Abstract
Biochar is an effective soil amendment for improving soil function; however, the effects of biochar produced at different pyrolysis temperatures on soil microbial community structure and enzyme activities remain insufficiently studied. A field experiment was conducted from 2023 to 2024 in the arid and semi-arid region of Northern China to investigate the effects of biochar produced at different pyrolysis temperatures (T1: 300 °C; T2: 500 °C; T3: 700 °C) and application rates (C1: 10 t ha−1; C2: 20 t ha−1; C3: 30 t ha−1) on soil chemical properties, microbial community structure, enzyme activity, and potato nutrient use efficiency. The results indicated that the C2T2 treatment was most effective in enhancing soil fungal and actinomycete populations, increasing total microbial biomass, significantly improving soil enzyme activities, and ultimately promoting crop yield. Structural equation modeling indicated that biochar regulates soil nutrient supply, drives microbial community succession toward functional specialization, prolongs microbial regeneration cycles, and ultimately enhances potato nutrient use efficiency. The results of this research provide scientific evidence to support the sustainable development of potato farming in the North China region.
1. Introduction
North China is an important grain production region, and potatoes, a key food crop in this area, are crucial in ensuring food security and improving nutrition [1]. However, in pursuit of high yields, farmers often over-irrigate and over-fertilize [2], leading to increased drought stress, nutrient leaching, loss of biodiversity, and soil degradation. This, in turn, exacerbates soil-borne diseases, threatening the sustainable development of potato cultivation [3]. Biochar, as a potential soil amendment, has been demonstrated to optimize microbial habitats and boost enzyme activity [4]. However, to fully realize the application potential of biochar in potato farming, additional studies are required to investigate its impact on soil chemical and biological properties, as well as the mechanisms that improve crop nutrient utilization efficiency.
Biochar significantly enhances soil carbon storage by improving its physical, chemical, and biological properties [5]. Additionally, it regulates soil pH and enhances nutrient use efficiency, thereby promoting plant productivity [6]. However, some studies have also suggested that biochar may have negative or negligible effects on soil nutrients and carbon storage potential. For example, its application can reduce soil mineral nitrogen availability in the short term [7] and may decrease crop productivity [8]. Several factors can affect the ability of biochar to enhance soil quality [9], with pyrolysis temperature being a key determinant of biochar’s physicochemical properties and a critical factor affecting the long-term effects of biochar on soil functions [10]. Biochar produced at higher temperatures generally exhibits greater specific surface area, porosity, pH, ash content, and carbon concentration, while also demonstrating lower toxicity to soil biota [11]. However, when the pyrolysis temperature exceeds a specific range, it may negatively affect crops’ ability to absorb soil nutrients [12]. The effects of biochar produced at different pyrolysis temperatures on microbial community composition and the associated metabolic changes in enzyme activity and crop nutrient use efficiency remain unclear.
The abundance and structure of soil microbial communities have been widely used as critical microbial indicators reflecting soil environmental changes and soil quality [13]. The physicochemical properties of biochar play a crucial role in determining its impact on microbial performance [14]. Biochar influences microbial community structure across different soils by modifying pH, organic carbon content, and the carbon-to-nitrogen ratio. Studies indicate that shifts in microbial composition driven by biochar–nitrogen interactions are closely linked to changes in soil pH and organic carbon levels [15]. Additionally, biochar can promote the growth of specific microorganisms, leading to an increased fungal-to-bacterial ratio and a higher proportion of Gram-negative to Gram-positive bacteria, thereby enhancing the activity of related organisms and improving the overall microbial community structure [16]. The impact of biochar on microbial communities is influenced not only by its properties but also by its application rate. Studies have demonstrated that microbial functions and community structure only change when the biochar application rate is sufficiently high, which is also a key factor affecting soil microbial diversity and composition [17]. A higher application rate of biochar provides microorganisms with more habitat space and pores, improving the soil environment, thereby significantly enhancing soil microbial activity [18]. However, excessive biochar application may also reduce the relative abundance of fungal and bacteria and decrease phospholipid fatty acid (PLFA) content [19]. Therefore, to fully explore biochar’s role in carbon storage and enhancing soil quality in agricultural ecosystems, additional research is required to examine how various biochar management approaches influence soil microbial community structure.
Soil enzymes are often seen as key microbial markers for assessing soil quality [20]. Applying biochar directly influences enzyme activity through the co-localization of enzymes and their interactions with the biochar surface [21]. Additionally, biochar improves soil physicochemical properties (such as specific surface area, water retention capacity, and unstable carbon compounds), enhancing microbial activity and biomass, which indirectly causes changes in enzyme activity [22]. Several studies have shown that pyrolysis temperature is a key factor determining the impact of biochar on soil enzyme activity [23]. Firstly, pyrolysis temperature affects the aromaticity, pH, mineral nutrient content, and active carbon pool of biochar, which in turn influences the abundance, composition, and activity of soil microorganisms [24], ultimately altering soil enzyme activity [25]. Secondly, biochar’s adsorption capacity is considered a significant factor leading to decreased enzyme activity in the soil [26], and pyrolysis temperature is a key factor affecting the ability of biochar particles to adsorb organic and inorganic molecules [27]. However, the threshold for the effect of biochar pyrolysis temperature on soil enzyme activity remains unclear. Research found that when biochar pyrolysis temperature was between 450 °C and 600 °C, extracellular enzyme activity related to carbon transformation first increased and then decreased [28]. The study also found that biochar produced at 350 °C in temperate sandy loam increased dehydrogenase activity, while biochar produced at 700 °C reduced dehydrogenase activity [29]. Current research indicates that the impact of biochar application on soil enzyme activity may exhibit a linear relationship with the application rate. However, it may also display a nonlinear or no relationship [23]. Therefore, how to combine pyrolysis temperature and the application rate of biochar to regulate soil enzyme activity, as well as the specific processes involved in this interaction, requires further investigation. Meanwhile, changes in microbial communities may indirectly or directly affect soil enzyme activity and crop nutrient uptake efficiency. However, the quantitative relationship between these processes has not yet been fully clarified.
