Abstract
Cereals are the basis of the human diet, and among them, after rice and corn, wheat is the most cultivated in the world. Drought, conflicts, and high prices affect food security in many countries. The CHANGE-UP project funded by the PRIMA program aims at redesigning agricultural systems for the Mediterranean area to make them more resilient to climate change, and includes, among other agronomic innovations, the cultivation and characterization of perennial wheat genotypes. In this study, four perennial wheat lines, 235a, 20238, OK72, and 11955, grown in Italy, were examined for their technological and chemical composition and rheological properties and compared with the perennial species Thinopyrum intemedium (Kernza®) and to a modern durum wheat variety, used as controls. On average, all the perennial genotypes presented very small kernels along with high protein content, total antioxidant capacity, and mineral content, and genotypes OK72 and 11955 presented good test weight values. Line 235a had the best gluten quality, whereas line 20238 reported the worst values for bread-making aptitude. Results indicate that perennial grains could adapt to the Italian environment and manifest their nutritional and technological potential, constituting promising raw materials for enhancing diversification in nutrition by sustainable agriculture based on agroecological principles.
1. Introduction
Over the years, breeding and innovative agriculture management have led to annual grain crops with high yields. Currently, annual grain crops are cultivated over 70% of total arable land providing 70–80% of the calories required. These annual crops resulted in continued soil tillage, and this approach has been considered among the main causes of soil degradation worldwide [1] and the source of a great quantity of greenhouse gases [2]. Moreover, intensive monoculture production entails other environmental concerns, such as soil erosion, often caused by water in areas with steep slopes that do not have vegetation cover or are subjected to plowing processes, loss in biodiversity, large quantities of fertilizers and pesticides, and need of ample labor to prepare the land [3].
In this context, the challenge to conciliate the 17 Sustainable Development Goals of the 2030 Agenda for Sustainable Development, adopted by all United Nations Member States in 2015 [4], along with the increasing world population (9 billion by 2050) and consequent food production, could not be achieved by expanding the land available for cultivation. A sustainable intensification of agriculture, through an environment-friendly and economically sustainable approach, achieving higher crop yields from the same amount of farmland with less environmental impact, could represent a valuable strategy to face this challenge [5].
The CHANGE-UP project (Innovative agroecological APProaches to achieving resilience to climate CHANGE in Mediterranean countries), part of the PRIMA (Partnership for Research and Innovation in the Mediterranean Area) program supported by the European Union, aims to redesign innovative farming systems for the Mediterranean Basin to be more resilient to climate change and able to face and overcome adverse and unpredictable events while ensuring food security and sustainable farmers’ income [6]. One of the strategies adopted in this project has been the introduction of perennial wheat genotypes (PGs); indeed, perennial grains are characterized by the recovery of the vegetative phase after harvesting; hence, thanks to their ability to regrow, they do not need replanting every year, thus leading to numerous ecosystem services [7]. The British Royal Society [8] stated that the transition from annual to perennial crops would be the ‘straightforward solution to reduce the overall impact of agriculture’. Thanks to their characteristics and potential, perennial grains could be a valuable agroecological approach according to the definition by FAO (2016) [9].
Perennial crops are emerging as an effective way of combining the adaptation strategy to climate change, with a mitigation strategy by reducing net greenhouse gas emissions from agriculture. Reductions in soil erosion, salinity, and acidification, as well as reduced cost of production, are some of the proposed benefits of this novel agronomic approach [10,11].
Avoiding soil tillage will reduce production costs and field management tasks [12,13]; perennial crops can favor soil fauna and microbial biodiversity, as well as improve soil health, high drought resilience, and long-term stability [14,15,16,17]. More importantly, they are able to develop a very extensive rooting system, which enables access to nutrients and water present in the deepest soil layers. As a result, when periodic drought and high temperatures occur, causing stress in annual crops, perennial ones can explore larger portions of soil, leading to more stable yields [18,19]. The below-ground biomass of perennial species could contribute not only to improving soil quality but also to increasing carbon sequestration and water infiltration [20].
However, despite the undoubted agroecological advantages, the widespread adoption of perennial wheat crops by farmers, processors, and consumers must also take into account quality-related parameters, such as high flour yield, good bread-making aptitude, processability, and the presence of both macro- and micronutrients. Nevertheless, currently, the main challenge relating to the large-scale cultivation of perennial cereals certainly concerns the grain yield, which is far from annual varieties. The objective of this work was to characterize four perennial wheat genotypes grown in Central Italy for several qualitative aspects and to compare them both with the perennial species Th. intermedium and with a modern durum wheat variety.
