Abstract
Rice root nematodes (Hirschmanniella spp.) cause serious damage to rice in various rice-producing countries in Asia. Nonetheless, there is limited information about the genetic diversity and structure of these nematodes, including in Thailand, where the current study explored the diversity and structure of Hirschmanniella spp. from 57 paddy fields in major rice-growing areas across the country. Using morphological characteristics and internal transcribed spacer rDNA sequencing, we identified all samples as Hirschmanniella mucronata. The use of the Inter-Simple-Sequence-Repeats-based delta K statistical test and principal coordinate analysis revealed two different genetic groups from three distinct geographical regions including the north and the northeast (Group 1) and the central (Group 2) regions. While the unbiased expected heterozygosity indicated low genetic diversity of H. mucronata in Thailand (0.149), the Shannon information index indicated there was higher diversity in Group 2 (0.259) than Group 1 (0.228). The AMOVA confirmed high genetic variation within such groups (76%), but low genetic variation between the two groups (24%). There was no clear correlation between genetic diversity and geographic distance. Given the presence of H. mucronata in almost all rice-growing countries, this first study of H. mucronata populations has provided fundamental knowledge that should help to combat this pest in the rice production system.
1. Introduction
Rice serves as a staple food for over one-half of the global population and is cultivated in more than 100 countries, with Asia accounting for 90% of the total worldwide production [1]. Global rice production is hampered by pests and pathogens, such as rice root nematodes that infest 58% of global rice fields and cause approximately 25% yield losses [2,3]. These migratory, endoparasitic nematodes penetrate rice roots, feed on the cells and create extensive necrotic lesions and cavities, rendering the root susceptible to secondary infections. As a result, the infected rice has stunted growth and reduced tillering. Among the parasitic nematodes of rice, Hirschmanniella mucronata is often found in paddy fields. This species has only been reported in Asia, specifically in the world’s top 10 rice-growing countries such as China, India, Indonesia, the Philippines, Vietnam and Thailand, where it can cause yield losses of up to 70% [4]. Even though the genus Hirschmanniella has a wide range of alternative hosts (more than 30 species), H. mucronata infects only two species, rice and sunflower (Helianthus annuus). Because rice serves as both a staple food and an economic crop for Thailand, it is very important to establish management strategies to contain this nematode [5].
The extent of genetic differentiation among local populations of a species is primarily influenced by gene flow and other processes, such as mutation, genetic drift and distinct local selection pressures that act independently in each subpopulation [6]. The significant genetic variation observed among plant-parasitic nematode (PPN) populations suggests that multiple inoculum sources may have been introduced to the region or that these populations may previously have existed in cultivated areas [7]. Intriguingly, various environmental factors, such as temperature, seasonal dynamics and farming practices, are known to influence the population structure of PPNs [8]. These factors may thus passively impact the adaptability of PPNs to the associated environments and thus contribute to variations in aggressiveness among different populations [9]. Therefore, a comprehensive understanding of the PPN population structure is crucial for designing effective control strategies. Despite the agricultural importance of nematodes, their genetic structure is not well understood [10,11]. Notably, the genetic structure and diversity of H. mucronata have not been yet determined anywhere in the world.
Inter-Simple Sequence Repeat (ISSR)-PCR is a technique requiring a single primer based on Simple Sequence Repeat (SSR; microsatellites) markers. SSRs are common in the genome, with changes in SSRs, such as deletion or insertion, leading to ISSR polymorphisms [12]. The technique is superior to random amplified polymorphic DNA (RAPD) and restriction fragment length polymorphism (RFLP) in generating informative and polymorphic bands, while maintaining reliability and cost-effectiveness [13]. To date, numerous studies have deployed and validated the reliability of ISSR markers for population studies. For example, ISSR markers have been instrumental in classifying the plant Dioscorea hispida into 10 distinct genetic groups, underscoring the substantial variation among germplasm collections. The high genetic diversity observed among D. hispida accessions further confirmed the efficacy of ISSR markers in detecting polymorphisms, reinforcing their usefulness in genetic diversity assessments [14]. Additionally, ISSR-PCR has been used for studying genetic diversity in various organisms, including lichen-forming fungi [15] and jasmine [16]. ISSR-PCR was also applied to various PPN species, such as Meloidogyne javanica [10], Globodera spp. [17] and Bursaphelenchus spp. [18], where the technique exhibited a high potential for studies of the PPN population. Considering the impact that H. mucronata may have on Thai rice production, the objective of the current study was to explore the distribution, genetic structure and diversity of H. mucronata in Thai paddy fields using combined morphometric-based conventional and ISSR-PCR-based molecular approaches. Given the ubiquitous production of rice in Thailand, the data will provide information useful for breeding rice resistant to Hirschmanniella spp.