This study aims to achieve the following: (1) to investigate the effects of biochar on the soil microbial community structure and related enzyme activity in potato fields; (2) to analyze the effects of biochar on soil biomass mortality rate and biomass turnover time; (3) to evaluate the impact of biochar on crop yield and nutrient use efficiency (PFP) and to explore the underlying regulatory mechanisms.
2. Materials and Methods
2.1. Experimental Site
Field trials were carried out from 2023 to 2024 at the Kebu’er Potato Science and Technology Backyard in Inner Mongolia (41°17′59.81″ N, 122°33′26.65″ E). The region has an average annual precipitation of 300 mm. The experimental area primarily comprises sandy loam soil, with a bulk density of 1.41 g cm−3 and organic matter content of 9.96 g kg−1 in the 0~100 cm soil layer. The soil contents of total nitrogen, phosphorus, and potassium in the 0~40 cm plow layer are 1.42 g kg−1, 12.22 mg kg−1, and 98.91 mg kg−1, respectively, with a soil pH of 7.95. Refer to Table S1 for details.
2.2. Experimental Design
2.2.1. Biochar
Biochar materials were prepared and provided by Zhengzhou Haosen Environmental Protection Technology Co., Ltd., Zhengzhou, China. Detailed information is presented in Table S2.
2.2.2. Experimental Arrangement
The planting dates for the potatoes were May 9, 2023, and May 10, 2024, with growing periods of 128 and 129 days, respectively. The variety used was Jinshu 16. The experiment included 10 treatments: a control without biochar application (CK) and nine biochar treatments formed by the combination of three pyrolysis temperatures (300 °C, 500 °C, and 700 °C) and three application rates (10, 20, and 30 t·ha−1). A completely randomized design was adopted with three replicates per treatment. Each plot measured 4 m × 5 m, resulting in a total of 30 plots.
Biochar for each treatment was first evenly applied to the soil surface and then incorporated into the 0–20 cm soil layer using a rotary tiller. To prevent cross-contamination between treatments, a 1.5 m-wide bare soil strip was left between adjacent plots as a buffer zone (Figure S1). The irrigation water source was groundwater, and irrigation was applied every 7 to 15 days during the study period. A water meter (accuracy of 0.001 m3) was installed to monitor water usage. Detailed information is provided in Table S2. Fertilizers included urea (46% N, 300 kg·ha−1) with a base ratio of 3:3:4, calcium superphosphate (46% P2O5, 180 kg·ha−1), and potassium sulfate (45% K2O, 300 kg·ha−1) as basal fertilizers.
2.3. Measurements and Calculations
2.3.1. Soil Chemical Properties
Soil pH was measured using a pH meter (Eutech pH 510, USA) [30]; organic carbon was determined by the potassium dichromate titrimetric method with external heating [31]; total nitrogen was measured using the Kjeldahl digestion method [32]; and nitrate and ammonium nitrogen were extracted with KCl solution and determined using an AA3 continuous-flow analyzer (Bran+Luebbe, Germany) [33].
2.3.2. Soil PLFA Extraction and Measurement
Lipid extraction and analysis were conducted by adding chloroform–methanol–citrate buffer (1:2:0.8, v:v:v) to 4.0 g of freeze-dried soil. Lipid classes were separated using a silica gel column and sequentially eluted with chloroform, acetone, and methanol to obtain neutral lipids, glycolipids, and phospholipid fatty acids (PLFAs), respectively. The purified PLFAs were methylated to fatty acid methyl esters (FAMEs), followed by the addition of an internal standard (19:0 FAME). Fatty acid identification was performed using gas chromatography (Agilent 7890B) combined with the Sherlock Microbial Identification System (MIS) version 4.5, and δ13C values of individual PLFAs were determined using gas chromatography–combustion–isotope ratio mass spectrometry (GC-C-IRMS, MAT 253, Thermo Fisher Scientific, USA) [34].
This study used the following fatty acids as biomarkers to represent the biomass of different microbial communities: 16:0, 18:0, i15:0, a15:0, i16:0, i17:0, a17:0, 16:1ω7c, 18:1ω7c, cy17:0ω7c, and cy19:0ω7c to represent bacterial biomass [35]; 18:2ω6c and 18:1ω9c to represent fungal biomass [35,36]; 10Me 16:0, 10Me 17:0, and 10Me 18:0 to represent actinobacterial biomass [36]; i15:0, a15:0, i16:0, i17:0, and a17:0 to represent Gram-positive bacterial biomass [37]; and 18:1ω7c, cy17:0ω7c, and cy19:0ω7c to represent Gram-negative bacterial biomass [36]. Additionally, the total sum of microbial PLFAs was used to represent the total microbial biomass in the soil [38].
2.3.3. Soil Enzyme Activity Soil Enzyme Dynamics
Soil samples at different depths were collected during different growth stages of potatoes to determine the activities of soil urease, alkaline phosphatase, sucrase, and catalase. Urease and alkaline phosphatase activities were measured using a spectrophotometric method [39,40], sucrase activity was determined using a colorimetric method [32], and catalase activity was measured by a titration method [41].
