Relationships Between Carbon Fractions and Soil Nutrients in Organic Cassava Cultivation in the Sandy Soil of Northeastern Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. The Study’s Experimental Design
2.3. Soil Sampling and Sample Distribution
2.4. Methodology for Soil and Organic Fertilizer Analysis
2.4.1. Soil Organic Carbon (SOC)
2.4.2. Permanganate Oxidizable Carbon (POXC)
2.4.3. Different Concentrations of H2SO4 Were Used to Chemically Oxidize Carbon [48] Using an Oxidizable Carbon Fraction Technique
2.4.4. Nutrient Analysis (NH4+, NO3−, Available Phosphorus), pH, and Particle Distribution
2.4.5. Organic Fertilizer and DDG Analysis
2.4.6. Nutrients in Organic Fertilizer and DDGs via Water Extraction
2.5. Statistical Analysis
2.6. Writing the Manuscript by Generative AI
3. Results
3.1. The Nutrient Content of Organic Fertilizer and DDGs as Soil Amendments
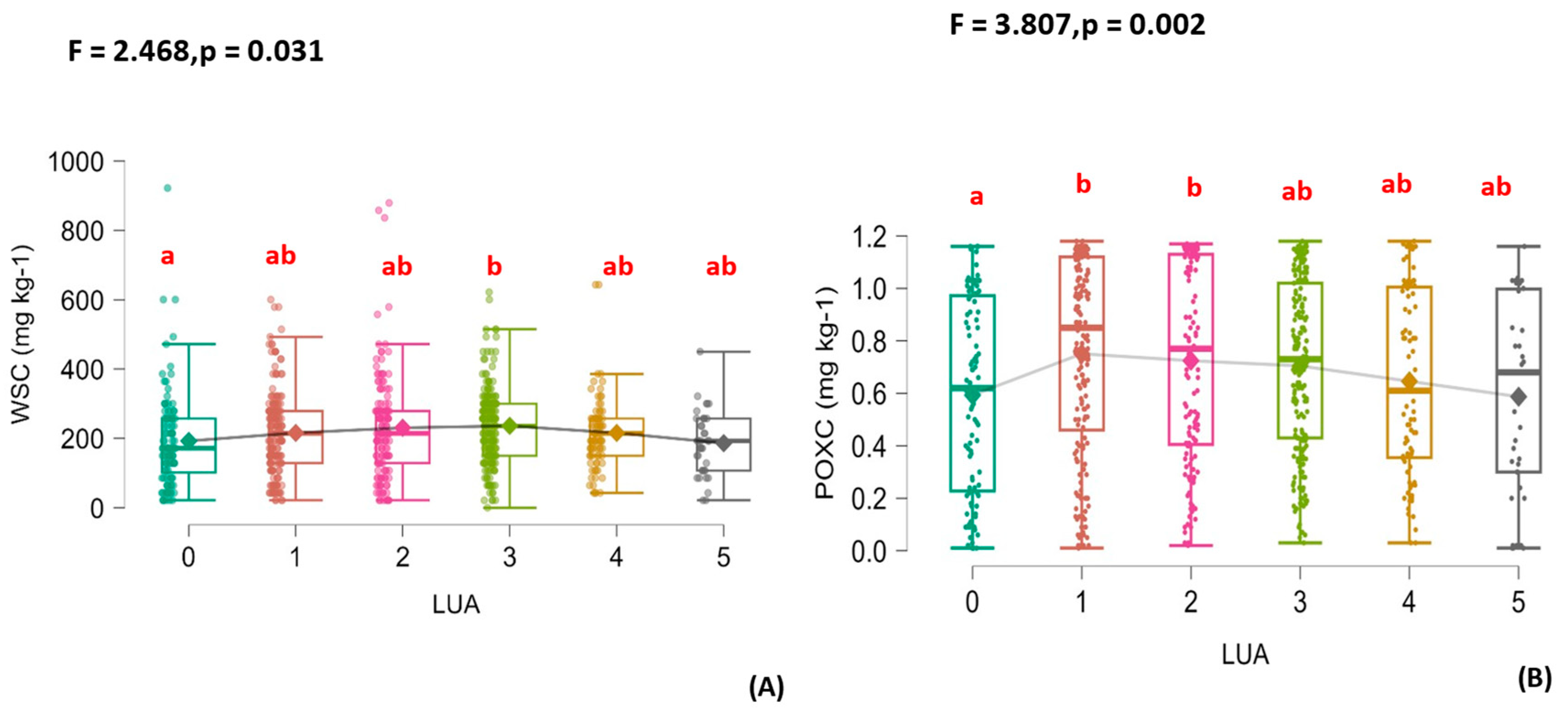
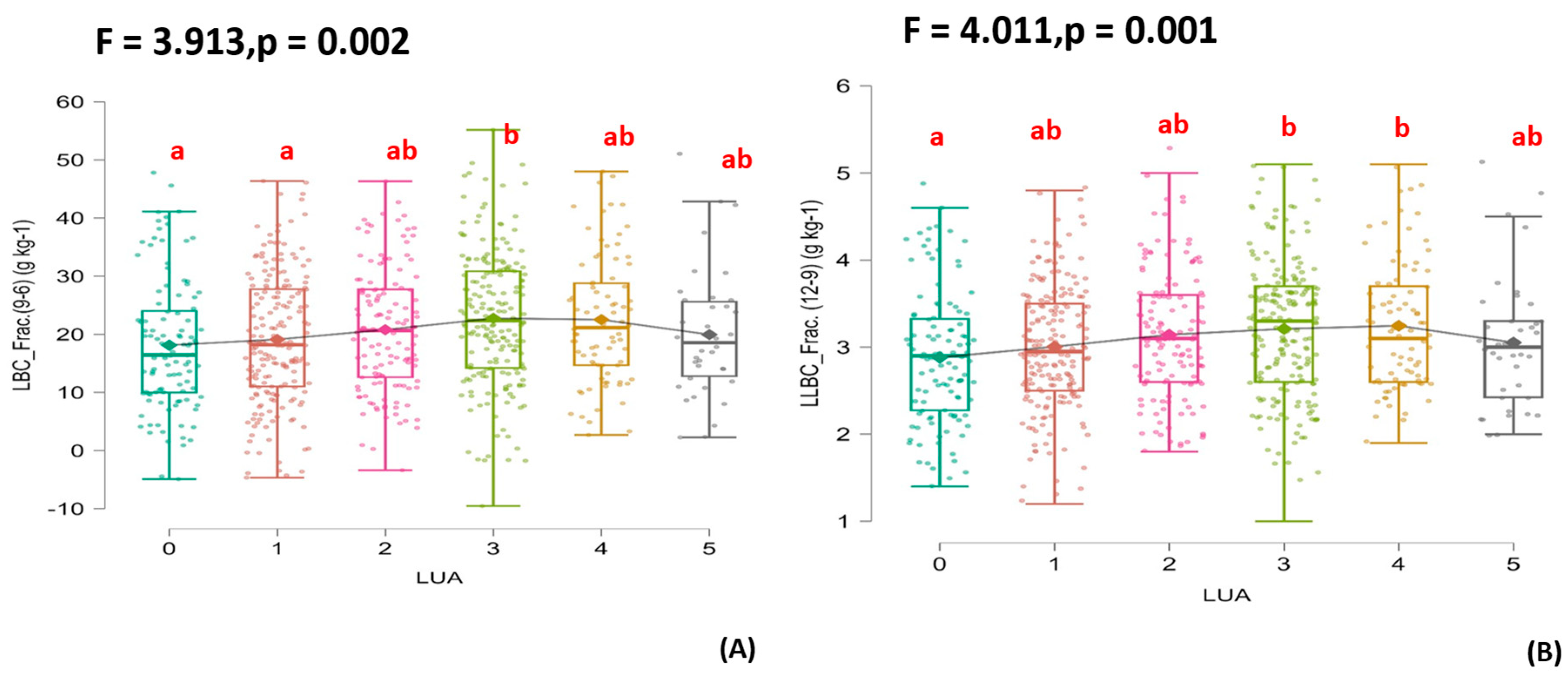
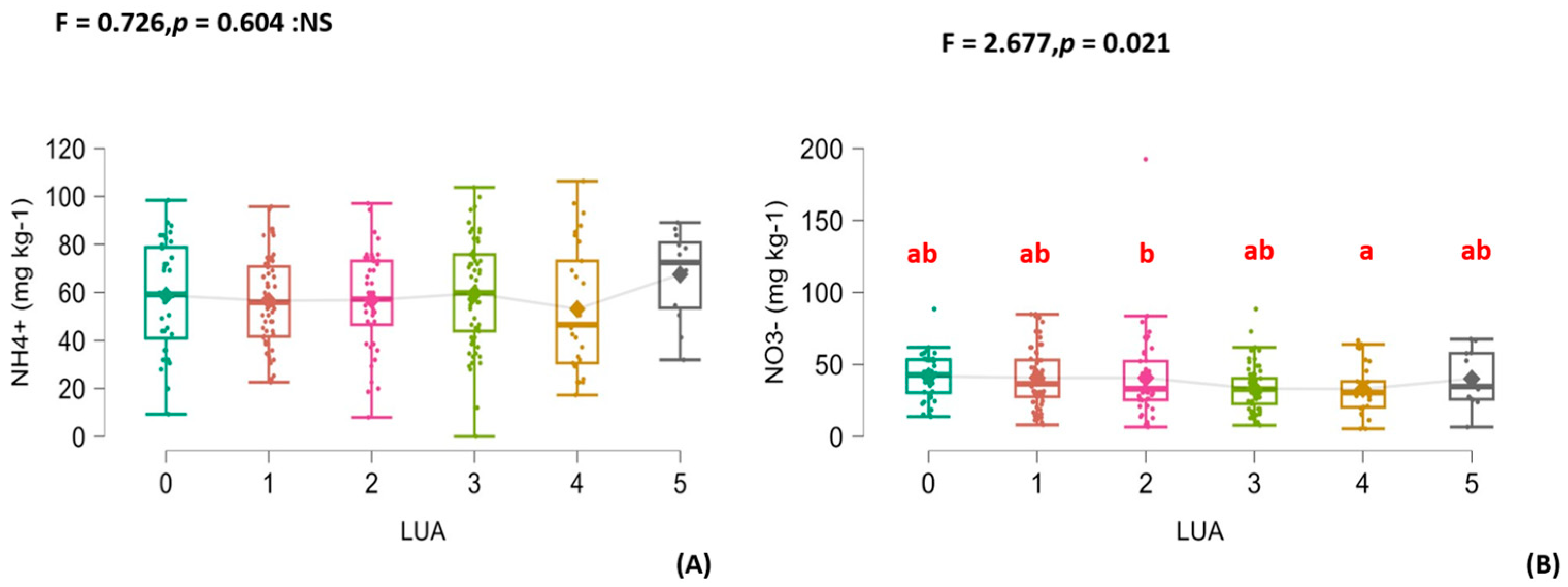
3.2. Organic Fertilization’s Effects on the Soil’s Physical Properties, Various Carbon Fractions, and N and P Changes
3.3. The Relationship Between Soil Carbon Fractions and Some of the Effects of the Availability of Nutrients on Carbon Stability
4. Discussion
4.1. The Carbon Fractions with the Combinations of Animal Manure with DDGs
4.2. Relationship Between the Soil Carbon Fraction and SOC According to the Mechanisms of Decomposition and Stabilization
4.3. The Relationship Between Carbon Fractions and Available Nitrogen and Its Effect on Soil Carbon in Sandy Soil
4.4. The Relationship Between the Carbon Fraction and P in Sandy Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yost, J.L.; Hartemink, A.E. Chapter Four—Soil organic carbon in sandy soils: A review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 158, pp. 217–310. [Google Scholar]
- Huang, J.; Hartemink, A.E. Soil and environmental issues in sandy soils. Earth-Sci. Rev. 2020, 208, 103295. [Google Scholar] [CrossRef]
- Miura, K.; Tulaphitak, T.; Kyuma, K. Pedogenetic studies on some selected soils in Northeast Thailand. Soil Sci. Plant Nutr. 1992, 38, 485–493. [Google Scholar] [CrossRef]
- Tancharoen, S.; Iwasaki, S.; Watanabe, T.; Luanmanee, S.; Nobuntou, W.; Amonpon, W.; Chumsuwan, N.; Paisancharoen, K.; Bumrung, S.; Matsumoto, N. Carbon Sequestration and Soil Fertility Management in Sandy and Clayey Soils Revealed by Over Four Decades of Long-Term Field Experiments in Thailand. Land Degrad. Dev. 2024, 35, 5488–5503. [Google Scholar] [CrossRef]
- Putthacharoen, S.; Howeler, R.H.; Jantawat, S.; Vichukit, V. Nutrient uptake and soil erosion losses in cassava and six other crops in a Psamment in eastern Thailand. Field Crops Res. 1998, 57, 113–126. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Puttaso, A.; Vityakon, P.; Saenjan, P.; Trelo-ges, V.; Cadisch, G. Relationship between residue quality, decomposition patterns, and soil organic matter accumulation in a tropical sandy soil after 13 years. Nutr. Cycl. Agroecosystems 2011, 89, 159–174. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, S.; Zhang, T.; Zhao, X.; Chen, S.; Wang, Q. Priming of soil organic carbon decomposition induced by exogenous organic carbon input: A meta-analysis. Plant Soil 2019, 443, 463–471. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Jiang, D. Soil exchangeable base cations along a chronosequence of Caragana microphylla plantation in a semi-arid sandy land, China. J. Arid. Land 2012, 5, 42–50. [Google Scholar] [CrossRef]
- Šimanský, V.; Juriga, M.; Jonczak, J.; Uzarowicz, Ł.; Stępień, W. How relationships between soil organic matter parameters and soil structure characteristics are affected by the long-term fertilization of a sandy soil. Geoderma 2019, 342, 75–84. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Bei, S.; Li, X.; Reinsch, S.; Zhang, H.; Zhang, J. Contrasting impacts of manure and inorganic fertilizer applications for nine years on soil organic carbon and its labile fractions in bulk soil and soil aggregates. CATENA 2020, 194, 104739. [Google Scholar] [CrossRef]
