Biocontrol Potential of Bacillus subtilis A3 Against Corn Stalk Rot and Its Impact on Root-Associated Microbial Communities
Abstract
1. Introduction
2. Methods and Materials
2.1. Isolation and Identification of Bacillus subtilis A3
2.2. Antibacterial Stability and Spectrum Tests of A3
2.3. Antibacterial Activity Test of the Antibacterial Substances from Strain A3
2.3.1. Isolation and Identification of Antibacterial Substances
2.3.2. Antibacterial Activity of Antibacterial Substances
2.4. Indoor Efficacy Test of A3
2.4.1. Efficacy of A3 Against Corn Stalk Rot
2.4.2. Effects of A3 on Corn Growth
2.5. Field Efficacy Test of Biocontrol Agent for Corn Stalk Rot
2.5.1. Seed Coating of Corn with A3
2.5.2. Field Experiment Design
2.5.3. Field Observation
2.6. Detection of Microbial Diversity in the Rhizosphere Soil and Root of Corn
2.7. Statistical Analysis
3. Results
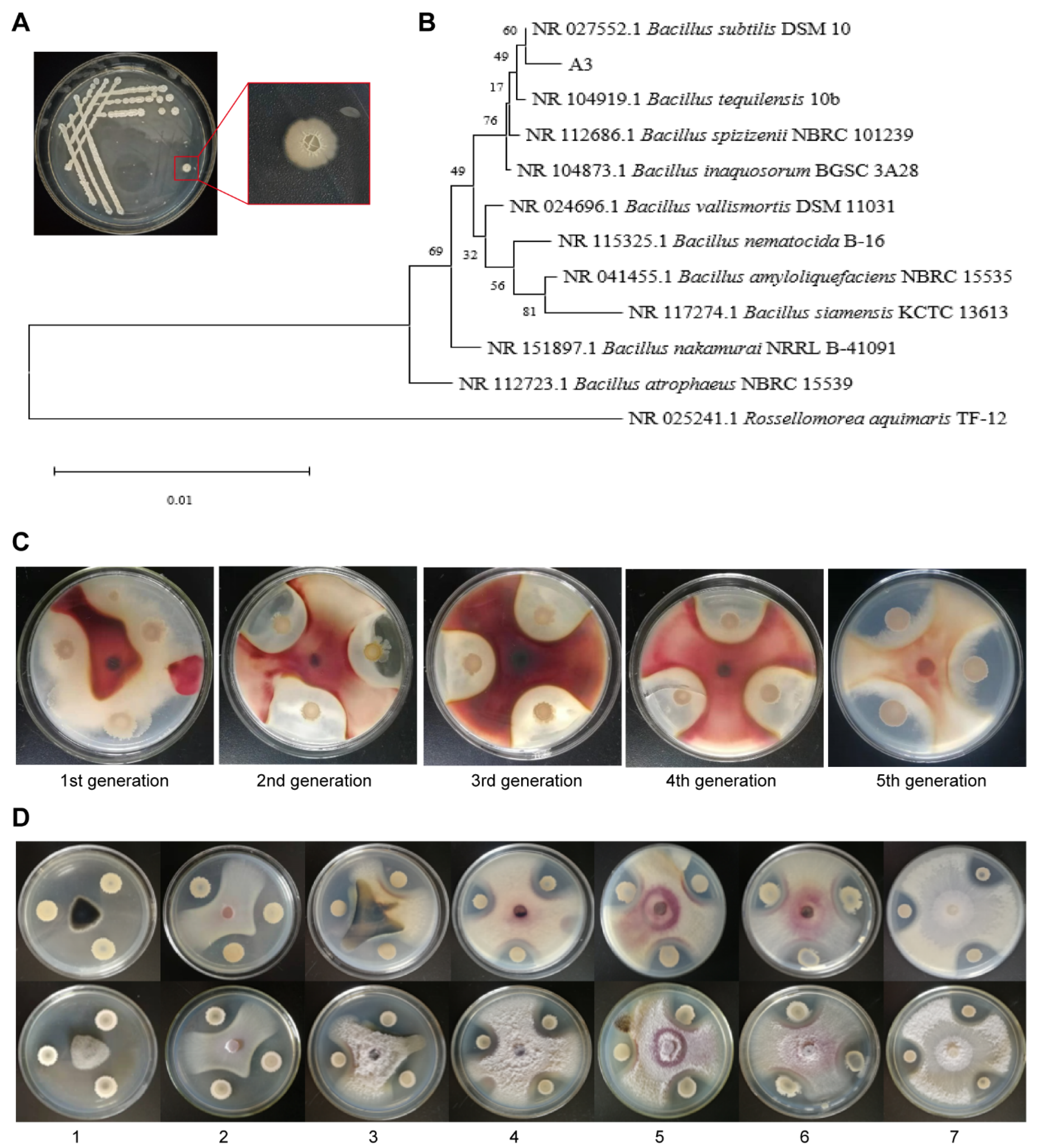
3.1. Identification of the Biocontrol Strain A3 for Corn Stalk Rot
3.2. Determination of the Stability and Antibacterial Spectrum of B. subtilis A3
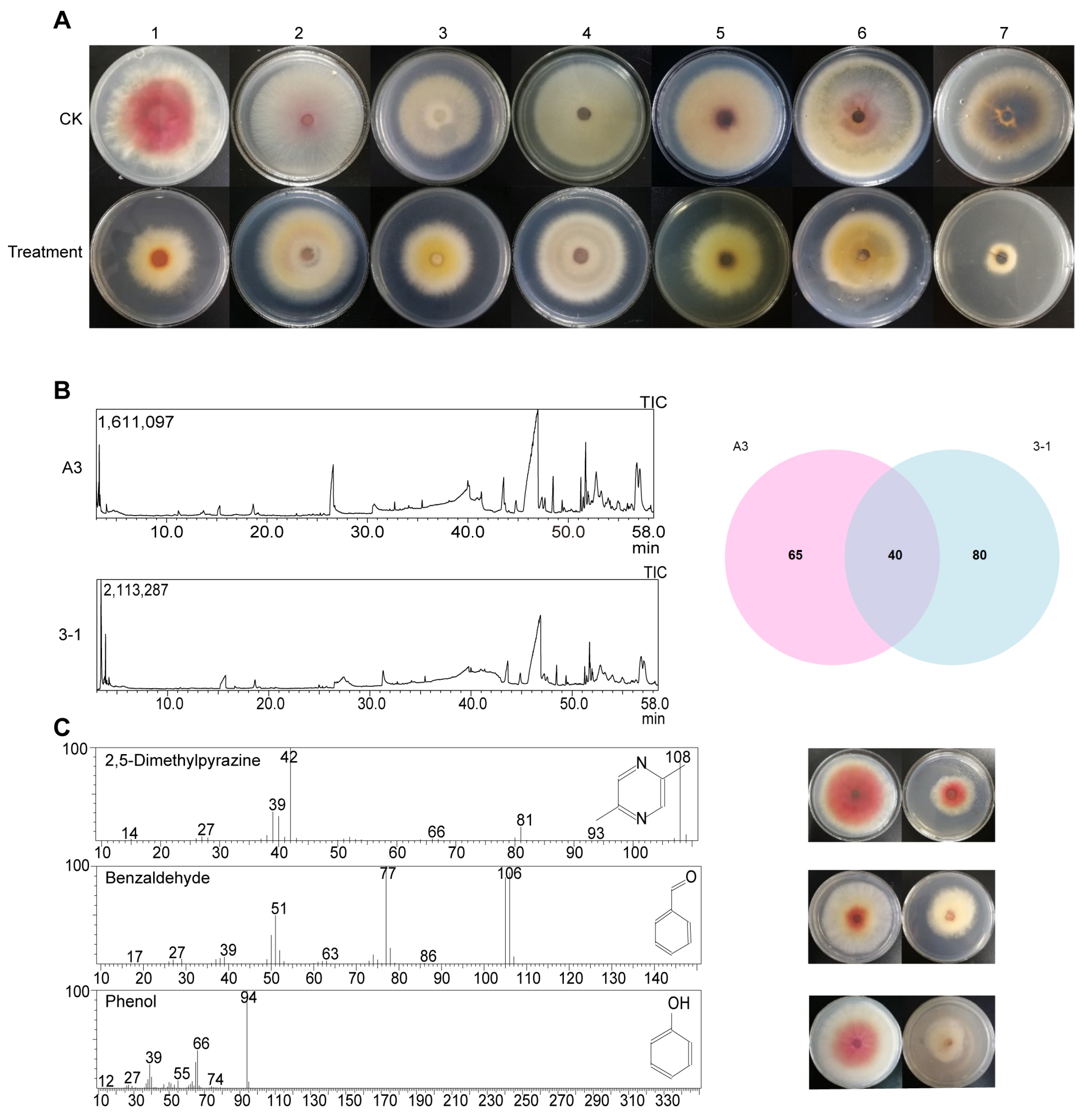
3.3. Inhibitory Effect of B. subtilis A3 on F. graminearum
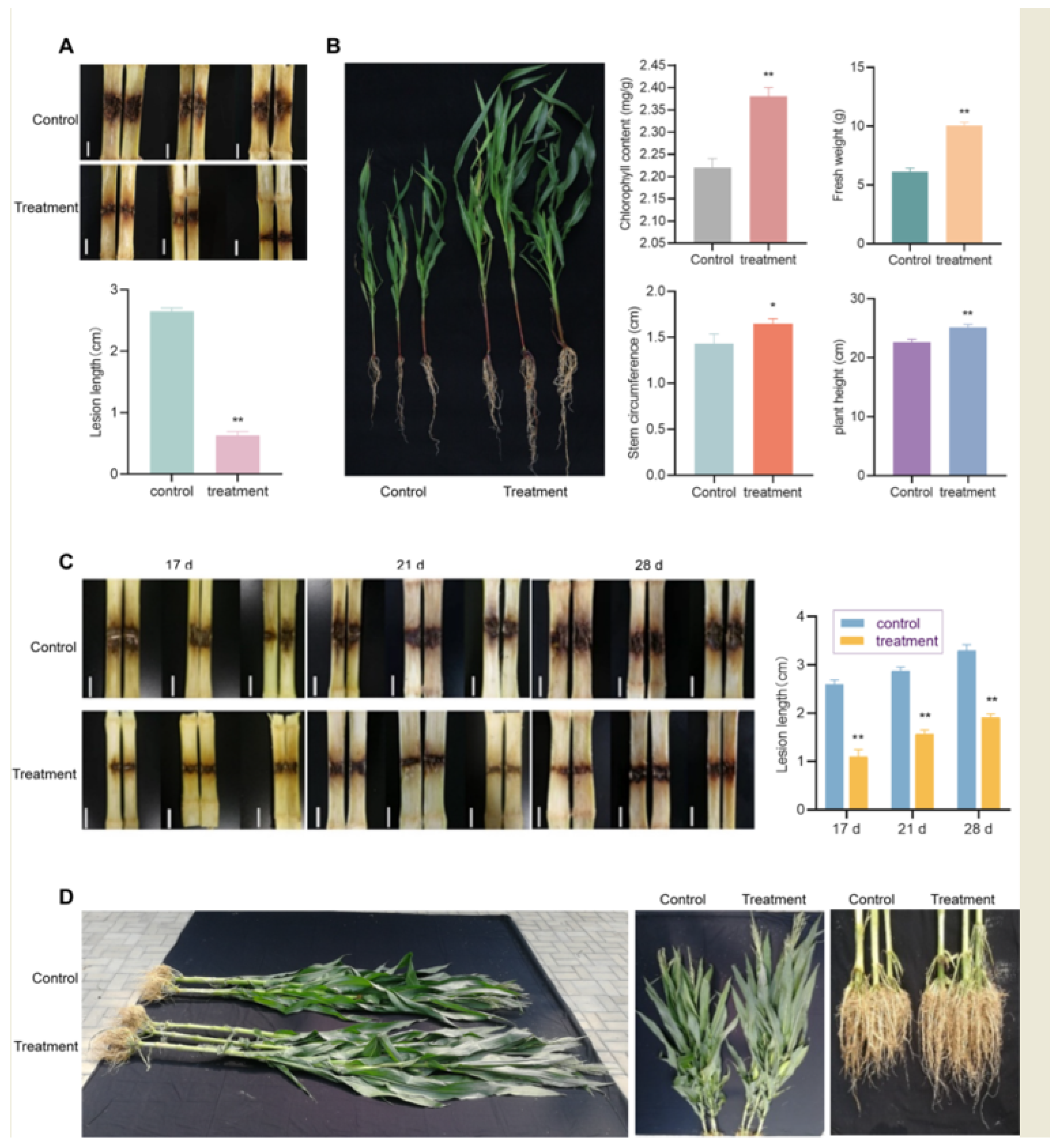
3.4. Indoor Control Effect of B. subtilis A3 Fermentation Solution Irrigation on Corn Stalk Rot and Plant Growth
3.5. Field Control Effect of Seed Coating with B. subtilis A3 on Corn Stalk Rot
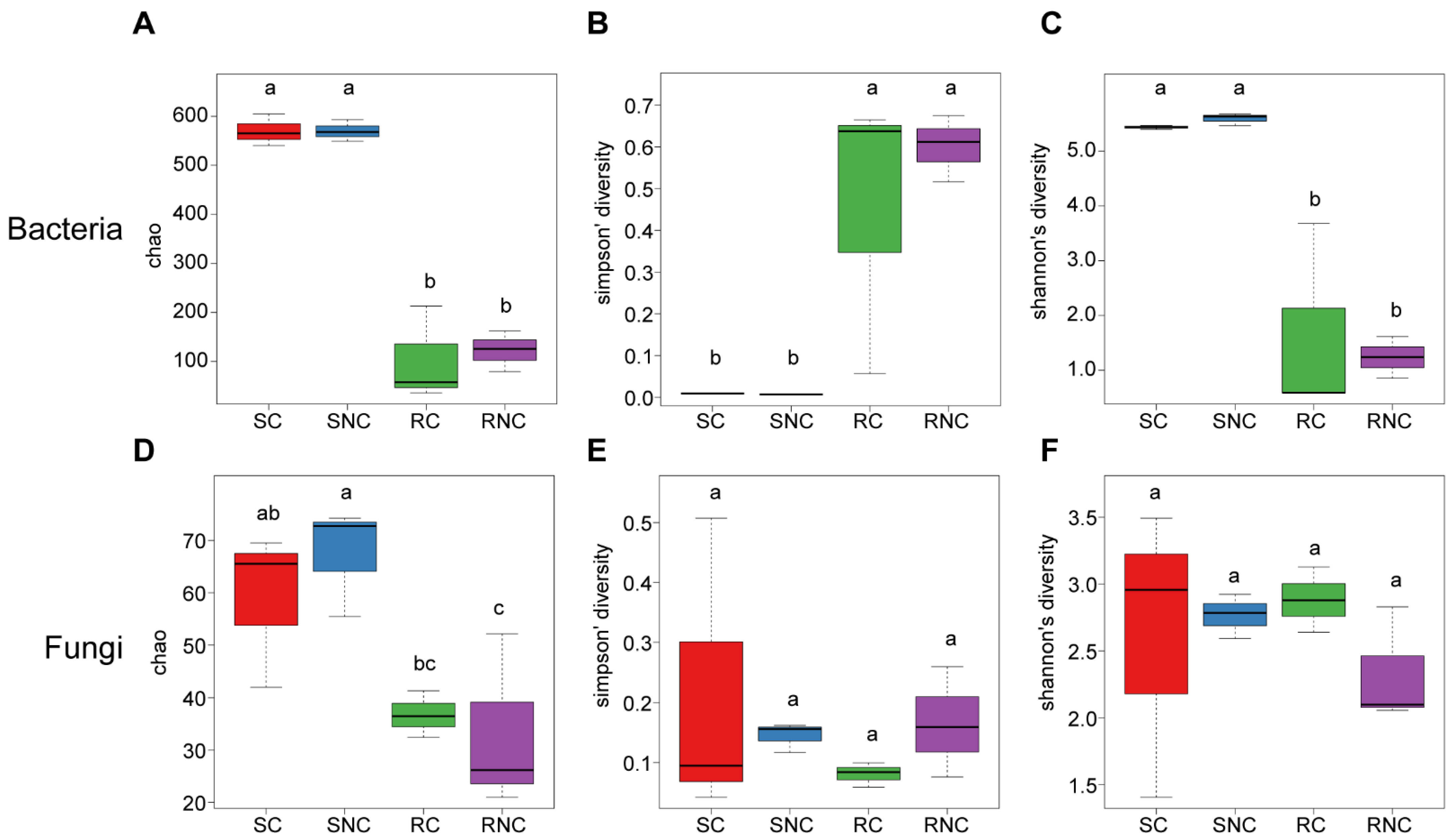
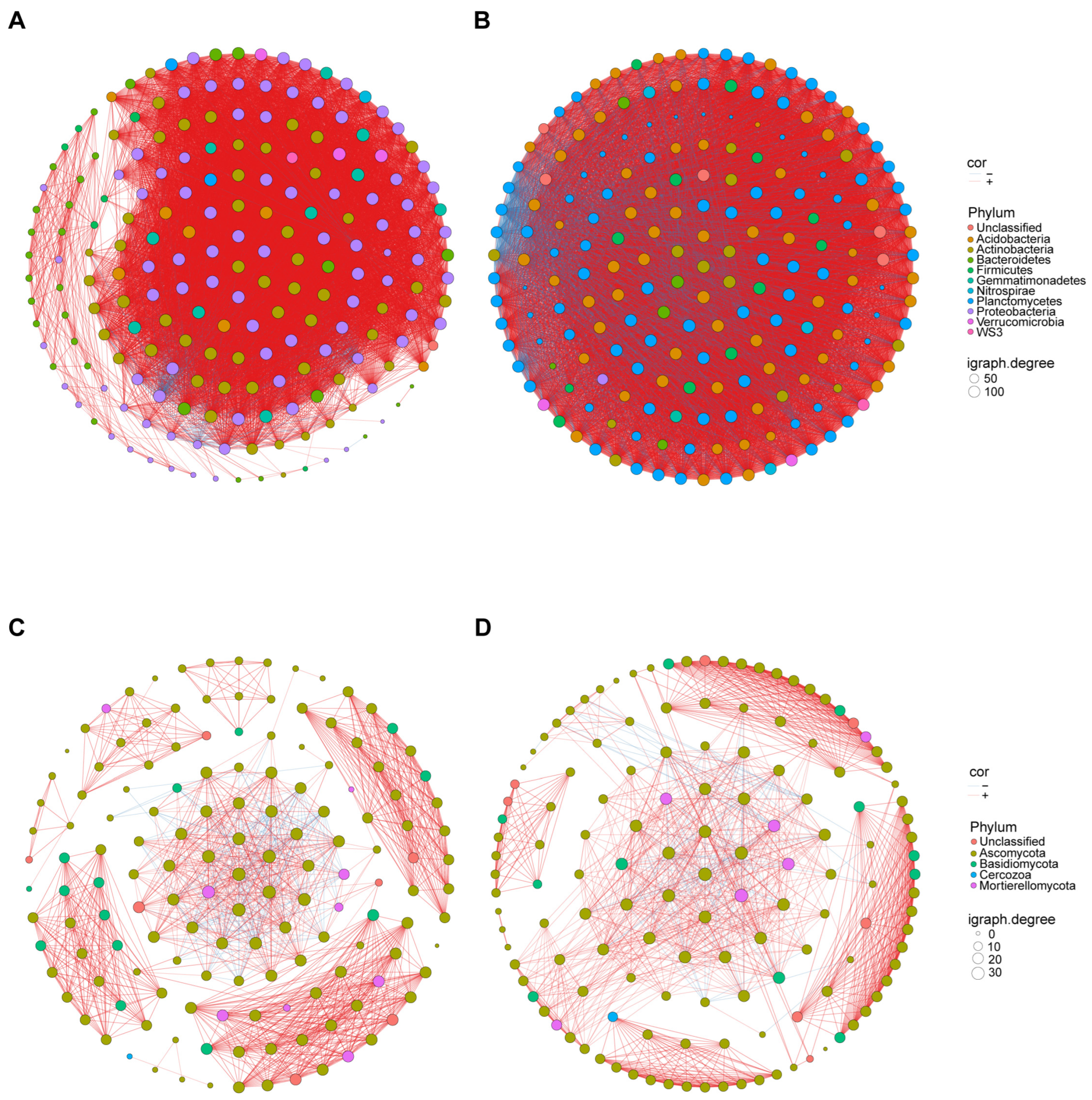
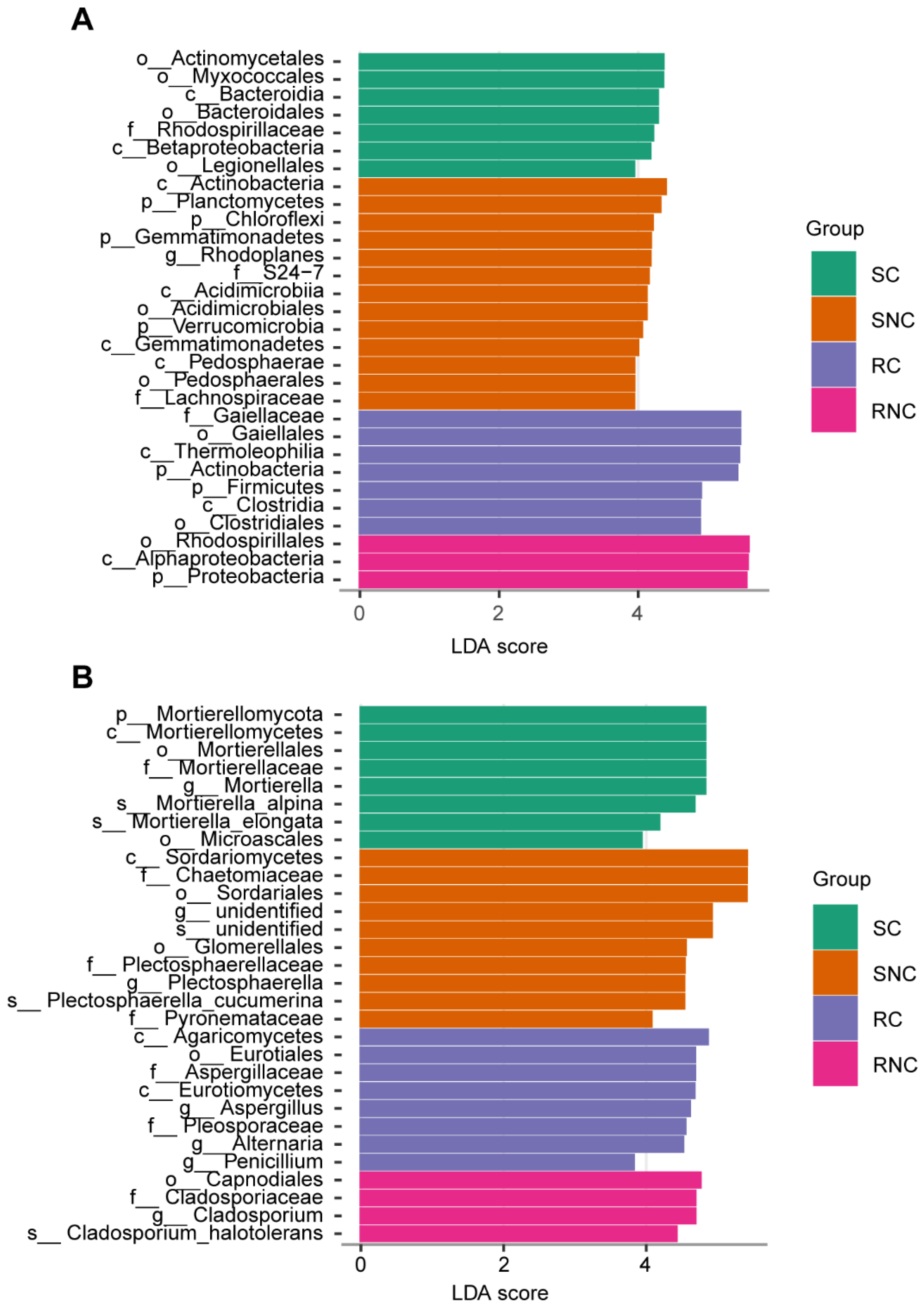
3.6. Effect of Seed Coating with A3 on Microbial Structure and Diversity of Corn Rhizosphere Soil and Root Endophyte
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kunst, F.; Ogasawara, N.; Moszer, I.; Albertini, A.M.; Alloni, G.; Azevedo, V.; Bertero, M.G.; Bessieres, P.; Bolotin, A.; Borchert, S.; et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Almasoudi, N.M.; Sallam, N.M.A.; Ali, E.F.; Alqurashi, A.S.; Issa, A.A.; Althobaiti, F.; Housny, M.; Alomari, H.; Abo-Elyousr, K.A.M. Development of Bacillus spp for controlling wilt disease and improving the growth of tomato. Eur. J. Plant Pathol. 2025. [Google Scholar] [CrossRef]