2. Materials and Methods
2.1. Nematode Collection and Extraction
In total, 57 rice root samples were collected from major rice-producing areas in the tillering stage throughout Thailand in September 2024, following the method of Nimnoi et al. [19]. Most sites were situated in remote areas and, thus, there was no record of which rice cultivars were grown. Details of the sampling sites and locations are shown in Figure S1. Briefly, the rice plants were uprooted randomly and the shoots removed. Samples consisted of varying numbers of plants at each location. The plants were pooled from one location (paddy field). All details are specified in Table S1. Then, the roots were transferred to plastic bags and immediately transported to the Agricultural Nematology and Microbiology Laboratory, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Kamphaeng Saen Campus, Nakhon Pathom, Thailand. In the laboratory, the root samples were carefully washed with running tap water and chopped into small pieces (approximately 1–2 cm) for nematode extraction using a modified Baermann funnel method [20]. After 48 h, the nematodes that had moved through the sieve into the water were gathered and concentrated in a 10 mL suspension. Twenty-five adult females obtained from each sampling site (primarily identified as Hirschmanniella based on morphological traits) were collected for further species identification under a stereomicroscope [21]. Simultaneously, one adult male and one female nematode from each location were randomly selected for propagation by co-inoculating the pair on 15-day-old rice seedlings, cv. Pathumthani 1. The derived offsprings were later used for DNA extraction.
2.2. Identification
2.2.1. Morphometrical Analyses
In total, 1425 Hirschmanniella females were collected from the 57 sites. They were euthanised using hot water (50 °C) before being mounted on separate glass slides using a drop of distilled water. Each nematode was examined and photographed using an Olympus DP26 camera (Olympus Corp., Tokyo, Japan) mounted on a compound microscope (Olympus Corp., Tokyo, Japan). The computer software CellSens Standard 1.6 (Olympus Corp., Tokyo, Japan) was used for morphometric measurements, according to the work of Ebsary and Anderson [22] and Loof [23]. The following measurements were made: L = total body length; a = body length/body width; b = body length based on anterior end to pharyngo-intestinal junction (PIJ); c = body length/tail length; V% = head to vulva length/body length × 100; stylet length; maximum body width; pharynx length; anterior end to PIJ; head to vulva length; maximum tail width; and tail length. The obtained morphometric data were compared with a polytomous key. The main characters for morphological identification were the contour of the nematode, the cephalic framework and the type and position of the oesophagus to the intestine [24].
Analysis of variance (ANOVA) was performed to examine the differences in morphometric characters among populations from the north, northeast and central regions. A post hoc least significant difference (LSD) test was performed (p < 0.05), based on the ANOVA. The ANOVA and LSD tests were conducted using the software R, version 4.2.2 [25], and the package agricolae, version 1.3-7 [26].
2.2.2. Phylogenetic Analysis
DNA was extracted from five H. mucronata adults from each location using worm lysis buffer (WLB), according to the method of Castagnone-Sereno et al. [27], with minor modifications. Briefly, the adults were immersed in distilled water for 10 min, transferred to a 75 μL WLB drop on a glass slide and cut into four pieces using a needle under a stereomicroscope [27]. The small pieces were transferred into a 1.5 mL Eppendorf tube containing 10 μL WLB buffer. Each tube was centrifuged at 17,115 x g for 2 min and incubated at −20 °C for 1 h. Then, 10 µL mineral oil was added to each tube, followed by incubation at 60 °C for 1 h and then at 90 °C for 10 min. The mineral oil was carefully removed and the DNA was kept at −20 °C until used.
The ITS rDNA region containing ITS1-5.8S-ITS2 was amplified with the primers TW81 and AB28 [28]. PCR was set up in 25 μL using 2.5 μL DNA template and 0.2 μL recombinant Taq DNA polymerase (Invitrogen, Carlsbad, USA). PCR cycling consisted of an initial denaturation for 5 min at 94 °C followed by 35 cycles at 94 °C for 1 min, annealing at 58 °C for 90 s, extension at 72 °C for 2 min and a final extension at 72 °C for 10 min. The PCR product was purified using a PCR purification kit (Qiagen, Hilden, Germany) and Sanger sequencing was performed at ATGC Co., Ltd. (Bangkok, Thailand).
Species identification of the obtained DNA sequences was performed using the BLASTn algorithm [29] against the NCBI nr/nt nucleotide database (accessed 12 December 2024). The verified ITS sequences were analysed by the MEGA software, version 7 [30] and the software MUSCLE 3.8.31 [31] was used to align the sequences with default settings. The ITS sequences of other rice root nematode species were downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 12 December 2024) and included in the analysis. The sequences of Tylenchorhynchus annulatus were used as an outgroup. A phylogenetic tree was constructed in MEGA v.7 using the maximum likelihood (ML) method and the Tamura 3-parameter + Gamma (T92 + G) model as the best substitution model. A bootstrap analysis with 1000 repetitions was also generated in MEGA v.7 to assess branch support. The phylogenetic tree was plotted and visualised using the online iTOL tool with default settings (Interactive Tree of Life, available on https://itol.embl.de, accessed on 17 December 2024), according to the method of Letunic et al. [32].
