Abstract
Years of employing the “one-shot” fertilization practice have led to low nutrient utilization efficiency and the degradation of soil structure in the black soil region during crop cultivation. Replacing a portion of chemical fertilizers with microbial agents can effectively solve these issues. In this study, we conducted a field plot experiment comparing eight different treatment combinations to investigate the effects of combining microbial agents with varying amounts of chemical fertilizers on black soil nutrients, soil ecology, and maize yield. The high-throughput absolute quantification 16S rRNA sequencing method was utilized to further investigate the effect of the various treatments on soil bacterial community structure and elucidate the interactions between environmental factors and microbial communities. The results showed that MC80 increased maize yield by 5.76% compared to RC, with an input–output ratio of 1:1.58. Additionally, soil nutrient levels in MC80 were higher than those in RC, increasing nutrient utilization efficiency, activating soil nutrients, and enhancing soil fertility. Meanwhile, the absolute quantification data of bacteria also indicated the highest bacterial abundance and diversity in MC80 samples. Among these, Acidobacteria was the main contributor to the changes in the bacterial community, showing significant enrichment in MC80. RDA and Spearman correlation analyses indicated that soil nutrients are the key factors influencing the bacterial community in this ecosystem, while the microbial community plays a crucial role in nutrient transformation processes. Principal component analysis (PCA) was used for comprehensive evaluation and ranking. Overall, the soil under the MC80 treatment was most conducive to microbial survival and maize growth. This study provides a high-yield and sustainable fertilization method for maize and offers a theoretical basis for applying microbial agents in sustainable agriculture.
1. Introduction
Northeast China is a significant grain production base, mainly because there is a “black soil region”. Black soil is the most precious and scarce soil resource in the world and the most suitable land for cultivation. The thickness of the black soil layer can reach 30~100 cm, and the content of organic matter and minerals is as high as 5~7%, which has a high utilization value [1]. Gu et al. evaluated the soil productivity in the black soil subregion of Northeast China, assigning an average index of 0.62, wherein more than half of the soil was rated at a high or the highest level of productivity [2]. In order to meet the needs of the consumer market and the growing world population, increasing crop yields depends on many unreasonable agricultural practices [3,4]. The behaviors, including intensive farming, years of continuous cropping, and excessive application of chemical fertilizers, will reduce the fertility of black soil [5]. Notably, excessive use of chemical fertilizers stands out as the most pervasive issue disrupting soil’s physical and chemical processes [6,7], altering the microbial community structure of microorganisms, and posing significant environmental threats to agricultural ecosystems [8]. Therefore, it is an urgent need to safeguard the fertility of black soil in Northeast China without delay.
The most commonly used approach to address this issue is to optimize integrated nutrient management. By substituting a portion of chemical fertilizers with microbial fertilizers, it is possible to maintain both yield and quality while reducing the amount of chemical fertilizers applied [9]. As a new type of soil amendment and biological fertilizer, microbial agents have been widely considered because of their advantages of minor limitation, good effect, and no pollution [4,10]. Previous studies have shown that applying plant growth-promoting microorganisms (PGPMs) can alleviate the inefficiency of synthetic fertilizers. Compared with the complete fertilization control (600 kg ha−1 compound fertilizer (N:P:K = 15:15:15) and 300 kg ha−1 Ca(NO3)2 fertilizer), PGPMs can improve nutrient use efficiency and plant growth while reducing fertilizer input by up to 50% without causing yield loss [11]. Win’s research shows that after inoculation with Bacillus pumilus strain TUAT-1, rice’s tiller biomass, yield, and nitrogen content were significantly increased [12].
Subterranean biota is a key regulator of plant growth and is very sensitive to changes in management patterns [13,14]. They dominate soil life and perform a range of important soil functions by regulating nutrient cycling [15], decomposing organic matter [12], determining soil structure, inhibiting plant diseases, and supporting plant productivity [16,17]. Studies have shown that long-term organic fertilization and fallow management can improve soil quality and maintain bacterial diversity. In particular, in combination with chemical fertilizers, it supports eutrophic ecosystems [7,18,19]. Microbial diversity is affected by various biotic and abiotic factors. For example, many studies have found that bacterial diversity is closely related to soil pH [20] and organic matter content [21]. So, microbial community structure and diversity can be used as soil health indicators. So far, studies have focused on how microbial communities respond to environmental changes [22]. However, there are relatively few studies on the relationship between bacterial community and soil nutrients and crop yield.
In recent years, high-throughput sequencing technology has greatly expanded our understanding of soil microbial communities in different ecosystems [23]. Relative abundance describes the composition of different taxa in a single community [24], while absolute abundance not only describes the characteristics of specific taxa in different samples but also calibrates the total abundance [25,26]. In this study, the absolute quantitative technique was used to study the changes in bacterial community in soil under different proportions of microbial agents and chemical fertilizers. To be more suitable for the actual production in the field, we chose maize (Zea mays L.) as the experimental crop because maize is one of the most important food crops in China and one of the most common crops in the black soil area of Northeast China. The microbial agent (Ft) commonly used by local farmers was selected.
This study aims to clarify the effect of chemical fertilizer reduction combined with microbial inoculants on the bacterial community structure of crop rhizosphere soil and to find the best ratio of chemical fertilizer to microbial inoculants to improve soil fertility and crop yield. We hypothesize that adding microbial agents at different fertilization levels will significantly alter the bacterial community structure, and different bacterial community structures will have different responses to soil physical and chemical properties. To achieve the goal, the following problems need to be solved: (1) To elucidate the interactions between bacterial communities and environmental factors; (2) to elucidate the interactions between bacterial communities and environmental factors; (3) to explicitly identify the changes in crop yield and economic benefits that accompany these interactions.
2. Materials and Methods
2.1. Field Experiment Site and Design
The field experiment was set up at Lishu County, Jilin Province, China (43°17′5″ N, 124°25′54″ E, Figure S1). The area enjoys a temperate continental monsoon climate and distinct seasons, with a mean annual rainfall of 572 mm, mean annual temperature of 5.8 °C, and mean annual sunshine of 2644.2 h. The main soil chemical properties are shown in Table S1.
The microbial agent used in this study is Ft, which is a liquid microbial agent independently developed by the research group. The dominant strain was Bacillus subtilis, with an effective viable count ≥ 2.0 × 109 g. The application rate of 20 kg/hm2 for the biofertilizer in this experiment was determined by integrating application rates reported in the existing literature with recommended doses from commercial product guidelines [27]. The inorganic fertilizer tested was a special compound fertilizer (N:P:K = 26:10:12) for maize used by local farmers. The tested maize variety was Jinongyu 1185.