2.3.4. Soil Microbial Activity
The soil microbial activity substrate experiment is used to determine the soil microbial mortality rate and regeneration cycle. First, 40 g of the soil sample is placed into a 1.5 L brown bottle and sealed with a rubber stopper for incubation. At the same time, 20 mL of 1 mmol L−1 sodium hydroxide (NaOH) solution is added to a 50 mL beaker. The soil is incubated under 50% field capacity conditions. The water lost during incubation is replenished whenever NaOH is extracted for analysis, and NaOH solution is re-added [42]. During the incubation, the beakers are placed at 25 °C for continuous cultivation. The CO2 emitted from the soil during incubation is measured using gas chromatography coupled with a thermal conductivity detector [43]. The soil microbial death rate (SDRB) is primarily related to substrate concentration (P) and the distribution coefficient (β) and can be calculated using a first-order kinetic equation [42].
where represents the rate of change in the distribution coefficient B with respect to time t; dB refers to the change in the distribution coefficient B; dt denotes the change in time t; MBC represents soil microbial biomass carbon (mg kg−1); P is the substrate concentration (mg kg−1); β is the distribution coefficient for the conversion of the substrate to soil microbial biomass carbon; and SDRB is the soil microbial death rate (mg kg−1 d−1).
When soil microbial activity reaches a stable state, the equation can be rewritten as follows:
where CER is the CO2 release rate, mg kg−1 d−1; α is the substrate conversion coefficient to CO2; and TT is the turnover time, d.
Adenosine triphosphate (ATP).
where ATP is adenosine triphosphate, mg kg−1. Since ATP is closely related to soil microbial biomass carbon content, this study estimated the soil ATP content using the equation proposed by Joergensen et al. [42].
Microbial biomass nitrogen and carbon were quantified using the chloroform fumigation–extraction technique [44].
2.3.5. Potato Yield and Nutrient Use Efficiency
Fresh potatoes from an area of 2 m × 1.8 m were harvested and weighed for measurement [45].
Nutrient use efficiency [46].
where Y represents the yield (kg ha−1); PFP is the fertilizer partial productivity (kg kg−1); and FT is the total amount of N, P2O5, and K2O applied (kg ha−1).
2.4. Data Processing and Analysis
The experimental data were processed in Excel 2019, followed by statistical analysis in SPSS 26.0 using LSD and Duncan’s multiple range tests. Data visualizations were generated with OriginPro 2021, while the Mantel test and structural analysis were performed in R Studio 1.1.
3. Results and Analysis
3.1. Soil Microbial Community Structure
As the growing season progressed, the total soil microbial community (total PLFAs), fungi, bacteria, Gram-positive (G+) bacteria, and Gram-negative (G−) bacteria exhibited a downward-opening parabolic trend, whereas actinomycetes showed a linear increase. Biochar application significantly altered the activity of total soil microorganisms (total PLFAs), fungi, and actinomycetes but had no significant effect on the activity of soil bacteria, G+ bacteria, or G− bacteria (Figure 1). Throughout the growing season, the activity of total soil microorganisms (total PLFAs) and actinomycetes was highest under the C2T2 treatment, significantly exceeding the other treatments by 7.29–18.35% and 7.02–16.21%, respectively (with no significant difference from C1T2 and C2T1 mean values across growth stages in 2023, p > 0.05). Similarly, fungal abundance was highest under the C2T2 treatment, significantly surpassing other treatments by 7.51–27.52% (with no significant difference from C1T2, mean values across growth stages in 2023, p > 0.05).
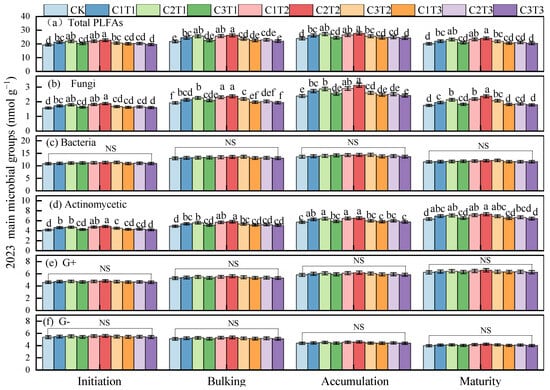
Figure 1.
Changes in total soil phospholipid fatty acids (a), fungi (b), bacteria (c), actinomycetes (d), Gram-positive bacteria (G+) (e), and Gram-negative bacteria (G−) (f) during the tuber formation, tuber bulking, starch accumulation, and maturity stages of potato farmland under different biochar treatments in 2023. NS indicates no significant difference. Different letters indicate significant differences between treatments (p < 0.05). Values are means ± standard errors (n = 3).
The temporal dynamics of soil microorganisms during the 2024 growing season were generally consistent with those observed in 2023. The total PLFAs and actinomycetes were highest under the C2T2 treatment, with no significant difference compared to C1T2 and C2T1. Compared to these treatments, C2T2 increased total microbial biomass and actinomycetes by 8.33–21.14% and 7.63–20.76%, respectively (p < 0.05, mean values across growth stages in 2024). Additionally, the fungal abundance in the C2T2 treatment was 8.62–32.87% higher than in other treatments, with no significant difference compared to C1T2 (p < 0.05, mean values across growth stages in 2024).
This study demonstrated that biochar application significantly altered soil F/B across different potato growth stages, whereas it had no significant effect on the G+/G− ratio (Figure 2 and Figure 3). Throughout the growing season, the impact of an increasing biochar pyrolysis temperature and application rate on F/B exhibited a unimodal trend, with the highest F/B value observed under the C2T2 treatment. There was no significant difference between C2T2 and C1T2 or C2T1; however, compared to other treatments, C2T2 significantly increased F/B by 10.42–22.93% (p < 0.05, mean values across growth stages in 2023 and 2024).