2.3. ISSR-PCR
Nine primers [6] with clear amplification and reproducible banding patterns were selected for ISSR-PCR analysis, as shown in Table 1. The ISSR patterns were amplified from 5 μL genomic DNA using one of the primers and recombinant Taq polymerase (Invitrogen, Carlsbad, CA, USA) in a PCR reaction of 25 μL. The PCR was carried out using the Sensoquest Labcycler Basic (SensoQuest GmbH, Gottingen, Germany) with the following cycling conditions: 5 min of initial denaturation at 94 °C followed by 35 cycles of denaturation at 94 °C, 1 min of annealing at 54 °C, 90 sec of extension at 72 °C and a final extension at 72 °C for 10 min. The amplified ISSR products were separated on a 2% agarose gel, visualised under UV light and digitally photographed using the Uvitec Fire Reader V10 package (UVITEC, Cambridge, UK).

Table 1.
List of ISSR primers used for analysis of H. mucronata populations in this study.
2.4. ISSR Data Analysis
2.4.1. Genetic Structure and Principal Coordinate Analysis (PCoA)
The ISSR bands were scored as either “1” or “0” for presence or absence, respectively, and used to construct a data matrix. Any weak or smeared bands were discarded from further analysis. The genetic structures of the 57 populations from different locations were analysed using the software Structure 2.3.4 [33] using the Bayesian method and setting the number of groups (k) as the most reliable adjustable parameter. The admixture model with independent allele frequencies was run with a burn-in period of 10,000 steps and a chain length of 100,000 replications based on the Markov Chain Monte Carlo algorithm. Simulations were performed for each k value in the range of 1–20.
The delta K (∆k) statistical test was performed using the Structure Selector online tool (https://lmme.ac.cn/StructureSelector/, accessed on 5 January 2025), according to the method used by Li and Liu [34]. The criterion for the selection of ∆k in the Structure Selector was based on the mean and standard deviation of the log probability of the data [lnP(D)] obtained for each value of k. Then, the ∆k value was estimated for each k to obtain the greatest value. The lower [lnP(D)] value was selected for each value of k following the chosen optimum ∆k value. A graph for each replication was generated, with different colours representing different groups of structured individuals [34].
A PCoA was used to visualise differences among the studied populations. The Jaccard coefficient was calculated using the software Paleontological Statistics (PAST), version 4.11, with the default settings [35]. The software was also used to construct and visualise the PCoA plot.
2.4.2. Genetic Diversity Analysis
The genetic diversity of the H. mucronata populations was evaluated using the software GenAlEx, version 6.5 [36]. Genetic parameters, i.e., the percentage of polymorphic loci (P), Shannon information index (I), expected heterozygosity (H) and unbiased expected heterozygosity (uHe), were computed as described by Khan et al. [37]. Analysis of molecular variance (AMOVA) and genetic differentiation (ϕPT) were computed using the same software. The ϕPT value was further used to estimate the number of migrants (Nm). A Mantel test between the genetic distance binary [36] and geographic distances calculated from the sampling latitudes was computed using the GenAlEx package to evaluate the correlation between the two factors [38].
3. Results
3.1. Identification of Hirschmanneilla mucronata
The following morphological features were observed from female specimens for classical identification (Figure 1a): body length of females 1426–2697 µm, stylet length 16–27 µm, position of vulva located at approximately 50% of body length, two ovaries, basal knob distinctly offset, mean values of anterior end to PIJ 109–153 µm, long overlap of oesophagus over the intestine, pharyngeal glands elongated, pharynx length 266–427 µm, tail length 69.4–104.5 µm, maximum tail width 15–26.1 µm and end of tail terminal as an obvious mucron. The examined morphometric characteristics (stylet length, maximum body length and maximum tail width) of the Hirschmanniella specimens were also measured (Table 2). Of the examined morphometric characteristics, there were significant differences in stylet length and maximum tail width between the northern and the central populations and between the northeastern and the central populations (p < 0.05 in both cases). However, there were no significant differences in the traits between the northern and northeastern populations. The observed morphological features were a good fit for Hirschmanniella mucronata, while the morphometric characteristics were also consistent with the descriptions of H. mucronata.
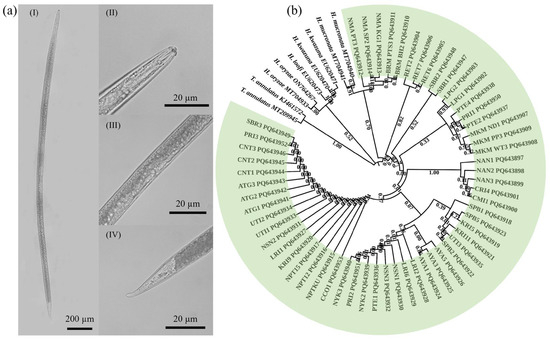
Figure 1.
Identification and classification of Hirschmanniella mucronata in this study: (a) morphological characteristics of representative H. mucronata female: (I) whole body, (II) anterior region, (III) middle region and (IV) tail; (b) maximum-likelihood-based phylogenetic tree constructed from ITS nucleotide sequences of 57 obtained samples (green colour), H. mucronata, H. oryzae, H. loofi and H. kwazuna. ITS sequence of Tylenchorhynchus annulatus used as outer group. Numbers beside branches represent ML bootstrap support values ≥ 90%.

Table 2.