- Heitkamp, F.; Raup, J.; Ludwig, B. Impact of fertilizer type and rate on carbon and nitrogen pools in a sandy Cambisol. Plant Soil 2009, 319, 259–275. [Google Scholar] [CrossRef]
- Banger, K.; Toor, G.; Biswas, A.; Sidhu, S.; Sudhir, A. Soil organic carbon fractions after 16-years of applications of fertilizers and organic manure in a Typic Rhodalfs in semi-arid tropics. Nutr. Cycl. Agroecosystems 2010, 86, 391–399. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; Nieder, R.; Richter, J. Sequestration of a Biologically Labile Organic Carbon in Soils by Humified Organic Matter. Clim. Change 2004, 67, 329–343. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef] [PubMed]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Sharma, S. Sensitivity of Labile Soil Organic Carbon Pools to Long-Term Fertilizer, Straw and Manure Management in Rice-Wheat System. Pedosphere 2015, 25, 534–545. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, W.; Luo, J.; Bolan, N.; Yu, H.; Zhu, J. Temporal responses of microorganisms and native organic carbon mineralization to 13C-glucose addition in a sandy loam soil with long-term fertilization. Eur. J. Soil Biol. 2016, 74, 16–22. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Cho, Y.-S.; Lee, J.-H.; Seo, H.-R.; Kim, B.-H.; Lee, D.-B.; Lee, Y.B.; Kim, K.-H. Short-Term Responses of Soil Organic Carbon Pool and Crop Performance to Different Fertilizer Applications. Agronomy 2022, 12, 1106. [Google Scholar] [CrossRef]
- Mustafa, A.; Xu, H.; Sun, N.; Liu, K.; Huang, Q.; Nezhad, M.T.; Xu, M. Long-Term Fertilization Alters the Storage and Stability of Soil Organic Carbon in Chinese Paddy Soil. Agronomy 2023, 13, 1463. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, J.; Li, J.; Huang, C.-Y.; Shen, Y.; Cheng, W.; Zhu, B. A distinct sensitivity to the priming effect between labile and stable soil organic carbon. New Phytol. 2023, 237, 88–99. [Google Scholar] [CrossRef]
- Aguilera-Huertas, J.; Parras-Alcántara, L.; González-Rosado, M.; Lozano-García, B. What Influence Does Conventional Tillage Have on the Ability of Soils to Sequester Carbon, Stabilise It and Become Saturated in the Medium Term? A Case Study in a Traditional Rainfed Olive Grove. Sustainability 2022, 14, 7097. [Google Scholar] [CrossRef]
- Vityakon, P. Degradation and restoration of sandy soils under different agricultural land uses in northeast Thailand: A review. Land Degrad. Dev. 2007, 18, 567–577. [Google Scholar] [CrossRef]
- de Alencar, G.V.; Gomes, L.C.; Barros, V.M.d.S.; Ortiz Escobar, M.E.; de Oliveira, T.S.; Mendonça, E.d.S. Organic farming improves soil carbon pools and aggregation of sandy soils in the Brazilian semi-arid region. Soil Use Manag. 2024, 40, e13097. [Google Scholar] [CrossRef]
- Amoakwah, E.; Frimpong, K.A.; Arthur, E. Corn Cob Biochar Improves Aggregate Characteristics of a Tropical Sandy Loam. Soil Sci. Soc. Am. J. 2017, 81, 1054–1063. [Google Scholar] [CrossRef]
- Luan, H.; Gao, W.; Huang, S.; Tang, J.; Li, M.; Zhang, H.; Chen, X. Partial substitution of chemical fertilizer with organic amendments affects soil organic carbon composition and stability in a greenhouse vegetable production system. Soil Tillage Res. 2019, 191, 185–196. [Google Scholar] [CrossRef]
- Sedlář, O.; Balík, J.; Černý, J.; Kulhánek, M.; Smatanová, M. Long-Term Application of Organic Fertilizers in Relation to Soil Organic Matter Quality. Agronomy 2023, 13, 175. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Abrar, M.M.; Xu, H.; Aziz, T.; Sun, N.; Mustafa, A.; Aslam, M.W.; Shah, S.A.A.; Mehmood, K.; Zhou, B.; Ma, X.; et al. Carbon, nitrogen, and phosphorus stoichiometry mediate sensitivity of carbon stabilization mechanisms along with surface layers of a Mollisol after long-term fertilization in Northeast China. J. Soils Sediments 2021, 21, 705–723. [Google Scholar] [CrossRef]
