Impact of Phosphorus Fertilization on Leaching, Accumulation, and Microbial Cycling in New Apple Orchards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Fertilization Design
2.3. Leaching Solution Analysis
2.4. Soil Physicochemical Property Analysis
2.5. DNA Isolation and Metagenomic Sequencing
2.6. Data Analysis
3. Results
3.1. P Leaching Loss
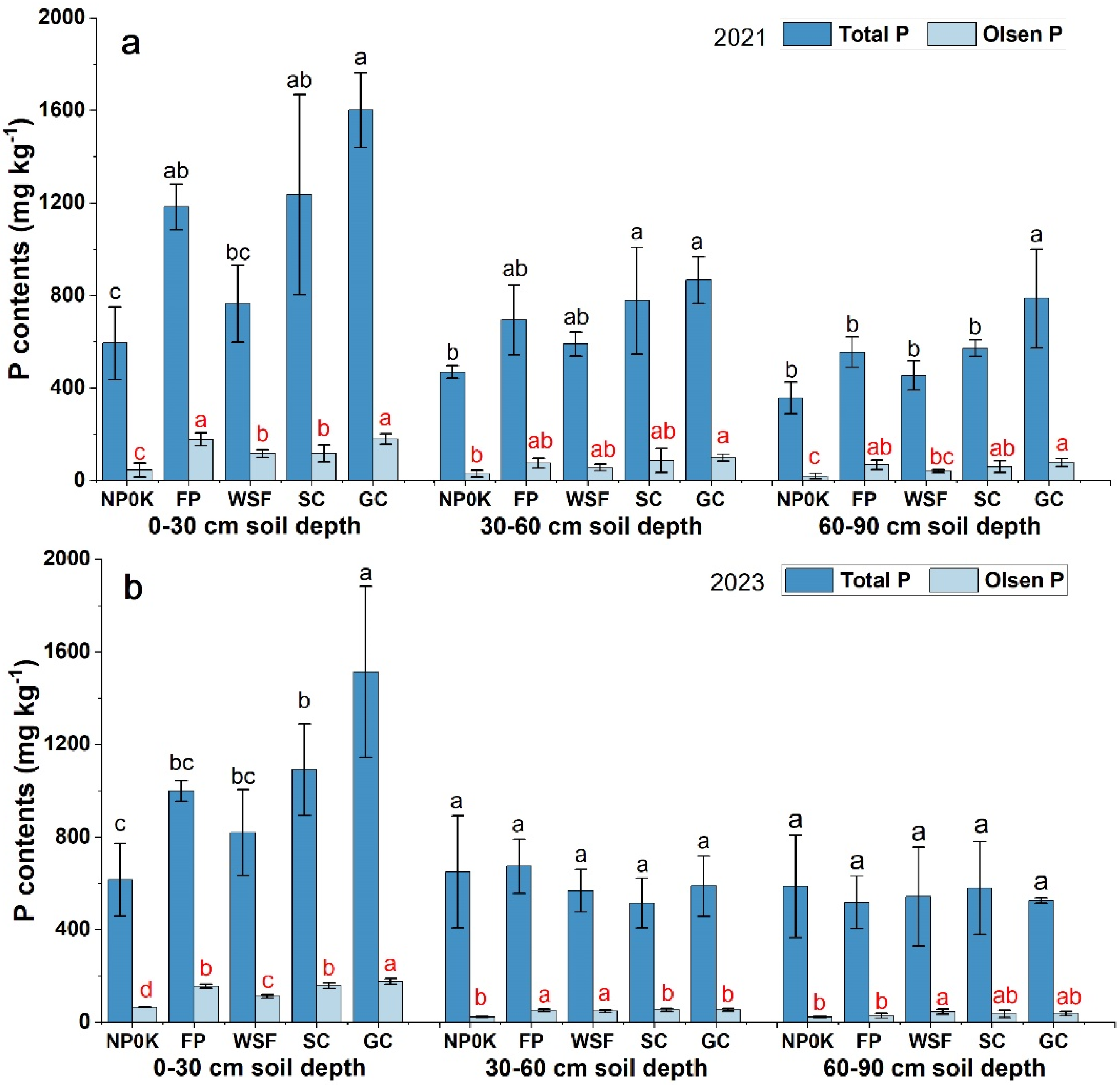
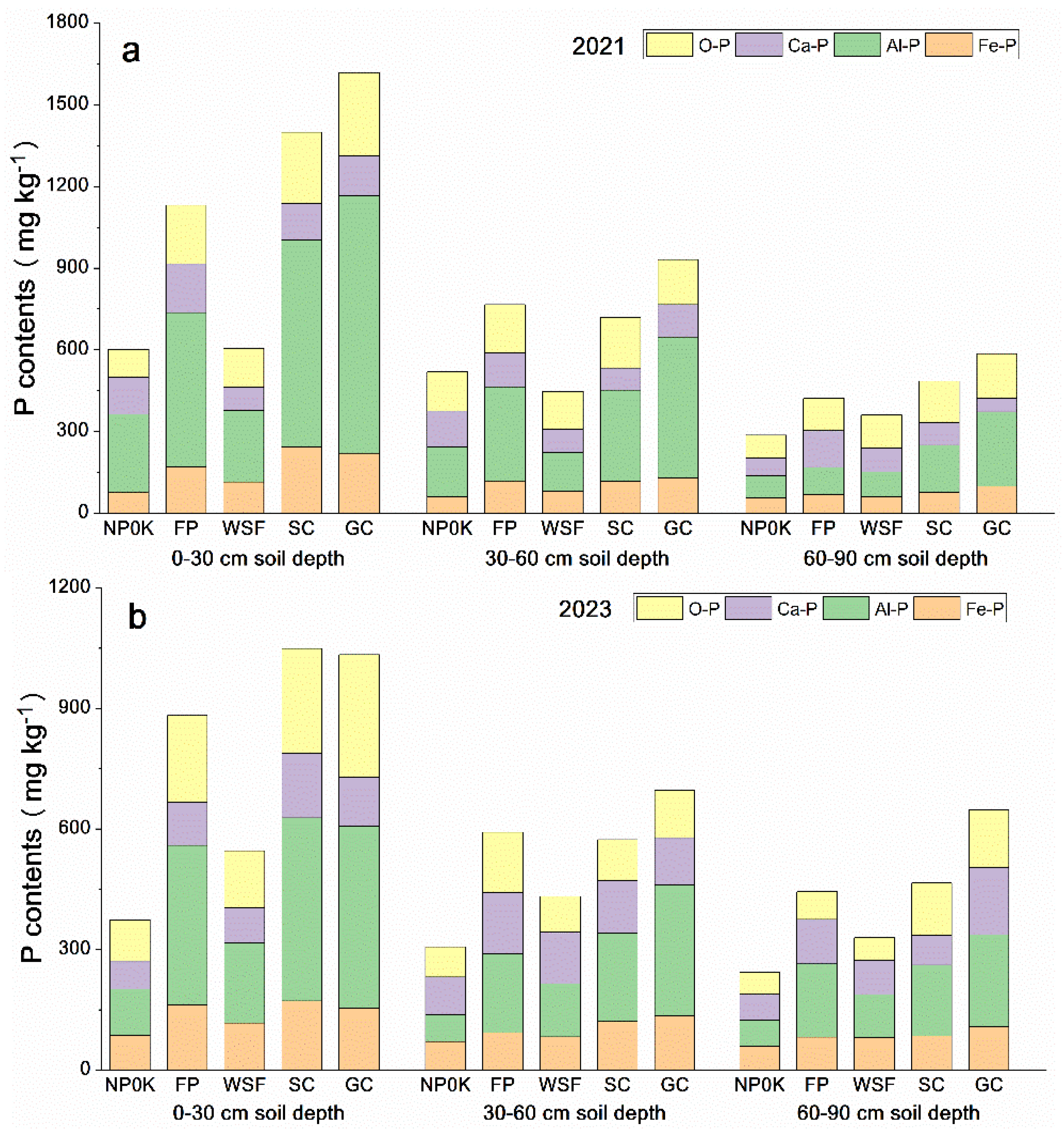
3.2. P Accumulation in Soil
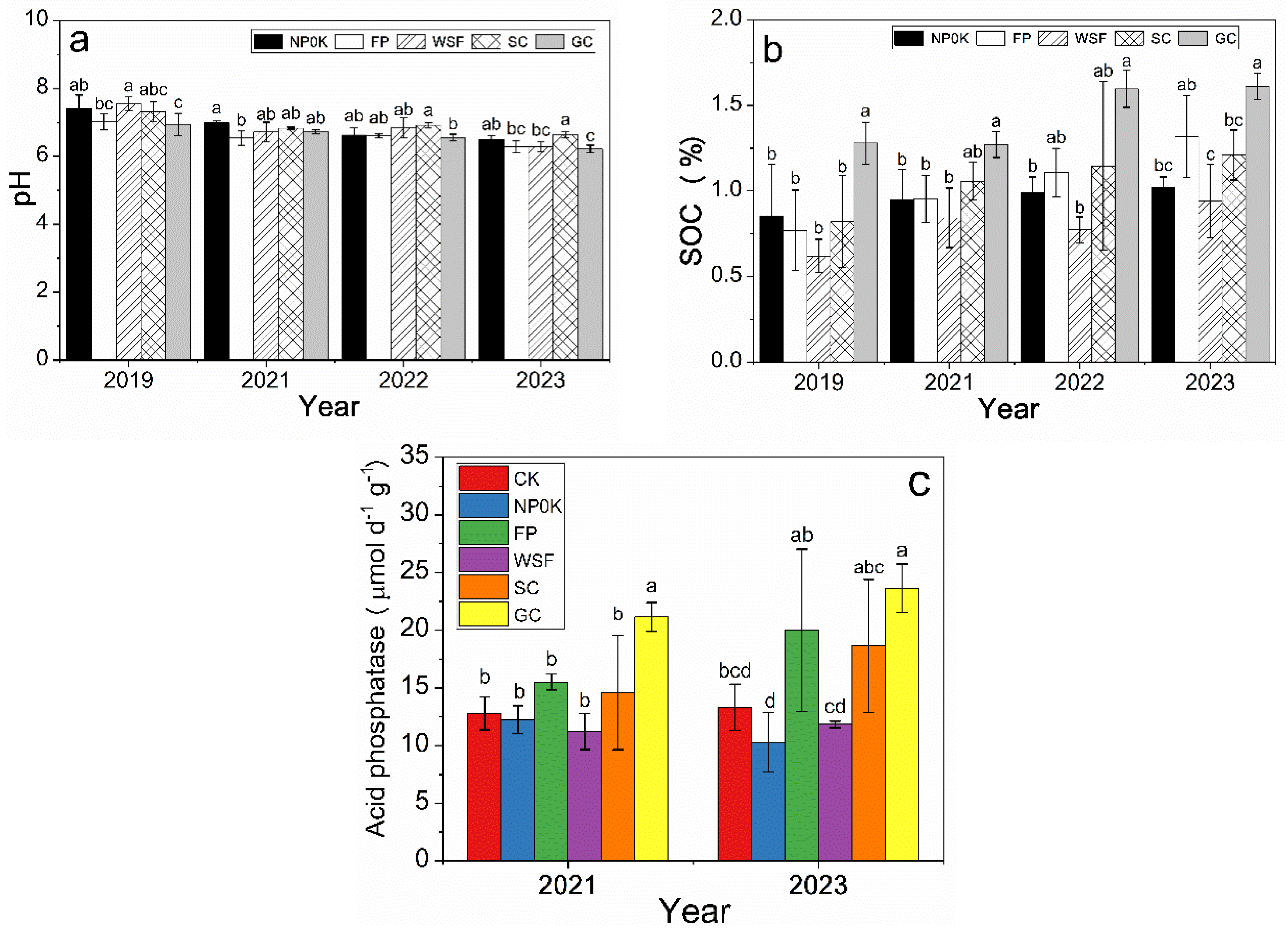
3.3. Soil Physicochemical Properties and Soil Acid Phosphatase Activity
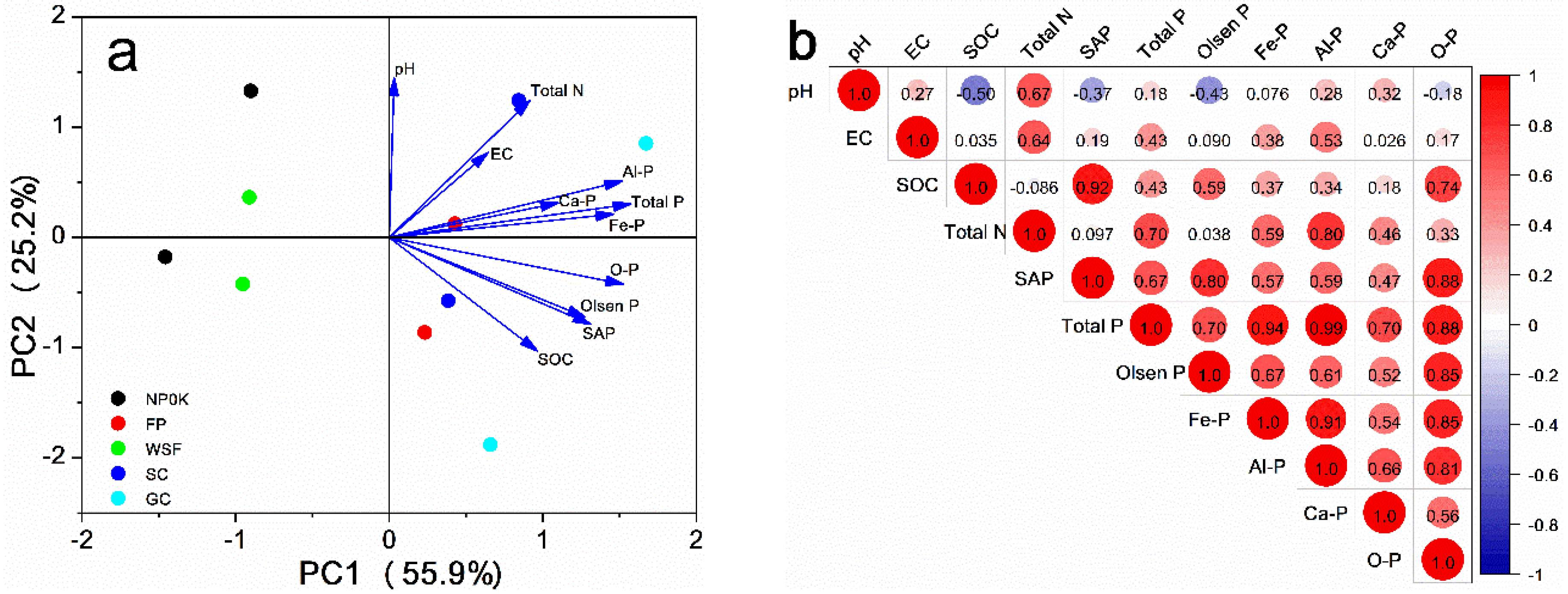
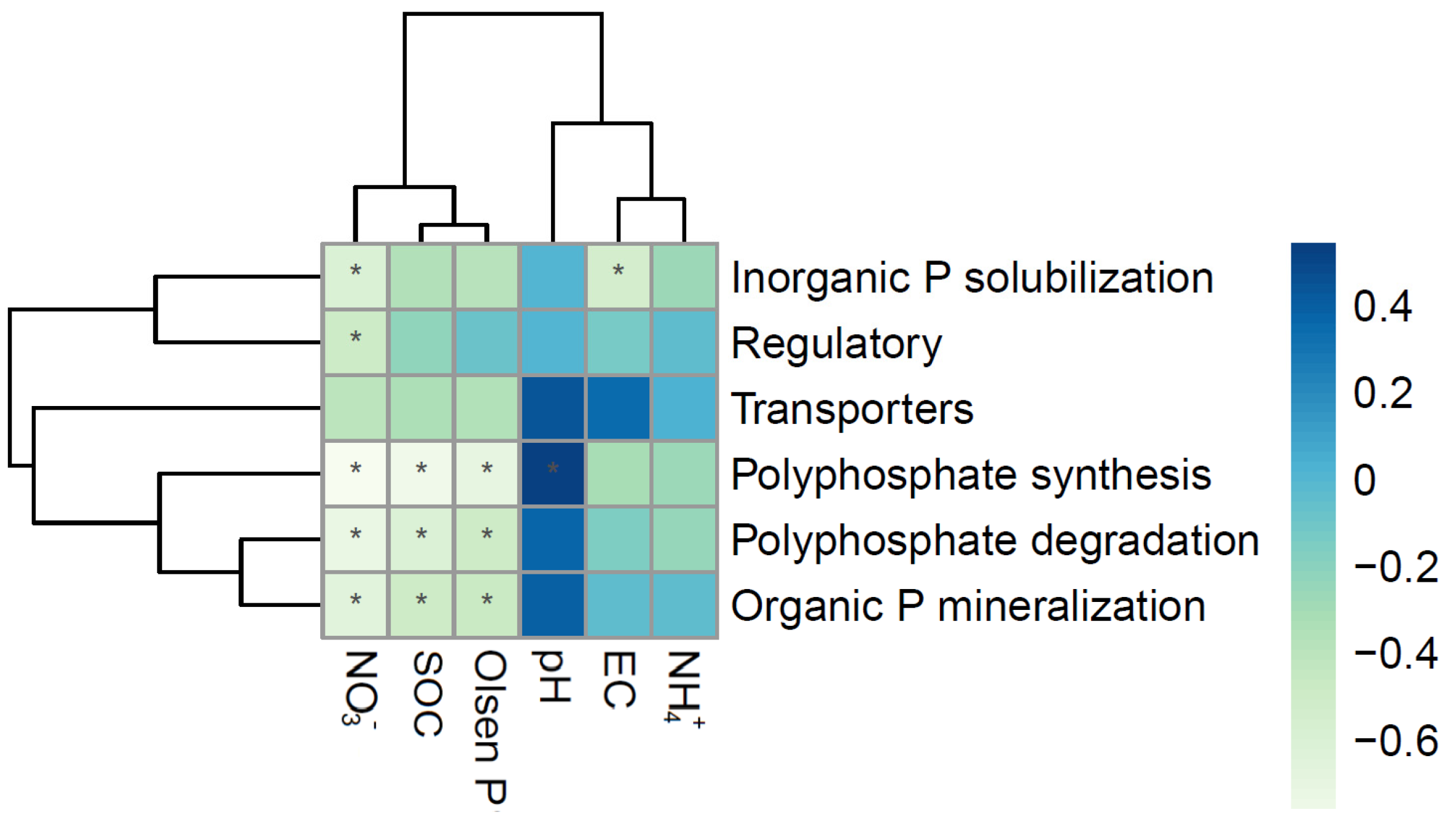
3.4. Correlations Between Soil P Fractions and Environmental Factors
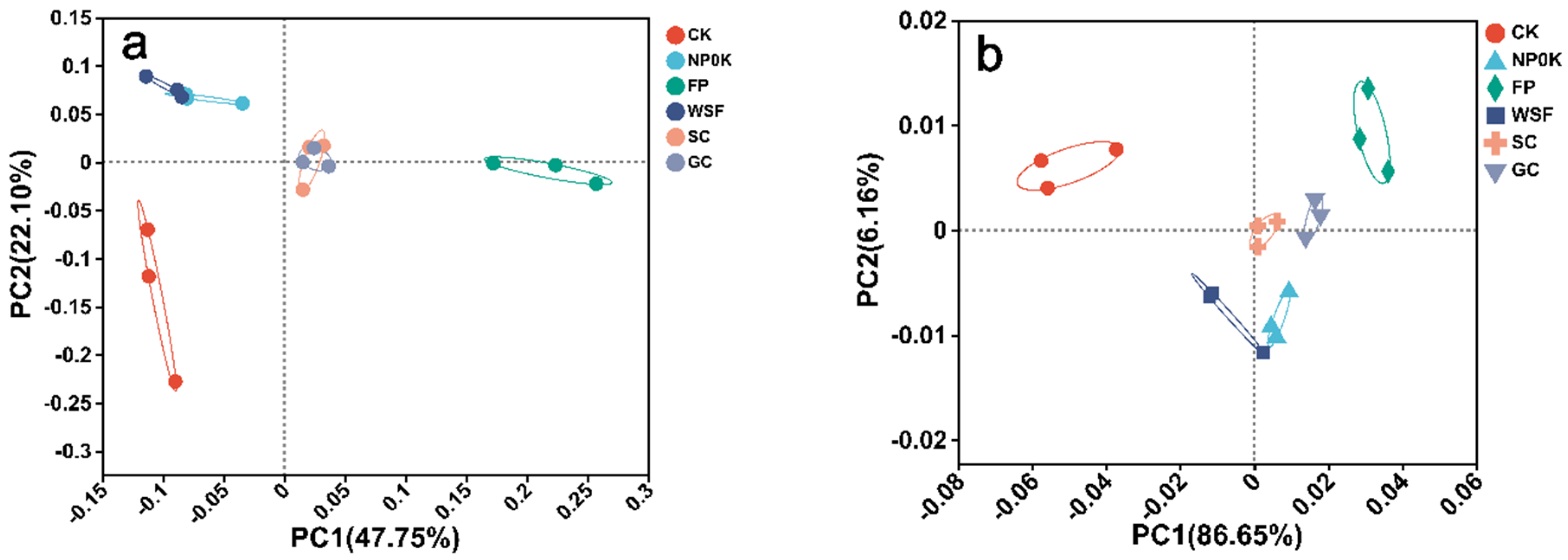
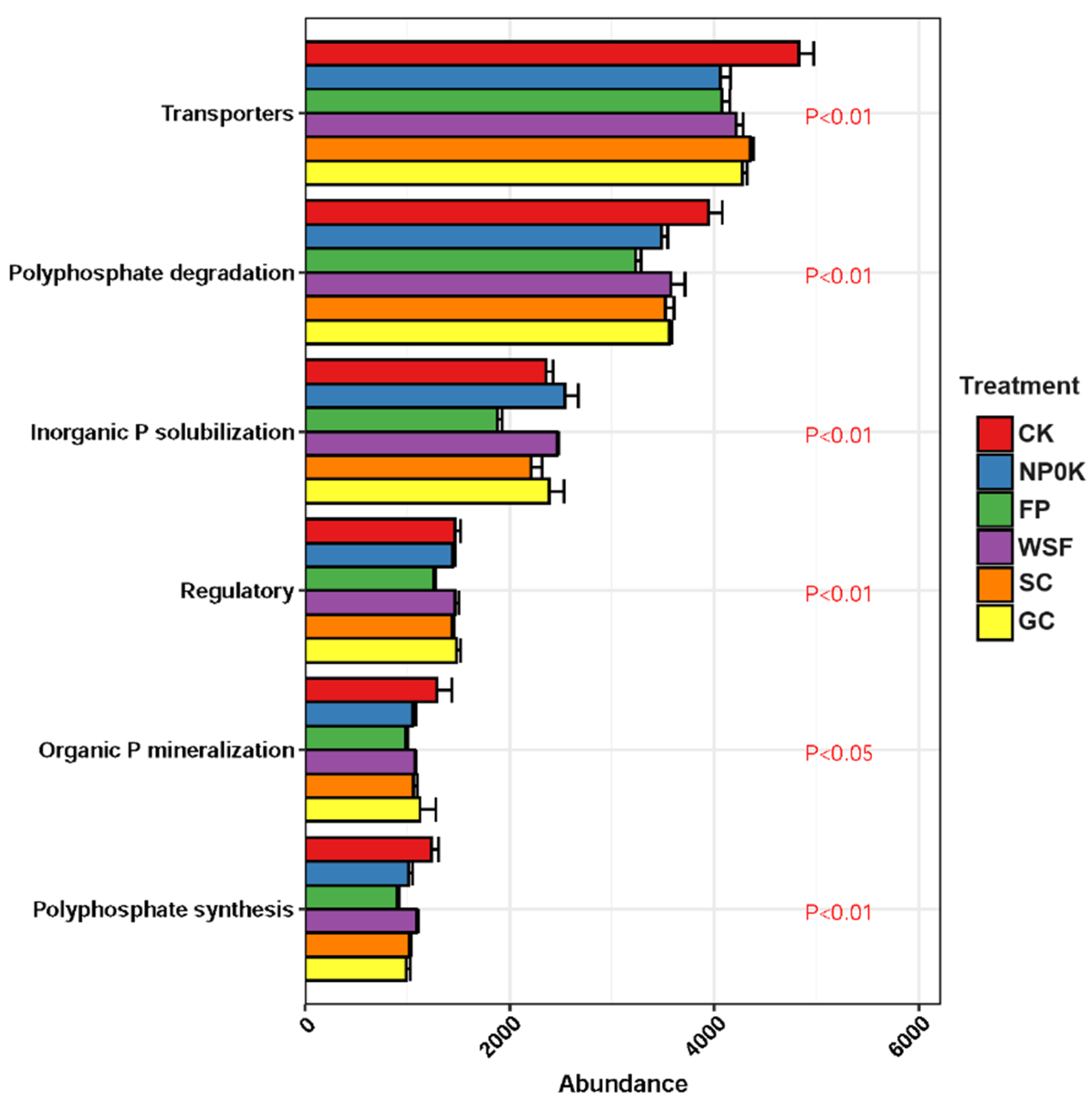
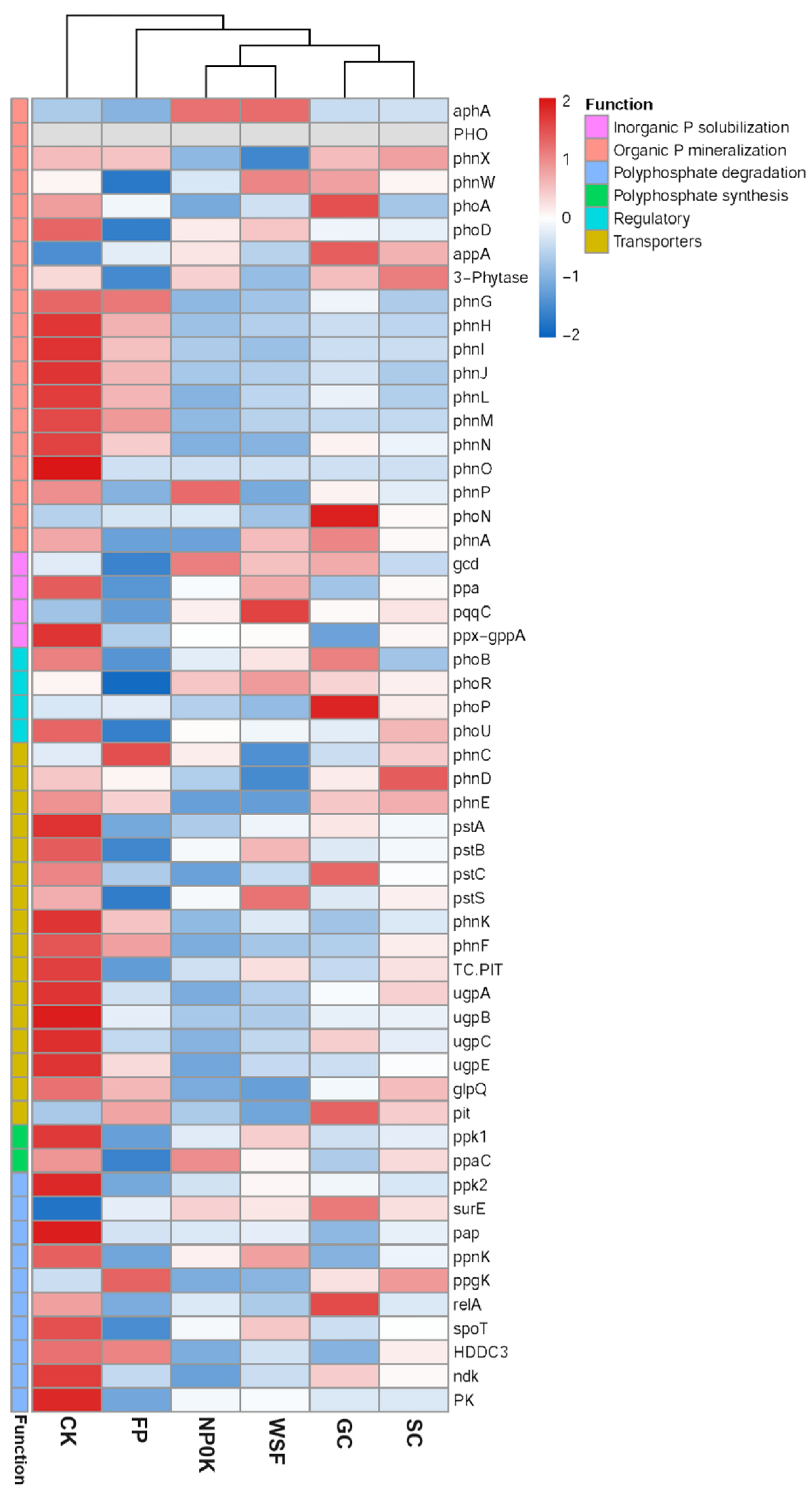
3.5. Soil Microbial Communities and the P Cycling Genes
4. Discussion
4.1. Planting Apple Trees Caused P Leaching Loss and Severe Accumulation of Inorganic P in Soil
4.2. The Inorganic P Fractions in Soils Were Closely Related to Environmental Factors
4.3. Planting Apple Trees Influenced the Microbial Genes and Functions Related to the Soil P Cycle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| N | nitrogen |
