Abstract
Incorporating temperate legumes is a strategy for increasing nitrogen (N) in tall fescue (Schedonorus arundinaceus (Schreb.) Dumort, nom. Cons) systems. However, when temperatures are elevated, biological N-fixation (BNF) by temperate legumes is limited. Sunn hemp (Crotalaria juncea L.), a warm-season annual legume, may provide greater N input during the warm season. This 2-year study aimed to (1) determine BNF in sunn hemp-tall fescue mixed pastures and (2) determine N transfer from sunn hemp to tall fescue. The experiment included four replicates of two treatments: tall fescue (TF) and tall fescue intercropped with sunn hemp (TF+SH), arranged in a randomized complete block design. Response variables included δ15N, N derived from the atmosphere (%NDFA), BNF, N concentration, N transferred (%Ntran), N stock, and herbage accumulation (HA). Herbage accumulation was 16% greater in TF+SH compared to TF (p < 0.05). Root mass was 43% greater for TF compared to both species combined in TF+SH (p < 0.05). Herbage N was 40% greater in sunn hemp shoots than tall fescue shoots in TF or TF+SH (p < 0.05). Sunn hemp root N was 34% greater than tall fescue (p < 0.05). NDFA by sunn hemp was 88% and 100% in 2017 and 2018, respectively. BNF by sunn hemp was greater (p < 0.05) in 2018 than in 2017 (53.8 and 44.3 kg ha−1, respectively). The %Ntran from sunn hemp to tall fescue was 13 and 20% in 2017 and 2018, respectively. Interseeding sunn hemp into tall fescue pastures can provide an alternate N source to tall fescue-based forage-livestock systems, increasing herbage accumulation during the summer grazing season.
1. Introduction
Pasture systems in the Mid-South United States are composed predominantly of a tall fescue (Schedonorus arundinaceus (Schreb.) Dumort, nom. Cons)–legume mixture [1,2]. In this region, the predominant legumes are bird’s-foot trefoil (Lotus corniculatus L.), red clover (Trifolium pratense L.), white clover (Trifolium repens L.), and alfalfa (Medicago sativa L.) [3,4]. The symbiotic relationship these legumes develop with rhizobium bacteria allows for converting atmospheric nitrogen (N) to plant-usable forms of N. Biological N fixation (BNF) reduces the production limitations associated with the N requirements of tall fescue. MacMillan et al. [5], in a literature review, reported BNF of between 40 and 112 kg N ha−1 in pure stands of cool-season legumes. The transfer of biologically fixed N from legumes to grasses can account for up to 80% of the total N in tall fescue when legumes are interseeded [6]. Though N transfer from legumes may increase tall fescue productivity, increased temperatures and reduced rainfall may decrease the symbiotic N inputs by temperate legumes through nodule damage and carbohydrate partitioning changes during the warm season [7,8]. Furthermore, BNF by legumes differs according to the legume species and proportion, soil properties and microbiology, agronomic practices, and environmental factors [5,9].
Herbage accumulation (HA) of legumes is another factor that directly affects the efficiency of BNF [10]. Temperatures exceeding 25–30 °C adversely affect critical physiological processes, including respiration and photosynthesis, in nearly all legume species [7,11]. This might result in a reduced leaf area and diminished photosynthate production and HA [7,12]. Reduced herbage growth during the warm season contributes to decreased BNF by temperate legumes [10]. The limitations imposed by elevated temperatures might result in similar HA between tall fescue–legume pastures and tall fescue in monoculture during the warm season [13]. Thus, research is needed to identify legumes that can be successfully intercropped in tall fescue and be productive under elevated temperatures.
The effect of intercropping a warm-season annual legume, such as sunn hemp (Crotalaria juncea L.), in tall fescue pastures on N dynamics is unknown. Sunn hemp is a tropical legume native to Southeastern Asia, typically described as relatively drought-tolerant and adapted to a wide range of soil types [14,15,16]. In the United States, sunn hemp has been used predominantly as a fiber, green manure, and cover crop because of its rapid growth rate, high N-fixation potential, and superior nutritive value [14,16,17]. Previous research has indicated that sunn hemp contributes to better agronomic performance than temperate legumes during the warm season [18]. Previous studies have also demonstrated the potential of sunn hemp intercropping tropical grasses to provide N, increasing HA and crude protein concentrations during the warm season [9,17]. Jaramillo et al. [15] found sunn hemp HA of 3200 kg DM ha−1 after 45 days of seeding, while Garzon et al. [19] reported sunn hemp HA ranging from 3300 to 5600 kg DM ha−1 after 60 days of seeding. Sunn hemp HA above 7000 kg DM ha−1 has been previously reported following 90 days of growth [20,21]. However, tropical legumes such as sunn hemp accumulate herbage proportionally to BNF, similarly to temperate legumes. Seasonal BNF of between 81 and 150 kg N ha−1 has previously been reported [19,21,22,23].
In grass–legume mixtures, legume N may be transferred to grasses via aboveground and belowground processes, directly affecting grass growth and accumulation [6]. The aboveground N transfer occurs via shoot litter or animal excreta decomposition. In contrast, the belowground N transfer may occur through different mechanisms: (I) decomposition of legume root tissue, (II) legume root exudation of soluble N compounds, and (III) transfer of N mediated by plant-associated mycorrhizae [6,24,25,26]. The literature indicates that N transfer in grass–legume swards can vary significantly due to biotic and abiotic factors, with estimates ranging from 0 to 80% of the N found in companion grasses coming from associated legumes [25,26,27,28]. Although previous research has demonstrated sunn hemp’s productivity and BNF potential, there is limited empirical data on BNF, N transfer, and the associated above and belowground responses of both sunn hemp and tall fescue when intercropped.
We hypothesized that intercropping tall fescue pastures with sunn hemp would result in N transfer from sunn hemp to tall fescue, increasing tall fescue herbage N and HA. The objectives of this experiment were to (1) determine BNF in a sunn hemp–tall fescue mixed system and (2) determine N transfer from sunn hemp to tall fescue. Associated above and belowground responses were also evaluated.
2. Materials and Methods
2.1. Experimental Site
The experiment was established and conducted at the Forage Systems Research Center (FSRC) in Linneus, MO, USA (39°51′ N, 93°6′ W) in 2017 and 2018. The 10-year average annual rainfall at this site is 1040.6 mm, and the annual rainfall during the experiment was 850.7 mm and 916.1 mm in 2017 and 2018, respectively (Table 1).

Table 1.
Total rainfall and temperature for the experimental site by month for 2017, 2018, and the 10-year mean. Data were collected at the University of Missouri Forage Systems Research Center near Linneus, MO (National Weather Service Location identifier CLLM7).
