Effect of Paulownia and Buckwheat Intercropping on Soil Microbial Biodiversity, Dehydrogenase Activity, and Glomalin-Related Soil Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Soil Sampling
2.2. Chemical-Physical Soil Analysis
- Content of bioavailable forms of P, K—assessed using the Egner–Riehm (DL) method;
- Soil Ca content—determined using the Sheibler method;
- Available magnesium content—measured using the Schachtschabel method;
- pH in distilled water and in 1 M KCl—determined using a potentiometric pH metre;
- Nitrogen content—determined using the modified Kjehdal method (total nitrogen determination).
2.3. Agrotechnology of the Experiment
2.4. Weather Conditions
2.5. Characteristics of Soil Microbiological Properties
2.5.1. DNA Extraction, PCR, and Illumina Amplicon Sequencing
2.5.2. Bioinformatic Analysis
2.5.3. Quantification of Cultivable Bacteria and Fungi
2.5.4. Soil Dehydrogenase Activity and T-GRSP Concentration
2.5.5. Colonisation of Buckwheat Roots by Filamentous Fungi
2.5.6. Analysis of Buckwheat Biometric Traits and Yield
3. Results
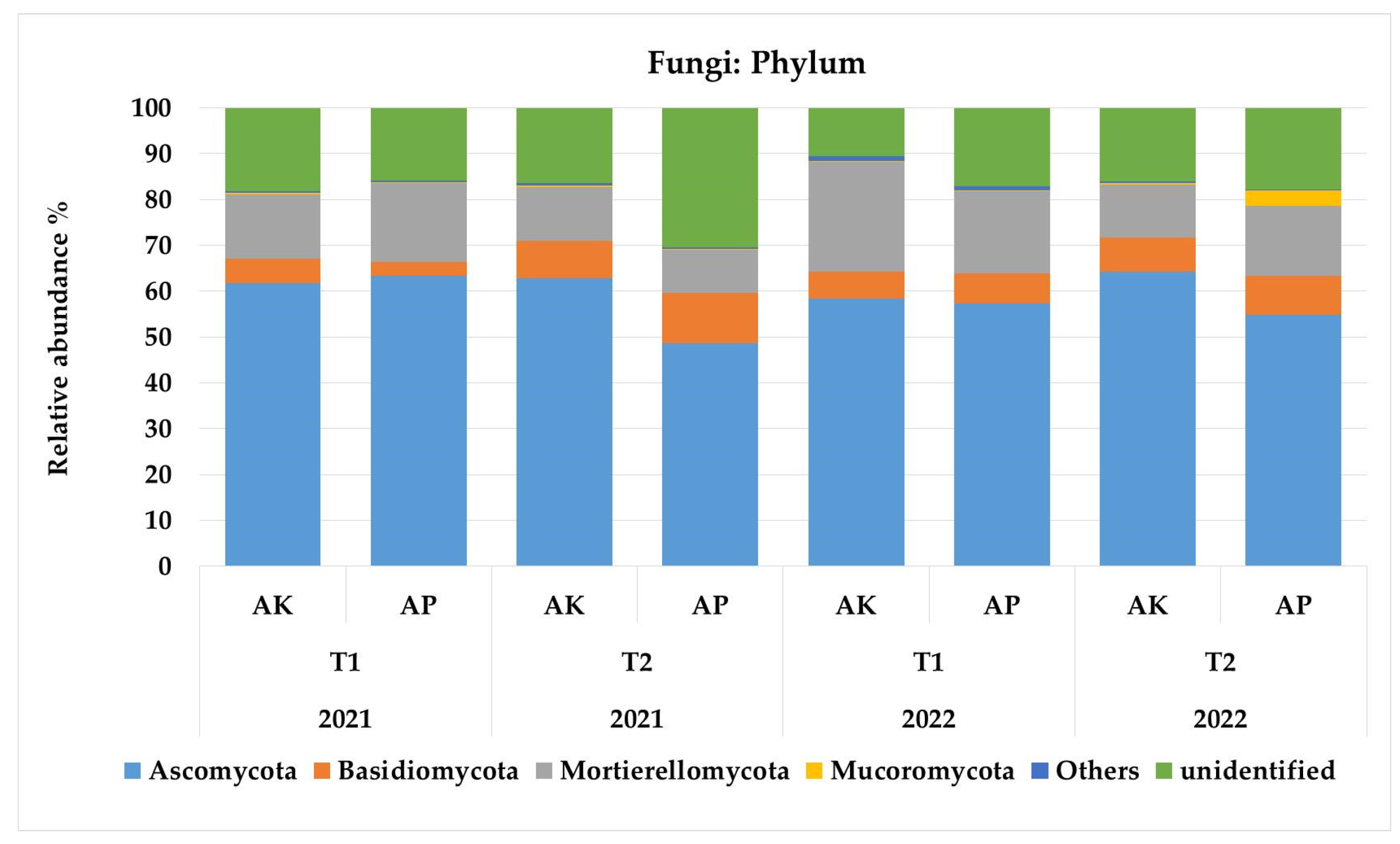