2. Materials and Methods
2.1. Plant Material
Perennial genotypes 235a, 11955, 20238, and OK72, obtained by crossing common wheat (T. aestivum) or durum wheat (T. durum) with different Thinopyrum species [21] (Table 1) selected in previous studies for their good agronomic performances [22] and nutritional quality [23], were grown in 2021–2022 at the experimental fields of CREA Research Centre for Engineering and Agro-Food Processing (CREA-IT) in Montelibretti (Rome, Italy), according to a randomized block design with three replications and plots of 10 m2. The perennial species Thinopyrum intemedium (Kernza®) and the modern durum wheat cultivar San Carlo were used as controls. The plots were harvested by hand, and the spikes were threshed and dehulled by two subsequent steps using a bench microthresher (Marelli SpA, Milan, Italy).

Table 1.
Genotype, pedigree, and origin of perennial wheat genotypes grown in the experimental fields of CREA-IT in Montelibretti (Rome).
2.2. Kernel Physical Analyses
The 1000 kernel weight (TKW) and test weight (TW) were determined according to ISO 520:2010 [24] and ISO 7971:2009 [25], respectively. The hardness index (HI) of the kernel was performed on 300 kernel samples by the Perten SKCS 4100 (Perten, Springfield, IL, USA), following the manufacturer’s operating procedure. The instrument was set at a range of hardness values between −40 and +120.
2.3. Chemical Characterization
All samples were milled to wholemeal flour using a laboratory mill (Cyclotec, FOSS, Hillerod, Denmark) at a 0.5 or 1.0 mm sieve, depending on the requirements of each analysis. All analyses were performed in triplicate. The sample moisture was measured using a thermobalance (Sartorius MA 40, Goettingen, Germany) at 120 °C just before the chemical analyses in order to express all data as dry weight (dw).
Protein content was determined by Dumas combustion method, according to AACC n. 46-30 [26], with the automatic instrument Leco FP528 (Leco Corp., St. Joseph, MI, USA), using N × 5.7 as the conversion factor. Ash content was determined according to the approved method AACC 08-01.01 [27]. The total starch (TS) content was determined by enzymatic method using the Megazyme (Bray, Ireland) kits K-TSTA according to McCleary et al. [28]. The total antioxidant capacity (TAC) was determined according to the direct method used by Martini et al. [29]. Total dietary fiber (TDF) content was measured using an enzymatic kit for fiber determination (Bioquant, Merck, Darmstadt, Germany) according to the AOAC 991.42 Official Method [30]. Storage proteins were extracted and fractionated by SDS-PAGE, as described by Pogna et al. [31].
2.4. Rheological and Technological Tests
Perennial wheat genotypes and durum wheat seeds were milled according to AACC Method 26-70.01 [32] by a Chopin CD1 and CD2 Laboratory Mill (Chopin Technologies, Villeneuve La Garenne, France), respectively; Th. intermedium whole flour was obtained by Fritsch miller laboratory (Fritsch, type pulverisette 14, Idar-Oberstein, Germany). The milling yield was considered as the percentage of the weight of flour obtained from 100 g of kernels.
The gluten index (GI) determination was conducted by the Glutomatic 2200 apparatus (Perten Instruments, Segeltorp, Sweden) according to AACC method 38-12 [33]. Alveograph parameters, dough strength (W; 10−4 J), and the ratio between dough tenacity and dough extensibility (P/L) were obtained by the Chopin Alveograph (Chopin, France) according to the manufacturer’s instructions. All the samples were also characterized by the SDS sedimentation test using a solution of 2% sodium dodecyl sulfate, as described by the standard method AACC 56-70 [34] and expressed in milliliters (mL). The AACC 56-81B method [35] was used for the determination of the falling number (FN) using the Perten 1500 system (Perten Instruments, Sweden). Color was evaluated by a Tristimulus colorimeter (ChromaMeter CR-400, Minolta, Milan, Italy) equipped with a D65 illuminant, using the CIELab color space coordinate b* (yellowness), a* (redness), and L* (lightness); brownness was expressed as 100-L*.
2.5. Statistical Analysis
Results were expressed as mean ± standard deviation. One-way analysis of variance was performed with MSTATC program (Michigan State University, East Lansing, MI, USA), followed by the Duncan multiple range test for post hoc comparison of means, applied to assess the significance of differences (p ≤ 0.05) for each parameter measured.