| P | phosphorus |
| K | potassium |
| Fe | iron |
| Al | aluminum |
| Ca | calcium |
| PUE | P use efficiency |
| PCGs | phosphorus cycling genes |
| WSF | water-soluble fertilizer |
| SC | soil conditioner |
| SOC | soil organic carbon |
| Al-P | aluminum phosphate |
| O-P | occluded phosphate |
| Ca-P | calcium phosphate |
| Fe-P | iron phosphate |
| SAP | soil acid phosphatase |
| PCA | principal component analysis |
| PCoA | Principal co-ordinates analysis |
| FDR | false discovery rate |
| EC | electrical conductivity |
References
- China Apple Industry Association. China Agricultural University Agricultural Planning and Design Institute 2021 China Apple Industry Development Report (General) Condensed version. China Fruit Veg. 2023, 44, 1–9. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, Z.L.; Wang, C.; Wu, J.M.; Guo, Y.; Li, L. Research on optimization of cultivated land protection policies based on dual objectives of development and protection. Nat. Resour. Econ. China 2024, 1–13. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Tracking phosphorus security: Indicators of phosphorus vulnerability in the global food system. Food Sec. 2015, 7, 337–350. [Google Scholar] [CrossRef]
- Haygarth, P.M.; Mezeli, M.M. Opportunity to improve global phosphorus governance. Nat. Food 2023, 4, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, G.M.; Mahboub Khomami, A.; Mammadov, T.G. Effect of fertilizer, furrow length, and irrigation flow rate on soil nutrient and yield of apple orchard. Appl. Environ. Soil Sci. 2024, 2024, 9088511. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, L.; Zhu, Y.; Xu, X. Vertical distribution and regulation of Olsen-phosphorus in 6-m soil profiles after farm Land-to-apple orchard conversion on the Chinese Loess Plateau. Catena 2021, 202, 105254. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, X.; Liu, Z.; Shi, H.; Zhai, B.; Zhu, Y. Divergent responses of soil physicochemical properties in 6-m profiles to long-term overfertilization in rainfed apple orchards on China’s Loess Plateau. Agric. Ecosyst. Environ. 2024, 361, 108817. [Google Scholar] [CrossRef]
- Sugihara, S.; Tomita, Y.; Nishigaki, T.; Kilasara, M.; Wasaki, J.; Funakawa, S. Effects of different phosphorus-efficient legumes and soil texture on fractionated rhizosphere soil phosphorus of strongly weathered soils. Biol. Fertil. Soils 2016, 52, 367–376. [Google Scholar] [CrossRef]
