Effects of Downy Mildew Infection and Potassium on Growth and Physiological Traits of Greenhouse Cucumber
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Treatment Details
2.2. Treatments and Experiment Design
2.3. Extraction and Inoculation of Pathogenic Fungi
2.4. Experimental Data Acquisition
2.5. Statistical Analyses
3. Results and Discussion
3.1. Leaf Gas Exchange Parameters
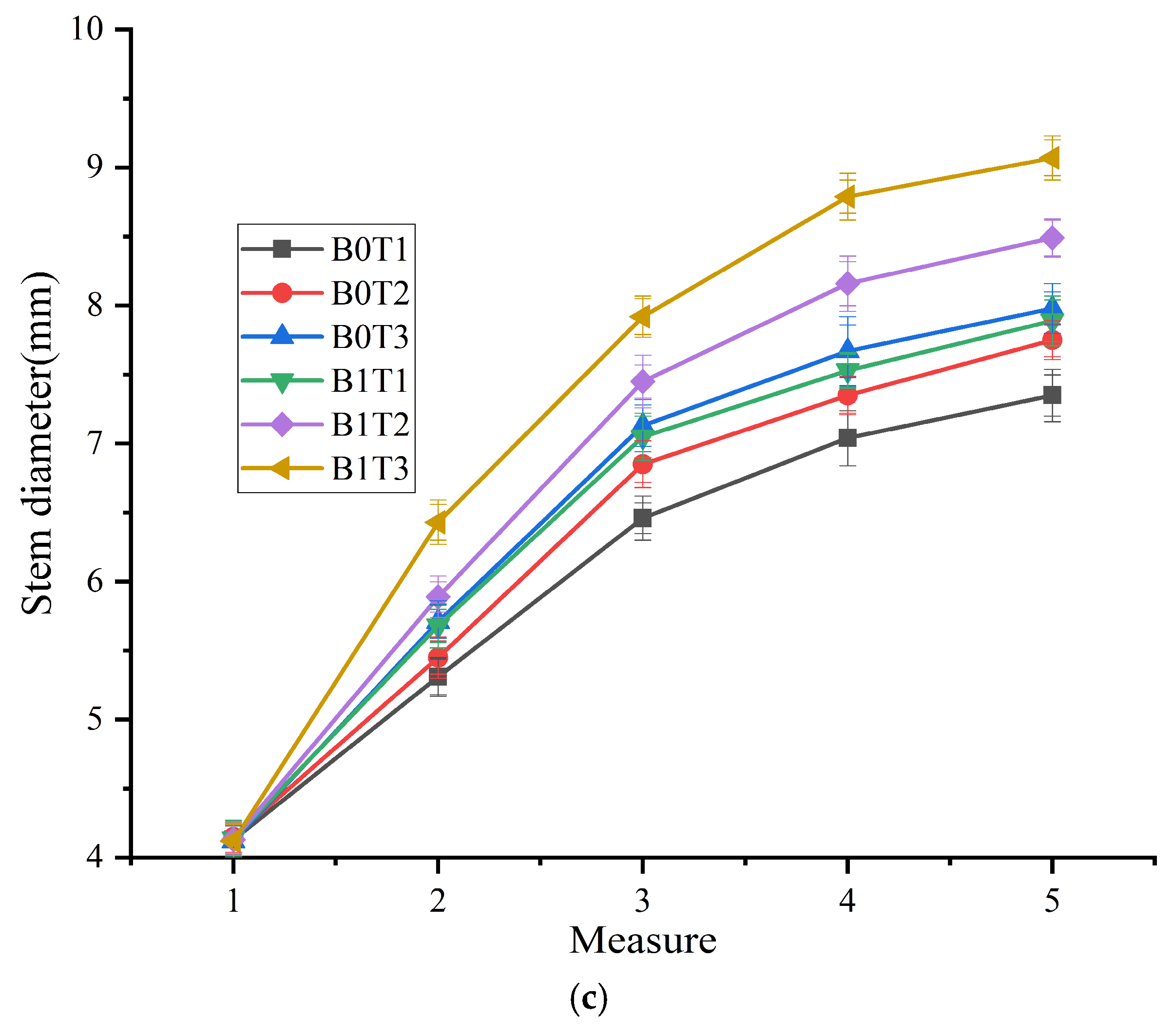
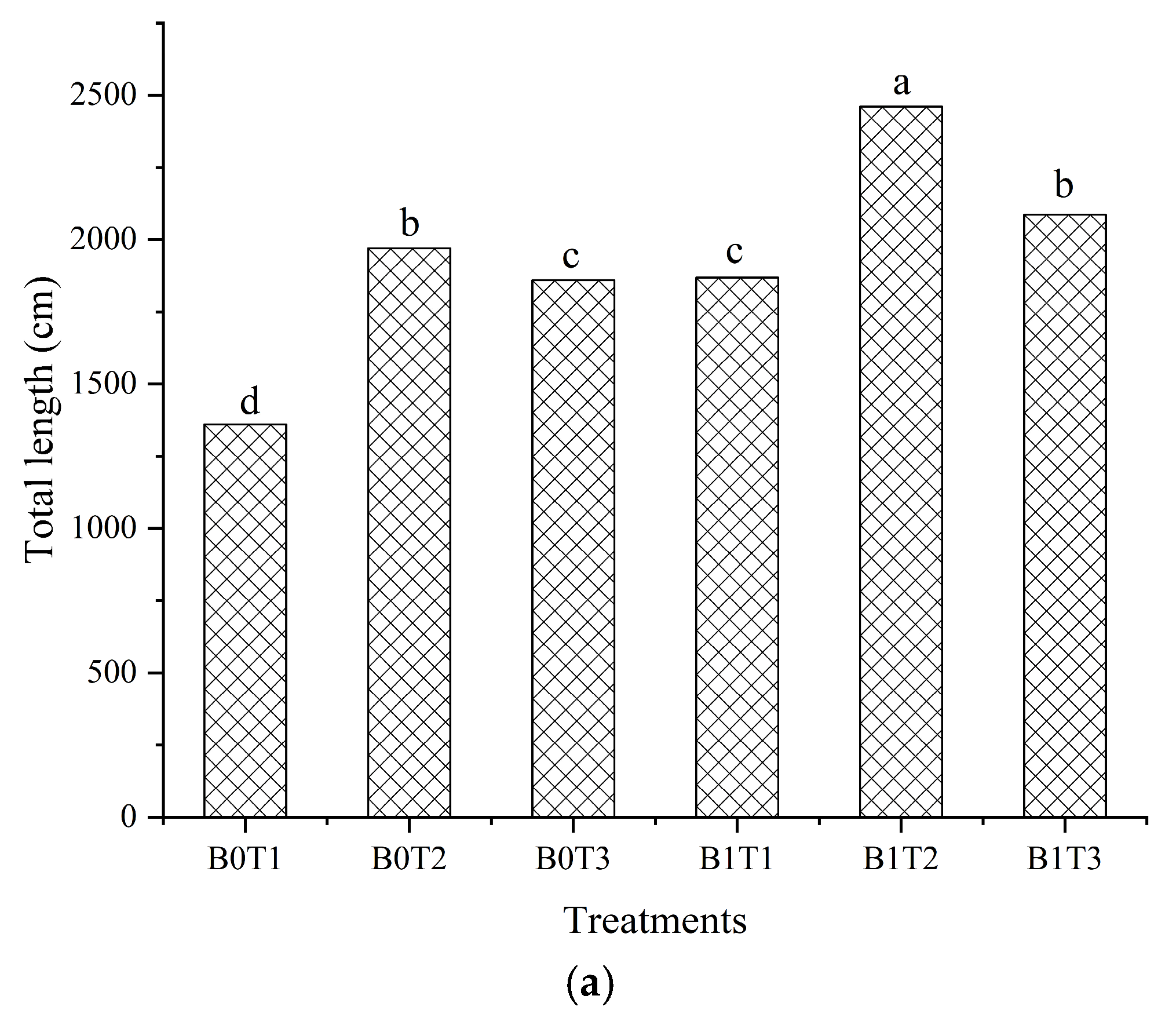
3.2. Cucumber Plants Growth Parameters
3.3. Parameters of Root Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, H.F.; Acquah, S.J.; Zhang, J.Y.; Wang, G.Q.; Zhang, C.; Darko, R.O. Overview of modelling techniques for greenhouse microclimate environment and evapotranspiration. Int. J. Agric. Biol. Eng. 2021, 14, 6. [Google Scholar] [CrossRef]
- Rasool, G.; Guo, X.P.; Wang, Z.C.; Ali, M.U.; Chen, S.; Zhang, S.X.; Wu, Q.; Ullah, M.S. Coupling fertigation and buried straw layer improves fertilizer use efficiency, fruit yield, and quality of greenhouse tomato. Agric. Water Manag. 2020, 239, 106239. [Google Scholar] [CrossRef]
- Yan, H.F.; Deng, S.S.; Zhang, C.; Wang, G.Q.; Zhao, S.; Li, M.; Liang, S.W.; Jiang, J.H.; Zhou, Y.D. Determination of energy partition of a cucumber grown Venlo-type greenhouse in southeast China. Agric. Water Manag. 2023, 276, 108047. [Google Scholar] [CrossRef]
- Yan, H.F.; Zhang, C.; Gerrits, M.C.; Acquah, S.J.; Zhang, H.N.; Wu, H.M.; Zhao, B.S.; Huang, S.; Fu, H.W. Parametrization of aerodynamic and canopy resistances for modeling evapotranspiration of greenhouse cucumber. Agric. For. Meteorol. 2018, 262, 370–378. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.Y.; Yan, H.F.; Ullah, I.; Zuo, Z.Y.; Li, L.L.; Yu, J.J. Effects of irrigation quantity and biochar on soil physical properties, growth characteristics, yield and quality of greenhouse tomato. Agric. Water Manag. 2020, 241, 106263. [Google Scholar] [CrossRef]
- Pan, L.X.; Zhou, C.G.; Jing, J.; Zhuang, M.; Zhang, J.C.; Wang, K.; Zhang, H.Y. Metabolomics analysis of cucumber fruit in response to foliar fertilizer and pesticides using UHPLC-Q-Orbitrap-HRMS. Food Chem. 2022, 369, 130960. [Google Scholar] [CrossRef]