- Xu, L.H.; Shang, Q.H.; Nicolaisen, M.; Zeng, R.; Gao, S.G.; Gao, P.; Song, Z.W.; Dai, F.M.; Zhang, J.Z. Biocontrol potential of rhizospheric Bacillus strains against Sclerotinia minor jagger causing lettuce drop. Microorganisms 2025, 13, 68. [Google Scholar] [CrossRef]
- Legrifi, I.; Al Figuigui, J.; Lahmamsi, H.; Taoussi, M.; Radi, M.; Belabess, Z.; Lazraq, A.; Barka, E.A.; Lahlali, R. Unlocking olive rhizobacteria: Harnessing biocontrol power to combat olive root rot and promote plant growth. Int. Microbiol. 2025. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Lan, X.; Xu, W.X.; Dong, W.B.; Ke, S.Y.; Wu, H.Q. Discovery, characterization, and application of broad-spectrum antimicrobial peptide AtR905 from Aspergillus terreus as a biocontrol agent. J. Agric. Food Chem. 2024, 12, 2793–2804. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Q.; Peng, Z.; Zhang, J.; Li, J. Biocontrol effect of Bacillus subtilis YPS-32 on potato common scab and its complete genome sequence analysis. J. Agric. Food Chem. 2022, 70, 5339–5348. [Google Scholar] [CrossRef]
- Wang, X.J.; Xie, S.Y.; Mu, X.Y.; Guan, B.; Hu, Y.Z.; Ni, Y.Q. Investigating the resistance responses to Alternaria brassicicola in ‘Korla’ fragrant pear fruit induced by a biocontrol strain Bacillus subtilis Y2. Postharvest Biol. Technol. 2023, 199, 112293. [Google Scholar] [CrossRef]
- Berlanga-Clavero, M.V.; Molina-Santiago, C.; Caraballo-Rodríguez, A.M.; Petras, D.; Díaz-Martínez, L.; Pérez-García, A.; de Vicente, A.; Carrión, V.J.; Dorrestein, P.C.; Romero, D. Bacillus subtilis biofilm matrix components target seed oil bodies to promote growth and anti-fungal resistance in melon. Nat. Microbiol. 2022, 7, 1001–1015. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Chen, H.; Yao, J. Characterization of Fusarium graminearum inhibitory lipopeptide from Bacillus subtilis IB. Appl. Microbiol. Biotechnol. 2007, 76, 889–894. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Selvaraj, J.N.; Xing, F.G.; Zhou, L.; Wang, Y.; Song, H.M.; Tan, X.X.; Sun, L.C.; Sangare, L.; Folly, Y.M.E.; et al. Antagonistic Action of Bacillus subtilis Strain SG6 on Fusarium graminearum. PLoS ONE 2014, 9, e92486. [Google Scholar] [CrossRef]