Morphometrics of 25 adult females of Hirschmanniella mucronata isolated from each of the 57 sampling sites in paddy fields in northern, northeastern and central Thailand and their comparison with those from Takeo, Cambodia [24]. All measurements are in microns and presented as average ± S.D. Values in brackets denotes the minimum to maximum range.
An ML-based phylogenetic tree was constructed from the full ITS sequences to confirm the classical identification. The tree showed that all isolates of Hirschmanniella collected in this study formed a cluster with H. mucronata, with strong bootstrap support (≥90%) for all of the samples (Figure 1b). Altogether, the data indicated that all the samples collected in this study were H. mucronata.
3.2. ISSR-Based Molecular Diversity
The use of nine ISSR primers produced 109 polymorphic bands (Table 1). The number of reproducible amplified fragments generated by such primers differed and ranged from 7 to 22 with an average of 12.11 fragments per primer. The primer (CA) 8C produced the highest number of bands (22) between 300 bp and 1600 bp, whereas the primer (ATG) 6 had the least (7) between 100 bp and 5000 bp.
The population structure of the 57 nematode populations was analysed and had a peak of ∆k = 300 at k = 2, suggesting the presence of two sub-populations (Group 1 and Group 2; Figure 2a,b). Likewise, the PCoA based on Jaccard dissimilarity index revealed two distinct groups (Figure 3). Group 1 comprised 18 populations, predominantly from the northern (7 populations) and northeastern (11 populations) regions of Thailand, whereas Group 2 comprised 39 populations, all from the central rice-growing region.
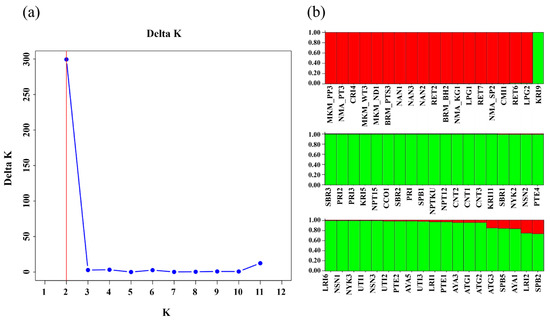
Figure 2.
Structure analysis of 57 nematode populations: (a) ∆k values corresponding to various numbers of groups (k), where the x-axis represents number of groups and the y-axis depicts ∆k values; (b) the populations were classified into 2 sub-groups, where the x-axis represents individual isolates and the y-axis shows the probability of membership of each isolate in a specific sub-group, with sub-groups distinguished by colour.
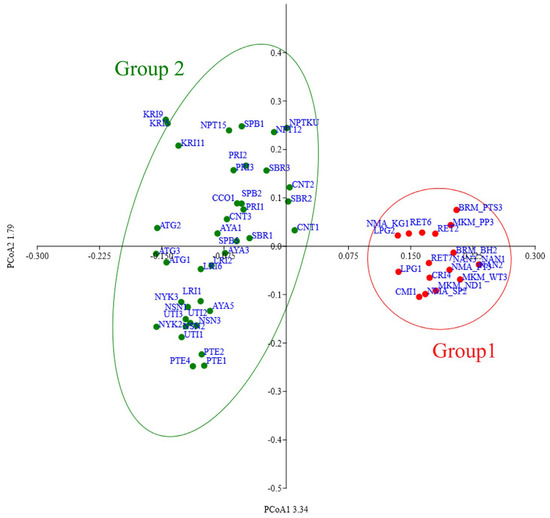
Figure 3.
PCoA analysis of Hirschmanniella mucronata groups in Thailand.
Further analysis of the diversity indices showed that Group 2, comprising the central populations, exhibited higher diversity than Group 1, comprising populations from the north and northeast regions (Table 3). For example, the percentage of polymorphic loci was 86.09% in Group 2 compared to 60.87% in Group 1. The Shannon information index in Group 2 was 0.259 compared to 0.228 in Group 1, while the values for the expected heterozygosity and unbiased expected heterozygosity were 0.152 and 0.154, respectively, in Group 2 compared to only 0.139 and 0.143, respectively, in Group 1.

Table 3.
Genetic diversity of Hirschmanniella mucronata populations in Thailand based on ISSR markers (groups defined based on k = 2).
Based on the results of the AMOVA, 76% of the molecular variation occurred within groups, while 22% originated from differences among groups. The ϕPT value, which explain the variations between the two groups, was 0.24 (p = 0.001), suggesting high genetic similarity between the populations. Furthermore, the number of migrants (Nm), indicating the level of gene flow among the H. mucronata populations in Thailand, was 0.80 (Table 4), supporting the previous finding of high similarity between the two groups. There was no obvious correlation between genetic distance and geographic distance between the two groups (Mentel test, R2 = 0.1318, Figure 4). Notably, the Nm value between the north and the northeast regions was 1.12 (Table 4), suggesting a high gene flow between the two regions, which may have contributed to the genetic similarity among the populations within Group 1.

Table 4.
Analysis of molecular variance (AMOVA) of the Hirschmanniella mucronata populations, based on ISSR markers.