- Shi, J.Y.; Wang, Y.Y.; Li, Z.H.; Huang, X.W.; Shen, T.T.; Zou, X.B. Simultaneous and nondestructive diagnostics of nitrogen/magnesium/potassium-deficient cucumber leaf based on chlorophyll density distribution features. Biosyst. Eng. 2021, 212, 458–467. [Google Scholar] [CrossRef]
- Yan, H.F.; Ma, J.M.; Zhang, J.Y.; Wang, G.Q.; Zhang, C.; Akhlaq, M.; Huang, S.; Yu, J.J. Effects of film mulching on the physiological and morphological parameters and yield of cucumber under insufficient drip irrigation. Irrig. Drain. 2022, 71, 897–911. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Li, Y.; Li, M. Effect of high-voltage electrostatic field on inorganic nitrogen uptake by cucumber plants. Trans. ASABE 2016, 59, 25–29. [Google Scholar]
- Abubaker, B.A.; Yan, H.F.; Hong, L.; You, W.Y.; Elshaikh, N.A.; Hussei, G.; Pandab, S.; Hassan, S. Enhancement of Depleted Loam Soil as Well as Cucumber Productivity Utilizing Biochar Under Water Stress. Commun. Soil Sci. Plant Anal. 2019, 50, 49–64. [Google Scholar] [CrossRef]
- Chauhdary, J.N.; Li, H.; Pan, X.W.; Zaman, M.; Anjum, S.A.; Yang, F.; Akbar, N.; Azamat, U. Modeling effects of climate change on crop phenology and yield of wheat-maize cropping system and exploring sustainable solutions. J. Sci. Food Agric. 2025, 105, 3679–3700. [Google Scholar] [CrossRef]
- Ali, A.B.; Elshaikh, N.A.; Hussien, G.; Abdallah, F.E.; Hassan, S. Biochar Addition For Enhanced Cucumber Fruit Quality Under Deficit Irrigation. Biosci. J. 2020, 36, 1930–1937. [Google Scholar] [CrossRef]
- Rasool, G.; Guo, X.P.; Wang, Z.C.; Chen, S.; Hamoud, Y.A.; Javed, Q. Response of Fertigation Under Buried Straw Layer on Growth, Yield, and Water-fertilizer Productivity of Chinese Cabbage Under Greenhouse Conditions. Commun. Soil Sci. Plant Anal. 2019, 50, 1030–1043. [Google Scholar] [CrossRef]
- Xu, Y.H.; Ma, Y.; Luz, C.M.; Angel, S.M.M.; Wang, Q.J. Compost biochemical quality mediates nitrogen leaching loss in a greenhouse soil under vegetable cultivation. Geoderma 2020, 358, 113984. [Google Scholar] [CrossRef]
- Yan, H.F.; Acquah, S.J.; Zhang, C.; Wang, G.Q.; Huang, S.; Zhang, H.N.; Zhao, B.S.; Wu, H.M. Energy partitioning of greenhouse cucumber based on the application of Penman-Monteith and Bulk Transfer models. Agric. Water Manag. 2019, 217, 201–211. [Google Scholar] [CrossRef]