Predominant soil types included Lagonda silt loam, Leonard silt loam, and Armstrong clay loam. Composite soil samples were collected on 28 October 2016, at a depth of 15 cm, indicating a pH of 6.2. Bray-I P, Ca, Mg, and K were 11, 3432, 365, and 127 kg ha−1, respectively. Soil organic matter was 34 g kg−1. The estimated cation exchange capacity was 12.9 cmol kg−1.
2.2. Experimental Design and Management
Treatments consisted of two forage systems arranged in a randomized complete block design with four replications (n = 8). Each replicate was subdivided into 13 paddocks of 0.16 ha (grazing cells). The first treatment was novel-endophyte-infected tall fescue (Schedonorus arundinaceus (Schreb.) Dumort, nom. Cons) (‘BarOptima’ cultivar, Barenbrug USA, Tangent, OR, USA) with no N inputs (TF). The second treatment was novel-endophyte-infected tall fescue intercropped with sunn hemp (crescent sun cultivar; TF+SH). Established tall fescue pastures were soil-tested in the fall of 2016. Pastures received commercial fertilizer (8-17-148; N-P-K, kg ha−1) to meet the University of Missouri Soil Testing Laboratory recommendations. Pastures were sprayed in November 2016 and May 2017 with a broadleaf-selective herbicide (Weedmaster, Nufarm Canada, Calgary, AB, Canada; 2.34 L ha−1) to remove existing legumes. Mechanical harvesting was conducted between 29 May and 31 May in 2017 and 2018 to remove reproductive growth and reduce competition for emerging sunn hemp. Thus, we consider that the effect of other plants on N input was negligible within the study conditions. Sunn hemp was no-till drilled into tall fescue sod at a rate of 45 kg ha−1 at a depth of 20 mm on 22 June 2017 and 1 June 2018. The establishment date of the TF+SH varied over the years due to weather conditions (Table 1).
For this study, grazing was used to achieve nutrient (N) cycling that would normally occur by urine and feces deposition and was imposed from July to October in 2017 and 2018. Animal management protocol #8925 was reviewed and approved by the University of Missouri Animal Care and Use Committee. Grazing commenced on 6 July 2017 and 3 July 2018. Fall-born, weaned red Angus calves were weighed on consecutive days before grazing. Cattle were stratified by the initial body weight (BW) into groups comprised of three steers and one heifer (304 kg ± 12). The stocking rate over the period was 3.7 and 4.1 AU ha−1 (UA = 350 kg BW) for TF and TF+SH, respectively. The stocking rate was adjusted based on the area allocated rather than the number of animals. Pastures were rotationally stocked from July through October in both years (117 and 120 days, respectively). All treatment groups received ad libitum water and trace minerals (MFA Inc., Columbia, MO, USA). A temporary aboveground water pipe provided water to a single 568-liter tank per group. Water and mineral troughs were moved in synchrony with cattle among paddocks. Single-day weights were measured 42 days after grazing began. Cattle were removed from the study on 31st October in both years. We did not focus on animal responses since grazing animals were merely a tool to distribute N within treatments, simulating real N cycling through animal excreta that would occur in real conditions. Each paddock had its own set of animals that were not mixed across experimental units to avoid N transfer between treatments.
2.3. Sampling and Evaluations
Forage canopy height was measured using a single sonic sensor (ToughSonic®, Senix, Hinesburg, VT, USA) mounted to an ATV to guide the grazing management and assess the HA. The sensor was operating at 50 hertz, moving at 10 km h−1. The position of the sensor on the ATV was unchanged throughout the study. However, the sensor-to-ground height varied due to the operator’s weight and ATV tire pressure. A concrete pad 30 m in length was used for daily tare to account for sensor-to-ground variability. Two north and south passes were performed before measuring the pastures. The average of those four passes was considered the daily tare. The tare was subtracted from canopy height to standardize measurements between dates. A single north and south pass were driven across each paddock. Software designed by the University of Missouri was used to record canopy height data. The canopy heights within the paddocks were extracted and georeferenced using SAS 9.4 (SAS Inst. Inc., Cary, NC, USA). Data points with a GPS track angle of 0° and 180° ± 20° were retained unless the speed was less than 7.5 km h−1. Repeated measurements were removed using the SAS lag function. Canopy heights were georeferenced and parsed into paddocks using PROC GMAP and PROC GINSIDE. The average canopy height of each paddock was calculated from parsed data using PROC MEANS. Average paddock heights were uploaded to the University of Missouri Division of Plant Science forage data management system to store and manage forage height.
The forage canopy height was measured using the sonic sensor every week, before and after grazing, from June to November of both years (2017 and 2018). Weekly measurements were used to develop grazing wedges for each group of cattle. Cattle rotation between paddocks was performed to maintain a minimum residual forage height of 10 cm. Cattle rotated from the grazed paddock with the lowest forage height to the paddock with the greatest forage height.
Calibration curves were generated to convert canopy height to herbage mass (kg ha−1). Strips of 10 m2 were previously delineated in each paddock for calibration, and the canopy height was measured before the mechanical harvest of the calibration strip. Average canopy heights were calculated for each calibration strip. Then, a single 10 m2 strip was harvested to 10 cm from the previously measured area using a custom-built flail harvester (University of Missouri-Columbia, Columbia, MO, USA). A subsample was collected from each harvested strip and dried at 55 °C until reaching a constant weight to obtain the herbage dry weight. A linear calibration curve was generated using the PROC REG procedure from SAS 9.4 (SAS Inst. Inc.). The response variable was the herbage dry mass of the calibration strip (kg ha−1) regressed against the forage canopy height. From the regression equation, β1 was multiplied by the forage canopy height obtained using the sensor, and β0 was added to get the actual herbage mass.
Pre- and post-grazing herbage mass was measured between grazing events. The post-grazing herbage mass was then subtracted from the subsequent pre-grazing herbage mass to estimate the HA in each paddock. The average rest periods (i.e., intervals between grazing events) were 36 days in 2017 and 45 days in 2018 (drought year). The average HA was estimated as the average accumulation (average of the replicates by treatment) from 1 June through October.
Each paddock was sampled before and after grazing events to evaluate N dynamics. At each sampling, two 0.2 m2 quadrats were placed, and the area within the quadrat was removed (above and below ground biomass) to a soil depth of 15 cm. Total shoot biomass was separated by plant species, and then shoots were removed from the roots. Soil was removed from the roots of both species using compressed air. A subsample of belowground biomass was hand-washed to account for residual soil in the samples. All samples were dried in a forced air oven at 55 °C until reaching a constant weight. Dry weights were recorded for above and belowground biomass. All samples were ground to pass a 1 mm screen using a Wiley mill (Thomas Scientific, Swedesboro, NJ, USA). Subsamples were ball-milled using a Mixer Mill MM 400 (Retsch) at 25 Hz for 9 min and analyzed for total N in a CHNS analyzer (Vario Micro Cube; Elementar) using the Dumas dry combustion method [29].
