Heat Stress Downregulates Photosystem I Redox State on Leaf Photosynthesis in Grapevine
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions and Treatments
2.2. Gas-Exchange Measurements
2.3. Measurements of PSI and PSII Parameters
2.4. Imaging-PAM Analysis
2.5. Chlorophyll Extraction and Measurement
2.6. Measurement of H2O2, Malondialdehyde (MDA) and Antioxidant Enzyme Activities
2.7. Statistical Analyses
3. Results
3.1. Temperature Monitoring and Changes of Chlorophyll and Carotenoid Contents Under Heat Stress
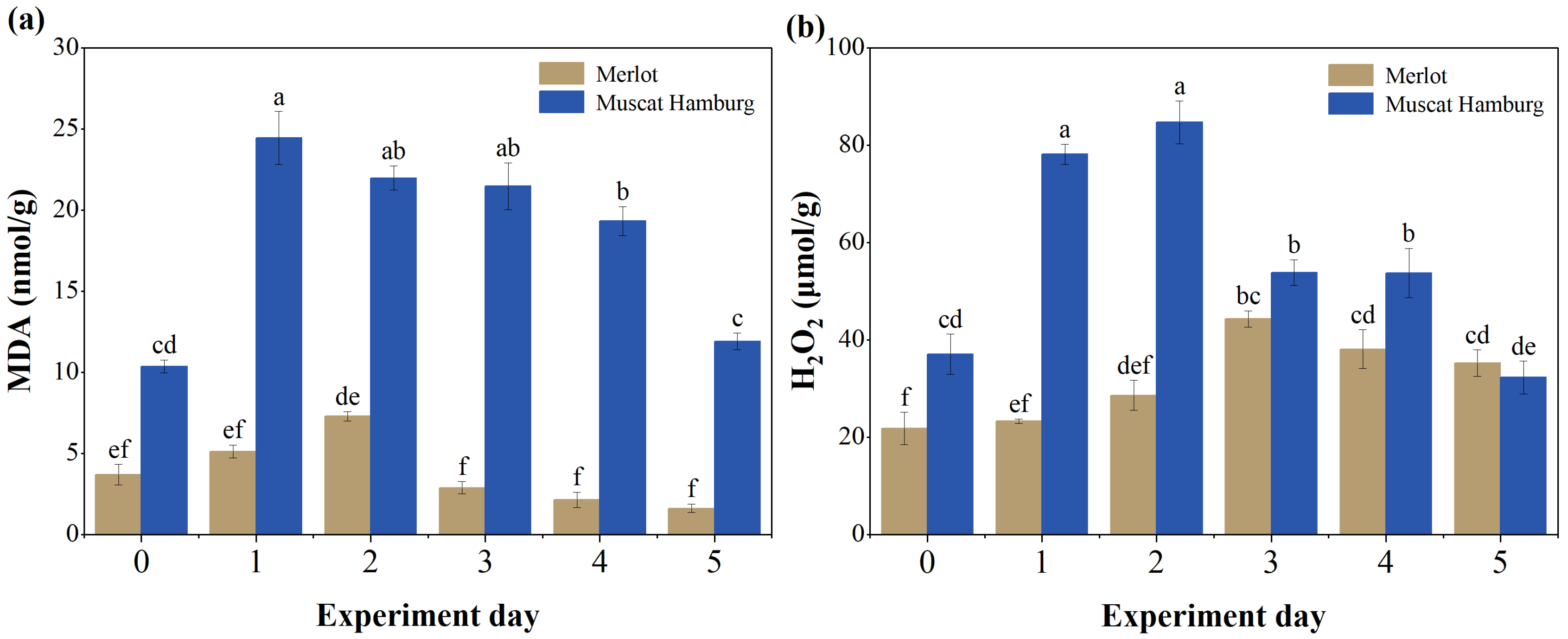
3.2. Changes in H2O2, MDA, and Antioxidant Enzyme Activities Under Heat Stress
3.3. Evolution of Leaf Gas Exchange Parameters Under Heat Stress
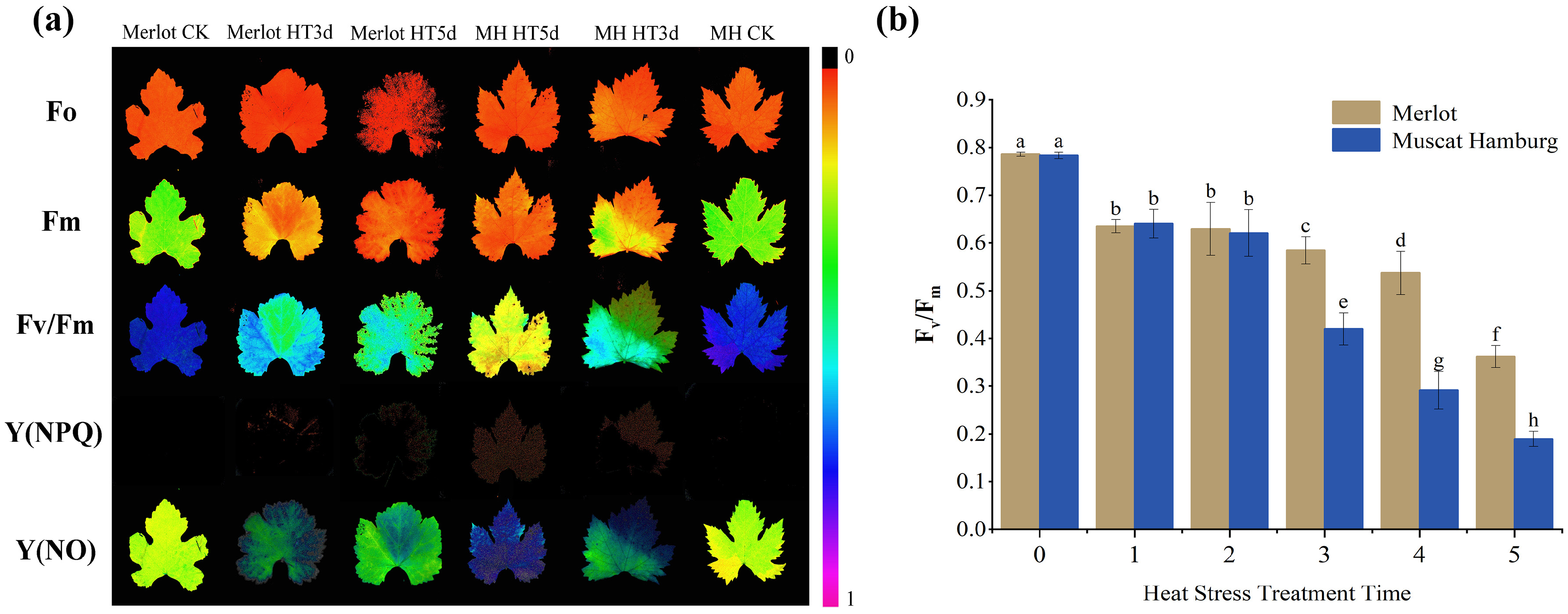
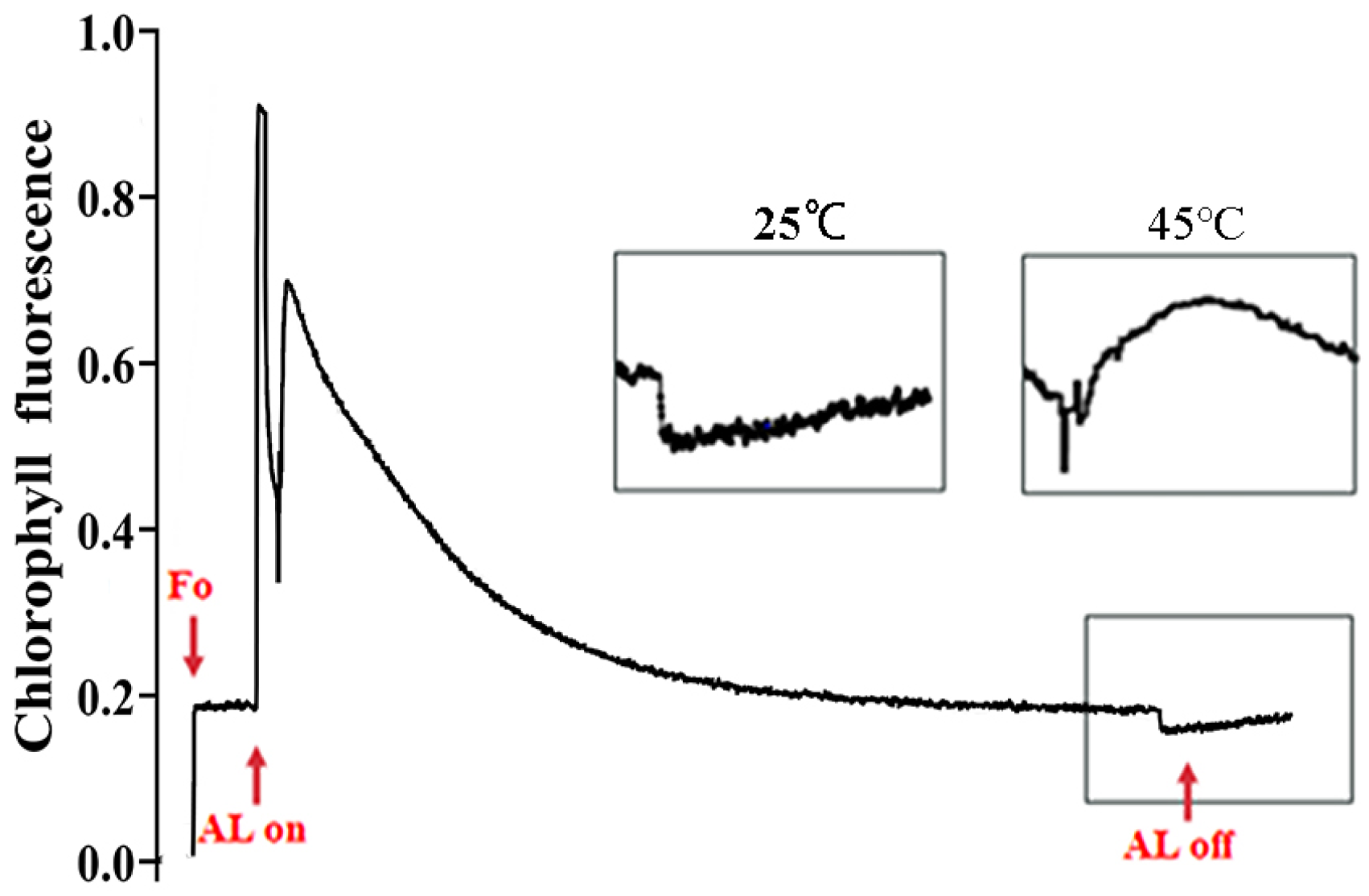