- Qian, P.; Schoenau, J.J.; King, T.; Fatteicher, C. Effect of Soil Amendment with Alfalfa Powders and Distillers Grains on Nutrition and Growth of Canola. J. Plant Nutr. 2011, 34, 1403–1417. [Google Scholar] [CrossRef]
- He, L.; Wu, H.; Chen, W.; Meng, Q.; Zhou, Z. Influence of sulfur on the fermentation characteristics of corn distiller’s dried grains with solubles in in vitro culture. Czech J. Anim. Sci. 2017, 62, 417–425. [Google Scholar] [CrossRef]
- Boydston, R.A.; Collins, H.P.; Vaughn, S.F. Response of Weeds and Ornamental Plants to Potting Soil Amended with Dried Distillers Grains. HortScience 2008, 43, 191–195. [Google Scholar] [CrossRef]
- Nelson, K.; Motavalli, P.; Smoot, R. Utility of Dried Distillers Grain as a Fertilizer Source for Corn. J. Agric. Sci. 2009, 1, 1–3. [Google Scholar] [CrossRef]
- Kader, M.A.; Sleutel, S.; Begum, S.A.; D’Haene, K.; Jegajeevagan, K.; De Neve, S. Soil organic matter fractionation as a tool for predicting nitrogen mineralization in silty arable soils. Soil Use Manag. 2010, 26, 494–507. [Google Scholar] [CrossRef]
- Elrys, A.S.; Wang, J.; Metwally, M.A.S.; Cheng, Y.; Zhang, J.-B.; Cai, Z.-C.; Chang, S.X.; Müller, C. Global gross nitrification rates are dominantly driven by soil carbon-to-nitrogen stoichiometry and total nitrogen. Glob. Change Biol. 2021, 27, 6512–6524. [Google Scholar] [CrossRef]
- Iqbal, S.; Xu, J.; Arif, M.S.; Worthy, F.R.; Jones, D.L.; Khan, S.; Alharbi, S.A.; Filimonenko, E.; Nadir, S.; Bu, D.; et al. Do Added Microplastics, Native Soil Properties, and Prevailing Climatic Conditions Have Consequences for Carbon and Nitrogen Contents in Soil? A Global Data Synthesis of Pot and Greenhouse Studies. Environ. Sci. Technol. 2024, 58, 8464–8479. [Google Scholar] [CrossRef]
- Chen, Y.; Camps-Arbestain, M.; Shen, Q.; Singh, B.; Cayuela, M.L. The long-term role of organic amendments in building soil nutrient fertility: A meta-analysis and review. Nutr. Cycl. Agroecosyst. 2018, 111, 103–125. [Google Scholar] [CrossRef]
- Bimüller, C.; Mueller, C.W.; von Lützow, M.; Kreyling, O.; Kölbl, A.; Haug, S.; Schloter, M.; Kögel-Knabner, I. Decoupled carbon and nitrogen mineralization in soil particle size fractions of a forest topsoil. Soil Biol. Biochem. 2014, 78, 263–273. [Google Scholar] [CrossRef]
- Tian, Q.; Yang, X.; Wang, X.; Liao, C.; Li, Q.; Wang, M.; Wu, Y.; Liu, F. Microbial community mediated response of organic carbon mineralization to labile carbon and nitrogen addition in topsoil and subsoil. Biogeochemistry 2016, 128, 125–139. [Google Scholar] [CrossRef]
- Sorrenti, G.; Buriani, G.; Gaggìa, F.; Baffoni, L.; Spinelli, F.; Di Gioia, D.; Toselli, M. Soil CO2 emission partitioning, bacterial community profile and gene expression of Nitrosomonas spp. and Nitrobacter spp. of a sandy soil amended with biochar and compost. Appl. Soil Ecol. 2017, 112, 79–89. [Google Scholar] [CrossRef]
- Manopkawee, P.; Mankhemthong, N.; Pattarakamolsen, C. Tectonic and lithologic controls on the landscape adjustment along the eastern terrain of the Mae Tha fault, northern Thailand. Geologica Acta 2023, 21, 1–21. [Google Scholar] [CrossRef]
- Cao, H.-b.; Xie, J.-y.; Hong, J.; Wang, X.; Hu, W.; Hong, J.-p. Organic matter fractions within macroaggregates in response to long-term fertilization in calcareous soil after reclamation. J. Integr. Agric. 2021, 20, 1636–1648. [Google Scholar] [CrossRef]
- Rakthai, S.; Fu, P.-L.; Fan, Z.-X.; Gaire, N.P.; Pumijumnong, N.; Eiadthong, W.; Tangmitcharoen, S. Increased Drought Sensitivity Results in a Declining Tree Growth of Pinus latteri in Northeastern Thailand. Forests 2020, 11, 361. [Google Scholar] [CrossRef]
- Department, T.M. Monthly Weather Summary in Thailand, January 2024. Available online: https://www.tmd.go.th/en/climate/summarymonthly/012024 (accessed on 19 March 2025).