- Zhao, Q.; Cao, J.; Cai, X.; Wang, J.; Kong, F.; Wang, D.; Wang, J. Antagonistic activity of volatile organic compounds produced by acid-tolerant Pseudomonas protegens CLP-6 as biological fumigants to control tobacco bacterial wilt caused by Ralstonia solanacearum. Appl. Environ. Microbiol. 2023, 89, e01892-22. [Google Scholar] [CrossRef]
- Raio, A. Diverse roles played by “Pseudomonas fluorescens complex” volatile compounds in their interaction with phytopathogenic microrganims, pests and plants. World J. Microb. Biot. 2024, 40, 80. [Google Scholar] [CrossRef]
- Zaid, D.S.; Li, W.; Yang, S.; Li, Y. Identification of bioactive compounds of Bacillus velezensis HNA3 that contribute to its dual effects as plant growth promoter and biocontrol against post-harvested fungi. Microbiol. Spectr. 2023, 11, e0051923. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Valencia, A.L.; Olivares, D.; Poblete-Morales, M.; Silva-Moreno, E.; Defilippi, B.G. Antifungal effect of volatile organic compounds (VOCs) release from Antarctic bacteria under postharvest conditions. Food Packag. Shelf 2023, 39, 101160. [Google Scholar] [CrossRef]
- Song, W.; Dai, M.; Gao, S.; Mi, Y.; Zhang, S.; Wei, J.; Zhao, H.; Duan, F.; Liang, C.; Shi, Q. Volatile organic compounds produced by Paenibacillus polymyxa J2-4 exhibit toxic activity against Meloidogyne incognita. Pest Manag. Sci. 2024, 80, 1289–1299. [Google Scholar] [CrossRef]
- Poveda, J. Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl. Soil Ecol. 2021, 168, 104118. [Google Scholar] [CrossRef]
- Song, G.C.; Ryu, C.M. Two volatile organic compounds trigger plant self-defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef]
- Giorgio, A.; De Stradis, A.; Lo Cantore, P.; Iacobellis, N.S. Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 2015, 6, 1056. [Google Scholar] [CrossRef]