- Rodrigues, M.; Withers, P.J.A.; Soltangheisi, A.; Vargas, V.; Holzschuh, M.; Pavinato, P.S. Tillage systems and cover crops affecting soil phosphorus bioavailability in Brazilian Cerrado Oxisols. Soil Till. Res. 2021, 205, 104770. [Google Scholar] [CrossRef]
- Wang, R.; Guo, S.; Li, N.; Li, R.; Zhang, Y.; Jiang, J.; Wang, Z.; Liu, Q.; Wu, D.; Sun, Q.; et al. Phosphorus Accumulation and Sorption in Calcareous Soil under Long-Term Fertilization. PLoS ONE 2015, 10, e0135160. [Google Scholar] [CrossRef]
- Weihrauch, C.; Weber, C.J. Comparative risk assessment of phosphorus loss from “deep phosphorus stocks” in floodplain subsoils to surface waters. Sci. Total Environ. 2021, 769, 149037. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yang, X.; Sun, X.; Huang, L.; Li, S.; Hu, Z. Effect of biochar application and irrigation rate on the soil phosphorus leaching risk of fluvisol profiles in open vegetable fields. Sci. Total Environ. 2021, 789, 147973. [Google Scholar] [CrossRef]
- Kopytko, P.; Karpenko, V.; Yakovenko, R.; Mostoviak, I. Soil fertility and productivity of apple orchard under a long-term use of different fertilizer systems. Agronomy Res. 2017, 15, 444–455. [Google Scholar]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Yuan, Z.; Jiang, S.; Sheng, H.; Liu, X.; Hua, H.; Liu, X.; Zhang, Y. Human perturbation of the global phosphorus cycle: Changes and consequences. Environ. Sci. Technol. 2018, 52, 2438–2450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Q.; Gao, H.; Zhang, R.; Yang, L.; Guo, Y.; Li, H.; Awasthi, M.K.; Li, G. Long-term cover crops improved soil phosphorus availability in a rain-fed apple orchard. Chemosphere 2021, 275, 130093. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Z.; Zhao, J.; Ma, P.; Xu, X. Comprehensive assessment of fertilization, spatial variability of soil chemical properties, and relationships among nutrients, apple yield and orchard age: A case study in Luochuan County, China. Ecol. Indic. 2021, 122, 107285. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Xie, X.; Chen, Y.; Lang, M.; Chen, X. Long-term moderate fertilization increases the complexity of soil microbial community and promotes regulation of phosphorus cycling genes to improve the availability of phosphorus in acid soil. Appl. Soil Ecol. 2024, 194, 105178. [Google Scholar] [CrossRef]