3. Results and Discussion
3.1. Kernel Physical Analyses
Milling performance and flour yield are influenced by the physical properties of grains. Kernels size analyses revealed, on average, that the perennial genotypes are characterized by small seeds, with an average thousand kernels weight (24.9 g) of approximately half that of durum wheat (56.2 g), while the mean test weight value observed in perennial genotypes was 67 kg/hL (Table 2). Kernel test weight (TW) is a quality parameter that indicates the degree of filling of the kernel, and it is mainly correlated with starch content [36]. The lower test weight present in the perennial genotypes indicated that they allocate a significant proportion of photosynthates to roots and green tissues, whereas annual species invest more of their photosynthetic energy in kernel development. The TW values (>70 Kg/hL) found in two perennial genotypes, OK72 and 11955, were just slightly lower than those established by the current TW requirement for the No.4 wheat class (TW ≥ 71 kg/hL) of Canadian Western Amber Durum [37].

Table 2.
Physical kernel traits of the four perennial genotypes, Th. intermedium, and durum wheat.
Kernel texture is a main determinant of the end-product quality because of its strong effects on technological and rheological quality traits, including milling conditions, granularity of flour, starch granule integrity, flour yield, bread volume, and crumb structure [38]. A detailed analysis of the hardness index (HI) values revealed considerable differences among perennial genotypes. In detail, as already observed by Gazza et al. [23], the genotypes OK72 and 11955 lines presented typical values of soft-textured kernels, and genotype 20238 derived from T. durum x Th. elongatum showed on average a medium-soft HI value [39] (Table 2). Indeed, the milling yield in perennial lines was found to be higher in the samples that had lower HI and higher TKW and TW, namely OK72 and 11955.
3.2. Chemical Characterization
Protein and total starch content represent the most important macronutrients in cereals. In the perennial wheat genotypes, an average protein content of 16.9% was observed (Table 3). Th. intermedium exhibited the highest value (21.4%), seven percentage units higher than the protein content of the annual counterpart (14.3%) (Table 3). The increment in protein content of PGs has already been reported in several studies, and this could be partly accounted for by the reduced kernel weight of the perennial wheat lines [23,40,41,42].

Table 3.
Chemical characterization of four perennial genotypes, Th. intermedium, and annual durum wheat.
As expected, the high protein content of perennial genotypes was associated with a lower amount of total starch content, since, in kernels, protein content and total starch are inversely proportional. Total starch (TS), the main energy source in food made by cereals, was higher in perennial genotypes 11955 and OK72 (66% on average), not significantly different from that of durum wheat (Table 3), while Thinopyrum and genotypes 20238 and 235a, with higher protein contents, on average did not reach 60% of TS.
Today, the health-beneficial properties provided by dietary fiber are well proven, and the market for products with high fiber content is constantly expanding due to increasing consumer demand [43]. Likely due to their very small kernel size, the total dietary fiber content (TDF) of the perennial genotypes was very high, especially in Th. intermedium (15.6%), followed by 235a and 20238 (on average 12.9%), significantly higher than the TDF found in the annual control (12.3%) (Table 3).
It is well known that wholegrain cereals are a rich source of unique bioactive compounds, such as antioxidants that significantly help to promote human health [44]. Antioxidant capacity is the most common in vitro parameter used to assess or predict potential benefits of plant phytochemical compounds; the antioxidant capacity in cereals is due to the presence, mostly in the outer layers of the kernels, of antioxidant compounds such as phenolic acids, carotenoids, and alkylresorcinols that help prevent cellular damage. The highest antioxidant capacity was observed in Th. intermedium (60 mmol TEAC/kg), while, on average, the perennial lines showed a value of 50 mmol TEAC/kg (Table 3). The reduced kernel weight of the perennial wheat genotypes also influenced the ash content in wholemeal samples, with all values exceeding 2%, higher than the 1.65% found in durum wheat. Ash content is correlated to microelements and mineral levels; indeed, wholemeal flour with high ash content offers high nutritional value [45].
A health-promoting phytochemical profile is a factor that could play an important role in promoting the introduction of perennial wheat crops in the food market.
3.3. Rheological and Technological Tests and Analysis of Storage Proteins Composition
Gluten-related analyses are extremely relevant in determining the baking quality, as they provide useful information on the flour’s ability to produce the elastic and strong doughs necessary for the production of baked products.