Biological N fixation was determined using the δ15N natural abundance method [30]. This technique assumes that an N-fixing legume growing in soil containing mineral N should have a δ15N between the values of the two possible N sources: soil and atmospheric N. A non-N-fixing reference plant is then used to assess the δ15N of the soil mineral N [30]. Unfertilized tall fescue samples were collected from grazed TF within each sampling period and used as a reference to estimate BNF. The proportion of N derived from the atmosphere (%NDFA) was calculated using the equation described by Unkovich et al. [30] (Equation (1)) for both sunn hemp and tall fescue herbage in TF+SH, where the δ15N of the reference plant is the δ15N value for the non-fixing reference plant (tall fescue grown in monoculture), the δ15N of the N-fixing legume is the δ15N value for the N-fixing sunn hemp grown in TF+SH, and B is the δ15N value for the N-fixing plant grown in the absence of inorganic N.
For this experiment, B = −1.35‰, as reported by Okito et al. [31] for sunn hemp not inoculated with rhizobium strains. The δ15N value from the reference plants equaled 2.31‰ (±0.21, SEM).
The percentage of N transferred from the sunn hemp to the tall fescue (%Ntran) in TF+SH was calculated according to (Frankow-Lindberg and Dahlin, [32]; Equation (2)), where the δ15N non-fixing species in the pure stand was the grass component of the TF treatment and the δ15N non-fixing species in the mixed stand was the grass component of the TF+SH treatment:
Nitrogen stock was estimated by multiplying the above and belowground biomass by the total nitrogen concentration. The aboveground BNF was estimated by multiplying the nitrogen stock by %NDFA.
2.4. Statistical Analysis
Data were analyzed using the PROC GLIMMIX procedure of SAS 9.4 (SAS Inst. Inc.). Response variables included the δ15N, %NDFA, %Ntran, plant N concentration, root mass, HA, N stock, and biologically fixed N stock of above and belowground biomass. Harvest timing was considered a fixed effect for sunn hemp, and year, block, and block × treatment interactions were considered random effects. For tall fescue, treatment, harvest timing, and their interactions were considered fixed effects, and year, block, and block × treatment interactions were considered random effects. The LS-Means were compared using PDIFF. Differences were considered significant when p ≤ 0.05.
3. Results
The herbage accumulation and root mass of tall fescue and sunn hemp grown in TF and TF+SH are shown in Figure 1A,B, respectively. Herbage accumulation was 16% greater (p = 0.02) for tall fescue grown in TF+SH (2772 kg ha−1) compared to tall fescue grown in monoculture (TF; 2386 kg ha−1; Figure 1A). The sunn hemp herbage accumulation in TF+SH was 1864 kg ha−1 (Figure 1A), totaling a 94% increase in total herbage accumulation in TF+SH compared to TF (2386 vs. 4636 kg ha−1). The tall fescue root mass in TF (71,848 kg ha−1) was 50% greater (p < 0.0001) than tall fescue grown in TF+SH (48,030 kg ha−1) and 43% greater compared to tall fescue and sunn hemp combined in TF+SH (50,270 kg ha−1; Figure 1B). The sunn hemp root mass (2239 kg ha−1) was less than that of tall fescue with either treatment (Figure 1B).
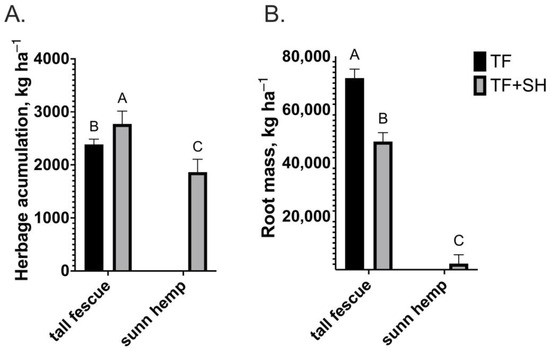
Figure 1.
(A) Herbage accumulation (p = 0.02; SEM = 99.3) of tall fescue monoculture (TF) and tall fescue or sunn hemp intercropped in tall fescue pasture (TF+SH) between two subsequent grazing cycles; (B) Root mass (p < 0.0001; SEM 335) of tall fescue monoculture (TF) and tall fescue or sunn hemp intercropped in tall fescue pasture (TF+SH). Data are averaged over cycles for each treatment. Values are LS-means ± SEM. LS-means with the same letter are not significantly different (p < 0.05).
Herbage N concentration was 34% greater (p = 0.0001) in sunn hemp shoots (1.9%) compared to tall fescue shoots in TF or TF+SH (1.42%; Figure 2A), resulting in an N stock of 92 kg N ha−1 in TF+SH compared to 35 kg N ha−1 in TF (Figure 3A). The sunn hemp root N concentration (0.94%) was 34% greater (p = 0.0004) than that of tall fescue in either TF or TF+SH (0.7%; Figure 2B). The root N stock from the sunn hemp component of TF+SH was only 16 kg N ha−1 due to the low root mass (Figure 3B). The root N stock produced by tall fescue in TF (489 kg ha−1) was 48% greater (p < 0.0001) than that of tall fescue produced in TF+SH (342 kg ha−1; Figure 3B).
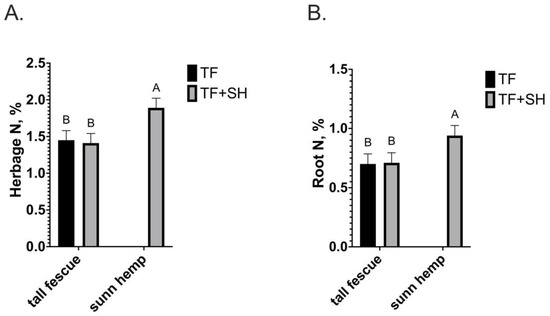
Figure 2.
(A) Herbage nitrogen concentration of tall fescue monoculture (TF) and tall fescue or sunn hemp intercropped in tall fescue pasture (TF+SH; p < 0.0001; SEM = 0.13); (B) Root nitrogen concentration of tall fescue monoculture (TF) and tall fescue or sunn hemp intercropped in tall fescue pasture (TF+SH; p = 0.0004; SEM 0.08). Values are LS-means ± SEM. LS-means (within plant fraction) with the same letter are not significantly different (p < 0.05).
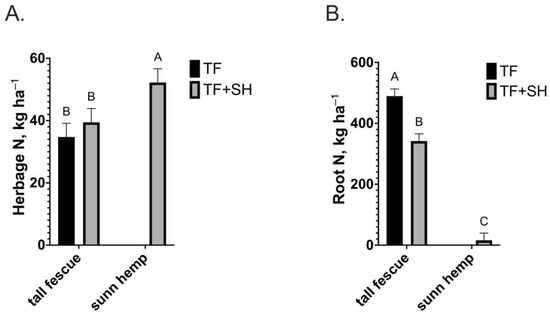
Figure 3.