- Mao, H.P.; Wang, Y.F.; Yang, N.; Liu, Y.; Zhang, X.D. Effects of nutrient solution irrigation quantity and powdery mildew infection on the growth and physiological parameters of greenhouse cucumbers. Int. J. Agric. Biol. Eng. 2022, 15, 68–74. [Google Scholar] [CrossRef]
- Rasool, G.; Guo, X.P.; Wang, Z.C.; Hassan, M.; Aleem, M.; Javed, Q.; Chen, S. Effect of Buried Straw Layer Coupled with Fertigation on Florescence and Yield Parameters of Chinese Cabbage Under Greenhouse Environment. J. Soil Sci. Plant Nutr. 2020, 20, 598–609. [Google Scholar] [CrossRef]
- Acquah, S.J.; Yan, H.F.; Zhang, C.; Wang, G.Q.; Zhao, B.S.; Wu, H.M.; Zhang, H.N. Application and evaluation of Stanghellini model in the determination of crop evapotranspiration in a naturally ventilated greenhouse. Int. J. Agric. Biol. Eng. 2018, 11, 95–103. [Google Scholar] [CrossRef]
- Zhu, W.J.; Li, J.Y.; Li, L.; Wang, A.C.; Wei, X.H.; Mao, H.P. Nondestructive diagnostics of soluble sugar, total nitrogen and their ratio of tomato leaves in greenhouse by polarized spectra-hyperspectral data fusion. Int. J. Agric. Biol. Eng. 2020, 13, 189–197. [Google Scholar] [CrossRef]
- Huang, S.; Yan, H.F.; Zhang, C.; Wang, G.Q.; Acquah, S.J.; Yu, J.J.; Li, L.L.; Mam, J.M.; Darko, R.O. Modeling evapotranspiration for cucumber plants based on the Shuttleworth-Wallace model in a Venlo-type greenhouse. Agric. Water Manag. 2020, 228, 105861. [Google Scholar] [CrossRef]
- Gokulnath, B.V.; Devi, U.G. A Survey on Plant Disease Prediction using Machine Learning and Deep Learning Techniques. Intel. Intel. Artif. 2020, 23, 136–154. [Google Scholar] [CrossRef]
- Zhu, W.D.; Sun, J.; Wang, S.M.; Shen, J.F.; Yang, K.F.; Zhou, X. Identifying Field Crop Diseases Using Transformer-Embedded Convolutional Neural Network. Agriculture 2022, 12, 1083. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Zhou, C.S. Novel assisted/unassisted ultrasound treatment: Effect on respiration rate, ethylene production, enzymes activity, volatile composition, and odor of cherry tomato. LWT-Food Sci. Technol. 2021, 149, 111779. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, T.Z.; Chen, T.H.; Zhang, X.D.; Taha, M.F.; Yang, N.; Mao, H.P.; Shi, Q. Cucumber Downy Mildew Disease Prediction Using a CNN-LSTM Approach. Agriculture 2024, 14, 1155. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Q.; Xu, G.; Yang, N.; Chen, T.; Taha, M.F.; Mao, H. Transmission Route of Airborne Fungal Spores for Cucumber Downy Mildew. Horticulturae 2025, 11, 336. [Google Scholar] [CrossRef]