- Bailly, A.; Groenhagen, U.; Schulz, S.; Geisler, M.; Eberl, L.; Weisskopf, L. The inter-kingdom volatile signal indole promotes root development by interfering with auxin signalling. Plant J. 2014, 80, 758–771. [Google Scholar] [CrossRef]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef]
- Xue, J.; Gao, S.; Hou, L.Y.; Li, L.L.; Ming, B.; Xie, R.Z.; Wang, K.R.; Hou, P.; Li, S.K. Physiological influence of stalk rot on maize lodging after physiological maturity. Agronomy 2021, 11, 2271. [Google Scholar] [CrossRef]
- Pammel, L.H. Serious root and stalk diseases of corn. IOWA Agric. 1914, 15, 156–158. [Google Scholar]
- Khokhar, M.K.; Hooda, K.S.; Sharma, S.S.; Singh, V. Post flowering stalk rot complex of maize—Present status and future prospects. Maydica 2014, 59, 226–242. [Google Scholar]
- Ledečan, T.; Šumić, D.; Brkić, I.; Jambrović, A.; Zdunić, Z. Resistance of maize inbreds and their hybrids to Fusarium stalk rot. Czech J. Genet. Plant Breed. 2003, 39, 15–20. [Google Scholar] [CrossRef]
- Sumarah, M.W. The deoxynivalenol challenge. J. Agric. Food Chem. 2022, 70, 9619–9624. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef]
- Bonito, G.; Reynolds, H.; Robeson, M.S., 2nd; Nelson, J.; Hodkinson, B.P.; Tuskan, G.; Schadt, C.W.; Vilgalys, R. Plant host and soil origin influence fungal and bacterial assemblages in the roots of woody plants. Mol. Ecol. 2014, 23, 3356–3370. [Google Scholar] [CrossRef]
- Müller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The plant microbiota: Systems-level insights and perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef]
- Bai, B.; Liu, W.; Qiu, X.; Zhang, J.; Zhang, J.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking plant secondary metabolites and plant microbiomes: A review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.R.; Tang, C.; Salvato, F.; Clouse, K.M.; Bartlett, A.; Vintila, S.; Phillips, L.; Sermons, S.; Hoffmann, M.; Balint-Kurti, P.J.; et al. Microbe-dependent heterosis in maize. Proc. Natl. Acad. Sci. USA 2021, 118, e2021965118. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013–E5020. [Google Scholar] [CrossRef]