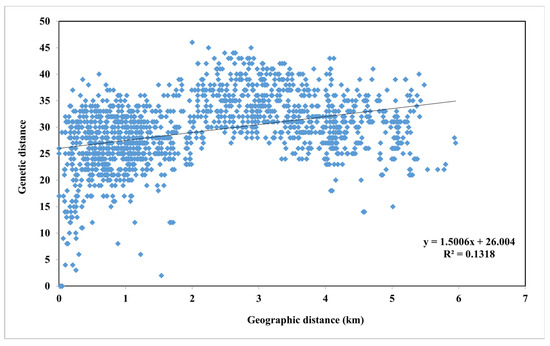
Figure 4.
Mantel test illustrating the correlation between genetic distance and geographic distance among total populations of Hirschmanniella mucronata in Thailand.
4. Discussion
Rice root nematodes are one of the major pests in rice production causing large losses, but there are currently no resistant cultivars. Based on the results of the present study, rice root nematodes were widely distributed across the major rice-growing areas in Thailand. Of the 57 populations investigated, only a single species (H. mucronata) was identified. There are five species of Hirschmanniella in Thailand, but only two species (H. oryzae and H. mucronata) are prevalent in rice fields [39,40]. The fact that only H. mucronata was isolated over a widespread area in the current study emphasises the importance of this species as a rice pest. Beesa et al. [39] reported the presence of H. mucronata in rice roots in rice fields in Pathum Thani, a province in the central rice-growing area. Furthermore, this species was considered to be the predominant PPN in lowland rice fields in Cambodia [40], raising the question of whether the Thai populations of H. oryzae have been replaced by H. mucronata.
One of the possibilities for the apparent distribution difference is species misidentification. Previously, this has been a concern because species identification has relied only on morphology and morphometric information, with some of the characteristics considered being continuous in nature. For example, H. mucronata and H. oryzae have highly similar morphological characteristics with only two distinguishable morphometric features, i.e., stylet length and tail width [24]. However, the morphometric information of stylet length for H. oryzae has been reported to be in the range 16–31 µm, with notable variations among populations from different countries, including 26–31 µm from South Korea [41] and 16–19 µm and 17–20 µm from Indonesia and India, respectively [42]. For comparison, the stylet length was reported to be in the range of 20–29 µm for H. mucronata [24]. A similar phenomenon was observed in the tail width, resulting in confusion and possibly misidentification between the two species. Consequently, it is important to incorporate DNA barcoding in the identification procedure, with ITS being one of the DNA barcoding regions/genes widely used for species identification. Bogale et al. [43] suggested that ITS sequences serve as reliable markers for nematode species differentiation. In the current study, the phylogenetic analysis, based on ITS sequences, showed that the Thai isolates clustered distinctly with H. mucronata, aligning well with the identification using the complementary classical approach.
Little is known about the genetic variation in H. mucronata. The current in-depth study is the first to investigate the genetic structure of the species. The ISSR marker is one of the most powerful markers for population studies as it provides useful information for resolving phylogenetic relationships among closely related species and relationships at or below the species level [44]. To date, this type of marker has been applied extensively to study the genetic diversity of nematodes [18,45,46]. In the current study, the use of ISSR markers revealed a high level of polymorphisms among the H. mucronata populations, with all primers generating polymorphic bands. Despite the extensive number of sampling sites, the population structure analysis indicated the presence of only two groups, from different regions where different groups of rice cultivars are grown. Sun et al. [47] reported that differences in physiology and root structure between these rice types may play a role in genetic differentiation among populations. It has been shown that rice cultivars can exert selection pressure on the populations of the rice root-knot nematode Meloidogyne graminicola [3,48]. The percentage of glutinous rice (O. sativa var. glutinosa) cultivation was found to be is much higher in the north and northeast regions of Thailand (13.6% and 24.9%, respectively) compared to only 0.1% in the central region [49]. Notably, there were significant differences in stylet length between the two nematode groups. The function of the stylet is to penetrate root cells for feeding and, therefore, it is very likely that the rice cultivar is one of the contributing factors contributing to group differentiation in the Thai populations of H. mucronata, in addition to the differences in cultivation practices between the three regions studied in the current study. In fact, our preliminary screening showed that glutinous rice cultivars were generally more tolerant to rice root-knot nematodes than non-glutinous cultivars [50]. It would be interesting to investigate, in detail, the effects of different cultivars on populations of rice root nematodes.
Derycke et al. [51] reported that the genetic structure of free-living marine nematodes did not always correlate with geographic distance. Instead, populations may exhibit “chaotic genetic patchiness”, where substantial genetic differences exist between nearby populations, while distant populations may be genetically similar. This pattern suggests that hydrodynamic currents and ecological factors influence gene flow, leading to increased genetic variation within populations and reduced genetic differences between them. In the current study, there was no clear correlation between genetic and geographic distances. It is most likely that, in this case, genetic differentiation was not solely driven by spatial factors, but also plausibly influenced by other factors such as host plant interactions, soil conditions and human-mediated dispersal.