- Dhillon, N.P.S.; Masud, M.A.; Pruangwitayakun, S.; Natheung, M.; Lertlam, S.; Jarret, R.L. Evaluation of Loofah Lines for Resistance to Tomato Leaf Curl New Delhi Virus and Downy Mildew, as well as Key Horticultural Traits. Agriculture 2020, 10, 298. [Google Scholar] [CrossRef]
- Nostar, O.; Ozdemir, F.; Bor, M.; Turkan, I.; Tosun, N. Combined effects of salt stress and cucurbit downy mildew (Pseudoperospora cubensis Berk. and Curt. Rostov.) infection on growth, physiological traits and antioxidant activity in cucumber (Cucumis sativus L.) seedlings. Physiol. Mol. Plant Pathol. 2013, 83, 84–92. [Google Scholar] [CrossRef]
- Wallace, E.C.; D’Arcangelo, K.N.; Quesada-Ocampo, L.M. Population Analyses Reveal Two Host-Adapted Clades of Pseudoperonospora cubensis, the Causal Agent of Cucurbit Downy Mildew, on Commercial and Wild Cucurbits. Phytopathology 2020, 110, 1578–1587. [Google Scholar] [CrossRef]
- Holmes, G.; Ojiambo, P.; Hausbeck, M.; Quesada-Ocampo, L.; Keinath, A. Resurgence of cucurbit downy mildew in the United States: A watershed event for research and extension. Plant Dis. 2015, 99, 428–441. [Google Scholar] [CrossRef]
- Wen, D.M.; Chen, M.X.; Zhao, L.; Ji, T.; Li, M.; Yang, X.T. Use of thermal imaging and Fourier transform infrared spectroscopy for the pre-symptomatic detection of cucumber downy mildew. Eur. J. Plant Pathol. 2019, 155, 405–416. [Google Scholar] [CrossRef]
- Wang, Y.F.; Ma, G.X.; Du, X.X.; Liu, Y.; Wang, B.; Xu, G.L.; Mao, H.P. Effects of Nutrient Solution Irrigation Quantity and Downy Mildew Infection on Growth and Physiological Traits of Greenhouse Cucumber. Agronomy 2020, 10, 1921. [Google Scholar] [CrossRef]
- Zhu, J.J.; Sun, L.Y.; Ju, F.Y.; Wang, Z.; Xiong, C.; Yu, H.L.; Yu, K.; Huo, Y.Y.; Khattak, W.A.; Hu, W.; et al. Potassium Application Increases Cotton (Gossypium hirsutum L.) Fiber Length by Improving K+/Na+ Homeostasis and Potassium Transport Capacity in the Boll-Leaf System under Moderate Salinity. Agronomy 2020, 12, 2962. [Google Scholar] [CrossRef]
- Li, M.Q.; Li, J.Y.; Wei, X.H.; Zhu, W.J. Early diagnosis and monitoring of nitrogen nutrition stress in tomato leaves using electrical impedance spectroscopy. Int. J. Agric. Biol. Eng. 2017, 10, 194–205. [Google Scholar] [CrossRef]