The experiment was designed for 8 treatments: Regional control—Apply 100% compound fertilizer (RC); 90% compound fertilizer (C90); 80% compound fertilizer (C80); 70% compound fertilizer (C70); 100% compound fertilizer + 20 kg hm−2 inoculant (MRC); 90% compound fertilizer + 20 kg hm−2 inoculant (MC90); 80% compound fertilizer + 20 kg hm−2 inoculant (MC80); 70% compound fertilizer + 20 kg hm−2 inoculant (MC70). The RC was set as the nutrient standard by applying 900 kg hm−2 of compound fertilizer to the field. Each treatment had three replicates (plots) randomly arranged in the field. Each plot has three ridges, and one ridge has two rows. Each plot was 78 m2 (20 m × 3.9 m). Before sowing, inorganic fertilizer was applied once, and microbial agents were sprayed once at the four-leaf stage of maize [28,29]. Soil sampling was conducted, and maize yield was quantified on 15 October 2023.
2.2. Sample Preparation and Analyses
The soil samples were collected at the maturity stage of maize, and then the yield was measured. Non-rhizosphere soil samples were collected by a five-point sampling method and dried naturally after being brought back to the laboratory. The rhizosphere soil was collected by the shaking root method, put into the incubator, brought back to the laboratory, and put into the −80 °C refrigerator.
Soil nutrient measurements, including available N, available P, available K, and organic matter, were determined for the field, as mentioned in the book by Bao [30]. The activities of soil catalase, phosphatase, urease, and sucrase were measured using potassium permanganate titration, disodium phenyl phosphate colorimetry, phenol-sodium hypochlorite colorimetry, and 3,5-dinitrosalicylic acid colorimetry, respectively [31,32]. Additionally, soil pH was detected in a soil-water slurry (1:2.5, w/v).
Grain yield data were obtained from the central two rows. The grain yield (kg ha−1) was calculated using the following formula [33]:
2.3. Advanced Absolute Quantification 16S-Seq
To observe the microbiome shifts of soils before and after microbial agent application, a total of 24 rhizosphere soil samples collected from the eight treatments (RC, C90, C80, C70, MRC, MC90, MC80, MC70) were sent to G&C Biotechnology Co., Ltd., Shanghai, 201315 (China), for absolute quantification 16S rRNA amplicon sequencing by Miseq. The total genomic DNA was extracted using the FastDNA® SPIN Kit for Soil DNA Extraction (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s instructions. The integrity of genomic DNA was detected through agarose gel electrophoresis, and the concentration and purity of genomic DNA were detected via Qubit 3.0 Spectrophotometer (Purchased from Thermo Fisher Scientific, Waltham, MA, USA). The V3-V4 hypervariable regions of the 16S rRNA gene and spike-ins were amplified with the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) [34]. The PCR amplification reaction was performed in triplicate in a total volume of 10 μL. The reaction mixture consisted of 1 μL of 10 × Toptaq Buffer, 0.2 μL of Toptaq DNA Polymerase, 0.2 μL of each primer (10 μM), and 3 μL of template DNA. Microbial diversity metrics were obtained on 19 June 2024.
2.4. Statistical Analyses
2.4.1. Alpha Diversity
The means and standard deviations of the data were calculated and statistically examined by one-way analysis of variance (ANOVA) and multiple comparison analysis using IBM SPSS 21.0 (IBM Corporation, New York, NY, USA). The heatmap, redundancy analysis (RDA), and average linkage hierarchical clustering were performed by R (version 4.3.2). The significance level was set at p < 0.05 unless otherwise stated.
The calculation formula of the community richness index:
Chao1:
Sobs: The actual number of OTUs measured. : The number of OTUs containing only one sequence. : The number of OTUs containing only two sequences.
The calculation formula of the community diversity index:
Shannon:
Simpson:
Sobs: The actual number of OTUs measured. ni: The number of sequences contained in the i-th OTU. N: The number of all sequences.
2.4.2. PLS-PM (Partial Least Squares Path Model) Analysis
A partial least squares path model (PLS-PM) was constructed using the “plspm” R package to elucidate the potential direct and indirect effects of chemical fertilizers, inoculants, soil microbes, soil enzymes, and available nutrients on crop yield. The quality of the PLS-PM was evaluated by examining the goodness of fit (GOF) index, with >0.7 indicating a good overall prediction performance of the model, and by examining the coefficients of determination (R2) of the latent variables. The significance level was set at p < 0.05 for all statistical analyses unless stated otherwise. All data were presented as the means and standard errors [35].
2.4.3. Principal Component Analysis
First, the data underwent KMO and Bartlett’s sphericity tests to determine their suitability for principal component analysis (PCA). Following confirmation of suitability, PCA was applied to calculate the communalities of various soil indicators [36]. Subsequently, the proportion of each communality to the total communality sum was calculated, which served as the weight coefficient for the evaluation indicators [37,38].
The comprehensive soil fertility score S was calculated using the following formula:
where is the weight coefficient for the -th indicator, is the principal component score for the -th indicator, and is the number of principal components considered.
2.4.4. Difference Analysis
Using SPSS 26, a one-way ANOVA was performed on the experimental data, and the LSD (Least Significant Difference) method was employed to analyze the significance of differences between treatments (p < 0.05).
3. Results
3.1. Soil Physicochemical Properties
The soil samples from different treatments were tested for nutrient content, and the results are presented in Figure 1. With the same fertilization, treatments with microbial agents had higher available nutrient levels than those without. The AN, AP, and AK contents in the treatment combining microbial agents with 100% chemical fertilizers (MRC) were significantly higher (p < 0.05) than those in other treatments, increasing by 35.00%, 18.53%, and 14.33%, respectively, compared to the control (RC). For treatments with reduced fertilizer levels (MC90, MC80, MC70), the available nutrients decreased with the reduction in fertilizer application, with MC70 showing values similar to RC. Specifically, the available nitrogen in MC70 was 5.43% higher than in RC, while available phosphorus and potassium were only 2.12% and 2.15% lower than in RC, respectively, which were not statistically significant differences. The organic matter content across different treatments showed minimal variation. However, the four treatments involving microbial inoculants’ application exhibited significantly higher organic matter content than those without inoculants. This indicates that applying microbial inoculants can increase soil nutrients and organic matter, potentially substituting for a portion of chemical fertilizers. Interestingly, the C90 treatment represents a unique case, as its readily available nutrient content was noticeably lower than other treatments. The outcome could be due to the environment, with a 10% reduction in chemical fertilizers being more conducive to the growth of plants and microorganisms, leading to an increased nutrient uptake rate and, consequently, fewer residual nutrients in the soil. This hypothesis warrants further validation through the analysis of bacterial communities to confirm the interactions between reduced fertilizer use and microbial activity in soil.