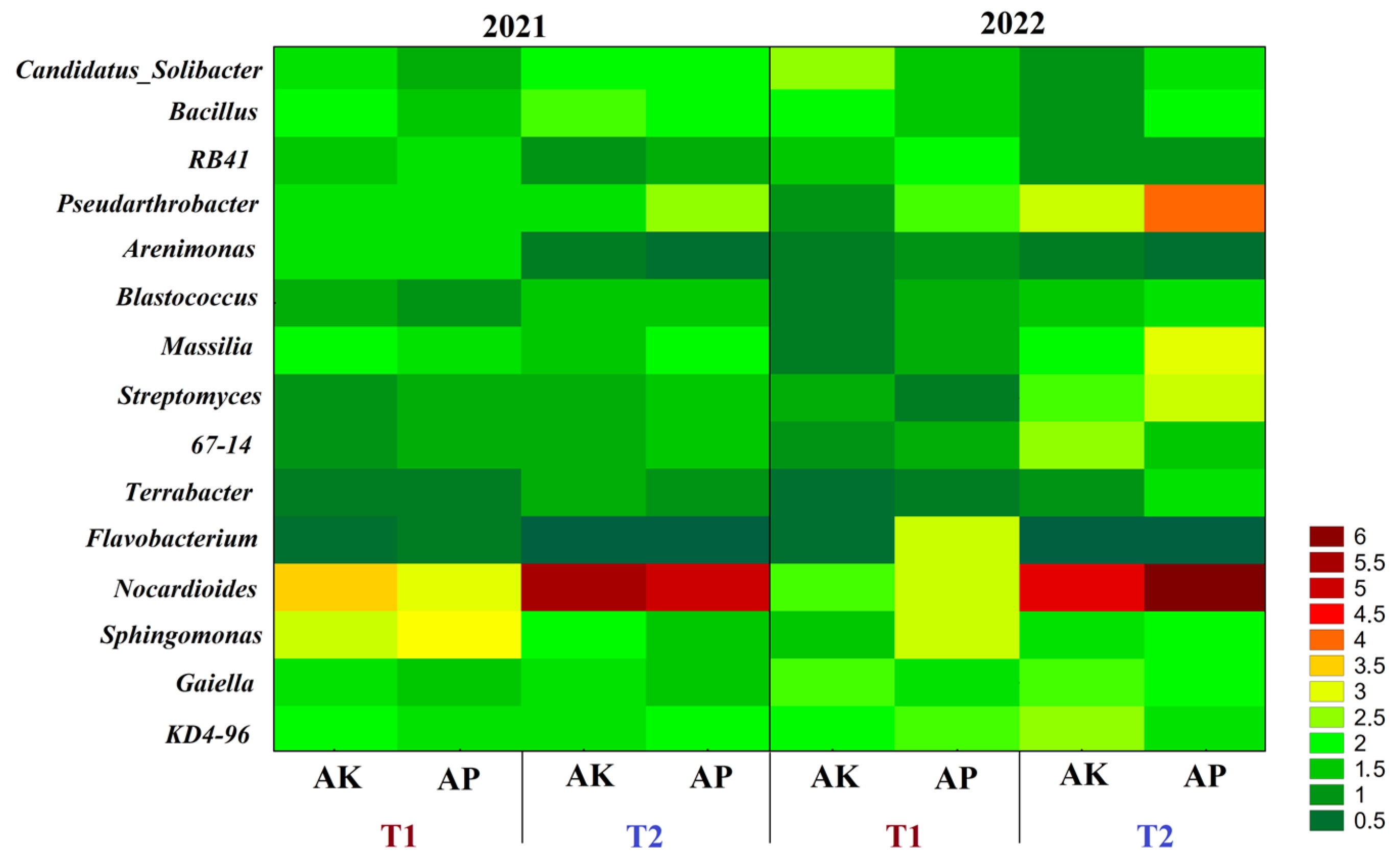
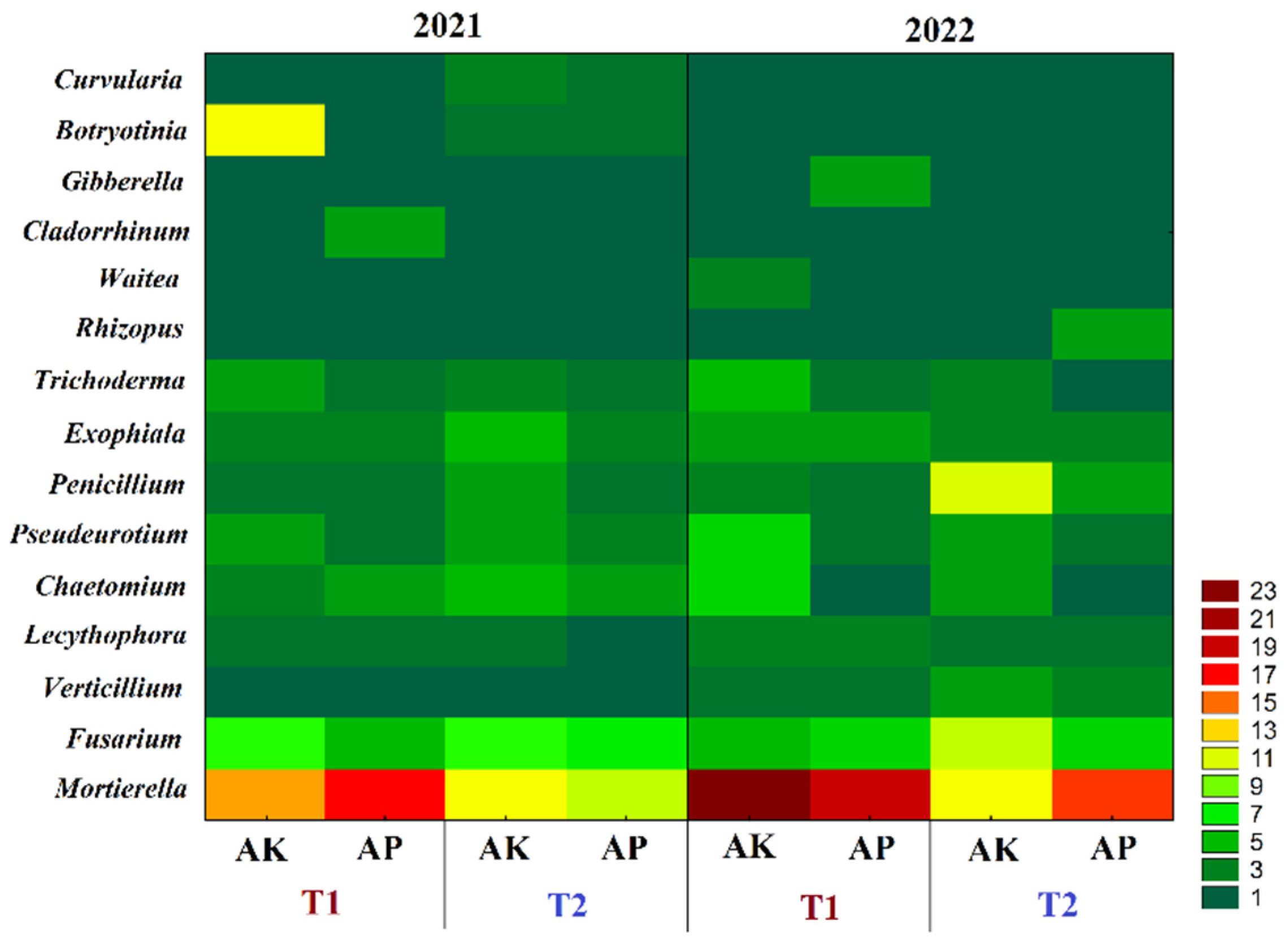
3.1. Diversity and Structure of the Microbiome and Mycobiome
3.2. Total Abundance of Cultivable Bacteria and Fungi
3.3. Soil Biological Activity
3.4. Correlation of Microbiological Analysis (Bacteria, Fungi, DHA, and T-GRSP)
3.5. Buckwheat Root Colonisation by Fungi
3.6. Biometric Traits and Yield of Buckwheat
4. Discussion
4.1. Effect of Soil Properties on Microbiological Changes
4.2. Effect of Intercropping System on DHA and T-GRSP Activity
4.3. Effect of Intercropping Cultivation on the Yield of Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AK | control (without Paulownia) |
| AP | intercropping (with Paulownia) |
| CFU | colony forming units |
| DHA | dehydrogenase activity |
| T1, T2 | the first and second soil sampling date |
| TBI | tree-based intercropping systems |
| T-GRSP | total glomalin-related soil proteins |
References
- Nair, P.K.R.; Gordone, A.M.; Mosquera-Losadac, M.R. Agroforest; Elsevier: Amsterdam, The Netherlands, 2008; pp. 101–110. [Google Scholar]