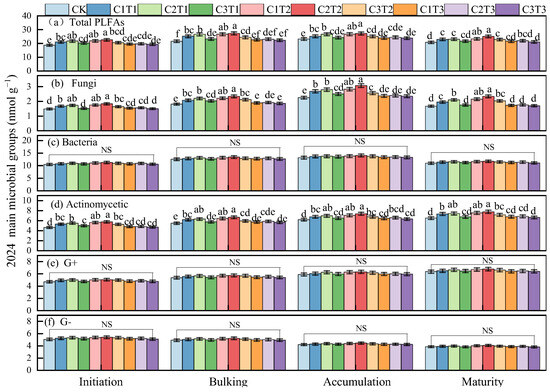
Figure 2.
Changes in total soil phospholipid fatty acids (a), fungi (b), bacteria (c), actinomycetes (d), Gram-positive bacteria (G+) (e), and Gram-negative bacteria (G−) (f) during the tuber formation, tuber bulking, starch accumulation, and maturity stages of potato farmland under different biochar treatments in 2024. The same procedure was followed as described above.
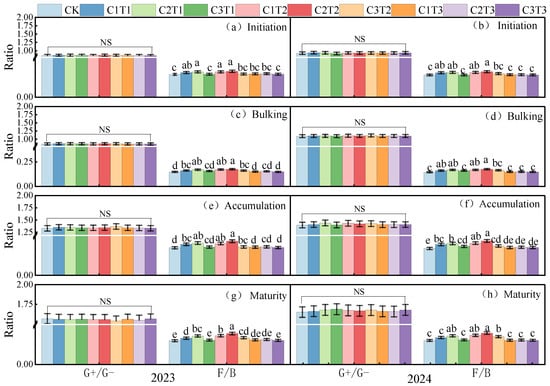
Figure 3.
Changes in soil G+/G− and F/B ratios in potato farmland under different biochar conditions during the tuber formation stage, tuber bulking stage, starch accumulation stage, and maturation stage in 2023 (a,c,e,g) and 2024 (b,d,f,h). G+/G− and F/B represent the ratio of Gram-positive to Gram-negative bacteria and the ratio of fungi to bacteria, respectively. The same procedure was followed as described above.
3.2. Microbial Death Rate and Regeneration Cycle
Microorganisms with longer regeneration cycles can gradually release nutrients, providing a stable nutrient supply, supporting continuous crop growth, and enhancing nutrient use efficiency. During the maturation period of potatoes, the C2T2 treatment showed the highest soil microbial carbon and nitrogen content, significantly higher by 6.46–31.93% and 6.21–30.51% compared to other treatments (Table 1, 2023 and 2024 average). The microbial biomass primarily influenced soil microorganisms’ death rate and corresponding regeneration cycle. Considering the energy release and regeneration cycle of microorganisms, the C2T2 treatment performed the best, with a CO2 release rate of 11.57 mg kg−1 d−1, ATP content of 2.59 mmol kg−1, death rate of 0.039 mg kg−1 d−1, and regeneration cycle of 25.37 days (2023 and 2024 average).

Table 1.
Effects of biochar on soil microbial activity in potato farmland in 2023 and 2024.
3.3. Soil Enzyme Activity
As there were no significant differences in soil enzyme activity between 2023 and 2024, the analysis was conducted using 2023 as a representative year. The activities of soil urease, alkaline phosphatase, sucrase, and catalase exhibited a trend of increasing first and then decreasing throughout the potato growing season (Figure 4). Overall, biochar application significantly enhanced soil enzyme activities. Across all growth stages, the highest activities of urease, catalase, alkaline phosphatase, and sucrase were observed under the C2T2 treatment, with values ranging from 349.1to 652.8, 42.6 to 67.6, 2.78 to 4.59, and 264.6 to 473.0, respectively. While no significant differences were detected between C2T2, C1T2, and C2T1, C2T2 significantly outperformed the other treatments, with increases of 6.58–17.35%, 10.64–19.32%, 9.91–26.20%, and 7.82–22.90%, respectively (mean values across growth stages in 2023).
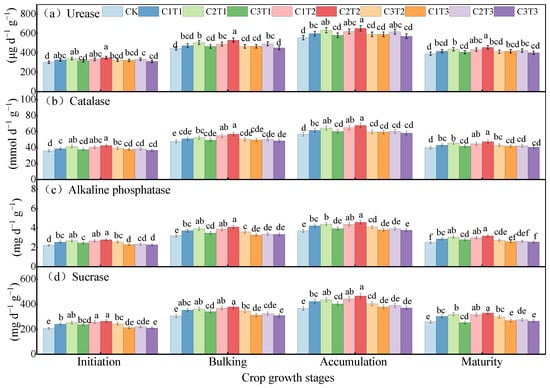
Figure 4.
Effects of biochar on soil urease (a), catalase (b), alkaline phosphatase (c), and sucrase (d) activities in potato farmland during the tuber formation stage, tuber bulking stage, starch accumulation stage, and maturation stage in 2023. The same procedure was followed as described above.
3.4. Nutrient Use Efficiency of Potatoes and Its Relationship with Soil Chemical Properties, Microbial Community Structure, and Enzyme Activity
Both potato yield and nutrient use efficiency were highest under the C2T2 treatment, with values 8.44% to 27.15% higher than those of other treatments, and the differences were significant (p < 0.05) (Figure 5 and Figure 6, p < 0.05, mean values for 2023 and 2024). In addition, this study found that biochar application notably influenced the contents of soil nitrate nitrogen, organic carbon, and total nitrogen, while having little impact on ammonium nitrogen and pH (Table S4). Mantel test analysis (Figure 7) showed that microbial community structure was significantly positively correlated with soil organic carbon, total nitrogen, enzyme activity, CO2 release rate (CER), soil microbial death rate (SDRB), and adenosine triphosphate (ATP) (p < 0.05). The regeneration cycle was strongly positively correlated with soil enzyme activity, microbial biomass carbon and nitrogen, and ATP (p < 0.001), while it was significantly negatively correlated with CER and SDRB (p < 0.001). Soil organic carbon and total nitrogen were significantly positively correlated with soil enzyme activity (p < 0.001) and significantly negatively correlated with CER (p < 0.05), while soil nitrate nitrogen showed the opposite trend.