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; SSSA Book Series; Wiley: Hoboken, NJ, USA, 1996; pp. 961–1010. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Weil, R.; Stine, M.; Gruver, J.; Samson-Liebig, S. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Culman, S.; Snapp, S.; Schipanski, M.; Freeman, M.; Beniston, J.; Drinkwater, L.; Franzluebbers, A.; Glover, J.; Grandy, S.; Lal, R.; et al. Permanganate Oxidizable Carbon Reflects a Processed Soil Fraction that is Sensitive to Management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Chan, K.; Bowman, A.; Oates, A. Oxidizible Organic Carbon Fractions and Soil Quality Changes in An Oxic Paleustalf Under Different Pasture Leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Maia, S.M.F.; Xavier, F.A.S.; Oliveira, T.S.; Mendonça, E.S.; Araújo Filho, J.A. Organic carbon pools in a Luvisol under agroforestry and conventional farming systems in the semi-arid region of Ceará, Brazil. Agrofor. Syst. 2007, 71, 127–138. [Google Scholar] [CrossRef]
- Miller, A.P.; Arai, Y. Comparative Evaluation of Phosphate Spectrophotometric Methods in Soil Test Phosphorus Extracting Solutions. Soil Sci. Soc. Am. J. 2016, 80, 1543–1550. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic Forms. In Methods of Soil Analysis; Agronomy Monographs; Wiley: Hoboken, NJ, USA, 1982; pp. 643–698. [Google Scholar]
- Ohno, T.; Zibilske, L.M. Determination of Low Concentrations of Phosphorus in Soil Extracts Using Malachite Green. Soil Sci. Soc. Am. J. 1991, 55, 892–895. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall, Inc.: Englewood Cliffs, NJ, USA, 1964. [Google Scholar]
- Bouyoucos, G.J. A Recalibration of the Hydrometer Method for Making Mechanical Analysis of Soils. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; Agronomy Monographs; Wiley: Hoboken, NJ, USA, 1965; pp. 1149–1178. [Google Scholar]
- Hoffman, W.M. AOAC Methods for the Determination of Phosphorus in Fertilizers. J. Assoc. Off. Agric. Chem. 1964, 47, 420–428. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwáth, W.R. Spectrophotometric Determination of Nitrate with a Single Reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Verdouw, H.; Van Echteld, C.J.A.; Dekkers, E.M.J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Walkley, A. A Critical Examination of a Rapid Method for Determining Organic Carbon in Soils: Effect of Variations in Digestion Conditions and of Inorganic Soil Constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Hines, A.R.; Gray, K.; Muir, J.P.; Bellows, B.; Rouquette, F.; Smith, W.B. PSIII-19 Soil nutrient profile from cool-season forages fertilized with manure from steers supplemented with titrated levels of dried distillers grains. J. Anim. Sci. 2021, 99, 340–341. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.; Yu, S.; Gu, X.; Cai, J.; Zhang, X. Physicochemical characterization and pyrolysis kinetic analysis of Moutai-flavored dried distiller’s grains towards its thermochemical conversion for potential applications. J. Anal. Appl. Pyrolysis 2021, 155, 105046. [Google Scholar] [CrossRef]
- Das, R.; Purakayastha, T.J.; Das, D.; Ahmed, N.; Kumar, R.; Biswas, S.; Walia, S.S.; Singh, R.; Shukla, V.K.; Yadava, M.S.; et al. Long-term fertilization and manuring with different organics alter stability of carbon in colloidal organo-mineral fraction in soils of varying clay mineralogy. Sci. Total Environ. 2019, 684, 682–693. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Escanhoela, A.S.B.; Pitombo, L.M.; Brandani, C.B.; Navarrete, A.A.; Bento, C.B.; do Carmo, J.B. Organic management increases soil nitrogen but not carbon content in a tropical citrus orchard with pronounced N2O emissions. J. Environ. Manag. 2019, 234, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Ai, Z.; Wu, Y.; Xu, H.; Li, Q.; Xue, S.; Liu, G. 16-Year fertilization changes the dynamics of soil oxidizable organic carbon fractions and the stability of soil organic carbon in soybean-corn agroecosystem. Agric. Ecosyst. Environ. 2018, 265, 320–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, Y.; Huang, X.; Zhang, W.; Jiang, T. Responses of Soil Labile Organic Carbon and Carbon Management Index to Different Long-Term Fertilization Treatments in a Typical Yellow Soil Region. Eurasian Soil Sci. 2021, 54, 605–618. [Google Scholar] [CrossRef]