- Borek, R. Agroforestry Systems in Poland a preliminary identification. Pap. Glob. Change IGBP 2015, 22, 37–51. [Google Scholar] [CrossRef][Green Version]
- Liszewski, M.; Chorbiński, P. The influence of foliar buckwheat fertilization with copper, manganese and iron on selected parameters of its nectar production and seed yield. Polish J. Agron. 2021, 46, 23–30. [Google Scholar]
- Jimenez, L.; Rodrigez, A.; Ferrer, J.R.; Perez, A.; Angulo, V. Paulownia, a fast-growing plant, as a raw material for paper manufacturing. Afinida-Barcelona 2005, 62, 100–105. [Google Scholar]
- Halarewicz, A.; Liszewski, M.; Bąbelewski, P.; Bączek, P. Allelopathic effects of Paulownia tomentosa and hybryd P. elongate × P. fortune on Sinapis alba, Festuca pratensis and Poa pratensis. Allelopathy J. 2018, 43, 83–92. [Google Scholar] [CrossRef]
- Valdivia, C.; Barbieri, C.; Gold, M.A. Between Forestry and Farming: Policy and Environmental Implications of the Barriers to Agroforestry Adoption. C. J. Agric. Econom. 2012, 60, 155–175. Available online: http://www4.ncsu.edu/~cebarbie/papers/valdivia_agroforestry_2012.pdf (accessed on 12 May 2023).