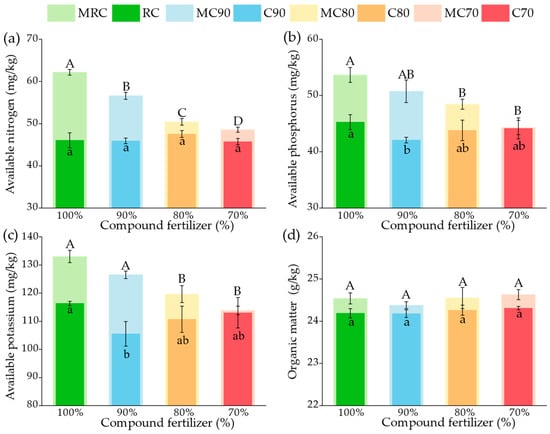
Figure 1.
Effects of different treatments on soil chemical properties: (a) available nitrogen, (b) available phosphorus, (c) available potassium, and (d) organic matter. Uppercase letters denote significant differences between fertilizer application rates under inoculant-treated conditions, while lowercase letters indicate significant differences between fertilizer application rates under non-inoculant conditions, ANOVA, p < 0.05.
3.2. Absolute Quantification of Soil Microbial Community
Quality control was performed on the original ASVs obtained from all samples. After filtration, noise reduction, splicing, and chimera removal, a total of 6,726,992 reads were retained from 24 soil samples for bacterial community analysis. Spike-in reads accounted for 18.63 ± 3.22% (ranging from 13.20% to 24.70%) of total reads in a given library. Finally, the number of ASVs annotated per sample varied from 2489 to 3316 and were further classified by the RDP database. Among them, MC70 has the highest values, indicating the highest species diversity. The Venn diagram of the generated ASVs in Figure S2 demonstrated the core microbiome, showing that there were still 1031 ASVs shared across all samples. With the same fertilization, ASVs were more abundant in treatments with microbial agents than in those without. This suggests that the application of microbial agents not only avoided antagonistic interactions with indigenous microorganisms but also effectively increased the soil bacterial population.
To determine the types and quantities of bacteria in the samples accurately, absolute and relative quantification tests were conducted on samples from eight treatments. The results at the phylum level are shown in Figure 2. Figure 2a demonstrates that among the four treatments without microbial agents, C90 exhibits the highest bacterial count at 2.98 × 107. In contrast, among the four treatments with microbial agents, MC80 shows the highest bacterial count, reaching 3.84 × 107. The ranking of bacterial quantities across all treatments is as follows: MC80 > MC70 > C90 > MC90 > RC > C70 > MRC > C80, indicating that reducing fertilizer application and supplementing with microbial agents can increase bacterial populations in the soil. Specifically, compared to C80, MC80 shows an increase of 80.29%, and compared to C70, MC70 shows an increase of 31.26%. However, when fertilizer application is high, the bacterial count in MRC decreases by 9.83% compared to RC, and MC90 decreases by 3.24% compared to C90.
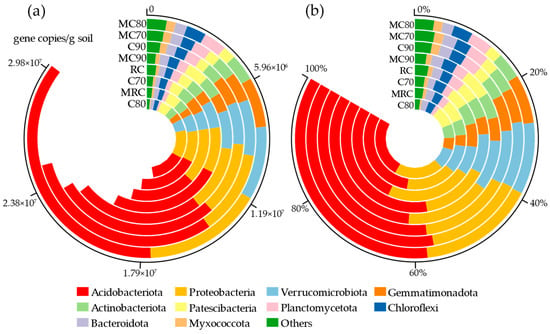
Figure 2.
(a) Absolute abundances (16S rRNA gene copies per g of soil) and (b) relative abundances (%) of the major bacterial phyla present in all soil samples.
The corresponding relative abundances at the phylum level are illustrated in Figure 2b. Upon observation, it was noted that the relative abundance of two bacterial phyla, namely Proteobacteria and Acidobacteria, collectively surpassed 50% of the total population. In the absence of bacterial agents, as the level of fertilizer reduction increased, the proportion of Proteobacteria correspondingly rose, whereas Acidobacteria experienced a decline. However, upon the introduction of bacterial agents, the impact on these phyla appeared to be negligible. When both figures are considered in tandem, it becomes evident that despite the Proteobacteria encompassing a multitude of types, their overall numbers are relatively low, whereas the Acidobacteria, though fewer in variety, are present in vast quantities.
The bacterial phyla responded differently to the fertilizer loads in the eight treatments, resulting in communities with different α diversity indexes (Figure 3). A 95% sequence similarity was used as the threshold for ASV classification. The rarefaction curve of the ASV number against the reads number indicated that the sequencing efforts covered nearly all of the diversity that was expected to be found in these 24 samples, and the trend is consistent with the Chao1 index value for each group. The Chao1, Shannon, and Simpson indexes of each sample were calculated to test whether there was a significant difference in α diversity between groups. The analysis of the α diversity index showed that under the same fertilization level, whether or not the application of microbial agents had a significant effect on the community diversity, the community richness was improved to varying degrees. Reducing chemical fertilizer application is a key factor affecting community diversity. Among the samples, MC70 had the highest flora richness of 3306.52. MRC had the highest Shannon index (6.61) and the lowest Simpson index (0.00457).
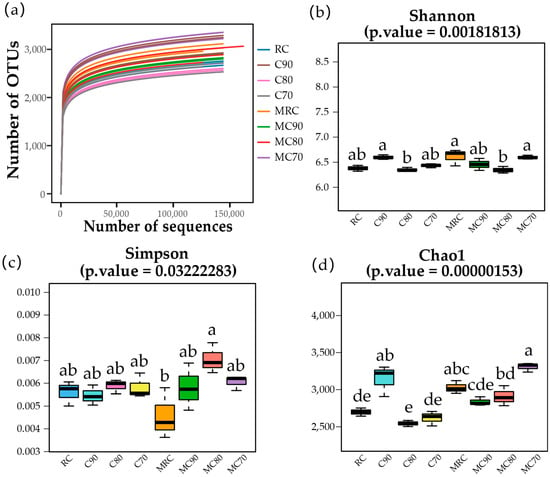
Figure 3.
(a) Sequencing depth and (b–d) α diversity of the sampled soils. Lowercase letters denote significant differences between different treatments, ANOVA, p < 0.05.