The genetic diversity indices revealed that Group 2 (central populations) had a higher level of polymorphisms, genetic diversity and Shannon information index than Group 1 (northern/northeastern populations). In the north and northeast, rice is cultivated only once a year and only a few cultivars are preferred. In contrast, the central rice-growing region is a hub of commercial rice production, benefiting from intensive farming and modern irrigation systems. Therefore, rice is cultivated up to three times a year in the central area and multiple rice cultivars are grown. It is possible that continuous cultivation and multiple cultivars may have enhanced the adaptation of the central populations, resulting in increased genetic diversity compared to the north and northeast populations. The same phenomenon was reported in rice attacked by the insect pest Chlorops oryzae, where continuous growing of rice allows C. oryzae to reproduce continuously and maintain larger population sizes with higher genetic diversity [52]. However, the low unbiased expected heterozygosity (only 14.9%) indicated that the Thai population of H. mucronata may have originally consisted of only a single group before adapting to specific environmental factors and diverging into two groups. Notably, the farmers in the north and northeast tend to use seedlings from a nearby source, which may limit the movement and distribution of nematode populations in the regions, causing lower genetic diversity [8]. Nazareno et al. [53] showed that the increased human-mediated dispersal of soil, plant material and water could promote genetic mixing, contributing to higher diversity levels.
The current study highlighted the presence and distribution of rice root nematodes in Thailand. Notably, Thailand is a major grower of rice and this is the first population study of H. mucronata. Consequently, the information acquired from the current study should provide valuable information on the genetic diversity and population structure of H. mucronata in Thailand, with possible applicability globally. As such, the current findings should be important in developing nematode management strategies, especially for breeding nematode-resistant rice cultivars that are not available at the moment. The understanding of the genetic variability within populations can therefore help to guarantee food security, particularly in light of the increasing world population.
5. Conclusions
The observed genetic differentiation and diversity patterns of H. mucronata in Thailand have been shaped by multiple interacting factors, including geography, host plant diversity, climate, agricultural practices and human activities. The studied populations in the north and the northeast were in the same genetic group, which was distinctly separated from the central population. The higher genetic diversity in the central region suggested a dynamic and heterogeneous population structure, likely driven by frequent gene flow, ecological variation and anthropogenic influences. The genetic variation in H. mucronata was mainly due to variation within, rather than between, different geographic groups. There was no substantial correlation between genetic distance and geographical distribution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15040919/s1, Figure S1: Geographic location of the studied populations of Hirschmanniella mucronata in 21 provinces of Thailand. Table S1: Details of collection site locations of Hirschmanniella mucronata populations.
Author Contributions
Conceptualization, S.S. and P.R.; methodology, P.R.; validation, S.S., H.J.L.J. and P.R.; formal analysis, S.S.; investigation, H.J.L.J.; resources, H.J.L.J.; writing—original draft preparation, S.S.; writing—review and editing, P.R. and H.J.L.J.; visualisation, S.S. and P.R.; supervision, P.R.; funding acquisition, P.R. and H.J.L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Council of Thailand (NRCT). The funding body had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
The DNA sequences generated in this study are available at the National Center for Biotechnology Information (NCBI) under the accession numbers PQ643897–PQ643955.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMOVA | Analysis of molecular variance |
| ANOVA | Analysis of variance |
| ISSR | Inter-Simple Sequence Repeats |
| ITS | Internal transcribed spacer |
| LSD | Least significant difference |
| Nm | Number of migrants |
| PCoA | Principal coordinate analysis |
| PPN | Plant-parasitic nematode |
| SSRs | Simple Sequence Repeats |
References
- Fukagawa, N.K.; Ziska, L.H. Rice Importance for global nutrition. J. Nutr. Sci. Vitaminol. 2019, 65, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Bridge, J.; Plowright, R.A.; Peng DeLiang, P.D. Nematode parasites of rice. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; CABI Publishing: Wallingford UK, 2005; pp. 87–130. [Google Scholar]