- Sørensen, J.; Sessitsch, A. Plant-associated bacterial-lifestyle and molecular interactions. In Modern Soil Microbiology; Van Elsas, J.D., Jansson, J.K., Trevors, J.T., Eds.; CRC: New York, NY, USA, 2007; pp. 221–236. [Google Scholar]
- Siebielec, S.; Siebielec, G.; Urbaniak, M.; Smreczek, M.; Grzęda, E.; Wywricka, A.; Susan Kidd, P. Impact of rhizobacterial inoculants on plant growth and enzyme activities in soil treated with contaminated bottom sediments. Int. J. Phytoremed. 2017, 21, 325–333. [Google Scholar]
- Woźniak, M.; Gałązka, A.; Siebielec, G.; Frąc, M. Can the Biological Activity of Abandoned Soils Be Changed by the Growth of Paulownia elongata × Paulownia fortunei?—Preliminary Study on a Young Tree Plantation. Agriculture 2022, 12, 128. [Google Scholar] [CrossRef]
- Chu, H.; Lin, X.; Fujii, T.; Morimoto, S.; Yagi, K.; Hu, J.; Zhang, J. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biol. Biochem. 2007, 39, 2971–2976. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Maiti, S.K. Different soil factors influencing dehydrogenase activity in mine degraded lands—State-of-art review. Water Air Soil Pollut. 2021, 232, 360. [Google Scholar] [CrossRef]
- Ma, Y.H.; Fu, S.L.; Zhang, X.P.; Zhao, K.; Chen, H.Y. Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Appl. Soil Ecol. 2017, 119, 171–178. [Google Scholar]
- Zhao, X.; Dong, Q.; Han, Y.; Zhang, K.; Shi, X.; Yang, X.; Yuan, Y.; Zhou, D.; Wang, K.; Wang, X.; et al. Maize/peanut intercropping improves nutrient uptake of side-row maize and system microbial community diversity. BMC Microbiol. 2022, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Z.Y.; Ling, W.T. Distribution and environmental function of glomalin-related soil protein: A review. J. Appl. Ecol. 2016, 27, 634–642. [Google Scholar]
- Liu, G.; Duan, X.; Yan, G.; Sun, X.; Jiang, S.; Xing, Y.; Wang, Q. Changes in Soil Aggregates and Glomalin-Related Soil Protein Stability During the Successional Process of Boreal Forests. J. Soil. Sci. Plant. Nutr. 2024, 24, 1335–1348. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, M.C.; Carrillo-Gonzalez, R.; Wright, S.F.; Nichols, K.A. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar]
- Nam, N.N.; Do, H.D.K.; Thrin, K.T.L.; Lee, N.Y. Metagenomics: An Effective Approach for Exploring Microbial Diversity and Functions. Foods 2023, 12, 2140. [Google Scholar] [CrossRef]
- Banerjee, S.; Bahh-Acheamfour, M.; Carlyle, C.N.; Bisset, A.; Richardson, A.E.; Siddique, T.; Bork, E.W.; Chang, S.X. Determinants of bacterial communities in Canadian agroforestry systems. Environ. Microbiol. 2016, 18, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- IUSS Working Group WRB World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Polish Soil Classification, Systematyka Gleb Polski. Polskie Towarzystwo Gleboznawcze, Komisja Genezy Klasyfikacji i Kartografii Gleb; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu, Polskie Towarzystwo Gleboznawcze: Wrocław/Warszawa, Poland, 2019; Volume 29. (In Polish) [Google Scholar]
- Kalbarczyk, R. Próba wydzielenia regionów termiczno-opadowych na obszarze Polski. Folia Univ. Agric. Stetinens. 2003, 231, 27–38. [Google Scholar]
- Bunt, J.S.; Rovira, A.D. Microbiological studies of some subantarctic soil. J. Soil Sci. 1995, 6, 119–128. [Google Scholar]
- Martin, J.P. Use of acid, rose Bengal, and streptomycin in the plate method for estimting soil fungi. Soil Sci. 1950, 69, 215–233. [Google Scholar]
- Wright, S.F.; Upadhyaya, A. Quantification of arbuscular mycorrhizal fungi activity by the glomalin concentration on hyphal traps. Mycorrhiza 1990, 8, 283–285. [Google Scholar]