- Shi, L.H.; Li, C.; Tang, H.M.; Cheng, K.K.; Li, W.Y.; Wen, L.; Xiao, X.P. Effects of long-term fertilizer management on soil labile organic carbon fractions and hydrolytic enzyme activity under a double-cropping rice system of southern China. Ying Yong Sheng Tai Xue Bao 2021, 32, 921–930. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Kundu, S.; Srivastva, A.K.; Gupta, H.S.; Prakash, V.; Bhatt, J.C. Long term fertilization effects on soil organic carbon pools in a sandy loam soil of the Indian sub-Himalayas. Plant Soil 2011, 341, 109–124. [Google Scholar] [CrossRef]
- Karabcová, H.; Pospíšilová, L.; Mrak-Fiala, P.; Škarpa, P.; Bjelková, M. Effect of Organic Fertilizers on Soil Organic Carbon and Risk Trace Elements Content in Soil under Permanent Grassland. Soil Water Res. 2015, 10, 2015–2228. [Google Scholar] [CrossRef]
- Shi, J.; Song, M.; Yang, L.; Zhao, F.; Wu, J.; Li, J.; Yu, Z.; Li, A.; Shangguan, Z.; Deng, L. Recalcitrant organic carbon plays a key role in soil carbon sequestration along a long-term vegetation succession on the Loess Plateau. CATENA 2023, 233, 107528. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0211163. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mäder, P.; Brussaard, L.; de Goede, R. Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Rousk, J.; Hill, P.W.; Jones, D.L. Priming of the decomposition of ageing soil organic matter: Concentration dependence and microbial control. Funct. Ecol. 2015, 29, 285–296. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Shi, L.; Marshall, M.R.; Kuzyakov, Y.; Blagodatskaya, E.; Zang, H. Strong priming of soil organic matter induced by frequent input of labile carbon. Soil Biol. Biochem. 2021, 152, 108069. [Google Scholar] [CrossRef]
- Zhang, D.; Gong, C.; Zhang, W.; Zhang, H.; Zhang, J.; Song, C. Labile carbon addition alters soil organic carbon mineralization but not its temperature sensitivity in a freshwater marsh of Northeast China. Appl. Soil Ecol. 2021, 160, 103844. [Google Scholar] [CrossRef]
- Rousk, K.; Michelsen, A.; Rousk, J. Microbial control of soil organic matter mineralization responses to labile carbon in subarctic climate change treatments. Glob. Change Biol. 2016, 22, 4150–4161. [Google Scholar] [CrossRef]
- Wu, H.; Cai, A.; Xing, T.; Huai, S.; Zhu, P.; Xu, M.; Lu, C. Fertilization enhances mineralization of soil carbon and nitrogen pools by regulating the bacterial community and biomass. J. Soils Sediments 2021, 21, 1633–1643. [Google Scholar] [CrossRef]
- Liu, G.; Xie, M.; Zhang, S. Effect of organic fraction of municipal solid waste (OFMSW)-based biochar on organic carbon mineralization in a dry land soil. J. Mater. Cycles Waste Manag. 2017, 19, 473–482. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, W.; Hu, P.; Xiao, D.; Yang, R.; Ye, Y.; Wang, K. The formation of large macroaggregates induces soil organic carbon sequestration in short-term cropland restoration in a typical karst area. Sci. Total Environ. 2021, 801, 149588. [Google Scholar] [CrossRef]
- Mikhael, J.; Ferraz-Almeida, R.; Franco, F.; Camargo, R.; Wendling, B. Recalcitrant carbon and nitrogen in agriculture soils with residue accumulation and fertilization under tropical conditions. Biosci. J. 2019, 35, 732–740. [Google Scholar] [CrossRef]
- Ye, G.; Lin, Y.; Liu, D.; Chen, Z.; Luo, J.; Bolan, N.; Fan, J.; Ding, W. Long-term application of manure over plant residues mitigates acidification, builds soil organic carbon and shifts prokaryotic diversity in acidic Ultisols. Appl. Soil Ecol. 2019, 133, 24–33. [Google Scholar] [CrossRef]
- Ye, G.; Lin, Y.; Kuzyakov, Y.; Liu, D.; Luo, J.; Lindsey, S.; Wang, W.; Fan, J.; Ding, W. Manure over crop residues increases soil organic matter but decreases microbial necromass relative contribution in upland Ultisols: Results of a 27-year field experiment. Soil Biol. Biochem. 2019, 134, 15–24. [Google Scholar] [CrossRef]
- Huang, J.; Rinnan, Å.; Bruun, T.B.; Engedal, T.; Bruun, S. Identifying the fingerprint of permanganate oxidizable carbon as a measure of labile soil organic carbon using Fourier transform mid-infrared photoacoustic spectroscopy. Eur. J. Soil Sci. 2021, 72, 1831–1841. [Google Scholar] [CrossRef]
- Thapa, B.; Mowrer, J. Soil carbon and aggregate stability are positively related and increased under combined soil amendment, tillage, and cover cropping practices. Soil Sci. Soc. Am. J. 2024, 88, 730–744. [Google Scholar] [CrossRef]
