10 (B), Zm4CL25 (C), Zm4CL26 (D), Zm4CL31 (E), and Zm4CL32 (F) in response to salt stress at 0, 1, 3, 6, and 12 h post-treatment, as determined by RT-qPCR analysis. (G–K) Transcriptional profiles of Zm4CL8 (G), Zm4CL13 (H), Zm4CL21 (I), Zm4CL26 (J), and Zm4CL31 (K) under drought stress conditions at 0, 1, 3, 6, and 12 h post-treatment, as assessed by RT-qPCR. Data are expressed as mean ± SD (n = 3). Statistical significance was determined by Student’s t-test, with * indicating p < 0.05 and ** indicating p < 0.01.
3.9. Silencing of Zm4CL8 Impairs Drought and Salt Stress Tolerance in Maize Seedlings
Zm4CL8 is located within the 4CL clade of the phylogenetic tree and is induced by both drought and salt stress. To analyze the function of Zm4CL8 in maize under drought and salt stress conditions, we employed the TRV vector to silence Zm4CL8 gene expression through virus-induced gene silencing (VIGS) experiments (Figure S2). Additionally, we used the TRV vector to interfere with the ZmPDS gene as a positive control for the VIGS assay. The maize lines inoculated with TRV-ZmPDS exhibited a distinct albino phenotype during the seedling stage (Figure S3). Phenotypic analysis revealed that after 12 days of drought treatment, TRV-Zm4CL8 plants exhibited weaker drought resistance compared to the control lines (Figure 9A), with reductions observed in chlorophyll content, survival rate, and leaf relative water content (RWC) (Figure 9B–D). Similarly, after 12 days of salt treatment, TRV-Zm4CL8 plants showed weaker salt tolerance compared to the control lines (Figure 9E). After 14 days of salt treatment, the phenotypic differences became more pronounced (Figure 9E), with significant reductions in chlorophyll content and survival rate (Figure 9F,G). The above results suggest that Zm4CL8 may play a positive regulatory role in resistance to both salt and drought stress.
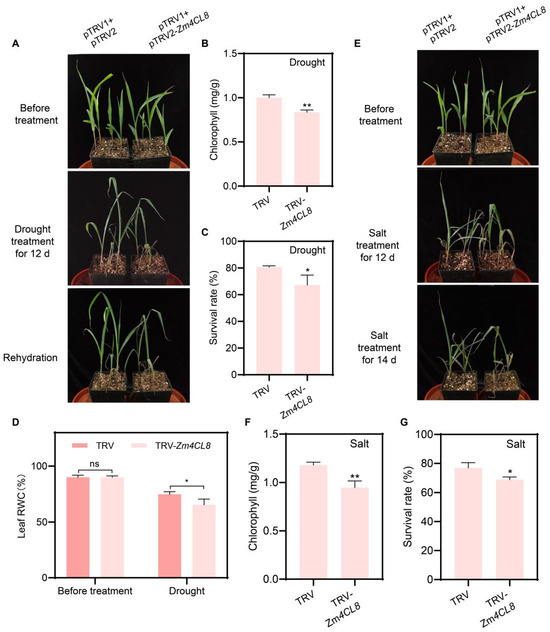
Figure 9.
Analysis of drought and salt tolerance in Zm4CL8-silenced maize plants. (A) Representative phenotypes of control (CK) and TRV-Zm4CL8 (VIGS) plants after 12 days of drought treatment and subsequent rewatering. (B,C) Chlorophyll content (B) and survival rate (C) in control and TRV-Zm4CL8 maize plants after 12 days of drought and rewatering. (D) Leaf RWC in control and TRV-Zm4CL8 maize plants before and after drought treatment. (E) Representative phenotypes of control and TRV-Zm4CL8 plants after 12 and 14 days of salt treatment. (F,G) Chlorophyll content (F) and survival Rate (G) in control and TRV-Zm4CL8 maize plants after salt treatment. All data were obtained from three independent replicates. Statistical significance was assessed using Student’s t-test. * and ** denote significance at p < 0.05 and p < 0.01, respectively.