- Germain, G.; Summerbell, R. Identifying Filamentous Fungi: A Clinical Laboratory Handbook; Star Publishing Company: Belmont, CA, USA, 1996. [Google Scholar]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C. Wprowadzenie Do Grzybów Przenoszonych Przez Żywność i Powietrze; Centraalbureau voor Schimmelcultures: Utrecht, The Netherland, 2004. [Google Scholar]
- Neina, D. The role of soil pH; in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 3, 5794869. [Google Scholar] [CrossRef]
- Ahmad, I.; Cheng, Z.; Meng, H.; Liu, T.; Wang, M.; Ejaz, M. Wasila H Effect of pepper-garlic intercropping system on soil microbial and bio-chemical properties. Pak. J. Bot. 2013, 45, 695–702. [Google Scholar]
- Makinde, E.A.; Oluwatoyinbo, F.I.; Ayoola, O.T. Intercropping and crop residue incorporation: Effects on soil nutrient status. J. Plant Nutr. 2006, 29, 235–244. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Jaillard, B. Rhizosphere: A new frontier for soil biogeochemistry. J. Geochem. Explor. 2006, 88, 210–213. [Google Scholar] [CrossRef]
- Hagen-Thorn, A.; Callesen, I.; Armolaitis, K.; Nihlgård, B. The impact of six European tree species on the chemistry of mineral topsoil in forest plantations on former agricultural land. For. Ecol. Manag. 2004, 195, 373–384. [Google Scholar]
- Zhu, Y.G.; He, Y.Q.; Smith, S.E.; Smith, F.A. Buckwheat (Fagopyrum esculentum Moench) has high capacity to take up phosphorus (P) from a calcium (Ca)-bound source. Plant Soil 2002, 239, 1–8. [Google Scholar] [CrossRef]
- Tang, X.; Zhong, R.; Jiang, J.; He, L.; Huang, Z.; Shi, G.; Wu, H.; Liu, J.; Xiong, F.; Han, Z.; et al. Cassava/peanut intercropping improves soil quality via rhizospheric microbes increased available nitrogen contents. BMC Biotechnol. 2020, 20, 13. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Shi, F.; Liu, Y.; Wang, F.; Dong, S.; Li, M. Composition and diversity of soil microbial community associated with land use types in the agro–pastoral area in the upper yellow river basin. Front. Plant Sci. 2022, 13, 819661. [Google Scholar] [CrossRef]
- Clivot, H.; Petitjean, C.; Marron, N.; Dalle, E.; Genestier, J.; Błaszczyk, N.; Santennoise, P.; Lafloptte, A.; Piutti, S. Early effects of temperate agroforestry practices on soil organic matter and microbial enzyme activity. PSE 2020, 453, 189–207. [Google Scholar] [CrossRef]
- Beule, L.; Karlovsky, P. Tree rows in temperate agroforestry croplands alter the composition of soil bacterial communities. PLoS ONE 2021, 16, e0246919. [Google Scholar] [CrossRef]
- Woźniak, M.; Grządziel, J.; Gałązka, A.; Frąc, M. Metagenomic analysis of bacterial and fungal community composition associated with Paulownia elongata × Paulownia fortunei. Bio Res. 2019, 14, 8511–8529. [Google Scholar] [CrossRef]
- Wang, D.; Han, P.; Ren, H.; Lin, W.; Chrn, J. Bacterial and fungal diversity in the rhizosphere of buckwheat under different mulching techniques. Curr. Sci. 2022, 123, 1365–1371. [Google Scholar] [CrossRef]
- Castro, H.F.; Classen, A.T.; Austin, E.E.; Norby, R.J.; Schadt, C.W. Soil microbial community responses to multiple experimental climate change drivers. AEM 2010, 76, 999–1007. [Google Scholar] [CrossRef]
- Gottel, N.R.; Castro, H.F.; Kerley, M.; Yang, Z.; Pelletier, D.A.; Potar, M.; Karpinets, T.; Uberbacher, E.D.; Tuskan, G.A.; Vilgalys, R.; et al. Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types. AEM 2011, 77, 5934–5944. [Google Scholar] [CrossRef]
- Zhang, K.; Bonito, G.; Hsu, C.H.M.; Hameed, K.; Vilgalys, R.; Laio, H.L. Mortierella elongata increases plant biomass among non-leguminous crop species. Agronomy 2020, 10, 754. [Google Scholar] [CrossRef]