3.4. Impact of Heat Stress on Chlorophyll a Fluorescence in Grapevine Leaves
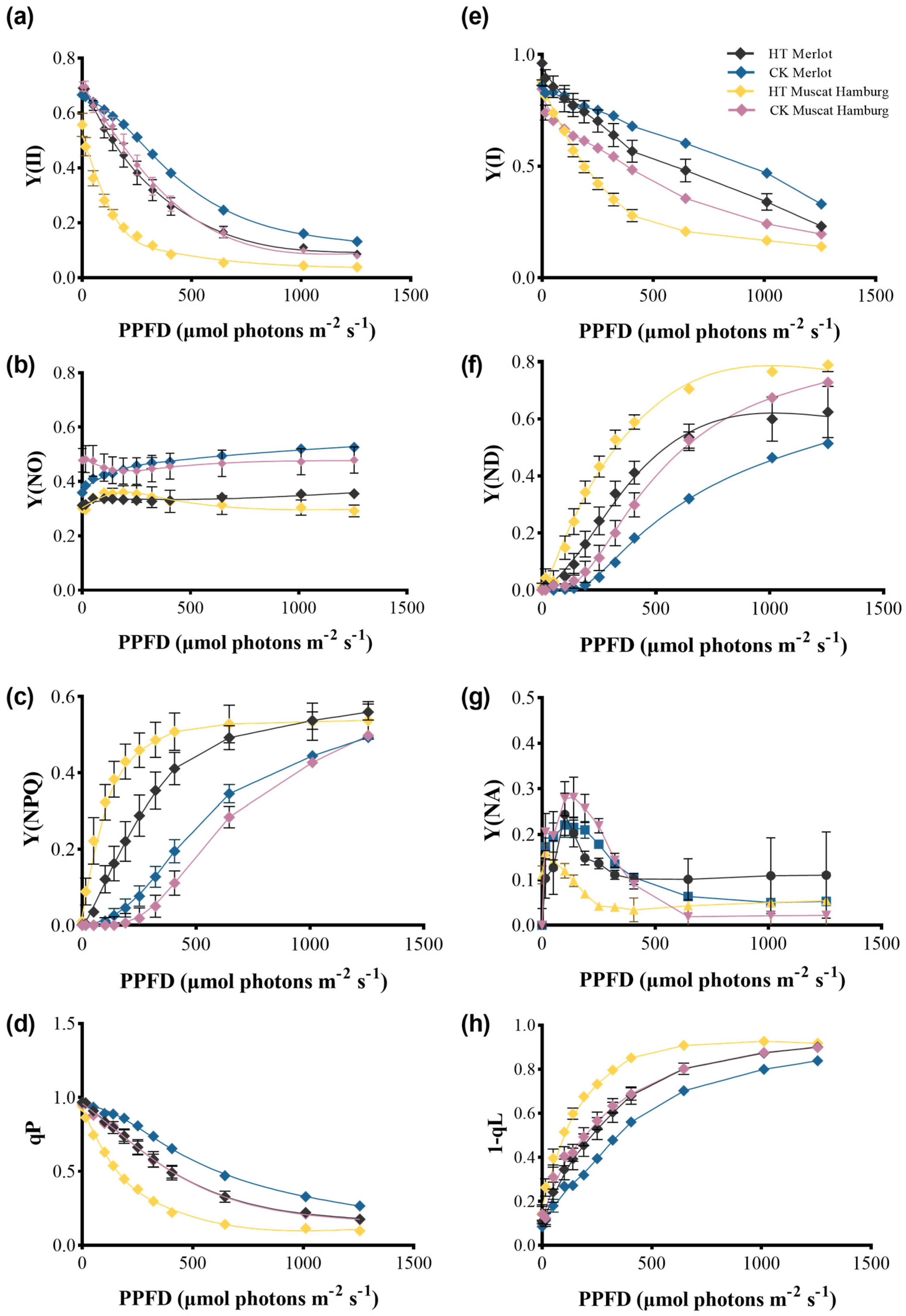
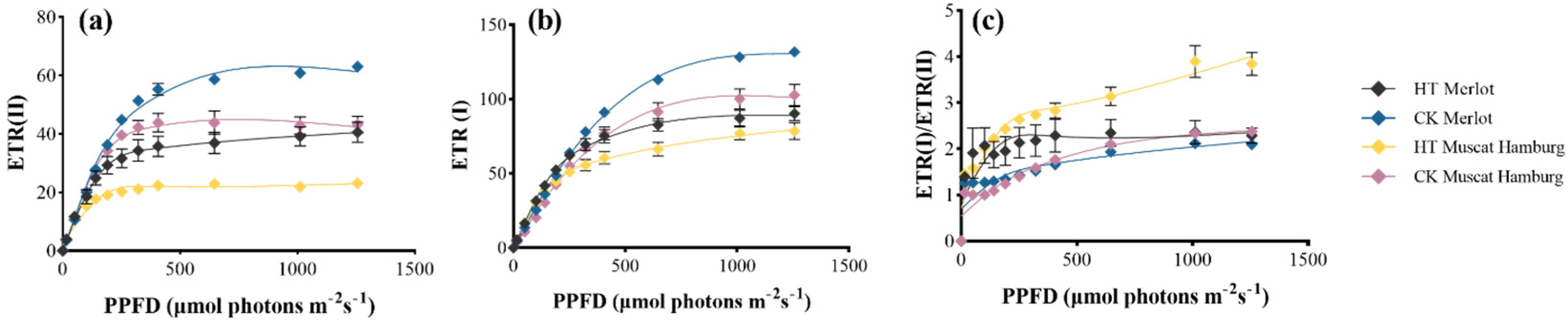
3.5. PSI and PSII Performance of Grapevine Leaves Under Heat Stress
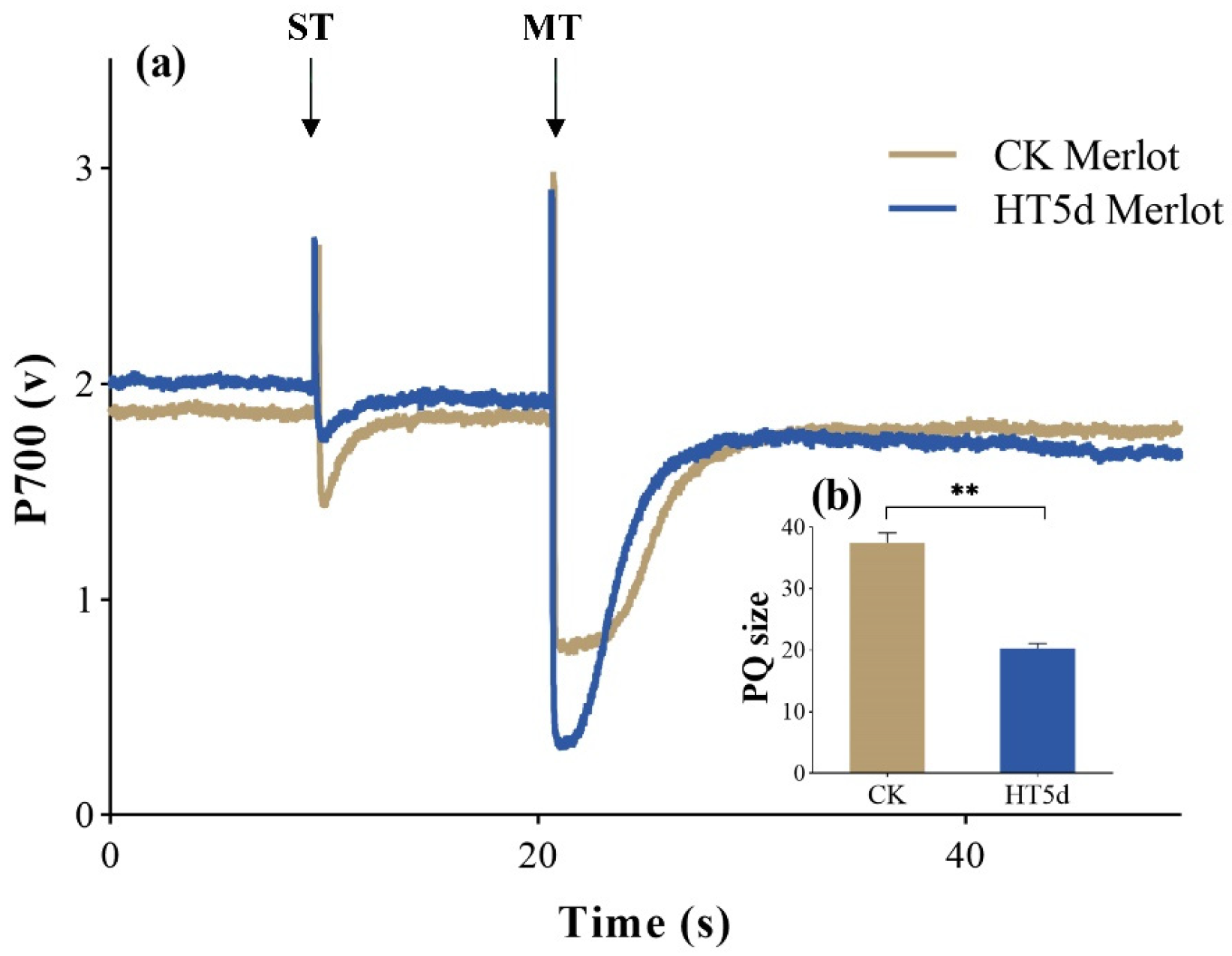
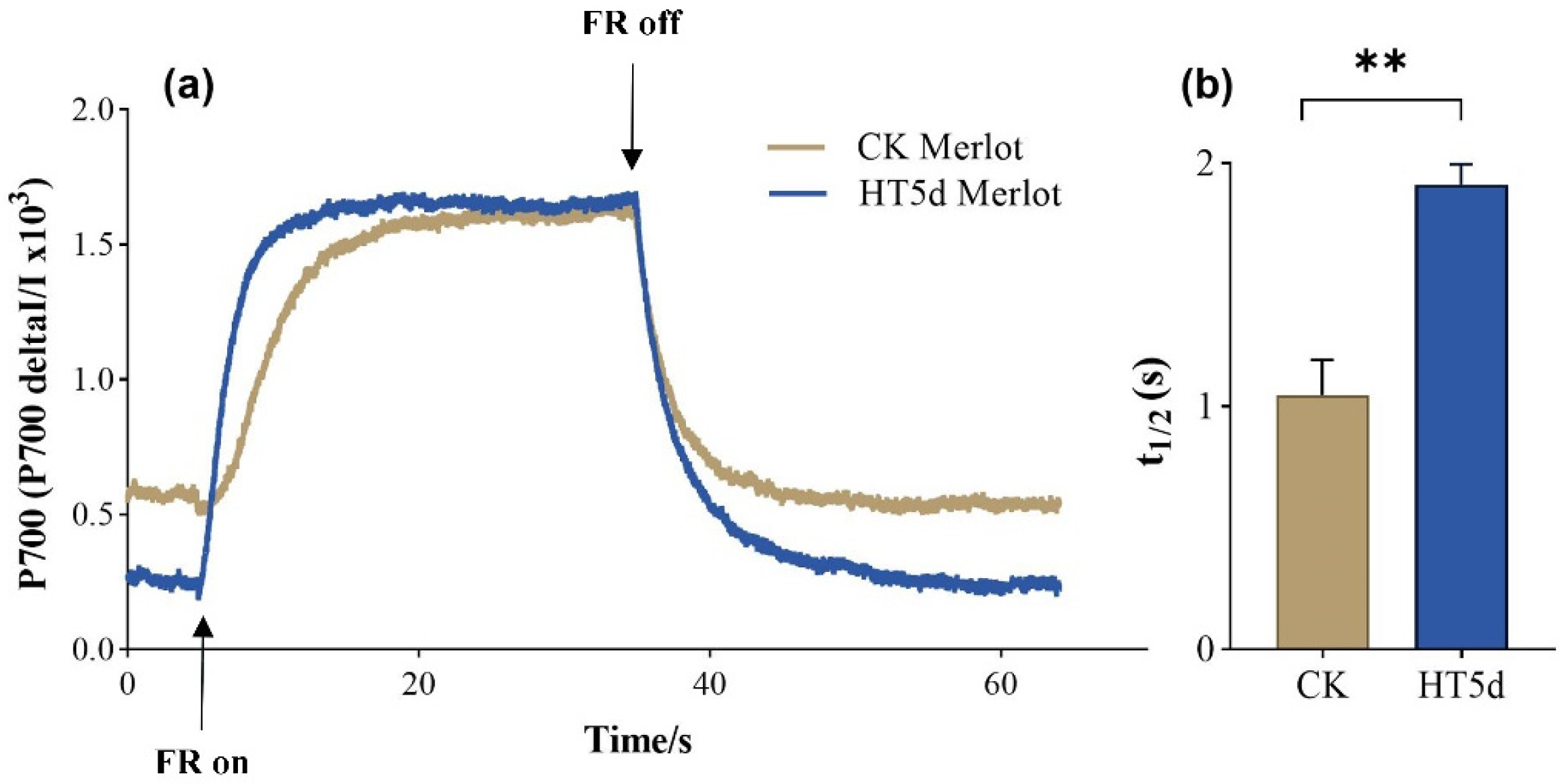
3.6. Impacts on Cyclic Electron Flow of PSI Under Heat Stress
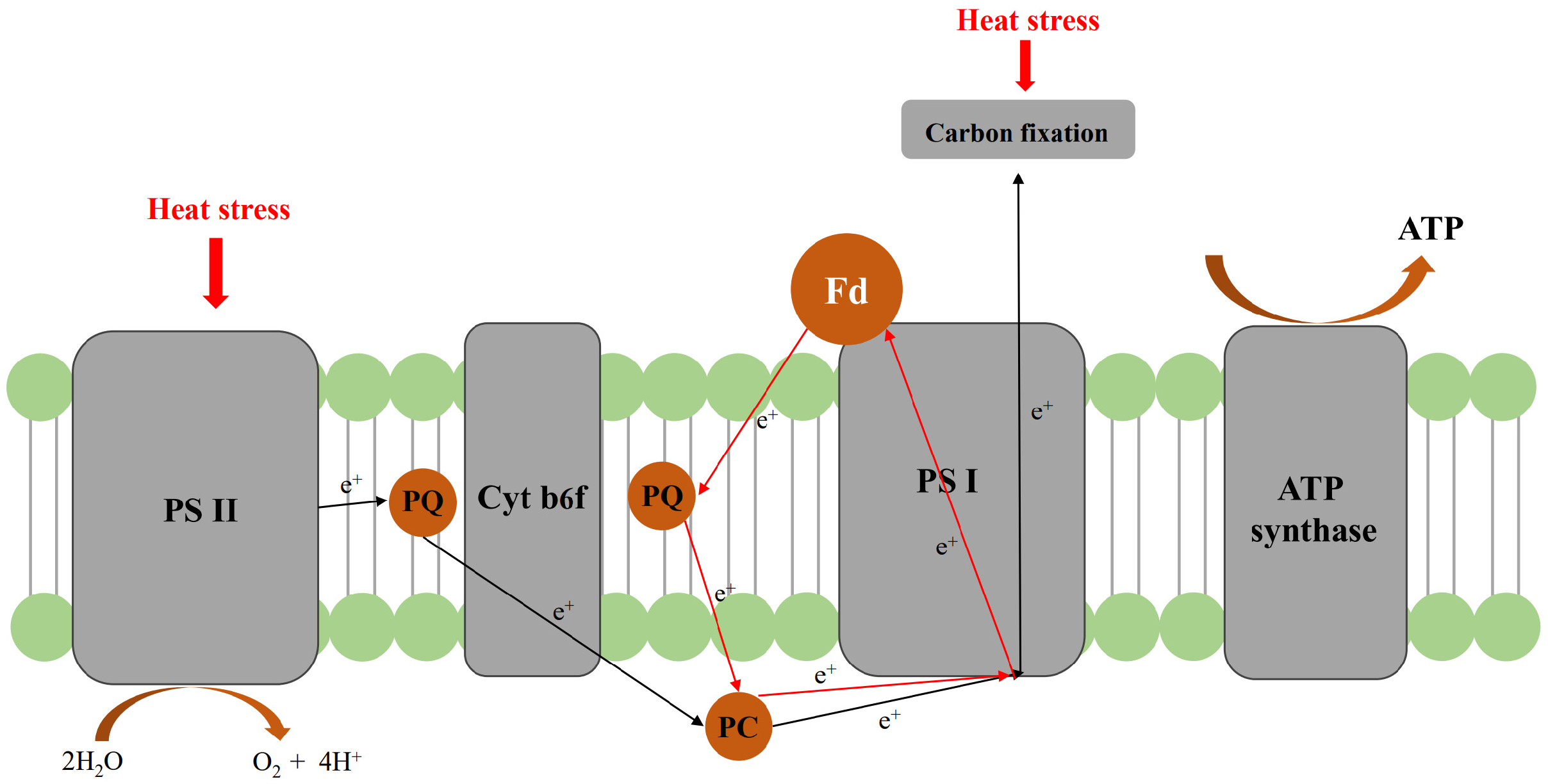
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lobell, D.B.; Field, C.B.; Cahill, K.N.; Bonfils, C. Impacts of future climate change on California perennial crop yields: Model projections with climate and crop uncertainties. Agric. For. Meteorol. 2006, 141, 208–218. [Google Scholar] [CrossRef]
- IPCC. Special Report-Climate Change on 1.5 °C; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; 616p. [Google Scholar]