4. Discussion
The evolution of the phenylpropanoid pathway plays a pivotal role in enabling terrestrial plants to colonize land [16]. Within this context, 4CL plays a pivotal role as one of the crucial enzymes in the phenylpropanoid metabolic pathway [38]. The magnitude of 4CL enzyme activity exerted a profound influence on the accumulation of compounds, notably flavonoids and lignin, which are essential for protecting plants from UV light damage and providing structural support [39]. In this study, we conducted a comprehensive genome-wide analysis to identify 4CL genes in maize and explored their expression patterns across various tissues and under diverse stress conditions, with the objective of pinpointing 4CL genes that may play a pivotal role in conferring stress tolerance [18]. A total of 32 Zm4CL members were identified within the maize genome (Table 1). In accordance with De Azevedo Souza’s delineation [12], the entirety of Zm4CL gene members were systematically classified into seven distinct clades, denoted as Clade 4CL and A-F (Figure 1). In Arabidopsis, At4CL1-4 is located in the clade 4CL branch and further divides into two types [14]. At4CL1, At4CL2, and At4CL4 are categorized under type I (associated with lignin biosynthesis), while At4CL3 falls within the type II cluster (related to phenylpropanoid biosynthesis other than lignin) [14,16]. Zm4CL16 and At4CL3 are located in the same sub-branch, indicating a closer homologous relationship. This suggests that Zm4CL16 may be involved in phenylpropanoid biosynthesis other than lignin, such as flavonoids. On the other hand, Zm4CL8, Zm4CL15, Zm4CL18, and Zm4CL30 form an independent sub-branch in clade 4CL. These genes are likely to play crucial roles in phenylpropanoid biosynthesis. The Zm4CLs located in clade A-F shares a similarity with clade 4CL proteins, as they all possess AMP-binding and AMP-binding_C domains [24], as well as 4CL and AFD_class_I superfamily domains. These genes are likely to be involved in the regulation of other complex metabolic pathways.
The chromosomal distribution of Zm4CL genes was uneven (Figure S1), yet collinear relationships were identified among specific members of this gene family (Figure 2A). For instance, Zm4CL12 and Zm4CL19, Zm4CL18 and Zm4CL30, as well as Zm4CL21 and Zm4CL29. This implies that the process of segmental duplication played a crucial and influential role in driving the evolutionary progression of the maize 4CL genes. Furthermore, a single maize 4CL gene and two Arabidopsis 4CL genes were found to form two syntenic gene pairs, while a total of fourteen maize 4CL genes and sixteen rice 4CL genes formed sixteen syntenic gene pairs (Figure 2B). The significant level of synteny observed between the maize and rice 4CL genes suggests a conservation of function that may be indicative of their closer evolutionary relationship as members of the Poaceae family. In contrast, the limited synteny observed between the maize and Arabidopsis 4CL genes suggests a heightened level of functional diversification since their divergence from a common ancestor. Genetic variability is contingent upon the structure of genes and their conserved domains [40]. In this study, the majority of Zm4CL genes within the same class exhibit a comparable intron-exon structure, characterized by an identical number and distribution of conserved motifs (Figure 3B,D). All members are found to possess the AMP-binding and AMP-binding_C domains (Figure 3C), signifying a remarkable level of conservation in both evolutionary history and functional attributes.
cis-Acting elements play a crucial role in the intricate regulation of gene expression [41]. Within gene promoters, a diverse array of cis-acting elements may be intricately linked to various gene functions, thereby contributing to the complexity and specificity of genetic regulation. A substantial proportion of identified Zm4CLs harbored cis-acting elements responsive to Abscisic Acid (ABA) hormone and drought (Figure 4). ABA plays a crucial role in the regulation of non-biological stress in plants [42]. This underscores the pivotal function of Zm4CLs in imparting resistance to abiotic stress. Analysis of the promoter regions of Zm4CL genes within the 4CL clade revealed a diverse array of upstream transcription factors (Figure 6A), including Zm1 and AP2/EREBP105, that potentially regulate the expression of these Zm4CL genes. Notably, the expression levels of Zm1 showed a remarkably strong correlation with those of Zm4CL18 and Zm4CL30 (Figure 6B,C). Both Zm1 and 4CL are known to be involved in the biosynthesis of anthocyanins [16,43]. Additionally, a significant correlation was observed between AP2/EREBP105 and Zm4CL30 (Figure 6D). As a member of the ERF class of transcription factors, AP2/EREBP105 is likely to play a pivotal role in mediating responses to abiotic stresses such as salt or drought [44]. These findings further suggest that Zm4CL genes may play significant roles in regulating maize development and stress response processes.