(A) Herbage nitrogen stock of tall fescue monoculture (TF) and tall fescue or sunn hemp intercropped in tall fescue pasture (TF+SH; p = 0.0001; SEM = 4.2); (B) Root N stock of tall fescue monoculture (TF) and tall fescue or sunn hemp intercropped in tall fescue pasture (TF+SH; p < 0.0001; SEM 2.4). Values are LS-means ± SEM. LS-means (within plant fraction) with the same letter are not significantly different (p < 0.05).
The shoot δ15N was depleted (−1.2‰) in sunn hemp herbage compared to that of tall fescue in TF or TF+SH (2.2‰; p < 0.0001; Figure 4A). Sunn hemp root δ15N values were positive but depleted compared to that of tall fescue roots with either treatment (p = 0.01; Figure 4B).
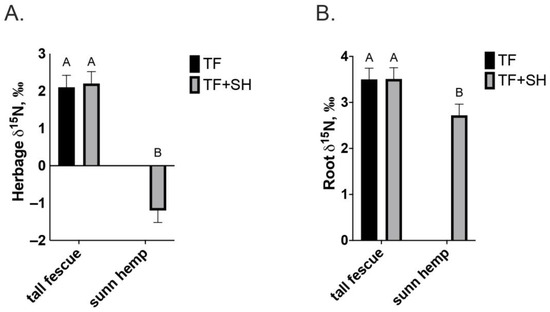
Figure 4.
(A) Herbage δ15N of tall fescue monoculture (TF) and tall fescue or sunn hemp intercropped in tall fescue pasture (TF+SH; p < 0.0001; SEM = 0.20); (B) Root δ15N of tall fescue monoculture (TF) and tall fescue or sunn hemp intercropped in tall fescue pasture (TF+SH; p = 0.01; SEM 0.24). Values are LS-means ± SEM. LS-means with the same letter are not significantly different (p < 0.05).
There was no difference in the percentage of NDFA in sunn hemp in 2017 compared to 2018 (88 and 100%, respectively; p > 0.05; Figure 5). The percentage of Ntran from sunn hemp to tall fescue in 2017 compared to 2018 also did not differ (13 and 20%, respectively; p > 0.05; Figure 5). Biological N fixation by sunn hemp was 21% greater (p < 0.0001) in 2018 compared to 2017 (53.8 and 44.3 kg ha−1, respectively; Figure 5).
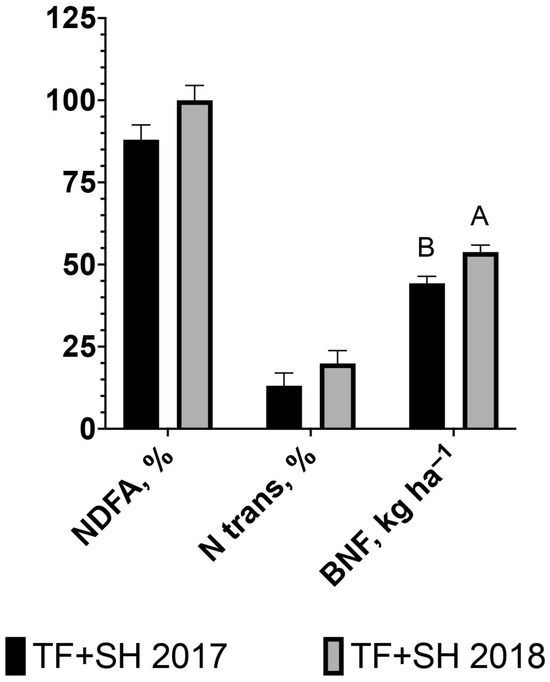
Figure 5.
Herbage percent nitrogen derived from the atmosphere (NDFA, %) in sunn hemp, percent nitrogen transferred (N trans, %) from sunn hemp to tall fescue, and biological nitrogen fixation (BNF, kg ha−1) by sunn hemp intercropped in tall fescue pasture (TF+SH). Values are LS-means ± SEM. LS-means with the same letter are not significantly different (p < 0.05). NDFA, % (p = 0.11; SEM 4.6); N trans, % (p = 0.24; SEM 3.8); BNF kg ha−1, (p < 0.0001; SEM 2.1).
4. Discussion
The increased HA of TF+SH compared to TF monoculture observed in this study corroborates Corbin et al. [18,33], who reported increased TF herbage mass when mixed with N-fixing legumes compared to TF monoculture in the transition zone of the USA. The same authors suggested red clover as an alternative source to synthetic N in TF swards. Although classified as a cool-season legume, red clover has demonstrated the potential to provide forage in late spring and summer in the transition zone of the USA due to favorable climatic conditions and a high rate of BNF [18,34]. Including legumes into tall fescue pastures also demonstrated the potential to increase animal performance compared to unfertilized tall fescue monoculture [35], or to keep the same animal performance as N fertilized tall fescue pastures [18]. Conversely, Sheaffer et al. [36] reported that temperate legumes interseeded in tall fescue pastures did not result in greater HA than tall fescue monocultures. Cool-season legumes may reduce shoot and root growth in temperatures above 25–30 °C [7,11,37], likely decreasing the BNF and HA. Thus, warm-season legumes might be options at temperatures above 25 °C. In this study, intercropping sunn hemp into tall fescue pastures positively affected the HA of the forage system. Much of this response was likely due to the HA resulting from the branching re-growth habit of sunn hemp following grazing, similar to previous reports by Lepcha et al. [38] in a sunn hemp monoculture. Besides this, using a warm-season legume such as sunn hemp might enhance BNF during the warm season, increasing the HA of the companion grass in grass–legume mixed swards [18]. The average temperature over the experimental period in both years ranged from 21 to 25 °C (Table 1), with maximum daily temperatures above 25 °C, indicating that warm-season legumes may be more productive than temperate species during the summer in the study region. Herbage accumulation of up to 7000 kg DM ha−1 has been reported in a pure stand of sunn hemp during a 90 d growing season [20,21]. Lesser values (3000 kg DM ha−1) have been reported for sunn hemp grown in monoculture in Missouri [38]. Likewise, lesser values were observed in this study, likely due to competition in a mixed sward and below-average rainfall during the 2018 growing season, which was 82% and 197% below the norm in June and July, respectively (Table 1). Our result shows that intercropping sunn hemp in TF pastures can increase the HA during the warm season, despite the competition between legumes and grasses in mixed pastures for photosynthetic radiation, soil moisture, and soil nutrients that normally limit the HA and affect the spatial distribution of each species [39,40]. The lower root mass in TF+SH than that in TF is associated with a much lower root mass of sunn hemp compared to TF. Tall fescue typically has a high root density and depth compared to other cool-season grasses, such as perennial ryegrass [41], while low sunn hemp root mass (<3000 kg ha−1) was also reported by Abdul-baki et al. [42]. Despite the low root mass, a root N of only 1% could result in as much as 22 kg of N per hectare released into the soil following the sunn hemp growing season, based on the sunn hemp root mass and N concentration observed in the current study. This might contribute to an increase in the HA in TF+SH systems.