- Zha, Q.; Xi, X.; Jiang, A.; Wang, S.; Tian, Y. Changes in the protective mechanism of photosystem II and molecular regulation in response to high temperature stress in grapevines. Plant Physiol. Biochem. 2016, 101, 43–53. [Google Scholar] [CrossRef]
- Schultz, H. Climate change and viticulture: A European perspective on climatology, carbon dioxide and UV-B effects. Aust. J. Grape Wine Res. 2000, 6, 2–12. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.; Jiang, A.; Tian, Y. High Temperature Affects Photosynthetic and Molecular Processes in Field-Cultivated Vitis vinifera L. × Vitis labrusca L. Photochem. Photobiol. 2016, 92, 446–454. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, S.; Liao, W.; Lu, Q.; Wen, X.; Lu, C. Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. J. Plant Physiol. 2010, 167, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.M.; Evans, J.R. The importance of energy balance in improving photosynthetic productivity. Plant Physiol. 2011, 155, 70–78. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Nicol, L.; Croce, R. The PsbS protein and low pH are necessary and sufficient to induce quenching in the light-harvesting complex of plants LHCII. Sci. Rep. 2021, 11, 7415. [Google Scholar] [CrossRef]
- Munekage, Y.; Shikanai, T. Cyclic electron transport through photosystem I. Plant Biotechnol. 2005, 22, 361–369. [Google Scholar] [CrossRef]
- Takizawa, K.; Kanazawa, A.; Kramer, D.M. Depletion of stromal Pi induces high ‘energy–dependent’ antenna exciton quenching (qE) by decreasing proton conductivity at CFO–CF1 ATP synthase. Plant Cell Environ. 