As one of the most important staple crops globally, maize production faces significant challenges from various abiotic stress factors that severely threaten its yield potential [45]. While a substantial number of abiotic stress-related genes have been discovered in maize to date [46], our understanding of the regulatory networks governing these stress responses remains incomplete. Consequently, continued investigation into the comprehensive understanding of maize’s abiotic stress response network remains crucial. Extensive research has demonstrated that 4CL genes play a crucial role in mediating plant responses to various environmental stresses [16]. The differential expression patterns of Zm4CL genes in response to diverse abiotic stresses (drought, salt, cold, heat, and UV radiation) strongly indicate their pivotal involvement in mediating maize’s stress adaptation responses (Figure 7). RT-qPCR results demonstrated consistent upregulation of Zm4CL8, Zm4CL26, and Zm4CL31 transcripts under both salinity and drought stress treatments, suggesting their crucial involvement in maize’s abiotic stress response mechanisms (Figure 8). 4CL catalyzes the production of various phenolic secondary metabolites in plants, particularly lignin and flavonoids, which play crucial roles in the regulation of plant stress responses [19,47]. In Fraxinus mandshurica, ectopic expression of Fm4CL-like1 in transgenic tobacco (Nicotiana tabacum) significantly enhanced drought tolerance, potentially through promoting lignification and modulating ROS homeostasis [48]. Similarly, Zm4CL-like9 in maize was shown to confer drought resistance via ROS scavenging regulation [25]. These parallel findings demonstrate an evolutionarily conserved role of 4CL family members in coordinating stress-responsive metabolic pathways in higher plants. Zm4CL8 is located within the 4CL branch (Figure 1) and is potentially involved in the synthesis of these phenolic secondary metabolites. Additionally, its expression is induced under salt and drought stress (Figure 7 and Figure 8). To further investigate its function, the Zm4CL8 gene was silenced using VIGS technology. Compared to the control, TRV-Zm4CL8 plants exhibited reduced tolerance to salt and drought stress (Figure 9), further highlighting the critical role of Zm4CL8 in abiotic stress responses in maize. These results suggest that similar to other plants [16,22,23], Zm4CL genes may also play a role in regulating maize’s response to abiotic stress.
5. Conclusions
In this study, we conducted a comprehensive genome-wide identification of the 4CL gene family members in maize. Although variations were observed among different Zm4CLs, those within the same phylogenetic clade exhibited conserved characteristics. Promoter element analysis revealed that Zm4CLs respond to stress-related signals, consistent with findings in other plant species. Transcriptional analysis demonstrated that multiple Zm4CLs are influenced by various abiotic stresses. Notably, Zm4CL8, located within the 4CL clade, was significantly induced under both drought and salt stress conditions. Furthermore, Zm4CL8-silenced lines generated via VIGS exhibited reduced tolerance to salt and drought treatments compared to controls. Although the stress-responsive mechanisms of the identified Zm4CL genes remain to be fully characterized, particularly the functional validation of Zm4CL8 in transgenic maize and detailed mechanistic studies, this systematic analysis of the maize 4CL gene family establishes their important potential in abiotic stress adaptation and provides promising genetic targets for maize improvement.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15051100/s1, Figure S1. Distribution of the Zm4CL gene family on maize chromosomes. Figure S2. RT-qPCR analysis of Zm4CL8 expression in control and Zm4CL8-silenced lines. Figure S3. Phenotypic observation of control and TRV-ZmPDS-silenced maize plants. Table S1. Ka, Ks, and Ka/Ks ratios of Zm4CL paralogous gene pairs. Table S2. Conserved motif sequences of maize 4CL family proteins. Table S3. Primers used in this study.