- Zarattini, M.; Farjad, M.; Launay, A.; Cannella, D.; Soulié, M.C.; Bernacchia, G.; Fagard, M. Every cloud has a silver lining: How abiotic stresses affect gene expression in plant-pathogen interactions. J. Exp. Bot. 2021, 72, 1020–1033. [Google Scholar] [CrossRef]
- Li, C.; Zeng, Q.P.; Han, Y.Z.; Zhou, X.F.; Xu, H.W. Effects of Bacillus subtilis on Cucumber Seedling Growth and Photosynthetic System under Different Potassium Ion Levels. Biology 2024, 13, 348. [Google Scholar] [CrossRef]
- Dabu, X.; Li, S.; Cai, Z.; Ge, T.; Hai, M. The effect of potassium on photosynthetic acclimation in cucumber during CO2 enrichment. Photosynthetica 2019, 57, 640–645. [Google Scholar] [CrossRef]
- Mao, H.P.; Liu, Y.; Wang, Y.F.; Ma, G.X.; Wang, B.; Du, X.X.; Shi, Q.; Ni, J.H. Response of growth, photosynthesis, dry matter partition and roots to combined nitrogen-potassium stress in cucumber. Qual. Assur. Saf. Crops Foods 2022, 14, 45–53. [Google Scholar] [CrossRef]
- Alotaibi, M.M.; El Nagy, M.M.M.; Almuziny, M.; Alsubeie, M.S.; Abo-Zeid, A.A.I.; Alzuaibr, F.M.; Alasmari, A.; Albalawi, B.F.; Abd-Elwahed, A.H.M.; Ismail, K.A.; et al. The Effect of Foliar Application with Naphthalene Acetic Acid and Potassium Nitrate on the Growth, Sex Ratio, and Productivity of Cucumbers (Cucumis sativas L.) under High Temperatures in Semi-Arid Areas. Agronomy 2024, 14, 1202. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.X.; Xue, Z.J.; Li, Q.Y. Water and potassium utilization efficiency and yield and quality of cucumber (Cucumis sativus L.). Sci. Hortic. 2024, 330, 113025. [Google Scholar] [CrossRef]
- Lee, N.R.; Kim, Y.X.; Lee, Y.; Lee, C.; Song, Y.; Park, H.; Lee, C.H.; Lee, Y. Metabolomics Reveals the Effects of Nitrogen/Phosphorus/Potassium (NPK) Fertilizer Levels on Cucumber Fruit Raised in Different Nutrient Soils. Metabolites 2024, 14, 102. [Google Scholar] [CrossRef]
- GB/T 17980.26-2000; Pesticide—Guidelines for the Field Efficacy Trials(I)—Fungicides Against Downy Mildew of Cucumber. National Standards of the People’s Republic of China: Beijing, China, 2000.