- Cha, J.Y.; Han, S.; Hong, H.J.; Cho, H.; Kim, D.; Kwon, Y.; Kwon, S.K.; Crüsemann, M.; Bok Lee, Y.; Kim, J.F.; et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016, 10, 119–129. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef]
- Helfrich, E.J.N.; Vogel, C.M.; Ueoka, R.; Schäfer, M.; Ryffel, F.; Müller, D.B.; Probst, S.; Kreuzer, M.; Piel, J.; Vorholt, J.A. Bipartite interactions, antibiotic production and biosynthetic potential of the Arabidopsis leaf microbiome. Nat. Microbiol. 2018, 3, 909–919. [Google Scholar] [CrossRef]
- Parratt, S.R.; Laine, A.L. Pathogen dynamics under both bottom-up host resistance and top-down hyperparasite attack. J. Appl. Ecol. 2018, 55, 2976–2985. [Google Scholar] [CrossRef]
- Zelezniak, A.; Andrejev, S.; Ponomarova, O.; Mende, D.R.; Bork, P.; Patil, K.R. Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc. Natl. Acad. Sci. USA 2015, 112, 6449–6454. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing causality: Opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.; Garrity, G.M.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H.; Whitman, W.B. Bergey’s Manual of Systematic Bacteriology; Springer: New York, NY, USA, 2009; Volume 3. [Google Scholar]
- Geng, S.; Zhang, Z.; Zhao, Y.; Zhao, R.; Li, J.; Liu, Y.; Cao, Z.; Zhao, B.; Dong, J. Identification of novel Bacillus velezensis zm026 in corn diseases control and fumonisin inhibition. J. Integr. Agric. 2025; in press. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef]
- Beirinckx, S.; Viaene, T.; Haegeman, A.; Debode, J.; Amery, F.; Vandenabeele, S.; Nelissen, H.; Inzé, D.; Tito, R.; Raes, J.; et al. Tapping into the maize root microbiome to identify bacteria that promote growth under chilling conditions. Microbiome 2020, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef]