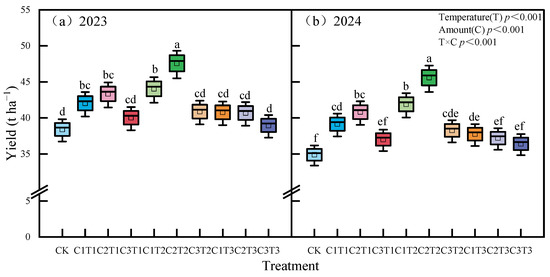
Figure 5.
Effect of different biochar treatments on potato yield in 2023 (a) and 2024 (b). The same procedure was followed as described above.
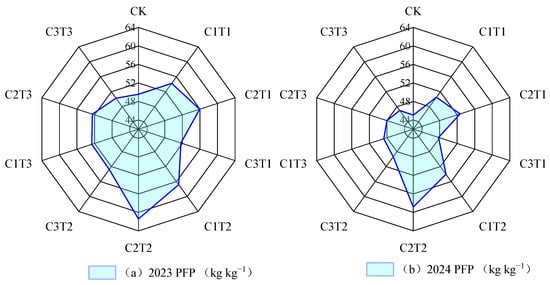
Figure 6.
Effect of different biochar treatments on potato nutrient use efficiency in 2023 (a) and 2024 (b).
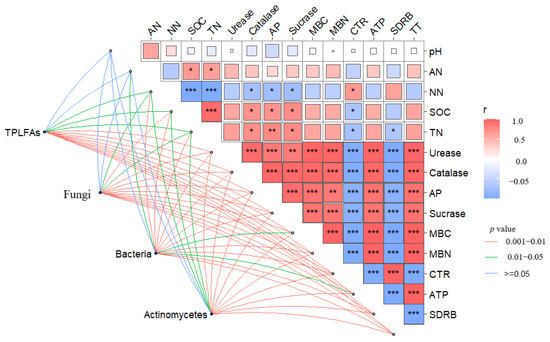
Figure 7.
The relationship between microbial community and soil environmental factors under different biochar conditions in 2023–2024. AN represents ammonium nitrogen; NN represents nitrate nitrogen; SOC represents organic carbon; TN represents total nitrogen; and AP represents alkaline phosphatase. * p < 0.05, ** p < 0.01, *** p < 0.001. The same procedure was followed as described above.
Based on the structural equation model (SEM) analysis (Figure 8), soil nutrients had a direct positive effect on soil microbial community structure, enzyme activity, microbial regeneration cycle, and PFP, with path coefficients of 0.638 (p < 0.01), 0.630 (p < 0.01), 0.580 (p < 0.05), and 0.665 (p < 0.01), respectively. Soil microbial community structure directly affected the soil enzyme activity and microbial regeneration cycle (path coefficient 0.986, p < 0.001; path coefficient 0.999, p < 0.001). The soil enzyme activity and soil microbial regeneration cycle directly affected PFP (path coefficient 0.683, p < 0.01; path coefficient 0.735, p < 0.01). This indicates that soil nutrients not only directly improve nutrient use efficiency but also, through enhancing soil microbial community structure, indirectly increase soil enzyme activity and the microbial regeneration cycle, further enhancing nutrient use efficiency.
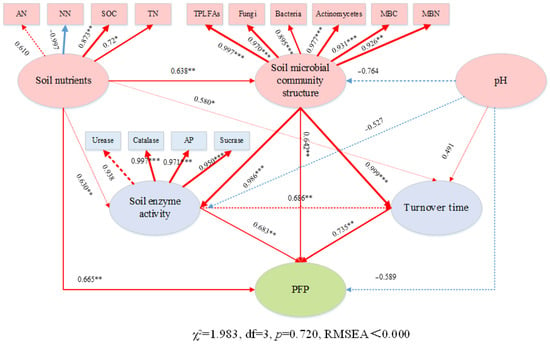
Figure 8.
Direct and indirect effects of biochar on soil pH, nutrients, enzyme activity, microbial community structure, and soil microbial regeneration cycle on soil nutrient use efficiency (PFP) in 2023 and 2024. The numbers beside the arrows indicate path coefficients, with arrow width reflecting the magnitude of these coefficients. Red arrows denote positive correlations, while blue arrows indicate negative correlations. Solid arrows represent significant relationships, and dashed arrows represent non-significant ones. * p < 0.05, ** p < 0.01, *** p < 0.001. The same procedure was followed as described above.
4. Discussion
4.1. The Effect of Biochar on Soil Microbial Abundance and Community Composition
Research has demonstrated that the application of biochar can increase the activity of total soil microorganisms [47]. However, most studies on the regulation of soil microbial communities by biochar have focused on humid and sub-humid regions, while research in potato farmlands of arid and semi-arid areas remains limited.
In this study, under the same biochar application rate, a pyrolysis temperature of 500 °C was most effective in enhancing the activity of total microorganisms, fungi, and actinomycetes in the soil. (Figure 1 and Figure 2). This pattern can be attributed to the fact that medium-temperature biochar, unlike low- and high-temperature biochar, contains a balanced level of organic nutrients and has a more optimal pore structure and surface area, both of which support increased microbial abundance and activity [18]. Additionally, an optimal biochar pyrolysis temperature can activate microbial activity, accelerate organic matter decomposition and transformation, promote organic carbon stability, and reduce its mineralization rate, thereby enhancing soil carbon sequestration and providing a more stable and sustained nutrient supply for potato growth [48].