The lower root mass in TF+SH than in TF is associated with a much lower root mass of sunn hemp compared to TF. Tall fescue typically has a high root density and depth compared to other cool-season grasses, such as perennial ryegrass [41], while a low sunn hemp root mass (<3000 kg ha−1) was also reported by Abdul-baki et al. [42]. Despite the low root mass, a root N of only 1% could result in as much as 22 kg of N per hectare being released to the soil following the sunn hemp growing season, based on the sunn hemp root mass and N concentration observed in the current study. This might contribute to an increase in the HA in TF+SH systems.
The herbage N concentration of sunn hemp grown in this study (1.9%) was within the range observed by Lepcha et al. [38] (1.5–2.1%) during the same season for sunn hemp under different harvesting management, and by Garzon et al. [19] for five different genotypes over two harvesting dates (1.7–2.5%). On the other hand, it was lower than what was observed by Jaramillo et al. [15] for different sunn hemp cultivars (2.8–3.0%) during spring/summer in north Florida. This is likely because of the greater cumulative precipitation and temperatures during that study compared to our experimental period. The greater herbage N concentration in sunn hemp shoots and roots than in tall fescue shoots and roots, associated with the increased HA, led to much greater aboveground N stock in TF+SH systems compared to TF.
The average N concentration of sunn hemp shoot in the current study (1.9%) was similar to the range observed by Garzon et al. [43] over two years (1.9–2.4%). Similarly, the average annual N stock in a system consisting of tall fescue interseeded with sunn hemp (91 kg N ha−1) was within the range observed by Garzon et al. [43] and Sant’Anna et al. [44] in a pure stand of sunn hemp (62–133 kg N ha−1), while the N stock in TF monoculture was considerably less than that of sunn hemp or TF+SH (35 kg N ha−1). Our findings suggest that intercropping sunn hemp in tall fescue pastures can increase the N stock in the aboveground biomass due to the increased HA and N concentration, but might reduce the belowground N stock due to the lower root mass of sunn hemp. Despite the lower belowground N stock, the greater aboveground N stock in TF+SH than in TF may be recycled and help build soil organic matter over time.
Jaramillo et al. [15] reported sunn hemp herbage δ15N values of 0.733 ‰, while Sant’Anna et al. [44] reported 3.3‰, considerably less depleted than the sunn hemp grown in this study. The sunn hemp herbage δ15N observed in this study was similar in depletion to a warm-season perennial legume, rhizoma peanut [45]. This might be associated with the soil (e.g., texture or soil organic matter %) and weather conditions, since the studies were conducted in different seasons and regions.
The percent NDFA in sunn hemp observed in this study (88–100%) appears to be considerably greater than that of sunn hemp varieties evaluated by Jaramillo et al. [15], ranging from 48 to 56%. Ashworth et al. [9] found a high variability across sites, estimation methods, and years in NDFA% in sunn hemp (37–86%). The inconsistency between studies indicates that estimation methods and environmental factors affect the percent NDFA in sunn hemp swards, requiring site-specific evaluations and standardized methodological approaches. In the present study, as much as 100% of the N acquired by sunn hemp was NDFA. The results from this study confirm that N was transferred from the intercropped sunn hemp to tall fescue in mixed pastures. As much as 20% of N acquired by sunn hemp was transferred to tall fescue, indicating cycling of N between sunn hemp and tall fescue throughout the experimental period that resulted in increased HA.
There are multiple mechanisms for N transfer from sunn hemp to tall fescue in a mixed pasture system. Transfer mechanisms include nutrient cycling via cattle excreta, litter decomposition, and belowground transfer [25,26,46]. Previous research findings have suggested that 70 to 95% of nitrogen ingested during grazing is excreted as urine and feces [6,47]. Direct observations from this study suggest that cattle generally removed sunn hemp leaf material, resulting in a reduced leaf-to-stem residue ratio following grazing events. This is not surprising, considering that sunn hemp leaves have previously been reported to have greater N concentrations compared to stems [48]. Mansoer et al. [20] reported 70% greater nitrogen concentrations in sunn hemp leaves compared to stems 12 weeks after planting. The utilization of N-dense sunn hemp by cattle likely resulted in N deposition following grazing. Despite the low root mass, the high root N concentration suggests that sunn hemp root turnover and decomposition may have also contributed to greater N transferred from sunn hemp to tall fescue. Stallings et al. [49] reported nitrogen mineralization from sunn hemp residues greater than 20% of the total nitrogen present 20 d after harvest.
Nitrogen transfer from legumes to cool-season grasses has been well documented. This large body of research has focused on cool-season grasses interseeded with perennial legumes such as red clover, white clover, and alfalfa [24,40,50,51]. Biological N fixation by these legumes has been reported as 55 to 296 kg N ha−1 annually in monoculture [52], with a seasonal BNF ranging from 81 to 150 kg N ha−1 (Balkcom and Reeves, 2005; Garzon et al., 2021; Rotar and Joy, 1983; Schomberg et al., 2007). Legume-N transfer to grasses in mixed pastures has resulted in up to 80% of the nitrogen accumulation in the grass component. Perennial legumes demonstrate greater accumulation and transfer of biologically fixed N compared to annual legumes due to increases in aboveground biomass, belowground biomass, and soil fertility [53,54,55,56]. Schipanski and Drinkwater [57] reported reduced N transfer in annual field pea intercropped in orchard grass compared to red clover, and that N transferred was positively correlated with legume biomass. Limited literature is available to compare N transferred in cool-season grass pastures intercropped with warm-season annual legumes.
Though sunn hemp in mixed pastures has not been thoroughly researched, BNF in sunn hemp has been reported when used as a green manure or cover crop [21,22,23]. Biological N fixation is proportional to the herbage mass [10]. Aboveground biologically fixed N observed in this experiment ranged from 44 to 54 kg N ha−1, within the range previously reported by Jaramillo et al. [15] and Garzon et al. [19] in pure stands of different sunn hemp varieties (25–41 and 26–81 kg N ha−1, respectively). Belowground N accounted for an additional 16 kg N ha−1.
Interseeding sunn hemp into tall fescue resulted in greater forage-system N concentrations compared to a pure stand of tall fescue. Due to greater N concentrations in the sunn hemp intercropped system and subsequent nutrient cycling, greater HA would be expected since N is the first limiting nutrient for herbage growth [58]. However, other pasture systems intercropped with annuals have demonstrated declines as great as 30% in tall fescue herbage mass [59,60], likely due to competition, low growth rates, and the limited BNF of some species, especially in the warm season.