- Wen, T.; Xie, P.; Yang, S.; Niu, G.; Liu, X.; Ding, Z.; Xue, C.; Liu, Y.-X.; Shen, Q.; Yuan, J. ggClusterNet: An R package for microbiome network analysis and modularity-based multiple network layouts. iMeta 2022, 1, e32. [Google Scholar] [CrossRef]
- Abuduaini, X.; Aili, A.; Lin, R.R.; Song, G.G.; Huang, Y.; Chen, Z.Y.; Zhao, H.P.; Luo, Q.; Zhao, H.X. The lethal effect of Bacillus subtilis Z15 secondary metabolites on Verticillium dahliae. Nat. Prod. Commun. 2021, 16, 1934578X20986728. [Google Scholar] [CrossRef]
- Wang, K.; Qin, Z.; Wu, S.Y.; Zhao, P.Y.; Zhen, C.Y.; Gao, H.Y. Antifungal mechanism of volatile organic compounds produced by Bacillus subtilis CF-3 on Colletotrichum gloeosporioides assessed using omics technology. J. Agric. Food Chem. 2021, 69, 5267–5278. [Google Scholar] [CrossRef]
- Zhu, H.J.; Wu, S.L.; Tang, S.J.; Xu, J.; He, Y.L.; Ren, Z.H.; Liu, E.R. Isolation, identification and characterization of biopotential cyclic lipopeptides from Bacillus subtilis strain JN005 and its antifungal activity against rice pathogen Magnaporthe oryzae. Biol. Control 2023, 182, 105241. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, X.; Wang, J.; Wu, L.J.; Zheng, Z.M.; Yu, Z.L. Antagonistic effects of volatiles generated by Bacillus subtilis on spore germination and hyphal growth of the plant pathogen, Botrytis cinerea. Biotechnol. Lett. 2008, 30, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Kong, H.; Buyer, J.S.; Lakshman, D.K.; Lydon, J.; Kim, S.D.; Roberts, D.P. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Appl. Microbiol. Biotechnol. 2008, 80, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Guevara-Avendaño, E.; Bejarano-Bolívar, A.A.; Kiel-Martínez, A.-L.; Ramírez-Vázquez, M.; Méndez-Bravo, A.; von Wobeser, E.A.; Sánchez-Rangel, D.; Guerrero-Analco, J.A.; Eskalen, A.; Reverchon, F. Avocado rhizobacteria emit volatile organic compounds with antifungal activity against Fusarium solani, Fusarium sp. associated with Kuroshio shot hole borer, and Colletotrichum gloeosporioides. Microbiol. Res. 2019, 219, 74–83. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Tong, J.; Xu, Y. An alkylpyrazine synthesis mechanism involving l-Threonine-3-Dehydrogenase describes the production of 2,5-Dimethylpyrazine and 2,3,5-Trimethylpyrazine by Bacillus subtilis. Appl. Environ. Microbiol. 2019, 85, e01807-19. [Google Scholar] [CrossRef]