Furthermore, at the same pyrolysis temperature, applying biochar at a rate of 20 t ha−1 was found to be the most effective in enhancing soil microbial biomass and activity. This could be due to the increased habitat surface area and porosity, which lower soil bulk density and improve water and nutrient retention. However, excessive biochar application may disrupt the microenvironment required for microbial growth, leading to a decline in microbial diversity due to selective pressure on microbial populations [49]. In addition, high biochar application rates may introduce toxic compounds that negatively affect certain soil microorganisms, further reducing microbial diversity [50]. This impact may be more pronounced in arid and semi-arid environments with limited water availability, as microbial resilience is weaker, and potentially harmful biochar-derived compounds may persist in the soil for extended periods.
4.2. Effect of Biochar on Soil Enzyme Activity
Urease, alkaline phosphatase, and sucrase play crucial roles in the soil nitrogen, phosphorus, and carbon cycles, respectively, influencing soil nitrogen supply intensity, plant-available phosphorus content, and the bioavailability of soil organic matter [12]. Catalase activity reflects soil oxidation intensity and is closely associated with soil organic matter and microbial activity [15]. Adding biochar to the soil not only serves as an energy source for microbial activity but also offers abundant substrates for enzymatic reactions [51].
This study found that soil urease, alkaline phosphatase, sucrase, and catalase activities were highest when biochar produced at a medium pyrolysis temperature was applied (Figure 4). This could be because biochar pyrolyzed at 500 °C contains a higher proportion of oxygen-containing functional groups, a lower aromatic structure content, and a greater abundance of readily degradable compounds, which collectively enhance enzyme activity [52]. In contrast, high-temperature biochar (700 °C) exhibits greater aromaticity and hydrophobicity, increasing its stability in soil but reducing its bioavailability, thereby suppressing enzymatic activity [53]. In addition, biochar produced at higher pyrolysis temperatures typically has a smaller pore size (S2), which not only increases its specific surface area and enhances its adsorption capacity but may also reduce microbial abundance and enzyme activity in the soil [54,55], potentially limiting potato’s uptake of essential nutrients. However, some studies have reported that biochar pyrolysis temperature has no significant effect on soil enzyme activity [56], while others have even found negative effects [57]. These discrepancies could be due to differences in experimental conditions and the properties of biochar.
This study further showed that, at the same pyrolysis temperature, applying biochar at a rate of 20 t ha−1 led to the highest soil enzyme activity (Figure 4). This could be because the optimal biochar application rate enhances soil water retention and improves the microbial environment, thereby supplying ample substrates for enzymatic reactions [51], thereby enhancing enzymatic efficiency, promoting nutrient uptake by potato roots, and improving drought tolerance and yield. However, excessive biochar application may increase its surface area and porosity, which could restrict substrate accessibility to enzyme active sites, ultimately reducing soil enzyme activity [53].
4.3. Impact and Mechanisms of Biochar on Potato Nutrient Use Efficiency
In arid and semi-arid environments, soil moisture scarcity and nutrient deficiency are the primary factors limiting potato growth. The application of biochar improves soil physicochemical properties and regulates microbial communities, thereby enhancing the soil’s ability to retain moisture and nutrients. This, in turn, increases nutrient availability, promotes crop growth, and boosts yield [58]. Our analysis through the Mantel test (Figure 7) and structural equation modeling (Figure 8) indicates that biochar directly improves soil microbial community structure by regulating soil nutrient status, which in turn indirectly affects soil enzyme activity and turnover cycles, ultimately enhancing soil nutrient use efficiency. This mechanism not only improves soil fertility but also contributes to the stability and sustainability of the soil ecosystem.
Based on the results of this study, applying 20 t ha−1 biochar, in addition to local fertilization and irrigation practices, can achieve increased yield and efficiency. However, the application of biochar inevitably increases farmers’ investment costs. Therefore, to promote the large-scale application of biochar technology, further consideration should be given to the resource use efficiency and cost-effectiveness of biochar as a partial substitute for chemical fertilizers. By constructing a green potato production system that balances both ecological and economic benefits, this study provides scientific support for sustainable agricultural development in arid and semi-arid regions and offers practical evidence for reducing fertilizer dependence and minimizing environmental impact.
5. Conclusions
This study systematically investigated the impact of biochar on the microbial community environment in potato fields in North China through a two-year field trial conducted from 2023 to 2024. The results were as follows:
(1) In the potato planting system of arid and semi-arid regions, biochar application had no significant effect on soil bacterial, G+ bacterial, or G− bacterial activity or the G+/G− ratio. However, biochar application significantly affected the activity of total soil microorganisms, fungi, actinomycetes, and soil enzymes, with the highest sensitivity observed under a medium pyrolysis temperature (500 °C) and medium application rate (20 t·ha−1).
(2) Structural equation modeling indicated that biochar improved nutrient supply, which drove the optimization of microbial community structure, subsequently enhancing soil enzyme activity, slowing down microbial turnover cycles, and ultimately increasing soil nutrient availability.
This study confirms that applying biochar produced at a pyrolysis temperature of 500 °C at a rate of 20 t ha−1 is an appropriate strategy for synergistically enhancing the soil microenvironment, yield, and nutrient use efficiency in potato farmland in the dryland regions of North China. Future research should further assess the resource use efficiency and economic benefits of partial biochar substitution for chemical fertilizers, providing scientific support for the green and sustainable development of potato agriculture in arid regions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15040945/s1. Figure S1: A schematic diagram of the traditional potato system (left) and example image (right). Table S1: Physical and chemical properties of the experimental soils in 0–100 cm soil layer. Table S2: Physicochemical characteristics of biochar under different pyrolysis temperatures; Table S3. Irrigation amounts for each growth stage of potato; Table S4: Effects of biochar on soil chemical properties in 2023–2024.