- Eroshin, V.K.; Dedyukhina, E.G.; Chistyakova, T.I.; Zhelifonova, V.P.; Kurtzman, C.P.; Bothast, R.J. Arachidonic-acid production by species of Mortierella. World J. Microbiol. Biotechnol. 1996, 12, 91–96. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.H.; Huang, E.; Huang, C.C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Ham, S.H.; Yoon, A.R.; Oh, H.E.; Park, Y.G. Plant growth-promoting microorganism Pseudarthrobacter sp. NIBRBAC000502770 enhances the growth and flavonoid content of Geum aleppicum. Microorganisms 2022, 10, 1241. [Google Scholar] [CrossRef]
- Peng, Z.; Guo, X.; Xiang, Z.X.; Liu, D.; Yu, K.; Sun, K.; Yan, B.; Wang, S.; Kang, C.; Xu, Y.; et al. Maize intercropping enriches plant growth-promoting rhizobacteria and promotes both the growth and volatile oil concentration of Atractylodes lancea. Front. Plant Sci. 2022, 13, 1029722. [Google Scholar] [CrossRef]
- Lu, M.; Lu, Z.; Li, M.; Yang, J.; Fullen, M.; Li, Y.; Fan, M. Maize–soybean intercropping increases soil nutrient availability and aggregate stability. IJP 2023, 506, 441–456. [Google Scholar] [CrossRef]
- Cai, H.; Yu, N.; Liu, Y.; Wei, X.; Guo, C. Meta-analysis of fungal plant pathogen Fusarium oxysporum infection-related gene profiles using transcriptome datasets. Front. Microbiol. 2022, 13, 970477. [Google Scholar] [CrossRef]
- Beule, L.; Lehtasaar, E.; Corre, M.D.; Schmidt, M.; Veldkamp, E.; Karlovsky, P. Poplar rows in temperate agroforestry croplands promote bacteria, fungi, and denitrification genes in soils. Front. Microbiol. 2020, 10, 3108. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Jose, S. Soil Respiration and Microbial Biomass in a Pecan—Cotton Alley Cropping System in Southern USA. Agrofor. Syst. 2003, 58, 45–54. [Google Scholar] [CrossRef]
- Li, X.; Mu, Y.; Cheng, Y.; Liu, X.; Nian, H. Effects of intercropping sugarcane and soybean on growth, rhizosphere soil microbes, nitrogen and phosphorus availability. Acta Physiol. Plant. 2013, 35, 1113–1119. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, Z.Z.; Zhao, Q.; Huang, X.Y.; Fuang, K.F. Effect of continuous cropping on the rhizosphere soil and growth of common buckwheat. Plant Prod. Sci. 2019, 23, 81–90. [Google Scholar] [CrossRef]
- Wan, F.; Chen, P. Soil enzyme activities under agroforestry systems in northern Jiangsu Province. For. Stud. China 2004, 6, 21–26. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, B.X.; Xu, C.H.Y.; Raza, M.; Wang, Q.; Wang, Q.Z.; Fu, Y.N.; Hu, J.H.; Imoulan, H.; Hussain, M.; et al. Intercropping walnut and tea: Effects on soil nutrients, enzyme activity, and microbial communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef]
- Staunton, S.; Saby, N.P.; Arrouays, D.; Quiquampoix, H. Can soil properties and land use explain glomalin-related soil protein (GRSP) accumulation? A nationwide survey in France. Catena 2020, 193, 104620. [Google Scholar] [CrossRef]
- Wang, Q.; Hong, H.; Laio, R.; Yuan, B.; Li, H.; Lu, H.; Liu, J.; Yan, C.H. Glomalin-related soil protein: The particle aggregation mechanism and its insight into coastal environment improvement. Ecotoxicol. Environ. Saf. 2021, 227, 112940. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Yuan, J.C.; Hou, Y.T.; Li, T.; Liao, Y.C. Tempo-spatial dynamics of AMF under maize soybean intercropping. Chin. J. Eco-Agric. 2020, 28, 631–642. [Google Scholar] [CrossRef]
- Dang, K.; Gong, X.; Zhao, G.; Wang, H.; Ivanistau, A.; Feng, B. Intercropping Alters the Soil Microbial Diversity and Community to Facilitate Nitrogen Assimilation: A Potential Mechanism for Increasing Proso Millet Grain Yield. Front. Microbiol. 2020, 11, 601054. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; He, Q. The spatial and temporal effects of Paulownia intercropping: The case of northern China. Agrofor. Sys. 1997, 37, 91–109. [Google Scholar] [CrossRef]
- Chirko, C.P.; Gold, M.A.; Nguyen, P.V.; Jiang, J.P. Influence of direction and distance from trees on wheat yield and photosynthetic photon density (Q p) in a Paulownia and wheat intercropping system. For. Ecol. Manag. 1996, 83, 171–180. [Google Scholar]