According to UNI 10709 [46] and UNI 10940 [47], good values for bread making begin at W > 170. The ratio P/L between tenacity and extensibility of dough is a very important indicator of the quality of the gluten and the processability of the dough (>0.7 for tough and strong flours; <0.4 for very extensible flours; and 0.4–0.7 for balanced flours). Therefore, based on the alveographic analyses (Table 4 and Figure 1), genotype 235a could be more suitable for bread making, while genotype OK72 could be more suitable for the biscuit manufacturing industry; on the contrary, the low W values and the P/L ratios higher than 2 found in the genotypes 20238 and 11955 flours indicate very tenacious doughs.

Table 4.
Gluten-quality-related parameters and falling number of four perennial genotypes, Th. intermedium, and durum wheat.
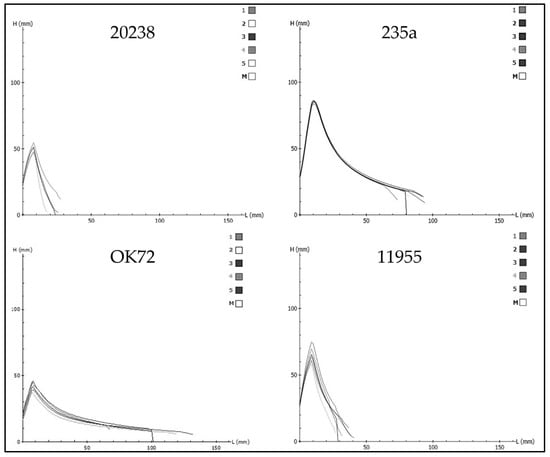
Figure 1.
Alveographic diagrams of perennial wheat genotypes 20238, 235a, OK72, and 11955.
Regarding the gluten index, according to the standard quality classes UNI10940 [47], genotypes 235a and 11955 showed values within the range of medium gluten quality (GI = 40–80%). On the contrary, line 20238 showed a weak gluten index (GI < 30%) and, despite the high protein content, had the worst values among all the perennial genotypes. Noteworthily, it was not possible to determine the gluten index for the Thinopyrum sample, as gluten was not formed with either the normal or semi-automatic method. The low gluten quality of Thinopyrum has also been reported by Cetiner et al., who performed a quality evaluation of the flour and obtained a GI value of 28% [40].
The falling number (FN), used to evaluate the baking quality of wheat flour in relation to amylase activity, reported values higher than 300 s in all samples, except for Thinopyrum (248 s) (Table 4), indicating a relatively low amylase activity, which could determine a delay in fermentation and products with hard bread crust [48]. These results are in line with the study conducted by Trevisan et al., who analyzed several blends of bread wheat and Thinopyrum flour at different percentages and concluded that FN values of the flour blend decreased as the Thinopyrum substitution level increased [41].
On average, the perennial wheat genotypes revealed poor gluten quality as also determined by the SDS test, with the exception of line 235a, which showed an SDS sedimentation volume as high as 54 mL. Common wheat cultivars with good gluten quality show sedimentation volumes higher than 50 mL [49]. Overall, genotype 235a showed the best performance in terms of gluten-quality-related parameters (W, SDS, and gluten index), as reported in Table 4. The low gluten quality of perennial genotypes, despite their high average protein content, was likely due to their high-molecular-weight glutenin subunits (HMW-GS), which are known to play an important role in the viscoelastic properties of dough.
Analysis of storage protein composition was performed on perennial wheat lines by SDS-PAGE fractionation, both on single seeds and on a mix of 30 seeds, also to confirm the identity and uniformity of the genotypes. As expected, line 20238, deriving from a cross between Thinopyrum and tetraploid durum wheat, lacked the genome D HMW glutenin subunits (Figure 2, lanes 6 and 7).
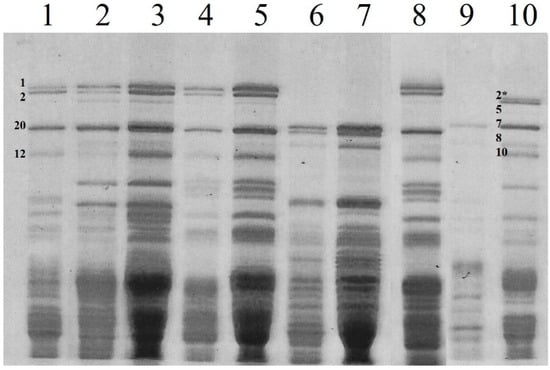
Figure 2.