Beta diversity analysis was performed on the eight sample groups, with PCoA (calculated on Bray–Curtis, Figure 4a) and the average linkage hierarchical clustering (UPGMA, calculated on Bray–Curtis, Figure 4b) shown in the figure. The results showed that the amount of fertilization had a significant influence on the soil bacterial community. PCoA clearly separated the samples from the eight treatments, indicating that the bacterial community structures among replicate treatments were relatively similar, thereby confirming the reliability of the data. The first and second principal coordinates explained 46.39% and 12.83%, respectively, of the differences among the eight treatments. It can be seen in Figure 4b that the treatments with more similar levels of comprehensive fertilization had more similar community structures. Moreover, fertility levels that are too high and too low will also affect the community structure, resulting in a significant difference from the medium fertility level group (MC80, MC70, C90).
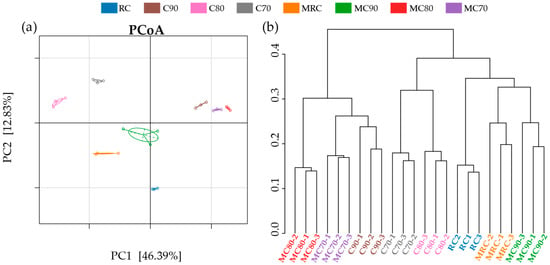
Figure 4.
(a) Principal coordinate analysis (PCoA) and (b) hierarchical cluster tree of bacterial communities (calculated on Bray–Curtis).
3.3. Relationship Between Rhizosphere Microbial Communities and Soil Environmental Factors
To elucidate the interactions between microorganisms and soil environmental factors, RDA and Spearman correlation analyses were performed at the phylum level (Figure 5), and the abundance threshold was set to 0.05. The first axis (RDA1) explained 47.15% of the variation, and the second axis (RDA2) explained 18.13% of the variation, together explaining 65.28% of the variation in bacterial communities. The three dominant bacterial phyla—Proteobacteria, Actinobacteriota, and Patescibacteria—showed a positive correlation with three available nutrients and a negative correlation with pH, OM, and three types of soil enzyme activities. In contrast, Acidobacteriota, Verrucomicrobiota, and Gemmatimonadota showed the opposite results, indicating that they are more sensitive to environmental conditions. Among the eight tested soil parameters, the importance of CAT was higher than the other seven parameters; RDA1 was −1.87, and RDA2 was −0.10. Additionally, the driving effect of pH is also strong; RDA1 is −1.58, and RDA2 is 0.15.
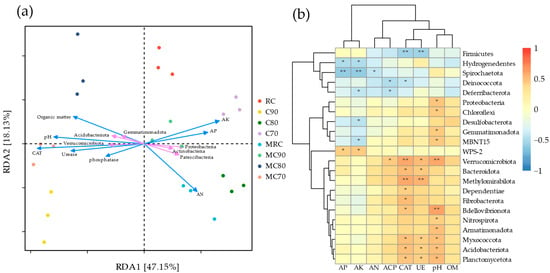
Figure 5.
(a) Redundancy analysis (RDA) and (b) Spearman correlation analysis for dominant soil bacterial communities from all samples associated with environmental variables at the phylum level. ACP, acid phosphatase; UE, urease; CAT, catalase. Asterisks represent significant effects: *, p < 0.05; **, p < 0.01.
Analysis of the correlation between bacterial communities under different treatments, environmental factors, and dominant bacterial phyla reveals that the distances between the C90, MC80, and MC70 treatments are relatively close. This indicates a similarity in the structure of bacterial communities among these three groups, which aligns with the results depicted in Figure 4b. Additionally, the direction of the environmental variable arrows in Figure 5a corresponds with the colors and significance levels in the heatmap (Figure 5b), revealing the correlation between these chemical indicators and microbial taxa. The bacterial communities from these three groups show a positive correlation with various soil enzyme activities and a negative correlation with soil available nutrients. Based on the absolute quantification of bacteria shown in Figure 2, it is reasonable to speculate that the higher bacterial count leads to increased enzyme activity, enhancing the ability to activate soil and exogenous nutrients. This, in turn, promotes plants’ absorption of more available nutrients during their growth.
3.4. Economic Benefit and Comprehensive Evaluation
The maize yield and economic benefits were calculated (Table 1). It can be seen from the table that the yield of maize decreased gradually with the decrease in chemical fertilizer application. When the amount of chemical fertilizer was the same, the yield of maize treated with microbial agents was significantly higher than that without. The yields of the MRC, MC90, and MC80 treatments significantly increased by 9.89%, 6.74%, and 5.76%, respectively, compared with RC, while there was no significant difference for MC70 (p < 0.05). It shows that the 70% chemical fertilizer combined with the Ft microbial agent can achieve the same effect as conventional fertilization, and the effect is the best when the reduction is 20%. The net income and production ratio of each treatment were higher than that of RC, and MC80 reached the maximum value, indicating that excessive fertilization would not only cause a waste of resources but also reduce farmers’ income. The application of 80% chemical fertilizer combined with the Ft microbial agent can maximize the economy, improve the utilization rate of chemical fertilizer, and achieve the effect of reducing fertilizer without reducing production.

Table 1.
Effect of Ft microbial fertilizers on maize yield and income.
These significant drivers of yield were further used for the partial least squares path model (PLS-PM) to investigate the direct and indirect impacts of both soil nutrient and microbial factors on the yield (Figure 6). The combined effects of chemical fertilizers, inoculants, soil microbes, soil enzymes, and available nutrients collectively explained 81.8% of the variance in crop yield. The inoculant directly impacted soil microbes, and microbes had a direct effect on soil enzymes, and soil enzymes had a direct effect on soil nutrients. The PLS-PM analysis demonstrated that soil AN, AK, and microbial communities exerted direct effects on yield, with path coefficients of 0.531, 0.460, and 0.302, respectively (p < 0.001). Thus, the inoculant was the strongest driving factor of yield.
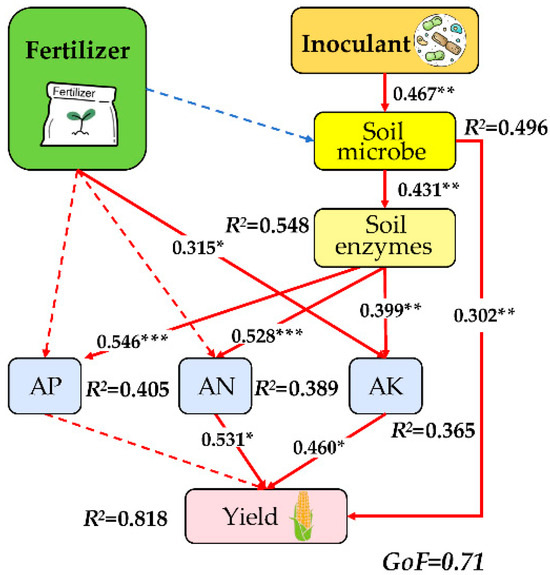
Figure 6.