- Yan, C.; An, D.; Zhao, B.; Ma, Z.; Ma, H.; Zhao, Q.; Kong, R.; Su, J. Intercropping herbage promoted the availability of soil phosphorus and improved the bacterial genus structure and the abundance of key bacterial taxa in the acidic soil of mango (Mangifera indica L.) orchards. Soil Use Manag. 2024, 40, e13046. [Google Scholar] [CrossRef]
- Zeng, Q.; Mei, T.; Wang, M.; Tan, W. Linking phosphorus fertility to soil microbial diversity and network complexity in citrus orchards: Implications for sustainable agriculture. Appl. Soil Ecol. 2024, 200, 105441. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, H.; Lin, H.; Liu, P.; Song, X.; Ma, Y.; Li, M.; Gao, W.; Song, L. Severe nitrogen leaching and marked decline of nitrogen cycle-related genes during the cultivation of apple orchard on barren mountain. Agric. Ecosyst. Environ. 2024, 367. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of Available P by Sequential Extraction; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. Soc. Am. J. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Wi American Society of Agronomy Inc. & Soil Science Society of America Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabae, F.S.; Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Dept. Agric. Circ. 1954, 939, 19. [Google Scholar]
- Williams, J.D.H.; Syers, J.K.; Walker, T.W. Fractionation of soil inorganic phosphate by modification of Chang and Jackson’s procedure. Soil Sci. Soc. Am. J. 1967, 3, 736–739. [Google Scholar] [CrossRef]
- Jiang, B.; Gu, Y. A suggested fractionation scheme of inorganic phosphorus in calcareous soils. Sci. Agr. Sin. 1989, 22, 58–66. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Guan, S. Soil Enzymes and Their Research Methods; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Huson, D.H.; Beier, S.; Flade, I.; Górska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.; Tappu, R. MEGAN community edition-interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using diamond. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Epp Schmidt, D.J.; Pouyat, R.; Szlavecz, K.; Setälä, H.; Kotze, D.J.; Yesilonis, I.; Cilliers, S.; Hornung, E.; Dombos, M.; Yarwood, S.A. Urbanization erodes ectomycorrhizal fungal diversity andmay cause microbial communities to converge. Nat. Ecol. Evol. 2017, 1, 0123. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R Package Version 2.5-2. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 6 March 2024).