SDS-PAGE fractionation of HMW glutenin subunits (HMW-GS) from both single seeds and a mix of 30 seeds: 235a (lane 2–3), 11955 (lane 4–5), 20238 (lane 6–7), OK72 (lane 8), and durum wheat cv San Carlo (lane 9). Common wheat, cvs San Pastore (lane 1) and Bologna (lane 10), were used as controls. HMW-GS are numbered according to Payne and Lawrence [50], 2* is the correct definition of this specific HMW-GS.
The HMW glutenin subunits have been analyzed in detail in common wheat because of their effects on dough strength and grain processing quality [51]. In particular, the expression of different HMW glutenin subunits such as 2 + 12 (Glu-D1 locus) and 20 (Glu-B1), occurring in perennial genotypes derived from T. aestivum (Table 5), resulted in glutenin aggregates of smaller molecular weights than other allelic glutenin subunits, leading to low or scarce gluten quality [51]. In the case of perennial line 20238, which presented HMW glutenin subunits 7 + 8 (Glu-B1) correlated with good baking quality, other factors probably derived by the perennial parental contribute to the low quality of its gluten.

Table 5.
HMW glutenin subunit composition at Glu-A1, Glu-B1, and Glu-D1 loci in four perennial wheat derivatives and durum wheat cv San Carlo.
3.4. Color Indexes Determination
Low values of the yellow (b*) parameter (Table 6) were found in all perennial wheat flour, mainly in OK72 and 11955 genotypes, which presented a b* value equal to half of the value found in durum wheat semolina (22.1). Genotypes 20238 and 235a exhibited the highest values of yellow and brown (100-L*) indexes among the perennial genotypes, while genotype 11955 was not statistically different from the durum wheat for the red index (a*) (Table 6). Among all samples examined, durum wheat semolina and Thinopyrum wholemeal flour showed the highest yellow and brown index, respectively. Color, besides being an important trade parameter, is positively correlated with carotenoid content, which represents an important nutritional component [52].

Table 6.
Color indexes of four perennial genotypes, Th. intermedium, and durum wheat.
To summarize, among the perennial genotypes, line 235a was the best in terms of nutritional and technological properties but showed the worst agronomic performance, in terms of post-harvest regrowth percentage, i.e., N regrown plants/N original plants [22] (23.0 vs. 43.4%, average of the other three perennial lines), number of fertile tillers/plants (5.1 vs. 9.8, average of the other three perennial lines), and of TKW (13.3 vs. 28.7 g average of the other three perennial lines). Quite oppositely, the 20238 genotype showed good agronomic traits, i.e., PHR (48.8%), number of fertile tillers (11.2), and TKW (23.2 g). The OK72 and 11955 perennial genotypes represent the best combination of good agronomic, nutritional, and technological traits.
4. Conclusions
In comparison with durum wheat, the perennial genotypes exhibited higher protein content and increased antioxidant capacity, coupled with poor breadmaking qualities, likely due to the presence of determined HMW glutenin subunits inherited from their parents and associated with poor processability aptitude.
Improving the end-use quality of these perennial genotypes could be achieved by backcrossing them with common or durum wheat cultivars known for superior breadmaking properties, followed by selecting for the presence of good-quality HMW and LMW glutenin subunits in the resulting perennial progeny. Ameliorated milling, bread-making aptitude, and nutritional characteristics will facilitate the successful development of perennial wheat cultivars and their widespread adoption by millers, bakers, and consumers. Finally, safeguarding genetic variability within plant species is a way not only to protect the species against diseases or adverse environmental effects but also to assure a wide nutritional profile in terms of macro- and micro-nutrients for humans.