2008, 31, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sharkey, T.D. Photosynthetic electron transport and proton flux under moderate heat stress. Photosynth. Res. 2009, 100, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, T. Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr. Opin. Biotechnol. 2014, 26, 25–30. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shikanai, T. PGR5-Dependent Cyclic Electron Flow Protects Photosystem I under Fluctuating Light at Donor and Acceptor Sides. Plant Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef]
- Tan, S.L.; Yang, Y.J.; Huang, W. Moderate heat stress accelerates photoinhibition of photosystem I under fluctuating light in tobacco young leaves. Photosynth. Res. 2020, 144, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Wang, H.; Zhang, B.; Gao, S.; Wang, Z.; Wang, Y.; Zhang, H.; Sun, G. Elevated air temperature damage to photosynthetic apparatus alleviated by enhanced cyclic electron flow around photosystem I in tobacco leaves. Ecotoxicol. Environ. Saf. 2020, 204, 111136. [Google Scholar]
- Takagi, D.; Miyake, C. PROTON GRADIENT REGULATION 5 supports linear electron flow to oxidize photosystem I. Physiol. Plant. 2018, 164, 337–348. [Google Scholar] [CrossRef]
- Chaux, F.; Peltier, G.; Johnson, X. A security network in PSI photoprotection: Regulation of photosynthetic control, NPQ and O2 photoreduction by cyclic electron flow. Front. Plant Sci. 2015, 6, 875. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine. Available online: https://www.oiv.int/what-we-do/country-report?oiv (accessed on 10 April 2025).