- He, A.-L.; Zhao, L.-Y.; Ren, W.; Li, H.-R.; Paré, P.W.; Zhao, Q.; Zhang, J.-L. A volatile producing Bacillus subtilis strain from the rhizosphere of Haloxylon ammodendron promotes plant root development. Plant Soil 2023, 486, 661–680. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus solubilization by Bacillus species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef]
- Xie, S.-S.; Wu, H.-J.; Zang, H.-Y.; Wu, L.-M.; Zhu, Q.-Q.; Gao, X.-W. Plant growth promotion by spermidine-producing Bacillus subtilis OKB105. Mol. Plant Microbe Interact. 2014, 27, 655–663. [Google Scholar] [CrossRef]
- Woo, O.-G.; Kim, H.; Kim, J.-S.; Keum, H.L.; Lee, K.-C.; Sul, W.J.; Lee, J.-H. Bacillus subtilis strain GOT9 confers enhanced tolerance to drought and salt stresses in Arabidopsis thaliana and Brassica campestris. Plant Physiol. Biochem. 2020, 148, 359–367. [Google Scholar] [CrossRef]
- Dai, Z.; Ahmed, W.; Yang, J.; Yao, X.; Zhang, J.; Wei, L.; Ji, G. Seed coat treatment by plant-growth-promoting rhizobacteria Lysobacter antibioticus 13–6 enhances maize yield and changes rhizosphere bacterial communities. Biol. Fert. Soils 2023, 59, 317–331. [Google Scholar] [CrossRef]
- Berbee, M.L. The phylogeny of plant and animal pathogens in the Ascomycota. Physiol. Mol. Plant Pathol. 2001, 59, 165–187. [Google Scholar] [CrossRef]
- Morrow, C.A.; Fraser, J.A. Sexual reproduction and dimorphism in the pathogenic basidiomycetes. FEMS Yeast Res. 2009, 9, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Jin, L.; Sang, Y.; Hu, S.; Wang, B.T.; Jin, F.J. Characterization of potassium-solubilizing fungi, Mortierella spp., isolated from a poplar plantation rhizosphere soil. Arch. Microbiol. 2024, 206, 157. [Google Scholar] [CrossRef]
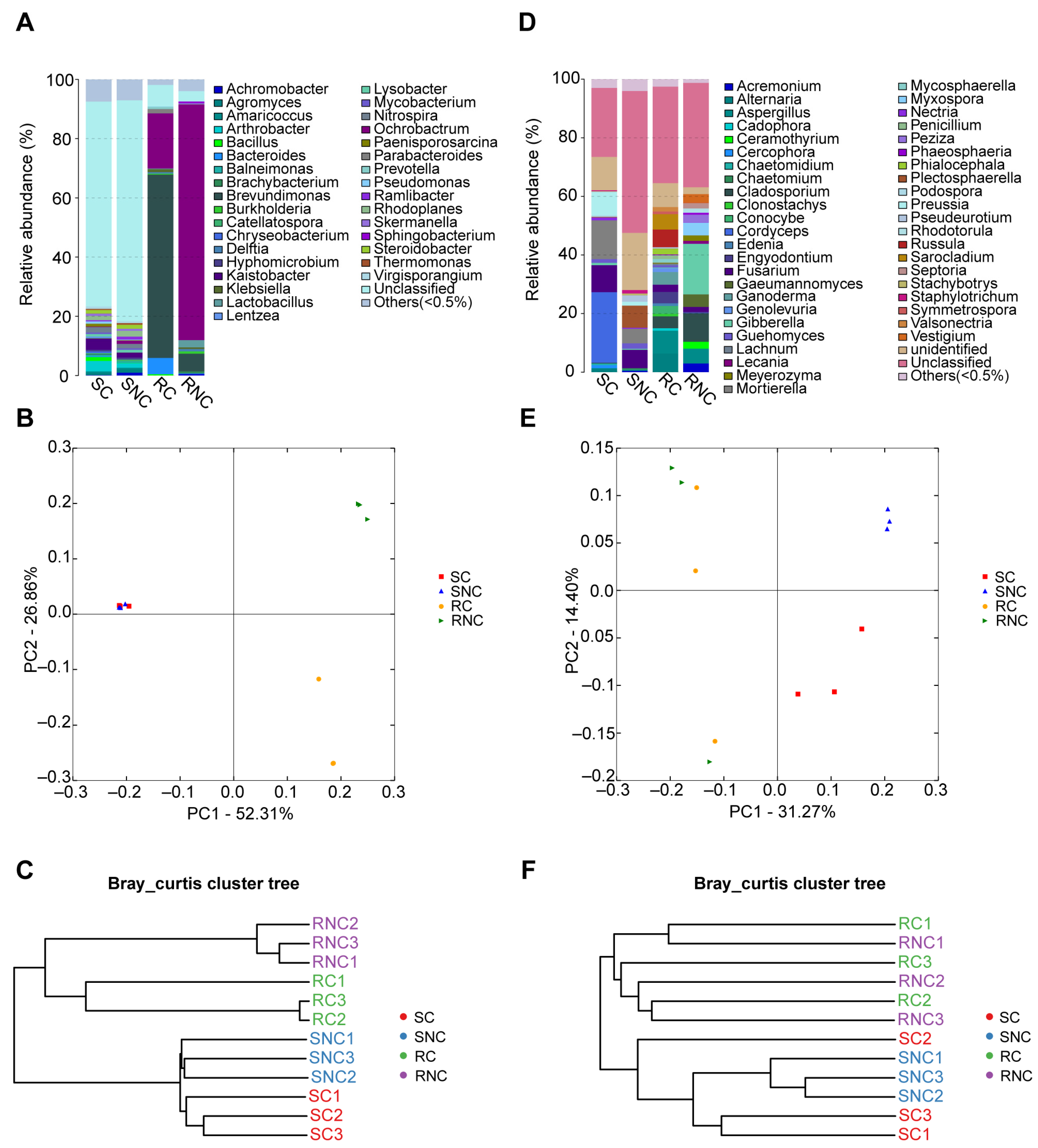