5. Conclusions
The potential of N transfer from sunn hemp to tall fescue was confirmed in this experiment. Under the evaluated conditions, sunn hemp can fix as much as 54 kg ha−1 of atmospheric N during the warm season in intercropped systems with tall fescue. The grazing of sunn hemp combined with the decomposition of belowground biomass contributes to transferring of up to 20% of the N from sunn hemp to tall fescue. Intercropping sunn hemp in tall fescue pastures increases herbage accumulation and N yield. Sunn hemp intercropped into tall fescue pasture can provide an alternative N source to tall fescue-based forage-livestock systems. However, it is important to note that biological nitrogen fixation and the transfer of nitrogen from legumes to companion grasses can widely vary depending on herbage accumulation, legume species and proportion, soil characteristics and microbiology, agronomic practices, and environmental conditions. Additionally, nitrogen transfer from sunn hemp to tall fescue in mixed pasture systems involves multiple mechanisms, highlighting the need for further research to better understand the primary factors influencing this process.
Author Contributions
Conceptualization, H.D.N. and J.C.B.D.J.; methodology, J.A.T. and J.A.L.; validation, H.D.N., J.C.B.D.J. and J.A.T.; formal analysis, H.D.N.; investigation, J.A.T.; resources, H.D.N. and J.A.L.; data curation, J.A.T. and I.L.B.; writing—original draft preparation, H.D.N.; writing—review and editing, J.C.B.D.J. and I.L.B.; visualization, I.L.B.; supervision, H.D.N.; project administration, H.D.N.; funding acquisition, H.D.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be available upon request to the corresponding author.
Acknowledgments
The authors acknowledge the University of Missouri for the field investigation and data analysis and the University of Florida, North Florida Research and Education Center in Marianna, FL, for collaborating on this project.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BNF | Biological nitrogen fixation |
| HA | Herbage accumulation |
| N | Nitrogen |
| NDFA | Nitrogen derived from the atmosphere |
| Ntran | Nitrogen transfer |
| SH | Sunn hemp |
| TF | Tall fescue |
References
- Kubesch, J.O.C.; Nave, R.L.G.; Cui, S.; Bates, G.E.; Butler, D.M.; Pantalone, V. Transitional Organic Forage Systems in the Southeastern U.S.: Production and Nutritive Value. Agron. J. 2022, 114, 1269–1283. [Google Scholar] [CrossRef]
- Min, D.-H.; Moyer, J.L. Establishing Legumes in a Tall Fescue Sward. Am. J. Plant Sci. 2015, 06, 355–361. [Google Scholar] [CrossRef]
- Mullenix, K.; Dillard, S.; Severino da Silva, L.; Dubeux, J., Jr.; Tucker, J.; Baxter, L.; Prevatt, C.; Santos, E.; Garcia, L. Forage Legumes in the Southeast; Clemson University Cooperative Extension Service: Pickens County, SC, USA, 2023. [Google Scholar]
- Evers, G. The Interaction of Annual Ryegrass and Nitrogen on Arrowleaf Clover in the Southeastern United States. Crop Sci. 2011, 51, 1353–1360. [Google Scholar] [CrossRef]
- MacMillan, J.; Adams, C.B.; Hinson, P.O.; DeLaune, P.B.; Rajan, N.; Trostle, C. Biological Nitrogen Fixation of Cool-season Legumes in Agronomic Systems of the Southern Great Plains. Agrosyst. Geosci. Environ. 2022, 5, e20244. [Google Scholar] [CrossRef]
- Rouquette, F.M.; Smith, G.R. Review: Effects of Biological Nitrogen Fixation and Nutrient Cycling on Stocking Strategies for Cow-Calf and Stocker Programs. Prof. Anim. Sci. 2010, 26, 131–141. [Google Scholar] [CrossRef]
- Dave, K.; Kumar, A.; Dave, N.; Jain, M.; Dhanda, P.S.; Yadav, A.; Kaushik, P. Climate Change Impacts on Legume Physiology and Ecosystem Dynamics: A Multifaceted Perspective. Sustainability 2024, 16, 6026. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Randall, H.C. Nitrogen and Biomass Accumulation by Alfalta under High Temperatures of Late Summer. Field Crops Res. 1993, 31, 287–294. [Google Scholar] [CrossRef]
- Ashworth, A.J.; West, C.P.; Allen, F.L.; Keyser, P.D.; Weiss, S.A.; Tyler, D.D.; Taylor, A.M.; Warwick, K.L.; Beamer, K.P. Biologically Fixed Nitrogen in Legume Intercropped Systems: Comparison of Nitrogen-Difference and Nitrogen-15 Enrichment Techniques. Agron. J. 2015, 107, 2419–2430. [Google Scholar] [CrossRef]
- Blesh, J. Functional Traits in Cover Crop Mixtures: Biological Nitrogen Fixation and Multifunctionality. J. Appl. Ecol. 2018, 55, 38–48. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The Effect of Increasing Temperature on Crop Photosynthesis: From Enzymes to Ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology and Development, 6th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2015; ISBN 978-1-60535-255-8. [Google Scholar]
- Gadberry, M.S.; Hawley, J.; Beck, P.A.; Jennings, J.A.; Kegley, E.B.; Coffey, K.P. BILL E. KUNKLE INTERDISCIPLINARY BEEF SYMPOSIUM: A Meta-Analysis of Research Efforts Aimed at Reducing the Impact of Fescue Toxicosis on Cattle Weight Gain and Feed Intake1. J. Anim. Sci. 2015, 93, 5496–5505. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.G.; White, G.A. Crotalaria Juncea: A Potential Multi-Purpose Fiber Crop. In Progress in New Crops; ASHS Press: Arlington, VA, USA, 1996; pp. 389–394. [Google Scholar]
- Jaramillo, D.M.; Dubeux, J.C.B.; Vendramini, J.M.B.; Queiroz, L.M.D.; Santos, E.R.S.; Ruiz-Moreno, M.; Garcia, L.; De Abreu, D.S.; De Miranda, L.R.; De Siqueira, M.C.F. Establishment Techniques Affect Productivity, Nutritive Value and Atmospheric N2 Fixation of Two Sunn Hemp Cultivars. Grass Forage Sci. 2020, 75, 153–158. [Google Scholar] [CrossRef]