- Fenoll, J.; Manso, A.; Hellín, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Tamizheezham, U.; Muthuvel, I.; Subbiah, A. Effect of Temperature on Shelf life of Muscat Hamburg Grapes under Storage. Madras Agric. J. 2018, 105, 176. [Google Scholar] [CrossRef]
- de Rosas, I.; Deis, L.; Baldo, Y.; Cavagnaro, J.B.; Cavagnaro, P.F. High Temperature Alters Anthocyanin Concentration and Composition in Grape Berries of Malbec, Merlot, and Pinot Noir in a Cultivar-Dependent Manner. Plants 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Bejerano, P.; Santa María, E.; Torres-Pérez, R.; Royo, C.; Lijavetzky, D.; Bravo, G.; Aguirreolea, J.; Sánchez-Díaz, M.; Antolín, M.C.; Martínez-Zapater, J.M. Thermotolerance Responses in Ripening Berries of Vitis vinifera L. cv Muscat Hamburg. Plant Cell Physiol. 2013, 54, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, B. Drought priming-induced heat tolerance: Metabolic pathways and molecular mechanisms. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 149–160. [Google Scholar]
- Lu, H.-C.; Chen, W.-K.; Wang, Y.; Bai, X.-J.; Cheng, G.; Duan, C.-Q.; Wang, J.; He, F. Effect of the seasonal climatic variations on the flavonoid accumulation in Vitis vinifera cv ‘Muscat hamburg’ and ‘Victoria’ grapes under the double cropping system. Foods 2021, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Weng, J.; Zhang, Q.; Yu, L.; Yao, Q.; Chang, L.; Niu, Q. Physiological and biochemical responses of Cucumis melo L. chloroplasts to low-phosphate stress. Front. Plant Sci. 2018, 9, 1525. [Google Scholar] [CrossRef]
- Iwai, M.; Yokono, M.; Kono, M.; Noguchi, K.; Akimoto, S.; Nakano, A. Light-harvesting complex Lhcb9 confers a green alga-type photosystem I supercomplex to the moss Physcomitrella patens. Nat. Plants 2015, 1, 14008. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 1994, 192, 261–268. [Google Scholar] [CrossRef]
- Hendrickson, L.; Furbank, R.T.; Chow, W.S. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth. Res. 2004, 82, 73–81. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Backes, A.; Vaillant-Gaveau, N.; Esmaeel, Q.; Ait Barka, E.; Jacquard, C. A biological agent modulates the physiology of barley infected with Drechslera teres. Sci. Rep. 2021, 11, 8330. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.W.; Nothnagel, E.A.; Huffaker, R.C. Plant Senescence: Its Biochemistry and Physiology; California University: Riverside, CA, USA, 1987. [Google Scholar]
- Xu, Q.; Paulsen, A.Q.; Guikema, J.A.; Paulsen, G.M. Functional and ultrastructural injury to photosynthesis in wheat by high temperature during maturation. Environ. Exp. Bot. 1995, 35, 43–54. [Google Scholar] [CrossRef]
- Kamatchi, K.M.; Anitha, K.; Kumar, K.A.; Senthil, A.; Kalarani, M.; Djanaguiraman, M. Impacts of combined drought and high-temperature stress on growth, physiology, and yield of crops. Plant Physiol. Rep. 2024, 29, 28–36. [Google Scholar] [CrossRef]
- Annadurai, M.K.K.; Alagarsamy, S.; Karuppasami, K.M.; Ramakrishnan, S.; Subramanian, M.; Venugopal, P.R.; Muthurajan, R.; Vellingiri, G.; Dhashnamurthi, V.; Veerasamy, R. Melatonin decreases negative effects of combined drought and high temperature stresses through enhanced antioxidant defense system in tomato leaves. Horticulturae 2023, 9, 673. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Cao, Z.; Gao, Z.; Du, Y. Effects of Long-Term High Temperatures in the Root Zone on the Physiological Characteristics of Grapevine Leaves and Roots: Implications for Viticulture Practices. Horticulturae 2024, 10, 245. [Google Scholar] [CrossRef]
- Choudhary, S.; Bhat, T.M.; Alwutayd, K.M.; Abd El-Moneim, D.; Naaz, N. Salicylic acid enhances thermotolerance and antioxidant defense in Trigonella foenum graecum L. under heat stress. Heliyon 2024, 10, e27227. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Wang, X.; Chen, J. Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci. 2006, 170, 151–158. [Google Scholar] [CrossRef]
- Zeng, C.-Q.; Liu, W.-X.; Hao, J.-Y.; Fan, D.-N.; Chen, L.-M.; Xu, H.-N.; Li, K.-Z. Measuring the expression and activity of the CAT enzyme to determine Al resistance in soybean. Plant Physiol. Biochem. 2019, 144, 254–263. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Amorim, L.; Filho, A.B.; Berger, R.D. Gas Exchange and Emission of Chlorophyll Fluorescence during the Monocycle of Rust, Angular Leaf Spot and Anthracnose on Bean Leaves as a Function of their Trophic Characteristics. J. Phytopathol. 2002, 150, 37–47. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Li, J.; Chang, Y.; Du, Q.; Pan, T. Regulation of vapor pressure deficit by greenhouse micro-fog systems improved growth and productivity of tomato via enhancing photosynthesis during summer season. PLoS ONE 2015, 10, e0133919. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sunaga, M.; Ito, M.; Yuchen, Q.; Matsushima, Y.; Sakoda, K.; Yamori, W. Minimizing VPD Fluctuations Maintains Higher Stomatal Conductance and Photosynthesis, Resulting in Improvement of Plant Growth in Lettuce. Front. Plant Sci. 2021, 12, 646144. [Google Scholar] [CrossRef]