Author Contributions
Conceptualization, G.G. and L.G.; data curation, E.G., C.N., F.N., F.N. and L.G.; formal analysis, E.G.; funding acquisition, G.G. and L.G.; investigation, E.G., C.N., F.N. and F.T.; methodology, E.G., C.N., F.N., F.T. and L.G.; project administration, G.G. and L.G.; resources, G.G. and L.G.; supervision, L.G.; validation, E.G., C.N., F.N. and F.T.; visualization, E.G. and C.N.; writing—original draft, E.G., C.N. and L.G.; writing—review and editing, E.G., C.N., F.N., F.T., G.V., S.C., G.G. and L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study is part of the project CHANGE-UP–Innovative agroecological APProaches to achieving resilience to climate CHANGE in Mediterranean countries, funded by MUR (DD n.16787, 19/11/2021) within the PRIMA EU Sect. 2–Multi-topic 2020 (Partnership for Research and Innovation in the Mediterranean Area) CALL 2020.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors would like to thank Ester Gosparini, Alessandra Arcangeli, Viviana Del Frate, and Cristina Cecchini of CREA-IT of Rome for their technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Glover, J.D. The necessity and possibility of perennial grain production systems. Renew. Agric. Food Syst. 2005, 20, 1–4. [Google Scholar] [CrossRef]
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.A.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; et al. Agriculture, Forestry and Other Land Use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Soto-Gómez, D.; Pérez-Rodríguez, P. Sustainable agriculture through perennial grains: Wheat, rice, maize, and other species. Agric. Ecosyst. Environ. 2022, 325, 107747. [Google Scholar] [CrossRef]
- United Nation. Available online: https://sdgs.un.org/goals (accessed on 10 December 2024).
- Reddy, P.P. Agro-Ecological Approaches to Pest Management for Sustainable Agriculture; Springer: Singapore, 2017; pp. 295–309. [Google Scholar]
- CHANGE-UP Project. Available online: https://changeupproject.com/ (accessed on 18 December 2024).
- Ryan, M.R.; Crews, T.E.; Culman, S.W.; DeHaan, L.R.; Hayes, R.C.; Jungers, J.M.; Bakker, M.G. Managing for multifunctionality in perennial grain crops. Bioscience 2018, 68, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Royal Society Policy Statements and Reports. Reaping the Benefits: Science and the Sustainable Intensification of Global Agriculture. Ref 11/09; The Royal Society: London, UK, 2009; Available online: https://royalsociety.org/news-resources/publications/2009/reaping-benefits/ (accessed on 10 October 2024).
- FAO. Achieving Sustainable Rural Development Through Agricultural Innovation: COAG/2016/6; Food and Agriculture Organization of the United Nations, Committee on Agriculture: Italy, Rome, 2019; Available online: http://www.fao.org/3/a-mr236e.pdf (accessed on 10 October 2024).
- Larkin, P.J.; Newell, M.T.; Hayes, R.C.; Aktar, J.; Norton, M.R.; Moroni, S.J.; Wade, L.J. Progress in developing perennial wheat for grain and grazing. Crop Pasture Sci. 2014, 65, 1147–1164. [Google Scholar] [CrossRef]
- Crews, T.E.; Carton, W.; Olsson, L. Is the future of agriculture perennial? Imperatives and opportunities to reinvent agriculture by shifting from annual monocultures to perennial polycultures. Glob. Sustain. 2018, 1, 11. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Botella, J.R.; Zhu, J.K. Generation of new glutinous rice by CRISPR/Cas9-targeted mutagenesis of the Waxy gene in elite rice varieties. J. Integr. Plant Biol. 2018, 60, 369–375. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, L.; Huang, G.; Zhang, J.; Zhang, Y.; Hu, F. Perennial Rice: Sustainable Rice Production System. In Scaling Up Agroecology to Achieve the Sustainable Development Goals, Proceedings of the Second FAO International Symposium, Italy, Rome, 3–5 April 2018; Food and Agriculture Organization of the United Nations: Italy, Rome, 2018; pp. 216–217. [Google Scholar]
- Burmeister, J. Promotion of ground beetles by integrating perennial energy crops into existing agricultural landscapes. Biomass Bioenergy 2021, 146, 105973. [Google Scholar] [CrossRef]
- Sanford, G.R.; Jackson, R.D.; Booth, E.G.; Hedtcke, J.L.; Picasso, V. Perenniality and diversity drive output stability and resilience in a 26-year cropping systems experiment. Field Crops Res. 2021, 263, 108071. [Google Scholar] [CrossRef]