- Dou, X.; He, P.; Cheng, X.; Zhou, W. Long-term fertilization alters chemically-separated soil organic carbon pools: Based on stable C isotope analyses. Sci. Rep. 2016, 6, 19061. [Google Scholar] [CrossRef]
- Yu, H.; Ding, W.; Chen, Z.; Zhang, H.; Luo, J.; Bolan, N. Accumulation of organic C components in soil and aggregates. Sci. Rep. 2015, 5, 13804. [Google Scholar] [CrossRef]
- Quan, Z.; Huang, B.; Lu, C.; Shi, Y.; Chen, X.; Zhang, H.; Fang, Y. The fate of fertilizer nitrogen in a high nitrate accumulated agricultural soil. Sci. Rep. 2016, 6, 21539. [Google Scholar] [CrossRef]
- Mousavi, H.; Cottis, T.; Solberg, S.Ø. Nitrogen Enriched Organic fertilizer (NEO) elevates nitrification rates shortly after application but has no lasting effect on nitrification in agricultural soils. Agric. Food Sci. 2023, 32, 179–194. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Yang, T.; Huang, F.; Fu, S.; Li, L. Changes in soil labile and recalcitrant carbon pools after land-use change in a semi-arid agro-pastoral ecotone in Central Asia. Ecol. Indic. 2020, 110, 105925. [Google Scholar] [CrossRef]
- Zang, H.; Mehmood, I.; Kuzyakov, Y.; Jia, R.; Gui, H.; Blagodatskaya, E.; Xu, X.; Smith, P.; Chen, H.; Zeng, Z.; et al. Not all soil carbon is created equal: Labile and stable pools under nitrogen input. Glob. Change Biol. 2024, 30, e17405. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Xu, Z.; Zhang, Y.; Wang, Y.Y. Responses of soil microbial biomass nitrogen to organic fertilizer with different degrees of maturity and regulation to soil mineral nitrogen. Ying Yong Sheng Tai Xue Bao 2023, 34, 137–144. [Google Scholar] [CrossRef]
- Ma, H.; Yin, Y.; Gao, R.; Taqi, R.; He, X. Response of nitrogen transformation to glucose additions in soils at two subtropical forest types subjected to simulated nitrogen deposition. J. Soils Sediments 2019, 19, 2166–2175. [Google Scholar] [CrossRef]
- Strauss, E.A.; Lamberti, G.A. Regulation of nitrification in aquatic sediments by organic carbon. Limnol. Oceanogr. 2000, 45, 1854–1859. [Google Scholar] [CrossRef]
- Fanin, N.; Alavoine, G.; Bertrand, I. Temporal dynamics of litter quality, soil properties and microbial strategies as main drivers of the priming effect. Geoderma 2020, 377, 114576. [Google Scholar] [CrossRef]
- Jegajeevagan, K.; Sleutel, S.; Ameloot, N.; Kader, M.A.; De Neve, S. Organic matter fractions and N mineralization in vegetable-cropped sandy soils. Soil Use Manag. 2013, 29, 333–343. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, M.; Chen, X.; Wang, J.; Zhang, Y.; Xiao, X.; Hu, C.; Liu, J.; Zhang, R.; Xu, D.; et al. Nitrifiers drive successions of particulate organic matter and microbial community composition in a starved macrocosm. Environ. Int. 2021, 157, 106776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Q.; Qin, Y.; Li, Z.-Z.; Song, Z.-Z. Mixed application of biochar, maize straw, and nitrogen can improve organic carbon fractions and available nutrients of a sandy soil. Arid Land Res. Manag. 2023, 37, 115–133. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi Binu, M.; Chu, H. Salinity Is a Key Determinant for Soil Microbial Communities in a Desert Ecosystem. mSystems 2019, 4, e00225-18. [Google Scholar] [CrossRef]
- Aumtong, S.; Foungyen, P.; Kanchai, K.; Chuephudee, T.; Chotamonsak, C.; Lapyai, D. Impact of Reduced Nitrogen Inputs on Soil Organic Carbon and Nutrient Dynamics in Arable Soil, Northern Thailand: Short-Term Evaluation. Agronomy 2024, 14, 2587. [Google Scholar] [CrossRef]
- Mehnaz, K.R.; Corneo, P.E.; Keitel, C.; Dijkstra, F.A. Carbon and phosphorus addition effects on microbial carbon use efficiency, soil organic matter priming, gross nitrogen mineralization and nitrous oxide emission from soil. Soil Biol. Biochem. 2019, 134, 175–186. [Google Scholar] [CrossRef]
- Cheng, Y.; Elrys, A.S.; Merwad, A.-R.M.; Zhang, H.; Chen, Z.; Zhang, J.; Cai, Z.; Müller, C. Global Patterns and Drivers of Soil Dissimilatory Nitrate Reduction to Ammonium. Environ. Sci. Technol. 2022, 56, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhao, H.; Wang, Y.; Butterly, C.R.; Chen, H.; Yuan, J.; Liu, M.; Chen, Q.; Zhang, L.; Wang, L. Optimal organic fertilization enhances the phytoavailability of phosphorus in the root zone of rice. Eur. J. Soil Sci. 2024, 75, e13588. [Google Scholar] [CrossRef]