- Li, F.; Meng, P.; Fu, D.; Wang, B. Light distribution, photosynthetic rate and yield in a Paulownia-wheat intercropping system in China. Agrofor. Sys. 2008, 74, 163–172. [Google Scholar] [CrossRef]
- Shukla, P.R.; Skeg, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. 2019. Available online: https://www.ipcc.ch/srccl/cite-report/ (accessed on 15 March 2022).

| Detailed Determination | 2019 | 2022 | ||
|---|---|---|---|---|
| AK | AP | AK | AP | |
| pH in KCl (soil pH) | 6.2 (slightly acidic) | 5.8 (slightly acidic) | 5.7 (slightly acidic) | 5.7 (slightly acidic) |
| Phosphorus P2O5 (mg 100 g−1 of soil) | 48.5 (very high) | 27.6 (very high) | 22.6 (very high) | 18.8 (high) |
| Potassium K2O (mg 100 g−1 of soil) | 33.5 (very high) | 33.5 (very high) | 28.9 (very high) | 24.1 (medium) |
| Magnesium Mg (mg 100 g−1 of soil) | 7.6 (very high) | 7.6 (very high) | 6.7 (medium) | 5.4 (medium) |
| N min. (kg ha−1) | 43.8 (very low) | 48.0 (very low) | 56.8 (low) | 21.4 (very low) |
| Month | Temperature (°C) | Rainfall (mm) | HTC (K) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | Mean 1990–2020 | 2019 | 2020 | 2021 | 2022 | Mean 1990–2020 | 2019 | 2020 | 2021 | 2022 | |
| IV | 10.8 | 9.5 | 7.5 | 6.4 | 9.6 | 24.2 | 6.4 | 32.5 | 32.7 | 32.8 | 0.7 | 0.2 | 1.4 | 1.5 |
| V | 12.1 | 11.6 | 12.4 | 12.7 | 14.3 | 76.8 | 77.2 | 59.0 | 20.9 | 58.9 | 2.1 | 2.1 | 1.5 | 0.4 |
| VI | 22.1 | 18.5 | 19.8 | 20.1 | 17.8 | 27.0 | 94.5 | 38.4 | 39.7 | 74.6 | 0.4 | 1.2 | 0.6 | 0.6 |
| VII | 19.3 | 19.5 | 20.5 | 20.7 | 19.7 | 44.5 | 53.2 | 41.1 | 132.5 | 86.6 | 0.7 | 1.3 | 0.6 | 2.1 |
| VIII | 20.3 | 22.5 | 17.3 | 17.7 | 19.2 | 59.8 | 6.2 | 11.0 | 92.5 | 63.6 | 0.9 | 0.1 | 0.2 | 1.4 |
| IX | 14.4 | 14.7 | 15.0 | 14.8 | 14.2 | 42.0 | 92.9 | 14.5 | 82.0 | 50.6 | 1.0 | 2.1 | 0.3 | 2.1 |
| X | 10.4 | 10.7 | 9.5 | 9.1 | 9.3 | 32.1 | 100.5 | 10.2 | 8.6 | 40.8 | 3.2 | 3.0 | 0.3 | 0.2 |
| Mean/sum (IV–X) | 15.6 | 15.3 | 14.6 | 14.5 | 14.5 | 306.4 | 430.9 | 206.7 | 408.9 | 377.0 | - | - | - | - |
| Year | Period | Samples | Total Bacterial Count (CFU g−1) | Total Fungal Count (CFU g−1) |
|---|---|---|---|---|
| 2021 | T1 | AK | 2.4 ± 0.05 × 104 c | 3.8 ± 0.05 × 104 abc |
| AP | 4.3 ± 0.05 × 104 c | 3.3 ± 0.08 × 104 abc | ||
| T2 | AK | 2.1 ± 0.17 × 106 ab | 1.6 ± 0.01 × 104 cd | |
| AP | 1.6 ± 0.18 × 106 b | 1.0 ± 0.02 × 104 d | ||
| 2022 | T1 | AK | 2.9 ± 0.45 × 106 ab | 1.8 ± 0.02 × 104 bcd |
| AP | 2.4 ± 0.51 × 106 ab | 1.7 ± 0.03 × 104 cd | ||
| T2 | AK | 3.1 ± 0.14 × 106 ab | 5.0 ± 0.02 × 104 a | |
| AP | 4.6 ± 0.27 × 106 a | 3.8 ± 0.02 × 104 ab |
| Year | Period | Samples | DHA (µg TPF g−1 h−1) | T-GRSP (µg g−1) |
|---|---|---|---|---|
| 2021 | T1 | AK | 3.8 ± 0.34 c | 1699.0 ± 0.53 a |
| AP | 7.9 ± 0.16 b | 1522.9 ± 1.82 a | ||
| T2 | AK | 2.9 ± 0.16 c | 1492.1 ± 2.10 a | |
| AP | 3.0 ± 0.14 c | 1533.2 ± 1.91 a | ||
| 2022 | T1 | AK | 7.7 ± 0.53 b | 1460.2 ± 1.13 ab |
| AP | 12.8 ± 0.40 a | 1471.6 ± 1.33 ab | ||
| T2 | AK | 2.0 ± 0.13 c | 1088.0 ± 0.89 c | |
| AP | 2.5 ± 0.08 c | 1233.2 ± 1.10 bc |
| Correlations (Microbiology) The Indicated Correlation Coefficients Are Significant with p < 0.05000, N = 40 | |||||
|---|---|---|---|---|---|
| Variable | SD | Bacteria (CFU g−1) | Fungi (CFU g−1) | DHA | T-GRSP |
| Bacteria (CFU g−1) | 1,763,107 | 1.00 | 0.09 | −0.19 | −0.54 |
| Fungi (CFU g−1) | 16,296 | 0.09 | 1.00 | −0.21 | −0.36 |
| DHA | 4 | −0.19 | −0.21 | 1.00 | 0.24 |
| GRSP | 212 | −0.53 | −0.36 | 0.24 | 1.00 |
| 2021 | 2022 | |||
|---|---|---|---|---|
| AK | AP | AK | AP | |
| Total number of colonies (sum) | 271 | 248 | 327 | 306 |
| Colletotrichum sp. | 0.4 (1 ± 0.22) c | – | – | – |