- Bertola, M.; Righetti, L.; Gazza, L.; Ferrarini, A.; Fornasier, F.; Cirlini, M.; Lolli, V.; Galaverna, G.; Visioli, G. Perenniality, more than genotypes, shapes biological and chemical rhizosphere composition of perennial wheat lines. Front. Plant Sci. 2023, 14, 1172857. [Google Scholar] [CrossRef]
- Giannelli, G.; Del Vecchio, L.; Cirlini, M.; Gozzi, M.; Gazza, L.; Galaverna, G.; Potestio, S.; Visioli, G. Exploring the rhizosphere of perennial wheat: Potential for plant growth promotion and biocontrol applications. Sci. Rep. 2024, 14, 22792. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M. Is there a need for a more sustainable agriculture? Crit. Rev. Plant Sci. 2011, 30, 6–23. [Google Scholar] [CrossRef]
- Vico, G.; Brunsell, N.A. Tradeoffs between water requirements and yield stability in annual vs. perennial crops. Adv. Water Resour. 2018, 112, 189–202. [Google Scholar] [CrossRef]
- Snapp, S.; Rogé, P.; Okori, P.; Chikowo, R.; Peter, B.; Messina, J. Perennial grains for africa: Possibility or pipedream? Exp. Agric. 2019, 55, 251–272. [Google Scholar] [CrossRef]
- Hayes, R.C.; Newell, M.T.; DeHaan, L.R.; Murphy, K.M.; Crane, S.; Norton, M.R.; Wade, L.J.; Newberry, M.; Fahim, M.; Jones, S.S.; et al. Perennial cereal crops: An initial evaluation of wheat derivatives. Field Crops Res. 2012, 133, 68–89. [Google Scholar] [CrossRef]
- Baronti, S.; Galassi, E.; Ugolini, F.; Miglietta, F.; Genesio, L.; Vaccari, F.P.; Cacciatori, P.; Gazza, L. Agronomic and ecophysiological evaluation of an early establishment of perennial wheat lines in central Italy. Genet. Resour. Crop Evol. 2022, 69, 619–633. [Google Scholar] [CrossRef]
- Gazza, L.; Galassi, E.; Ciccoritti, R.; Cacciatori, P.; Pogna, N.E. Qualitative traits of perennial wheat lines derived from different Thinopyrum species. Genet. Resour. Crop Evol. 2016, 63, 209–219. [Google Scholar] [CrossRef]
- ISO 520:2010; Cereals and Pulses-Determination of the Mass of 1000 Grains. ISO: Geneva, Switzerland, 2010; p. 10.
- ISO 7971-1:2009; Determination of Bulk Density, Called Mass per Hectolitre-Part 1: Reference: Method. ISO: Geneva, Switzerland, 2009; p. 8.
- American Association of Cereal Chemists. 46–30 Crude Protein-Combustion Method. In Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- American Association of Cereal Chemists. Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2009. [Google Scholar]
- McCleary, B.V.; Gibson, T.S.; Lugford, D.C. Measurement of total starch in cereal products by amyloglucosidase-α-amylase method: Collaborative study. J. AOAC Int. 1997, 80, 571–579. [Google Scholar] [CrossRef]
- Martini, D.; Taddei, F.; Nicoletti, I.; Ciccoritti, R.; Corradini, D.; D’Egidio, M.G. Effects of genotype and environment on phenolic acids content and total antioxidant capacity in durum wheat. Cereal Chem. 2014, 91, 310–317. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis 991, 16th ed.; Cunniff, P., Ed.; AOAC: Gaithersburg, MD, USA, 1995; p. 42. [Google Scholar]
- Pogna, N.E.; Autran, J.C.; Mellini, F.; Lafiandra, D.; Feillet, P. Chromosome 1B-encoded gliadins and glutenin subunits in durum wheat: Genetics and relationship to gluten strength. J. Cereal Sci. 1990, 11, 15–34. [Google Scholar] [CrossRef]
- AACC International. The AACC Approved Methods of Analysis, 11th ed.; per AACC Approved Method 26-70.01 (AACC, 2000); AACC International: Saint Paul, MN, USA, 2010. [Google Scholar]
- American Association of Cereal Chemists. 38-12, Wet Gluten, Dry Gluten, Water-Binding Capacity, and Gluten Index. In Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- American Association of Cereal Chemists. 56-70.01 Sodium Dodecyl Sulfate Sedimentation Test for Durum Wheat. In Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- American Association of Cereal Chemists. Approved Methods, 11th ed.; 56-81B Determination of Falling Number; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Fox, G.P.; Osborne, B.; Bowman, J.; Kelly, A.; Cakir, M.; Poulsen, D.; Inkerman, A.; Henry, R. Measurement of genetic and environmental variation in barley (Hordeum vulgare) grain hardness. J. Cereal Sci. 2007, 46, 82–92. [Google Scholar] [CrossRef]
- Canadian Grain Commission. Wheat: Export Grade Determinants Tables for Canada Western Amber Durum (CWAD) Wheat 2020. Available online: https://www.grainscanada.gc.ca/en/grain-quality/official-graingrading-guide/04-wheat/export-gradedeterminants/ (accessed on 12 February 2025).