- Mengmeng, C.; Shirong, Z.; Lipeng, W.; Chao, F.; Xiaodong, D. Organic Fertilization Improves the Availability and Adsorptive Capacity of Phosphorus in Saline-Alkaline Soils. J. Soil Sci. Plant Nutr. 2021, 21, 487–496. [Google Scholar] [CrossRef]
- Khan, K.; Ali, M.M.; Naveed, M.; Rehmani, M.I.A.; Shafique, M.; Ali, H.; Abdelsalam, N.; Ghareeb, Y.; Feng, G. Co-application of organic amendments and inorganic P increase maize growth and soil carbon, phosphorus availability in calcareous soil. Front. Environ. Sci. 2022, 10, 949371. [Google Scholar] [CrossRef]
- Guera, K.C.S.; da Fonseca, A.F. Phosphorus fractions and their relationships with soil chemical attributes in an integrated crop-livestock system under annual phosphates fertilization. Front. Sustain. Food Syst. 2022, 6, 1–22. [Google Scholar] [CrossRef]
- Vermeiren, C.; Kerckhof, P.; Reheul, D.; Smolders, E. Increasing soil organic carbon content can enhance the long-term availability of phosphorus in agricultural soils. Eur. J. Soil Sci. 2022, 73, e13191. [Google Scholar] [CrossRef]
- Shu, X.; Liu, W.; Huang, H.; Ye, Q.; Zhu, S.; Peng, Z.; Li, Y.; Deng, L.; Yang, Z.; Chen, H.; et al. Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems. Plants 2023, 12, 3801. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kuzyakov, Y. Soil organic matter priming: The pH effects. Glob. Change Biol. 2024, 30, e17349. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Yang, X.; Cui, J.; Sui, P. The effect of different organic materials amendment on soil bacteria communities in barren sandy loam soil. Environ. Sci. Pollut. Res. 2017, 24, 24019–24028. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Yuan, D.; Li, W.; He, W.; Raza, S.; Kuzyakov, Y.; Zamanian, K.; Zhao, X. Soil Chemical Properties Depending on Fertilization and Management in China: A Meta-Analysis. Agronomy 2022, 12, 2501. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Zheng, S.-R.; Yang, M.-M.; Wan, J.-M.; Huang, Y.; Yu, K.; Tong, X.-G. Stability characteristics of soil organic carbon pool following development of sand-fixing forest in Mu Us sandy land, China. Chin. J. Appl. Ecol. 2019, 30, 2567–2574. [Google Scholar] [CrossRef]
- Sosulski, T.; Srivastava, A.K.; Ahrends, H.E.; Smreczak, B.; Szymańska, M. Carbon Storage Potential and Carbon Dioxide Emissions from Mineral-Fertilized and Manured Soil. Appl. Sci. 2023, 13, 4620. [Google Scholar] [CrossRef]
- Gross, A.; Glaser, B. Meta-analysis on how manure application changes soil organic carbon storage. Sci. Rep. 2021, 11, 5516. [Google Scholar] [CrossRef]
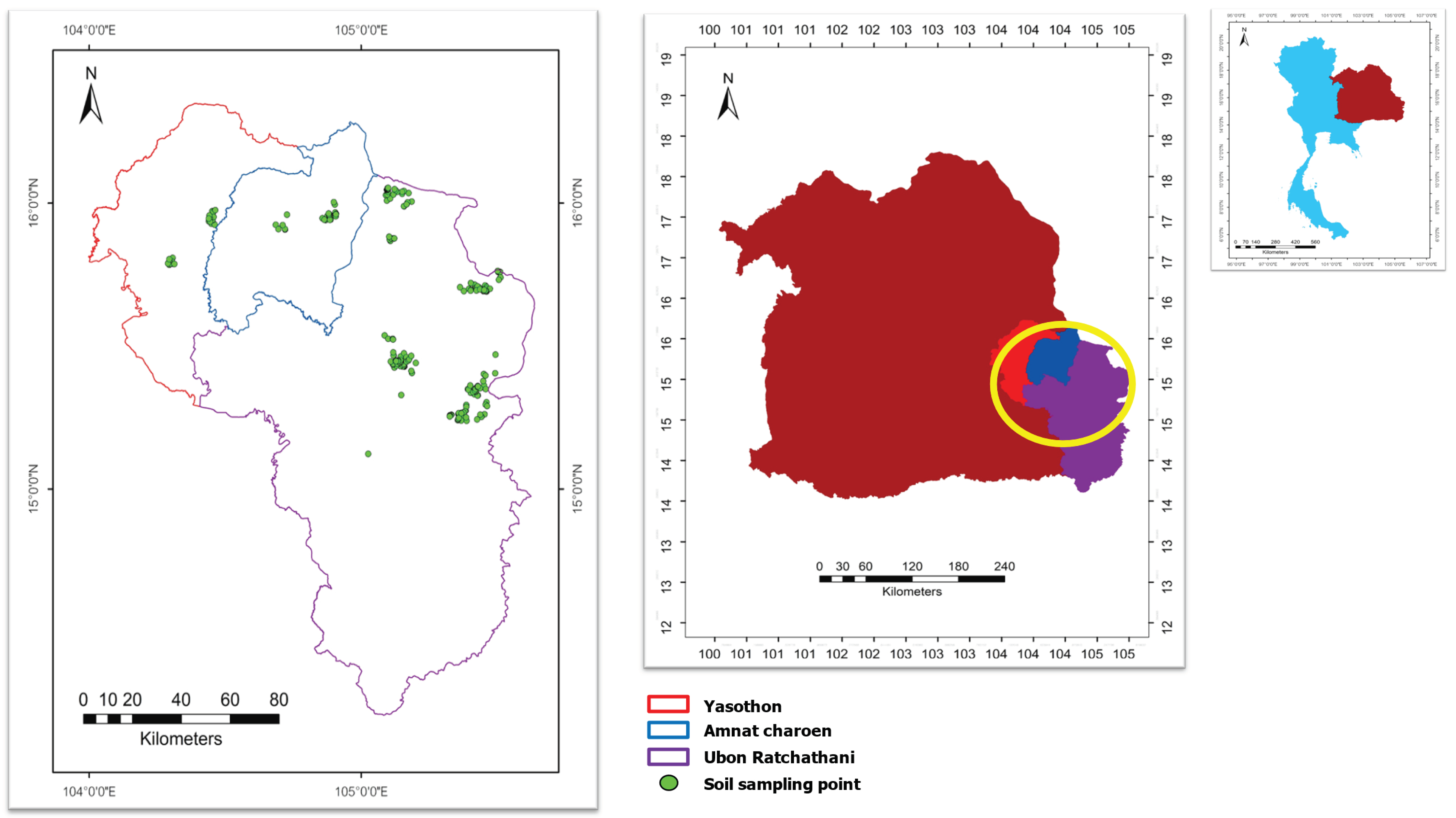


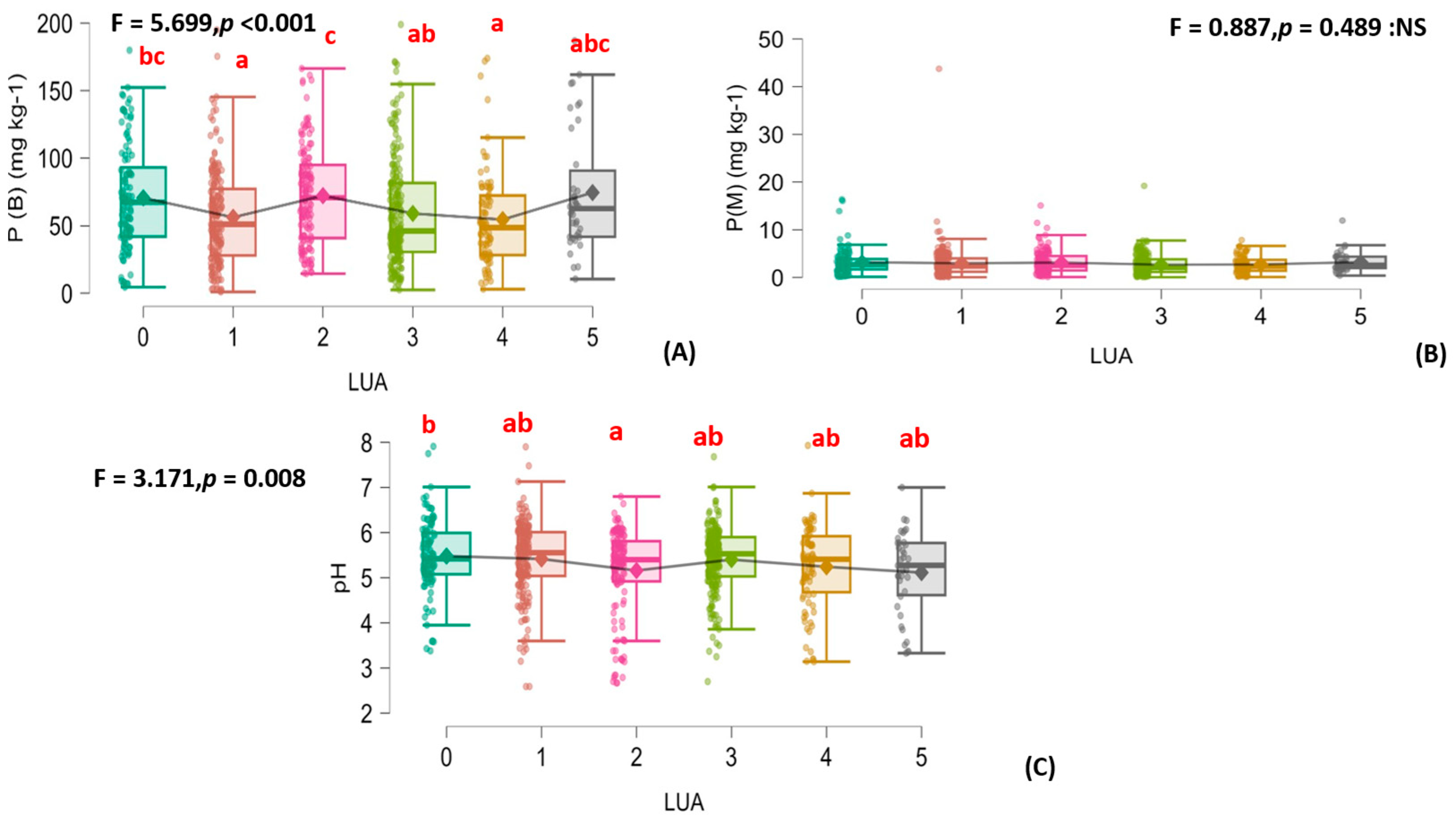
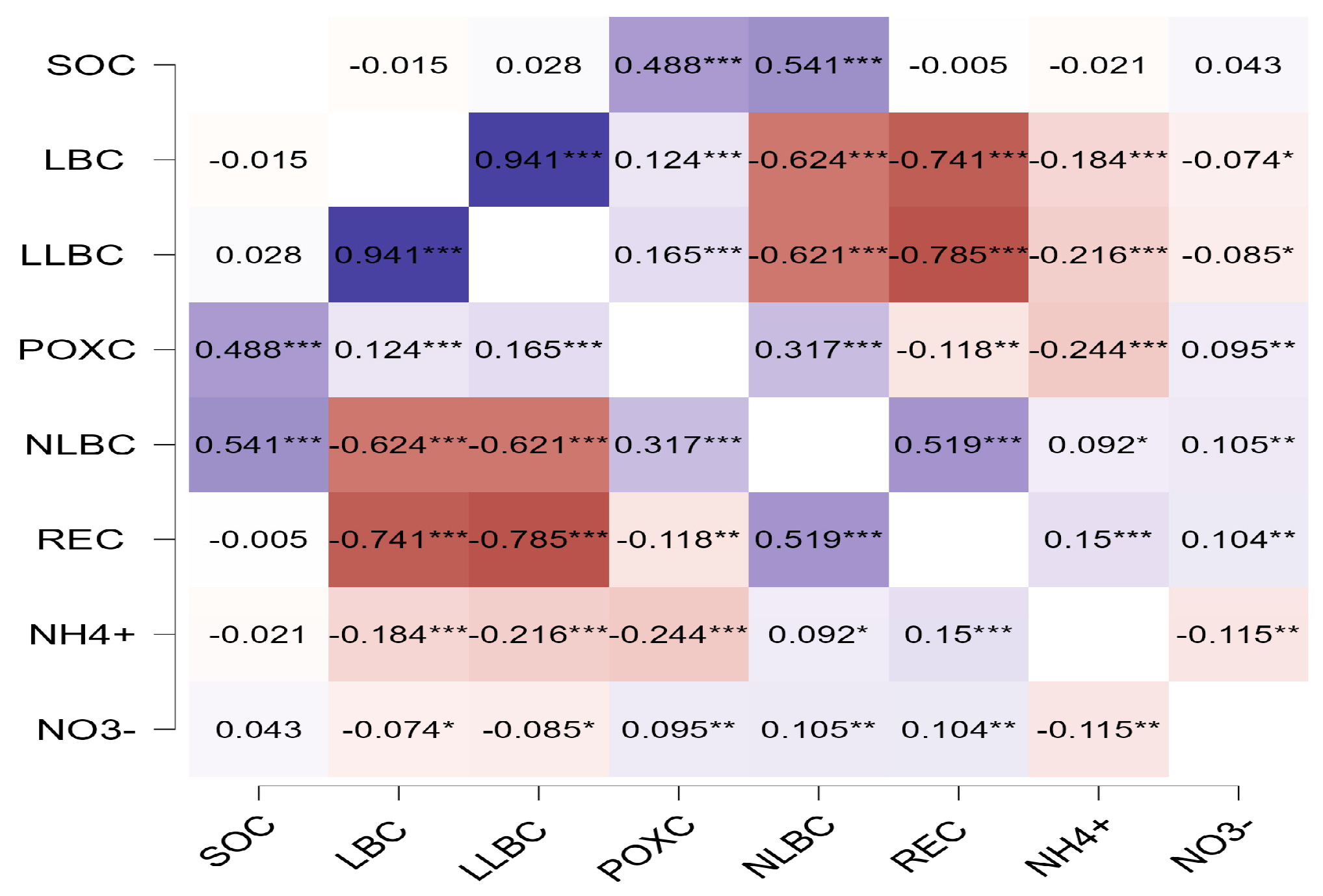
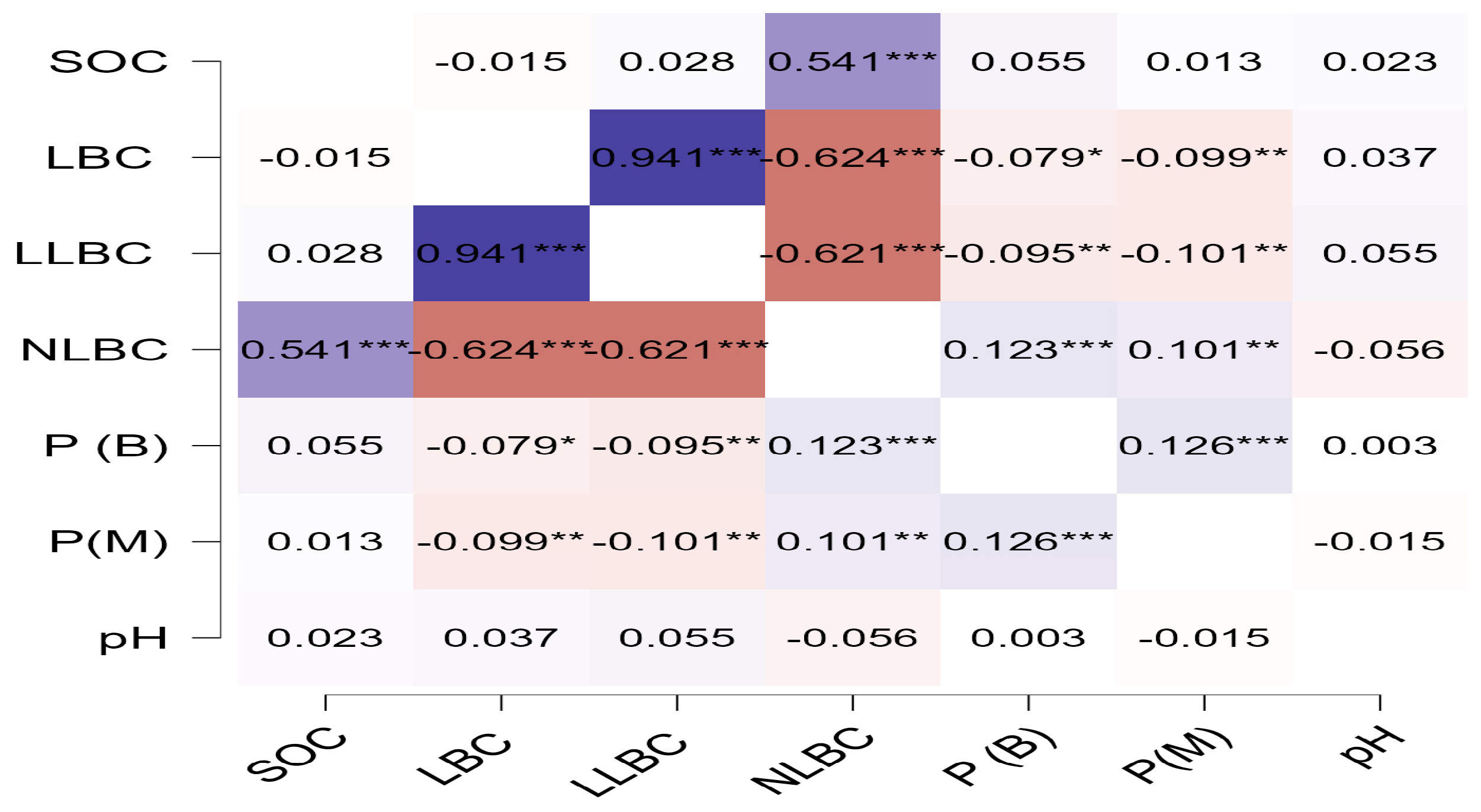

| Land-Use Age (Year) | Information on the Combinations Used in Organic Fertilizer Management |
|---|---|
| LUA5 | These soils only received DDGs, varying, on average, 811, 681, 594, 4420, and 4351 kg ha−1 year−1 from 2018 to 2022. |
| LUA4 | These soils received an average combination of cow manure, chicken manure, and DDGs (i.e., 1389, 3125, and 4410 kg ha−1 year−1, respectively) from 2019 to 2022. |
| LUA3 | These soils received an average combination of chicken manure and DDGs (i.e., 2500 and 625–833 kg ha−1 year−1, respectively) from 2020 to 2022. |
| LUA2 | These soils received an average combination of cow manure, chicken manure, and DDGs (i.e., 13,021, 2600, and 572 kg ha−1 year−1, respectively) from 2021 to 2022. |
| LUA1 | These soils received an average combination of chicken manure and DDGs (i.e., 888 and 636 kg ha−1 year−1, respectively) in 2022. |
| LUA0 | The soil and land underwent pre-organic fertilization and entered a period of transition. |
| Indicator | Amendments. | Number of Samples | Median | Mean | F-Value | p-Value | Std. Deviation | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|---|
| pH | Chicken | 10 | 7.25 | 7.14 a | 4.39 | 0.037 | 0.42 | 6.49 | 7.63 |
| pH | Cow | 2 | 7.91 | 7.91 ab | 0.09 | 7.84 | 7.98 | ||
| pH | DDF | 3 | 8.5 | 8.11 b | 1.01 | 6.97 | 8.87 | ||
| C (%) | Chicken | 10 | 30.50 | 29.80 b | 40.20 | <0.001 | 4.80 | 19.00 | 36.00 |
| C (%) | Cow | 2 | 10.50 | 10.50 a | 7.77 | 5.00 | 16.00 | ||
| C (%) | DDF | 3 | 4.39 | 4.35 a | 0.86 | 3.48 | 5.20 | ||
| N (%) | Chicken | 10 | 3.38 | 3.49 b | 17.17 | <0.001 | 1.12 | 1.73 | 5.10 |