Partial least squares path model showing the effects of soil and microbial properties on yields. Asterisks represent significant effects: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Principal component analysis (PCA) was used to evaluate 10 soil fertility indicators comprehensively. The KMO test result was 0.761 (>0.6), and the significance coefficient of Bartlett’s sphericity test was 0.000 (<0.05), indicating that the data were suitable for principal component analysis. The eigenvalues, variance contribution rates, and loading matrix of the principal components are shown in Table S1. Three principal components were extracted, with eigenvalues of 4.125, 2.593, and 2.413, accounting for a cumulative variance contribution rate of 91.312%. Based on the communalities, weight coefficients were determined, and the comprehensive soil fertility evaluation values were calculated (Table 2). The comprehensive scores ranged from −0.627 to 0.713, in descending order: MC80 > MRC > MC90 > MC70 > C90 > C80 > RC > C70.

Table 2.
Comprehensive evaluation of soil fertility.
4. Discussion
4.1. Microbial Inoculants Can Partially Replace Chemical Fertilizers and Improve Soil Fertility
The physicochemical properties of soil are crucial for agricultural production and the sustainable use of agricultural land. Microorganisms can not only regulate the fate of nutrients in the soil but also respond to nutrient input through changes in biomass and community structure [39]. This study measured the content of available nutrients in soil under different proportions of reduced chemical fertilizer combined with microbial inoculants. The results for the MC70 treatment were similar to those of the RC treatment, indicating that microbial inoculants can replace approximately 30% of chemical fertilizers. This substitution improves fertilizer use efficiency and reduces economic costs. Relevant studies have shown that the application of microbial agents has an ‘aftereffect’ on the activation of phosphorus and potassium in the soil of greenhouse tomatoes. Compared with conventional fertilization, applying bacteria increased available phosphorus by 24.10% and potassium by 37.22% during the vigorous bearing stage [40]. In a study by Rose et al., the estimated yield of rice treated with biofertilizers was significantly higher than that of rice grown using conventional methods. Nitrogen fertilizer usage could also be reduced by 23% to 52% [41].
4.2. The Combined Application of Chemical Fertilizers and Microbial Inoculants Can Increase Crop Yields and Enhance Economic Benefits
It is a feasible but difficult challenge to reduce farmland’s fertilizer load without causing productivity loss [42]. However, with the emergence of microbial agents as a new type of fertilizer, this problem has been well solved. The field experiment results confirmed our hypothesis that the combination of 80% chemical fertilizer + 20 kg/hm2 microbial agent (MC80) had the highest input–output ratio of 1:1.58, and the maize yield increased by 5.76% compared with the full amount of chemical fertilizer (RC). The maize yield of the combination of 70% chemical fertilizer + 20 kg/hm2 microbial agent (MC70) was similar to that of the full amount of chemical fertilizer (RC), and the production ratio was reduced by 10.16%. Similar results also appeared in other research experiments. The experimental results of reducing chemical fertilizers combined with bio-organic fertilizers show that the tomato yield after the combination is comparable to that achieved with 100% chemical fertilizers. However, a single application of inoculum (SS) or organic fertilizer (OF) resulted in a 6–38% and 9–35% decrease in yield, respectively, compared to the control [11]. In the case of reducing the recommended amount of fertilizer and applying inoculants, the plant height, shoot dry weight, root dry weight, yield, and nutrient uptake were comparable to those of full fertilization without inoculants [43].
4.3. Fertilizer Application Rates Can Affect the Diversity and Richness of Microorganisms
When performing absolute quantitative sequencing of microbes in the rhizosphere soil of eight treatments, we found that the microbial biomass in RC was higher than in C80 and C70. This indicates that too little inorganic fertilizer can lead to a reduction in microbial biomass. Interestingly, C90 was significantly higher than RC, indicating that excessive and insufficient fertilizer application can impact microbial communities. These findings are similar to those reported in other studies, which examined different nutrients and soil types. Studies have shown that applying varying amounts of urea to semi-arid grassland soils [44] and nitrogen fertilizers to black soils planted with different crops [45] reduced bacterial diversity and altered microbial communities and functions. Similar investigations have been conducted across diverse soil types. In Calcaric Ochri-Aquic Cambosol, a maize–wheat rotation system subjected to four distinct potassium fertilization gradients demonstrated that moderate potassium application regimes exhibited higher fungal and bacterial diversity compared to both deficient and excessive potassium treatments [46]. A gradient experiment on phosphorus fertilization was conducted in the dryland of the Loess Plateau, concluding that phosphorus input thresholds can reduce phosphorus fertilizer application and regulate microbial communities to maintain soil multifunctionality and network complexity. However, long-term high phosphorus input significantly reduced soil bacteria diversity and the richness index [47].
In this experiment, Acidobacteria is a phylum that warrants attention, as its variation influences the overall structure of the bacterial community. The variations in the abundance of Acidobacteria across different treatments are not coincidental. Fierer’s study showed that Acidobacteria have high 16S rRNA gene sequence abundance and diversity, especially in soil habitats [48]. Through previous studies, it can be determined that Acidobacteria are mostly chemo-organic heterotrophic thermophilic bacteria [49], which are microaerophilic [50], able to reduce iron [51], participate in the sulfur cycle [52], and can play a role in the global carbon and nitrogen cycles [53]. In soil with high organic matter and available phosphorus content, the proportion of Acidobacteriota was higher [54]. Therefore, to a certain extent, the abundance of Acidobacteria reflects soil quality, which is highly consistent with the results of our study.
4.4. Interplay Between Rhizosphere Microenvironment Characteristics and Microbial Community
Changes in the soil environment affect the composition of soil microbial communities. Soil microorganisms not only participate in nutrient cycling and organic matter transformation but also alter the soil habitat through various biochemical and biophysical mechanisms [55]. In this study, three microbial communities were positively correlated with organic matter content. Organic matter provides a rich carbon source for microorganisms, improves soil structure, and enhances water retention, indirectly promoting microbial growth [56]. Increasing the application of organic fertilizer can significantly enhance soil microbial diversity and metabolic activity [57]. Recent studies have indicated that most organic acids entering the soil solution are likely produced by microorganisms rather than plants [58]. Conversely, bacterial oxalate metabolism is associated with an increase in soil pH by 2.5 units [59]. Bargaz et al. stated that phosphorus fertilizer can alter microbial community composition, affecting biodiversity, activity, and functional diversity [60]. Moreover, microbial phosphorus transformation can change the levels of phosphorus in the environment [61]. Biochar or Bio can improve soil pH, total nitrogen, exchangeable NH4-N, and potassium levels, indicating that changes in soil microbial communities may be directly or indirectly related to changes in physical and chemical properties [12].