- Eberle, C.; Shortnacy, L. Sunn Hemp Planting Date Effect on Growth, Biomass Accumulation, and Nutritive Value in Southeastern Wyoming. Crop Sci. 2021, 61, 4447–4457. [Google Scholar] [CrossRef]
- Kaneko, M.; Da Silva, H.M.S.; Vendramini, J.M.B.; Sanchez, J.M.D.; De Sousa, J.L.; De Oliveira, R.A. Hybrid Brachiariagrass (Urochloa Spp.) Establishment with Annual Sorghum Sudangrass (Sorghum bicolor (L.) Moench) and Sunn Hemp (Crotalaria juncea L.) Mixtures at Half and Full Recommended Seeding Rates. Grassl. Sci. 2023, 69, 87–95. [Google Scholar] [CrossRef]
- Corbin, M.D.; Nave, R.L.G.; Naumann, H.D.; Bates, G.E.; Boyer, C.; De Almeida, O.G. Inclusion of Cool- and Warm-season Species in Tall Fescue Swards for Increased Productivity. Agron. J. 2024, agj2.21690. [Google Scholar] [CrossRef]
- Garzon, J.; Vendramini, J.M.B.; Silveira, M.L.; Moriel, P.; Da Silva, H.M.S.; Dubeux, J.C.B.; Kaneko, M.; Carnelos, C.C.; Mamede, P.A. Harvest Management and Genotype Effects on Sunn Hemp Forage Characteristics. Agron. J. 2021, 113, 298–307. [Google Scholar] [CrossRef]
- Mansoer, Z.; Reeves, D.W.; Wood, C.W. Suitability of Sunn Hemp as an Alternative Late-Summer Legume Cover Crop. Soil Sci. Soc. Amer. J. 1997, 61, 246–253. [Google Scholar] [CrossRef]
- Balkcom, K.S.; Reeves, D.W. Sunn-Hemp Utilized as a Legume Cover Crop for Corn Production. Agron. J. 2005, 97, 26–31. [Google Scholar] [CrossRef]
- Rotar, P.P.; Joy, R.J. “Tropic Sun” Sunn Hemp; Crotalaria Juncea L.; Research Extension Series; RES-36; University of Hawaii: Honolulu, HI, USA, 1983; 7p. [Google Scholar]
- Schomberg, H.H.; Martini, N.L.; Diaz-Perez, J.C.; Phatak, S.C.; Balkcom, K.S.; Bhardwaj, H.L. Potential for Using Sunn Hemp as a Source of Biomass and Nitrogen for the Piedmont and Coastal Plain Regions of the Southeastern USA. Agron. J. 2007, 99, 1448–1457. [Google Scholar] [CrossRef]
- Brophy, L.S.; Heichel, G.H.; Russelle, M.P. Nitrogen Transfer from Forage Legumes to Grass in a Systematic Planting Design1. Crop Sci. 1987, 27, 753–758. [Google Scholar] [CrossRef]
- Luo, F.; Mi, W.; Liu, W. Legume–Grass Mixtures Improve Biological Nitrogen Fixation and Nitrogen Transfer by Promoting Nodulation and Altering Root Conformation in Different Ecological Regions of the Qinghai–Tibet Plateau. Front. Plant Sci. 2024, 15, 1375166. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; McElroy, M.S.; Chapagain, T.; Papadopoulos, Y.A.; Raizada, M.N. Belowground Nitrogen Transfer from Legumes to Non-Legumes under Managed Herbaceous Cropping Systems. A Review. Agron. Sustain. Dev. 2016, 36, 58. [Google Scholar] [CrossRef]
- Chalk, P.M.; Peoples, M.B.; McNeill, A.M.; Boddey, R.M.; Unkovich, M.J.; Gardener, M.J.; Silva, C.F.; Chen, D. Methodologies for Estimating Nitrogen Transfer between Legumes and Companion Species in Agro-Ecosystems: A Review of 15N-Enriched Techniques. Soil Biol. Biochem. 2014, 73, 10–21. [Google Scholar] [CrossRef]
- Moyer-Henry, K.A.; Burton, J.W.; Israel, D.W.; Rufty, T.W. Nitrogen Transfer Between Plants: A 15N Natural Abundance Study with Crop and Weed Species. Plant Soil 2006, 282, 7–20. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon and Organic Matter. In Methods of Soil Analysis. Part 2; Page, A.L., Miller, R.H., Kenney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 539–579. [Google Scholar]
- Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; Unkovich, M., Cadisch, G., Australian Centre for International Agricultural Research, Eds.; ACIAR monograph series; ACIAR: Canberra, Australia, 2008; ISBN 978-1-921531-26-2. [Google Scholar]
- Okito, A.; Alves, B.R.J.; Urquiaga, S.; Boddey, R.M. Isotopic Fractionation during N2 Fixation by Four Tropical Legumes. Soil Biol. Biochem. 2004, 36, 1179–1190. [Google Scholar] [CrossRef]
- Frankow-Lindberg, B.E.; Dahlin, A.S. N2 Fixation, N Transfer, and Yield in Grassland Communities Including a Deep-Rooted Legume or Non-Legume Species. Plant Soil 2013, 370, 567–581. [Google Scholar] [CrossRef]
- Corbin, M.D.; Nave, R.L.G.; Bates, G.E.; Butler, D.M.; Hawkins, S.A. Alternatives to Conventional Nitrogen Fertilization on Tall Fescue and Bermudagrass. Agron. J. 2019, 111, 275–286. [Google Scholar] [CrossRef]
- Dhamala, N.R.; Eriksen, J.; Carlsson, G.; Søegaard, K.; Rasmussen, J. Highly Productive Forage Legume Stands Show No Positive Biodiversity Effect on Yield and N2-Fixation. Plant Soil 2017, 417, 169–182. [Google Scholar] [CrossRef]
- Kubesch, J.O.C.; Greiner, S.P.; Pent, G.J.; Reid, J.L.; Tracy, B.F. Diversifying Tall Fescue Pastures with Native Warm-season Grasses and White Clover Improves Beef Cattle Performance. Crop Sci. 2025, 65, e70030. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Seguin, P.; Cuomo, G.J. Sward Characteristics and Management Effects on Cool-Season Grass Forage Quality. In Grass for Dairy Catrtle; CABI Publishing: New York, NY, USA, 1998; pp. 73–100. ISBN 978-0-85199-288-4. [Google Scholar]
- Kendall, W.A.; Shaffer, J.A.; Hill, R.R. Effect of Temperature and Water Variables on the Juvenile Growth of Lucerne and Red Clover. Grass Forage Sci. 1994, 49, 264–269. [Google Scholar] [CrossRef]
- Lepcha, I.; Naumann, H.D.; Fritschi, F.B.; Kallenbach, R.L. Herbage Accumulation, Nutritive Value, and Regrowth Potential of Sunn Hemp at Different Harvest Regimens and Maturity. Crop Sci. 2019, 59, 413–421. [Google Scholar] [CrossRef]