| N (%) | Cow | 2 | 0.36 | 0.37 a | 0.05 | 0.34 | 0.41 | ||
| N (%) | DDF | 3 | 0.31 | 0.37 a | 0.12 | 0.30 | 0.51 | ||
| C/N ratio | Chicken | 10 | 8.85 | 9.05 a | 10.82 | 0.002 | 1.89 | 6.5 | 11.5 |
| C/N ratio | Cow | 2 | 27.90 | 27.90 b | 16.97 | 15.9 | 39.9 | ||
| C/N ratio | DDF | 3 | 11.60 | 11.98 a | 2.01 | 10.19 | 14.16 | ||
| P (%) | Chicken | 10 | 1.21 | 1.22 b | 70.02 | <0.001 | 0.18 | 0.97 | 1.56 |
| P (%) | Cow | 2 | 0.105 | 0.11 a | 0.04 | 0.08 | 0.13 | ||
| P (%) | DDF | 3 | 0.16 | 0.20 a | 0.08 | 0.15 | 0.3 | ||
| K (%) | Chicken | 10 | 3.71 | 3.82 ns | 3.82 | 0.052 | 1.43 | 2.22 | 6.98 |
| K (%) | Cow | 2 | 0.39 | 0.39 ns | 0.23 | 0.23 | 0.55 | ||
| K (%) | DDF | 3 | 0.58 | 2.13 ns | 2.93 | 0.30 | 5.50 |
| Indicator | Organic Amendments | Number of Sample | Mean | F-Value | p-Value | SD | SE | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|---|
| soluble C (mg kg−1) | Chicken | 4 | 613.13 b | 12,134.89 | <0.001 | 9.44 | 4.72 | 600.00 | 622.50 |
| Cow | 4 | 16.88 a | 3.75 | 1.88 | 15.00 | 22.50 | |||
| DDG | 4 | 16.88 a | 3.75 | 1.88 | 15.00 | 22.50 | |||
| H2PO4 (mg kg−1) | Chicken | 4 | 50.04 c | 12,425.43 | <0.001 | 0.74 | 0.37 | 49.18 | 50.97 |
| Cow | 4 | 7.69 b | 0.20 | 0.10 | 7.45 | 7.93 | |||
| DDG | 4 | 5.38 a | 0.16 | 0.08 | 5.18 | 5.56 | |||
| NH4+ (mg kg−1) | Chicken | 4 | 25.93 a | 29.51 | <0.001 | 7.75 | 3.87 | 16.30 | 35.20 |
| Cow | 4 | 61.25 b | 10.00 | 5.00 | 48.60 | 71.00 | |||
| DDG | 4 | 25.83 a | 3.10 | 1.55 | 21.40 | 28.50 | |||
| NO3− (mg kg−1) | Chicken | 4 | 338.10 ns | 0.84 | 0.461 | 19.03 | 9.52 | 321.29 | 364.87 |
| Cow | 4 | 351.18 ns | 9.27 | 4.63 | 338.72 | 361.14 | |||
| DDG | 4 | 338.10 ns | 19.03 | 9.52 | 321.29 | 364.87 | |||
| pH | Chicken | 4 | 7.06 ns | 0.68 | 0.528 | 0.02 | 0.01 | 7.03 | 7.08 |
| Cow | 4 | 7.16 ns | 0.23 | 0.11 | 7.03 | 7.50 | |||
| DDG | 4 | 7.08 ns | 0.03 | 0.01 | 7.05 | 7.11 |
| Particle Size (g kg−1) | LUA | Valid | Mean | F-Value | p-Value | Std. Error | Std. Deviation | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|---|
| Sand | 0 | 108 | 821.6 a | 6.576 | <0.001 | 6.1 | 63.4 | 661.6 | 921.6 |
| Sand | 1 | 192 | 836.7 ab | 5.3 | 72.9 | 661.6 | 961.6 | ||
| Sand | 2 | 135 | 817.9 a | 5.5 | 63.6 | 681.6 | 921.6 | ||
| Sand | 3 | 197 | 845.9 b | 3.9 | 55.4 | 681.6 | 981.6 | ||
| Sand | 4 | 83 | 858.7 b | 5.9 | 54.1 | 741.6 | 961.6 | ||
| Sand | 5 | 38 | 845.4 ab | 10.4 | 64.2 | 721.6 | 941.6 | ||
| Silt | 0 | 108 | 98.9 ab | F = 3.690 | p = 0.003 | 5.4 | 55.8 | 5.6 | 245.6 |
| Silt | 1 | 192 | 97.7 a | 4.2 | 57.7 | 25.6 | 245.6 | ||
| Silt | 2 | 135 | 105.3 b | 4.4 | 50.8 | 25.6 | 225.6 | ||
| Silt | 3 | 197 | 90.0 ab | 3.3 | 45.9 | 5.6 | 205.6 | ||
| Silt | 4 | 83 | 78.4 a | 6.0 | 55.0 | 5.6 | 205.6 | ||
| Silt | 5 | 38 | 81.9 ab | 8.3 | 51.3 | 25.6 | 185.6 | ||
| Clay | 0 | 108 | 79.5 b | F = 3.422 | p = 0.004 | 4.5 | 46.9 | 12.8 | 172.8 |
| Clay | 1 | 192 | 65.6 ab | 3.2 | 44.0 | 12.8 | 192.8 | ||
| Clay | 2 | 135 | 76.8 ab | 3.5 | 40.7 | 12.8 | 152.8 | ||
| Clay | 3 | 197 | 64.2 a | 2.8 | 38.9 | 12.8 | 132.8 | ||
| Clay | 4 | 83 | 62.9 a | 3.6 | 32.8 | 12.8 | 132.8 | ||
| Clay | 5 | 38 | 72.8 ab | 6.7 | 41.1 | 12.8 | 132.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aumtong, S.; Somyo, C.; Kanchai, K.; Chuephudee, T.; Chotamonsak, C. Relationships Between Carbon Fractions and Soil Nutrients in Organic Cassava Cultivation in the Sandy Soil of Northeastern Thailand. Agronomy 2025, 15, 1069. https://doi.org/10.3390/agronomy15051069
Aumtong S, Somyo C, Kanchai K, Chuephudee T, Chotamonsak C. Relationships Between Carbon Fractions and Soil Nutrients in Organic Cassava Cultivation in the Sandy Soil of Northeastern Thailand. Agronomy. 2025; 15(5):1069. https://doi.org/10.3390/agronomy15051069
Chicago/Turabian StyleAumtong, Suphathida, Chanitra Somyo, Kanokorn Kanchai, Thoranin Chuephudee, and Chakrit Chotamonsak. 2025. "Relationships Between Carbon Fractions and Soil Nutrients in Organic Cassava Cultivation in the Sandy Soil of Northeastern Thailand" Agronomy 15, no. 5: 1069. https://doi.org/10.3390/agronomy15051069
APA StyleAumtong, S., Somyo, C., Kanchai, K., Chuephudee, T., & Chotamonsak, C. (2025). Relationships Between Carbon Fractions and Soil Nutrients in Organic Cassava Cultivation in the Sandy Soil of Northeastern Thailand. Agronomy, 15(5), 1069. https://doi.org/10.3390/agronomy15051069