5. Conclusions
This study demonstrates that reducing chemical fertilizer application combined with microbial inoculants is an ideal fertilization strategy that maintains maize yield while improving soil ecological functions. Field plot experiments confirmed that reducing chemical fertilizer application and supplementing with microbial agents effectively improves soil physicochemical properties and increases soil nutrients. Meanwhile, an appropriate amount of fertilizer provides a favorable environment for microbial growth, enhancing soil bacterial abundance. Through experiments with different fertilization treatments, it was found that reducing chemical fertilizer by 10–30% and supplementing with microbial agents increased maize yields. The MC80 treatment increased yield and achieved the highest economic benefits and the most abundant soil microbial communities. Principal component analysis indicated that MC80 is the optimal combination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15051029/s1, Figure S1: Experimental location. Figure S2: Venn diagram for soil samples collected from different treatments showing shared and unique ASV numbers. Table S1: The chemical properties of the tested soil. Table S2: Principal component analysis of soil fertility indicators.
Author Contributions
Conceptualization, H.W. and X.Z.; methodology, X.Z., H.G., H.W. and N.H.; formal analysis, C.Z. and L.Y.; validation, H.G., F.Z. (Fugui Zhang), H.W. and N.H.; writing—original draft preparation, F.Z. (Fenglin Zhang); writing—review and editing, F.Z. (Fenglin Zhang) and N.W.; investigation, F.Z. (Fugui Zhang) and H.G.; supervision, C.Z., X.Z., H.W. and N.H.; visualization, F.Z. (Fenglin Zhang) and N.W.; data curation, N.W. and F.Z. (Fugui Zhang); software, C.Z. and L.Y.; resources, L.Y.; project administration, H.W.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Key Research and Development Program of China (Grant No. 2024YFD1500300) and Jilin Province Science and Technology Development Plan Item, China (Grant Nos. 20230303006SF and 20230203158SF).
Data Availability Statement
The data presented in this study are available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Teng, Y.; Pang, B.; Guo, X. Study on the Quality Improvement on Black Land in Northeast China under the Environment of Sustainable Agricultural Development. Kybernetes 2023, 52, 809–827. [Google Scholar] [CrossRef]
- Gu, Z.; Xie, Y.; Gao, Y.; Ren, X.; Cheng, C.; Wang, S. Quantitative Assessment of Soil Productivity and Predicted Impacts of Water Erosion in the Black Soil Region of Northeastern China. Sci. Total Environ. 2018, 637–638, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Dong, K.; Geisen, S.; Yang, W.; Yan, Y.; Gu, D.; Liu, N.; Borisjuk, N.; Luo, Y.; Friman, V.-P. The Effect of Microbial Inoculant Origin on the Rhizosphere Bacterial Community Composition and Plant Growth-Promotion. Plant Soil 2020, 452, 105–117. [Google Scholar] [CrossRef]
- Shen, M.; Li, J.; Dong, Y.; Zhang, Z.; Zhao, Y.; Li, Q.; Dang, K.; Peng, J.; Liu, H. The Effects of Microbial Inoculants on Bacterial Communities of the Rhizosphere Soil of Maize. Agriculture 2021, 11, 389. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms Underlying the Protective Effects of Beneficial Fungi against Plant Diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Rafiee, S.; Pan, J.; Zhang, Y.; Liu, H. A Multi-Criteria Evolutionary-Based Algorithm as a Regional Scale Decision Support System to Optimize Nitrogen Consumption Rate; a Case Study in North China Plain. J. Clean. Prod. 2020, 256, 120213. [Google Scholar] [CrossRef]
- Xun, W.; Zhao, J.; Xue, C.; Zhang, G.; Ran, W.; Wang, B.; Shen, Q.; Zhang, R. Significant Alteration of Soil Bacterial Communities and Organic Carbon Decomposition by Different Long-Term Fertilization Management Conditions of Extremely Low-Productivity Arable Soil in South China. Environ. Microbiol. 2016, 18, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, L.; Yang, S.; Wang, Z.; Tian, R.; Peng, Z.; Chen, Y.-L.; Zhang, X.; Kuang, J.; Ling, N.; et al. Critical Transition of Soil Bacterial Diversity and Composition Triggered by Nitrogen Enrichment. Ecology 2020, 101, e03053. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Kloepper, J.W. Plant–Microbes Interactions in Enhanced Fertilizer-Use Efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef]
- Cong, P.; Ouyang, Z.; Hou, R.; Han, D. Effects of Application of Microbial Fertilizer on Aggregation and Aggregate-Associated Carbon in Saline Soils. Soil Tillage Res. 2017, 168, 33–41. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-Organic Fertilizer with Reduced Rates of Chemical Fertilization Improves Soil Fertility and Enhances Tomato Yield and Quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Win, K.T.; Okazaki, K.; Ohkama-Ohtsu, N.; Yokoyama, T.; Ohwaki, Y. Short-Term Effects of Biochar and Bacillus Pumilus TUAT-1 on the Growth of Forage Rice and Its Associated Soil Microbial Community and Soil Properties. Biol. Fertil. Soils 2020, 56, 481–497. [Google Scholar] [CrossRef]
- Na, X.; Yu, H.; Wang, P.; Zhu, W.; Niu, Y.; Huang, J. Vegetation Biomass and Soil Moisture Coregulate Bacterial Community Succession under Altered Precipitation Regimes in a Desert Steppe in Northwestern China. Soil Biol. Biochem. 2019, 136, 107520. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Kent, A.D.; Brisson, V.L.; Gaudin, A.C.M. Agricultural Management and Plant Selection Interactively Affect Rhizosphere Microbial Community Structure and Nitrogen Cycling. Microbiome 2019, 7, 146. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Caruso, T. Soil Microbial Community Responses to Climate Extremes: Resistance, Resilience and Transitions to Alternative States. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190112. [Google Scholar] [CrossRef] [PubMed]