- Tsilo, T.J.; Hareland, G.A.; Chao, S.; Anderson, J.A. Genetic mapping and QTL analysis of flour color and milling yield related traits using recombinant inbred lines in hard red spring wheat. Crop Sci. 2011, 51, 237–246. [Google Scholar] [CrossRef]
- Mahesh, S.; Jayas, D.S.; Paliwal, J.; White, N.D.G. Comparison of partial least squares regression (PLSR) and principal components regression (PCR) methods for protein and hardness predictions using the near-infrared (NIR) hyperspectral images of bulk samples of Canadian wheat. Food Bioprocess Technol. 2005, 8, 31–40. [Google Scholar] [CrossRef]
- Cetiner, B.; Shamanin, V.P.; Tekin-Cakmak, Z.H.; Pototskaya, I.V.; Koksel, F.; Shepelev, S.S.; Amanzhol, N.A.; Bayram, O.; Morgounov, A.I.; Koksel, H. Utilization of intermediate wheatgrass (Thinopyrum intermedium) as an innovative ingredient in bread making. Foods 2023, 12, 2109. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, S.; Koksel, F.; Cetiner, B.; Shamanin, V.P.; Pototskaya, I.V.; Ozdemir, B.; Morgounov, A.I.; Koksel, H. Incorporation of intermediate wheatgrass (Thinopyrum intermedium) into bread wheat doughs: Effects on pasting and rheological properties. Cereal Chem. 2024, 101, 871–883. [Google Scholar] [CrossRef]
- Craine, E.B.; DeHaan, L.R. Nutritional quality of early-generation Kernza perennial grain. Agriculture 2024, 14, 919. [Google Scholar] [CrossRef]
- Norton, V.; Wagstaff, C.; Rodriguez Garcia, J.; Lovegrove, A.; Shewry, P.; Charlton, M.; Gillett, N.; Tindall, M.J.; Lignou, S. “Wait, Do I Need More Fiber?” Exploring UK Consumers’ Dietary Fiber-Related Awareness and White Bread as a Viable Solution to Promote Subsequent Intake. Curr. Develop. Nutr. 2024, 8, 104430. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.; Anderson, F.L.; Blomhoff, R. Wholegrain consumption is associated with a reduced risk of non-cardiovascular, non-cancer death attributed to inflammatory disease in the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2007, 85, 1606–1614. [Google Scholar] [CrossRef]
- Gooding, M.J.; Shewry, P.R. Wheat: Environment, Food and Health; Wiley Online Library: Hoboken, NJ, USA, 2022; p. 432. [Google Scholar]
- UNI10709; Durum Wheat Products for Pasta-Making—Definition, Characteristics and Quality Grades. UNI: Milan, Italy, 1998.
- UNI10940; Durum Wheat Products for Pasta-Making—Definition, Characteristics and Quality Grades. UNI: Milan, Italy, 2001.
- Galassi, E.; Taddei, F.; Ciccoritti, R.; Nocente, F.; Gazza, L. Biochemical and technological characterization of two C4 gluten-free cereals: Sorghum bicolor and Eragrostis tef. Cereal Chem. 2020, 97, 65–73. [Google Scholar] [CrossRef]
- AbuHammad, W.A.; Elias, E.M.; Manthey, F.A.; Alamri, M.S.; Mergoum, M.A. Comparison of methods for assessing dough and gluten strength of durum wheat and their relationship to pasta cooking quality. Int. J. Food Sci. Technol. 2012, 47, 2561–2573. [Google Scholar] [CrossRef]
- Payne, P.I.; Lawrence, G.J. Catalogue of alleles for the complex gene loci, Glu-A1, Glu-B1, and Glu-D1 which code for high-molecular-weight subunits of glutenin in hexaploid wheat. Cereal Res. Commun. 1983, 11, 29–35. [Google Scholar]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Requena-Ramírez, M.D.; Rodríguez-Suárez, C.; Flores, F.; Hornero-Méndez, D.; Atienza, S.G. Marker-Trait Associations for Total Carotenoid Content and Individual Carotenoids in Durum Wheat Identified by Genome-Wide Association Analysis. Plants 2022, 11, 2065. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).