- Souza, R.P.; Machado, E.C.; Silva, J.A.B.; Lagôa, A.M.M.A.; Silveira, J.A.G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Maxwell, D.P.; Falk, S.; Trick, C.G.; Huner, N. Growth at Low Temperature Mimics High-Light Acclimation in Chlorella vulgaris. Plant Physiol. 1994, 105, 535–543. [Google Scholar] [CrossRef]
- Li, Q.; Yao, Z.-J.; Mi, H. Alleviation of Photoinhibition by Co-ordination of Chlororespiration and Cyclic Electron Flow Mediated by NDH under Heat Stressed Condition in Tobacco. Front. Plant Sci. 2016, 7, 285. [Google Scholar] [CrossRef]
- Havaux, M.; Davaud, A. Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of Photosystem-II activity. Photosynth. Res. 2004, 40, 75–92. [Google Scholar] [CrossRef]
- Suggett, D.J.; MacIntyre, H.L.; Kana, T.M.; Geider, R.J. Comparing electron transport with gas exchange: Parameterising exchange rates between alternative photosynthetic currencies for eukaryotic phytoplankton. Aquat. Microb. Ecol. 2009, 56, 147–162. [Google Scholar] [CrossRef]
- Laisk, A.; Eichelmann, H.; Oja, V.; Peterson, R.B. Control of cytochrome b6f at low and high light intensity and cyclic electron transport in leaves. Biochim. Biophys. Acta (BBA) Bioenerg. 2005, 1708, 79–90. [Google Scholar] [CrossRef]
- Mattila, H.; Khorobrykh, S.; Hakala-Yatkin, M.; Havurinne, V.; Kuusisto, I.; Antal, T.; Tyystjärvi, T.; Tyystjärvi, E. Action spectrum of the redox state of the plastoquinone pool defines its function in plant acclimation. Plant J. 2020, 104, 1088–1104. [Google Scholar] [CrossRef]
- Hahn, A.; Vonck, J.; Mills, D.J.; Meier, T.; Kühlbrandt, W. Structure, mechanism, and regulation of the chloroplast ATP synthase. Science 2018, 360, eaat4318. [Google Scholar] [CrossRef] [PubMed]
- Alboresi, A.; Storti, M.; Morosinotto, T. Balancing protection and efficiency in the regulation of photosynthetic electron transport across plant evolution. New Phytol. 2019, 221, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, S.-B.; Xu, J.-C.; Liu, T. Plasticity in roles of cyclic electron flow around photosystem I at contrasting temperatures in the chilling-sensitive plant Calotropis gigantea. Environ. Exp. Bot. 2017, 141, 145–153. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Differential regulation of photorespiratory gene expression by moderate and severe salt and drought stress in relation to oxidative stress. Plant Sci 2011, 180, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.D.; Livingston, A.K.; Satoh-Cruz, M.; Froehlich, J.E.; Maurino, V.G.; Kramer, D.M. Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 5539–5544. [Google Scholar] [CrossRef]
- Havaux, M.; Greppin, H.; Strasser, R.J. Functioning of photosystems I and II in pea leaves exposed to heat stress in the presence or absence of light: Analysis using in-vivo fluorescence, absorbance, oxygen and photoacoustic measurements. Planta 1991, 186, 88–98. [Google Scholar] [CrossRef]
- Kono, M.; Noguchi, K.; Terashima, I. Roles of the Cyclic Electron Flow Around PSI (CEF-PSI) and O2-Dependent Alternative Pathways in Regulation of the Photosynthetic Electron Flow in Short-Term Fluctuating Light in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 990–1004. [Google Scholar] [CrossRef]
- Wang, P.; Duan, W.; Takabayashi, A.; Endo, T.; Shikanai, T.; Ye, J.-Y.; Mi, H. Chloroplastic NAD(P)H Dehydrogenase in Tobacco Leaves Functions in Alleviation of Oxidative Damage Caused by Temperature Stress. Plant Physiol. 2006, 141, 465–474. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Q.; Sun, Y.; Guo, D.; Wang, L.; Pagay, V.; Wang, S. Heat Stress Downregulates Photosystem I Redox State on Leaf Photosynthesis in Grapevine. Agronomy 2025, 15, 948. https://doi.org/10.3390/agronomy15040948
Qiu Q, Sun Y, Guo D, Wang L, Pagay V, Wang S. Heat Stress Downregulates Photosystem I Redox State on Leaf Photosynthesis in Grapevine. Agronomy. 2025; 15(4):948. https://doi.org/10.3390/agronomy15040948
Chicago/Turabian StyleQiu, Qian, Yanli Sun, Dinghan Guo, Lei Wang, Vinay Pagay, and Shiping Wang. 2025. "Heat Stress Downregulates Photosystem I Redox State on Leaf Photosynthesis in Grapevine" Agronomy 15, no. 4: 948. https://doi.org/10.3390/agronomy15040948
APA StyleQiu, Q., Sun, Y., Guo, D., Wang, L., Pagay, V., & Wang, S. (2025). Heat Stress Downregulates Photosystem I Redox State on Leaf Photosynthesis in Grapevine. Agronomy, 15(4), 948. https://doi.org/10.3390/agronomy15040948