- Luo, C.L.; Duan, H.X.; Wang, Y.L.; Liu, H.J.; Xu, S.X. Complementarity and Competitive Trade-Offs Enhance Forage Productivity, Nutritive Balance, Land and Water Use, and Economics in Legume-Grass Intercropping. Field Crops Res. 2024, 319, 109642. [Google Scholar] [CrossRef]
- Spasiani, P.P.; Homem, B.G.C.; de Lima, I.B.G.; Guimarães, B.C.; de Medeiros, E.S.; Muir, J.P.; de Oliveira, M.S.; Boddey, R.M.; Casagrande, D.R. Light Competition Is the Key Factor Determining Spatio-Temporal Variability in Legume Proportion within Marandu Palisadegrass–Forage Peanut Mixed Pastures. Crop Pasture Sci. 2023, 74, 898–910. [Google Scholar] [CrossRef]
- Huang, B.; Gao, H. Root Physiological Characteristics Associated with Drought Resistance in Tall Fescue Cultivars. Crop Sci. 2000, 40, 196–203. [Google Scholar] [CrossRef]
- Abdul-baki, A.A.; Bryan, H.H.; Zinati, G.M.; Klassen, W.; Codallo, M.; Heckert, N. Biomass Yield and Flower Production in Sunn Hemp: Effect of Cutting the Main Stem. J. Veg. Crop Prod. 2001, 7, 83–104. [Google Scholar] [CrossRef]
- Garzon, J.; Vendramini, J.M.B.; Silveira, M.L.; Dubeux, J.C.; Liao, H.; Sollenberger, L.E.; Moriel, P.; Silva, H.M.S.; Gomes, V.C.; Ferreira, I.M.; et al. Residue Management and Genotype Effect on Sunn Hemp Organic Matter and Nitrogen Decomposition. Agron. J. 2023, 115, 261–272. [Google Scholar] [CrossRef]
- Sant’Anna, S.A.C.; Martins, M.R.; Goulart, J.M.; Araújo, S.N.; Araújo, E.S.; Zaman, M.; Jantalia, C.P.; Alves, B.J.R.; Boddey, R.M.; Urquiaga, S. Biological Nitrogen Fixation and Soil N2O Emissions from Legume Residues in an Acrisol in SE Brazil. Geoderma Reg. 2018, 15, e00196. [Google Scholar] [CrossRef]
- Dubeux, J.C.B.; Blount, A.R.S.; Mackowiak, C.; Santos, E.R.S.; Pereira Neto, J.D.; Riveros, U.; Garcia, L.; Jaramillo, D.M.; Ruiz-Moreno, M. Biological N 2 Fixation, Belowground Responses, and Forage Potential of Rhizoma Peanut Cultivars. Crop Sci. 2017, 57, 1027–1038. [Google Scholar] [CrossRef]
- Dubeux, J.C.B.; Sollenberger, L.E.; Mathews, B.W.; Scholberg, J.M.; Santos, H.Q. Nutrient Cycling in Warm-Climate Grasslands. Crop Sci. 2007, 47, 915–928. [Google Scholar] [CrossRef]
- Russelle, M.P. Nitrogen Cycling in Pasture and Range. J. Prod. Agric. 1992, 5, 13–23. [Google Scholar] [CrossRef]
- Lepcha, I.; Naumann, H.D. Partitioning of Forage Mass and Nutritive Value in Sunn Hemp Leaf and Stem Components. Int. J. Agron. 2021, 2021, 5547120. [Google Scholar] [CrossRef]
- Stallings, A.M.; Balkcom, K.S.; Wood, C.W.; Guertal, E.A.; Weaver, D.B. Nitrogen Mineralization from ‘AU Golden’ Sunn Hemp Residue. J. Plant Nutr. 2017, 40, 50–62. [Google Scholar] [CrossRef]
- Høgh-Jensen, H.; Schjoerring, J.K. Interactions between White Clover and Ryegrass under Contrasting Nitrogen Availability: N2 Fixation, N Fertilizer Recovery, N Transfer and Water Use Efficiency. Plant Soil 1997, 197, 187–199. [Google Scholar] [CrossRef]
- Tracy, B.F.; Albrecht, K.; Flores, J.; Hall, M.; Islam, A.; Jones, G.; Lamp, W.; MacAdam, J.W.; Skinner, H.; Teutsch, C. Evaluation of Alfalfa–Tall Fescue Mixtures across Multiple Environments. Crop Sci. 2016, 56, 2026–2034. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Steele, K.W. Biological Nitrogen Fixation in Mixed Legume/Grass Pastures. In Biological Nitrogen Fixation for Sustainable Agriculture; Ladha, J.K., George, T., Bohlool, B.B., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1992; pp. 137–153. ISBN 978-90-481-4164-7. [Google Scholar]
- Carlsson, G.; Huss-Danell, K. Nitrogen Fixation in Perennial Forage Legumes in the Field. Plant Soil 2003, 253, 353–372. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Midega, C.A.O.; Awuor, R.; Nyagol, D.; Khan, Z.R. Perennial Legume Intercrops Provide Multiple Belowground Ecosystem Services in Smallholder Farming Systems. Agric. Ecosyst. Environ. 2021, 320, 107566. [Google Scholar] [CrossRef]
- Meza, K.; Vanek, S.J.; Sueldo, Y.; Olivera, E.; Ccanto, R.; Scurrah, M.; Fonte, S.J. Grass–Legume Mixtures Show Potential to Increase Above- and Belowground Biomass Production for Andean Forage-Based Fallows. Agronomy 2022, 12, 142. [Google Scholar] [CrossRef]
- Reilly, E.C.; Gutknecht, J.L.; Tautges, N.E.; Sheaffer, C.C.; Jungers, J.M. Nitrogen Transfer and Yield Effects of Legumes Intercropped with the Perennial Grain Crop Intermediate Wheatgrass. Field Crops Res. 2022, 286, 108627. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Drinkwater, L.E. Nitrogen Fixation in Annual and Perennial Legume-Grass Mixtures across a Fertility Gradient. Plant Soil 2012, 357, 147–159. [Google Scholar] [CrossRef]
- Santos, E.R.S.; Dubeux, J.C.B.; Mackowiak, C.L.; Blount, A.R.S.; Sollenberger, L.E.; Jaramillo, D.M.; Garcia, L.; Abreu, D.S.; Souza, R.T.; Ruiz-Moreno, M. Herbage Responses and Nitrogen Agronomic Efficiency of Bermudagrass–Legume Mixtures. Crop Sci. 2021, 61, 3815–3829. [Google Scholar] [CrossRef]
- Belesky, D.P.; Wilkinson, S.R.; Dawson, R.N.; Elsner, J.E. Forage Production of a Tall Fescue Sod Intercropped with Sorghum × Sudangrass and Rye. Agron. J. 1981, 73, 657–660. [Google Scholar] [CrossRef]
- Reinbott, T.M.; Blevins, D.G. Multiyear Use of Killed Strips for Forage and Grain Sorghum Production in a Tall Fescue Pasture. J. Prod. Agric. 1995, 8, 354–359. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).