Author Contributions
Conceptualization: J.G. and L.J.; Methodology: H.Z. and Y.W.; Investigation: M.F. and X.S.; Data analysis: H.Z. and Y.W.; Visualization: X.S. and J.G.; Writing—original draft: J.G. and M.F.; Writing—review and editing: L.J. and H.Z.; Manuscript verification: Y.W. and X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32360536) and the Basic Scientific Research Funds for Universities in Inner Mongolia (BR 22-13-01).
Data Availability Statement
All data generated during the manuscript analysis are provided within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Z.J.; Liu, H.; Zeng, F.K.; Yang, Y.C.; Xu, D.; Zhao, Y.C.; Singh, J. Potato processing industry in China: Current scenario, future trends and global impact. Potato Res. 2023, 66, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, P.; Xu, X.-P.; Qu, X.-J.; Zhou, W. Current situation and potential of potato fertilizer reduction in China. J. Plant Nutr. Fertilizer. 2023, 29, 2059–2070. [Google Scholar] [CrossRef]
- Nasir, M.W.; Toth, Z. Effect of drought stress on potato production: A review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Hou, J.; Xing, C.; Zhang, J.; Wang, Z.; Liu, M.; Duan, Y.; Zhao, H. Increase in potato yield by the combined application of biochar and organic fertilizer: Key role of rhizosphere microbial diversity. Front. Plant Sci. 2024, 15, 1389864. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 138–151. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Belal, E.E.; Rady, M.O.A.; Abd El-Mageed, S.A.; Mansour, E.; Awad, M.F.; Semida, W.M. Acidified biochar as a soil amendment to drought-stressed (Vicia faba L.) plants: Influences on growth and productivity, nutrient status, and water use efficiency. Agronomy 2021, 11, 1290. [Google Scholar] [CrossRef]
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Short-term effects of biochar on soil properties and wheat yield formation with meat bone meal and inorganic fertiliser on a boreal loamy sand. Agric. Ecosyst. Environ. 2014, 191, 108–116. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2009, 327, 235–246. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, S.; Yan, P.; Aurangzeib, M. Effect of biochar application method and amount on the soil quality and maize yield in Mollisols of Northeast China. Biochar 2022, 4, 180–194. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef]
- Cheng, H.; Jones, D.L.; Hill, P.; Bastami, M.S.; Tu, C.L. Influence of biochar produced from different pyrolysis temperatures on nutrient retention and leaching. Arch. Agron. Soil Sci. 2017, 64, 850–859. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Tahir, M.; Amjad, M.; Murtaza, B.; Yang, A.; Akhtar, S.S. Effect of wheat and rice straw biochar produced at different temperatures on maize growth and nutrient dynamics of a calcareous soil. Arch. Agron. Soil Sci. 2017, 63, 2048–2061. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Kong, L.; Tong, L.; Cao, H.; Zhou, H.; Lv, Y. Long-term application of bio-compost increased soil microbial community diversity and altered its composition and network. Microorganisms 2022, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Zhan, L.; Xu, X.; Bi, R.; Xiong, Z. Biochar addition stabilized soil carbon sequestration by reducing temperature sensitivity of mineralization and altering the microbial community in a greenhouse vegetable field. J. Environ. Manag. 2022, 313, 114972. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Xiang, Y.; Zhang, R.; Lu, H. Responses of soil microbial community structure changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935. [Google Scholar] [CrossRef]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 2013, 65, 28–39. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Wang, X.; Song, D.; Liang, G.; Zhang, Q.; Ai, C.; Zhou, W. Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl. Soil Ecol. 2015, 96, 265–272. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Potential use of soil enzymes as soil quality indicators in agriculture. In Frontiers in Soil and Environmental Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 57–64. [Google Scholar]
- Muneer, M.A.; Fatima, S.; Hussain, N.; Mashifana, T.; Sayed, A.; Boczkaj, G.; Rajoka, M.S.R. Enzyme Conjugation—A Promising Tool for Bio-catalytic and Biotransformation Applications—A Review. Top. Catal. 2024, 4, 1–7. [Google Scholar] [CrossRef]
- Amoakwah, E.; Arthur, E.; Frimpong, K.A.; Lorenz, N.; Rahman, M.A.; Nziguheba, G.; Islam, K.R. Biochar amendment impacts on microbial community structures and biological and enzyme activities in a weathered tropical sandy loam. Appl. Soil Ecol. 2022, 172, 104364. [Google Scholar] [CrossRef]
- Liao, X.; Kang, H.; Haidar, G.; Wang, W.; Malghani, S. The impact of biochar on the activities of soil nutrients acquisition enzymes is potentially controlled by the pyrolysis temperature: A meta-analysis. Geoderma 2022, 411, 115692. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio./Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Datt, N.; Singh, D. Enzymes in relation to soil biological properties and sustainability. Sustain. Manag. Soil Environ. 2019, 3, 383–406. [Google Scholar] [CrossRef]
- Lei, O.L.; Tang, Q.; Yu, L.; Zhang, R. Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation. Soil Res. 2014, 52, 706–716. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, W.; Liang, G.; Song, D.; Zhang, X. Characteristics of maize biochar with different pyrolysis temperatures and its effects on organic carbon, nitrogen and enzymatic activities after addition to fluvo-aquic soil. Sci. Total Environ. 2015, 538, 137–144. [Google Scholar] [CrossRef]