- Tang, Y.; Zhan, T.; Fan, G.; Huang, J.; Zhou, Y.; Yuan, X.; Li, K.; Chen, S.; Zhao, X.; Hu, C. Selenium combined with chitin reduced phosphorus leaching in soil with pomelo by driving soil phosphorus cycle via microbial community. J. Environ. Chem. Engin. 2022, 10, 107060. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.; Zeng, Q.; Zhang, S.; Liao, B.; Shu, W.; Li, J. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, S.; Li, J.; Alva, A.; Chen, Q. Manure and nitrogen application enhances soil phosphorus mobility in calcareous soil in greenhouses. J. Environ. Manag. 2016, 181, 26–35. [Google Scholar] [CrossRef]
- Do Nascimento, C.A.; Pagliari, P.H.; Faria, L.D.A.; Vitti, G.C. Phosphorus mobility and behavior in soils treated with calcium, ammonium, and magnesium phosphates. Soil Sci. Soc. Am. J. 2018, 82, 622–631. [Google Scholar] [CrossRef]
- Yan, X.; Shi, L.; Gong, L. Mechanism analysis of soil amelioration and phosphorus recovery by using a mineral soil conditioner in southern China. J. Soils Sediments 2018, 18, 1884–1895. [Google Scholar] [CrossRef]
- Liu, M.; Tan, X.; Zheng, M.; Yu, D.; Lin, A.; Liu, J.; Cui, J. Modified biochar/humic substance/fertiliser compound soil conditioner for highly efficient improvement of soil fertility and heavy metals remediation in acidic soils. J. Environ. Manag. 2023, 325, 116614. [Google Scholar] [CrossRef]
- Ge, N.; Wei, X.; Wang, X.; Liu, X.; Shao, M.; Jia, X.; Zhang, Q. Soil texture determines the distribution of aggregate-associated carbon, nitrogen and phosphorous under two contrasting land use types in the Loess Plateau. Catena 2019, 172, 148–157. [Google Scholar] [CrossRef]
- Grenon, G.; Singh, B.; De Sena, A.; Madramootoo, C.A.; von Sperber, C.; Goyal, M.K.; Zhang, T. Phosphorus fate, transport and management on subsurface drained agricultural organic soils: A review. Environ. Res. Lett. 2021, 16, 013004. [Google Scholar] [CrossRef]
- Schröder, J.J.; Smit, A.L.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 2011, 84, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, J.; Yao, R.; Cao, Y.; Zhu, H.; Sun, Y.; Wang, X.; Xie, W. Effects of Different Management on Phosphorus Fractions in Coastal Saline Soil and Phosphorus Absorption and Utilization by Crops. Soils 2020, 52, 691–698. [Google Scholar] [CrossRef]
- Lu, P.; Li, W.; Niu, J.; Batbayar, J.; Zhang, S.; Yang, X. Phosphorus Availability and Transformation of Inorganic Phosphorus Forms Under Different Organic Carbon Levels in a Tier Soil. Sci. Agric. Sin. 2022, 55, 111–122. [Google Scholar]
- Shao, W.; Zhu, J.; Teng, Z.; Zhang, K.; Liu, S.; Li, M. Distribution of inorganic phosphorus and its response to the physicochemical characteristics of soil in Yeyahu Wetland, China. Process Saf. Environ. Prot. 2019, 125, 1–8. [Google Scholar] [CrossRef]
- Yan, X.J.; Yang, W.H.; Chen, X.H.; Wang, M.K.; Wang, W.Q.; Ye, D.L.; Wu, L.Q. Soil phosphorus pools, bioavailability and environmental risk in response to the phosphorus supply in the red soil of Southern China. Int. J. Envrion. Res. Public Health 2020, 17, 7384. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Hui, D.; Li, J.; Yao, X.; Zhang, D.; Deng, Q. Soil acidification suppresses phosphorus supply through enhancing organomineral association. Sci. Total Environ. 2023, 905, 167105. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Hu, Y.; Zhang, J.; Jia, R.; Huang, Q.; Gao, H.; Awasthi, M.K.; Li, H.; Zhao, Z. The spatio-temporal change in soil P and P-solubilizing bacteria under clover mulching in apple orchards of Loess Plateau. Chemosphere 2022, 304, 135334. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Shen, R.F. Stronger effects of maize rhizosphere than phosphorus fertilization on phosphatase activity and phosphorus-mineralizing-related bacteria in acidic soils. Rhizosphere 2022, 23, 100555. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Lin, H.; Xue, R.; Ma, Y.; Song, Y. Impact of Phosphorus Fertilization on Leaching, Accumulation, and Microbial Cycling in New Apple Orchards. Agronomy 2025, 15, 952. https://doi.org/10.3390/agronomy15040952
Shen Y, Lin H, Xue R, Ma Y, Song Y. Impact of Phosphorus Fertilization on Leaching, Accumulation, and Microbial Cycling in New Apple Orchards. Agronomy. 2025; 15(4):952. https://doi.org/10.3390/agronomy15040952
Chicago/Turabian StyleShen, Yuwen, Haitao Lin, Rui Xue, Yanan Ma, and Yan Song. 2025. "Impact of Phosphorus Fertilization on Leaching, Accumulation, and Microbial Cycling in New Apple Orchards" Agronomy 15, no. 4: 952. https://doi.org/10.3390/agronomy15040952
APA StyleShen, Y., Lin, H., Xue, R., Ma, Y., & Song, Y. (2025). Impact of Phosphorus Fertilization on Leaching, Accumulation, and Microbial Cycling in New Apple Orchards. Agronomy, 15(4), 952. https://doi.org/10.3390/agronomy15040952