- Coban, O.; De Deyn, G.B.; Van Der Ploeg, M. Soil Microbiota as Game-Changers in Restoration of Degraded Lands. Science 2022, 375, abe0725. [Google Scholar] [CrossRef]
- Domene, X.; Hanley, K.; Enders, A.; Lehmann, J. Short-Term Mesofauna Responses to Soil Additions of Corn Stover Biochar and the Role of Microbial Biomass. Appl. Soil Ecol. 2015, 89, 10–17. [Google Scholar] [CrossRef]
- Sun, L.; Gao, J.; Huang, T.; Kendall, J.R.A.; Shen, Q.; Zhang, R. Parental Material and Cultivation Determine Soil Bacterial Community Structure and Fertility. FEMS Microbiol. Ecol. 2015, 91, 1–10. [Google Scholar] [CrossRef]
- Ye, L.; Wu, X.; Wu, C.; Zhang, Y.; Meng, L.; Bao, E.; Cao, K. Response of Soil Bacterial Community to Agricultural Reclamation in the Tengger Desert, Northwestern China. Appl. Soil Ecol. 2022, 169, 104189. [Google Scholar] [CrossRef]
- Tian, J.; He, N.; Hale, L.; Niu, S.; Yu, G.; Liu, Y.; Blagodatskaya, E.; Kuzyakov, Y.; Gao, Q.; Zhou, J. Soil Organic Matter Availability and Climate Drive Latitudinal Patterns in Bacterial Diversity from Tropical to Cold Temperate Forests. Funct. Ecol. 2017, 32, 61–70. [Google Scholar] [CrossRef]
- Fierer, N.; Wood, S.A.; Bueno De Mesquita, C.P. How Microbes Can, and Cannot, Be Used to Assess Soil Health. Soil Biol. Biochem. 2021, 153, 108111. [Google Scholar] [CrossRef]
- Yang, L.; Lou, J.; Wang, H.; Wu, L.; Xu, J. Use of an improved high-throughput absolute abundance quantification method to characterize soil bacterial community and dynamics. Sci. Total Environ. 2018, 633, 360–371. [Google Scholar] [CrossRef]
- Jiang, S.-Q.; Yu, Y.-N.; Gao, R.-W.; Wang, H.; Zhang, J.; Li, R.; Long, X.-H.; Shen, Q.-R.; Chen, W.; Cai, F. High-Throughput Absolute Quantification Sequencing Reveals the Effect of Different Fertilizer Applications on Bacterial Community in a Tomato Cultivated Coastal Saline Soil. Sci. Total Environ. 2019, 687, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Jin, X.-Y.; Zhang, X.-C.; Chen, L.; Liu, J.-L.; Zhang, H.-M.; Zhang, X.; Zhang, Y.-F.; Zhao, J.-H.; Ma, Z.-S.; et al. Comparative Metagenomics of Two Distinct Biological Soil Crusts in the Tengger Desert, China. Soil Biol. Biochem. 2020, 140, 107637. [Google Scholar] [CrossRef]
- Smets, W.; Leff, J.W.; Bradford, M.A.; McCulley, R.L.; Lebeer, S.; Fierer, N. A Method for Simultaneous Measurement of Soil Bacterial Abundances and Community Composition via 16S rRNA Gene Sequencing. Soil Biol. Biochem. 2016, 96, 145–151. [Google Scholar] [CrossRef]
- Pysarenko, P.; Samojlik, M.; Galytskaya, M.; Tsova, Y.; Mostoviak, I. Influence of Bacillus Subtilis on Soil Microbiocenosis. Ecol. Quest. 2023, 34, 127–133. [Google Scholar] [CrossRef]
- Malvestiti, G.S. Resposta Técnica e Econômica para Adubação Com N, P e K em Milho Convencional e Geneticamente Modificado; Mestrado em Qualidade e Produtividade Animal; Universidade de São Paulo: São Paulo, Brazil, 2014. [Google Scholar]
- Paolinelli, A.; Dourado Neto, D.; Mantovani, E.C. (Eds.) Agricultura Irrigada no Brasil: Ciência e Tecnología; Universidade de São Paulo. Escola Superior de Agricultura Luiz de Queiroz; Universidade Federal de Viçosa; Universidade de São Paulo: São Paulo, Brazil, 2022; ISBN 978-65-87391-23-6. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000; ISBN 7-109-06644-4. [Google Scholar]
- Ma, Z.; Xu, W.; Chen, Y.; Liu, M.; Wen, J. A Study of the Influence of the Type of Land Use on the Enzymatic Activity of Soils in Southwestern China. Forests 2024, 15, 581. [Google Scholar] [CrossRef]
- Sun, J.; Ma, J.; Zhu, M.; Yuan, Z.; Yao, J. Effects of Nitrogen Application on the Soil Microbial Activity, Enzyme Activities and Properties and Their Relationships in a Maize Field. Fresenius Environ. Bull. 2016, 25, 852–861. [Google Scholar]
- Mekonnen, T.W. Effects of Planting Date, Environments and Their Interaction on Grain Yield and Quality Traits of Maize Hybrids. Heliyon 2023, 9, e21660. [Google Scholar] [CrossRef]
- Kataoka, T.; Ooki, A.; Nomura, D. Production of Dibromomethane and Changes in the Bacterial Community in Bromoform-Enriched Seawater. Microbes Environ. 2019, 34, 215–218. [Google Scholar] [CrossRef]
- Su, X.; Zhang, L.; Meng, H.; Wang, H.; Zhao, J.; Sun, X.; Song, X.; Zhang, X.; Mao, L. Long-Term Conservation Tillage Increase Cotton Rhizosphere Sequestration of Soil Organic Carbon by Changing Specific Microbial CO2 Fixation Pathways in Coastal Saline Soil. J. Environ. Manag. 2024, 358, 120743. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, D.; Ren, Y.; Wang, Z.; Zhou, Y. Soil Quality Assessment of Croplands in the Black Soil Zone of Jilin Province, China: Establishing a Minimum Data Set Model. Ecol. Indic. 2019, 107, 105251. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Soil Quality Response to Long-Term Nutrient and Crop Management on a Semi-Arid Inceptisol. Agric. Ecosyst. Environ. 2007, 118, 130–142. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Alternative Soil Quality Indices for Evaluating the Effect of Intensive Cropping, Fertilisation and Manuring for 31 Years in the Semi-Arid Soils of India. Environ. Monit. Assess. 2007, 136, 419–435. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and Mechanisms of Responses by Soil Microbial Communities to Nitrogen Addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, Y.; Yang, Y.; Zhang, M.; Mao, X.; Guo, Y.; Li, X.; Tao, B.; Qi, Y.; Ma, L.; et al. Co-Application of Biochar and Microbial Inoculants Increases Soil Phosphorus and Potassium Fertility and Improves Soil Health and Tomato Growth. J. Soils Sediments 2023, 23, 947–957. [Google Scholar] [CrossRef]