Author Contributions
Conceptualization, Z.Z. and R.L.; data curation, Z.Z. and Y.W.; formal analysis, R.L.; funding acquisition, Z.Z.; investigation, Y.W. and R.L.; methodology, Z.Z.; project administration, Z.Z.; resources, Y.W.; software, Z.Z.; supervision, R.L.; validation, Y.W. and R.L.; visualization, Z.Z.; writing—original draft, Z.Z.; writing—review and editing, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Scientific Research Initiation Project for Introduced Talents at Shanxi Agricultural University (Grant No. 2023BQ113) and the Scientific Research Project of Incentive Funds for Doctoral Graduates and Postdoctoral Researchers Coming to Work in Shanxi Province (Grant No. SXBYKY2024033).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tzin, V.; Galili, G. The Biosynthetic Pathways for Shikimate and Aromatic Amino Acids in Arabidopsis thaliana. Arabidopsis Book 2010, 8, e0132. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Jayaramaiah, R.H.; Beedkar, S.D.; Singh, P.A.; Joshi, R.S.; Mulani, F.A.; Dholakia, B.B.; Punekar, S.A.; Gade, W.N.; Thulasiram, H.V.; et al. Comparative functional characterization of eugenol synthase from four different Ocimum species: Implications on eugenol accumulation. Biochim. Biophys. Acta 2016, 1864, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Hamberger, B.; Hahlbrock, K. The 4-coumarate:CoA ligase gene family in Arabidopsis thaliana comprises one rare, sinapate-activating and three commonly occurring isoenzymes. Proc. Natl. Acad. Sci. USA 2004, 101, 2209–2214. [Google Scholar] [CrossRef]
- Allina, S.M.; Pri-Hadash, A.; Theilmann, D.A.; Ellis, B.E.; Douglas, C.J. 4-Coumarate:coenzyme A ligase in hybrid poplar. Properties of native enzymes, cDNA cloning, and analysis of recombinant enzymes. Plant Physiol. 1998, 116, 743–754. [Google Scholar] [CrossRef]
- Hahlbrock, K.; Scheel, D. Physiology and Molecular Biology of Phenylpropanoid Metabolism. Annu. Rev. Plant Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Schneider, K.; Hövel, K.; Witzel, K.; Hamberger, B.; Schomburg, D.; Kombrink, E.; Stuible, H.P. The substrate specificity-determining amino acid code of 4-coumarate:CoA ligase. Proc. Natl. Acad. Sci. USA 2003, 100, 8601–8606. [Google Scholar] [CrossRef]
- Fulda, M.; Heinz, E.; Wolter, F.P. The fadD gene of Escherichia coli K12 is located close to rnd at 39.6 min of the chromosomal map and is a new member of the AMP-binding protein family. Mol. Gen. Genet. 1994, 242, 241–249. [Google Scholar] [CrossRef]
- Stuible, H.; Büttner, D.; Ehlting, J.; Hahlbrock, K.; Kombrink, E. Mutational analysis of 4-coumarate:CoA ligase identifies functionally important amino acids and verifies its close relationship to other adenylate-forming enzymes. FEBS Lett. 2000, 467, 117–122. [Google Scholar] [CrossRef]
- Stuible, H.P.; Kombrink, E. Identification of the substrate specificity-conferring amino acid residues of 4-coumarate:coenzyme A ligase allows the rational design of mutant enzymes with new catalytic properties. J. Biol. Chem. 2001, 276, 26893–26897. [Google Scholar] [CrossRef]
- Zhang, C.H.; Ma, T.; Luo, W.C.; Xu, J.M.; Liu, J.Q.; Wan, D.S. Identification of 4CL Genes in Desert Poplars and Their Changes in Expression in Response to Salt Stress. Genes 2015, 6, 901–917. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo Souza, C.; Barbazuk, B.; Ralph, S.G.; Bohlmann, J.; Hamberger, B.; Douglas, C.J. Genome-wide analysis of a land plant-specific acyl:coenzyme A synthetase (ACS) gene family in Arabidopsis, poplar, rice and Physcomitrella. New Phytol. 2008, 179, 987–1003. [Google Scholar] [CrossRef]
- Costa, M.A.; Collins, R.E.; Anterola, A.M.; Cochrane, F.C.; Davin, L.B.; Lewis, N.G. An in silico assessment of gene function and organization of the phenylpropanoid pathway metabolic networks in Arabidopsis thaliana and limitations thereof. Phytochemistry 2003, 64, 1097–1112. [Google Scholar] [CrossRef]