- Kyndt, T.; Fernandez, D.; Gheysen, G. Plant-parasitic nematode infections in rice: Molecular and cellular insights. Annu. Rev. Phytopathol. 2014, 52, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, N.K.; Rao, Y.S. Seasonal prevalence of the rice root nematode Hirschmanniella mucronata Das 1960. Proc. Indian Acad. Sci. 1980, 89, 485–489. [Google Scholar] [CrossRef]
- Youssef, M.; Eissa, M. The rice root nematode, Hirschmanniella oryzae, its identification, economic importance and control measures in Egypt: A review. Arch. Phytopathol. Plant Prot. 2014, 47, 2340–2351. [Google Scholar] [CrossRef]
- Lax, P.; Dueñas, J.R.; Gardenal, C.N.; Doucet, M.E. Assessment of genetic variability in populations of Nacobbus aberrans (Thorne, 1935) Thorne & Allen, 1944 (Nematoda: Pratylenchidae) from Argentina. Nematology 2007, 9, 261–270. [Google Scholar] [CrossRef]
- Devran, Z.; Söğüt, M.A. Distribution and identification of root-knot nematodes from Turkey. J. Nematol. 2009, 41, 128. [Google Scholar]
- Yang, Z.; Zhang, L.; Li, X.; Lin, Y.; Ye, S.; Ding, Z. Population dynamics of Meloidogyne graminicola in soil in different types of direct-seeded rice agroecosystems in Hunan Province, China. J. Nematol. 2023, 55, 20230040. [Google Scholar] [CrossRef]
- Mondal, S.; Purohit, A.; Hazra, A.; Das, S.; Chakrabarti, M.; Khan, M.R.; Lopez-Nicora, H.; Chakraborti, D.; Mukherjee, A. Intraspecific variability of rice root knot nematodes across diverse agroecosystems for sustainable management. Sci. Rep. 2024, 14, 1–14. [Google Scholar] [CrossRef]
- Hesar, A.; Rostami, M.; Ghaderi, R.; Danesh, Y.; Jalal, A.; da Silva Oliveira, C.; Teixeira Filho, M. Population genetic structure of Meloidogyne javanica recovered from different regions of Iran. Agriculture 2022, 12, 1374. [Google Scholar] [CrossRef]
- Plantard, O.; Porte, C. Population genetic structure of the sugar beet cyst nematode Heterodera schachtii: A gonochoristic and amphimictic species with highly inbred but weakly differentiated populations. Mol. Ecol. 2004, 13, 33–41. [Google Scholar] [CrossRef]
- Wang, X.-M. Optimization of DNA isolation, ISSR-PCR system and primers screening of genuine species of rhubarb, an important herbal medicine in China. J. Med. Plants Res. 2010, 4, 904–908. [Google Scholar] [CrossRef]
- Prevost, A.; Wilkinson, M. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor. Appl. Genet. 1999, 98, 107–112. [Google Scholar] [CrossRef]
- Nudin, N.F.H.; Ali, A.M.; Ngah, N.; Mazlan, N.Z.; Mat, N.; Ghani, M.N.A.; Alias, N.; Zakaria, A.J.; Jahan, M.S. ISSR marker-assisted genetic diversity analysis of Dioscorea hispida and selection of the best variety for sustainable production. C. R. Biol. 2017, 340, 359–366. [Google Scholar] [CrossRef]
- Lindblom, L.; Ekman, S. Genetic variation and population differentiation in the lichen-forming ascomycete Xanthoria parietina on the island Storfosna, central Norway. Mol. Ecol. 2006, 15, 1545–1559. [Google Scholar] [CrossRef]
- Akhtar, N.; Hafiz, I.A.; Hayat, M.Q.; Potter, D.; Abbasi, N.A.; Habib, U.; Hussain, A.; Hafeez, H.; Bashir, M.A.; Malik, S.I. ISSR-based genetic diversity assessment of genus Jasminum L. (Oleaceae) from Pakistan. Plants 2021, 10, 1270. [Google Scholar] [CrossRef]
- Trayanov, K.; Kostova, M. ISSR molecular markers for the study of the genetic diversity in Bulgarian populations of PCN from genus Globodera. Agric. Sci. 2020, 12, 25–28. [Google Scholar]
- Zhou, L.; Chen, F.; Xie, L.; Pan, H.; Ye, J. Genetic diversity of pine-parasitic nematodes Bursaphelenchus xylophilus and Bursaphelenchus mucronatus in China. For. Pathol. 2017, 47, e12334. [Google Scholar] [CrossRef]
- Nimnoi, P.; Pirankham, P.; Srimuang, K.; Ruanpanun, P. Insights into soil nematode diversity and bacterial community of Thai jasmine rice rhizosphere from different paddy fields in Thailand. PeerJ 2024, 12, e17289. [Google Scholar] [CrossRef]
- Prot, J.-C.; Gergon, E.; Matias, D. Influence of extraction procedures from root samples on the recovery and infectivity of Pratylenchus zeae and Hirschmanniella oryzae. Nematol. Mediterr. 1993, 21, 133–137. [Google Scholar]
- Tarjan, A.C.; Esser, R.P.; Chang, S.L. An illustrated key to nematodes found in fresh water. J. Water Pollut. Control Fed. 1977, 49, 2318–2337. [Google Scholar]
- Ebsary, B.; Anderson, R. Two new species of Hirschmanniella Luc and Goodey, 1963 (Nematoda Pratylenchidae) with a key to the nominal species. Can. J. Zool. 1982, 60, 530–535. [Google Scholar] [CrossRef]
- Loof, P.A. The family Pratylenchidae Thorne, 1949. In Manual of Agricultural Nematology; Nickle, W.R., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 363–422. [Google Scholar]
- Khun, K.; Decraemer, W.; Couvreur, M.; Karssen, G.; Steel, H.; Bert, W. Deceptive morphological variation in Hirschmanniella mucronata (Nematoda: Pratylenchidae) and a polytomous key to the genus. Nematology 2015, 17, 377–400. [Google Scholar] [CrossRef]
- Team R.C. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 18 December 2024).
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 20 December 2024).