- Ameloot, N.; De Neve, S.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Prins, W.; Bouckaert, L.; Sleutel, S. Short-term CO₂ and N₂O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013, 57, 401–410. [Google Scholar] [CrossRef]
- Pansu, M. Handbook of Soil Analysis; Springer: Berlin/Heidelberg, Germany, 2006; pp. 551–579. [Google Scholar]
- Islam, K.R. Organic carbon content assessment methods. In Encyclopedia of Soil Science, 2nd ed.; Taylor & Francis: New York, NY, USA, 2006; pp. 1164–1168. [Google Scholar]
- Lu, R.K. Methods of Soil and Agro-Chemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 127–332. (In Chinese) [Google Scholar]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, H.; Luo, X.; Xing, B. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 2017, 41, 517–532. [Google Scholar] [CrossRef]
- Yao, H.; Chapman, S.J.; Thornton, B.; Paterson, E. 13C PLFAs: A key to open the soil microbial black box? Plant Soil 2014, 392, 3–15. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Pan, F.; Li, Y.; Chapman, S.J.; Khan, S.; Yao, H. Microbial utilization of rice straw and its derived biochar in a paddy soil. Sci. Total Environ. 2016, 559, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Xu, Q.; Fuhrmann, J.J.; Li, L.; Pan, G.; Li, Y.; Qin, H.; Liang, C.; Sun, X. Organic carbon quality, composition of main microbial groups, enzyme activities, and temperature sensitivity of soil respiration of an acid paddy soil treated with biochar. Biol. Fertil. Soils 2018, 55, 185–197. [Google Scholar] [CrossRef]
- Kandeler, E.G. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Guo, L. Effect and Mechanism of Biochar on Improving Water and Fertilizer Utilization of Tomato in Greenhouse; Northwest AF University: Xianyang, China, 2021; pp. 44–54, (In Chinese). [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Camina, F.; Leiros, M.C.; Gil-Sotres, F. An improved method to measure catalase activity in soils. Soil Biol. Biochem. 1999, 31, 483–485. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Brookes, P.C.; Jenkinson, D.S. Survival of the soil microbial biomass at elevated temperatures. Soil Biol. Biochem. 1990, 22, 1129–1136. [Google Scholar] [CrossRef]
- Montagnolli, R.N.; Lopes, P.R.M.; Bidoia, E.D. Fluorinated waste and firefighting activities: Biodegradation of hydrocarbons from petrochemical refinery soil co-contaminated with halogenated foams. Environ. Sci. Pollut. Res. 2018, 25, 36002–36013. [Google Scholar] [CrossRef]
- Jenkinson, D.S. Determination of microbial biomass carbon and nitrogen in soil. In Advances in Nitrogen Cycling in Agricultural Ecosystems; Wilson, J.R., Ed.; CAB International: Wallingford, UK, 1988; pp. 368–386. [Google Scholar]
- Wang, H.; Cheng, M.; Zhang, S.; Fan, J.; Feng, H.; Zhang, F.; Wang, X.; Sun, L.; Xiang, Y. Optimization of irrigation amount and fertilization rate of drip-fertigated potato based on Analytic Hierarchy Process and Fuzzy Comprehensive Evaluation methods. Agric. Water Manag. 2021, 256, 107130. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, T.; Jiang, W.; Li, P.; Shi, P.; Xu, G.; Cheng, S.; Cheng, Y.; Fan, Z.; Wang, X. Effects of irrigation and fertilization on different potato varieties’ growth, yield and resource use efficiency in Northwest China. Agric. Water Manag. 2022, 261, 107351. [Google Scholar] [CrossRef]
- Chen, K.; Peng, J.; Feng, X.; Li, Y.; Zhan, X.; Han, X. Effects of biochar and carbon-based fertilizer on soil microbial community structure. Chin. J. Agric. Sci. 2018, 51, 1920–1930. [Google Scholar] [CrossRef]
- Hou, J.; Pugazhendhi, A.; Sindhu, R.; Vinayak, V.; Thanh, N.C.; Brindhadevi, K.; Yuan, D. An assessment of biochar as a potential amendment to enhance plant nutrient uptake. Environ. Res. 2022, 214, 113909. [Google Scholar] [CrossRef]
- McCormack, S.A.; Ostle, N.; Bardgett, R.D.; Hopkins, D.W.; Vanbergen, A.J. Biochar in bioenergy cropping systems: Impacts on soil faunal communities and linked ecosystem processes. GCB Bioenergy 2013, 5, 81–95. [Google Scholar] [CrossRef]
- Muhammad, N.; Hussain, M.; Ullah, W.; Khan, T.A.; Ali, S.; Akbar, A.; Aziz, R.; Rafiq, M.K.; Bachmann, R.T.; Al-Wabel, M.I.; et al. Biochar for sustainable soil and environment: A comprehensive review. Arab. J. Geosci. 2018, 11, 4074. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Anand, A.; Gautam, S.; Ram, L.C. Feedstock and pyrolysis conditions affect the suitability of biochar for various sustainable energy and environmental applications. J. Anal. Appl. Pyrolysis. 2023, 170, 105881. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F. Influence of biochar on potential enzyme activities in two calcareous soils of contrasting texture. Geoderma 2017, 308, 149–158. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Cai, L.; Hussain, S.; Cheema, S.A.; Wu, J.; Zhang, R. An overview on biochar production, its implications, and mechanisms of biochar-induced amelioration of soil and plant characteristics. Pedosphere 2022, 32, 107–130. [Google Scholar] [CrossRef]
- Huang, H.; Reddy, N.G.; Huang, X.; Chen, P.; Wang, P.; Zhang, Y.; Garg, A. Effects of pyrolysis temperature, feedstock type and compaction on water retention of biochar amended soil. Sci. Rep. 2021, 11, 7419. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Pan, G.; Liu, X.; Zhang, X.; Li, L.; Bian, R.; Cheng, K.; Jinwei, J. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci. Total Environ. 2016, 571, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).