| Treatment | Barren Ear Tip Length (cm) | Ear Length (cm) | Ear Diameter (cm) | Kernel Rows | Kernel Number per Row | Seed Yield Rate (%) | Hundred-Grain Weight (g) | Production (kg) | Disease Index (%) | Control Effect (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1.11 ± 0.21 | 16.57 ± 0.12 | 4.34 ± 0.18 | 15.37 ± 0.08 | 31.04 ± 0.61 | 84.46 ± 051 | 36.95 ± 1.71 | 636 ± 0.26 | 56.71 | – |
| A3 | 0.55 ± 0.24 | 18.39 ± 0.23 | 4.13 ± 0.33 | 15.31 ± 0.07 | 35.55 ± 0.31 | 85.37 ± 0.05 | 37.77 ± 02.02 | 659 ± 0.21 | 30.76 | 45.75 |
| Property | Bacteria | Fungi | ||
|---|---|---|---|---|
| TRT | CK | TRT | CK | |
| Number of nodes | 200 | 196 | 145 | 138 |
| Number of edges | 9201 | 12,066 | 1168 | 1067 |
| Number of positive correlations | 8808 | 9700 | 1050 | 963 |
| Number of negative correlations | 393 | 2366 | 15 | 104 |
| Mean degree | 92.01 | 123.12 | 16.11 | 15.46 |
| Connectance | 0.46 | 0.63 | 0.11 | 0.11 |
| Network diameter | 6.68 | 5.13 | 5.25 | 5.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Jia, S.; Du, Y.; Cao, H.; Zhang, K.; Xing, J.; Dong, J. Biocontrol Potential of Bacillus subtilis A3 Against Corn Stalk Rot and Its Impact on Root-Associated Microbial Communities. Agronomy 2025, 15, 706. https://doi.org/10.3390/agronomy15030706
Wang L, Jia S, Du Y, Cao H, Zhang K, Xing J, Dong J. Biocontrol Potential of Bacillus subtilis A3 Against Corn Stalk Rot and Its Impact on Root-Associated Microbial Communities. Agronomy. 2025; 15(3):706. https://doi.org/10.3390/agronomy15030706
Chicago/Turabian StyleWang, Liming, Shiqi Jia, Yue Du, Hongzhe Cao, Kang Zhang, Jihong Xing, and Jingao Dong. 2025. "Biocontrol Potential of Bacillus subtilis A3 Against Corn Stalk Rot and Its Impact on Root-Associated Microbial Communities" Agronomy 15, no. 3: 706. https://doi.org/10.3390/agronomy15030706
APA StyleWang, L., Jia, S., Du, Y., Cao, H., Zhang, K., Xing, J., & Dong, J. (2025). Biocontrol Potential of Bacillus subtilis A3 Against Corn Stalk Rot and Its Impact on Root-Associated Microbial Communities. Agronomy, 15(3), 706. https://doi.org/10.3390/agronomy15030706