- Raes, J.; Rohde, A.; Christensen, J.H.; Van de Peer, Y.; Boerjan, W. Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiol. 2003, 133, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Shockey, J.M.; Fulda, M.S.; Browse, J. Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme a synthetases. Plant Physiol. 2003, 132, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Lavhale, S.G.; Kalunke, R.M.; Giri, A.P. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta 2018, 248, 1063–1078. [Google Scholar] [CrossRef]
- Chowdhury, M.E.K.; Choi, B.; Cho, B.-K.; Kim, J.B.; Park, S.U.; Natarajan, S.; Lim, H.-S.; Bae, H. Regulation of 4CL, encoding 4-coumarate: Coenzyme A ligase, expression in kenaf under diverse stress conditions. Plant Omics 2013, 6, 254–262. [Google Scholar]
- Sun, H.; Li, Y.; Feng, S.; Zou, W.; Guo, K.; Fan, C.; Si, S.; Peng, L. Analysis of five rice 4-coumarate:coenzyme A ligase enzyme activity and stress response for potential roles in lignin and flavonoid biosynthesis in rice. Biochem. Biophys. Res. Commun. 2013, 430, 1151–1156. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Cesarino, I. Structural features and regulation of lignin deposited upon biotic and abiotic stresses. Curr. Opin. Biotechnol. 2019, 56, 209–214. [Google Scholar] [CrossRef]
- Moura, J.C.; Bonine, C.A.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Xiong, X.P.; Zhang, X.L.; Feng, H.J.; Zhu, Q.H.; Sun, J.; Li, Y.J. Characterization of the Gh4CL gene family reveals a role of Gh4CL7 in drought tolerance. BMC Plant Biol. 2020, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Qing, J.; Wang, Q.; Liu, C.; Du, H.; Liu, P.; Du, Q.; Du, L.; Wang, L. Genome-Wide Identification and Expression Analyses of the 4-Coumarate: CoA Ligase (4CL) Gene Family in Eucommia ulmoides. Forests 2022, 13, 1253. [Google Scholar] [CrossRef]
- Nie, T.; Sun, X.; Wang, S.; Wang, D.; Ren, Y.; Chen, Q. Genome-Wide Identification and Expression Analysis of the 4-Coumarate: CoA Ligase Gene Family in Solanum tuberosum. Int. J. Mol. Sci. 2023, 24, 1642. [Google Scholar] [CrossRef]
- Fan, J.; Luo, Z.; Wang, Y.; Jiao, P.; Wang, Q.; Dai, Y.; Guan, S.; Ma, Y.; Yu, H.; Liu, S. Maize 4-coumarate coenzyme A ligase Zm4CL-like9 gene positively regulates drought stress response in Arabidopsis thaliana. GM Crops Food 2025, 16, 199–215. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32, W327–W331. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, D.; Zhang, Y.; Liu, K.; Xu, K.; Zhang, F.; Wang, J.; Tan, G.; Nie, X.; Ji, Q.; et al. Vacuum and Co-cultivation Agroinfiltration of (Germinated) Seeds Results in Tobacco Rattle Virus (TRV) Mediated Whole-Plant Virus-Induced Gene Silencing (VIGS) in Wheat and Maize. Front. Plant Sci. 2017, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Vinocur, B.J.; Diber, A.; Shatil, A.; Ronen, G.; Nissan, H.; Wallach, R.; Karchi, H.; Moshelion, M. Improving plant stress tolerance and yield production: Is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol. 2009, 181, 651–661. [Google Scholar] [CrossRef]
- Lee, D.; Ellard, M.; Wanner, L.A.; Davis, K.R.; Douglas, C.J. The Arabidopsis thaliana 4-coumarate:CoA ligase (4CL) gene: Stress and developmentally regulated expression and nucleotide sequence of its cDNA. Plant Mol. Biol. 1995, 28, 871–884. [Google Scholar] [CrossRef]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014, 217–218, 109–119. [Google Scholar] [CrossRef]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Franken, P.; Schrell, S.; Peterson, P.A.; Saedler, H.; Wienand, U. Molecular analysis of protein domain function encoded by the myb-homologous maize genes C1, Zm 1 and Zm 38. Plant J. 1994, 6, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Wang, D. AP2/ERF Transcription Factors for Tolerance to Both Biotic and Abiotic Stress Factors in Plants. Trop. Plant Biol. 2023, 16, 105–112. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Salika, R.; Riffat, J. Abiotic stress responses in maize: A review. Acta Physiol. Plant. 2021, 43, 130. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Li, X.; Ma, K.; Zhan, Y.; Zeng, F. Molecular cloning and functional analysis of 4-Coumarate:CoA ligase 4(4CL-like 1)from Fraxinus mandshurica and its role in abiotic stress tolerance and cell wall synthesis. BMC Plant Biol. 2019, 19, 231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).