- Castagnone-Sereno, P.; Esparrago, G.; Abad, P.; Leroy, F.; Bongiovanni, M. Satellite DNA as a target for PCR-specific detection of the plant-parasitic nematode Meloidogyne hapla. Curr. Genet. 1995, 28, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Driver, F.; Ballard, J.; Milner, R. Phylogeny of Metarhizium: Analysis of ribosomal DNA sequence data. Mycol. Res. 1994, 98, 547–552. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+ Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7 Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, J.X. StructureSelector: A web-based software to select and visualize the optimal number of clusters using multiple methods. Mol. Ecol. Resour. 2018, 18, 176–177. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.I.; Jusoh, M.; Al Mamun, M.; Halidu, J. DNA fingerprinting, fixation-index (Fst), and admixture mapping of selected Bambara groundnut (Vigna subterranea [L.] Verdc.) accessions using ISSR markers system. Sci. Rep. 2021, 11, 14527. [Google Scholar] [CrossRef]
- Guo, Q.; Cao, S.; Dong, L.; Li, X.; Zhang, J.; Zhang, Y.; Zhang, Z.; Sun, Y.; Long, C.; Fan, Y. Genetic diversity and population structure of Robinia pseudoacacia from six improved variety bases in China as revealed by simple sequence repeat markers. J. For. Res. 2022, 33, 611–621. [Google Scholar] [CrossRef]
- Beesa, N.; Sasnarukkit, A.; Jindapunnapat, K.; Chinnasri, B.; Chairin, T. Incidence and characterization of rice root nematodes, Hirschmanniella mucronata, from rice fields in Pathum Thani province, Thailand. Trends Sci. 2021, 18, 486. [Google Scholar] [CrossRef]
- Beesa, N.; Sasnarukkit, A.; Jindapunnapat, K.; Tivet, F.; Bellafiore, S.; Chinnasri, B. Species characterization and population dynamics of Hirschmanniella mucronata in lowland rice fields managed under conservation agriculture in Cambodia. J. Saudi Soc. Agric. Sci. 2021, 20, 137–145. [Google Scholar] [CrossRef]
- 41 Mwamula, A.O.; Lim, T.H.; Kim, Y.; Lee, H.-w.; Kim, Y.H.; Lee, D.W. Morphological plasticity in the rice root nematode, Hirschmanniella oryzae (van Breda de Haan, 1902) Luc & Goodey, 1964 from Korea, with inferences from its ribosomal and mitochondrial DNA. Eur. J. Plant Pathol. 2022, 164, 337–352. [Google Scholar] [CrossRef]
- Sher, S. Revision of the Genus Hirschmanniella Luc & Goodey, 1963 (Nematoda: Tylenchoidea); University of California Press: Berkeley, CA, USA, 1968. [Google Scholar]
- Bogale, M.; Baniya, A.; DiGennaro, P. Nematode identification techniques and recent advances. Plants 2020, 9, 1260. [Google Scholar] [CrossRef]
- Metge, K.; Bürgermeister, W. Intraspecific variation in isolates of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) revealed by ISSR and RAPD fingerprints. J. Plant Dis. Prot. 2006, 113, 275–282. [Google Scholar] [CrossRef]
- Feng, T.; Jia, Q.; Meng, X.; Chen, X.; Wang, F.; Chai, W.; Liang, Z. Evaluation of genetic diversity and construction of DNA fingerprinting in Polygonatum Mill. based on EST-SSR and SRAP molecular markers. 3 Biotech 2020, 10, 322. [Google Scholar] [CrossRef]
- Huang, W.-K.; Peng, D.-L.; Zhang, D.-S.; Jiang, H.-Y.; Ding, Z.; Peng, H.; Long, H.-B. Assessement of genetic variability in population of Ditylenchus destructor (Thorne 1945) (Tylenchida: Anguinidae) from China. Russ. J. Nematol. 2010, 18, 19–30. [Google Scholar]
- Sun, X.; Zhang, L.; Tang, Z.; Shi, X.; Ma, J.; Cui, R. Transcriptome analysis of roots from resistant and susceptible rice varieties infected with Hirschmanniella mucronata. FEBS Open Bio 2019, 9, 1968–1982. [Google Scholar] [CrossRef]
- Cabasan, M.T.N.; Kumar, A.; De Waele, D. Comparison of migration, penetration, development and reproduction of Meloidogyne graminicola on susceptible and resistant rice genotypes. Nematology 2012, 14, 405–415. [Google Scholar] [CrossRef]
- Office of Agricultural Economics. Type of rice in Thailand. Available online: https://www.oae.go.th/view/1/ตารางแสดงรายละเอียดข้าวนาปรัง/TH-TH (accessed on 14 January 2025).
- Pirankham, P.; Ruanpanun, P. Evaluation of resistance to root-knot nematode (Meloidogyne graminicola) in Thai rice germplasm. 2023; Department of Plant Pathology, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Kamphaeng Saen Campus: Nakhon Pathom, Thailand, (unpublished work). [Google Scholar]
- Derycke, S.; Backeljau, T.; Moens, T. Dispersal and gene flow in free-living marine nematodes. Front. Zool. 2013, 10, 1. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.; Fan, J.; Wang, Y.; Li, Y.; Chen, J.; Fan, J.; Yang, S.; Hu, L.; Leung, H. Genetic diversity and disease control in rice. Nature 2000, 406, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Nazareno, A.G.; Knowles, L.L.; Dick, C.W.; Lohmann, L.G. By animal, water, or wind: Can dispersal mode predict genetic connectivity in riverine plant species? Front. Plant Sci. 2021, 12, 626405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).