- Rose, M.T.; Phuong, T.L.; Nhan, D.K.; Cong, P.T.; Hien, N.T.; Kennedy, I.R. Up to 52% N Fertilizer Replaced by Biofertilizer in Lowland Rice via Farmer Participatory Research. Agron. Sustain. Dev. 2014, 34, 857–868. [Google Scholar] [CrossRef]
- Da Costa, P.B.; Beneduzi, A.; De Souza, R.; Schoenfeld, R.; Vargas, L.K.; Passaglia, L.M.P. The Effects of Different Fertilization Conditions on Bacterial Plant Growth Promoting Traits: Guidelines for Directed Bacterial Prospection and Testing. Plant Soil 2013, 368, 267–280. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant Growth-Promoting Rhizobacteria Allow Reduced Application Rates of Chemical Fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Z.; Zhuang, D.; Wang, J.; Xie, S.; Liu, G. Urea Fertilization Decreases Soil Bacterial Diversity, but Improves Microbial Biomass, Respiration, and N-Cycling Potential in a Semiarid Grassland. Biol. Fertil. Soils 2019, 55, 229–242. [Google Scholar] [CrossRef]
- Xia, Z.; Yang, J.; Sang, C.; Wang, X.; Sun, L.; Jiang, P.; Wang, C.; Bai, E. Phosphorus Reduces Negative Effects of Nitrogen Addition on Soil Microbial Communities and Functions. Microorganisms 2020, 8, 1828. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fang, F.; Wu, L.; Gao, F.; Li, M.; Li, B.; Wu, K.; Hu, X.; Wang, S.; Wei, Z.; et al. The Microbial Community, Nutrient Supply and Crop Yields Differ along a Potassium Fertilizer Gradient under Wheat–Maize Double-Cropping Systems. J. Integr. Agric. 2024, 23, 3592–3609. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.; Liu, W.; Li, H.; Wang, Z.; Liu, J. Phosphorus Fertilizer Input Threshold Shifts Bacterial Community Structure and Soil Multifunctionality to Maintain Dryland Wheat Production. Soil Tillage Res. 2024, 243, 106174. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Huber, K.J.; Pester, M.; Eichorst, S.A.; Navarrete, A.A.; Foesel, B.U. Editorial: Acidobacteria—Towards Unraveling the Secrets of a Widespread, Though Enigmatic, Phylum. Front. Microbiol. 2022, 13, 960602. [Google Scholar] [CrossRef]
- Trojan, D.; Garcia-Robledo, E.; Meier, D.V.; Hausmann, B.; Revsbech, N.P.; Eichorst, S.A.; Woebken, D. Microaerobic Lifestyle at Nanomolar O2 Concentrations Mediated by Low-Affinity Terminal Oxidases in Abundant Soil Bacteria. mSystems 2021, 6, e0025021. [Google Scholar] [CrossRef]
- Falagán, C.; Foesel, B.; Johnson, B. Acidicapsa ferrireducens Sp. Nov., Acidicapsa acidiphila Sp. Nov., and Granulicella acidiphila Sp. Nov.: Novel Acidobacteria Isolated from Metal-Rich Acidic Waters. Extremophiles 2017, 21, 459–469. [Google Scholar] [CrossRef]
- Flieder, M.; Buongiorno, J.; Herbold, C.W.; Hausmann, B.; Rattei, T.; Lloyd, K.G.; Loy, A.; Wasmund, K. Novel Taxa of Acidobacteriota Implicated in Seafloor Sulfur Cycling. ISME J. 2021, 15, 3159–3180. [Google Scholar] [CrossRef]
- Ward, N.L.; Challacombe, J.F.; Janssen, P.H.; Henrissat, B.; Coutinho, P.M.; Wu, M.; Xie, G.; Haft, D.H.; Sait, M.; Badger, J.; et al. Three Genomes from the Phylum Acidobacteria Provide Insight into the Lifestyles of These Microorganisms in Soils. Appl. Environ. Microbiol. 2009, 75, 2046–2056. [Google Scholar] [CrossRef]
- Shelyakin, P.V.; Semenkov, I.N.; Tutukina, M.N.; Nikolaeva, D.D.; Sharapova, A.V.; Sarana, Y.V.; Lednev, S.A.; Smolenkov, A.D.; Gelfand, M.S.; Krechetov, P.P.; et al. The influence of kerosene on microbiomes of diverse soils. Life 2022, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The Interplay between Microbial Communities and Soil Properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Wang, S.; Heal, K.V.; Zhang, Q.; Yu, Y.; Tigabu, M.; Huang, S.; Zhou, C. Soil Microbial Community, Dissolved Organic Matter and Nutrient Cycling Interactions Change along an Elevation Gradient in Subtropical China. J. Environ. Manag. 2023, 345, 118793. [Google Scholar] [CrossRef]
- Wang, X.; Bian, Q.; Jiang, Y.; Zhu, L.; Chen, Y.; Liang, Y.; Sun, B. Organic Amendments Drive Shifts in Microbial Community Structure and Keystone Taxa Which Increase C Mineralization across Aggregate Size Classes. Soil Biol. Biochem. 2021, 153, 108062. [Google Scholar] [CrossRef]
- Huet, S.; Romdhane, S.; Breuil, M.-C.; Bru, D.; Mounier, A.; Spor, A.; Philippot, L. Experimental Community Coalescence Sheds Light on Microbial Interactions in Soil and Restores Impaired Functions. Microbiome 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; Buddolla, V.; Lian, B. Oxalate Carbonate Pathway—Conversion and Fixation of Soil Carbon—A Potential Scenario for Sustainability. Front. Plant Sci. 2020, 11, 591297. [Google Scholar] [CrossRef]
- Bargaz, A.; Elhaissoufi, W.; Khourchi, S.; Benmrid, B.; Borden, K.A.; Rchiad, Z. Benefits of Phosphate Solubilizing Bacteria on Belowground Crop Performance for Improved Crop Acquisition of Phosphorus. Microbiol. Res. 2021, 252, 126842. [Google Scholar] [CrossRef]
- Ikoyi, I.; Fowler, A.; Schmalenberger, A. One-Time Phosphate Fertilizer Application to Grassland Columns Modifies the Soil Microbiota and Limits Its Role in Ecosystem Services. Sci. Total Environ. 2018, 630, 849–858. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).