- Okazaki, K.; Tanahashi, T.; Kato, Y.; Suzuki, I.; Tanaka, F.; Ohwaki, Y. Metabolic indices related to leaf marginal necrosis associated with potassium deficiency in tomato using GC/MS metabolite profiling. J. Biosci. Bioeng. 2020, 130, 520–524. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Li, D.; Wang, P.; Li, J.H.; Lu, X.C. Transcriptome and ionome analysis of nitrogen, phosphorus and potassium interactions in sorghum seedlings. Theor. Exp. Plant Physiol. 2020, 32, 271–285. [Google Scholar] [CrossRef]
- Sarah, M.M.D.; Prado, R.D.; de Souza, J.P.; Teixeira, G.C.M.; Duarte, J.C.D.; de Medeiros, R.L.S. Silicon supplied via foliar application and root to attenuate potassium deficiency in common bean plants. Sci Rep. 2021, 11, 19690. [Google Scholar] [CrossRef]
- Thornburg, T.E.; Liu, J.; Li, Q.; Xue, H.Y.; Wang, G.; Li, L.J.; Fontana, J.E.; Davis, K.E.; Liu, W.Y.; Zhang, B.H.; et al. Potassium Deficiency Significantly Affected Plant Growth and Development as Well as microRNA-Mediated Mechanism in Wheat (Triticum aestivum L.). Front. Plant Sci. 2020, 11, 1219. [Google Scholar] [CrossRef]
- Helena Ramirez-Solet, C.; Magnitskiy, S.; Melo Martinez, S.E.; Alvarez-Florez, F.; Marina Melgarejo, L. Photosynthesis, biochemical activity, and leaf anatomy of tree tomato (Solanum betaceum Cav.) plants under potassium deficiency. J. Appl. Bot. Food Qual. 2021, 94, 75–81. [Google Scholar] [CrossRef]
- Hu, W.; Coomer, T.D.; Loka, D.A.; Oosterhuis, D.M.; Zhou, Z.G. Potassium deficiency affects the carbon-nitrogen balance in cotton leaves. Plant Physiol. Biochem. 2017, 115, 408–417. [Google Scholar] [CrossRef]
- Fontana, J.E.; Wang, G.; Sun, R.R.; Xue, H.Y.; Li, Q.; Liu, J.; Davis, K.E.; Thornburg, T.E.; Zhang, B.H.; Zhang, Z.Y.; et al. Impact of potassium deficiency on cotton growth, development and potential microRNA-mediated mechanism. Plant Physiol. Biochem. 2020, 153, 72–80. [Google Scholar] [CrossRef]
- Ma, N.N.; Dong, L.N.; Lu, W.; Lu, J.; Meng, Q.W.; Liu, P. Transcriptome analysis of maize seedling roots in response to nitrogen-, phosphorus-, and potassium deficiency. Plant Soil 2020, 447, 637–658. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Q.; Dai, B.J.; Wang, K.L.; Lu, L.; Qaseem, M.F.; Wang, J.X.; Li, H.L.; Wu, A.M. The key physiology and molecular responses to potassium deficiency in Neolamarckia cadamba. Ind. Crops Prod. 2021, 162, 113260. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Jin, Y.M.; Kan, L.P.; Shen, C.W.; Malladi, A.; Nambeesan, S.; Xu, Y.C.; Dong, C.X. Transcriptome Analysis of Pyrus betulaefolia Seedling Root Responses to Short-Term Potassium Deficiency. Int. J. Mol. Sci. 2020, 21, 8857. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Feng, H.M.; Hu, Q.D.; Qu, H.Y.; Chen, A.Q.; Yu, L.; Xu, G.H. Improving rice tolerance to potassium deficiency by enhancing OsHAK16p:WOX11-controlled root development. Plant Biotechnol. J. 2015, 13, 833–848. [Google Scholar] [CrossRef] [PubMed]





| Nutrient Solution Group | Chemical Reagent | K-50% | K-100% | K-150% |
|---|---|---|---|---|
| A | Ca(NO3)2·4H2O | 826 | 826 | 826 |
| KNO3 | 303 | 606 | 606 | |
| B | NH4H2PO4 | 114 | 114 | 114 |
| MgSO4·7H2O | 492 | 492 | 492 | |
| NaNO3 | 255 | 0 | 0 | |
| KCl | 0 | 0 | 223 | |
| C | NaFe-EDTA | 7 | 7 | 7 |
| MnSO4 | 1.7 | 1.7 | 1.7 | |
| ZnSO4 | 1.45 | 1.45 | 1.45 | |
| CuSO4 | 0.19 | 0.19 | 0.19 | |
| Na2MoO4 | 0.12 | 0.12 | 0.12 | |
| Na2B4O7 | 2.45 | 2.45 | 2.45 |
| Disease Level | Symptoms Described |
|---|---|
| Level 0 | Asymptomatic |
| Level 1 | Diseased spot area occupies less than 5% of the leaf area |
| Level 3 | Diseased area accounts for 6% to 10% of the leaf area |
| Level 5 | Diseased area accounts for 11% to 25% of the leaf area |
| Level 7 | Diseased area accounts for 26% to 50% of the leaf area |
| Level 9 | Diseased spot area accounts for more than 50% of the leaf area |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; You, L.; Wang, Y.; Du, X.; Chen, T. Effects of Downy Mildew Infection and Potassium on Growth and Physiological Traits of Greenhouse Cucumber. Agronomy 2025, 15, 1017. https://doi.org/10.3390/agronomy15051017
Shi Q, You L, Wang Y, Du X, Chen T. Effects of Downy Mildew Infection and Potassium on Growth and Physiological Traits of Greenhouse Cucumber. Agronomy. 2025; 15(5):1017. https://doi.org/10.3390/agronomy15051017
Chicago/Turabian StyleShi, Qiang, Lu You, Yafei Wang, Xiaoxue Du, and Tianhua Chen. 2025. "Effects of Downy Mildew Infection and Potassium on Growth and Physiological Traits of Greenhouse Cucumber" Agronomy 15, no. 5: 1017. https://doi.org/10.3390/agronomy15051017
APA StyleShi, Q., You, L., Wang, Y., Du, X., & Chen, T. (2025). Effects of Downy Mildew Infection and Potassium on Growth and Physiological Traits of Greenhouse Cucumber. Agronomy, 15(5), 1017. https://doi.org/10.3390/agronomy15051017