| Cylindrocarpon sp. | 1.1 (3 ± 0.16) c | – | – | – |
| Exserohilum pedicellatum | – | 1.6 (4 ± 0.21) c | 5.9 (18 ± 0.11) b | 9.5 (29 ± 0.12) b |
| Fusarium avenaceum | – | – | 6.4 (20 ± 0.14) b | 8.8 (27 ± 0.31) b |
| Fusarium culmorum | 10.0 (27 ± 0.11) b | 6.5 (169 ± 0.22) b | 4.9 (15 ± 0.09) bc | 3.9 (12 ± 0.16) c |
| Fusarium oxysporum | 66.0 (179 ± 0.16) a | 54.4 (135 ± 0.22) a | 65.7 (201 ± 0.07) a | 66.7 (204 ± 0.18) a |
| Fusarium solani | – | – | 0.7 (2 ± 0.19) c | – |
| Fusarium sporotrichoides | – | 0.4 (1 ± 0.22) c | 2.3 (7 ± 0.22) c | – |
| Mucor mucedo | 1.1 (3 ± 0.16) c | – | 0.7 (2 ± 0.19) c | – |
| Penicillium notatum | – | 12.9 (32 ± 0.10) b | – | – |
| Penicillium purpurogeum | 0.7 (2 ± 0.19) c | 0.8 (2 ± 0.19) | 0.7 (2 ± 0.19) c | 0.3 (1 ± 0.22) c |
| Penicillium vermiculatum | – | 0.4 (1 ± 0.22) | – | – |
| Penicillium sp. | 6.3 (17 ± 0.14) b | – | 7.2 (22 ± 0.16) b | 0.3 (1 ± 0.22) c |
| Phoma sp. | – | – | 0.3 (1 ± 0.22) c | – |
| Pythium sp. | – | – | – | 0.3 (1 ± 0.22) c |
| Rhizoctonia solani | – | – | 0.3 (1 ± 0.22) c | 0.7 (2 ± 0.19) c |
| Rhizopus nigricans | 0.4 (1 ± 0.22) c | – | 2.0 (6 ± 0.09) c | 2.6 (8 ± 0.16) c |
| Trichoderma hamatum | 1.8 (5 ± 0.22) c | 7.3 (18 ± 0.18) bc | – | 1.6 (5 ± 0.16) c |
| Trichoderma harzianum | 7.4 (20 ± 0.14) bc | 11.7 (29 ± 0.37) b | 0.7 (2 ± 0.19) c | 3.6 (11 ± 0.22) c |
| Trichoderma viride | 0.4 (1 ± 0.22) c | 0.4 (1 ± 0.22) c | 2.3 (7 ± 0.17) c | 1.6 (5 ± 0.16) c |
| Other fungi | 4.4 (12 ± 0.16) bc | 3.6 (9 ± 0.14) bc | – | – |
| Specification | Plant Height (cm) | Number of Branches on Main Shoot | Number of Branches | Number of Inflorescences | Number of Full Seeds | Total Seed Mass per Plant (g) | Buckwheat Yield (t ha−1) |
|---|---|---|---|---|---|---|---|
| Cultivation | |||||||
| AP | 46.7 ± 2.2 | 1.22 ± 0.2 | 2.99 ± 0.3 | 5.95 ± 0.5 | 22.5 ± 2.7 | 0.26 ± 0.03 | 0.65 ± 0.1 |
| AK | 47.2 ± 1.3 | 1.57 ± 0.1 | 3.59 ± 0.4 | 6.44 ± 0.5 | 21.8 ± 4.5 | 0.20 ± 0.05 | 0.51 ± 0.1 |
| LSD | ns | ns | ns | ns | ns | ns | ns |
| Years | |||||||
| 2021 | 47.3 ± 1.3 | 1.4 ± 0.1 | 2.4 ± 0.4 | 6.8 ± 0.5 | 15.5 ± 3.6 | 0.16 ± 0.04 | 0.39 ± 0.10 |
| 2022 | 47.1 ± 1.5 | 1.4 ± 0.1 | 4.2 ± 0.2 | 5.5 ± 0.3 | 28.8 ± 3.1 | 0.31 ± 0.04 | 0.77 ± 0.1 |
| LSD | ns | ns | 0.69 | ns | 9.1 | 0.100 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, M.; Liszewski, M.; Jama-Rodzeńska, A.; Gębarowska, E.; Siebielec, S.; Kaczmarek, A.; Gałka, B.; Zalewski, D.; Bąbelewski, P. Effect of Paulownia and Buckwheat Intercropping on Soil Microbial Biodiversity, Dehydrogenase Activity, and Glomalin-Related Soil Protein. Agronomy 2025, 15, 888. https://doi.org/10.3390/agronomy15040888
Woźniak M, Liszewski M, Jama-Rodzeńska A, Gębarowska E, Siebielec S, Kaczmarek A, Gałka B, Zalewski D, Bąbelewski P. Effect of Paulownia and Buckwheat Intercropping on Soil Microbial Biodiversity, Dehydrogenase Activity, and Glomalin-Related Soil Protein. Agronomy. 2025; 15(4):888. https://doi.org/10.3390/agronomy15040888
Chicago/Turabian StyleWoźniak, Małgorzata, Marek Liszewski, Anna Jama-Rodzeńska, Elżbieta Gębarowska, Sylwia Siebielec, Agata Kaczmarek, Bernard Gałka, Dariusz Zalewski, and Przemysław Bąbelewski. 2025. "Effect of Paulownia and Buckwheat Intercropping on Soil Microbial Biodiversity, Dehydrogenase Activity, and Glomalin-Related Soil Protein" Agronomy 15, no. 4: 888. https://doi.org/10.3390/agronomy15040888
APA StyleWoźniak, M., Liszewski, M., Jama-Rodzeńska, A., Gębarowska, E., Siebielec, S., Kaczmarek, A., Gałka, B., Zalewski, D., & Bąbelewski, P. (2025). Effect of Paulownia and Buckwheat Intercropping on Soil Microbial Biodiversity, Dehydrogenase Activity, and Glomalin-Related Soil Protein. Agronomy, 15(4), 888. https://doi.org/10.3390/agronomy15040888








